Abstract
Obesity and overeating have become fundamental problems in modern society. This article studies the inhibition of food-seeking behavior, and how contextual cues can control it. Rats that had free food in the home cage nevertheless learned to lever press for sucrose or high-fat pellets in a distinctive context (a Skinner box). Lever pressing was then inhibited by extinction, in which lever presses no longer produced food. After extinction, inhibited responding was “renewed” when the rats were switched to a different context: in the new context, the rats lever-pressed again, and worked more for food when food was made available. These effects were observed when conditioning, extinction and testing occurred in contexts A, B, and A (respectively) or in A, A, and B. Thus, mere removal from the context in which food-seeking was inhibited initiated a return to food-seeking. The contextual control of extinction may help explain why food seeking and consumption seem so persistent.
Keywords: Appetite, Extinction, Renewal, Relapse, Context, Operant conditioning, Instrumental learning
Introduction
Human appetite and food intake is controlled by a variety of complex processes (e.g., Woods, 2009). However, one especially important process is simple conditioning (see Bouton, 2011; Holland & Petrovich, 2005), in which organisms learn to associate their actions or environmental cues with food. For example, Weingarten (1983) sounded a buzzer every time rats were given a liquid diet based on evaporated milk. During subsequent tests while the rats were satiated, the sound of the buzzer caused the rats to initiate meal intake (see also Boggiano, Dorsey, Thomas, & Murdaugh, 2009; Galarce & Holland, 2009; Holland & Petrovich, 2005; Petrovich, Ross, Gallagher, & Holland, 2007). Similar effects have been demonstrated with children, where rooms associated with snack food have been shown to prompt eating, even when the children have been pre-fed ice cream (Birch, 1991). Overweight children may be particularly vulnerable to cues that prompt overeating (Jansen et al., 2003). The fact that humans are motivated to eat in the presence of food cues is especially relevant to modern society, where palatable foods, and cues that predict them, are so prevalent (see Kessler, 2009).
The present article is concerned with the inhibition of appetitive behavior. One procedure that is known to produce inhibition is extinction. In Pavlovian appetitive conditioning, extinction occurs when a cue associated with food is presented repeatedly alone, in the absence of food. Although extinction causes responding to decrease, one important fact about extinction is that it does not reflect an erasure of the original learning (Bouton, 2002, 2004). Instead, extensive research suggests that extinction results in the inhibition of responding, and equally important, that this inhibition can be especially dependent on the context for expression (e.g., Bouton, 2004). For example, consider the renewal effect. In one form of renewal, so-called ABA renewal, extinguished responding returns following conditioning in one context (A) and extinction in a second context (B) when the cue is presented again in Context A. In another form of renewal, so-called AAB renewal, conditioning and extinction occur in Context A, and testing then occurs in Context B (e.g., Bouton & Ricker, 1994). The renewal of extinguished responding in the latter case is especially significant because it indicates that the inhibition learned in extinction is more context-specific than the original learning (conditioning appears to generalize more to the new Context B than extinction does). Responding recovers in a new situation, where the food cue has never been associated with food before.
Renewal effects have been demonstrated in a wide variety of Pavlovian conditioning preparations, including both fear conditioning and appetitive conditioning in rats (e.g., Bouton, 2002) and humans (Van Gucht, Vansteenwegen, Beckers, & Van den Bergh, 2008; Van Gucht, Vansteenwegen, Van den Bergh, & Beckers, 2008). Recent research has also begun to extend it to the domain of appetitive operant or instrumental learning (e.g., Bouton, Todd, Vurbic, & Winterbauer, 2011; Nakajima, Tanaka, Urushihara, & Imada, 2000). Bouton et al. (2011) trained food-deprived rats to lever press for grain-based food pellets. Responding was then extinguished by withholding the pellets. When testing then occurred in a different context, the inhibited response returned: ABA, AAB, and ABC (where testing occurs in a third context) forms of renewal were all observed. In the latter two cases, responding renewed following removal from the extinction context. The context specificity of the extinction of instrumental food-seeking behavior might be one reason why activities like overeating are so persistent.
The idea behind the present experiments was to extend these results to a situation in which rats could work for sweet and fatty food pellets while they were fed freely on ad lib food in the home cage. Rats that are fed on ad lib food will still work for palatable foods, such as sucrose (e.g., Brennan, Roberts, Anisman, & Merali, 2001), a phenomenon that is reminiscent of human behavior in the modern, food-saturated world. The new question posed by the present experiments was whether food-seeking in ad lib fed rats would demonstrate lapse and relapse in the form of the renewal effect after food-seeking had been extinguished. We examined the contextual control of response inhibition in sated rats in both the ABA and AAB renewal paradigms. Evidence of ABA renewal would suggest that return to a context in which food-seeking had been reinforced would lead to a return of food-seeking behavior. Evidence of AAB renewal would suggest that mere removal from a context recently associated with extinction may be sufficient to generate a relapse—even though the new context is not extensively associated with food. In the present experiments, renewal testing occurred both in extinction (when renewed lever pressing was not reinforced) and when lever pressing earned sweet or sweet/fatty food pellets again. The latter “reacquisition” condition may be especially relevant to the human situation, where a lapse in inhibited appetitive behavior would normally lead to more food.
Experiment 1
In Experiment 1 we examined ABA renewal. Half the rats learned to lever press for sucrose pellets while the other half learned to lever press for sweet, high-fat pellets. This phase (“acquisition”) occurred in one of two counterbalanced Skinner boxes that we will call Context A. Following acquisition, all rats received extinction, in which lever pressing was no longer associated with pellets. In this experiment, extinction occurred in a different (again counterbalanced) set of Skinner boxes, Context B. In a with-in- subject renewal test, all rats were then tested, with no food available, in both Context B and Context A (test order was counterbalanced). We predicted that rats would respond again for food in Context A despite extinction in Context B. Following the renewal test, we conducted a second test in which the rats could now earn pellets again in either Context A or Context B. Would extinguished food-seeking be reacquired more quickly in the original context than in the extinction context?
Methods
Subjects
The subjects were 32 female Wistar rats purchased from Charles River Laboratories (St. Constance, Quebec). They had previously participated in an experiment conducted in a different apparatus in which a light stimulus had been associated with a different 45-mg grain-based food pellet (TestDiet; Richmond, IN, USA). The rats had been food-deprived to 80% of their free-feeding weights in that experiment, but were returned to ad lib feeding on home chow (Prolab RMH 3000, LabDiet; Richmond, IN, USA) for 16 days prior to the beginning of the current experiment.
Apparatus
For all rats, two sets of four Skinner boxes served as Contexts A and B (counterbalanced). Both sets of boxes were slightly modified versions of Med Associates (St. Albans, VT) model ENV-007-VP. They measured 31.8 × 24.1 × 29.2 cm (l × w × h) and had food cups (where the food was delivered) centered in the front wall. A 4.8-cm retractable operant lever protruded 1.9 cm from the front wall when extended and was positioned 6.2 cm above the grid floor to the right of the food cup. In one set of boxes, the floor was made of stainless steel rods (0.48 cm in diameter) spaced 1.6 cm apart from center to center and mounted parallel to the front wall. In the other set of boxes the floor consisted of alternating rods of different diameters (0.48 and 1.27 cm), spaced 1.6 cm apart from center to center. Each set of boxes also had unique visual markings on the walls and ceiling. Finally, the different boxes were scented with Anise or Coconut odors, respectively. Thus, the two sets of four boxes differed in visual, tactile and olfactory respects.
In each set of boxes, half were equipped to deliver 45-mg sucrose pellets (Sucrose Tablet 45 mg, TestDiet, Richmond, IN, USA) and half were equipped to deliver 45-mg sweet, high-fat pellets (38% kcal Omnitreat Tab 45 mg, TestDiet, Richmond, IN, USA).
Procedure
Rats were first allowed to consume 15 pellets of the type they were assigned while in the home cage. On the next day (i.e., Day 1), rats learned to retrieve the food pellets from the food cup at the sound of pellet delivery in separate sessions in Contexts A and B. Half the rats received sucrose pellets, and half the rats received high-fat pellets. Approximately 30 pellets were randomly delivered during magazine training on average every 30 s in each session.
On the following day, the first of five daily sessions of lever press training occurred in Context A. Two minutes after placement in the box, the lever was inserted and the reinforcement schedule was in effect for 30 min. On the first day, lever presses were reinforced on a variable interval (VI) 10-second schedule (pellets were available, and delivered upon a response, on average every 10 s). On the next day the schedule was increased to VI-20. Finally, on acquisition days 3, 4, and 5, the schedule was VI-30. No additional shaping was necessary. Throughout training, half the rats pressed for sucrose pellets and half pressed for fatty pellets.
Following acquisition, rats received three daily sessions of extinction in the alternative context (Context B). Extinction was identical to acquisition except that lever presses did not result in the delivery of a food pellet. On the day following the last session of extinction, each rat was tested for renewal in a within-subject fashion. All rats were tested in Context A and Context B, in a counterbalanced order. As usual, the lever was inserted 2 min after placement in the box; lever presses were then recorded for the next 10 min with pellets never being delivered. The two test sessions were separated by approximately 1 h.
On the next and last day of the experiment, a single 12-min reacquisition session occurred. Half the rats were returned to the acquisition context (Context A), and half were returned to the extinction context (Context B). Following 2 min the lever was inserted and every 5th lever press produced a pellet of the type the rat had received before.
The results were evaluated with analyses of variance (ANOVAs) using a rejection criterion of p < .05.
Results and discussion
Acquisition, extinction, and renewal
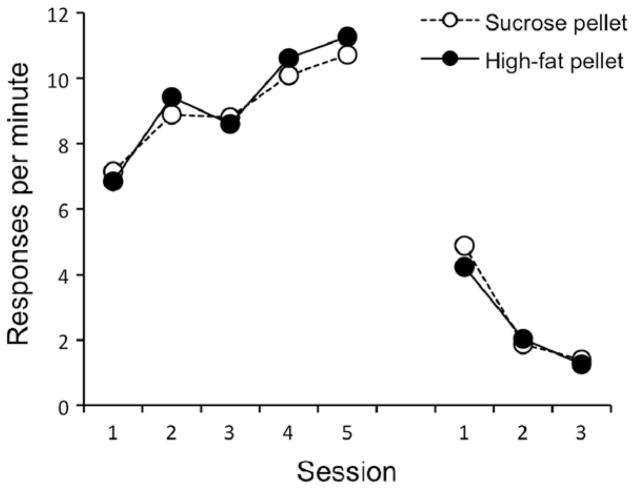
The results of the acquisition and extinction phases of the experiment are presented in the left panel of Fig. 1. The rats readily learned to lever press, and reached a final response rate that was roughly half the rate typically achieved by food-deprived rats working for grain-based pellets (e.g., Bouton et al., 2011). Throughout the experiment, there was no difference in the performance of the sucrose and high-fat pellet groups. As expected, lever pressing increased over the five acquisition sessions, F(4, 120) = 22.88, MSE = 5.36, p < .001. This effect did not interact with pellet type nor was the main effect of pellet type significant, Fs < 1. During extinction, the rate of lever pressing decreased over sessions, F(2, 60) = 179.16, MSE = .24, p < .001. No other main effects or interactions were significant, largest F(2, 60) = 1.45, MSE = .24, p = .24. Although the comparison is not made here, we have previously observed extinction of free operant behavior to be more rapid in Context B than in Context A (Bouton et al., 2011, Experiment 1).
Fig. 1.
Results of Experiment 1. Left: mean responding during each 30-min session of acquisition and extinction. Right: mean responding during the 10-min test session in the extinction context (B) and the renewal context (A).
The data from the renewal test are presented in the right panel of Fig. 1. Responding increased when testing occurred in Context A relative to Context B, F(1, 30) = 34.30, MSE = 3.55, p < .001, indicating an overall renewal of responding in Context A. This effect did not interact with pellet type nor was the main effect of pellet type significant, largest F(1, 30) = 1.14, MSE = 3.55, p = .29. Thus, after food-seeking had been extinguished, responding returned when the rats were returned to the context in which they had originally worked for food.
Reacquisition
The data from the session in which rats could earn pellets again in either Context A or B are presented in Fig. 2. Three rats tested in Context B failed to lever press enough to earn a pellet during the session; that is, they pressed the lever less than five times. Responding generally increased over the 10 min of reacquisition, F(9, 252) = 9.78, MSE = 33.61, p < .001. No other main effects or interactions, including those with pellet type, were significant, largest F(1, 28) = 2.37, MSE = 576.24, p = .14. However, responding was greater in Context A than in Context B during each of the first four time bins, minimum F(1, 30) = 5.58, MSE = 52.67, p = .03. Thus, responding over the first 4 min of reacquisition was higher for rats in the original acquisition context than in the extinction context.
Fig. 2.
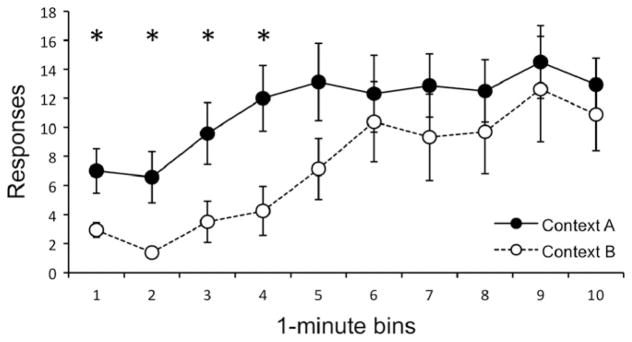
Results of the reacquisition test for Experiment 1. Mean responses (± SEM) as a function of 1-minute time bins. Groups are differentiated based on reacquisition context (A vs. B).* indicates significant effect of context (p < .05).
Overall, these data confirm that responding for sweet and fatty pellets renews when the rat is returned to the context of acquisition after extinction in another context. They thus suggest that inhibition (extinction) of voluntary actions is context specific. Importantly, this experiment is the first to suggest that this is true with rats that are not food deprived. The amount of recovery in the present method (to approximately 40% of the response rate evident at the end of acquisition) is entirely consistent with the amount seen in operant ABA renewal in food-deprived rats (Bouton et al., 2011). Another new aspect of the present results is that higher responding was also evident in the original context during a reacquisition test. One way to view that result is that the extinction context provided a modest degree of protection against relapse when food was made contingent on responding again. Reinforced food-seeking was quicker to reemerge in the original acquisition context (Context A).
Experiments 2a and 2b
Experiment 1 involved a return to the original learning context after extinction in a second context. To determine if removal from the extinction context is alone sufficient for responding to relapse, Experiment 2a assessed AAB renewal, in which acquisition and extinction occurred in the same context (A) and testing then occurred in a second context (B). Experiment 2a tested AAB renewal in extinction, whereas Experiment 2b examined reacquisition in which lever pressing led to food again in either Context B or Context A.
Methods
Subjects
The subjects for each experiment were 32 female Wistar rats. As before, they had previously participated, while food-deprived, in an experiment in which visual and/or auditory stimuli were paired with a 45-mg grain-based food pellet (TestDiet; Richmond, IN, USA). The current experiments began a minimum of 7 days after they had been returned to ad lib feeding on the home chow described in Experiment 1.
Apparatus
Two new sets of four Skinner boxes housed in separate rooms of the laboratory served as Contexts A and B (counterbalanced). All boxes were of the same design (Med Associates model ENV-008-VP). They had the same recessed food cups and lever as the boxes from Experiment 1. In one set of boxes, the floor was made of stainless steel grids (0.48 cm diameter) staggered such that odd- and even-numbered grids were mounted in two separate planes, one 0.5 cm above the other. In the other set of boxes, the grids of the floor were mounted on the same plane and were spaced 1.6 cm apart (center-to-center). Each set of boxes was also unique based on visual cues on the ceiling and walls. Finally, each set of boxes was scented with lemon or pine odor respectively. As usual, the two sets of boxes were fully counterbalanced in the roles of Contexts A and B.
Procedure
All phases of the procedure (pellet preexposure, magazine training, acquisition, extinction, renewal testing, and reacquisition) were identical to Experiment 1 with the following exceptions. In Experiment 2a, acquisition and extinction both took place in Context A, followed by renewal testing in Contexts A and B (in a counterbalanced order). Lever presses were not reinforced during testing. In Experiment 2b, the first test was omitted, and a reacquisition test occurred in its place. Half the rats were placed in Context A and half the rats were placed in Context B, where they could earn a pellet for every fifth response. As before, half the rats in each experiment earned sucrose pellets and the other half earned sweet-fatty pellets.
Results and discussion
Acquisition, extinction, and renewal (Experiment 2a)
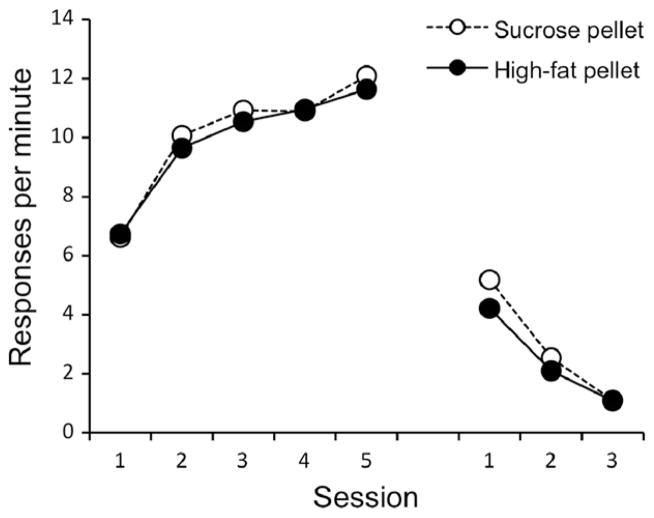
As expected, lever pressing increased over the course of acquisition (Fig. 3), F(4, 120) = 20.02, MSE = 3.86, p < .001. No other main effects or interactions were significant, Fs < 1. During extinction (also Fig. 3), the rate of lever pressing decreased, F(2, 60) = 97.1, MSE = .97, p < .001. No other main effects or interactions were significant, largest F(2, 60) = 1.38, MSE = .97, p = .26.
Fig. 3.
Results of Experiment 2a. Mean responding during each 30-min session of acquisition and extinction.
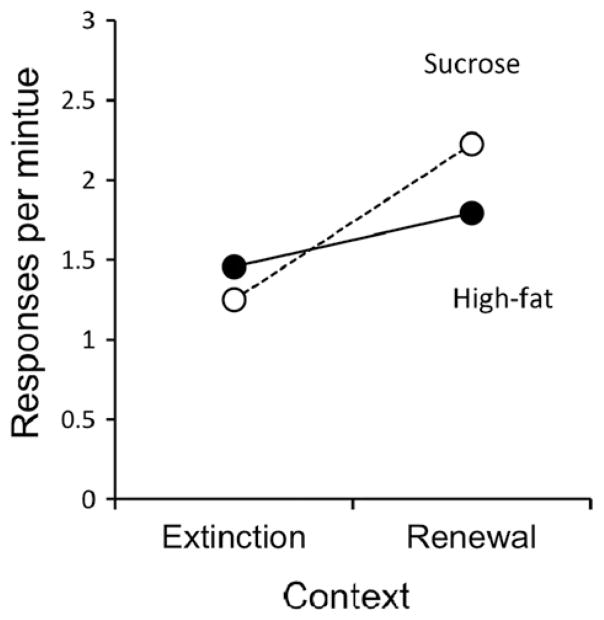
Figure 4 displays the data from the crucial renewal test. Responding was significantly stronger in Context B, the relatively new context, than in Context A, the context in which both acquisition and extinction occurred, F(1, 30) = 13.54, MSE = .51, p = .001. The pattern indicates an AAB renewal effect. The interaction between context and pellet type was well short of significance, F(1, 28) = 3.20, MSE = .51, p = .084. The amount of recovery observed in Context B reached approximately 20% of the response rate observed at the end of acquisition.
Fig. 4.
Results of the renewal test for Experiment 2a. Mean responding during the 10-min test session in the extinction context (A) and the renewal context (B).
Reacquisition (Experiment 2b)
The acquisition and extinction phases of Experiment 2b also proceeded as expected (see Fig. 5). Lever pressing first increased during acquisition, F(4, 120) = 32.29, MSE = 3.95, p < .001. No other main effects or interactions were significant, Fs < 1. Responding then decreased during extinction, F(2, 60) = 136.90, MSE = .79, p < 001. Once again, no other main effects or interactions were significant, largest F(2, 60) = 2.32, MSE = .79, p = .11.
Fig. 5.
Results of Experiment 2b. Mean responding during each 30-min session of acquisition and extinction.
The results of the reacquisition test are presented in Fig. 6 (collapsed over pellet type). Not surprisingly, responding increased during the reacquisition session, F(9, 252) = 8.75, MSE = 65.41, p < .001. However, the extent of this increase depended on the context, as represented in a significant Context × bin interaction, F(9, 252) = 2.38, MSE = 65.41, p = .013. Responding in Context B increased more, relative to Context A, over the later minutes of the test. Responding was higher in Context B during time bin 8, F(1, 30) = 5.25, MSE = 128.71, p = .03, and time bin 10, F(1, 30) = 6.10, MSE = 141.73, p = .02. No other main effects or interactions were significant, largest F(1, 28) = 1.24, MSE = 665.93, p = .28.
Fig. 6.
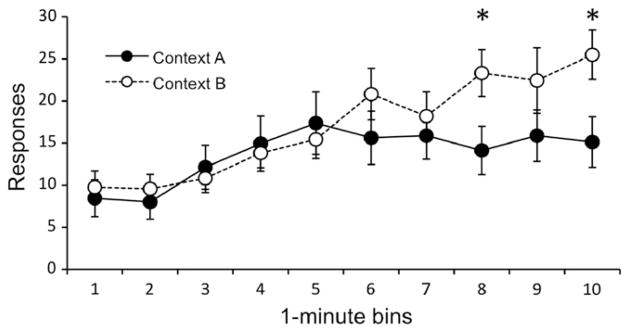
Results of the reacquisition test for Experiment 2b. Mean responses (±SEM) as a function of 1-minute time bins. Groups are differentiated based on reacquisition context (A vs. B). * indicates significant effect of context (p < .05).
These data extend the results of Experiment 1, where renewal occurred upon a return to the original context, to show that simple removal from the context of extinction is itself sufficient to cause a renewal of food-seeking. This result is especially interesting, because it requires that the rats lever pressed more in a context (Context B) in which lever pressing had never been reinforced before. The fact that the between-subject difference between responding in Contexts A and B emerged late in the reacquisition session (Fig. 6) suggests that inhibition that accrued prior to reacquisition persisted for some time even after the reinforcer was available again. It is possible that both extinction in Context A, plus exposure to the original interval schedule of reinforcement in that context, interfered with reacquisition on the ratio schedule. Even though it had been associated with both the extinction and reinforcement of food seeking, Context A appeared to provide some protection from relapse when food was made available again.
General discussion
Rats that ate ad lib food in the home cage learned to lever press for sweet and sweet-fatty pellets, a pattern that may resemble the behavior of humans who consume food in the absence of an actual “need” for it. The present experiments uncovered several new features of such nonobligatory food-seeking behavior. First, in Experiment 1, responding for sucrose or high-fat pellets was renewed after extinction upon return to the original context. Further, when the food was made available again, food seeking resumed faster in the original context (Context A) than in the context where inhibition had been learned (Context B). The reacquisition result is consistent with recent research in which food-deprived rats were reinforced for nose poking with alcoholic beer (Willcocks & McNally, 2011). In that method, reacquisition after extinction was faster than initial acquisition (in naïve controls); in addition, the latency to the first response was shorter in Context A than in Context B. In the present case, with satiated animals working for sweet or sweet-fatty food pellets, renewal may be analogous to the overeater who learns to inhibit responding in a new context and then finds it difficult to resist the food in the original context. A person might learn to refrain from eating dessert when out at a new restaurant, but cannot resist investigating the freezer for ice cream when at home. Although ABA renewal is a well-studied phenomenon (e.g., Bouton & Woods, 2008), Experiment 1 provides its first demonstration in ad lib fed rats, which may be particularly relevant to modeling human food intake. Consistent with the applicability to humans, Van Gutch and colleagues have shown that, following extinction, human’s expectation to eat chocolate in the presence of a chocolate-associated stimulus shows renewal upon a return to the original acquisition context (Van Gucht, Vansteenwegen, Beckers, et al., 2008; Van Gucht, Vansteenwegen, Van den Bergh, et al., 2008).
The second crucial new finding is that removal from the extinction context alone may be sufficient to cause a renewal of food seeking (Experiments 2a and 2b). In Experiment 2a, when rats were removed from the context in which food seeking had been both reinforced and extinguished (Context A) and placed in a relatively new context (Context B), food seeking once again returned. Experiment 2b extended this finding to show that the rats also reacquired the response more rapidly in Context B than Context A. Individuals thus do not need to return to a context originally associated with food for relapse to occur. Indeed, in both experiments, there was paradoxically more responding in a context that was less associated with food. This counter-intuitive result may help further explain why eating and overeating may be so persistent and difficult to control. To extend the analogy introduced above, a person might learn to stop eating ice cream while at home (where many trips to the freezer have previously been reinforced), but still find it difficult to resist ice cream when eating at a new restaurant, or a new friend’s dinner party. In principle, the context- specificity of the inhibition of appetitive behavior might allow appetitive behavior to re-emerge, seemingly “spontaneously,” in any new context.
The current experiments extend our previous work on the extinction of appetitive conditioning (e.g., Bouton & Peck, 1989; Bouton et al., 2011). In either Pavlovian (cue-food) or instrumental (action-food) learning, the extinction of behavior does not erase or destroy appetitive learning. Instead, extinguished responding readily returns when the organism is removed from the context of extinction. Importantly, our previous work was conducted exclusively with food-deprived rats. It is significant that renewal occurred in the present experiments in the absence of explicit food deprivation. The results thus extend previous studies showing that Pavlovian cues for food can prompt appetitive and consumption behaviors in satiated humans and animals (Birch, 1991; Boggiano et al., 2009; Galarce & Holland, 2009; Jansen et al., 2003; Petrovich et al., 2007; Weingarten, 1983). Coupled with this previous research, our findings indicate that presence of excitatory food cues or the removal of inhibitory cues can prompt appetitive behavior under conditions of satiety.
How do contexts modulate instrumental appetitive behavior? One way contexts might work is to excite (or inhibit) appetitive behavior via their simple associations with food. For example, a direct connection between a context and food (either excitatory or inhibitory) would be expected to motivate (or de-motivate) instrumental actions through a process known as “Pavlovian-instrumental transfer.” One view of Pavlovian-instrumental transfer is that the presentation of a cue associated with a goal creates a goal expectancy that serves to motivate goal-directed behavior (e.g., Hogarth, Dickinson, Wright, Kouvaraki, & Duka, 2007; Rescorla & Solomon, 1967). Another view is that the cue increases a kind of behavioral arousal (e.g., Balleine, 2005). There is evidence that context-food associations can modulate instrumental food-seeking behavior in hungry rats (Baker, Steinwald, & Bouton, 1991; Pearce & Hall, 1979). However, a recent study from our laboratory (Bouton et al., 2011; Experiment 4) demonstrated that repeated exposure to Context A alone (without the lever present) did not attenuate the ABA renewal effect, suggesting that Context A did not excite responding through a simple, direct association with food. (Repeated exposure to the context alone should have weakened any direct context-food association.) Alternatively, it is possible that the context acts as an “occasion setter”, and modulates the response-food association, more than being directly associated with food itself (e.g., Rescorla, 1991). Importantly, the occasion setting power of stimuli is not weakened by simple exposure to the occasion setter itself (Rescorla, 1986). Occasion setters can be external stimuli, such as contexts and discrete cues, but they can also be internal stimuli, such as feelings of hunger. In fact, Davidson (1993, 2000) has argued that feeding behavior is regulated by such an occasion-setting mechanism. Further, Wilfley et al. (2010) have suggested that context-related processes and extinction may contribute to obesity-related behaviors.
The control of appetite is arguably a major issue in modern society (Kessler, 2009). From a learning perspective, there are several ways that cues in the environment can influence appetitive behavior. The ideas developed here are twofold. First, previous research has shown that food-associated cues can encourage eating even when the organism is sated. The ABA renewal observed in Experiment 1 can be considered a new example of that principle. Second, the inhibition (extinction) of appetitive behaviors does not erase the originally learned association between the behavior and food. Instead, extinction should be considered a new form of inhibitory learning that is relatively context specific. And although stimuli associated with food will prompt eating, the AAB renewal observed in Experiments 2a and 2b suggest that the removal of contextual inhibition will do the same. The combination of these learned excitatory and inhibitory influences might help explain why eating and overeating can seem so resistant to change.
Footnotes
This research was supported by Grant No. R01 MH064847 from the National Institute of Mental Health. TPT was partially supported by a graduate scholarship from the Natural Sciences and Engineering Research Council of Canada (NSERC).
References
- Baker AG, Steinwald H, Bouton ME. Contextual conditioning and reinstatement of extinguished instrumental responding. The Quarterly Journal of Experimental Psychology. 1991;43B:199–218. [Google Scholar]
- Balleine BW. Neural bases of food-seeking: Affect, arousal and reward in corticostriatolimbic circuits. Physiology & Behavior. 2005;86:717–730. doi: 10.1016/j.physbeh.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Birch LL. Obesity and eating disorders: A developmental perspective. Bulletin of the Psychonomic Society. 1991;29:265–272. [Google Scholar]
- Boggiano MM, Dorsey JR, Thomas TM, Murdaugh DL. The Pavlovian power of palatable food: Lessons for weight-loss adherence from a new rodent model of cue-induced overeating. International Journal of Obesity. 2009;33:693–701. doi: 10.1038/ijo.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Learning and the persistence of appetite: Extinction and the motivation to eat and overeat. Physiology & Behavior. 2011;103:51–58. doi: 10.1016/j.physbeh.2010.11.025. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Peck CA. Context effects on conditioning, extinction, and reinstatement in an appetitive conditioning preparation. Animal Learning & Behavior. 1989;17:188–198. [Google Scholar]
- Bouton ME, Ricker ST. Renewal of extinguished responding in a second context. Animal Learning & Behavior. 1994;22:317–324. [Google Scholar]
- Bouton ME, Todd TP, Vurbic D, Winterbauer NE. Renewal after the extinction of free-operant behavior. Learning & Behavior. 2011;39:57–67. doi: 10.3758/s13420-011-0018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Woods AM. Extinction: Behavioral mechanisms and their implications. In: Byrne JH, Sweatt D, Menzel R, Eichenbaum H, Roediger H, editors. Learning, memory: A comprehensive reference. Vol. 1. Oxford, UK: Elsevier; 2008. pp. 151–171. [Google Scholar]
- Brennan K, Roberts DC, Anisman H, Merali Z. Individual differences in sucrose consumption in the rat: Motivational and neurochemical correlates of hedonia. Psychopharmacology. 2001;157:269–276. doi: 10.1007/s002130100805. [DOI] [PubMed] [Google Scholar]
- Davidson TL. The nature and function of interoceptive signals to feed: Toward integration of physiological and learning perspectives. Psychological Review. 1993;100:640–657. doi: 10.1037/0033-295x.100.4.640. [DOI] [PubMed] [Google Scholar]
- Davidson TL. Pavlovian occasion setting: A link between physiological change and appetitive behavior. Appetite. 2000;35:271–272. doi: 10.1006/appe.2000.0355. [DOI] [PubMed] [Google Scholar]
- Galarce EM, Holland PC. Effects of cues associated with meal interruption on eating behavior. Appetite. 2009;52:693–702. doi: 10.1016/j.appet.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogarth L, Dickinson A, Wright A, Kouvaraki M, Duka T. The role of drug expectancy in the control of human drug seeking. Journal of Experimental Psychology: Animal Behavior Processes. 2007;33:484–496. doi: 10.1037/0097-7403.33.4.484. [DOI] [PubMed] [Google Scholar]
- Holland PC, Petrovich GD. A neural systems analysis of the potentiation of feeding by conditioned stimuli. Physiology & Behavior. 2005;86:747–761. doi: 10.1016/j.physbeh.2005.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A, Theunissen N, Slechten K, Nederkoorn C, Boon B, Mulkens S, Roefs A. Overweight children overeat after exposure to food cues. Eating Behaviors. 2003;4:197–209. doi: 10.1016/S1471-0153(03)00011-4. [DOI] [PubMed] [Google Scholar]
- Kessler DA. The end of overeating: Taking control of the insatiable American appetite. New York: Rodale; 2009. [Google Scholar]
- Nakajima S, Tanaka S, Urushihara K, Imada H. Renewal of extinguished lever-press responses upon return to the training context. Learning and Motivation. 2000;31:416–431. [Google Scholar]
- Pearce JM, Hall G. The influence of context-reinforcer associations on instrumental performance. Animal Learning & Behavior. 1979;7:504–508. [Google Scholar]
- Petrovich GD, Ross CA, Gallagher M, Holland PC. Learned contextual cue potentiates eating in rats. Physiology & Behavior. 2007;90:362–367. doi: 10.1016/j.physbeh.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA. Extinction of facilitation. Journal of Experimental Psychology: Animal Behavior Processes. 1986;12:16–24. [Google Scholar]
- Rescorla RA. Associative relations in instrumental learning: The Eighteenth Bartlett Memorial Lecture. The Quarterly Journal of Experimental Psychology. 1991;43B:1–23. [Google Scholar]
- Rescorla RA, Solomon RL. Two-process learning theory: Relationships between Pavlovian conditioning and instrumental learning. Psychological Review. 1967;74:151–182. doi: 10.1037/h0024475. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Vansteenwegen D, Beckers T, Van den Bergh O. Return of experimentally induced chocolate craving after extinction in a different context: Divergence between craving for and expecting to eat chocolate. Behaviour Research and Therapy. 2008;46:375–391. doi: 10.1016/j.brat.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Van Gucht D, Vansteenwegen D, Van den Bergh O, Beckers T. Conditioned craving cues elicit an automatic approach tendency. Behaviour Research and Therapy. 2008;46:1160–1169. doi: 10.1016/j.brat.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220:431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- Woods SC. The control of food intake: Behavioral versus molecular perspective. Cell Metabolism. 2009;9:489–498. doi: 10.1016/j.cmet.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcocks AN, McNally GP. The role of context in re-acquisition of extinguished alcoholic beer-seeking. Behavioral Neuroscience. 2011;125:541–550. doi: 10.1037/a0024100. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Van Buren DJ, Theim KR, Stein RI, Saelens BE, Ezzet F, Russian AC, Perri MG, Epstein LH. The use of biosimulation in the design of a novel multilevel weight loss maintenance program for overweight children. Obesity. 2010;18:S91–S98. doi: 10.1038/oby.2009.437. [DOI] [PMC free article] [PubMed] [Google Scholar]