Tumor blood volume assessment by using perfusion MR imaging with ferumoxytol may help differentiate true tumor progression from pseudoprogression in patients with glioblastoma multiforme who show disease progression at conventional MR imaging after chemotherapy and radiation therapy.
Abstract
Purpose:
To compare gadoteridol and ferumoxytol for measurement of relative cerebral blood volume (rCBV) in patients with glioblastoma multiforme (GBM) who showed progressive disease at conventional magnetic resonance (MR) imaging after chemo- and radiation therapy (hereafter, chemoradiotherapy) and to correlate rCBV with survival.
Materials and Methods:
Informed consent was obtained from all participants before enrollment in one of four institutional review board–approved protocols. Contrast agent leakage maps and rCBV were derived from perfusion MR imaging with gadoteridol and ferumoxytol in 19 patients with apparently progressive GBM on conventional MR images after chemoradiotherapy. Patients were classified as having high rCBV (>1.75), indicating tumor, and low rCBV (≤1.75), indicating pseudoprogression, for each contrast agent separately, and with or without contrast agent leakage correction for imaging with gadoteridol. Statistical analysis was performed by using Kaplan-Meier survival plots with the log-rank test and Cox proportional hazards models.
Results:
With ferumoxytol, rCBV was low in nine (47%) patients, with median overall survival (mOS) of 591 days, and high rCBV in 10 (53%) patients, with mOS of 163 days. A hazard ratio of 0.098 (P = .004) indicated significantly improved survival. With gadoteridol, rCBV was low in 14 (74%) patients, with mOS of 474 days, and high in five (26%), with mOS of 156 days and a nonsignificant hazard ratio of 0.339 (P = .093). Five patients with mismatched high rCBV with ferumoxytol and low rCBV with gadoteridol had an mOS of 171 days. When leakage correction was applied, rCBV with gadoteridol was significantly associated with survival (hazard ratio, 0.12; P = .003).
Conclusion:
Ferumoxytol as a blood pool agent facilitates differentiation between tumor progression and pseudoprogression, appears to be a good prognostic biomarker, and unlike gadoteridol, does not require contrast agent leakage correction.
© RSNA, 2012
Introduction
Radiation therapy in combination with temozolomide chemotherapy (hereafter, chemoradiotherapy) shows a significant survival benefit in patients with newly diagnosed glioblastoma multiforme (GBM) and has become the standard of care (1). However, radiologic deterioration is seen in patients more frequently after chemoradiotherapy than in those who receive radiation alone. Radiologic deterioration appears as an enlarged area of enhancement on contrast material–enhanced T1-weighted magnetic resonance (MR) images in up to 50% of all patients who have received chemoradiotherapy (2–4). Radiologic deterioration with or without clinical worsening can reflect either tumor progression or treatment-induced inflammatory change with increased permeability of the blood-brain barrier, which is known as pseudoprogression (5–8). Apparent tumor progression on MR images may actually represent pseudoprogression in up to 64% of cases (4). Patients with pseudoprogression, unlike those with tumor progression, recover or stabilize spontaneously, generally without any changes in their treatment paradigm. Moreover, pseudoprogression is associated with a favorable prognosis and with O-6-methylguanine-DNA methyltransferase methylation status (4,5).
Conventional T2-weighted and contrast material–enhanced T1-weighted MR imaging sequences do not allow differentiation of tumor progression from pseudoprogression (5,9). Therefore, there is an urgent need to develop imaging tools that will assist clinicians in evaluating treatment effects and allow them to make appropriate therapeutic decisions. One of these imaging modalities is dynamic susceptibility-weighted contrast-enhanced (DSC) MR imaging measurement of relative cerebral blood volume (rCBV), which has been used for glioma grading (10–13), assessment of prognosis for patients with gliomas (13–17), and differentiation of recurrent tumor from radiation necrosis (18–20). High rCBV values indicate active neovascularization and a viable tumor (16,21,22). Accurate measurement of tumor rCBV by using standard DSC modeling approaches requires intravascular localization of contrast agent, which is compromised by the leaky blood-brain barrier present in patients with malignant brain tumors, especially after chemoradiotherapy. Rapid extravasation of a low-molecular-weight gadolinium-based contrast agent (GBCA) can result in underestimation of tumor rCBV (19–21,23).
Ferumoxytol, a very small superparamagnetic iron oxide nanoparticle that is approved by the U.S. Food and Drug Administration for iron replacement therapy in adults with chronic kidney disease, has shown utility in brain imaging (24–26). Because ferumoxytol does not undergo renal elimination, it may serve as an alternative to GBCA in patients who are at risk for nephrogenic systemic fibrosis (27). Ferumoxytol acts as a blood pool agent shortly after administration because the iron nanoparticles are large (approximately 30 nanometers), and vascular localization is not compromised by the leaky blood-brain barrier (25). In addition, unlike other iron oxide nanoparticle contrast agents, ferumoxytol is administered as a fast bolus injection in therapeutic use (26). For these reasons, we hypothesized that ferumoxytol has the potential to allow measurement of rCBV more reliably than does GBCA.
The goal of this study was to compare gadoteridol versus ferumoxytol for measurement of relative cerebral blood volume (rCBV) in patients with glioblastoma multiforme who showed progressive disease at conventional magnetic resonance (MR) imaging after chemoradiotherapy and to correlate rCBV with survival.
Materials and Methods
Between April 2007 and October 2009, 19 patients with GBM were prospectively studied in one of four different research imaging protocols that were sponsored by the National Institutes of Health and approved by the institutional review board. The four protocols were designed to compare anatomic and dynamic MR imaging by using GBCA versus ferumoxytol. The number of patients from each protocol that were included in our analysis and the objective of each protocol were as follows: six patients from protocol 2753 (http://clinicaltrials.gov/ct2/show/NCT006-60543?term=neuwelt+and+portland&-rank=3), which was a study of serial imaging changes of GBM patients before and after chemoradiotherapy; five patients from protocol 2864 (http://clinicaltrials.gov/ct2/show/NCT00659126?term=neuwelt+and+portland&rank=8), a study of sequential imaging with gadoteridol and ferumoxytol at 3-T and 7-T MR imaging in patients with primary or metastatic brain tumors either before or after therapy; seven patients from protocol 1562 (http://clinicaltrials.gov/ct2/show/NCT00659776?term=neuwelt+and+portland&rank=6), a study of MR imaging of central nervous system inflammatory lesions with intravenous gadoteridol and ferumoxytol; and one patient from protocol 3678 (http://clinicaltrials.gov/ct2/show/NCT007690-93?term=neuwelt+and+portland&rank=10), a comparison of imaging changes induced by bevacizumab versus dexamethasone in patients with recurrent high-grade glioma. As of October 2009, 84 patients were imaged in these four protocols, 27 (32%) had a diagnosis of GBM, and 19 patients with GBM met the inclusion criteria for our study. Informed written consent was obtained from all patients.
Inclusion criteria for this analysis included histologically proved GBM (World Health Organization classification, grade IV) and apparent tumor progression at conventional MR imaging after standard postsurgical treatment of radiation therapy and chemotherapy with temozolomide. No patients who met the inclusion criteria were excluded from analysis. Inclusion criteria common to all protocols were Karnofsky Performance Score greater than 50 and absence of contraindication for GBCA or ferumoxytol administration. Collected patient data included age, sex, extent of surgery, radiation dose, date of chemoradiotherapy, date of first conventional MR imaging examination that was suggestive of progression, date of DSC MR imaging examination of the study, ferumoxytol and gadoteridol dose, dexamethasone dose, and antiangiogenic treatment after study. The patients were followed up until death or the date of their last follow-up examination. Patients who were still alive at the time of analysis were treated as censored cases for the data analysis.
Magnetic Resonance Imaging
Nineteen patients who underwent conventional MR imaging after chemoradiotherapy and received results that suggested progression of the disease were included. Patients underwent a total of 19 MR imaging sessions on two consecutive days. On the first day, unenhanced and contrast-enhanced T1-weighted images and DSC MR images were acquired by using gadoteridol gadolinium (III) chelate (ProHance, Bracco Diagnostic, Princeton, NJ). On the following day, the same MR imaging sequences were performed by using ferumoxytol (AMAG Pharmaceuticals, Cambridge, Mass). AMAG Pharmaceuticals provided ferumoxytol free of charge. The authors had full control of the data and the information submitted for publication. No patient had any complications during the MR imaging examinations.
MR imaging sessions for 18 of the 19 patients were conducted by using a 3-T whole-body MR imaging system (Tim Trio; Siemens, Erlangen, Germany) with a body radiofrequency coil transmitter and a 12-channel matrix head coil signal receiver. One patient underwent MR imaging sessions with a 7-T MR imaging system (Magnetom 7 T, Siemens) equipped with an eight-channel phased-array transmit-receive radiofrequency head coil (Rapid Biomedical, Rimpar, Germany).
For DSC MR imaging, dynamic T2*-weighted images were acquired by using a gradient-echo echo-planar imaging pulse sequence (repetition time msec/echo time msec, 1500/20; flip angle, 45°; field of view, 192 × 192 mm; matrix, 64 × 64; and 27 interleaved sections at 3 T or 13 sections at 7 T; section thickness, 3 mm; and section gap, 0.9 mm). After patients underwent a baseline period of seven imaging volumes (11 seconds), a rapid bolus of contrast agent was administered intravenously through an 18-gauge intravenous catheter at a rate of 3 mL per second by using a power injector (Spectris Solaris-Medrad, Warrendale, Pa), immediately followed by 20 mL of saline flush at the same rate. DSC data collection comprised a total of 90 series (2 minutes 21 seconds). Gadoteridol was injected at a dose of 0.1 mmol/kg of body weight. The ferumoxytol dose was dependent on the protocol in which the patient was enrolled and was administered at either 2 mg/kg, 1 mg/kg, or in a constant volume of 2.5 mL diluted with 2.5 mL of saline (75 mg) regardless of body weight. T1-weighted anatomic MR imaging was performed before and 20 minutes after gadoteridol administration by using a two-dimensional spin-echo sequence (900/10; field of view, 180 × 240 mm; matrix, 256 × 192; sections, 44; section thickness, 2 mm, gapless).
Imaging Analysis
All data were acquired (S.G. and C.V., with 4 and 8 years of experience, respectively), processed (S.G., C.V., and B.H., with 10 years of experience) and analyzed (D.K. and R.F., with 15 and 10 years of experience, respectively). The neuroradiologists (S.G., C.V., and B.H.) were blinded to survival data. All DSC MR imaging data were processed with a dedicated software package (NordicICE; NordicNeuroLab, Bergen, Norway). The rCBV maps were generated by using established tracer kinetic models applied to the first-pass data (28,29) without and with application of the mathematic leakage correction method (30). Leakage correction was applied only when gadoteridol was used because ferumoxytol is an intravascular contrast agent and eliminates the need for correction, and all correction methods were developed for GBCAs. On a pixel-by-pixel basis, the rCBV maps were normalized by dividing every rCBV value by the unaffected white matter rCBV value. The normalized rCBV maps were coregistered and displayed as color overlays on gadoteridol-enhanced T1-weighted images. In the enhancing lesion, a 2 × 2 pixel (6 × 6 mm) region of interest with the highest rCBV value on the ferumoxytol rCBV parametric map and the same region of interest on the gadoteridol rCBV parametric map were analyzed. Areas showing major vessels, obvious hemorrhage, and visible cystic and necrotic changes were excluded from regions of interest. Previous studies showed that a threshold rCBV ratio of 1.75, which was measured by using DSC MR imaging with GBCA, was predictive of time to progression or survival (14,16). Our prior study results showed the feasibility of this cutoff value for ferumoxytol as well (21). Therefore, in this study, we considered an rCBV greater than 1.75 to be high (active tumor) and rCBV less than or equal to 1.75 to be low (pseudoprogression). However, we also tested alternative cutoff values. By using the NordicICE software, we generated first-order estimates of vascular permeability or leakage maps, referred to as K2 in the Boxerman et al study (30), from DSC MR imaging data for both contrast agents for contrast agent extravasation visualization and assessment.
Statistical Analysis
To assess whether rCBV measurements allow differentiation of active tumor from pseudoprogression and to predict prognosis by using DSC MR imaging with ferumoxytol or GBCA, we classified each patient into one of two groups on the basis of rCBV value (rCBV > 1.75 and rCBV ≤ 1.75) for ferumoxytol, gadoteridol, and gadoteridol with leakage correction. Mean and standard deviation of rCBV measurements were obtained for each group. Differences between groups were assessed by using the Student paired t test and were graphed by using Bland-Altman plots.
For each contrast agent, median overall survival (mOS) and 95% confidence interval (CI) were assessed in each group by using Kaplan-Meier product limit estimates, and the groups were compared by using the log-rank test. When the upper limit of the 95% CI was not estimable, the highest observed survival time was reported. In addition, Cox proportional hazards models were fitted to estimate the hazard ratios separately for each contrast agent. These survival analyses were performed by using four different rCBV cutoff values (1.5, 1.75, 2.0, 2.5) for ferumoxytol, gadoteridol, and gadoteridol with leakage correction. A P value less than .05 was considered to indicate a significant difference. All analyses were performed by using SAS Version 9.2 for Windows (SAS Institute, Cary, NC).
Results
Nineteen patients with GBM showed apparent tumor progression on conventional MR images obtained within one year of chemoradiotherapy. In 16 of 19 (84%) patients, DSC MR imaging studies were performed within 6 months after completion of chemoradiotherapy, and three (16%) patients underwent DSC MR imaging 9, 10, and 11 months after chemoradiotherapy.
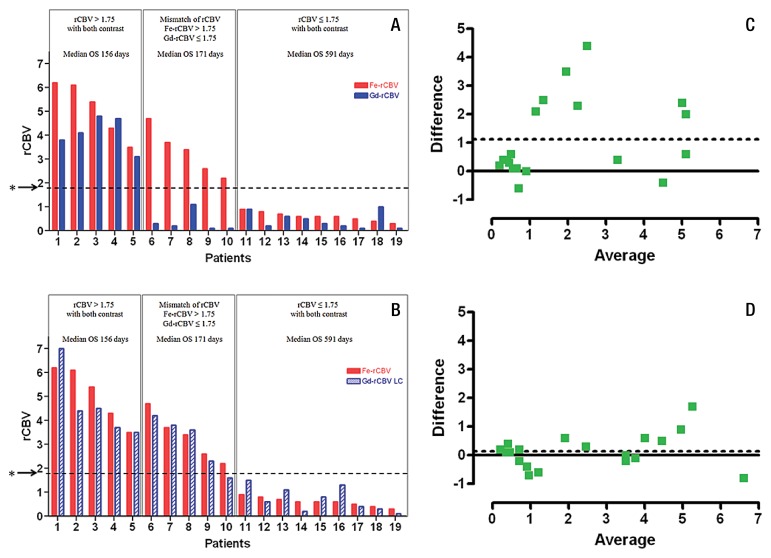
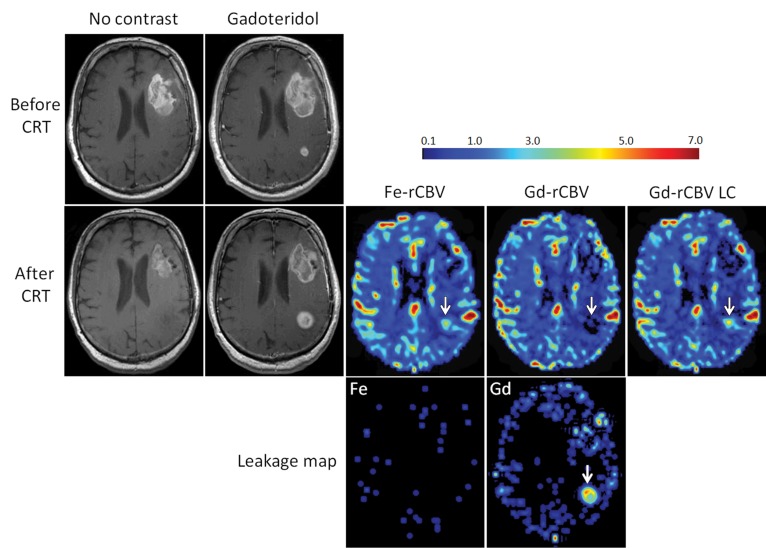
The mean and standard deviation for rCBV values in the entire group measured by using ferumoxytol, gadoteridol, and gadoteridol with leakage correction were 2.5 ± 2.1, 1.38 ± 1.73, and 2.36 ± 1.94, respectively, with a statistically significant difference between ferumoxytol and gadoteridol (P = .003), and between ferumoxytol and gadoteridol with leakage correction (P = .008). When leakage correction was applied, rCBV values measured with gadoteridol were not different from those measured with ferumoxytol (P = .33). On the basis of DSC MR imaging with ferumoxytol, nine (47%) patients had rCBV values less than or equal to 1.75 (0.6 ± 0.19) and 10 (53%) patients had rCBV values greater than 1.75 (4.21 ± 1.38) (Fig 1A and 1B). In the same patients, DSC MR imaging by using gadoteridol without leakage correction showed rCBV less than or equal to 1.75 in 14 (74%) patients (0.41 ± 0.36) and rCBV greater than 1.75 in five (26%) patients (4.1 ± 0.7). With leakage correction, the rCBV was less than or equal to 1.75 in 10 (53%) patients (0.79 ± 0.55) and greater than 1.75 in nine (47%) patients (4.1 ± 1.26) (Fig 1A and 1B). All nine patients who had low rCBV with ferumoxytol also had low rCBV with gadoteridol without and with leakage correction. Figure 2 shows a representative patient in whom conventional MR imaging suggested tumor progression but in whom DSC MR imaging with both ferumoxytol and gadoteridol showed low rCBV. Such results were unambiguously classified as pseudoprogression. All five patients who had high rCBV with gatoderidol without leakage correction and nine patients with leakage correction also had high ferumoxytol rCBV (Fig 1A and 1B). Figure 3 shows a patient who had high rCBV with both contrast agents, particularly at the tumor margin, which is indicative of true tumor progression. In five patients, rCBV values for ferumoxytol and gadoteridol were mismatched, where rCBV was greater than 1.75 with ferumoxytol and less than 1.75 with gadoteridol. However, after leakage correction application, only one patient had mismatched values (Fig 1A and 1B). The representative patient images in Figure 4 show progression in the posterior lesion with ferumoxytol and pseudoprogression with gadoteridol. With the application of leakage correction, accuracy of gadoteridol rCBV estimation improved and was suggestive of tumor progression. Bland-Altman plots showed that the average of the differences between rCBV values obtained by using ferumoxytol and those obtained by using gadoteridol was 1.12, and between rCBV values with ferumoxytol and those with gadoteridol with leakage correction, the difference was reduced to 0.14 (Fig 1C and 1D).
Figure 1:
Bar graphs and scatterplots show summary of rCBV values estimated at perfusion MR imaging with both contrast agents. Bar graphs A and B show tumor rCBVs with ferumoxytol (red bars, Fe rCBV), gadoteridol (blue bars, Gd rCBV) and gadoteridol with leakage correction (striped blue bars, Gd-rCBV LC) for each patient. Patients with high rCBV (>1.75) with both contrast agents had poor overall survival (OS). Patients with low rCBV (≤1.75) with both contrast agents had significantly longer overall survival. Patients with high rCBV with ferumoxytol and low rCBV with gadoteridol had poor prognosis. Asterisks indicate rCBV cutoff value of 1.75. Bland-Altman plots show average differences between rCBV values for, C, ferumoxytol and gadoteridol as 1.12 and, D, ferumoxytol and gadoteridol with leakage correction as 0.14.
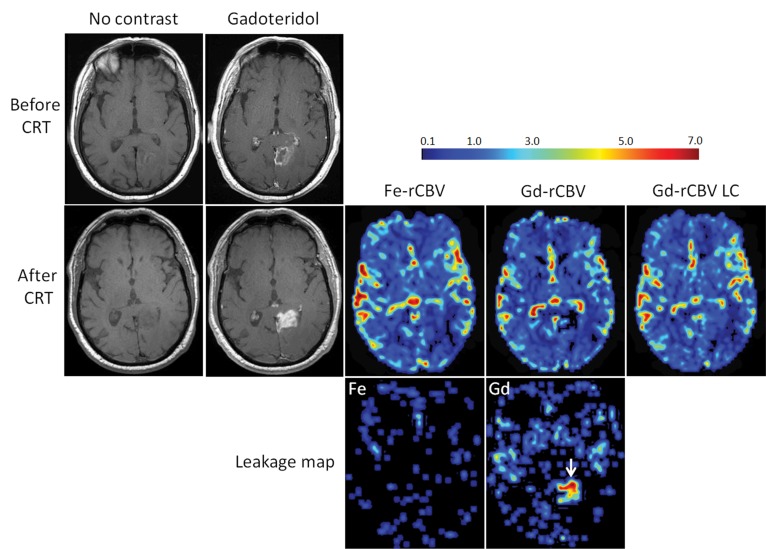
Figure 2:
Axial images of 73-year-old man with GBM show pseudoprogression of disease. T1-weighted MR images without contrast enhancement and with gadoteridol (Gd) obtained before and 3 months after chemoradiotherapy (CRT) show increased contrast enhancement after treatment. Low rCBV (≤1.75) is apparent on parametric maps obtained by using ferumoxytol (Fe-rCBV), gadoteridol (Gd-rCBV), and gadoteridol with leakage correction (Gd-rCBV LC), which indicates pseudoprogression. Leakage map shows absence of contrast extravasation when ferumoxytol (Fe) was used and contrast leakage with gadoteridol (arrow).
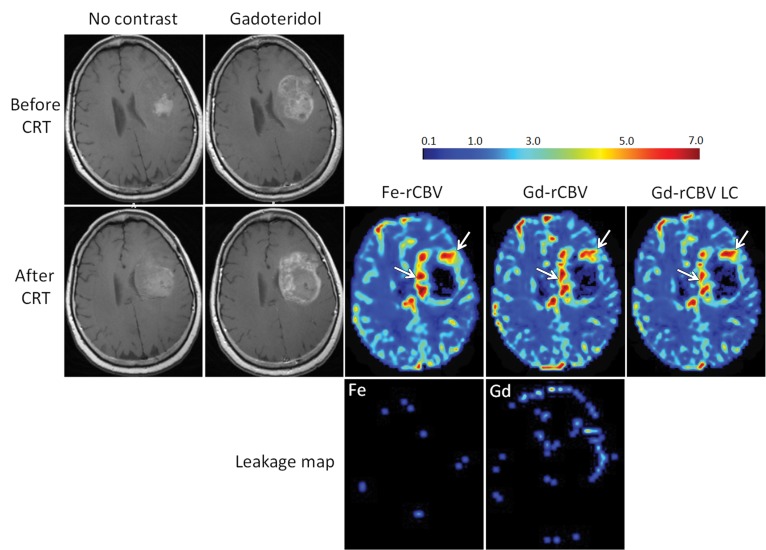
Figure 3:
Axial images of 59-year-old woman with GBM show progression of disease. T1-weighted MR images without contrast agent and with gadoteridol (Gd) obtained before and 2 weeks after chemoradiotherapy (CRT) show increased contrast enhancement after treatment. High rCBV (> 1.75) is apparent on parametric maps obtained by using ferumoxytol (Fe-rCBV), gadoteridol (Gd-rCBV), and gadoteridol with leakage correction (Gd-rCBV LC), which indicates true tumor progression (arrows). No contrast agent extravasation is seen on leakage map with ferumoxytol (Fe) but small leakage in the lateral aspect of the tumor is seen with gadoteridol.
Figure 4:
Axial images in 68-year-old man with GBM show discordance between rCBV values measured with ferumoxytol (Fe) and gadoteridol (Gd). T1-weighted MR images without contrast agent and with gadoteridol obtained before and 2 weeks after chemoradiotherapy (CRT) show increased contrast enhancement after treatment. High rCBV (> 1.75) on parametric maps obtained by using ferumoxytol (Fe-rCBV) and gadoteridol with leakage correction (Gd-rCBV) indicates active tumor, and low rCBV (≤1.75) on gadoteridol parametric map (Gd-rCBV) indicates pseudoprogression (arrows). Leakage map indicates absence of contrast extravasation when ferumoxytol was used and contrast extravasation with gadoteridol (arrow).
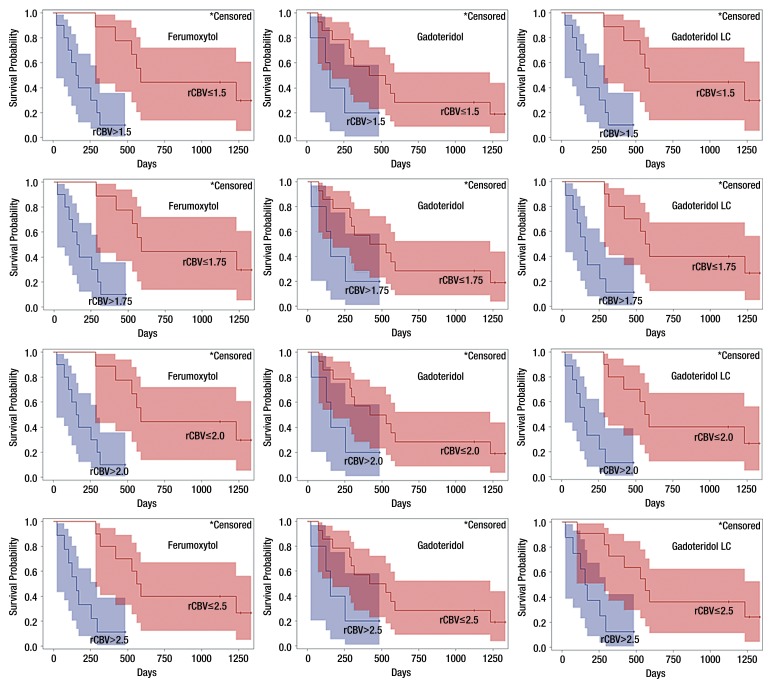
At the time of analysis, 15 of 19 (79%) patients had died and four of 19 (21%) patients were still alive at the time of analysis (mean survival, 1049 days; range, 483–1331 days). The mOS in all patients from DSC MR imaging to death or last follow-up examination was 314 days (95% CI: 156, 591 days). The Kaplan-Meier estimates of survival for patients with high and low rCBV values are shown in Figure 5.
Figure 5:
Charts show survival of GBM patients according to rCBV by using four cutoff values. Tumor rCBV was measured by using ferumoxytol versus gadoteridol and gadoteridol with leakage correction (LC). Kaplan-Meier survival curves show best survival prediction by using rCBV values obtained with ferumoxytol (P < .001) when cutoff range is between 1.5 and 2.0, and the same result was achieved with gadoteridol leakage correction with cutoff of 1.5. By using gadoteridol, survival prediction is similar but not statistically significant (P = .079) for all cutoff values. Colored areas indicate 95% CIs.
For ferumoxytol, the mOS was 591 days (95% CI: 286, 1232 days) for patients with rCBV values less than or equal to 1.75 and 163 days (95% CI: 24, 295 days) for those with values greater than 1.75 (log-rank P value < .001). The estimated hazard ratio for rCBV values less than or equal to 1.75 measured by using ferumoxytol was 0.098 (95% CI: 0.020, 0.481; P = .004), indicating significantly improved survival in patients with rCBV values less than or equal to 1.75. The same results were obtained by using an rCBV cutoff value of 1.5 or 2.0 for ferumoxytol, but by using a cutoff of 2.5, the mOS was 576 days (95% CI: 286–1232 days) in patients with rCBV values less than or equal to 2.5 and 156 days (95% CI: 24, 295 days) in patients with rCBV values greater than 2.5 (P < .001), with an estimated hazard ratio of 0.120 (95% CI: 0.030, 0.482; P = .003) for rCBV values less than or equal to 2.5.
For gadoteridol without leakage correction, the mOS was 474 days (95% CI: 171, 1232 days) in patients with rCBV less than or equal to 1.75 and 156 days (95% CI: 24, 484 days) in patients with rCBV greater than 1.75 (P = .079). The estimated hazard ratio for rCBV values less than or equal to 1.75 with gadoteridol was 0.339 (95% CI: 0.096, 1.197, P = .093) also indicating improved survival in patients with rCBV values less than or equal to 1.75, but it was not a statistically significant difference. Testing rCBV cutoff values of 1.5, 2.0, and 2.5 for gadoteridol resulted in the same outcome as that for 1.75.
For gadoteridol with leakage correction, the mOS was 576 days (95% CI: 286, 1232 days) in patients with rCBV less than or equal to 1.75 and 156 days (95% CI: 24, 295 days) in patients with rCBV greater than 1.75 (P < .001). The estimated hazard ratio for rCBV values less than or equal to 1.75 was 0.12 (95% CI: 0.030, 0.482; P = .003) also indicating significantly improved survival in patients with rCBV less than or equal to 1.75. These were the same as they were when using a cutoff value of 2.0. The mOS was 561 days (95% CI: 286, 1232 days) in patients with rCBV less than or equal to 2.5 and 163 days (95% CI: 24, 295 days) in patients with rCBV greater than 2.5 (P = .004) and the estimated hazard ratio for rCBV less than or equal to 2.5 was 0.182 (95% CI: 0.050, 0.659; P = .009), and by using the rCBV cutoff of 1.5, the mOS was 591 days (95% CI: 286, 1232 days) in patients with rCBV less than or equal to 1.5 and 163 days (95% CI: 24, 295 days) with rCBV greater than 1.5 (P < .001) and the estimated hazard ratio for rCBV less than or equal to 1.5 was 0.098 (95% CI: 0.020, 0.481; P = .004), which was the same as for rCBV measured with ferumoxytol with cutoff values between 1.5 and 2.0.
The mOS in five patients with discordance between ferumoxytol and gadoteridol rCBV was 171 days (95% CI: 73, 315 days) indicating poor survival due to tumor progression (Fig 1, A); however, after leakage correction application only one patient had rCBV mismatch, with survival of 315 days. None of the leakage maps showed ferumoxytol extravasation, but gadoteridol leakage was apparent in all cases (Figs 2–4).
Discussion
Our study results showed the role of rCBV measurement in differentiating tumor progression from pseudoprogression and predicting survival in GBM patients who appeared to have progressive disease at conventional MR imaging after chemoradiotherapy. There is a significant difference in survival between patients with low versus high rCBV with ferumoxytol, and this binary cutoff set in a range between 1.5 and 2.0 returns the same prediction capacity. Absence of ferumoxytol extravasation shown on leakage maps indicated that leakage correction is not necessary with ferumoxytol. The results based on rCBV with gadoteridol without leakage correction are similar but not statistically significant for all tested cutoff values. In addition, rCBV values obtained with gadoteridol that suggested pseudoprogression were not correct in five patients with a short survival, and rCBV with ferumoxytol indicated a progressive tumor in these patients. Use of leakage correction resulted in improvement of gadoteridol rCBV estimation, which was not significantly different from ferumoxytol rCBV values. Moreover, leakage-corrected gadoteridol rCBV values with a cutoff of 1.5 were significantly associated with survival and matched the ferumoxytol rCBV cutoff range of 1.5–2.0. This finding shows the effect of GBCA extravasation and rCBV underestimation, which can potentially lead to incorrect diagnosis, treatment, and prognosis and necessity of leakage correction.
The issue of GBCA extravasation during perfusion MR imaging of lesions with a disrupted blood-brain barrier and the resulting underestimation of rCBV is well known and has been shown by multiple investigators (18–20). To improve the diagnostic accuracy of DSC MR imaging, several methods have been proposed for leakage correction, such as the use of small flip-angle gradient-echo (11,31,32) or dual-echo (33,34) perfusion acquisitions, a preload method (33,34), or postprocessing with multiple mathematic correction algorithms (30,35,36), but these still have a lack of consistency and reproducibility. Magnetic field strength differences and MR pulse sequence details must be taken into account on correction methods for proper leakage correction with GBCA. Leakage correction works best with limited extravasation rates, above which the blood space and extravascular extracellular space becomes intrinsically indistinguishable when GBCA is used, and the derived rCBV value and leakage rate are no longer independent. The results of our preclinical rodent study also showed that brain tumor rCBV was underestimated when GBCA was used in a human high-grade glioma xenograft, and the accuracy of the measurement was improved by using the preload leakage correction method; however, rCBV values depended on the dose of contrast agent preload (37). By using ferumoxytol for DSC MR imaging in the same animals, we obtained consistent rCBV estimation regardless of the permeability of the tumor vasculature and independent of contrast agent preload.
Current treatment response evaluation criteria, such as the McDonald criteria, Response Evaluation Criteria in Solid Tumors, and Response Assessment in Neuro-Oncology (RANO) Working Group, are based on conventional MR imaging sequences and cannot allow reliable differentiation of progression from pseudoprogression (38–40). This is the major reason that there was no significant difference in survival shown in patients with progressive disease versus those with no progression (stable or partial response) based on McDonald criteria 1 month after chemoradiotherapy in two studies (41,42). The current standard-of-practice recommendation in patients with increased or new enhancement within 3 months after completion of chemoradiotherapy is to continue adjuvant temozolomide treatment for one to three cycles (40,43,44). If repeated MR imaging 1–3 months after the first MR imaging examination shows no further progression or even a decrease of enhancement and the patient is on adjuvant temozolomide treatment with a stable dose of steroids and without antiangiogenic therapy, then diagnosis of pseudoprogression can be made retrospectively. This approach has limitations because (a) ineffective therapy may be continued in patients with true progressive disease; (b) patients with true progressive disease may not be included in second line or experimental therapy without delay; (c) retrospective diagnosis of pseudoprogression cannot be made if a patient’s condition required an increase in steroid dose or antiangiogenic therapy to decrease mass effect; (d) new or increased enhancement more than 3 months after chemoradiotherapy does not necessarily indicate true tumor progression because pseudoprogression can be seen beyond this time frame; (e) further increases of enhancement on the second follow-up MR imaging examination could be caused not only by tumor progression but also by continuation of treatment-related inflammatory reaction; (f) the inability to reliably differentiate tumor progression from pseudoprogression can cause inclusion of patients with pseudoprogression in experimental protocols, with further false-positive response to experimental treatment.
Our study indicated that perfusion MR imaging in conjunction with conventional MR imaging can assist clinicians in avoiding continuation of ineffective therapy, starting second line or experimental therapy without delay in patients with active tumors, and establishing the patients who have a better prognosis and who will benefit from continuation of adjuvant temozolomide. These findings are in agreement with those of other investigations regarding the role of perfusion MR imaging in this dilemma (14,41). Unlike authors of other studies, we compared DSC MR imaging by using low-molecular-weight GBCA versus high-molecular-weight ferumoxytol in the patients with apparent progression at conventional MR imaging after chemoradiotherapy and assessed the ability of mathematic leakage correction to improve accuracy of rCBV estimation.
One of the limitations of this study was the inclusion of only 19 patients. Even so, 70% of patients with high rCBV values with ferumoxytol died before the first death among patients with low rCBV with ferumoxytol. Thus, rCBV values with ferumoxytol appeared to be a strong predictor of prognosis and similar to rCBV values with leakage-corrected gadoteridol. In addition, survival prediction with rCBV with ferumoxytol was robust in a wide range of rCBV cutoff values. Larger confirmatory studies are required to validate the findings of this study. A second limitation is that only one type of leakage correction method was tested. Paulson et al (35) found variability in tumor rCBV depending on the choice of five different mathematic correction algorithms. Moreover, our preclinical study (37) results showed the inconsistency of preload methods for leakage correction. The most commonly used of these two methods required either sophisticated postprocessing or high contrast-agent dose exposure but was still inconsistent. The third potential limitation was the difference in the treatment after the study, which may have affected the overall survival rates. However, most of the patients in this study received antiangiogenic treatment, and there was no marked survival benefit from the use of different chemotherapy regimens in patients with recurrent GBM. The use of three different ferumoxytol doses for DSC MR imaging was the fourth limitation of our study. MR imaging signal intensity may be affected by ferumoxytol dose. Because there is not a recommended standard dose of ferumoxytol for DSC MR imaging, we tested a variety of doses in different protocols. Ferumoxytol has a greater apparent transverse relaxivity than GBCA and causes more signal decrease on T2* sequences, and therefore less contrast agent is required for perfusion MR imaging. We found no correlation between ferumoxytol dose and rCBV value. It is likely that within a detectable dosage range the findings of the study were independent of ferumoxytol dose. The fifth limitation of this study was the difference in timing of DSC MR imaging among patients. Although most patients (84%) were studied within 6 months after chemoradiotherapy, which is the time period when pseudoprogression is thought to occur, three patients were included in the study 9, 10, and 11 months after chemoradiotherapy. Nonetheless, ferumoxytol DSC MR images showed that one of these patients had pseudoprogression rather than true tumor progression. Although this study included only patients with GBM, the diagnostic dilemma of pseudoprogression may be valid for any brain tumor requiring radiation without or with concomitant chemotherapy.
On the basis of our findings, we propose that tumor blood volume assessment by using perfusion MR imaging with ferumoxytol may help clinicians to differentiate true tumor progression from pseudoprogression in patients with GBM who show radiologic progression at conventional MR imaging after chemoradiotherapy. Unlike GBCA, ferumoxytol does not require leakage correction application. The rCBV values obtained by using ferumoxytol and leakage-corrected gadoteridol appear to be good prognostic biomarkers and may improve disease management by allowing clinicians to choose the correct treatment strategy without delay. Leakage correction is necessary to improve accuracy of rCBV estimation when GBCA is used, and despite that misestimation of rCBV, still can occur in some cases. Furthermore, the effectiveness of leakage correction may depend on the rate constant of GBCA extravasation (ie, Ktrans), a dimension that we did not investigate. Ferumoxytol, because of the absence of extravasation during DSC acquisition, greatly simplifies modeling and holds promise for evaluation of tumor behavior and response to therapy in patients with brain tumors.
Advances in Knowledge.
• The results of this study confirm that brain tumor blood volume measurements are underestimated when gadoteridol, a low-molecular-weight gadolinium-based contrast agent, is used, most likely because of extravasation artifacts, and leakage correction is necessary to improve the accuracy of rCBV estimation.
• Ferumoxytol as a blood pool agent offers reliable and simplified modeling of tumor blood volume assessment with no need of leakage correction.
• Tumor blood volume estimated by using perfusion MR imaging with ferumoxytol or leakage-corrected perfusion MR imaging with gadoteridol, unlike nonleakage-corrected perfusion MR imaging with gadoteridol, may facilitate diagnosis of pseudo-progression and is significantly associated with survival.
Implication for Patient Care.
• Perfusion MR imaging with ferumuoxytol can assist clinicians in establishing which patients have active glioblastoma multiforme after chemoradiotherapy and should begin second line or experimental therapy without delay, and which patients have a better prognosis and would benefit from continuation of adjuvant temozolomide.
Disclosures of Conflicts of Interest: S.G. No relevant conflicts of interest to disclose. L.L.M. No relevant conflicts of interest to disclose. C.G.V. No relevant conflicts of interest to disclose. X.L. No relevant conflicts of interest to disclose. D.F.K. No relevant conflicts of interest to disclose. R.F. No relevant conflicts of interest to disclose. B.E.H. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: Received payment for consultancy and royalties from Amirsys. Other relationships: none to disclose. W.D.R. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: Institution has patent pending related to DCE MR imaging for cancer. Author has stock/stock options for DeltaPoint in relation to patent. Other relationships: none to disclose. E.A.N. Financial activities related to the present article: none to disclose. Financial activities not related to the present article: none to disclose. Other relationships: none to disclose.
Acknowledgments
The authors thank Aliana Culp and Lisa Bennett for help with manuscript preparation.
Received July 25, 2011; revision requested September 8; revision received June 5, 2012; accepted June 25; final version accepted August 2.
Current address: Department of Neuroradiology, Universitätsklinikum Würzburg, Würzburg, Germany.
Funding: This research was supported by the National Institutes of Health (grants 5 R01 NS053468, 5 R01 CA137488, 3 R01 CA137488-15S1, and 5 R01 NS044687).
Abbreviations:
- CI
- confidence interval
- DSC
- dynamic susceptibility-weighted contrast-enhanced
- GBCA
- gadolinium-based contrast agent
- GBM
- glioblastoma multiforme
- mOS
- median overall survival
- rCBV
- relative cerebral blood volume
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352(10):987–996 [DOI] [PubMed] [Google Scholar]
- 2.Gerstner ER, McNamara MB, Norden AD, Lafrankie D, Wen PY. Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol 2009;94(1):97–101 [DOI] [PubMed] [Google Scholar]
- 3.de Wit MC, de Bruin HG, Eijkenboom W, Sillevis Smitt PA, van den Bent MJ. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology 2004;63(3):535–537 [DOI] [PubMed] [Google Scholar]
- 4.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 2008;26(13):2192–2197 [DOI] [PubMed] [Google Scholar]
- 5.Brandsma D, Stalpers L, Taal W, Sminia P, van den Bent MJ. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol 2008;9(5):453–461 [DOI] [PubMed] [Google Scholar]
- 6.Tofilon PJ, Fike JR. The radioresponse of the central nervous system: a dynamic process. Radiat Res 2000;153(4):357–370 [DOI] [PubMed] [Google Scholar]
- 7.Wong CS, Van der Kogel AJ. Mechanisms of radiation injury to the central nervous system: implications for neuroprotection. Mol Interv 2004;4(5):273–284 [DOI] [PubMed] [Google Scholar]
- 8.Nordal RA, Nagy A, Pintilie M, Wong CS. Hypoxia and hypoxia-inducible factor-1 target genes in central nervous system radiation injury: a role for vascular endothelial growth factor. Clin Cancer Res 2004;10(10):3342–3353 [DOI] [PubMed] [Google Scholar]
- 9.Brandes AA, Tosoni A, Spagnolli F, et al. Disease progression or pseudoprogression after concomitant radiochemotherapy treatment: pitfalls in neurooncology. Neuro-oncol 2008;10(3):361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aronen HJ, Gazit IE, Louis DN, et al. Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 1994;191(1):41–51 [DOI] [PubMed] [Google Scholar]
- 11.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast-enhanced T2*-weighted MR imaging. Radiology 1999;211(3):791–798 [DOI] [PubMed] [Google Scholar]
- 12.Sugahara T, Korogi Y, Kochi M, et al. Correlation of MR imaging-determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR Am J Roentgenol 1998;171(6):1479–1486 [DOI] [PubMed] [Google Scholar]
- 13.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol 2004;25(2):214–221 [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y, Tsien CI, Nagesh V, et al. Survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT [corrected]. Int J Radiat Oncol Biol Phys 2006;64(3):876–885 [DOI] [PubMed] [Google Scholar]
- 15.Danchaivijitr N, Waldman AD, Tozer DJ, et al. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation? Radiology 2008;247(1):170–178 [DOI] [PubMed] [Google Scholar]
- 16.Law M, Young RJ, Babb JS, et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2008;247(2):490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh J, Henry RG, Pirzkall A, et al. Survival analysis in patients with glioblastoma multiforme: predictive value of choline-to-N-acetylaspartate index, apparent diffusion coefficient, and relative cerebral blood volume. J Magn Reson Imaging 2004;19(5):546–554 [DOI] [PubMed] [Google Scholar]
- 18.Barajas RF, Jr, Chang JS, Segal MR, et al. Differentiation of recurrent glioblastoma multiforme from radiation necrosis after external beam radiation therapy with dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2009;253(2):486–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoefnagels FW, Lagerwaard FJ, Sanchez E, et al. Radiological progression of cerebral metastases after radiosurgery: assessment of perfusion MRI for differentiating between necrosis and recurrence. J Neurol 2009;256(6):878–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu LS, Baxter LC, Smith KA, et al. Relative cerebral blood volume values to differentiate high-grade glioma recurrence from post-treatment radiation effect: direct correlation between image-guided tissue histopathology and localized dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging measurements. AJNR Am J Neuroradiol 2009;30(3):552–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gahramanov S, Raslan AM, Muldoon LL, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys 2011;79(2):514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu LS, Eschbacher JM, Dueck AC, et al. Correlations between perfusion MR imaging cerebral blood volume, microvessel quantification, and clinical outcome using stereotactic analysis in recurrent high-grade glioma. AJNR Am J Neuroradiol 2012;33(1):69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sorensen AG. Perfusion MR imaging: moving forward. Radiology 2008;249(2):416–417 [DOI] [PubMed] [Google Scholar]
- 24.Neuwelt EA, Várallyay CG, Manninger S, et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery 2007;60(4):601–611; discussion 611–612 [DOI] [PubMed] [Google Scholar]
- 25.Varallyay CG, Muldoon LL, Gahramanov S, et al. Dynamic MRI using iron oxide nanoparticles to assess early vascular effects of antiangiogenic versus corticosteroid treatment in a glioma model. J Cereb Blood Flow Metab 2009;29(4):853–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinstein JS, Varallyay CG, Dosa E, et al. Superparamagnetic iron oxide nanoparticles: diagnostic magnetic resonance imaging and potential therapeutic applications in neurooncology and central nervous system inflammatory pathologies, a review. J Cereb Blood Flow Metab 2010;30(1):15–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuwelt EA, Hamilton BE, Varallyay CG, et al. Ultrasmall superparamagnetic iron oxides (USPIOs): a future alternative magnetic resonance (MR) contrast agent for patients at risk for nephrogenic systemic fibrosis (NSF)? Kidney Int 2009;75(5):465–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med 1990;14(2):249–265 [DOI] [PubMed] [Google Scholar]
- 29.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 1996;36(5):715–725 [DOI] [PubMed] [Google Scholar]
- 30.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 2006;27(4):859–867 [PMC free article] [PubMed] [Google Scholar]
- 31.Maeda M, Itoh S, Kimura H, et al. Tumor vascularity in the brain: evaluation with dynamic susceptibility-contrast MR imaging. Radiology 1993;189(1):233–238 [DOI] [PubMed] [Google Scholar]
- 32.Cha S. Perfusion MR imaging of brain tumors. Top Magn Reson Imaging 2004;15(5):279–289 [DOI] [PubMed] [Google Scholar]
- 33.Heiland S, Benner T, Debus J, Rempp K, Reith W, Sartor K. Simultaneous assessment of cerebral hemodynamics and contrast agent uptake in lesions with disrupted blood-brain-barrier. Magn Reson Imaging 1999;17(1):21–27 [DOI] [PubMed] [Google Scholar]
- 34.Uematsu H, Maeda M. Double-echo perfusion-weighted MR imaging: basic concepts and application in brain tumors for the assessment of tumor blood volume and vascular permeability. Eur Radiol 2006;16(1):180–186 [DOI] [PubMed] [Google Scholar]
- 35.Paulson ES, Schmainda KM. Comparison of dynamic susceptibility-weighted contrast-enhanced MR methods: recommendations for measuring relative cerebral blood volume in brain tumors. Radiology 2008;249(2):601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu LS, Baxter LC, Pinnaduwage DS, et al. Optimized preload leakage-correction methods to improve the diagnostic accuracy of dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging in post-treatment gliomas. AJNR Am J Neuroradiol 2010;31(1):40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gahramanov SML, Muldoon LL, Li X, Neuwelt EA. Improved perfusion MR imaging assessment of intracerebral tumor blood volume and antiangiogenic therapy efficacy in a rat model with ferumoxytol. Radiology 2011;261(3):796–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 1990;8(7):1277–1280 [DOI] [PubMed] [Google Scholar]
- 39.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92(3):205–216 [DOI] [PubMed] [Google Scholar]
- 40.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010;28(11):1963–1972 [DOI] [PubMed] [Google Scholar]
- 41.Mangla R, Singh G, Ziegelitz D, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology 2010;256(2):575–584 [DOI] [PubMed] [Google Scholar]
- 42.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer 2008;113(2):405–410 [DOI] [PubMed] [Google Scholar]
- 43.Mason WP, Maestro RD, Eisenstat D, et al. Canadian recommendations for the treatment of glioblastoma multiforme. Curr Oncol 2007;14(3):110–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roldán GB, Scott JN, McIntyre JB, et al. Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci 2009;36(5):617–622 [DOI] [PubMed] [Google Scholar]