Abstract
Diseases affecting hemoglobin synthesis and function are extremely common worldwide. More than 1000 naturally occurring human hemoglobin variants with single amino acid substitutions throughout the molecule have been discovered, mainly through their clinical and/or laboratory manifestations. These variants alter hemoglobin structure and biochemical properties with physiological effects ranging from insignificant to severe. Studies of these mutations in patients and in the laboratory have produced a wealth of information on hemoglobin biochemistry and biology with significant implications for hematology practice. More generally, landmark studies of hemoglobin performed over the past 60 years have established important paradigms for the disciplines of structural biology, genetics, biochemistry, and medicine. Here we review the major classes of hemoglobin variants, emphasizing general concepts and illustrative examples.
More than 1000 natural variants of hemoglobin are known. They have different structures and biochemical properties (e.g., oxygen affinity), and their physiological effects range from insignificant to severe.
Globin gene mutations affecting hemoglobin (Hb), the major blood oxygen (O2) carrier, are common, affecting an estimated 7% of the world’s population (Weatherall and Clegg 2001; Kohne 2011). These mutations are broadly subdivided into those that impair globin protein subunit production (thalassemias) and those that produce structurally abnormal globin proteins (Hb variants). The latter class is mainly composed of missense mutations that cause single amino acid substitutions in the globin protein, resulting in an abnormal, or “variant” Hb tetramer. Less commonly, Hb variants are associated with deletions, multiple amino acid substitutions, antitermination mutations, and altered posttranslational processing (Table 1).
Table 1.
Hb variants that are discussed in this article
| Name | Globin site (fold) | Amino acid substitution | Molecular mechanism | Clinical phenotype | Other biochemical and laboratory findings |
|---|---|---|---|---|---|
| Unstable Mutants | |||||
| Brockton | β138 (H16) | Ala > Pro | Altered secondary structure | Hemolytic anemia, reticulocytosis | |
| Philly | β35 (C1) | Tyr > Phe | Altered α1β1 interface | Hemolytic anemia, reticulocytosis | Decreased cooperativity, increased oxygen affinity |
| Peterborough | β111 (G13) | Val > Phe | Altered α1β1 interface | Hemolytic anemia, reticulocytosis | Decreased oxygen affinity |
| Stanmore | β111 (G13) | Val > Ala | Altered α1β1 interface | Hemolytic anemia | Decreased oxygen affinity |
| J-Guantanamo | β128 (H6) | Ala > Asp | Altered α1β1 interface | Hemolytic anemia | Target cells |
| Khartoum | β124 (H2) | Pro > Arg | Altered α1β1 interface | Normal | |
| Prato | α1 or α2 31 (B12) | Arg > Ser | Altered α1β1 interface | Anisocytosis, hypochromia | Mildly unstable in isopropanol |
| Lombard | α2 103 (G10) | His > Tyr | Altered α1β1 interface | Anemia | |
| Contaldo | α1 or α2 103 (G10) | His > Arg | Altered α1β1 interface | Hemolytic anemia | |
| Foggia | α2 117 (GH5) | Phe > Ser | Altered α1β1 interface | Microcytosis | Rapidly degraded α chains |
| Groene Hart | α1 119 (H2) | Pro > Ser | Altered α1β1 interface, disrupted AHSP binding | Hemolytic anemia, microcytosis | |
| Turriff | α1 or α2 99 (G6) | Lys > Glu | Altered α1β1 interface, disrupted AHSP binding | Normal | Comigrates with HbA1C, rapidly degraded α chains |
| Beziers | α1 99 (G6) | Lys > Asn | Altered α1β1 interface, disrupted AHSP binding | Normal | Comigrates with HbA1C |
| Hirosaki | α2 43 (CE1) | Phe > Leu | Altered heme pocket | Heinz body hemolytic anemia | Hyperunstable |
| Terre Haute | β106 (G8) | Leu > Arg | Altered heme pocket | Heinz body hemolytic anemia, dominant inclusion body thalassemia | Hyperunstable |
| High Affinity Variants | |||||
| Kempsey | β99 (G1) | Asp > Asn | Unstable T state | Erythrocytosis | Decreased cooperativity |
| Hiroshima | β146 (HC3) | His > Asp | Mutated Bohr proton donor | Erythrocytosis | Decreased cooperativity, decreased Bohr effect |
| York | β146 (HC3) | His > Pro | Mutated Bohr proton donor | Erythrocytosis | Decreased cooperativity, decreased Bohr effect |
| Cowtown | β146 (HC3) | His > Leu | Mutated Bohr proton donor | Erythrocytosis | Decreased Bohr effect |
| Rahere | β82 (EF6) | Lys > Thr | Altered 2,3DPG binding site | Erythrocytosis | |
| Providence | β82 (EF6) | Lys > Asn | Altered 2,3DPG binding site | Erythrocytosis | Low oxygen affinity |
| Helsinki | β82 (EF6) | Lys > Met | Altered 2,3DPG binding site | Erythrocytosis | Decreased Bohr effect |
| Low Affinity Variants | |||||
| Kansas | β102 (G4) | Asn > Thr | Unstable R state | Cyanosis | Decreased cooperativity |
| Beth Israel | β102 (G4) | Asn > Ser | Unstable R state | Cyanosis | Decreased cooperativity |
| St. Mandé | β102 (G4) | Asn > Tyr | Unstable R state | Cyanosis | |
| Methemoglobin Variants | |||||
| M-Iwate | α1 or α2 (F8) | His > Tyr | Oxidized heme | Pseudocyanosis (Methemoglobinemia) | Abnormal visible spectrum |
| M-Saskatoon | β63 (E7) | His > Tyr | Oxidized heme | Pseudocyanosis (Methemoglobinemia) | Abnormal visible spectrum |
| Globin Chain Elongation Variants | |||||
| Constant Spring | α2 142 (HC3) | Stop > Gln | Antitermination mutant | Microcytosis | Decreased mRNA stability |
| Cranston | β145 (HC3) | +CT | Frameshift, elongated globin | Hemolytic anemia | Increased oxygen affinity, decreased cooperativity |
| Variants with Multiple Effects | |||||
| HbE | β26 (B8) | Glu > Lys | Unstable, reduced synthesis | Microcytosis | |
| Bruxelles | β41 (C7) or β42 (CD1) | Phe > 0 | Altered heme pocket | Hemolytic anemia, cyanosis, splenomegaly, reticulocytosis | Heinz bodies, decreased cooperativity |
| Warsaw | β42 (CD1) | Phe > Val | Altered heme pocket | Hemolytic anemia, cyanosis | Heinz bodies, decreased cooperativity |
| Hammersmith | β42 (CD1) | Phe > Ser | Altered heme pocket | Hemolytic anemia, cyanosis | Heinz bodies, decreased cooperativity |
| Buccuresti-Louisville | β42 (CD1) | Phe > Leu | Altered heme pocket | Hemolytic anemia, cyanosis | Heinz bodies, decreased cooperativity |
| Zurich | β63 (E7) | His > Arg | Altered heme pocket | Normal, but hypersensitive to oxidative stress | Decreased cooperativity, increased oxygen affinity, increased CO affinity |
| Jamaica Plain | β6 (A3) and β68 (E12) | Glu > Val and Leu > Phe | Altered secondary structure | Hemolytic anemia, cyanosis, splenomegaly, splenic sequestration | Unstable, Heinz bodies, sickle cell phenotype, decreased oxygen affinity |
| Quebec-Chori | β87 (F3) | Thr > Ile | Altered interaction with HbS polymer | Normal | Promotes HbS polymerization |
| D-Ibadan | β87 (F3) | Thr > Lys | Altered interaction with HbS polymer | Normal | Inhibits HbS polymerization |
| Bristol-Alesha | β67 (E11) | Val > Met | Altered heme pocket | Hemolytic anemia, reticulocytosis | Heinz bodies, decreased cooperativity, decreased Bohr effect, decreased oxygen affinity |
| Toms River | γ67 (E11) | Val > Met | Altered heme pocket | Anemia, cyanosis | Unstable, low oxygen affinity |
For a full listing of hemoglobin variants, see The Globin Gene Server (http://globin.bx.psu.edu; Hardison et al. 2002; Giardine et al. 2011).
Naturally occurring Hb mutations cause a range of biochemical abnormalities, some of which produce clinically significant symptoms. The most common and medically important Hb variants include HbS (Cao and Kan 2012; Lettre 2012; Schechter and Elion 2012; Serjeant and Rodgers 2012; Williams and Weatherall 2012), HbC (Cao and Kan 2012; Lettre 2012; Schechter and Elion 2012; Serjeant and Rodgers 2012; Williams and Weatherall 2012), HbE (see the sections on Selected Variants that Illustrate Important Aspects of Hemoglobin Biology and Variants that Affect Multiple Hemoglobin Functions; see also Musallam et al. 2012), and some thalassemias (e.g., “thalassemic hemoglobinopathies”), all of which are under positive genetic selection because they confer survival advantages in areas where malaria is endemic (Weatherall and Clegg 2001). In addition to these prevalent mutant proteins, there are also >1000 other known naturally occurring Hb variants, which are rare individually but common collectively. Most Hb mutants are cataloged on the Globin Gene database (HbVar, http://globin.bx.psu.edu; Hardison et al. 2002; Giardine et al. 2011). By convention, these variants are named after the geographic origin of the affected individual. Although many Hb variants are clinically silent, some produce clinical manifestations of varying severity. Analyses of these variants, which can be considered to be “experiments of nature” (Garrod 1928), have generated valuable insights into structure–function relationships within the Hb molecule, with interesting and important clinical consequences.
The goal of this work is to provide a succinct conceptual framework for understanding the biology and clinical implications of Hb variants. We explore major concepts of Hb biology followed by a discussion of selected Hb variants that reinforce basic principles. For more extensive reviews of this topic, see Nathan and Oski’s Hematology of Infancy and Childhood (Nathan et al. 2009); Hemoglobin: Molecular, Genetic, and Clinical Effects (Bunn and Forget 1986); Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (Steinberg et al. 2001); and the Globin Gene database (Hardison et al. 2002; Giardine et al. 2011).
BASIC PRINCIPLES
Hemoglobin Synthesis, Structure, and Function
Hemoglobin is a heterotetramer composed of α-like and β-like globin subunits, each bound to a heme prosthetic group. The major functions of Hb are to transport oxygen (O2) from the lungs to peripheral tissues and carbon dioxide (CO2) from the tissues to the lungs. The kinetics of Hb-O2 binding and release are fine-tuned for this purpose and adaptable according to developmental ontogeny and metabolic perturbations. Moreover, the Hb molecule must limit potential problems caused by its associated iron and free O2, reactive molecules capable of inflicting damage through the production of reactive oxygen species. Efforts to understand how Hb structure imparts these critical functions have been ongoing for more than 50 years. Hemoglobin was one of the first proteins to be sequenced (Konigsberg et al. 1961; Schroeder et al. 1961; Watson and Kendrew 1961) and the globin genes were among the earliest to be cloned (Rabbitts 1976). In the late 1950s, Perutz and colleagues determined the three-dimensional structure of Hb through X-ray crystallography (Perutz 1960; Perutz et al. 1960). More recent studies refined this structure to high resolution (Paoli et al. 1996; Park et al. 2006). In addition, O2 and other ligand-binding properties have been measured in detail for native Hb and many naturally occurring mutants. All of this work provides a substantial framework for defining the O2 delivery properties of Hb, as well as the molecular consequences of variant mutations (see, for example, Lehmann 1957; Konigsberg and Lehmann 1965; Shimizu et al. 1965; Perutz and Lehmann 1968; Perutz 1970; Bunn and Forget 1986).
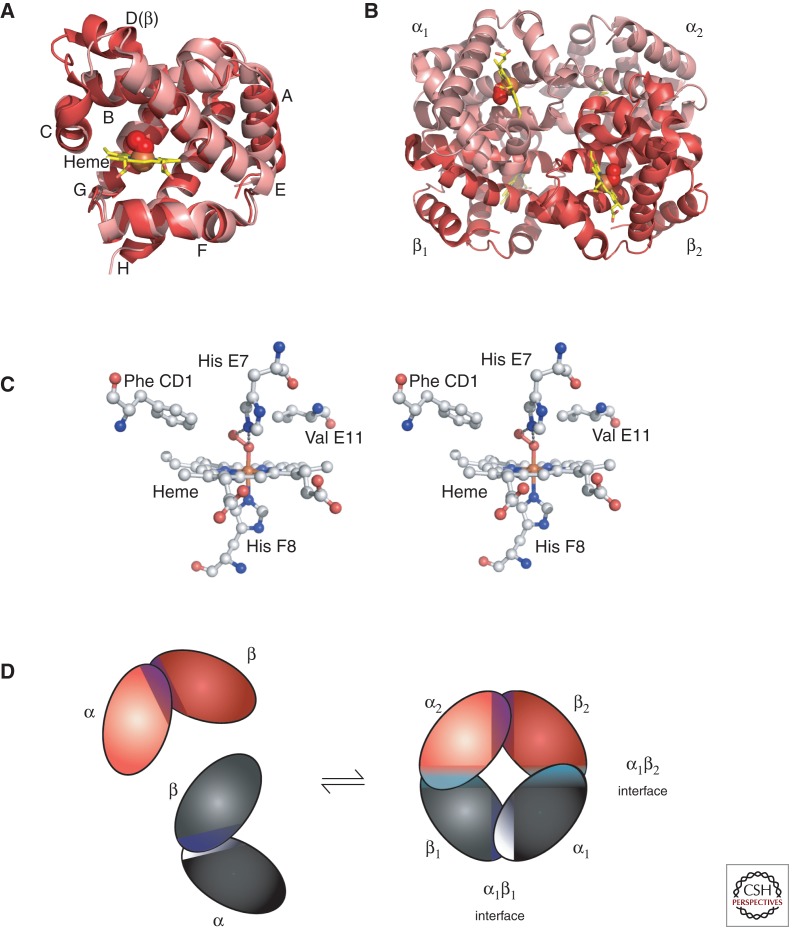
Globin polypeptides are synthesized from separate α-like and β-like globin gene clusters located on human chromosomes 16 and 11, respectively. Nascent globin chains rapidly incorporate heme, which stabilizes their native folding into Hb subunits composed of seven or eight α helices named A–H, which fold together into a globular structure (Fig. 1A). Hb subunits bind O2 and other ligands via the heme iron buried within an evolutionarily conserved hydrophobic pocket that faces the outside of HbA tetramers (Fig. 1B). Heme iron is axially coordinated to globin proteins by an invariant histidine residue in helix F8, termed the “proximal” histidine (Fig. 1C). The opposite axial position binds O2, which is stabilized by interaction with the conserved “distal” histidine in helix E7 (Fig. 1C). Multiple additional amino acids within the globin proteins stabilize heme binding through noncovalent interactions (Fig. 1C). Iron must be in its reduced (ferrous, Fe2+) state for Hb to bind O2. Oxidized or “met” Hb (ferric, Fe3+) cannot bind O2 and is relatively unstable, tending to lose hemin and denature. Thus, red blood cells have evolved elaborate mechanisms to maintain Hb in its reduced state (Bunn and Forget 1986; Ganz and Nemeth 2012; Schechter 2012). For example, the methemoglobin reductase system converts methemoglobin (metHb) to its reduced form. Not surprisingly, globin mutations that alter amino acids within the ligand pocket frequently produce strong functional effects, including destabilization, altered affinity for O2, and increased rates of metHb formation and heme loss (see the section on Selected Variants that Illustrate Important Aspects of Hemoglobin Biology). Variants promoting autoxidation are termed “M-Hbs” (see the sections on Selected Variants that Illustrate Important Aspects of Hemoglobin Biology and Methemoglobin (“M-Type”) Variants). Because Hb gains its distinctive color from the heme group, alterations that affect the environment of the heme iron, including changes in the surrounding amino acids, different gas ligands, or redox state, produce characteristic changes in visible light absorption. These color changes are used clinically to assess Hb-O2 saturation, metHb formation and the effects of Hb variants.
Figure 1.
The structure of Hb. (A) The α (pink) and β (red) Hb subunits have conserved α-helical folds. Helices are labeled A–H from the amino terminus. The α subunit lacks helix D. (B) The high O2 affinity R state quaternary structure of Hb with O2 (red spheres) bound at all four heme sites (protoporphyrin-IX as yellow sticks, with central iron atom as orange sphere). (C) Stereo (wall-eye) diagram of the heme pocket of β showing the proximal (F8) and distal (E7) histidines and selected residues in the distal heme pocket that influence ligand binding and autoxidation. (D) Hb tetramer is assembled from two identical αβ dimers (shown in red and gray for clarity). In the tetramer, each subunit makes contact with the unlike chain through a high affinity dimerization α1β1 interface and a lower affinity α1β2 dimer–tetramer interface (cyan).
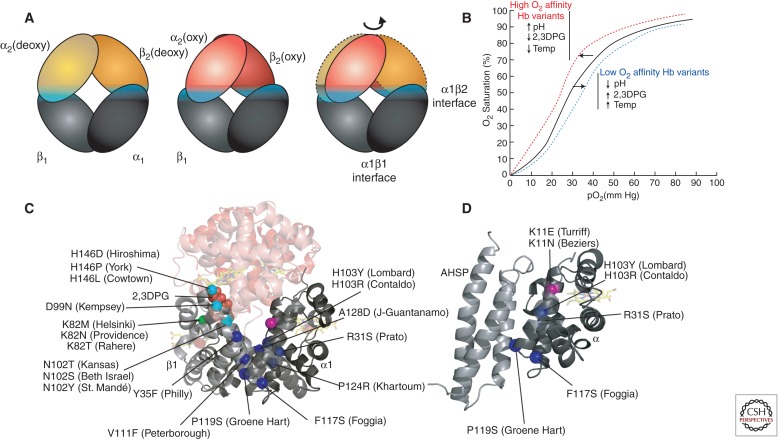
Within the Hb tetramer, each globin subunit binds the unlike chain through two distinct interfaces, termed α1β1 and α1β2 (Fig. 1D). Individual globin subunits form dimers through the extremely high affinity α1β1 interaction. Globin chain monomers are relatively unstable compared with dimers, with a tendency to form intracellular precipitates that damage erythrocytes, causing hemolytic anemia. Thus, mutations that impair the α1β1 interaction can cause erythrotoxicity by favoring the accumulation of monomeric subunits (Fig. 2C). The α1β2 interaction, which is lower affinity, mediates tetramerization. Oxygen binding destabilizes the α1β2 interaction, resulting in a transition of the quaternary structure from the “T” (tense, low affinity, deoxygenated) to “R” (relaxed, high affinity, oxygenated) state, which facilitates O2 binding to additional subunits (Fig. 2A). This process causes cooperative O2 binding, which is illustrated by the characteristic sigmoidal shape of the Hb-O2 equilibrium curve (Fig. 2B). Cooperativity allows maximal O2 release over relatively small drops in O2 tension. Mutations within the α1β2 interaction region can alter the functional properties of Hb, mainly by perturbing O2-binding characteristics (Fig. 2C, cyan spheres; Table 1).
Figure 2.
Hb variants with altered subunit interactions. (A) Conversion from the low O2 affinity (deoxy, T state) to high O2 affinity (oxy, R state) involves a relative rotation of the α1β1 and α2β2 dimers, with changes in contacts across the α1β2 (and α2β1) interface (cyan). In this cartoon, the α1β1 dimer is held stationary to reveal the relative motion of the α2β2 dimer in going from the deoxy (orange) to the oxy (red) states. (B) The sigmoidal shape of the Hb-O2 saturation curve shows allosteric regulation by changes in pH, temperature, and 2,3DPG. These regulators, as well as Hb variants, influence the shape of the curve. High oxygen affinity variants, high pH, low 2,3DPG, or low temperature induce a “left shift” in the saturation curve (red line). Conversely, low oxygen affinity variants, low pH, high 2,3DPG, or high temperature induce a “right shift” (blue line). (C) Hb sequence variants at the allosteric α1β2 interface (cyan spheres) show an impaired response to O2 binding. Some sequence variants disrupt binding to other allosteric regulators, e.g., substitutions at βK82 (green) disrupt interactions with 2,3DPG that normally stabilize the low O2 affinity T state. Mutations that disrupt α1β1 (and α2β2) dimerization (blue spheres) increase the concentration of free monomers, which are unstable. (D) Some α mutations that disrupt binding to β may also disturb binding to the chaperone, AHSP. Other α variants, such as Turriff and Beziers (pink sphere) may inhibit only AHSP binding.
Cooperativity represents a general phenomenon termed allosteric regulation, in which effector molecules control the properties of enzymes or other proteins by binding to regions that are distinct from the active sites. Several allosteric regulators, in addition to O2, influence the properties of Hb. For example, H+ binds Hb to promote O2 release in a process termed the Bohr effect (Perutz et al. 1984). In peripheral tissues, abundant CO2 is taken up by red blood cells and metabolized by carbonic anhydrase to carbonic acid, resulting in the production of H+ and consequent O2 release. In contrast, relatively low CO2 and high pH in the lung favors O2 binding to Hb. Additionally, CO2 forms carbamino adducts with the amino termini of both α and β globins in vivo. These interactions produce minor effects on whole blood O2 affinity compared to the impact of CO2 on pH (Bunn and Forget 1986). The compound 2,3-diphosphoglycerate (2,3DPG), formed as a by-product of glycolysis and present at relatively high concentrations in red blood cells, is another important allosteric regulator of Hb. 2,3DPG binds and stabilizes T state Hb to facilitate O2 release. Hemoglobin binding sites for H+ and 2,3DPG have been identified (Perutz et al. 1969, 1984; Perutz 1970; Arnone 1972). Together, these interactions allow Hb to sense metabolic activity and modulate O2 binding accordingly. This environmental sensory function can be altered by mutations that affect the affinity of Hb for its allosteric regulators (Fig. 2C, green sphere; Table 1).
Identification of Hb Variants and Their Clinical Implications
Many Hb variants are readily ascertained through physical examination and/or routine laboratory testing, which explains why so many have been discovered. Uncommonly, some variants are identified through evaluation of ill patients with severe anemia or clinically significant cyanosis. Many amino acid substitutions alter surface charge and are thus detected by electrophoresis or chromatography, techniques which are routinely performed on neonates in North America and Europe. Interestingly, a few Hb variants migrate similarly to HbA1c, a glycosylated form of Hb that reflects long-term control of blood glucose levels in diabetic patients (Table 1) (reviewed in Little and Roberts 2009). In this way, specific Hb amino acid substitutions can artificially elevate HbA1c measurement and interfere with diabetic management. Other Hb variants are clinically benign but produce obvious changes in skin color. For example, mutations that increase Hb-O2 affinity typically stimulate erythropoietic drive by inhibiting O2 tissue delivery, causing erythrocytosis associated with a ruddy complexion (Nathan et al. 2009). Mutations that reduce O2 affinity produce a bluish hue to the skin (cyanosis) caused by abnormally high levels of deoxyHb. Mutations that favor oxidation of Hb iron to the met form (M-Hbs), also cause blue-tinged skin, whereas the blood itself appears brown. Studies of one family with congenital cyanosis led Horlein and Weber to describe the first known hemoglobinopathy, caused by the variant Hb-M Saskatoon (Horlein and Weber 1948). The M-Hb variant Iwate, which causes “black blood” or “hereditary nigremia,” was first described more than 200 years ago in Japan (Shibata et al. 1960).
It is important to note that many Hb variants affecting skin color are not clinically damaging beyond their cosmetic effects. However, these conditions can mimic more life-threatening problems such as cardiopulmonary and myeloproliferative disorders, which must be excluded. Thus, patients with cyanotic or polycythemic Hb variants may mistakenly undergo unnecessary and potentially dangerous medical procedures. Historical examples include cyanotic patients undergoing surgery or catheterization for presumed heart defects and polycythemic patients receiving radioactive 32P for presumed polycythemia vera (Steinberg et al. 2001; Nathan et al. 2009). As stated, “the primary reason for establishing the diagnosis [of M Hbs] is to prevent iatrogenic misadventures that might arise under the mistaken impression that the patient has a cardiac or pulmonary disorder” (Bunn and Forget 1986). Most Hb variants with altered O2 affinity are relatively simple to diagnose through history, physical examination, and laboratory testing (Wajcman et al. 2001; Wajcman and Moradkhani 2011).
Globin Gene Synthesis Is Developmentally Regulated
Developmental regulation of the α-like and β-like globin gene families is of great medical significance (Sankaran and Orkin 2012). Hemoglobin F (α2γ2) is the most highly expressed form during late fetal gestation. After birth, expression gradually switches to HbA (α2β2) over several months. Thus, symptomatic mutations affecting α or γ globins are present prenatally or at birth, whereas the manifestations of β-globin mutations are typically delayed until a few months after birth. Interestingly, γ-globin gene mutations that are apparent at birth, most typically reflected by cyanosis or hemolytic anemia (e.g., Hb-F Toms River, sections on Selected Variants that Illustrate Important Aspects of Hemoglobin Biology and Variants that Affect Multiple Hemoglobin Functions, and Table 1), fade over a few weeks as Hb production switches from F (α2γ2) to A (α2β2).
Laboratory Testing for Hb Variants
In many countries, routine testing of all newborns is performed to identify common hemoglobinopathies such as some thalassemias and HbS. Isoelectric focusing or high-performance liquid chromatography (HPLC), the most commonly used tests, identify most structurally abnormal Hbs (Wajcman et al. 2001). In this way, many benign Hb variants are discovered incidentally. Clinical indications for laboratory testing to investigate potential Hb variants are listed in Table 2.
Table 2.
Clinical indications for laboratory testing to diagnose Hb variants
| Indications for hemoglobin testing |
|---|
| Routine newborn testing for common hemoglobinopathies (i.e., HbS, HbC, thalassemias) |
| Cyanosis with adequate arterial oxygenation and no apparent cardiopulmonary disease |
| Erythrocytosis with normal or elevated erythropoietin levels |
| Unexplained hemolytic anemia |
| Unexplained thalassemia phenotype |
| Family history consistent with an Hb variant |
Specific laboratory tests to investigate Hb variants include:
Physical methods of Hb separation. These include electrophoretic and chromatographic techniques that examine the physical properties of α1β1 dimers or individual globin subunits. Specific standards, such as HbA, HbS, HbC, HbF, and HbA2, are typically examined as controls. Hb variants may show altered migration in these assays. Historically, cellulose acetate and citrate agar electrophoresis were most commonly used to detect variant Hbs. Current clinical testing more typically uses isoelectric focusing and HPLC, which are more sensitive.
Hb-O2 binding curve. This test, performed on whole red blood cells or hemolysate, indicates the percent (%) oxygenated Hb at a given O2 partial pressure (Fig. 2B). Hemoglobin variants with an abnormally high O2 affinity (see sections on Selected Variants that Illustrate Important Aspects of Hemoglobin Biology and High Oxygen Affinity Variants) will become saturated at lower O2 pressures producing a “left-shifted” O2 equilibrium curve, whereas mutations that reduce O2 affinity (see sections on Selected Variants that Illustrate Important Aspects of Hemoglobin Biology and Low Oxygen Affinity Variants) will cause the opposite “right shift.” Determination of Hb-O2 affinity responses to allosteric regulators, particularly 2,3DPG or H+ (pH changes), can provide insight into the structural causes of the observed phenotypes. Unfortunately, few clinical laboratories currently offer this assay.
Visible wavelength spectroscopy. Hemoglobin variants with amino acid substitutions in the heme pocket affect visible light absorbance. For example, M-type Hbs show characteristic spectra that can distinguish them from methemoglobinemia caused by an enzyme deficiency in the metHb reductase system (Bunn and Forget 1986; Dailey and Meissner 2012; Ganz and Nemeth 2012; Schechter 2012). Pulse oximetry is a noninvasive spectrophotometric test that measures absorbance ratios at specific wavelengths for oxygenated (660 nm) and deoxygenated (940 nm) blood (Tremper and Barker 1989). This can produce confusing and sometimes misleading results in patients with variant Hbs that show unique light absorbance properties (Verhovsek et al. 2010). In these cases, analysis of arterial blood O2 concentration may be required to rule out hypoxia. Analyzing these variant Hbs using light absorbance throughout the full visible wavelength spectrum can provide useful diagnostic information.
Hemoglobin stability testing. Typically, Hb stability is impaired in variants that are associated with hemolytic anemia. Hemoglobin stability tests measure the propensity for Hb to denature on exposure to various stresses including heat (Carrell and Kay 1972), isopropanol (Bender et al. 1981), mechanical agitation (Asakura et al. 1975), and zinc acetate (Roth et al. 1976). The Heinz body test uses supravital stains, such as methylene blue or crystal violet, to detect aggregated globins within erythrocytes (Eisinger et al. 1985).
Specialized testing. Mass spectrometry analysis of patient hemolysate and DNA sequencing of globin genes are specialized confirmatory tests to identify amino acid and nucleotide alterations associated with suspected Hb variants (Wajcman et al. 2001; Wajcman and Moradkhani 2011). DNA sequencing may readily elucidate the presence of an Hb variant. However, additional biochemical and structural studies, including those discussed in this section, are required to determine how the variant affects Hb function. Crystallographic analysis is the highest resolution approach to determine the effects of globin amino acid substitutions on molecular structure. Crystallography has been used historically as a research tool to assess the effects of some interesting Hb variants (see, for example, Pulsinelli et al. 1973; Perutz et al. 1984).
SELECTED VARIANTS THAT ILLUSTRATE IMPORTANT ASPECTS OF HEMOGLOBIN BIOLOGY
Unstable Variants
Unstable variants frequently cause congenital Heinz body hemolytic anemia detected by laboratory screening and clinical symptoms (see sections on Basic Principles and Laboratory Testing for Hb Variants and Table 2). Mutations that alter any step in globin processing, including subunit folding, heme interaction, dimerization, or tetramerization, can destabilize Hb. Bunn and Forget note five general mechanisms that destabilize Hbs: amino acid substitutions within the heme pocket, disruption of secondary structure, substitution in the hydrophobic interior of the subunit, amino acid deletions, and elongation of the subunit (Bunn and Forget 1986).
More than 75% of Hb is α helical (Perutz et al. 1960; Park et al. 2006). This structure is particularly susceptible to disruptions by proline substitutions (Levitt 1981). For example, in Hb Brockton (β138 [H16] Ala > Pro) the substituted proline disrupts intermolecular hydrogen bonding between β138Ala and β134Val in helix H (Russu and Ho 1986; Moo-Penn et al. 1988). This produces an unstable variant with a propensity to precipitate and aggregate, thereby damaging erythrocytes and predisposing to hemolysis. Hb Brockton does not show altered O2 binding affinity or electrophoretic mobility shifts. This variant was identified by HPLC analysis of patient globin chains and its altered X-ray crystallography diffraction pattern shows local disruption of helix H (Moo-Penn et al. 1988).
Mutations at the α1β1 interface can cause hemolytic anemia by inhibiting heterodimer formation, favoring the accumulation of free globin subunits, which themselves are unstable, particularly α chains (Fig. 2C, blue spheres). Examples include Hb Philly (β35 [C1] Tyr > Phe) (Rieder et al. 1969), Hb Peterborough (β111 [G13] Val > Phe) (King et al. 1972), Hb Stanmore (β111 [G13] Val > Ala) (Como et al. 1991), and Hb J-Guantanamo (β128 [H6] Ala > Asp) (Martínez et al. 1977). Hb Khartoum (β124 [H2] Pro > Arg) contains a substitution at the α1β1 interface that is mildly destabilizing in vitro, but does not cause clinical symptoms (Clegg et al. 1969; Argos et al. 1979).
Interestingly, some α-globin (HBA) gene mutations affecting the α1β1 interface may also destabilize free α chains by inhibiting their binding to α-hemoglobin stabilizing protein (AHSP), an erythroid molecular chaperone that facilitates Hb assembly (Fig. 2D, blue spheres) (reviewed in Weiss et al. 2005; Favero and Costa 2011). These α-globin variants include Hbs Prato (α1 or α2 31 [B12] Arg > Ser) (Marinucci et al. 1979), Lombard (α2 103 [G10] His > Tyr) (Hoyer et al. 2002), Contaldo (α1 or α2 103 [G10] His > Arg) (Sciarratta et al. 1984), Foggia (α2 117 [GH5] Phe > Ser) (Lacerra et al. 2008), Groene Hart (α1 119 [H2] Pro > Ser) (Harteveld et al. 2002; Vasseur-Godbillon et al. 2006; Giordano et al. 2007; Vasseur et al. 2009), and others (Wajcman et al. 2008; Yu et al. 2009). Naturally occurring α-globin variants with amino acid substitutions at position 99 including Hb Turriff (α1 or α2 99 [G6] Lys > Glu) (Langdown et al. 1992) and Hb Beziers (α1 99 [G6] Lys > Asn) (Lacan et al. 2004) bind β globin normally but show impaired interaction with AHSP and may be mildly destabilizing (Fig. 2D, violet spheres) (see also Yu et al. 2009; Khandros et al. 2012; Mollan et al. 2012). Antitermination mutations can also destabilize α globin in part by impairing its binding to AHSP (Turbpaiboon et al. 2006).
Hyperunstable Hb variants precipitate shortly after synthesis and are not incorporated into Hb tetramers. These ephemeral proteins are difficult to isolate. In this case, electrophoresis may be falsely negative due to the rapid turnover of these variants, making DNA sequencing a critical diagnostic test. Affected patients show a dominantly inherited “inclusion body thalassemia” resulting from both the precipitated variant globin and consequent chain imbalance with accumulation of the unaffected globin, which is unstable in its free form (Stamatoyannopoulos et al. 1974). Patient erythrocytes typically display abnormal morphology with microcytosis, hypochromia, moderate to severe anisopoikilocytosis, basophilic stippling, and inclusions that may be become particularly prominent following splenectomy (Steinberg et al. 2001). Weatherall, Thein, and colleagues characterized several hyperunstable mutations in exon 3 of the β-globin gene (Thein et al. 1990). All of the mutations were frameshifts or nonsense codons that produced relatively long (>120 amino acid) proteins with carboxy-terminal truncations. The investigators proposed that truncated globins causing dominantly inherited thalassemia are long enough to bind heme posttranslationally, which rendered them relatively resistant to proteolytic degradation, allowing for subsequent precipitation of heme-containing aggregates detected as Heinz bodies. Missense mutations also cause hyperunstable Hb variants. Hb Hirosaki (α2 43 [CE1] Phe > Leu) was discovered in a family with hemolytic anemia (Ohba et al. 1975; Tanaka et al. 2005). After several tests failed to identify a soluble variant Hb within erythrocytes, DNA sequencing was used to characterize the mutation. Hb Terre Haute (β106 [G8] Leu > Arg) is another hyperunstable variant associated with a severe Heinz body hemolytic anemia and globin chain imbalance (Coleman et al. 1991). In initial studies of patient erythroid cells, performed in 1979, abnormal Hb tetramers were not detected and peptide mapping of radiolabeled nascent globins identified a β112 (G14) Cys > Arg substitution, originally termed Hb Indianapolis (Adams et al. 1979). However, the β112 Cys > Arg mutation was subsequently identified in unrelated individuals with much less severe disease. In 1991, reevaluation of the original pedigree by DNA analysis discovered a β106 Leu > Arg mutation, which was renamed Hb Terre Haute (Coleman et al. 1991). Most likely, incomplete tryptic cleavage of the abnormal β-globin peptide in the earlier studies led to misidentification of the causative mutation. This work reflects the interesting historical point that many Hb variants were identified through laborious and technically challenging protein studies performed some time ago, before DNA sequence analysis of patient globin genes was feasible. Reevaluation of these mutations through genetic testing has yielded some interesting surprises (see also the discussion on Hb Bristol-Alesha and the section on Variants that Affect Multiple Hemoglobin Functions).
High Oxygen Affinity Variants
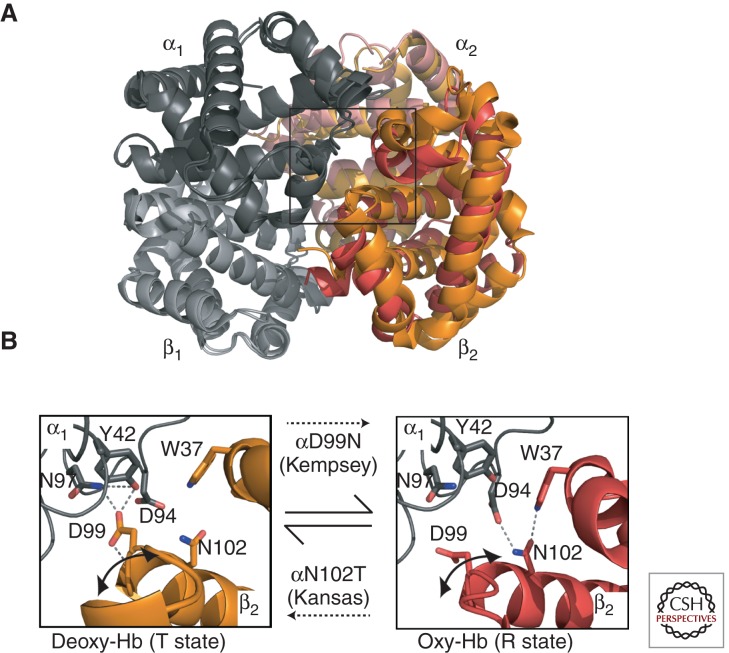
Hemoglobin variants with increased O2 affinity cause erythrocytosis by stimulating erythropoietic drive (see Hebbel et al. 1978 for a description of the associated physiology). This commonly results from amino acid substitutions that stabilize the R (high O2 affinity) state relative to the T (low O2 affinity) state and/or inhibit responses to environmental allosteric regulators that stimulate O2 release, including H+ (Bohr effect) or 2,3DPG (see the sections on Basic Principles and Hemoglobin Synthesis, Structure, and Function). Because T to R state transitions are mediated largely through α1β2 interactions, high affinity variants frequently result from substitutions that alter this interface (Fig. 2C, cyan spheres). For example, the amino acid replacement in Hb Kempsey (β99 [G1] Asp > Asn) perturbs α1β2 interactions by preventing the formation of a hydrogen bond between β99 Asp and α42 Tyr, which normally stabilizes the deoxygenated low O2 affinity T state (Fig. 3) (Reed et al. 1968; Lindstrom et al. 1973; Bunn et al. 1974). This structural change shifts quaternary equilibrium toward the oxygenated R form, which impairs O2 release to peripheral tissues and thus increases erythropoietic drive. The carboxyl termini of globin chains are also involved in α1β2 interactions that stabilize the low O2 affinity T state and numerous substitutions within these regions cause high O2 affinity variants. In addition, β146 His at the carboxyl terminus contributes significantly to the Bohr effect by forming a salt bridge with β94 Asp (Perutz et al. 1984). This interaction is disrupted in several high O2 affinity variants with β146 substitutions: Hb Hiroshima (β146 [HC3] His > Asp) (Hamilton et al. 1969; Perutz et al. 1971; Imai et al. 1972; Olson et al. 1972), Hb York (β146 [HC3] His > Pro) (Bare et al. 1976), and Hb Cowtown (β146 [HC3] His > Leu) (Fig. 2C) (Schneider et al. 1979; Perutz et al. 1984). These variants show reduced Bohr effect with impaired release of O2 under acidic conditions.
Figure 3.
Hb variants that affect allosteric regulation. (A) The α2 and β2 subunits indicated on the right side of the figure show an overlay of the R-state (red/pink) and T-state (orange/light orange) quaternary structures of Hb. The allosteric α1β2 interface is boxed. (B) Detail of the α1β2 interface in the deoxy T state (β2 chain in orange, PDB 2DN2) and oxy R state (β2 chain in red, PDB 2DN1) showing selected H-bonding interactions. Note that the two H-bonding networks use nonoverlapping sets of side chains, hence mutations in these residues affect only one state. Mutation of Asp99 to Asn (Hb Kempsey) compromises electrostatic interactions in the deoxygenated state, thereby favoring the R state and causing impaired O2 release. Mutation of Asn102 to Thr (Hb Kansas) abrogates interactions with Asp94, favoring the T state and O2 binding inhibition.
Several high affinity Hb variants are caused by substitutions that inhibit interaction with 2,3DPG, which normally binds globin chains to stimulate O2 release (Fig. 2C). For example, Hb Rahere (β82 [EF6] Lys > Thr) replaces an invariant lysine in the 2,3DPG binding site of β globin, thereby reducing the affinity for this allosteric regulator (Lorkin et al. 1975; Sugihara et al. 1985). Consequently, Hb Rahere shows blunted O2 release in response to added 2,3DPG in vitro. In vivo, O2 release in peripheral tissues is inhibited, resulting in elevated blood Hb levels. Similarly, Hb Providence (β82 [EF6] Lys > Asn) (Bonaventura et al. 1976; Moo-Penn et al. 1976) and Hb Helsinki (β82 [EF6] Lys > Met) (Ikkala et al. 1976; Charache et al. 1977) are high O2 variants caused by different amino acid substitutions at the 2,3DPG binding site on β82.
Low Oxygen Affinity Variants
Low O2 affinity Hb variants typically present with cyanosis. In general, these variants are caused by globin amino acid substitutions that tip the quaternary equilibrium of Hb tetramers from the high affinity oxygenated R state to the low affinity deoxygenated T state; more or less the opposite of what occurs for high O2 affinity variants (see the sections on Selected Variants that Illustrate Important Aspects of Hemoglobin Biology and High Oxygen Affinity Variants). This does not inhibit Hb-O2 release in tissue capillaries, but rather, interferes with Hb-O2 uptake if the P50 has increased to ≥50 mm Hg. Paradoxically, low O2 affinity Hb variants can be associated with mild anemia thought to be caused by increased O2 tissue delivery with reduced erythropoietic drive (Stamatoyannopoulos et al. 1969). In addition, some low O2 affinity mutants are unstable and therefore associated with not only cyanosis but also Heinz body hemolytic anemia (see the sections on Selected Variants that Illustrate Important Aspects of Hemoglobin Biology and Unstable Variants).
Several low affinity variants involve replacements at the α1β2 interface, which plays an important role in Hb cooperativity. Hb Kansas (β102 [G4] Asn > Thr) is a well-studied low O2 affinity variant (Fig. 3B) (Reissmann et al. 1961; Bonaventura and Riggs 1968; Gibson et al. 1973; Riggs and Gibson 1973). Affected individuals are markedly cyanotic, although clinically well. Replacement of Asn102 at the α1β2 interface inhibits the formation of a hydrogen bond with Asp94 that normally stabilizes the oxygenated R structure. A similar mechanism causes low O2 affinity in two other Hb variants through different substitutions of the same amino acid residue (B102 [G4] Asn) in Hb Beth Israel (β102 [G4] Asn > Ser) (Nagel et al. 1976) and Hb St. Mandé (β102 [G4] Asn > Tyr) (Arous et al. 1981; Poyart et al. 1990).
Methemoglobin (“M-Type”) Variants
Hemoglobin iron must be in its reduced (Fe2+, ferrous) state to bind O2. Moreover, oxidized (Fe3+, ferric, met) Hb is intrinsically unstable with a tendency to release heme. Hemoglobin reduction is maintained through intrinsic features of the Hb protein and extrinsic antioxidant pathways within red blood cells (see the sections on Basic Principles and Hemoglobin Synthesis, Structure, and Function). Exposure to oxidant drugs or toxins, genetic alterations in erythroid metHb reductase enzyme systems (Ganz and Nemeth 2012; Schechter 2012), or globin chain variants can predispose to methemoglobinemia. These disorders present as “pseudocyanosis,” (i.e., low Hb-O2 saturation), despite adequate arterial oxygenation. Detailed in vitro analyses of the red cell and isolated Hb samples can usually distinguish wild-type metHb resulting from toxins or defective reductase systems and M-type Hb variants that are predisposed to spontaneous oxidation (Bunn and Forget 1986; Steinberg et al. 2001; Nathan et al. 2009). For example, various M-Hbs show characteristic visible absorbance spectra.
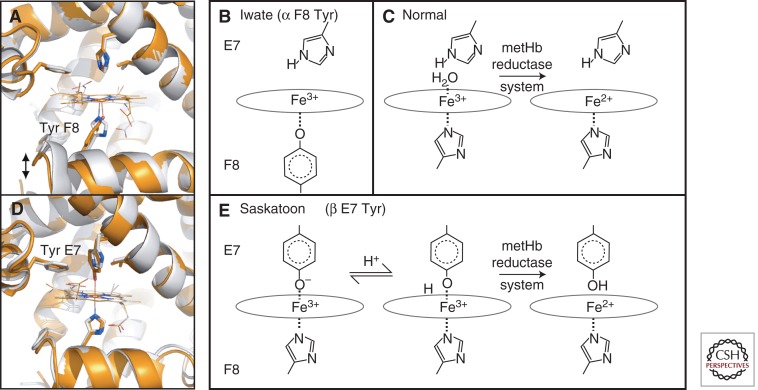
Globin variants associated with metHb formation are typically caused by amino acid substitutions within the heme pocket. For example, four different M-Hbs occur when tyrosine replaces the α- or β-globin proximal or distal histidine residues that interact with heme (Fig. 1C) (reviewed in Adachi et al. 2011). In Hb M-Iwate (α1 or α2 87 [F8] His > Tyr), the proximal histidine is replaced by tyrosine (Fig. 4A), which deprotonates and coordinates to the heme iron (Fig. 4B) (Konigsberg and Lehmann 1965; Shimizu et al. 1965). Ferric heme, bound through the native His F8, is readily reduced by metHb reductase (Fig. 4C). Tyrosine (F8) coordination stabilizes the oxidized ferric state and decreases reactivity with metHb reductases. This interaction also distorts the position of heme and helix F within the altered α subunits. In native Hb, movement of the proximal His F8 and F helix away from the heme group stabilizes the deoxygenated T state and reduces the O2 affinity of the native β subunit partners. Hence, substitution of the normal His F8 side chain for a longer Tyr F8 (Fig. 4A) also stabilizes the deoxygenated T state and reduces O2 affinity of the native β subunit in Hb M-Iwate (Nagai et al. 2000; Jin et al. 2004). These biochemical and structural alterations underlie the lack of cooperativity and severe cyanosis in patients with Hb M-Iwate, in which metHb levels can exceed 20% (normal <2%) (Ameri et al. 1999). In contrast, Hb M-Saskatoon (β63 [E7] His > Tyr) replaces the distal His with Tyr (Fig. 4D) (Horlein and Weber 1948; Hayashi et al. 1966). In this variant, the protonated form of mutant Tyr can bind ferric heme iron to generate a hexacoordinate structure that is relatively easily reduced by cellular metHb reductases (Fig. 4E). Consequently, patients with Hb M-Saskatoon have lower levels of circulating oxidized Hb compared with those with Hb M-Iwate. Comparative studies of these patients and their variant M-Hbs have contributed greatly to understanding the biochemical properties of the heme iron, including its interactions with various ligands and nearby amino acids such as the proximal and distal histidines.
Figure 4.
Examples of M-type hemoglobins. (A) The heme pocket of wild-type Mb (gray) and the F8 His > Tyr mutation (orange, PDB 1HRM), which serves as a model for Hb M-Iwate. Effects on the protein fold are to increase the distance from the heme to the F helix, recapitulating features of the deoxy-α (T state) structure. (B) In Hb M-Iwate, α 87 Tyr F8 is deprotonated and favors Fe3+ oxidation state, resulting in rapid autoxidation. This ferric form is not reduced by met-Hb reductase. (C) With the normal His F8 present, ferric heme is readily reduced. (D) Substituting the distal His E7 side chain in Mb for a larger Tyr E7 (orange, PDB 1MGN), as also occurs in Hb Saskatoon, brings the Tyr hydroxyl group within binding distance of the iron, forming a hexacoordinate iron site. (E) In Hb Saskatoon the hexacoordinate ferric iron in the effected β chains can be reduced by met-Hb reductase, possibly owing to a transient protonation of Tyr E7. Note that Mb is used as a model for Hb subunits in A and D, whereas B and E are based on Hb spectroscopy.
Globin Chain Elongation Mutants
Antitermination and frameshift mutations that add irrelevant amino acids to the carboxyl terminus of globin proteins produce interesting variants that can damage erythrocytes (Nathan et al. 2009). The most clinically significant example is Hb Constant Spring (α2 142 [HC3] Stop > Gln), caused by an antitermination mutation at the α2 stop codon (Clegg et al. 1971; Efremov et al. 1971; Milner et al. 1971; Clegg and Weatherall 1974). This elongates the protein by 31 amino acids, generating an unstable protein that is relatively underrepresented in hemolysates, but can be detected by physical methods (see sections on Basic Principles, Laboratory Testing for Hb Variants, and Physical methods of Hb separation). In addition, Hb Constant Spring mRNA is rapidly degraded in developing erythrocytes, owing to ribosomal entry into the 3′UTR, causing displacement of RNA-bound stabilizing proteins with a resultant thalassemia syndrome (Hunt et al. 1982; Derry et al. 1984; Weiss and Liebhaber 1994; Morales et al. 1997).
Hb Constant Spring contributes to α-thalassemia syndromes, particularly when combined with two α-globin deletional alleles (–/αCSα), which produces a severe form of HbH disease (Viprakasit and Tanphaichitr 2002). Isolated Hb Constant Spring, in its heterozygous (αα/αCSα) or homozygous (αCSα/αCSα) forms, results in more severe anemia than occurs when the same α alleles are deleted (αα/−α) or (−α/−α) (Schrier et al. 1997). This is due to the cytotoxic effects of the unstable Constant Spring protein. Although most common in Southeast Asia, Hb Constant Spring is increasingly identified in other geographic regions, largely through global migration (Lal et al. 2011). In fact, it was first discovered in a Chinese family living in Constant Spring, Jamaica.
One example of a β-globin chain elongation mutant is Hb Cranston (β 145 [HC3] +CT) (Bunn et al. 1975). This mutation introduces a frameshift at the normal stop codon to generate a β chain that is extended by 11 amino acids. This results in an unstable Hb tetramer with high O2 affinity and diminished cooperativity (McDonald et al. 1980; Shaeffer et al. 1980). Affected patients show compensated hemolytic anemia with the variants accounting for 30% of total Hb in the hemolysate. Interestingly, the structure of Hb Cranston was investigated simultaneously with studies to determine the β-globin mRNA 3′ untranslated sequence (Forget et al. 1975). Cross-comparison of the protein and mRNA sequencing data allowed Bunn, Forget, and colleagues to more rapidly define normal β-globin gene structures and ascertain that the Hb Cranston mutation likely arose by nonhomologous crossover of two normal β-globin genes.
Variants that Affect Multiple Hemoglobin Functions
Not surprisingly, amino acid substitutions within critical regions of globin proteins can produce multiple effects. For example, HbE (β26 [B8] Glu > Lys), a common variant in Southeast Asia, contains an amino acid substitution that renders β chains mildly unstable in vitro with minimal clinical significance (Frischer and Bowman 1975; Huisman 1997; Rees et al. 1998; see also Musallam et al. 2012). However, this mutation also creates an alternate splice site in the β-globin mRNA, leading to reduced synthesis of productive transcripts with resultant thalassemia (Orkin et al. 1982). HbE is particularly deleterious when coinherited with a more severe β-thalassemic allele, which happens commonly in Southeast Asia.
Mutations that alter the heme pocket commonly produce multiple biochemical effects. For example, deletion or substitution of the conserved Phe residue at the CD1 helical region in the heme pocket markedly destabilizes the affected globin and also alters its O2 affinity. Thus, Hb Bruxelles (β42 [CD1] Phe > 0) (Blouquit et al. 1989; Griffon et al. 1996), Hb Warsaw (β42 [CD1] Phe > Val) (Honig et al. 1990), Hb Hammersmith (β42 [CD1] Phe > Ser) (Dacie et al. 1967), and Hb Buccuresti-Louisville (β42 [CD1] Phe > Leu) (Bratu et al. 1971; Keeling et al. 1971) cause both congenital Heinz body hemolytic anemia and cyanosis. These combined effects arise from severely reduced cooperativity, rapid rates of autoxidation and hemin loss, and unfolding of these unstable globin variants (Griffon et al. 1996).
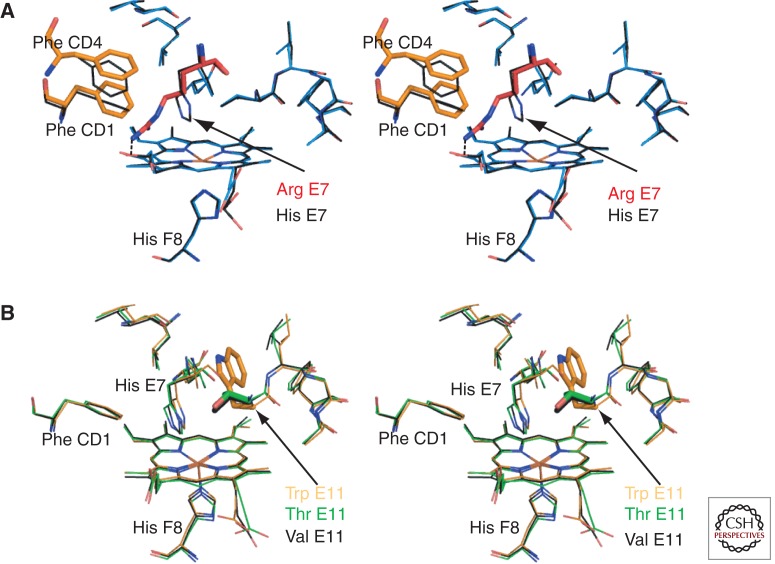
Another interesting heme pocket variant is Hb Zurich (β63 [E7] His > Arg) in which the distal His is replaced by Arg (Huisman et al. 1961; Bachmann and Martihr 1962; Frick et al. 1962; Tucker et al. 1978; Phillips et al. 1981; Springer et al. 1989). The large, highly polar variant His side chain swings out of the distal heme pocket, and the positively charged guanidino group forms a salt bridge with a heme propionate (Fig. 5A). This results in an enlarged ligand-binding pocket, destabilizing O2 binding and causing iron autoxidation via exposure to water. Affected individuals show increased sensitivity to oxidant agents, including sulfa drugs, which more easily enter the expanded heme pocket. Loss of the distal histidine markedly decreases O2 affinity but has little effect on carbon monoxide (CO) binding. As a result, individuals with Hb Zurich tend to have supranormal levels of CO-Hb, which ironically protects the heme iron from oxidation and the globin from denaturation. Affected subjects who are cigarette smokers accumulate even higher levels of CO-Hb, which tends to protect against hemolysis. Thus, “the pathology of a mutant protein is ameliorated by a normally toxic pollutant” (Bunn and Forget 1986).
Figure 5.
Hb variants with amino acid changes in the heme pocket. (A) Stereo diagram of a model of the deoxy Hb Zurich β heme pocket (blue) overlaid with the wild-type β heme pocket (black, PDB 2DN2). This model of Hb Zurich is based on the structures solved by Phillips et al. (1981) and Tucker et al. (1978). It was generated with the macromolecular modeling program Coot (Emsley et al. 2010) by mutating the distal His of deoxy-β (PDB 2DN2) to Arg. The distal Arg E7 (red) is oriented toward the CD corner, disturbing the position of Phe CD1 and Phe CD4 (orange). The heme pocket entrance is much wider allowing increased access to ligands. However, unlike normal His E7, mutant Arg is unable to stabilize bound O2 via hydrogen bonding. (B) Stereo diagram showing the structural changes associated with substitution of β Val E11. The wild-type structure carrying the branched hydrophobic side chain Val (black bonds, PDB 2DN2) is overlaid with structures carrying the largest aromatic side chain Trp E11 (orange, PDB 101K) or a polar side chain Thr E11 (green PDB, 1HDB). It is clear that no major backbone or side chain repacking occurs. Thus, mutations in this position are likely to have specific effects in changing the volume of the heme pocket accessible to solvent or diatomic ligands and the electrostatic properties of the pocket. These changes will manifest as differences in O2 binding, ligand selectivity, autoxidation, and heme loss.
Two other recently identified Hb variants illustrate how multiple biochemical defects produce unique phenotypes. Hb Jamaica Plain (β6 [A3] Glu > Val and β68 [E12] Leu > Phe) contains two defects in the same β chain, a β6 Glu to Val substitution that causes sickle cell anemia (Serjeant and Rodgers 2012) and a β68 amino acid substitution that reduces O2 affinity, probably by destabilizing the oxygenated conformation through steric effects introduced into helix E (Geva et al. 2004). The affected patient, who was heterozygous for the mutant allele, showed symptoms of sickle cell anemia that were precipitated by infection or airplane travel. Thus, an amino acid substitution that reduces O2 affinity exacerbates the effects of a sickling mutation in the same globin chain.
Several other Hb variants modulate the severity of sickle cell anemia (see also Cao and Kan 2012; Schechter and Elion 2012; Serjeant and Rodgers 2012). For example, γ globin inhibits polymerization of HbS (Nagel et al. 1979). This effect is attributable to differences in several amino acid residues compared with the corresponding β chain, including γ80 and γ87 (Adachi and Asakura 1979; Nagel et al. 1979; Adachi et al. 1996). Hb D-Ibadan (β87 [F3] Thr > Lys), which introduces a lysine residue at the β87 position, is presumed to have decreased interaction with the mutant Val residue at HbS β6 (Watson-Williams et al. 1965; Nagel et al. 1979). Thus, Hb D-Ibadan inhibits HbS polymerization. In contrast, Hb Quebec-Chori (β87 [F3] Thr > Ile) was identified in a compound heterozygous patient with mild to moderately severe sickle cell anemia (Witkowska et al. 1991). The introduction of an isoleucine at β87 causes Hb Quebec-Chori to promote deoxygenated HbS polymerization.
Hb Bristol-Alesha (β67 [E11] Val > Met) (Molchanova et al. 1993; Rees et al. 1996) and Hb Toms River (γ67 [E11] Val > Met) (Crowley et al. 2011), which contain analogous amino acid substitutions in β and γ chains, respectively, represent interesting globin variants with multiple biochemical defects. Hb Bristol-Alesha was initially observed in patients with moderately severe hemolytic anemia. Studies of the mutant protein in patient erythrocytes revealed a β67 Val > Asp substitution, predicted to render the protein unstable by introducing a highly charged polar residue into the hydrophobic heme pocket. However, subsequent DNA analysis of the same patient identified a Val > Met codon substitution (Rees et al. 1996). The investigators concluded that the mutant Met residue was converted to Asp posttranslationally, probably through oxidative mechanisms. More recently, the analogous variant was identified in fetal (γ) globin (Hb Toms River) (Crowley et al. 2011). The affected patient was a newborn presenting with both cyanosis and anemia. DNA testing revealed the codon change (Val > Met at E11). Mass spectrometry of patient hemolysate indicated a mixture of variant γ globins containing either Met or Asp at position E11. Although no structural studies have been performed with Hb chains carrying Met or Asp E11, structures with polar (Thr) or large aromatic (Trp) substitutions are available. These indicate that a range of amino acids can be accommodated without gross changes in the heme pocket structure (Fig. 5B). Instead, altered steric and electrostatic interactions with the distal His and diatomic ligands entering the heme pocket are likely to be functionally significant. Biochemical studies indicated that the Hb Toms River Met substitution produced a stable, low O2 affinity variant γ globin, causing cyanosis. Its gradual posttranslational conversion to Asp destabilized the molecule, causing hemolytic anemia. This provides an example of how posttranslational modifications of variant globins can modify phenotypes. The reason that Hb Bristol-Alesha causes predominantly hemolytic anemia whereas Hb Toms River causes mainly cyanosis probably reflects different rates of Met conversion to Asp in the variant globin chains.
CONCLUDING REMARKS
More than 1000 Hb variants are known to exist (Globin Gene Server; Hardison et al. 2002; Giardine et al. 2011). These are mainly missense mutations that destabilize Hb, alter Hb-O2 affinity, or most commonly, alter Hb function minimally. Moreover, variants that do alter Hb biochemistry are rarely life threatening or health compromising. Nonetheless, studies of these variants have been of great benefit to science and medicine for two main reasons. First, identification of Hb gene mutations as a cause for cyanosis, erythrocytosis, or mild hemolysis in otherwise healthy patients provides reassurance and minimizes additional diagnostic procedures, sparing expense and risk. Second, efforts to understand how Hb variants produce their structural, biochemical, and clinical effects has generated important insights into red blood cell function and also created general paradigms for the study of protein biology. Despite hundreds of studies over more than 50 years, new Hb variants continue to emerge, yielding new insights into this important molecule.
ACKNOWLEDGMENTS
We thank Drs. Franklin Bunn, Kazuhiko Adachi, and John Olson for comments on the manuscript. Hemoglobin research in Dr. Weiss’s laboratory is funded through National Institutes of Health (NIH) grants DK061692, HL087427, and P30DK090969. The authors declare no competing financial interests.
Footnotes
Editors: David Weatherall, Alan N. Schechter, and David G. Nathan
Additional Perspectives on Hemoglobin and Its Diseases available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Adachi K, Asakura T 1979. The solubility of sickle and non-sickle hemoglobins in concentrated phosphate buffer. J. Biol Chem 254: 4079–4084 [PubMed] [Google Scholar]
- Adachi K, Pang J, Konitzer P, Surrey S 1996. Polymerization of recombinant hemoglobin F γE6V and hemoglobin F γE6V, γQ87T alone, and in mixtures with hemoglobin S. Blood 87: 1617–1624 [PubMed] [Google Scholar]
- Adachi K, Surrey S, Nagai M 2011. Hemoglobinopathies due to amino acid mutation/deletion: HbS and HbM. In Hemoglobin: Recent developments and topics, pp. 179–210 Research Signpost, Kerala, India [Google Scholar]
- Adams J, Boxer L, Baehner R, Forget B, Tsistrakis G, Steinberg M 1979. Hemoglobin Indianapolis (β112 [G14] arginine). An unstable β-chain variant producing the phenotype of severe β-thalassemia. J Clin Invest 63: 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameri A, Fairbanks V, Yanik G, Mahdi F, Thibodeau S, McCormick D, Boxer L, McDonagh K 1999. Identification of the molecular genetic defect of patients with methemoglobin M-Kankakee (M-Iwate), α87 (F8) His→ Tyr: Evidence for an electrostatic model of αM hemoglobin assembly. Blood 94: 1825–1826 [PubMed] [Google Scholar]
- Argos P, Rossman MG, Grau UM, Zuber H, Frank G, Tratschin JD 1979. Thermal stability and protein structure. Biochemistry 18: 5698–5703 [DOI] [PubMed] [Google Scholar]
- Arnone A 1972. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature 237: 146–149 [DOI] [PubMed] [Google Scholar]
- Arous N, Braconnier F, Thillet J, Blouquit Y, Galacteros F, Chevrier M, Bordahandy C, Rosa J 1981. Hemoglobin Saint Mandé [β102 (G4) Asn→Tyr]: A new low oxygen affinity variant. FEBS Lett 126: 114–116 [DOI] [PubMed] [Google Scholar]
- Asakura T, Adachi K, Shapiro M, Friedman S, Schwartz E 1975. Mechanical precipitation of hemoglobin köln. Biochim Biophys Acta 412: 197–201 [DOI] [PubMed] [Google Scholar]
- Bachmann F, Marti HR 1962. Hemoglobin Zürich. II. Physicochemical properties of the abnormal hemoglobin. Blood 20: 272–86 [PubMed] [Google Scholar]
- Bare GH, Bromberg PA, Alben JO, Brimhall B, Jones RT, Mintz S, Rother I 1976. Altered C-terminal salt bridges in haemoglobin York cause high oxygen affinity. Nature 259: 155–156 [DOI] [PubMed] [Google Scholar]
- Bender JW, Adachi K, Asakura T 1981. Precipitation of oxyhemoglobins A and S by isopropanol. Hemoglobin 5: 463–474 [DOI] [PubMed] [Google Scholar]
- Blouquit Y, Bardakdjian J, Lena-Russo D, Arous N, Perrimond H, Orsini A, Rosa J, Galacteros F 1989. Hb Bruxelles: α 2A β (2)41 or 42(C7 or CD1)Phe deleted. Hemoglobin 13: 465–474 [DOI] [PubMed] [Google Scholar]
- Bonaventura J, Riggs A 1968. Hemoglobin Kansas, a human hemoglobin with a neutral amino acid substitution and an abnormal oxygen equilibrium. J. Biol Chem 243: 980–991 [PubMed] [Google Scholar]
- Bonaventura J, Bonaventura C, Sullivan B, Ferruzzi G, McCurdy PR, Fox J, Moo-Penn WF 1976. Hemoglobin providence. Functional consequences of two alterations of the 2,3-diphosphoglycerate binding site at position β82. J. Biol Chem 251: 7563–7571 [PubMed] [Google Scholar]
- Bratu V, Lorkin PA, Lehmann H, Predescu C 1971. Haemoglobin Buccureşti 42(CD1) Phe-Leu, a cause of unstable haemoglobin haemolytic anaemia. Biochim Biophys Acta 251: 1–6 [DOI] [PubMed] [Google Scholar]
- Bunn HF, Forget BG 1986. Hemoglobin: Molecular, genetic and clinical aspects. W.B. Saunders, Philadelphia [Google Scholar]
- Bunn HF, Wohl RC, Bradley TB, Cooley M, Gibson QH 1974. Functional properties of hemoglobin Kempsey. J Biol Chem 249: 7402–7409 [PubMed] [Google Scholar]
- Bunn HF, Schmidt GJ, Haney DN, Dluhy RG 1975. Hemoglobin Cranston, an unstable variant having an elongated β chain due to nonhomologous crossover between two normal β chain genes. Proc Natl Acad Sci 72: 3609–3613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Cao A, Kan YW 2012. The prevention of thalassemia. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrell RW, Kay R 1972. A simple method for the detection of unstable haemoglobins. Br J Haematol 23: 615–619 [DOI] [PubMed] [Google Scholar]
- Charache S, Fox J, McCurdy P, Kazazian H Jr, Winslow R, Hathaway P, van Beneden R, Jessop M 1977. Postsynthetic deamidation of hemoglobin Providence (β82 Lys replaced by Asn, Asp) and its effect on oxygen transport. J. Clin Invest 59: 652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg JB, Weatherall DJ 1974. Hemoglobin Constant Spring, and unusual α-chain variant involved in the etiology of hemoglobin H disease. Ann NY Acad Sci 232: 168–178 [DOI] [PubMed] [Google Scholar]
- Clegg J, Weatherall D, Boon W, Mustafa D 1969. Two new haemoglobin variants involving proline substitutions. Nature 222: 379–380 [DOI] [PubMed] [Google Scholar]
- Clegg JB, Weatherall DJ, Milner PF 1971. Haemoglobin Constant Spring—A chain termination mutant? Nature 234: 337–340 [DOI] [PubMed] [Google Scholar]
- Coleman MB, Steinberg MH, Adams JG 3rd. 1991. Hemoglobin Terre Haute arginine β106. A posthumous correction to the original structure of hemoglobin Indianapolis. J Biol Chem 266: 5798–5800 [PubMed] [Google Scholar]
- Como PF, Wylie BR, Trent RJ, Bruce D, Volpato F, Wilkinson T, Kronenberg H, Holland RA, Tibben EA 1991. A new unstable and low oxygen affinity hemoglobin variant: Hb Stanmore (β111[G13]Val→Ala). Hemoglobin 15: 53–65 [DOI] [PubMed] [Google Scholar]
- Crowley M, Mollan T, Abdulmalik O, Butler AD, Goodwin E, Sarkar A, Stolle C, Gow A, Olson J, Weiss M 2011. A hemoglobin variant associated with neonatal cyanosis and anemia. N Engl J Med 364: 1837–1843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacie JV, Shinton NK, Gaffney PJ Jr, Lehmann H 1967. Haemoglobin Hammersmith (β-42 [CDI] Phe replaced by ser). Nature 216: 663–665 [DOI] [PubMed] [Google Scholar]
- *.Dailey HA, Meissner PN 2012. Erythroid heme biosynthesis and its disorders. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derry S, Wood WG, Pippard M, Clegg JB, Weatherall DJ, Wickramasinghe SN, Darley J, Fucharoen S, Wasi P 1984. Hematologic and biosynthetic studies in homozygous hemoglobin Constant Spring. J. Clin Invest 73: 1673–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efremov GD, Wrightstone RN, Huisman TH, Schroeder WA, Hyman C, Ortega J, Williams K 1971. An unusual hemoglobin anomaly and its relation to α-thalassemia and hemoglobin-H disease. J. Clin Invest 50: 1628–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger J, Flores J, Tyson JA, Shohet SB 1985. Fluorescent cytoplasm and Heinz bodies of hemoglobin Köln erythrocytes: Evidence for intracellular heme catabolism. Blood 65: 886–893 [PubMed] [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K 2010. Features and development of Coot. Acta Cryst 66: 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero ME, Costa FF 2011. α-Hemoglobin-stabilizing protein: An erythroid molecular chaperone. Biochem Res Int 2011: 373859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget BG, Marotta CA, Weissman SM, Cohen-Solal M 1975. Nucleotide sequences of the 3′-terminal untranslated region of messenger RNA for human β globin chain. Proc Natl Acad Sci 72: 3614–3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick PG, Hitzig WH, Betke K 1962. Hemoglobin Zurich. I. A new hemoglobin anomaly associated with acute hemolytic episodes with inclusion bodies after sulfonamide therapy. Blood 20: 261–271 [PubMed] [Google Scholar]
- Frischer H, Bowman J 1975. Hemoglobin E, an oxidatively unstable mutation. J Lab Clin Med 85: 531–539 [PubMed] [Google Scholar]
- *.Ganz T, Nemeth E 2012. Iron metabolism: Interactions with normal and disordered erythropoiesis. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod A 1928. The lessons of rare maladies: Annual oration before the medical society of London. Lancet 1: 914–915 [PMC free article] [PubMed] [Google Scholar]
- Geva A, Clark JJ, Zhang Y, Popowicz A, Manning JM, Neufeld EJ 2004. Hemoglobin Jamaica Plain—A sickling hemoglobin with reduced oxygen affinity. N Engl J Med 351: 1532–1538 [DOI] [PubMed] [Google Scholar]
- Giardine B, Borg J, Higgs D, Peterson K, Philipsen S, Maglott D, Singleton B, Anstee D, Basak A, Clark B, et al. 2011. Systematic documentation and analysis of human genetic variation in hemoglobinopathies using the microattribution approach. Nat Genet 43: 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson QH, Riggs A, Imamura T 1973. Kinetic and equilibrium properties of hemoglobin Kansas. J. Biol Chem 248: 5976–5986 [PubMed] [Google Scholar]
- Giordano PC, Zweegman S, Akkermans N, Arkesteijn SGJ, van Delft Peter, Versteegh FGA, Wajcman Henri, Harteveld CL 2007. The first case of Hb Groene Hart (α119[H2]Pro→Ser, CCT→TCT [α1]) homozygosity confirms that a thalassemia phenotype is associated with this abnormal hemoglobin variant. Hemoglobin 31: 179–182 [DOI] [PubMed] [Google Scholar]
- Griffon N, Badens C, Lena-Russo D, Kister J, Bardakdjian J, Wajcman H, Marden MC, Poyart C 1996. Hb Bruxelles, deletion of Pheβ42, shows a low oxygen affinity and low cooperativity of ligand binding. J. Biol Chem 271: 25916–25920 [DOI] [PubMed] [Google Scholar]
- Hamilton HB, Iuchi I, Miyaji T, Shibata S 1969. Hemoglobin Hiroshima (β143 histidine→aspartic acid): A newly identified fast moving β chain variant associated with increased oxygen affinity and compensatory erythremia. J. Clin Invest 48: 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardison R, Chui D, Giardine B, Riemer C, Patrinos G, Anagnou N, Miller W, Wajcman H 2002. HbVar: A relational database of human hemoglobin variants and thalassemia mutations at the globin gene server. Hum Mutat 19: 225–233 [DOI] [PubMed] [Google Scholar]
- Harteveld C, van Delft P, Plug R, Versteegh F, Hagen B, van Rooijen I, Kok P, Wajcman H, Kister J, Giordano PC 2002. Hb Groene Hart: A new Pro→Ser amino acid substitution at position 119 of the α1-globin chain is associated with a mild α-thalassemia phenotype. Hemoglobin 26: 255–60 [DOI] [PubMed] [Google Scholar]
- Hayashi A, Shimizu A, Yamamura Y, Watari H 1966. Hemoglobins M: Identification of Iwate, Boston, and Saskatoon variants. Science 152: 207–208 [DOI] [PubMed] [Google Scholar]
- Hebbel RP, Eaton JW, Kronenberg RS, Zanjani ED, Moore LG, Berger EM 1978. Human llamas: Adaptation to altitude in subjects with high hemoglobin oxygen affinity. J Clin Invest 62: 593–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig GR, Vida LN, Rosenblum BB, Perutz MF, Fermi G 1990. Hemoglobin Warsaw (Pheβ42(CD1)→Val), an unstable variant with decreased oxygen affinity. Characterization of its synthesis, functional properties, and structure. J. Biol Chem 265: 126–132 [PubMed] [Google Scholar]
- Horlein H, Weber G 1948. Ueber chronische familiäre methämoglobinämie und eine neue modifikation des methämoglobins. Dtsch Med Wochenschr 73: 476–478 [DOI] [PubMed] [Google Scholar]
- Hoyer JD, McCormick DJ, Snow K, Kwon JH, Booth D, Duarte M, Grayson G, Kubik KS, Holmes MW, Fairbanks VF 2002. Four new variants of the α2-globin gene without clinical or hematologic effects: Hb Park Ridge (α9[α7]Asn→Lys [α2]), Hb Norton (α72[EF1]His→Asp [α2]), Hb Lombard (α103[G10]His→Tyr [α2]), and Hb San Antonio (A113[GH2]Leu→Arg [A2]). Hemoglobin 26: 175–179 [DOI] [PubMed] [Google Scholar]
- Huisman TH 1997. Hb E and α-thalassemia; variability in the assembly of βE chain containing tetramers. Hemoglobin 21: 227–236 [DOI] [PubMed] [Google Scholar]
- Huisman TH, Horton B, Bridges MT, Betke K, Hitzig WH 1961. A new abnormal human hemoglobin-Hb: Zurich. Clin Chim Acta 6: 347–355 [DOI] [PubMed] [Google Scholar]
- Hunt DM, Higgs DR, Winichagoon P, Clegg JB, Weatherall DJ 1982. Haemoglobin Constant Spring has an unstable α chain messenger RNA. Br J Haematol 51: 405–413 [DOI] [PubMed] [Google Scholar]
- Ikkala E, Koskela J, Pikkarainen P, Rahiala EL, El-Hazmi MA, Nagai K, Lang A, Lehmann H 1976. Hb Helsinki: A variant with a high oxygen affinity and a substitution at a 2,3-DPG binding site (β82[EF6] Lys replaced by Met). Acta Haematol 56: 257–275 [DOI] [PubMed] [Google Scholar]
- Imai K, Hamilton HB, Miyaji T, Shibata S 1972. Physicochemical studies of the relation between structure and function in hemoglobin Hiroshima (HC3, histidine leads to aspartate). Biochemistry 11: 114–121 [DOI] [PubMed] [Google Scholar]
- Jin Y, Nagai M, Nagai Y, Nagatomo S, Kitagawa T 2004. Heme structures of five variants of hemoglobin M probed by resonance Raman spectroscopy. Biochemistry 43: 8517–8527 [DOI] [PubMed] [Google Scholar]
- Keeling MM, Ogden LL, Wrightstone RN, Wilson JB, Reynolds CA, Kitchens JL, Huisman TH 1971. Hemoglobin Louisville (β-42 [CD1] phe-leu): An unstable variant causing mild hemolytic anemia. J Clin Invest 50: 2395–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khandros E, Mollan TL, Yu X, Wang X, Yao Y, D’Souza J, Gell DA, Olson JS, Weiss MJ 2012. Insights into hemoglobin assembly through in vivo mutagenesis of α-hemoglobin stabilizing protein. J Biol Chem 287: 11325–11327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MA, Wiltshire BG, Lehmann H, Morimoto H 1972. An unstable haemoglobin with reduced oxygen affinity: Haemoglobin Peterborough, 3 (GI3) Valine lead to Phenylalanine, its interaction with normal haemoglobin and with haemoglobin Lepore. Br J Haematol 22: 125–134 [DOI] [PubMed] [Google Scholar]
- Kohne E 2011. Hemoglobinopathies: Clinical manifestations, diagnosis, and treatment. Dtsch Ärztebl Int 108: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konigsberg W, Lehmann H 1965. The amino acid substitution in hemoglobin M-Iwate. Biochim Biophys Acta 107: 266–269 [DOI] [PubMed] [Google Scholar]
- Konigsberg W, Guidotti G, Hill RJ 1961. The amino acid sequence of the α chain of human hemoglobin. J. Biol Chem 236: PC55–PC56 [PubMed] [Google Scholar]
- Lacan P, Aubry M, Couprie N, Francina A 2004. Two new α chain variants: Hb Die (α93[FG5]Val→Ala [α1]) and Hb Beziers (α99[G6]Lys→Asn [α1]). Hemoglobin 28: 59–63 [DOI] [PubMed] [Google Scholar]
- Lacerra G, Scarano C, Musollino G, Flagiello A, Pucci P, Carestia C 2008. Hb Foggia or α117(GH5)Phe→Ser: A new α2 globin allele affecting the αHb-AHSP interaction. Haematologica 93: 141–142 [DOI] [PubMed] [Google Scholar]
- Lal A, Goldrich ML, Haines DA, Azimi M, Singer ST, Vichinsky EP 2011. Heterogeneity of hemoglobin H disease in childhood. N. Engl J Med 364: 710–718 [DOI] [PubMed] [Google Scholar]
- Langdown JV, Davidson RJ, Williamson D 1992. A new α chain variant, Hb Turriff (α99[G6]Lys→Glu): The interference of abnormal hemoglobins in Hb A1c determination. Hemoglobin 16: 11–17 [DOI] [PubMed] [Google Scholar]
- Lehmann H 1957. Haemoglobin and its abnormalities. Practitioner 178: 198–214 [PubMed] [Google Scholar]
- *.Lettre G 2012. The search for genetic modifiers of disease severity in the β-hemoglobinopathies. Cold Spring Harb Perspect Med 10.1101/cshperspect.a015032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M 1981. Effect of proline residues on protein folding. J. Mol Biol 145: 251–263 [DOI] [PubMed] [Google Scholar]
- Lindstrom TR, Baldassare JJ, Bunn HF, Ho C 1973. Nuclear magnetic resonance and spin-label studies of hemoglobin Kempsey. Biochemistry 12: 4212–4217 [DOI] [PubMed] [Google Scholar]
- Little R, Roberts W 2009. A review of variant hemoglobins interfering with hemoglobin A1c measurement. J Diabetes Sci Technol 3: 446–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorkin P, Stephens A, Beard M, Wrigley P, Adams L, Lehmann H 1975. Haemoglobin Rahere (β Lys-Thr): A new high affinity haemoglobin associated with decreased 2,3-diphosphoglycerate binding and relative polycythaemia. Br Med J 4: 200–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinucci M, Mavilio F, Massa A, Gabbianelli M, Fontanarosa PP, Camagna A, Ignesti C, Tentori L 1979. A new abnormal human hemoglobin: Hb Prato (α2 31 [B12] Arg leads to Ser β2). Biochim Biophys Acta 578: 534–540 [DOI] [PubMed] [Google Scholar]
- Martínez G, Lima F, Colombo B 1977. Haemoglobin J Guantanamo (α 2 β 2 128 [H6] Ala replaced by Asp). A new fast unstable haemoglobin found in a Cuban family. Biochim Biophys Acta 491: 1–6 [DOI] [PubMed] [Google Scholar]
- McDonald MJ, Lund DP, Bleichman M, Bunn HF, De Young A, Noble RW, Foster B, Arnone A 1980. Equilibrium, kinetic and structural properties of hemoglobin Cranston, an elongated β chain variant. J. Mol Biol 140: 357–375 [DOI] [PubMed] [Google Scholar]
- Milner PF, Clegg JB, Weatherall DJ 1971. Haemoglobin-H disease due to a unique haemoglobin variant with an elongated α-chain. Lancet 1: 729–732 [DOI] [PubMed] [Google Scholar]
- Molchanova TP, Postnikov YuV, Pobedimskaya DD, Smetanina NS, Moschan AA, Kazanetz EG, Tokarev YuN, Huisman TH 1993. Hb Alesha or α 2 β (2)67 (E11)Val→Met: A new unstable hemoglobin variant identified through sequencing of amplified DNA. Hemoglobin 17: 217–225 [DOI] [PubMed] [Google Scholar]
- Mollan TL, Khandros E, Weiss MJ, Olson JS 2012. The kinetics of α-globin binding to α hemoglobin stabilizing protein (AHSP) indicate preferential stabilization of a hemichrome folding intermediate. J Biol Chem 287: 11338–11350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moo-Penn WF, Jue DL, Bechtel KC, Johnson MH, Schmidt RM 1976. Hemoglobin Providence. A human hemoglobin variant occurring in two forms in vivo. J. Biol Chem 251: 7557–7562 [PubMed] [Google Scholar]
- Moo-Penn W, Jue D, Johnson M, Olsen K, Shih D, Jones R, Lux S, Rodgers P, Arnone A 1988. Hemoglobin Brockton (β 138 [H16] Ala→Pro): An unstable variant near the C-terminus of the β-subunits with normal oxygen-binding properties. Biochemistry 27: 7614–7619 [DOI] [PubMed] [Google Scholar]
- Morales J, Russell JE, Liebhaber SA 1997. Destabilization of human α-globin mRNA by translation anti-termination is controlled during erythroid differentiation and is paralleled by phased shortening of the poly(A) tail. J Biol Chem 272: 6607–6613 [DOI] [PubMed] [Google Scholar]
- *.Musallam KM, Taher AT, Rachmilewitz EA 2012. β-Thalassemia intermedia: A clinical perspective. Cold Spring Harb Perspect Med 10.1101/cshperspect.a013482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai M, Aki M, Li R, Jin Y, Sakai H, Nagatomo S, Kitagawa T 2000. Heme structure of hemoglobin M Iwate (α87[F8]His→Tyr): A UV and visible resonance Raman study. Biochemistry 39: 13093–13105 [DOI] [PubMed] [Google Scholar]
- Nagel RL, Lynfield J, Johnson J, Landau L, Bookchin RM, Harris MB 1976. Hemoglobin Beth Israel. A mutant causing clinically apparent cyanosis. N. Engl J Med 295: 125–130 [DOI] [PubMed] [Google Scholar]
- Nagel RL, Bookchin RM, Johnson J, Labie D, Wajcman H, Isaac-Sodeye WA, Honig GR, Schilirò G, Crookston JH, Matsutomo K 1979. Structural bases of the inhibitory effects of hemoglobin F and hemoglobin A2 on the polymerization of hemoglobin S. Proc Natl Acad Sci 76: 670–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan D, Orkin S, Ginsburg D, Look A, Fisher D, Lux S 2009. Nathan and Oski’s hematology of infancy and childhood, 7th ed Saunders Elsevier, Philadelphia [Google Scholar]
- Ohba Y, Miyaji T, Matsuoka M, Yokoyama M, Numakura H 1975. Hemoglobin Hirosaki (α43 [CE 1] Phe replaced by Leu), a new unstable variant. Biochim Biophys Acta 405: 155–160 [DOI] [PubMed] [Google Scholar]
- Olson J, Gibson Q, Nagel R, Hamilton H 1972. The ligand-binding properties of hemoglobin Hiroshima (α2β2146 asp ). J Biol Chem 247: 7485–7493 [PubMed] [Google Scholar]
- Orkin S, Kazazian HJ, Antonarakis S, Ostrer H, Goff S, Sexton J 1982. Abnormal RNA processing due to the exon mutation of βE-globin gene. Nature 300: 768–769 [DOI] [PubMed] [Google Scholar]
- Paoli M, Liddington R, Tame J, Wilkinson A, Dodson G 1996. Crystal structure of T state haemoglobin with oxygen bound at all four haems. J Mol Biol 256: 775–792 [DOI] [PubMed] [Google Scholar]
- Park S, Yokoyama T, Shibayama N, Shiro Y, Tame JR 2006. 1.25 A resolution crystal structures of human haemoglobin in the oxy, deoxy and carbonmonoxy forms. J Mol Biol 360: 690–701 [DOI] [PubMed] [Google Scholar]
- Perutz M 1960. Structure of hemoglobin. Brookhaven Symp Biol 13: 165–183 [PubMed] [Google Scholar]
- Perutz M 1970. Stereochemistry of cooperative effects in haemoglobin. Nature 228: 726–739 [DOI] [PubMed] [Google Scholar]
- Perutz M, Lehmann H 1968. Molecular pathology of human haemoglobin. Nature 219: 902–909 [DOI] [PubMed] [Google Scholar]
- Perutz M, Rossman M, Cullis A, Muirhead H, Will G, North A 1960. Structure of haemoglobin: Three-dimensional Fourier synthesis at 5.5-A resolution, obtained by X-ray analysis. Nature 185: 416–422 [DOI] [PubMed] [Google Scholar]
- Perutz MF, Muirhead H, Mazzarella L, Crowther RA, Greer J, Kilmartin JV 1969. Identification of residues responsible for the alkaline Bohr effect in haemoglobin. Nature 222: 1240–1243 [DOI] [PubMed] [Google Scholar]
- Perutz MF, Pulsinelli P, Eyck LT, Kilmartin JV, Shibata S, Iuchi I, Miyaji T, Hamilton HB 1971. Haemoglobin Hiroshima and the mechanism of the alkaline Bohr effect. Nat New Biol 232: 147–149 [DOI] [PubMed] [Google Scholar]
- Perutz M, Fermi G, Shih TB 1984. Structure of deoxyhemoglobin Cowtown (His HC3[146] β→Leu): Origin of the alkaline Bohr effect and electrostatic interactions in hemoglobin. Proc Natl Acad Sci 81: 4781–4784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips SE, Hall D, Perutz MF 1981. Structure of deoxyhaemoglobin Zürich (HisE7[63 β]—greater than Arg). J Mol Biol 150: 137–141 [DOI] [PubMed] [Google Scholar]
- Poyart C, Schaad O, Kister J, Galacteros F, Edelstein SJ, Blouquit Y, Arous N 1990. Hemoglobin Saint Mandé [β102 (G4) Asn→Tyr]. Functional studies and structural modeling reveal an altered T state. Eur J Biochem 194: 343–348 [DOI] [PubMed] [Google Scholar]
- Pulsinelli PD, Perutz M, Nagel R 1973. Structure of hemoglobin M Boston, a variant with a five-coordinated ferric heme. Proc Natl Acad Sci 70: 3870–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabbitts TH 1976. Bacterial cloning of plasmids carrying copies of rabbit globin messenger RNA. Nature 260: 221–225 [DOI] [PubMed] [Google Scholar]
- Reed CS, Hampson R, Gordon S, Jones RT, Novy MJ, Brimhall B, Edwards MJ, Koler RD 1968. Erythrocytosis secondary to increased oxygen affinity of a mutant hemoglobin, hemoglobin Kempsey. Blood 31: 623–632 [PubMed] [Google Scholar]
- Rees DC, Rochette J, Schofield C, Green B, Morris M, Parker NE, Sasaki H, Tanaka A, Ohba Y, Clegg JB 1996. A novel silent posttranslational mechanism converts methionine to aspartate in hemoglobin Bristol (β67[E11] Val-Met→Asp). Blood 88: 341–348 [PubMed] [Google Scholar]
- Rees DC, Clegg JB, Weatherall DJ 1998. Is hemoglobin instability important in the interaction between hemoglobin E and β thalassemia? Blood 92: 2141–2146 [PubMed] [Google Scholar]
- Reissmann K, Ruth W, Nomura T 1961. A human hemoglobin with lowered oxygen affinity and impaired heme-heme interactions. J Clin Invest 40: 1826–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder RF, Oski FA, Clegg JB 1969. Hemoglobin Philly (β35 tyrosine phenylalanine): Studies in the molecular pathology of hemoglobin. J. Clin Invest 48: 1627–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A, Gibson QH 1973. Oxygen equilibrium and kinetics of isolated subunits from hemoglobin Kansas. Proc Natl Acad Sci 70: 1718–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth EF Jr, Elbaum D, Bookchin RM, Nagel RL 1976. The conformational requirements for the mechanical precipitation of hemoglobin S and other mutants. Blood 48: 265–271 [PubMed] [Google Scholar]
- Russu IM, Ho C 1986. Assessment of role of β 146-histidyl and other histidyl residues in the Bohr effect of human normal adult hemoglobin. Biochemistry 25: 1706–1716 [DOI] [PubMed] [Google Scholar]
- *.Sankaran VG, Orkin SH 2012. The switch from fetal to adult hemoglobin. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RG, Bremner JE, Brimhall B, Jones RT, Shih TB 1979. Hemoglobin Cowtown (β 146 HC3 His-Leu): A mutant with high oxygen affinity and erythrocytosis. Am J Clin Pathol 72: 1028–1032 [DOI] [PubMed] [Google Scholar]
- Schrier SL, Bunyaratvej A, Khuhapinant A, Fucharoen S, Aljurf M, Snyder LM, Keifer CR, Ma L, Mohandas N 1997. The unusual pathobiology of hemoglobin constant spring red blood cells. Blood 89: 1762–1769 [PubMed] [Google Scholar]
- Schroeder WA, Jones RT, Shelton JR, Shelton JB, Cormick J, McCalla K 1961. A partial sequence of the amino acid residues in the γ chain of human hemoglobin F. Proc Natl Acad Sci 47: 811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciarratta GV, Ivaldi G, Molaro GL, Sansone G, Salkie ML, Wilson JB, Reese AL, Huisman TH 1984. The characterization of hemoglobin Manitoba or α2102(G9)Ser→Arg β2 and hemoglobin Contaldo or α2103(G10)His→Arg β2 by high performance liquid chromatography. Hemoglobin 8: 169–181 [DOI] [PubMed] [Google Scholar]
- Shaeffer JR, Schmidt GJ, Kingston RE, Bunn HF 1980. Synthesis of hemoglobin Cranston, and elongated β chain variant. J Mol Biol 140: 377–389 [DOI] [PubMed] [Google Scholar]
- *.Schechter A 2012. Hemoglobin function. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011650 [DOI] [Google Scholar]
- *.Schechter A, Elion J 2012. Cold Spring Harb Perspect Med (to be published) [Google Scholar]
- *.Serjeant G, Rodgers G 2012. Natural history of sickle cell disease. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Tanuira A, Iuchi I 1960. Hemoglobin M1 demonstration of a new abnormal hemoglobin in hereditary nigremia. Acta Haematol Jpn 23: 96–105 [Google Scholar]
- Shimizu A, Tsugita A, Hayashi A, Yamamura Y 1965. The primary structure of hemoglobin M-Iwate. Biochim Biophys Acta 107: 270–277 [DOI] [PubMed] [Google Scholar]
- Springer BA, Egeberg KD, Sligar SG, Rohlfs RJ, Mathews AJ, Olson JS 1989. Discrimination between oxygen and carbon monoxide and inhibition of autooxidation by myoglobin. Site-directed mutagenesis of the distal histidine. J Biol Chem 264: 3057–3060 [PubMed] [Google Scholar]
- Stamatoyannopoulos G, Parer JT, Finch CA 1969. Physiologic implications of a hemoglobin with decreased oxygen affinity (hemoglobin seattle). N Engl J Med 281: 916–919 [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos G, Woodson R, Papayannopoulou T, Heywood D, Kurachi S 1974. Inclusion-body β-thalassemia trait. A form of β thalassemia producing clinical manifestations in simple heterozygotes. N Engl J Med 290: 939–943 [DOI] [PubMed] [Google Scholar]
- Steinberg M, Forget B, Higgs D, Nagel R 2001. Disorders of hemoglobin: Genetics, pathophysiology, and clinical management. Cambridge University Press, Cambridge [Google Scholar]
- Sugihara J, Imamura T, Nagafuchi S, Bonaventura J, Bonaventura C, Cashon R 1985. Hemoglobin Rahere, a human hemoglobin variant with amino acid substitution at the 2,3-diphosphoglycerate binding site. Functional consequences of the alteration and effects of bezafibrate on the oxygen bindings. J Clin Invest 76: 1169–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Matsui K, Matsuda K, Shinohara K, Haranob K 2005. A family with hemoglobin Hirosaki. Int J Hematol 82: 124–126 [DOI] [PubMed] [Google Scholar]
- Thein SL, Hesketh C, Taylor P, Temperley IJ, Hutchinson RM, Old JM, Wood WG, Clegg JB, Weatherall DJ 1990. Molecular basis for dominantly inherited inclusion body β-thalassemia. Proc Natl Acad Sci 87: 3924–3928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremper K, Barker S 1989. Pulse oximetry. Anesthesiology 70: 98–108 [DOI] [PubMed] [Google Scholar]
- Tucker PW, Phillips SE, Perutz MF, Houtchens R, Caughey WS 1978. Structure of hemoglobins Zürich (His E7[63]β replaced by Arg) and Sydney (Val E11[67]β replaced by Ala) and role of the distal residues in ligand binding. Proc Natl Acad Sci 75: 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turbpaiboon C, Limjindaporn T, Wongwiwat W, U-Pratya Y, Siritanaratkul N, Yenchitsomanus P-thai, Jitrapakdee S, Wilairat P 2006. Impaired interaction of α-haemoglobin-stabilising protein with α-globin termination mutant in a yeast two-hybrid system. Br J Haematol 132: 370–373 [DOI] [PubMed] [Google Scholar]
- Vasseur C, Domingues-Hamdi E, Brillet T, Marden Michael C, Baudin-Creuza V 2009. The α-hemoglobin stabilizing protein and expression of unstable α-Hb variants. Clin Biochem 42: 1818–1823 [DOI] [PubMed] [Google Scholar]
- Vasseur-Godbillon C, Marden M, Giordano P, Wajcman H, Baudin-Creuza V 2006. Impaired binding of AHSP to α chain variants: Hb Groene Hart illustrates a mechanism leading to unstable hemoglobins with α thalassemic like syndrome. Blood Cells Mol Dis 37: 173–179 [DOI] [PubMed] [Google Scholar]
- Verhovsek M, Henderson M, Cox G, Luo H, Steinberg M, Chui D 2010. Unexpectedly low pulse oximetry measurements associated with variant hemoglobins: A systematic review. Am J Hematol 85: 882–885 [DOI] [PubMed] [Google Scholar]
- Viprakasit V, Tanphaichitr VS 2002. Compound heterozygosity for α0-thalassemia (- -THAI) and Hb constant spring causes severe Hb H disease. Hemoglobin 26: 155–162 [DOI] [PubMed] [Google Scholar]
- Wajcman H, Moradkhani K 2011. Abnormal haemoglobins: Detection and characterization. Indian J Med Res 134: 538–546 [PMC free article] [PubMed] [Google Scholar]
- Wajcman H, Préhu C, Bardakdjian-Michau J, Promé D, Riou J, Godart C, Mathis M, Hurtrel D, Galactéros F 2001. Abnormal hemoglobins: Laboratory methods. Hemoglobin 25: 169–181 [DOI] [PubMed] [Google Scholar]
- Wajcman H, Traeger-Synodinos J, Papassotiriou I, Giordano PC, Harteveld CL, Baudin-Creuza V, Old J 2008. Unstable and thalassemic α chain hemoglobin variants: A cause of Hb H disease and thalassemia intermedia. Hemoglobin 32: 327–349 [DOI] [PubMed] [Google Scholar]
- Watson HC, Kendrew JC 1961. The amino-acid sequence of sperm whale myoglobin. Comparison between the amino-acid sequences of sperm whale myoglobin and of human hemoglobin. Nature 190: 670–672 [DOI] [PubMed] [Google Scholar]
- Watson-Williams EJ, Beale D, Irvine D, Lehmann H 1965. A new haemoglobin, D-Ibadan (β-87 threonine–lysine), producing no sickle-cell haemoglobin D disease with haemoglobin S. Nature 205: 1273–1276 [DOI] [PubMed] [Google Scholar]
- Weatherall D, Clegg J 2001. Inherited haemoglobin disorders: An increasing global health problem. Bull World Health Organ 79: 704–712 [PMC free article] [PubMed] [Google Scholar]
- Weiss IM, Liebhaber SA 1994. Erythroid cell-specific determinants of α-globin mRNA stability. Mol Cell Biol 14: 8123–8132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss MJ, Zhou S, Feng L, Gell DA, Mackay JP, Shi Y, Gow AJ 2005. Role of α-hemoglobin-stabilizing protein in normal erythropoiesis and β-thalassemia. Ann NY Acad Sci 1054: 103–117 [DOI] [PubMed] [Google Scholar]
- *.Williams TN, Weatherall DJ 2012. World distribution, population genetics, and health burden of the hemoglobinopathies. Cold Spring Harb Perspect Med 10.1101/cshperspect.a011692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkowska HE, Lubin BH, Beuzard Y, Baruchel S, Esseltine DW, Vichinsky EP, Kleman KM, Bardakdjian-Michau J, Pinkoski L, Cahn S 1991. Sickle cell disease in a patient with sickle cell trait and compound heterozygosity for hemoglobin S and hemoglobin Quebec-Chori. N Engl J Med 325: 1150–1154 [DOI] [PubMed] [Google Scholar]
- Yu X, Mollan TL, Butler Andrew, Gow AJ, Olson JS, Weiss MJ 2009. Analysis of human α globin gene mutations that impair binding to the α hemoglobin stabilizing protein. Blood 113: 5961–5969 [DOI] [PMC free article] [PubMed] [Google Scholar]