Abstract
Purpose
The present study was performed to determine the factor, either duration or the temperature of heat treatment, exerting maximal and significant influence on the composition and allergenicity of egg white (EW) proteins.
Methods
Raw EW and 4 kinds of heated EW (fried EW, boiled EW for 10 minutes, boiled EW for 30 minutes, and baked EW for 20 minutes at 170℃) were prepared, and subsequently protein extraction was carried out. The proteins were separated by SDS-PAGE, and then immunoglobulin E (IgE) immunoblots were performed with the sera of 7 egg-allergic patients. Furthermore, the antigenic activities of ovalbumin (OVA), ovomucoid (OM), and ovotransferrin (OT) in different EW samples were measured by inhibition enzyme-linked Immuno-sorbent assay (ELISA).
Results
In SDS-PAGE analysis, the intensity of the protein band at 45 kD (corresponding to OVA) decreased significantly in boiled EW (30 minutes) and baked EW, but no change was observed in the case of boiled EW for 10 minutes. In IgE immunoblots, the IgE response to 34-50 kD (OM and OVA) in boiled EW for 30 minutes decreased significantly, when compared with raw EW and other heated EWs. In inhibition ELISA, a significant decrease in the OVA antigenic activity was observed in boiled EW for 30 minutes amongst other heated EW samples. However, OM antigenic activity in all kinds of heated EW including boiled EW for 30 minutes did not reduce after heat treatment. The OT antigenic activity nearly disappeared in heated EWs except in the case of boiled EW for 10 minutes.
Conclusions
Amongst 4 kinds of heated EWs, the boiled EW for 30 minutes showed the most significant changes both in composition and reduction in allergenicity. Our results revealed that the duration of heat treatment had more influence on the composition and allergenicity of EW proteins than the temperature.
Keywords: Egg allergy, egg white, heat treatment, ovomucoid
INTRODUCTION
Hen eggs are one of the most frequent causes for adverse reactions to food1,2 affecting approximately 1.6% of children.3,4 Egg allergy has been shown to be the most common food allergy in children with atopic dermatitis.5,6
Allergic reactions are more frequently elicited by egg white (EW) proteins than egg yolk.7,8 EW contains 24 different types of glycoproteins. Ovomucoid (OM, Gal d1), ovalbumin (OVA, Gal d2), ovotransferrin (OT, Gal d3), and lysozyme (Gal d4), comprising 10, 54, 12, and 3.5% of total EW protein, respectively, have been identified as major allergens.9-11 OM is considered to be the dominant allergen in hen's EW.8,12-14 OM is resistant to heat and remains in the soluble form even after heating. In contrast, although OVA is the most abundant protein found in EW, it is sensitive to thermal denaturation, thus causing a resultant decrease in allergenicity.15
Heat treatment has been recognized as a simple way of reducing allergenicity.16 When a protein is denatured by heat, most of the original tertiary structure is lost, so that a number of sites recognized by antibodies on the native molecule are destroyed. There are many examples of heat induced reduction in allergenicity but not complete elimination. However, food allergy occurs when we usually eat heat-treated foods. Heat-denatured proteins can also present new antigenic sites, uncovered by the unfolding process or created by new chemical reactions with other molecules present in the food.17 Although it has been well known that the allergenicity of EW proteins is reduced to a great extent by heat treatment, it has not been studied about the factor which exerts more significant influence on the composition and allergenicity of EW proteins; the duration of heat treatment or the temperature of heat treatment.
In this study, we compared the composition and allergenicity of raw EW and several kinds of heat treated EW samples with different duration and temperature of heat treatment, and we sought to determine that amongst the duration of heat treatment and the temperature of heat treatment, which factor exerts more significant influence on the composition and allergenicity of EW proteins.
MATERIALS AND METHODS
Patient sera
We used the pooled sera from seven patients, who were allergic to EW. We diagnosed the egg allergy when the patients experienced evident aggravation of atopic dermatitis, urticaria and angioedema or anaphylaxis within 2 hours after the ingestion of EW, and having EW white-specific immunoglobulin E (IgE) (>0.7 kUA/L, immunoCAP; Pharmacia, Uppsala, Sweden). After informed consent was received, blood samples were obtained and all the serum samples were stored at -80℃ until use. This study was approved by the Samsung Medical Center Institutional Review Board.
Preparation of EW protein extracts
The time and temperature of boiling, frying and baking were determined considering the usual methods of cooking in daily life. We compared the allergenicity of EW boiled for 10 minutes and for 30 minutes to find whether or not prolonged boiling than usual could reduce the antigenic activity of EW. In addition, we compared the allergenicity of EW boiled at 100℃ and EW baked at 170℃ to know the effect of heat temperature.
Hen's eggs were purchased from a local grocery store, stored at 4℃ and used in this experiment within 2 days of purchase. Heated EW samples were prepared by frying the eggs in vegetable oil for 3 minutes without checking the temperature, boiling whole egg for 10 minutes and 30 minutes, baking EW at 170℃ for 20 minutes, respectively. After freeze drying, the preparations were milled into a powder by using a homogenizer. Raw EW was prepared by directly freeze-drying liquid EW before homogenization. Subsequently, the proteins were extracted from the raw EW and 4 EW samples prepared from different kinds of heating methods.
EW protein extract was prepared by adding 10 g of fresh EW to 90 mL of phosphate-buffered saline in sterile centrifuge tubes. The mixture was rotated for 90 minutes at 4℃, centrifuged at 9,000 rpm for 20 minutes, and then decanted and placed in sterile tubes. The supernatants were filter-sterilized through 0.45 µm filters (Millipore, Corringlwohill, Co. Cork, Ireland) and lyophilized. Protein concentrations were determined by using microplate reader (VersaMax) with a Bradford protein assay (Bio-Rad, catalog No. 5000006). All the extracts were stored at -80℃ until further use.
SDS-PAGE
The extracts and purified proteins were separated by SDS-PAGE according to Laemmli methods. Extracts and proteins were reduced by heating with 4×SDS-PAGE gel loading buffer (LDS sample Buffer, Invitrogen, catalog No. NP0007; mixed 1:10 with reducing agent, Invitrogen, catalog No. NP0004) and loaded into each well of the gel (4-12%Tris-Glycine gels, 1.0 mm×10 wells, Novex pre-cast gel; Invitrogen, catalog No. NC0321Box). Precision plus protein standards (Bio-Rad, product No. 161-0374) were used as molecular weight markers to estimate protein size. The separated proteins were transferred onto 0.45 µm pore-sized polyvinylidene fluoride (PVDF) membrane (Invitrogen, catalog No. IB4010-02) by means of the dry-blotting method using a iBlot® gel transfer device (Invitrogen Corporation, Carlsbad, CA, USA, catalog No. IB1001EU).
IgE immunoblots
Immunoblotting was carried out by electrophoretically transferring the samples subjected to SDS-PAGE to a supported PVDF membrane. Subsequently, IgE immunoblots were performed with the individual serum of 7 egg-allergic patients. Immunoblots were pre-incubated for 1 hour at room temperature with blocking reagent (phosphate buffered saline tween 20 [PBST] with 2% non-fat dry milk [NFDM]) and then incubated overnight with allergic and non-allergic sera (diluted to 1:10 v/v in blocking reagent). Unbound IgE was removed by performing repeated rinses with PBST. For detection of bound IgE, the membranes were incubated with biotin-labeled goat polyclonal IgG-anti-human IgE (KPL, Gaithersburg, MD, USA), followed by washing and then incubation in NeutrAvidin-HRP (Pierce Biotechnology, Rockford, IL, USA). Detection of bound IgE was achieved using enhanced chemiluminescence (ECL reagents [Amersham, Arlington Heights, IL, USA] or SuperSignal reagents [Pierce, Rockford, IL, USA]) with multiple exposure times per membrane to provide optimal signal to noise ratio on the X-ray films. Blotted membrane was then exposed to High performance chemiluminescence film (GE Healthcare Limited, Buckinghamshire, UK) and the film was then developed. As a control, one membrane was incubated with the secondary antibody and ECL detection, but without human serum to evaluate the specificity of the detection system. Immunoblots were compared with a reflective densitometer (Chemi documentation UVP [DDS-8000]) and Quantity One software (Bio-Rad).
Inhibition enzyme-linked Immuno-sorbent assay (ELISA)
We performed inhibition ELISA with the pooled sera of 7 egg-allergic patients. OVA (Sigma A-5503, purity approximatively 98%), OM (Sigma T-2011) and OT (Sigma C-7786 ) were used as the plate-coating antigen. The EW proteins were dissolved in deionized water (1 mg/mL for final conc.), aliquoted, and then frozen until required.
The coating buffer (0.1 M carbonate/bicarbonate, pH 9.1) containing OVA, OM or OT at a concentration of 50 µg/mL was introduced into each well of a plate (Nunc-Maxisorp NO FDF). The plate was covered with plate-sealing tape and was kept at 4℃ overnight. Preincubation of the sample or standard in the presence of pooled sera of egg allergic patients was utilized to optimize the competition. The sera were diluted in PBS, pH 7.4, containing 0.05% Tween 20 and 2% BSA (Sigma A-21536). One volume (55 µL) of antibody solution (anti-OVA, anti-OM, or anti-OT) was added to one volume (55 µL) (vol/vol) of the standard or unknown eggshell extracts that had been previously diluted in assay diluents. The pooled sera were used at a concentration of 1/100. The antibody-protein mixtures were kept in tubes at 37℃ for 1 hour. The optical density of each well was measured in a plate reader (VersaMax) at 595 nm.
RESULTS
SDS-PAGE
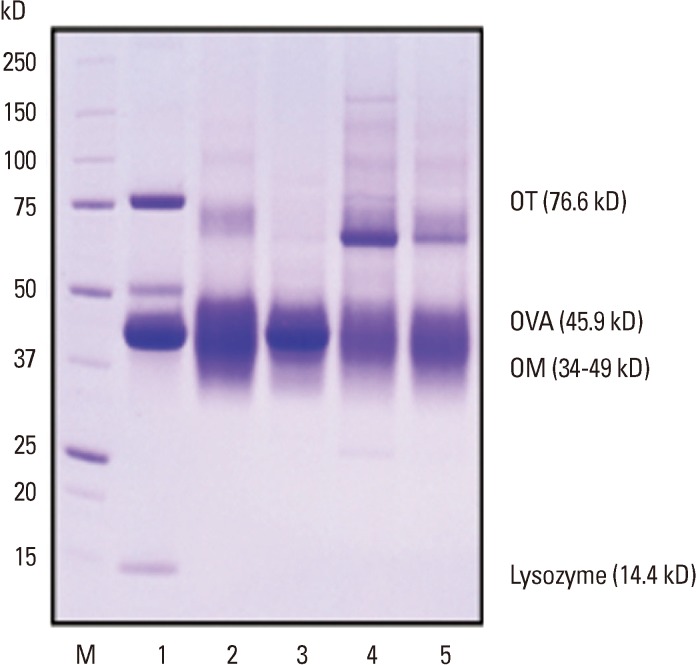
The gel electrophoretogram revealed each EW proteins had different changing patterns by heat treatment conditions (Fig. 1). Several dominant protein bands were observed at 37-50 kD (overlapped bands of OVA and OM), 14 kD (lysozyme), and 76 kD (OT) in raw EW (Fig. 1, lane 1). Based on a previous report,18 it was confirmed that the strong band around 45 kD in raw EW corresponded to OVA. Overall, protein bands became weaker and broader by heat treatment and the pattern of protein bands changed according to the heating time (Fig. 1, lane 3, 4). The protein band at 37-50 kD became broader by heating and it was weakest in boiled EW for 30 minutes. The intensity of the protein band at 45 kD decreased significantly in boiled EW (30 minutes) and baked EW, but not in boiled EW for 10 minutes. The bands corresponding to lysozyme completely disappeared by heating, which signifies that lysozyme is very heat-labile and gets degraded by heat treatment. The OT (75 kD) became weak by all kinds of heat treatment, and nearly disappeared in boiled EW for 10 minutes, as can be seen from the gel electrophoretogram. Several new weak protein bands were seen above 100 kD after heat treatment, whereas they were not seen in boiled EW for 10 minutes and got stronger with an increase in time of heating. The strongest band was observed in boiled EW for 30 minutes.
Fig. 1.
SDS-PAGE analysis of raw and heated EWs. Lane 1: raw egg white (EW), lane 2: fried EW, lane 3: boiled EW for 10 min, lane 4: boiled EW for 30 min, lane 5: baked EW for 20 min at 170℃. OT, ovotransferrin; OVA, ovalbumin; OM, ovomucoid.
IgE immunoblots
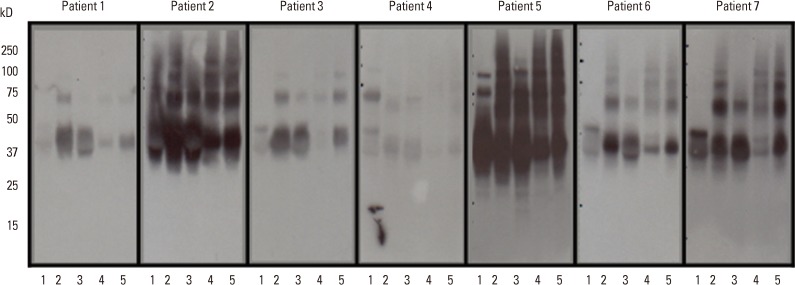
The IgE immunoblots against EW samples of 7 egg-allergic patients exposed to different heating conditions are shown in Fig. 2. Based on the results of SDS-PAGE, it is difficult to assess the separate profiles of OVA and OM because of not only diffused shape of OM, but also because of similar overlapping of the mobile phases of the two respective proteins in the gel. Consequently, we evaluated the IgE reactivity against OM and OVA, as one thing without differentiating the two. The IgE response to heated EW samples in all the patients showed similar patterns except for minor individual variations in the level of IgE responses. The IgE binding intensity to 37-50 kD (overlapped band of OM and OVA) of boiled EW for 30 minutes was weakest when compared with raw EW and other heated EWs in all the patients. In most of the patients, except in the case of patient '5', the IgE binding activity to 37-50 kD of fried EW and boiled EW for 10 minutes was even more intense than that of raw EW. In most of the patients (patient 1, 2, 3, 6, 7), an increase in IgE reactivity to 37-50 kD of baked EW was observed when compared with raw EW. The IgE responses against the proteins around 75 kD and above 100 kD increased by heat treatment irrespective of the level and type of heat treatment in most of the patients. None of the patients exhibited IgE reactivity against lysozyme regardless of the nature of heat treatment.
Fig. 2.
IgE Immunoblots against egg white (EW) proteins extracted from raw EW and heated EWs with the sera of 7 egg-allergic patients. The IgE response to 34-50 kD (overlapped band of ovomucoid and ovalbumin of boiled EW (30 min) decreased significantly, compared with raw EW and other heated EWs. Lane 1: raw EW, lane 2: fried EW, lane 3: boiled EW for 10 min, lane 4: boiled EW for 30 min, lane 5: baked EW at 170℃ for 20 min.
Inhibition ELISA
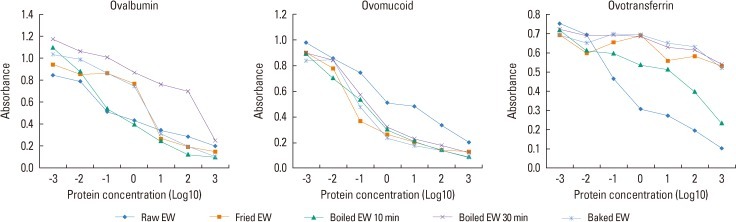
In inhibition ELISA, we could confirm the individual antigenic activities of 3 major EW allergens, especially differentiating the reactivity against OM from the reactivity against OVA. A decrease in the OVA antigenic activity was observed in all the heated EW samples except in the case of boiled EW for 10 minutes by heating, also a major decrease was observed in boiled EW (30 minutes). In boiled EW for 10 minutes, OVA antigenic activity did not decrease when compared with raw EW. On the other hand, no reduction in the OM antigenic activity in all kinds of heated EW including boiled EW for 30 minutes was observed. On the contrary, an increase in antigenic activity was observed after heat treatment. The OT antigenic activity nearly disappeared in heated EWs except in boiled EW for 10 minutes (Fig. 3).
Fig. 3.
Enzyme-linked Immuno-sorbent assay inhibition analysis for detecting changes in the antigenic activity of ovalbumin (OVA), ovomucoid (OM) and ovotransferrin in raw, fried, boiled (10 and 30 min), and baked EW samples using pooled sera of 7 egg-allergic patients. OVA antigenic activity mostly decreased in boiled EW (30 min) amongst the other heated EW samples. Nevertheless, no reduction in OM antigenic activity in all kinds of heated EW samples including boiled EW (30 min) was observed after heat treatment.
DISCUSSION
In the present investigation, we evaluated as how the composition and allergenicity of EW allergens are altered by the degree of heat processing and the cooking methods. And we confirmed that each EW protein had different changing patterns depending on the nature of heat treatment condition. Among 4 kinds of heated EWs, the boiled EW for 30 minutes exhibited the most significant changes in composition and reduction in allergenicity. A decrease in the IgE reactivity against OVA was observed in boiled EW for 30 minutes, but not in EW baked at 170℃ for 20 minutes and in EW boiled for 10 minutes. Heat treatment did not reduce the antigenic activity of OM regardless of the duration and temperature of the heat treatment. Although no reduction in IgE reactivity against OM was observed by boiling for 30 minutes, our results showed that the duration of heat treatment had more influence on the composition and allergenicity of EW proteins than heat temperature.
Generally, heating decreases protein allergenicity by destroying conformational epitopes. Heat denaturation of globular proteins may disrupt their tertiary structure and lead to random-coiled aggregation and insolubility. There are some reports, which suggest that the changes in the antigenicity of food proteins caused by heating can be different according to the duration of heating.16,19,20 There was a report showing that OVA denatured by heating at 80℃ for 10 minutes had only little reduction in allergenicity.16 Similarly, in the present study, no reduction in the allergenicity of OVA in boiled EW for 10 minutes was observed. On the contrary, a study has demonstrated reduction in IgE binding with OVA and OM by heating for 15 minutes at 95℃.20 In a previous study, EW proteins boiled for 5 or 60 minutes showed a decreased allergenicity, especially after prolonged boiling for 60 minutes when compared with unheated EW proteins.19 We found that the allergenicity of EW proteins other than OM could be minimized after intensive heat denaturation for 30 minutes.
Many studies have revealed that OM was stable to digestion and heat, and heated eggs can cause allergic reactions in OM allergic patients.13 Our study revealed that the antigenic activity of OM did not decrease after heating regardless of the duration and temperature of heating, and the results were similar to the previous studies. In ELISA inhibition, OM reactivity of boiled EW for 30 minutes did not decrease, showing that OM is even resistant to prolonged heating. The OVA has been known to be heat labile and easily lose its allergenicity by heating. However, in our study the allergenicity of OVA did not decrease after boiling for 10 minutes, and only a little decrease was seen after frying and baking for 20 minutes, showing that the allergenicity of OVA cannot be reduced by short-time and light heating.
The majority of milk and egg-allergic children can tolerate extensively heated milk and eggs.21,22 Nowak-Wegrzyn et al.21 showed that the majority of children with milk allergy tolerate heated milk, and subjects ingesting heated milk products had significantly smaller skin prick test wheals and higher casein-IgG4 when compared with baseline. Similar result was obtained in children with egg allergy. One recent study reported that the majority of 117 subjects with egg allergy were tolerant to heated egg, 64 tolerated extensively heated egg products (muffin baked at 350°F for 30 minutes or waffle baked at 500°F for 3 minutes), on the other hand only 23 patients tolerated regular egg (scrambled egg or French toast).22 Reaction to raw or lightly cooked egg, but tolerance to more extensively cooked egg-containing foods is a common finding.23,24
Heat treatment might also potentially increase protein allergenicity by formation of neoepitopes. In this study, the IgE binding activities to 34-50 kD of fried EW and boiled EW for 10 minutes were more intense than that of raw EW in most of the patients, demonstrating that insufficient heat treatment might increase the allergenicity of some EW allergen. Such an observation can be attributed to the presence of newly formed epitopes due to incomplete heating. Similarly, the IgE-binding capacity of soy 2S-globulin has been reported to be strengthened by heating.25 Moreover, roasting at high temperature can enhance allergenicity of peanut as a result of glycation.
Hen's egg is mostly ingested in a heated state, and certain EW allergens are especially labile to extensive prolonged heat treatment. It has been reported that 50-70% of children with egg allergy tolerated heated egg ingestion.13,22,24,26 Continued ingestion of heated egg was associated with decreased skin test reactivity and OVA-specific IgE levels and increased OVA- and OM-specific IgG4 levels.22 Continued ingestion of heated egg products would be better for the development of tolerance than strict egg restriction. It can also prove useful in practical terms for participation of the child in social activities.
Previously, it had been thought that the majority of children with egg allergy become egg tolerant by school age.2,27 However, a recent study in U.S.A. has suggested that egg allergy persists well into the adolescent years for many children with egg allergy.28 If egg allergy persists beyond the infancy, although the majority is not life-threatening, egg allergy can exert a bad influence on the quality of life of the patients who attend the day care center or a school due to the difficulty of exclusion of egg from the diet. Accordingly, the demand for oral immunotherapy might increase in the treatment of egg allergy. Introduction of extensively heated milk and egg proteins into the diet of allergic children may represent an alternative approach to oral tolerance induction. The result of our study demonstrated that prolonged boiling of eggs for 30 minutes most significantly reduced the allergenicity of EW allergen. The egg products with reduced allergenicity by prolonged heat treatment for 30 minutes can be used for oral immunotherapy of egg allergy. In the future, we are going to try oral immunotherapy with the egg products heated for more than 30 minutes in children with egg allergy.
In conclusion, the result of our study showed that boiling the eggs for 30 minutes most significantly reduced the allergenicity of EW amongst 4 kinds of heated EW proteins with different temperature and duration, indicating that the duration of heat treatment had more influence on the composition and allergenicity of EW proteins than the heat temperature.
Footnotes
There are no financial or other issues that might lead to conflict of interest.
References
- 1.Bock SA, Atkins FM. Patterns of food hypersensitivity during sixteen years of double-blind, placebo-controlled food challenges. J Pediatr. 1990;117:561–567. doi: 10.1016/s0022-3476(05)80689-4. [DOI] [PubMed] [Google Scholar]
- 2.Boyano-Martínez T, García-Ara C, Díaz-Pena JM, Martín-Esteban M. Prediction of tolerance on the basis of quantification of egg white-specific IgE antibodies in children with egg allergy. J Allergy Clin Immunol. 2002;110:304–309. doi: 10.1067/mai.2002.126081. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Sampson HA. 9. Food allergy. J Allergy Clin Immunol. 2006;117:S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 4.Eggesbø M, Botten G, Halvorsen R, Magnus P. The prevalence of allergy to egg: a population-based study in young children. Allergy. 2001;56:403–411. doi: 10.1034/j.1398-9995.2001.056005403.x. [DOI] [PubMed] [Google Scholar]
- 5.Sampson HA, McCaskill CC. Food hypersensitivity and atopic dermatitis: evaluation of 113 patients. J Pediatr. 1985;107:669–675. doi: 10.1016/s0022-3476(85)80390-5. [DOI] [PubMed] [Google Scholar]
- 6.Niggemann B, Sielaff B, Beyer K, Binder C, Wahn U. Outcome of double-blind, placebo-controlled food challenge tests in 107 children with atopic dermatitis. Clin Exp Allergy. 1999;29:91–96. doi: 10.1046/j.1365-2222.1999.00454.x. [DOI] [PubMed] [Google Scholar]
- 7.Anet J, Back JF, Baker RS, Barnett D, Burley RW, Howden ME. Allergens in the white and yolk of hen's egg. A study of IgE binding by egg proteins. Int Arch Allergy Appl Immunol. 1985;77:364–371. doi: 10.1159/000233846. [DOI] [PubMed] [Google Scholar]
- 8.Sampson HA, Ho DG. Relationship between food-specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444–451. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 9.Langeland T. A clinical and immunological study of allergy to hen's egg white. III. Allergens in hen's egg white studied by crossed radio-immunoelectrophoresis (CRIE) Allergy. 1982;37:521–530. doi: 10.1111/j.1398-9995.1982.tb02335.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoffman DR. Immunochemical identification of the allergens in egg white. J Allergy Clin Immunol. 1983;71:481–486. doi: 10.1016/0091-6749(83)90465-7. [DOI] [PubMed] [Google Scholar]
- 11.Holen E, Elsayed S. Characterization of four major allergens of hen egg-white by IEF/SDS-PAGE combined with electrophoretic transfer and IgE-immunoautoradiography. Int Arch Allergy Appl Immunol. 1990;91:136–141. doi: 10.1159/000235104. [DOI] [PubMed] [Google Scholar]
- 12.Bernhisel-Broadbent J, Dintzis HM, Dintzis RZ, Sampson HA. Allergenicity and antigenicity of chicken egg ovomucoid (Gal d III) compared with ovalbumin (Gal d I) in children with egg allergy and in mice. J Allergy Clin Immunol. 1994;93:1047–1059. doi: 10.1016/s0091-6749(94)70054-0. [DOI] [PubMed] [Google Scholar]
- 13.Urisu A, Ando H, Morita Y, Wada E, Yasaki T, Yamada K, Komada K, Torii S, Goto M, Wakamatsu T. Allergenic activity of heated and ovomucoid-depleted egg white. J Allergy Clin Immunol. 1997;100:171–176. doi: 10.1016/s0091-6749(97)70220-3. [DOI] [PubMed] [Google Scholar]
- 14.Cooke SK, Sampson HA. Allergenic properties of ovomucoid in man. J Immunol. 1997;159:2026–2032. [PubMed] [Google Scholar]
- 15.Joo K, Kato Y. Assessment of allergenic activity of a heat-coagulated ovalbumin after in vivo digestion. Biosci Biotechnol Biochem. 2006;70:591–597. doi: 10.1271/bbb.70.591. [DOI] [PubMed] [Google Scholar]
- 16.Coombs RR, McLaughlan P. Allergenicity of food proteins and its possible modification. Ann Allergy. 1984;53:592–596. [PubMed] [Google Scholar]
- 17.Davis PJ, Williams SC. Protein modification by thermal processing. Allergy. 1998;53:102–105. doi: 10.1111/j.1398-9995.1998.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 18.Desert C, Guérin-Dubiard C, Nau F, Jan G, Val F, Mallard J. Comparison of different electrophoretic separations of hen egg white proteins. J Agric Food Chem. 2001;49:4553–4561. doi: 10.1021/jf001423n. [DOI] [PubMed] [Google Scholar]
- 19.Peng HJ, Chang ZN, Tsai LC, Su SN, Shen HD, Chang CH. Heat denaturation of egg-white proteins abrogates the induction of oral tolerance of specific Th2 immune responses in mice. Scand J Immunol. 1998;48:491–496. doi: 10.1046/j.1365-3083.1998.00432.x. [DOI] [PubMed] [Google Scholar]
- 20.Mine Y, Zhang JW. Comparative studies on antigenicity and allergenicity of native and denatured egg white proteins. J Agric Food Chem. 2002;50:2679–2683. doi: 10.1021/jf0112264. [DOI] [PubMed] [Google Scholar]
- 21.Nowak-Wegrzyn A, Bloom KA, Sicherer SH, Shreffler WG, Noone S, Wanich N, Sampson HA. Tolerance to extensively heated milk in children with cow's milk allergy. J Allergy Clin Immunol. 2008;122:342.e1–347.e2. doi: 10.1016/j.jaci.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 22.Lemon-Mulé H, Sampson HA, Sicherer SH, Shreffler WG, Noone S, Nowak-Wegrzyn A. Immunologic changes in children with egg allergy ingesting extensively heated egg. J Allergy Clin Immunol. 2008;122:977–983.e1. doi: 10.1016/j.jaci.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Kemp AS. Egg allergy. Pediatr Allergy Immunol. 2007;18:696–702. doi: 10.1111/j.1399-3038.2007.00679.x. [DOI] [PubMed] [Google Scholar]
- 24.Eigenmann PA. Anaphylactic reactions to raw eggs after negative challenges with cooked eggs. J Allergy Clin Immunol. 2000;105:587–588. doi: 10.1067/mai.2000.104255. [DOI] [PubMed] [Google Scholar]
- 25.Burks AW, Jr, Brooks JR, Sampson HA. Allergenicity of major component proteins of soybean determined by enzyme-linked immunosorbent assay (ELISA) and immunoblotting in children with atopic dermatitis and positive soy challenges. J Allergy Clin Immunol. 1988;81:1135–1142. doi: 10.1016/0091-6749(88)90881-0. [DOI] [PubMed] [Google Scholar]
- 26.Des Roches A, Nguyen M, Paradis L, Primeau MN, Singer S. Tolerance to cooked egg in an egg allergic population. Allergy. 2006;61:900–901. doi: 10.1111/j.1398-9995.2006.01134.x. [DOI] [PubMed] [Google Scholar]
- 27.Wood RA. The natural history of food allergy. Pediatrics. 2003;111:1631–1637. [PubMed] [Google Scholar]
- 28.Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. J Allergy Clin Immunol. 2007;120:1413–1417. doi: 10.1016/j.jaci.2007.09.040. [DOI] [PubMed] [Google Scholar]