Abstract
Objective:
Estimation of baseline bone mineral density (BMD) at the time of instituting androgen deprivation therapy (ADT) for metastatic prostate cancer is recommended by several specialty groups and expert panels. The present study was carried out to analyze the practice pattern of Indian urologists with regard to bone densitometric assessment and management of fracture risk in men of prostate cancer on ADT, and their degree of adherence to currently available guidelines
Materials and Methods:
Telephonic interviews of 108 qualified urologists, randomly selected from the member database of Urological Society of India was carried out with a predefined questionnaire. The responses were analyzed and compared with the available evidences and recommendations.
Results:
Only 19.4% urologists routinely perform a baseline BMD before starting ADT. Although majority of them prescribe calcium and vitamin D supplementation, only few tell regarding fracture risk and life-style modification to their patients. While 59.6% of the respondents use Zoledronic acid (ZA) in their patients on ADT, half of them prescribe it without knowing the BMD status, which may lead to overuse of ZA.
Conclusion:
Majority of the urologists in India do not follow the guidelines for BMD measurement in prostate cancer. A baseline BMD may help in reducing the unnecessary use of ZA.
Keywords: Androgen deprivation therapy, bone mineral density, osteoporosis, prostate cancer, zoledronic acid
INTRODUCTION
Skeletal complications are a major cause of morbidity and mortality in men with prostate cancer (CaP). Several factors contribute to the adverse skeletal health in these patients, e.g. age related osteoporosis, accelerated bone turnover associated with the disease, metastatic bone lesions and androgen deprivation therapy (ADT) employed for CaP.[1] ADT is associated with progressive decline in bone mineral density (BMD) and an increased risk of fractures.[2–5] Skeletal fractures have been correlated with reduced overall survival in men with prostate cancer.[6] With earlier detection of prostate cancer and increased longevity of the patients, prevention and management of their skeletal related complications has become more important. Screening for the presence of osteoporosis at the time of starting long-term ADT is of paramount importance. Life style modification, supplementation of calcium and vitamin D, and bone-directed pharmacotherapy are vital for optimal skeletal care in this group of patients. Recommendations from several specialty groups and expert panels are available for prevention and treatment of ADT-associated-osteoporosis.[7–14] All of them recommend baseline BMD estimation before starting long-term ADT. Further treatment strategy and monitoring schedule of the patients is modified as per the baseline BMD values.[7] The present study was carried out to analyze the practice pattern of Indian urologists with regard to bone densitometric assessment and management of fracture risk in men of prostate cancer on androgen deprivation therapy, and their degree of adherence to currently available guidelines.
MATERIALS AND METHODS
The study was conducted after approval from our institutional ethics committee. The survey was designed to address physicians’ practice regarding: (a) monitoring of BMD in men starting ADT; (b) counselling of men regarding bone-related adverse effects of ADT; and (c) prevention and management of osteoporosis.
One hundred and thirty qualified urologists were randomly selected from the updated member database of Urological Society of India (USI) in September 2011. Telephonic interview was carried out by one author (M.R.P) with a predefined questionnaire (Appendix). The questionnaire was developed by us and was pilot tested in the Department of Urology and Renal Transplantation, SGPGIMS and was modified as per the suggestions. The questions focused on the different aspects of assessment, prevention and management of osteoporosis in men with CaP on ADT. Data regarding physician practice characteristics (place of practice, type of practice and number of years in practice) were also collected. Place of practice was classified into two categories – major cities (Delhi, Mumbai, Kolkata, Chennai and Bangalore) and non-major cities. Type of practice was categorized as academic (those working in institutions or hospitals imparting postgraduate degree in urology) and non-academic. Years in practice was classified as ≤5 years or >5 years of practice after the urology degree. The responses were recorded in a datasheet anonymously, and were analyzed and compared with the available evidences including the recommendations and guidelines pertaining to maintenance of bone health in men with CaP on ADT.
Descriptive statistics was used for data analysis and presentation of results using SPSS 17.0 for Windows statistical software (SPSS Inc., Chicago, Ill). For exploratory analyses, we used chi-square test to examine the impact of physician practice characteristics on their practice pattern. A P-value of less than 0.05 was considered statistically significant.
RESULTS
Out of 130 urologists contacted, 114 (87.6%) responded to the questionnaire. Six of them were no longer or very infrequently seeing cancer prostate patients and so were excluded from the study. The questionnaire was administered to rest 108 urologists. One fifth (24/108, 22.2%) of them practiced in major Indian cities and 40% (43/108) of practitioners resorted to academic centers. They constituted about 6% of the total number of practicing urologists. Using a 95% confidence interval, this response rate resulted in a 9.1% margin of error in estimating the practice of >1800 practicing urologists in India.
Only 19.4% (21/108) of urologists routinely consider the state of their patients’ bone mineralization and order baseline BMD estimation before starting ADT. Another 11% (12/108) of urologists advice baseline BMD in less than 50% of their patients starting ADT. Financial status of the patient and presence of skeletal metastasis determines their advice for baseline BMD. However, majority of the interviewed urologists (75/108, 69.6%) never order a baseline BMD prior to starting ADT. All the respondents measuring baseline BMD, use Zoledronic acid (ZA) in their patients when indicated; but 42.6% of urologists not doing BMD estimation also regularly use ZA in their patients on ADT [Figure 1]. Dual-energy X-ray absorptiometry (DEXA) remains the most commonly adopted method (95.3%) for BMD measurement. Sixty seven percent of the respondents doing baseline BMD estimation modify their treatment schedule according to the test result, i.e. T-score. BMD measurement in follow-up is done by only 24% of the urologists advising baseline BMD estimation.
Figure 1.
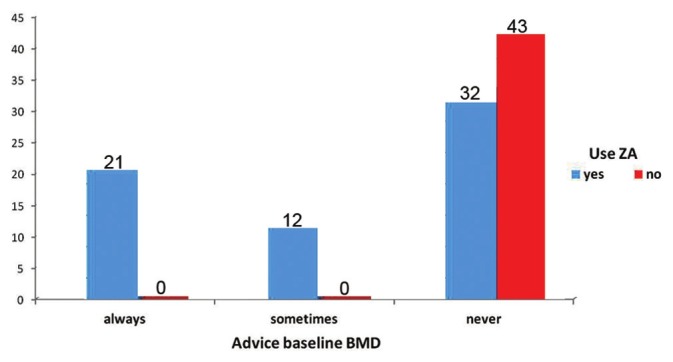
Physicians’ practice of baseline BMD estimation
Only 50% of the respondents routinely warn their patients of osteoporosis and adverse skeletal events at the time of starting ADT. Therapeutic modalities adopted by the urologists for prevention and treatment of osteoporosis in men on ADT are presented in Table 1. While most urologists give calcium and vitamin D supplementation, only few of them advice for life-style modification, e.g. regular weight-bearing exercises and avoiding use of tobacco and excessive alcohol intake. Majority (65/108, 59.6%) of the interviewed urologists frequently use ZA in their clinical practice. Of them, only one third urologists (21/65, 32.3%) routinely perform a baseline BMD and 18.4% (12/65) urologists do it in less than 50% of their patients. About half of them (32/65, 49.2%) prescribe ZA to men on ADT, without knowing their BMD status [Figure 2]. Monthly intravenous infusion of 4 mg of ZA for six months remains the most commonly adopted (40/65, 61.5%) treatment schedule.
Table 1.
Therapies used for prevention and treatment of osteoporosis in men on ADT
Figure 2.
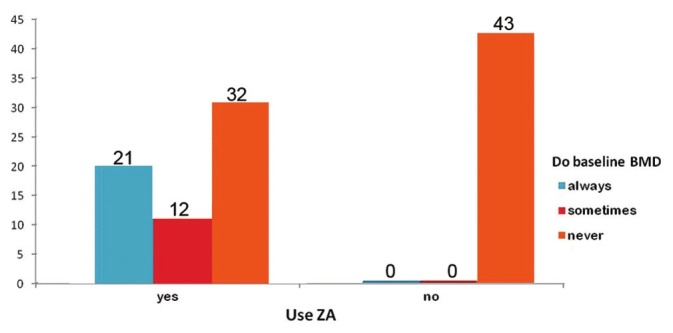
Physicians’ practice of bisphosphonate use in men on ADT
DISCUSSION
With better understanding of the disease, the survival of CaP patients has increased significantly over the last few decades due to early detection and improved therapy. Improved socioeconomic status and better access to health care has also played a major role in countries like India. Therefore, better care of the bone in such patients, has become increasingly important. Skeletal related events (SRE) tremendously affect the quality of life and fractures have been shown to increase mortality in men.[6] So, all practicing physicians should be aware of the need of good skeletal care in patients of CaP and should update themselves periodically with the available scientific evidences for better patient care. Various guidelines and recommendations proposed by different expert panels and specialty societies are very much helpful in this regard, as they are based on solid scientific background and meticulous scrutiny of the available evidences.
ADT is associated with significant decrease in BMD and an increased risk of fractures.[5,15,2–4,16] Baseline and periodical follow-up BMD measurement is recommended in patients on ADT.[7–14] In a study done on Indian patients, Agarwal et al. found significant loss of BMD after orchidectomy (13% at 6 months and 18% at 12 months), resulting in an increased incidence of osteoporosis from 24% to 48% at 6 months after orchidectomy.[17] Treatment and monitoring schedule of osteoporosis should be modified according to the T-score in the baseline BMD.[7] An expert panel comprising of urologists, medical oncologists, radiation oncologists, and endocrinologists have recommended for periodic clinical review and serial BMD measurements every 12-24 months in patients with normal BMD and every 6-12 months in patients with osteopenia.[12] They recommend use of ZA in patients with osteoporosis and in those with history of bone fracture (irrespective of BMD values), followed by yearly review with BMD estimation. DEXA-scan remains the gold standard for BMD estimation.[9] The compliance of medical practitioners for baseline BMD estimation at the start of ADT has been shown to be 5-36% in various studies.[18–21] In our study, only 19.4% of respondents advice it routinely. Though most of them (67%) would modify the treatment schedule as per the BMD report, only few (24%) of them would advice for follow-up BMD. In view of existence of several guidelines and expert panel recommends for baseline BMD measurement, the compliance for BMD estimation was expected to be more among clinicians than the previous reported studies. Multiple factors could be responsible for the discrepancy. First, they might not be aware of the severity and consequences of the treatment-related bone loss compared to the other adverse effects of ADT. Second, many of them might be unaware of the guidelines and expert panel recommends for baseline and follow-up BMD measurement. Unavailability of DEXA scan at local places may be another responsible factor, though only 17.6% of the urologists in our study, practised in places with no facility for DEXA scan within reasonable distance.
Before starting long-term ADT, all probable adverse effects including risk of osteoporosis and fractures should be discussed with the patient. It is done routinely by 50% of the urologists in our study, though previous studies have reported it between 15% to 38.5%.[21,22] It could be due to increased awareness among the doctors regarding the need of patient education for better treatment compliance. Guidelines on prostate cancer recommend encouraging the patients to adopt lifestyle changes, for e.g. increased physical activity, cessation of smoking, decreased alcohol consumption and normalisation of their body mass index (BMI).[7,8] National Comprehensive Cancer Network (NCCN) guidelines on prostate cancer endorses the guidelines of National osteoporosis foundation (NOF) and recommends supplemental calcium (1200 mg daily) and vitamin D3 (800-1000 IU daily) for all men over age 50 years.[8] Though most of the urologists (90.7%) in our study advice calcium and vitamin D supplementation to the patients, only 37.9% advice for life-style modification. In previous similar studies, physicians have been reported to advice calcium and vitamin D supplementation in 8.7-64% of patients and lifestyle modification in 11% of patients on ADT.[18–21] However, it is to be remembered that, though these two agents are an integral part of skeletal-care programme, they have not been shown to be potent enough for the preservation of BMD during ADT.[23]
Another way to circumvent the skeletal side effects of ADT is monotherapy with nonsteroidal antiandrogens like Bicalutamide. It has the advantage of maintenance of normal serum testosterone level and preservation of BMD. However, it is not very popular and as per the NCCN guidelines “androgen monotherapy appears to be less effective than medical or surgical castration and should not be recommended. The side effects are different but overall less tolerable.”[8]
Bisphosphonates have been shown to protect against ADT-related bone loss in both metastatic and non-metastatic prostate cancer.[24–26] ZA is the most potent drug among the bisphosphonates.[27] Though major volume of the work concerning ZA has been done in metastatic hormone refractory prostate cancer (HRPC), it has also been found beneficial in hormone sensitive and non-metastatic CaP.[6,7,13,14,28–30] From the available evidences, we infer that, the use of ZA in patients of CaP should primarily be based on the BMD and fracture risk of the patient, with modification of the dose as per the stage of the disease and hormonal status. The WHO absolute fracture risk model (FRAX®; www.NOF.org and www.shef.ac.uk/FRAX) is an important adjunct in decision making in such patients.[8] ZA should be administered as intravenous infusion under medical supervision. The drug concentrate (4 mg) should be diluted in 100 ml of 0.9% Sodium chloride or 5% Dextrose solution and should be infused over a period of 15 minutes. Patients should be adequately hydrated prior to administration of ZA. Serum levels of calcium, phosphate, magnesium and creatinine should be carefully monitored before and following ZA therapy. In case of hypocalcemia, hypophosphatemia or hypomagnesemia, short-term supplemental therapy may be necessary. Due to its nephrotoxic effects, it should be used with caution with other nephrotoxic drugs and the dose should be modified in patients with renal impairment (creatinine clearance < 60 ml/min).[31] It should not be used in patients with serum creatinine of more than 3 mg/dl. Common adverse events associated with ZA use include fever, flu-like symptoms (e.g. fever, chills, bone pain, arthralgia, myalgia), anemia, neutropenia, nausea, vomiting, constipation, hypotension, atrial fibrillation, hypertension, blurred vision, uveitis, scleritis, headache, dizziness, dyspnoea, bronchoconstriction, hypomagnesemia, hypophosphatemia and hypocalcemia. However, the most dreaded complications include osteonecrosis of the jaw (ONJ), atypical subtrochanteric and diaphyseal femoral fractures and renal impairment.[31] Cancer patients should maintain good oral hygiene and should have a dental examination prior to treatment with ZA. While on treatment, these patients should avoid invasive dental procedures if possible. For patients who develop ONJ while on bisphosphonate therapy, dental surgery may exacerbate the condition.[31]
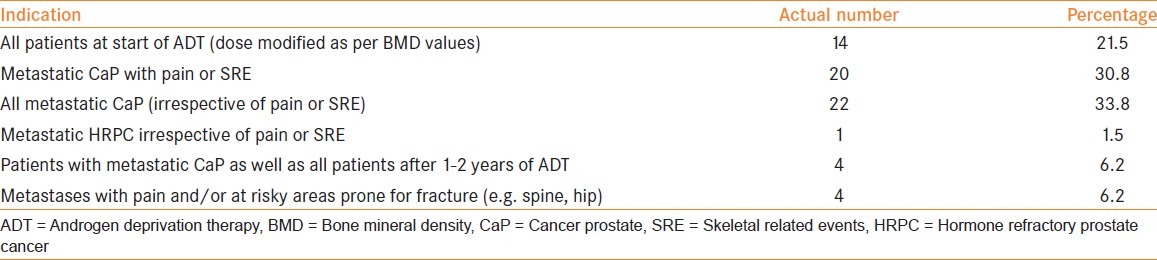
Different studies have reported the urologists and radiation oncologists to use bisphosphonates in 5-21% of their patients on ADT.[18–20,22] In our study, 60% of the interviewed urologists frequently use ZA in patients on ADT, with majority of them being sometimes (18.5%) or always (49.2%) unaware of the baseline BMD, at the start of therapy. The optimal dosage schedule and duration of therapy of ZA remains uncertain. In metastatic hormone refractory CaP, once-every-3-4 weeks infusion has been recommended.[7,8,12] For prevention or treatment of osteoporosis in men on ADT, quarterly IV infusion remains the most studied protocol, though monthly, biannual and annual injections have also been described.[12,25,26] In our study, monthly infusion of 4 mg of ZA for a period of six months was found to be the most commonly followed schedule (40/65, 61.5%). The various schedules adopted by the participating urologists and their indications for use of ZA have been presented in Table 2. Since monthly infusion of ZA is recommended only in metastatic hormone refractory CaP with quarterly or biannual injections sufficient for rest all scenarios, there appears an overuse of ZA by Indian urologists. Apart from financial burden, this may expose the patients to undue side-effects of the drug.
Table 2.
Interviewed urologists’ indications for use of Zoledronic acid
On exploratory analysis, years of clinical practice had no bearing on the use of ZA (P-0.31) or estimation of baseline BMD (P-0.32). Use of ZA was found to be significantly more among those practising in academic centres (P- 0.031); however, it was not affected by the place of practice (P- 0.072). Clinicians practising in major cities ordered for baseline BMD more often (P-0.002); however, it was not affected by the centre of practice (academic vs non-academic).
Strengths of our study include a high response rate and a structured telephonic interview enabling us to garner maximum possible information from the respondents. Direct verbal communication enabled us to clarify our questions, when sought by the interviewee and get clarifications of their answers, when required. We also ensured them to answer all our questions, which may not be possible with a mailed questionnaire.[32] Our sample size constituted about 6% of the practicing urologists in India and the results can be considered to closely represent the prevalent practice pattern. Our study has certain limitations. First, it's difficult to determine how closely the respondents’ answers reflected their actual practice. Second, India being a vast country with widely variable socio-cultural factors affecting physician practice patterns, some geographical areas might have been under-represented.
CONCLUSION
Majority of Indian urologists do not adhere to the current evidences and guidelines for BMD measurement in CaP. While majority of the respondents prescribe ZA to patients on ADT, most of them do so without knowing the baseline BMD, leading to apparent overuse of the drug. Though the awareness regarding bone-health in patients on ADT is better in our survey than the previously reported studies, urologists need to better counsel their patients regarding the bone-related adverse effects of ADT and encourage life-style modifications.
APPENDIX: Questionnaire
The salient points of the questionnaire used in the telephonic interview are mentioned below. The questionnaire included but was not always limited to the mentioned questions. Since it was a direct verbal communication, questions were not always asked in the given order and answer options were suggested only when necessary. Questions were further explained and clarifications to answers were sought whenever required.
Place of practice / Type of practice / Years in urology practice (≤5 years or >5 years)?
Do you see prostate cancer patients in your clinical practice? Do you prescribe hormonal treatment (androgen deprivation therapy when indicated) to any of them?
Do you always inform the patient regarding the risk of osteoporosis and adverse skeletal events at the time of starting ADT? Do you advice them for life-style modifications, e.g. regular weight-bearing exercises and avoidance of tobacco and alcohol? Do you prescribe calcium and vitamin D to such patients?
Do you use Bisphosphonates in your clinical practice? If yes, in which form - IV Zoledronic acid or oral bisphosphonates?
-
If “No”, Why?
- High Cost of Therapy
- Did not get desired result in own Clinical Experience
- Not sure of benefits
- Not much Idea about the molecule
- Refer the patient to medical oncologist for further treatment
- Any other reason
When do you use Zoledronic acid in cancer prostate patients in your clinical practice (i.e. indications)?
Your schedule of treatment (dose, frequency and duration) of Zoledronic acid? Do you use any other dosage schedule in your patients? If yes, please elaborate.
How often do you order quantitative bone mineral density assays like DEXA or QCT in your patients before starting hormonal therapy? Are they available at your place of practice? Which one you prefer – DEXA or QCT?
Do you modify your treatment schedule of Zoledronic acid based on the result of DEXA or QCT?
How often you order follow up DEXA or QCT in your patients on hormonal therapy?
Answer options for Q. 4, 8, 9 and 10 were:
-
(a)
Routinely or very often (50-100% of the times)
-
(b)
Less often (<50% of times)
-
(c)
Rarely or never (<10% of times)
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hussain SA, Weston R, Stephenson RN, George E, Parr NJ. Immediate dual energy X-ray absorptiometry reveals a high incidence of osteoporosis in patients with advanced prostate cancer before hormonal manipulation. BJU Int. 2003;92:690–4. doi: 10.1046/j.1464-410x.2003.04471.x. [DOI] [PubMed] [Google Scholar]
- 2.Stoch SA, Parker RA, Chen L, Bubley G, Ko YJ, Vincelette A, et al. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab. 2001;86:2787–91. doi: 10.1210/jcem.86.6.7558. [DOI] [PubMed] [Google Scholar]
- 3.Preston DM, Torrens JI, Harding P, Howard RS, Duncan WE, McLeod DG. Androgen deprivation in men with prostate cancer is associated with an increased rate of bone loss. Prostate Cancer Prostatic Dis. 2002;5:304–10. doi: 10.1038/sj.pcan.4500599. [DOI] [PubMed] [Google Scholar]
- 4.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 5.Diamond TH, Bucci J, Kersley JH, Aslan P, Lynch WB, Bryant C. Osteoporosis and spinal fractures in men with prostate cancer: Risk factors and effects of androgen deprivation therapy. J Urol. 2004;172:529–32. doi: 10.1097/01.ju.0000130508.61020.66. [DOI] [PubMed] [Google Scholar]
- 6.Oefelein MG, Ricchiuti V, Conrad W, Resnick MI. Skeletal fractures negatively correlate with overall survival in men with prostate cancer. J Urol. 2002;168:1005–7. doi: 10.1016/S0022-5347(05)64561-2. [DOI] [PubMed] [Google Scholar]
- 7.Heidenreich A, Bolla M, Joniau S, Mason MD, Matveev V, Mottet N, et al. Guidelines on prostate cancer. European Association of Urology. 2011. [Last accessed on 2011 Nov]. Available from: http://www.uroweb.org/gls/pdf/11_Prostate_Cancer.pdf .
- 8.National Comprehensive Cancer Network Inc. Clinical Practice Guidelines in Oncology: Prostate Cancer. V.4. 2011. [Last accessed on 2011 Nov]. Available from: http://www.nccn.org/ professionals/physician_gls/PDF/prostate.pdf .
- 9.Gralow JR, Biermann JS, Farooki A, Fornier MN, Gagel RF, Kumar RN, et al. NCCN Task Force Report: Bone Health in Cancer Care. J Natl Compr Canc Netw. 2009;7:S1–35. doi: 10.6004/jnccn.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, et al. Guidance on the use of bisphosphonates in solid tumours: Recommendations of an international expert panel. Ann Oncol. 2008;19:420–32. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 11.Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: Recommendations for diagnosis and therapies. Cancer. 2004;100:892–9. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 12.Saad F, Higano CS, Sartor O, Colombel M, Murray R, Mason MD, et al. The Role of Bisphosphonates in the Treatment of Prostate Cancer: Recommendations from an Expert Panel. Clin Genitourin Cancer. 2006;4:257–62. doi: 10.3816/CGC.2006.n.004. [DOI] [PubMed] [Google Scholar]
- 13.Grossmann M, Hamilton EJ, Gilfillan C, Bolton D, Joon DL, Zajac JD. Bone and metabolic health in patients with non-metastatic prostate cancer who are receiving androgen deprivation therapy. Med J Aust. 2011;194:301–6. doi: 10.5694/j.1326-5377.2011.tb02979.x. [DOI] [PubMed] [Google Scholar]
- 14.Carroll PR, Neal D, Scher H, Altwein J, Brawley O, Cockett A, et al. Management of disseminated prostate cancer. In: Denis L, Bartsch G, Khoury S, Murai M, Partin A, editors. Prostate cancer: 3rd International Consultation on Prostate Cancer—Paris. Paris (France): Health Publications; 2003. pp. 251–84. [Google Scholar]
- 15.Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–6. [PubMed] [Google Scholar]
- 16.Oefelein MG, Ricchuiti V, Conrad W, Seftel A, Bodner D, Goldman H, et al. Skeletal fracture associated with androgen suppression induced osteoporosis: The clinical incidence and risk factors for patients with prostate cancer. J Urol. 2001;166:1724–8. doi: 10.1016/s0022-5347(05)65661-3. [DOI] [PubMed] [Google Scholar]
- 17.Agarwal MM, Khandelwal N, Mandal AK, Rana SV, Gupta V, Chandra Mohan V, et al. Factors affecting bone mineral density in patients with prostate carcinoma before and after orchidectomy. Cancer. 2005;103:2042–52. doi: 10.1002/cncr.21047. [DOI] [PubMed] [Google Scholar]
- 18.Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103:237–41. doi: 10.1002/cncr.20766. [DOI] [PubMed] [Google Scholar]
- 19.Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: A survey of urologists and radiation oncologists. Urology. 2006;68:126–31. doi: 10.1016/j.urology.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 20.Yee EF, White RE, Murata GH, Handanos C, Hoffman RM. Osteoporosis management in prostate cancer patients treated with androgen deprivation therapy. J Gen Intern Med. 2007;22:1305–10. doi: 10.1007/s11606-007-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panju AH, Breunis H, Cheung AM, Leach M, Fleshner N, Warde P, et al. Management of decreased bone mineral density in men starting androgen-deprivation therapy for prostate cancer. BJU Int. 2009;103:753–7. doi: 10.1111/j.1464-410X.2008.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ngu WS, Byrne DJ. The practice of Scottish urologists in the assessment and management of fracture risk in the ageing male being treated for prostate cancer. ScientificWorldJournal. 2007;7:1590–5. doi: 10.1100/tsw.2007.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;354:948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 24.Eastham JA. Bone health in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2007;177:17–24. doi: 10.1016/j.juro.2006.08.089. [DOI] [PubMed] [Google Scholar]
- 25.Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 26.Bhoopalam N, Campbell SC, Moritz T, Broderick WR, Iyer P, Arcenas AG, et al. Intravenous zoledronic acid to prevent osteoporosis in a veteran population with multiple risk factors for bone loss on androgen deprivation therapy. J Urol. 2009;182:2257–64. doi: 10.1016/j.juro.2009.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Reid IR, Brown JP, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, et al. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–61. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 28.Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96:879–82. doi: 10.1093/jnci/djh141. [DOI] [PubMed] [Google Scholar]
- 29.Wong R, Wiffen PJ. Bisphosphonates for the relief of pain secondary to bone metastases. Cochrane Database Syst Rev. 2002;2:CD002068. doi: 10.1002/14651858.CD002068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saad F, Abrahamsson PA, Miller K. Preserving bone health in patients with hormone-sensitive prostate cancer: The role of bisphosphonates. BJU Int. 2009;104:1573–9. doi: 10.1111/j.1464-410X.2009.08952.x. [DOI] [PubMed] [Google Scholar]
- 31.Full prescribing information of Zometa (Novartis), updated March 2012. [Last accessed on 2011 Apr]. Available at http://www.pharma.us.novartis.com/product/pi/pdf/Zometa.pdf .
- 32.Warren KS, Osborn J, Chodak GW. Assessment of American urologists’ approach to hormonal management of prostate cancer. Urology. 2006;68:1305–7. doi: 10.1016/j.urology.2006.08.1068. [DOI] [PubMed] [Google Scholar]


