Abrupt substitution of gadobenate dimeglumine for gadopentetate dimeglumine resulted in a significant increase in reporting of allergic-like reactions that exhibited a temporal pattern suggestive of the Weber effect.
Abstract
Purpose:
To evaluate the effect of abruptly substituting gadobenate dimeglumine for gadopentetate dimeglumine on allergic-like reactions.
Materials and Methods:
The institutional review board approved and waived patient consent for this HIPAA-compliant retrospective study. Allergic-like reactions related to gadolinium-based contrast media were assessed 2 years before and 3.5 years after gadobenate dimeglumine was substituted for gadopentetate dimeglumine. Reaction rates and severity were compared by using χ2 tests, Fisher exact tests, odds ratios (ORs), and confidence intervals (CIs).
Results:
Allergic-like reactions (137 mild, 19 moderate, and six severe) occurred in 162 (0.15%) of 105 607 injections of gadolinium-based contrast media (gadopentetate dimeglumine, 31 540; gadobenate dimeglumine, 66 152; other, 7915). Gadobenate dimeglumine was associated with significantly more overall (0.19% [123 of 66 152] vs 0.08% [24 of 31 540]; OR, 2.4; 95% CI: 1.6, 3.8; P < .0001) and mild (0.16% [107 of 66 152] vs 0.06% [18 of 31 540]; OR, 2.8; 95% CI: 1.7, 4.7; P < .0001) allergic-like reactions than was gadopentetate dimeglumine. The reaction rate for gadobenate dimeglumine peaked (maximum per quarter, 0.38% [16 of 4262]; minimum per quarter, 0.07% [three of 4237]) in the 2nd year after it replaced gadopentetate dimeglumine (maximum per quarter, 0.10% [four of 4122]; minimum per quarter, 0.05% [two of 4222]) and then declined in the 3rd year. The final gadobenate dimeglumine reaction rate (last 3 quarters, 0.12% [17 of 14 387]) did not significantly differ from the original baseline reaction rate with gadopentetate dimeglumine.
Conclusion:
After gadobenate dimeglumine was substituted for gadopentetate dimeglumine, a significant transient increase occurred in the frequency of reported allergic-like reactions that demonstrated a temporal pattern suggestive of the Weber effect (a transient increase in adverse event reporting that tends to peak in the 2nd year after a new agent or indication is introduced).
© RSNA, 2012
Introduction
Although gadolinium-based contrast media have long been considered extremely safe, the relationship described in 2006 between exposure in patients with severe renal impairment and the subsequent development of nephrogenic systemic fibrosis (NSF) has led many to reconsider their policies toward these media (1–7). It is now recommended that exposure to gadolinium-based contrast media be minimized in patients with severely impaired renal function (eg, those with estimated glomerular filtration rate less than 30 mL/min/1.73 m2 and those with acute kidney injury) (1). In addition to implementing risk mitigation policies on the basis of estimated glomerular filtration rate, some institutions have changed the type of gadolinium-based contrast media they administer on the basis of estimates of relative NSF risk by the American College of Radiology (8), the U.S. Food and Drug Administration (FDA) (1), and the European Society of Urogenital Radiology (9).
Changing gadolinium-based contrast media in an attempt to reduce the incidence of NSF may have unintended consequences. For example, although allergic-like reactions are an infrequent complication of intravascular administration of gadolinium-based contrast media (10), studies have suggested that in addition to variation in NSF risk among the different types of gadolinium-based contrast media, risk of allergic-like reactions may also vary (11,12). In particular, two studies have shown data that suggest gadobenate dimeglumine (a gadolinium-based contrast agent approved by the FDA in 2004 that has a reportedly lower NSF risk) may have a higher rate of allergic-like reactions than does gadopentetate dimeglumine (a gadolinium-based contrast agent approved by the FDA in 1988 that has a reportedly higher NSF risk) (11,12). As institutions consider changing the type of gadolinium-based contrast media they use for their general patient population as part of an approach to reduce NSF risk, the overall risk of adverse events could increase if a replacement agent with a higher rate of allergic-like reactions is used.
Because of a change in a prime vendor contract, our institution abruptly switched from primarily using gadopentetate dimeglumine to primarily using gadobenate dimeglumine. This offered an opportunity to measure the differences in allergic-like reactions that might occur with such a change. The null hypothesis of our study was that the rate of allergic-like reactions associated with gadobenate dimeglumine would be similar to that associated with gadopentetate dimeglumine.
The purpose of our study was to evaluate the effect of abruptly substituting gadobenate dimeglumine for gadopentetate dimeglumine on allergic-like reactions.
Materials and Methods
Before the initiation of this investigation, approval of the institutional review board was obtained. The study complied with the Health Insurance Portability and Accountability Act. Patient informed consent was not required on the basis of institutional policy and the retrospective nature of this investigation. A portion of these data was shared with Bracco Diagnostics (Princeton, NJ) as part of phase IV postmarketing analysis. One author (J.R.D.) received partial salary support from Bracco Diagnostics for that effort. The remaining authors had control of all data that may present a conflict of interest for that author.
Participants
All 105 607 intravenous administrations of gadolinium-based contrast media between January 1, 2007, and June 30, 2012, were identified by means of a query of electronic billing records at the University of Michigan Health System. These dates were chosen to reflect a period 2 years before and 3.5 years after a change in prime vendor contract at our institution that resulted in the substitution of gadobenate dimeglumine for gadopentetate dimeglumine. A 3.5-year period (instead of a 2-year period) after this switch was chosen to account for the Weber effect, which can be responsible for transient elevations in the rates of adverse events after introduction of a new drug or changes in use of an existing drug (13–15) and which tends to peak in the 2nd year.
Examinations that used gadolinium-based contrast media were grouped by contrast agent and date (according to 3-month quarter and calendar year). Some patients underwent more than one examination involving gadolinium-based contrast media during the study period. All adverse event forms for allergic-like reactions related to gadolinium-based contrast media completed by radiologists from January 1, 2007, to June 30, 2012, were collected and analyzed (n = 163). One allergic-like reaction was due to intraarticular administration and was excluded from analysis. This resulted in a total of 162 allergic-like reactions related to intravenous administration of gadolinium-based contrast media during the study period.
Contrast Media
The following media were used during the study period: gadopentetate dimeglumine (Magnevist; Bayer Healthcare, Wayne, NJ), gadobenate dimeglumine (MultiHance; Bracco Diagnostics), gadoteridol (ProHance; Bracco Diagnostics), gadoxetate disodium (Eovist; Bayer Healthcare), gadofosveset trisodium (Ablavar; Lantheus Medical Imaging, North Billerica, Mass), and gadodiamide (Omniscan; GE Healthcare, Princeton, NJ). Most intravenous gadolinium-based contrast media were hand injected or power injected at doses of 0.1 mmol per kilogram of body weight, with the following exceptions. “Double-dose” injections (0.2 mmol/kg) were administered for cardiac viability studies and peripheral magnetic resonance (MR) angiography. Gadofosveset trisodium was administered at a dose of 0.03 mmol/kg, and gadoxetate disodium was administered at a dose of 0.05 mmol/kg. (Table 1)lists the number of administrations for each gadolinium-based contrast agent used at our institution during the study period.
Table 1.
Numbers and Characteristics of Allergic-like Reactions Resulting from Intravenous Administration of Various Gadolinium-based Contrast Media from January 1, 2007, to June 30, 2012
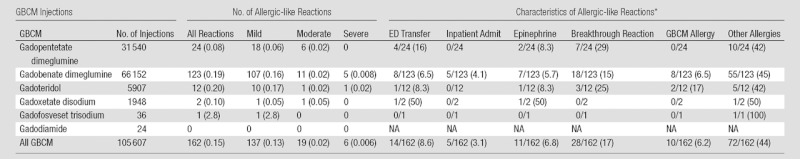
Note.—Allergic-like reaction rates were calculated according to the total number of injections for each gadolinium-based contrast medium. Rates of allergic-like reaction characteristics are calculated according to the total number of allergic-like reactions for each agent. The allergic-like reaction characteristics are not mutually exclusive. “Reactions” refers to allergic-like reactions. “ED transfer” refers to a transfer to the emergency department initiated as a direct result of the allergic-like reaction. “Inpatient admit” refers to an inpatient admission initiated as a direct result of the allergic-like reaction. “Epinephrine” refers to epinephrine administered as part of the management of the allergic-like reaction. “Breakthrough reaction” refers to allergic-like reaction occurring despite the presence of corticosteroid prophylaxis. “GBCM allergy” refers to allergic-like reaction occurring in a patient with a history of gadolinium-based contrast medium allergic-like reaction. “Other allergies” refers to allergic-like reaction occurring in a patient with a history of allergy to another substance. ED = emergency department, GBCM = gadolinium-based contrast medium, NA = not applicable.
Data are numbers of reactions, with percentages in parentheses.
Gadopentetate dimeglumine, which was approved by the FDA in 1988 (n = 31 540 total administrations during the study period), was the primary gadolinium-based contrast agent used during 2007 and 2008 because of an institutional prime vendor contract with Bayer Healthcare. This contract changed to Bracco Diagnostics in the first quarter of 2009. As a result, gadopentetate dimeglumine was replaced with gadobenate dimeglumine, which was approved by the FDA in 2004. This resulted in a precipitous decline in the number of gadopentetate dimeglumine–enhanced studies and a concomitant increase in the number of gadobenate dimeglumine–enhanced studies (66 152 total administrations during the study period). Gadodiamide was used rarely (24 administrations), and gadoteridol was used infrequently (5907 administrations) during the study period at the discretion of the supervising radiologist. Gadoteridol was not available for use until the 4th quarter of 2008. Gadoxetate disodium, which was approved by the FDA in 2008, was used for hepatobiliary imaging (1948 administrations), and gadofosveset trisodium was used for rare MR angiographic purposes (MR venography and MR arteriography) (36 administrations) at the discretion of the radiologist prescribing the study protocol. Gadoxetate disodium was not available for use until the 1st quarter of 2009, and gadofosveset trisodium was not available for use until the 3rd quarter of 2010.
Allergic-like Reactions
Radiologists (residents, fellows, or attending physicians) respond to every possible allergic-like reaction identified by a Department of Radiology technologist or nurse, evaluate and manage each possible reaction, and complete written documentation of the event. The written documentation during the study period included a standardized adverse event form that identified the type, dose, and route of the contrast material; the presence of corticosteroid prophylaxis; patient vital signs, symptoms, and signs; physician assessment; and treatment (including administration of medications, intravenous fluids, or oxygen, as well as disposition). A copy of each form was stapled to the paper requisition so that it was available to the radiologist at the time the study findings were dictated. A copy of the form was also hand delivered to the quality assurance manager (a registered nurse) for internal monitoring. This written documentation was always completed by the responding physician. Radiology nurses were also instructed to update each patient’s “allergy” history in the electronic medical record.
In addition to collecting the data on the reaction forms, the patient’s electronic medical record was queried to record the following for each allergic-like reaction: patient age and sex; type, dose, and route of the gadolinium-based contrast agent; history of allergy to gadolinium-based contrast media or other allergens; reaction severity (mild, moderate, or severe); manifestations; treatment characteristics (medications administered, emergency department transfer, inpatient hospital admission, outcome); and presence of corticosteroid prophylaxis. Allergic-like reactions occurring despite corticosteroid prophylaxis were termed “breakthrough reactions,” a term adopted by Freed et al in 2001 (16) and by others (17,18).
Allergic-like reactions were classified as mild, moderate, or severe according to previously published guidelines (10). Mild allergic-like reactions were characterized by one or more of the following: hives, pruritus, localized facial edema, nasal congestion, sneezing, and “scratchy throat.” Moderate allergic-like reactions were characterized by one or more of the following: diffuse erythema, dyspnea, wheezing, stridor, or emergency department transfer. Severe allergic-like reactions were characterized by one or more of the following: severe laryngeal edema, cardiopulmonary collapse, anaphylactoid shock, or hospital admission.
Physiologic reactions (eg, vasovagal reactions, nausea, vomiting) and contrast medium extravasations were not analyzed because they are not allergic-like reactions.
Power Calculation
A noninferiority power calculation was performed to determine the number of patients that would be needed to claim that the rates of severe reactions between two gadolinium-based contrast media were similar. The following assumptions were used: α, .05; power, 80%; prevalence of severe reactions in reference population, 0.01%; and noninferiority margin, 0.01%. This yielded a needed sample size of 123 639 administrations of gadolinium-based contrast media for each arm, indicating that a total of 247 278 total administrations would be needed for appropriate statistical power to claim that two gadolinium-based contrast agents were noninferior with respect to their incidence of severe allergic-like reactions.
Statistical Analysis
Continuous variables (eg, patient age, rates of allergic-like reactions) were summarized by using means and ranges. Categoric data (eg, patient sex, characteristics and treatment of allergic-like reactions, patient history of allergy) are presented as counts and percentages. Rates and characteristics of allergic-like reactions were compared between the different gadolinium-based contrast media (minimum total number of administrations, 1000) by using a χ2 or Fisher exact test (with odds ratios [ORs] and 95% confidence intervals [CIs]), as appropriate. We performed χ2 tests with ORs and 95% CIs to compare the rates of allergic-like reactions with gadolinium-based contrast media between sexes and among different age groups (pediatric [<18 years of age], adult [18–65 years of age], and elderly [>65 years of age]). The pediatric age range was chosen on the basis of commonly accepted age limits for this group. The age threshold differentiating adult from elderly patients was arbitrary. A P value of .05 or less was considered to represent a significant difference for all hypothesis tests. The preceding procedures were performed with statistical software (SAS, version 9.2; SAS Institute, Cary, NC).
Results
There were 162 allergic-like reactions (137 mild [12 with “scratchy throat”; 80 with hives or pruritus; 14 with localized erythema; 19 with localized facial swelling; five with sneezing, congestion, or watery eyes; and seven with self-limited transient dyspnea that resolved without medication], 19 moderate [16 with moderate dyspnea, two with diffuse erythema, and one with transient laryngospasm], and six severe [two with laryngospasm, two with severe dyspnea, one with angioedema, and one with anaphylactic shock, all resulting in inpatient hospitalization and/or emergency department admission]) associated with 105 607 injections of gadolinium-based contrast media, for an overall rate of allergic-like reactions of 0.15% (Table 1). There were 42 allergic-like reactions in 40 male patients (mean age, 46 years; range, 11–75 years) and 120 allergic-like reactions in 114 female patients (mean age, 43 years; range, 7–90 years). Table 2 summarizes the number of allergic-like reactions and number of administrations of each gadolinium-based contrast agent by age and sex during the study period. Although female patients (0.21% [120 of 57 524]) were significantly more likely than male patients (0.09% [42 of 47 892]) to experience an allergic-like reaction (OR, 2.4; 95% CI: 1.7, 3.4; P < .0001), there was no significant difference in the severity of allergic-like reactions (P = .74) between male patients (mild in 35, moderate in six, severe in one) and female patients (mild in 102, moderate in 13, severe in five). Pediatric patients (0.05% [eight of 15 706]; P < .0001) and patients older than 65 years of age (0.07% [15 of 20 074]; P = .0002) had a significantly lower rate of allergic-like reactions associated with gadolinium-based contrast media than did patients aged 18–65 years (0.20% [139 of 69 636]). The rates of allergic-like reactions to gadolinium-based contrast media for pediatric patients and those older than 65 years did not significantly differ (P = .50).
Table 2.
Allergic-like Reaction Rates for Different Gadolinium-based Contrast Media for a Variety of Age Group and Sex Combinations
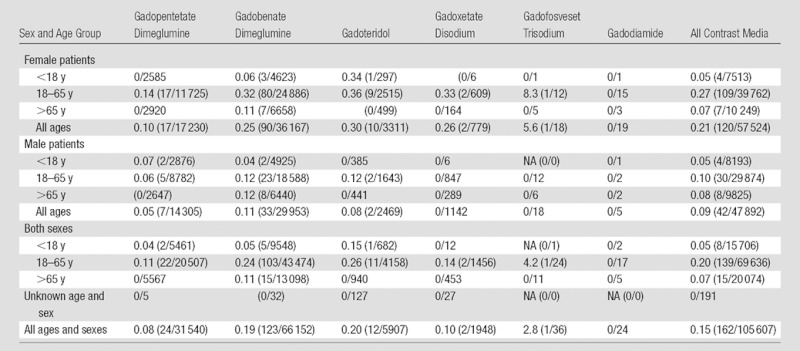
Note.—Data are percentages, with numerators/denominators in parentheses. Numerators are the numbers of allergic-like reactions, and denominators are the numbers of intravenous administrations of gadolinium-based contrast media. NA = not applicable.
The number and severity of allergic-like reactions that occurred with respect to each gadolinium-based contrast agent are listed in Table 1. Comparisons of the overall rates of allergic-like reactions for the gadolinium-based contrast media in this study are summarized in Table 3 (minimum total number of administrations per agent, 1000). Gadobenate dimeglumine was associated with significantly more overall (0.19% [123 of 66 152] vs 0.08% [24 of 31 540]; OR, 2.4 [95% CI, 1.6, 3.8]; P < .0001) and mild (0.16% [107 of 66 152] vs 0.06% [18 of 31 540]; OR, 2.8 [95% CI, 1.7, 4.7]; P < .0001) allergic-like reactions than gadopentetate dimeglumine. However, the frequencies of moderate (11 of 66 152 [0.02%] vs six of 31 540 [0.02%], respectively) and severe (five of 66 152 [0.008%] vs 0 of 31 540 [0.0%], respectively) allergic-like reactions for these two gadolinium-based contrast media were not significantly different. Three of the five severe reactions to gadobenate dimeglumine occurred in patients who were premedicated with corticosteroids (two for a history of prior reaction to gadolinium-based contrast media [now a listed contraindication to gadobenate dimeglumine exposure (19)] and one for a history of reactions to other drugs).
Table 3.
χ2 Statistics Comparing Overall Allergic-like Reaction Rates among the Various Intravenous Gadolinium-based Contrast Media Administered during the Study
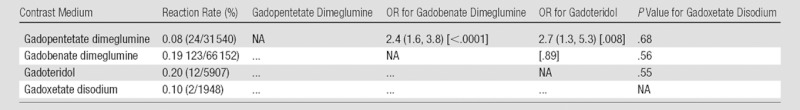
Note.—The minimum total number of administrations per agent was 1000. Data in parentheses are 95% CIs, and data in square brackets are P values.
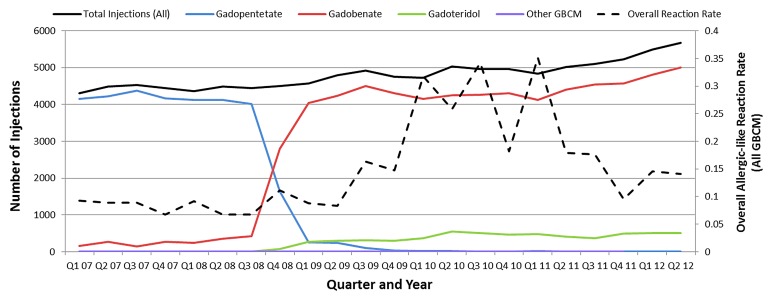
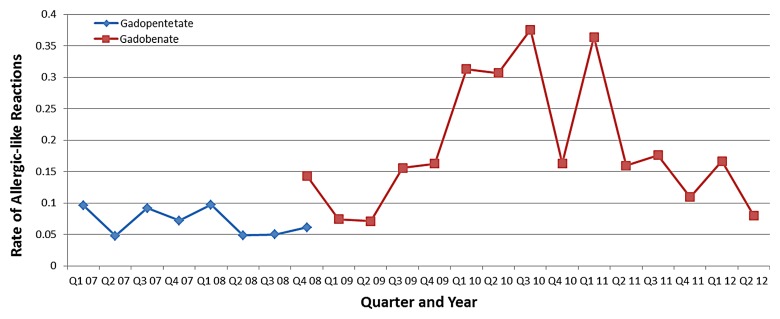
Table 4 compares biquarterly rates of allergic-like reactions to gadobenate dimeglumine to the overall reaction rate associated with gadopentetate dimeglumine. This, along with Figures 1 and 2, demonstrates a significant (P < .0001) peak in reaction rates approximately 2 years after gadobenate dimeglumine replaced gadopentetate dimeglumine at our institution. The final gadobenate dimeglumine reaction rate (last 3 quarters, 0.12% [17 of 14 387]) was not significantly different from the original baseline reaction rate to gadopentetate dimeglumine (0.08% [24 of 31 540]; OR, 1.6; 95% CI: 0.8, 2.9; P = .22).
Table 4.
χ2 Statistics Comparing Biquarterly Allergic-like Reaction Rates for Gadobenate Dimeglumine with the Aggregate Rate of Allergic-like Reactions for Gadopentetate Dimeglumine
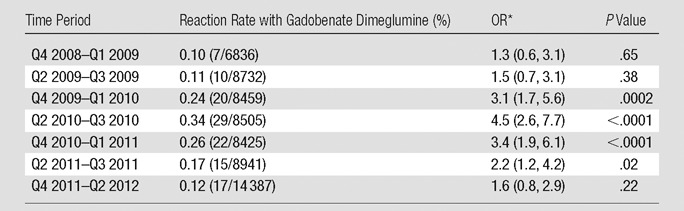
Note.—The minimum number of total administrations of gadobenate dimeglumine was 1000. The aggregate rate of allergic-like reaction with gadopentetate dimeglumine was 0.08% (24 of 31 540). The final coupling includes a total of 3 quarters (to incorporate the final uneven quarter of data). The temporal trend in the reaction rates supports the influence of the Weber effect. Q = quarter.
Data in parentheses are 95% CIs.
Figure 1:
Graph shows numbers of intravenous administrations of gadolinium-based contrast media (GBCM) according to 3-month quarter with respect to the aggregate allergic-like reaction rate for all contrast media.
Figure 2:
Graph shows rates of allergic-like reactions for gadopentetate dimeglumine and gadobenate dimeglumine according to 3-month quarter (minimum number of administrations per quarter, 500).
Allergic-like reactions to gadobenate dimeglumine and gadopentetate dimeglumine demonstrated no significant difference in the rates of epinephrine use (seven of 123 [4.1%] vs two of 24 [8.3%]; P = .64), frequency of transfer to the emergency department (eight of 123 [6.5%] vs four of 24 [16%]; P = .11), frequency of inpatient admission (five of 123 [4.1%] vs 0 of 24 [0%]; P = .59), breakthrough reaction rates (18 of 123 [15%] vs seven of 24 [29%], P = .13), or rates of allergy to other substances (55 of 123 [45%] vs 10 of 24 [42%]; P = .96). However, despite the large number of gadobenate dimeglumine and gadopentetate dimeglumine administrations in this study, the study is underpowered to detect differences in the rates of these uncommon associations.
Gadoteridol was associated with a significantly greater number of overall (OR, 2.7; 95% CI: 1.3, 5.3; P = .008) and mild (OR, 3.0; 95% CI: 1.4, 6.4; P = .008) allergic-like reactions when compared with gadopentetate dimeglumine. Additional associations and temporal patterns were difficult to analyze because of the relatively low number of gadoteridol administrations (n = 5907). Gadofosveset trisodium (n = 36) and gadodiamide (n = 24) were administered too infrequently to permit reasonable statistical analysis of their rates of allergic-like reaction.
Figure 1 demonstrates the aggregate rate of allergic-like reactions associated with gadolinium-based contrast media according to quarter year, as well as the number of injections of each agent over the same period. Figure 2 demonstrates the rate of allergic-like reactions according to 3-month quarter for gadobenate dimeglumine and gadopentetate dimeglumine. The increase in the number of allergic-like reactions beginning in 2009 corresponds temporally to the replacement of gadopentetate dimeglumine with gadobenate dimeglumine as our primary gadolinium-based contrast medium. The reaction rate for gadobenate dimeglumine peaked (maximum rate per quarter, 0.38% [16 of 4262]; minimum rate per quarter, 0.07% [three of 4237]) in the 3rd quarter of the 2nd year after it replaced gadopentetate dimeglumine (maximum rate per quarter, 0.10% [four of 4122]; minimum rate per quarter, 0.05% [two of 4222]), and then declined in the 3rd year of use.
No patients died of allergic-like reactions related to the administration of gadolinium-based contrast media at our institution during the study period.
Discussion
Gadolinium-based contrast media are widely used in MR imaging examinations and have an excellent safety profile overall (20,21). However, gadolinium-based contrast media are not identical in chemical structure, and some agents may be associated with different adverse event rates with respect to both NSF (1,8) and possibly allergic-like reactions (11,12). To determine the most suitable gadolinium-based contrast media for any specific patient, the diagnostic properties and benefits should be weighed against each unique risk profile. For example, if an agent is considered to be associated with a higher risk for the development of NSF but has a lower risk for allergic-like reactions, that agent may be preferable in a patient population at low risk for NSF (and vice versa). Because several major organizations (including the American College of Radiology, the FDA, and the European Society of Urogenital Radiology) have recently issued recommendations regarding the relative NSF risk among different gadolinium-based contrast media, we sought to compare the rate and severity of allergic-like reactions to gadobenate dimeglumine (an agent with a reportedly lower risk for NSF) after it replaced gadopentetate dimeglumine (an agent with a reportedly higher risk for NSF) at our institution.
The rates of allergic-like reaction with gadolinium-based contrast media reported in this study are similar to those of other published studies (10–12,22–24). All the gadolinium-based contrast media used in our study had good safety profiles, and no patient deaths were definitively attributed to these agents at our institution during the study period. Our results demonstrate that there were significantly more aggregate and mild allergic-like reactions for gadobenate dimeglumine (P < .0001 and P < .0001 for both) and gadoteridol (P = .008 and P = .008 for both) than for gadopentetate dimeglumine. The difference in reaction rates between gadobenate dimeglumine and gadopentetate dimeglumine was related primarily to a peak in the incidence of reported allergic-like reactions that occurred in the 2nd year after gadobenate dimeglumine replaced gadopentetate dimeglumine at our institution. This peak reaction rate was significantly different from the baseline rate of gadopentetate dimeglumine (0.34% [29 of 8505] vs 0.08% [24 of 31 540]; OR, 4.5; 95% CI: 2.6, 7.7; P < .0001). The reaction rate for gadobenate dimeglumine declined sharply in the 3rd year after the substitution, and by the last 3 documented quarters, the reaction rate was no longer significantly different from the baseline reaction rate of gadopentetate dimeglumine (0.12% [17 of 14 387] vs 0.08% [24 of 31 540]; OR, 1.6; 95% CI: 0.8, 2.9; P = .22). The rates of moderate and severe allergic-like reactions for each of these gadolinium-based contrast media were not significantly different, but unfortunately our study was underpowered to determine whether this is a meaningful finding (123 639 administrations would be needed per arm to confirm noninferiority for severe reactions, according to our power calculation).
We detected a significant difference (P < .0001) in allergic-like reaction rates by sex, with female patients having an aggregate rate of allergic-like reactions associated with gadolinium-based contrast media of 0.21% (120 of 57 524), compared with 0.09% (42 of 47 892) in male patients. This finding has been reported previously (10). There were similar numbers of moderate and severe reactions between male and female patients, although the number of such reactions was low. The rates of allergic-like reactions to gadolinium-based contrast media in our pediatric and elderly populations were less than that of the remaining adult population, with an aggregate pediatric rate of allergic-like reactions associated with gadolinium-based contrast media of 0.05% (eight of 15 706), compared with 0.20% (139 of 69 636) for patients aged 18–65 years and 0.07% (15 of 20 074) for patients older than 65 years. The rate of allergic-like reactions in children in our study is similar to data on pediatric use of gadolinium-based contrast media published in 2007 (0.04% [six of 13 344]) (10).
Our findings, which show a significant difference in allergic-like reaction rates between gadobenate dimeglumine and gadopentetate dimeglumine, appear to conflict with those of a previously published study by Shellock et al (21). Those authors reported that the adverse event rates of gadopentetate dimeglumine and gadobenate dimeglumine were similar (7.0% [33 of 472] vs 9.1% [51 of 561], respectively). However, differences between the two studies may help explain this. First, their study was underpowered and analyzed results from only 1033 administrations of gadolinium-based contrast media, whereas our study is approximately 100-fold greater. It is possible that because of the known very low rates of allergic-like reactions with gadolinium-based contrast media in general, differences among unique gadolinium-based contrast media could be obscured in a smaller patient population. Second, Shellock et al combined all adverse events into one category, whereas our study focused solely on allergic-like reactions. Finally, the study by Shellock et al used a randomized design in which patients received one or the other gadolinium-based contrast medium in parallel. Our study investigated the effects of abruptly substituting gadobenate dimeglumine for gadopentetate dimeglumine at a single institution. Our study design is subject to the influence of the Weber effect, whereas the study of Shellock et al might not be.
The Weber effect is a known phenomenon in the epidemiology literature (13–15) in which the number of spontaneously reported adverse events for a drug tends to peak around the 2nd year after its introduction. Although not all drugs exhibit a classic Weber effect, it is known to often occur (14). For example, Hartnell et al (14) demonstrated the classic Weber effect with fluvoxamine (a selective serotonin reuptake inhibitor), but not with fluoxetine, paroxetine, or sertraline (medications of the same class). In their study, the adverse event rates for fluoxetine, paroxetine, and sertraline instead peaked 1–2 years after each new indication was released for each respective drug. They suggested that approval of new indications might also result in transient increases in adverse event reporting in a pattern similar to the classic Weber effect. Another study by Hartnell and Wilson (25) investigating the Weber effect in the United States with respect to nonsteroidal antiinflammatory drugs showed that it occurred with all five drugs included in their report, with peak incidences of adverse events occurring in the 2nd year after each drug was introduced. A study by Wallenstein and Fife (15) showed that three of five nonsteroidal antiinflammatory drugs they studied followed a temporal pattern consistent with the Weber effect, whereas two of five did not.
The influence of the Weber effect suggests that the increase in adverse events associated with gadobenate dimeglumine in our study could be attributed to epidemiologic reporting bias and may not reflect a true difference in adverse events based on the pharmacologic structure of the contrast medium. The peak in adverse event reporting is predicted by the Weber effect to occur within the 2nd year after introduction of a new drug or 2 years after a new indication is provided for a preexisting drug (13–15,25). In our study, gadobenate dimeglumine was abruptly substituted for gadopentetate dimeglumine, and we observed a peak incidence of allergic-like reactions for this agent approximately 2 years later. During the 3rd year, the rate of allergic-like reactions declined sharply, as would be predicted by the Weber effect.
The reported rates of death for the dominant gadolinium-based contrast agent evaluated in this study (based on FDA data from 2004 to 2009) is 0.97 (gadopentetate dimeglumine), 0.7 (gadoteridol), and 2.7 (gadobenate dimeglumine) per million administrations, suggesting that there may be a difference in serious adverse events that was not detected in this study (11). However, it is important to note that the FDA approved gadobenate dimeglumine only in 2004, whereas gadopentetate dimeglumine was FDA approved in 1988. This difference in release dates may have affected FDA reporting data for gadobenate dimeglumine during this period because of the influence of the Weber effect. In our study, five severe reactions were related to gadobenate dimeglumine, and none were related to gadopentetate dimeglumine (not significantly different). Two of the five severe reactions to gadobenate dimeglumine occurred in patients with a history of reaction to gadolinium-based contrast media, which is now a listed contraindication to gadobenate dimeglumine exposure (19); both patients were premedicated before the study.
This study had several limitations. First, this was a retrospective study and relied on completed adverse event forms. Although our adverse event reporting system was rigorous and has been in place for more than a decade, it is subject to the possibility of data loss that could result in an underreporting of the total number of allergic-like reactions for any given gadolinium-based contrast media. However, this same bias was probably transmitted across all gadolinium-based contrast agents and is not expected to affect our comparison statistics. In addition, the method of recording adverse events was constant throughout the study dates, which should minimize the possibility of longitudinal systematic error. Second, although 105 607 injections of gadolinium-based contrast media were administered during the study, it was underpowered to confirm noninferiority among different gadolinium-based contrast media with respect to severe reactions, which are very uncommon. For such an analysis, at least 123 639 administrations of gadolinium-based contrast media would be needed in each arm.
In conclusion, abrupt substitution of gadobenate dimeglumine for gadopen-tetate dimeglumine resulted in a significant increase in reporting of allergic-like reactions that exhibited a temporal pattern suggestive of the Weber effect. This pattern can mimic true differences in reaction rates between gadolinium-based contrast media.
Advance in Knowledge.
• Abrupt substitution of gadobenate dimeglumine for gadopentetate dimeglumine resulted in a significant transient increase (0.34% [29 of 8505] vs 0.08% [24 of 31 540]; odds ratio, 4.5; 95% confidence interval: 2.6, 7.7; P < .0001) in reporting of allergic-like reactions in a temporal pattern suggestive of the Weber effect.
Implication for Patient Care.
• Changes in an institution’s prime vendor contract can result in a significant transient increase in rates of allergic-like reactions (0.34% [29 of 8505] vs 0.08% [24 of 31 540]; odds ratio, 4.5; 95% confidence interval: 2.6, 7.7; P < .0001) that might mimic a true difference in reaction rates among different gadolinium-based contrast media.
Disclosures of Conflicts of Interest: M.S.D. No relevant conflicts of interest to disclose. J.R.D. Financial activities related to the present article: institution received support from Bracco Diagnostics for collecting data as part of phase IV surveillance of multihance; received minimal salary support from Bracco Diagnostics for chart review; GE Healthcare provided institution with investigator-initiated grant to compare iohexol and iopamidol with respect to effect on kidney function at CT. Financial activities not related to the present article: institution has pending investigator-initiated grants from Bracco Diagnostics and Siemens Medical Solutions to study contrast-enhanced ultrasonography. Other relationships: none to disclose. R.H.C. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for GE Healthcare; has given expert testimony for GE Healthcare, the U.S. Department of Justice, LeClair Ryan Attorneys at Law, and John Hickey, Esq; receives royalties from Lippincott, Williams, and Wilkins. Other relationships: none to disclose. H.K.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for and is on the speakers bureau of Bayer Healthcare. Other relationships: none to disclose. S.K. No relevant conflicts of interest to disclose. J.B.M. No relevant conflicts of interest to disclose. J.H.E. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: is a consultant for GE Healthcare; has given expert testimony for a law firm representing GE Healthcare. Other relationships: none to disclose.
Received January 31, 2012; revision requested March 28; revision received August 14; final version accepted September 2.
Funding: This research was supported by the National Institutes of Health (grant UL1RR024986).
Abbreviations:
- CI
- confidence interval
- FDA
- Food and Drug Administration
- NSF
- nephrogenic systemic fibrosis
- OR
- odds ratio
References
- 1.Public health advisory: gadolinium-containing contrast agents for magnetic resonance imaging (MRI). http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm. Originally published June 8, 2006. Updated December 2010. Accessed August 18, 2011
- 2.Thomsen HS, Marckmann P, Logager VB. Nephrogenic systemic fibrosis (NSF): a late adverse reaction to some of the gadolinium based contrast agents. Cancer Imaging 2007;7:130–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 2007;243(1):148–157 [DOI] [PubMed] [Google Scholar]
- 4.Grobner T. Gadolinium: a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 2006;21(4):1104–1108 [DOI] [PubMed] [Google Scholar]
- 5.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 2006;17(9):2359–2362 [DOI] [PubMed] [Google Scholar]
- 6.Kuo PH, Kanal E, Abu-Alfa AK, Cowper SE. Gadolinium-based MR contrast agents and nephrogenic systemic fibrosis. Radiology 2007;242(3):647–649 [DOI] [PubMed] [Google Scholar]
- 7.Thomsen HS, Morcos SK, Dawson P. Is there a causal relation between the administration of gadolinium based contrast media and the development of nephrogenic systemic fibrosis (NSF)? Clin Radiol 2006;61(11):905–906 [DOI] [PubMed] [Google Scholar]
- 8.American College of Radiology Manual on contrast media. 7th ed. Reston, Va: American College of Radiology, 2011 [Google Scholar]
- 9.ESUR Guidelines on Contrast Media, v 7.0: very late adverse reactions. http://www.esur.org/Contrast-media.51.0.html; Accessed August 18, 2011
- 10.Dillman JR, Ellis JH, Cohan RH, Strouse PJ, Jan SC. Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am J Roentgenol 2007;189(6):1533–1538 [DOI] [PubMed] [Google Scholar]
- 11.Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 2011;196(2):W138–W143 [DOI] [PubMed] [Google Scholar]
- 12.Abujudeh HH, Kosaraju VK, Kaewlai R. Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: experience with 32,659 injections. AJR Am J Roentgenol 2010;194(2):430–434 [DOI] [PubMed] [Google Scholar]
- 13.Weber JCP. Epidemiology of adverse reactions to nonsteroidal antiinflammatory drugs. In: Rainsford KD, Velo GP, eds. Advances in inflammation research, Vol 6. New York, NY: Raven, 1984; 1–7 [Google Scholar]
- 14.Hartnell NR, Wilson JP, Patel NC, Crismon ML. Adverse event reporting with selective serotonin-reuptake inhibitors. Ann Pharmacother 2003;37(10):1387–1391 [DOI] [PubMed] [Google Scholar]
- 15.Wallenstein EJ, Fife D. Temporal patterns of NSAID spontaneous adverse event reports: the Weber effect revisited. Drug Saf 2001;24(3):233–237 [DOI] [PubMed] [Google Scholar]
- 16.Freed KS, Leder RA, Alexander C, DeLong DM, Kliewer MA. Breakthrough adverse reactions to low-osmolar contrast media after steroid premedication. AJR Am J Roentgenol 2001;176(6):1389–1392 [DOI] [PubMed] [Google Scholar]
- 17.Dillman JR, Ellis JH, Cohan RH, Strouse PJ, Jan SC. Allergic-like breakthrough reactions to gadolinium contrast agents after corticosteroid and antihistamine premedication. AJR Am J Roentgenol 2008;190(1):187–190 [DOI] [PubMed] [Google Scholar]
- 18.Davenport MS, Cohan RH, Caoili EM, Ellis JH. Repeat contrast medium reactions in premedicated patients: frequency and severity. Radiology 2009;253(2):372–379 [DOI] [PubMed] [Google Scholar]
- 19.Package insert MultiHance (gadobenate dimeglumine injection). Bracco Diagnostics, Princeton NJ. November 2010 [Google Scholar]
- 20.Knopp MV, Balzer T, Esser M, Kashanian FK, Paul P, Niendorf HP. Assessment of utilization and pharmacovigilance based on spontaneous adverse event reporting of gadopentetate dimeglumine as a magnetic resonance contrast agent after 45 million administrations and 15 years of clinical use. Invest Radiol 2006;41(6):491–499 [DOI] [PubMed] [Google Scholar]
- 21.Shellock FG, Parker JR, Pirovano G, et al. Safety characteristics of gadobenate dimeglumine: clinical experience from intra- and interindividual comparison studies with gadopentetate dimeglumine. J Magn Reson Imaging 2006;24(6):1378–1385 [DOI] [PubMed] [Google Scholar]
- 22.Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol 1996;167(4):847–849 [DOI] [PubMed] [Google Scholar]
- 23.Bleicher AG, Kanal E. Assessment of adverse reaction rates to a newly approved MRI contrast agent: review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol 2008;191(6):W307–W311 [DOI] [PubMed] [Google Scholar]
- 24.Morgan DE, Spann JS, Lockhart ME, Winningham B, Bolus DN. Assessment of adverse reaction rates during gadoteridol-enhanced MR imaging in 28,078 patients. Radiology 2011;259(1):109–116 [DOI] [PubMed] [Google Scholar]
- 25.Hartnell NR, Wilson JP. Replication of the Weber effect using postmarketing adverse event reports voluntarily submitted to the United States Food and Drug Administration. Pharmacotherapy 2004;24(6):743–749 [DOI] [PubMed] [Google Scholar]