Abstract
Background
Reports that patients with heart failure and anemia incur greater costs and medical resource use have relied largely on data with limited clinical detail.
Methods
HF-ACTION, a large trial of exercise training in heart failure, recorded hemoglobin at baseline. Medical resource use and hospital bills for inpatient and emergency department visits were collected throughout the study. We analyzed hemoglobin as a continuous variable to evaluate relationships with medical resource use and costs over 1 year.
Results
Among 1,763 patients with baseline hemoglobin levels, those with lower hemoglobin levels tended to be older, African American, and women and to have more severe heart failure. Lower hemoglobin was significantly associated with more hospital admissions, inpatient days, outpatient visits, and urgent care or emergency department visits (all P < .005, unadjusted). Although cost outliers influenced estimates, these observations were distributed across hemoglobin levels. Mean 1-year costs across hemoglobin levels defined as ≤11, >11–12, >12–13, >13–14, >14–15, and >15 g/dL were $21,106, $20,189, $16,249, $17,989, $13,216, and $12,492, respectively (P < .001, unadjusted). Significant associations remained after multivariable adjustment.
Conclusions
Patients with lower baseline hemoglobin levels experienced progressively greater resource use and higher costs.
Keywords: Anemia, health care costs, heart failure
Reports on the prevalence of anemia in patients with heart failure have ranged from 18% to 38%.1,2 Anemia has repeatedly been associated with poor health outcomes in patients with heart failure.3,4 There is ongoing debate about whether anemia independently contributes to poor outcomes or whether its relationship is confounded by coexisting risk factors that lead to adverse outcomes. Furthermore, it is unclear whether treatment of anemia improves health outcomes in heart failure. A meta-analysis of case-control, cohort, and randomized studies suggested that use of erythropoiesis-stimulating agents (ESAs) was associated with improvements in several markers of heart failure severity.5 Although publication bias and other biases associated with nonrandomized studies may have contributed to these findings, a secondary analysis of data from the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial demonstrated the need for further study of ESAs in heart failure,6 and such trials are ongoing (ClinicalTrials.gov identifiers NCT003582157 and NCT00286182).
Anemia in patients with heart failure is also associated with greater use of medical resources and higher costs.1,8–11 However, studies reporting these associations were largely based on retrospective analyses of administrative claims data using diagnosis codes representing anemia instead of actual hemoglobin levels and did not have disease-specific measures of heart failure severity or other clinical details.7,8 In addition, some of these studies were limited to hospitalized patients with heart failure,1,6 elderly patients with heart failure,8 patients undergoing cardiac catheterization in a single tertiary center, and patients treated outside the United States.11 Furthermore, with the exception of Nordyke et al.,8 who examined three groups, the studies compared patients with and without anemia instead of evaluating relationships between hemoglobin level and health resource use and associated costs.
A recently completed trial funded by the National Institutes of Health, Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION), provided an opportunity to use prospectively collected detailed clinical data to examine relationships between hemoglobin level and measures of medical resource use and costs in patients with heart failure.12,13 We hypothesized that patients with heart failure and lower hemoglobin levels incur more hospitalizations, inpatient days, inpatient procedures, emergency department visits, and outpatient visits than patients with heart failure and higher hemoglobin levels over 1 year of follow-up. We also hypothesized that 1-year total direct medical costs would be higher among patients with lower hemoglobin levels than among patients with higher hemoglobin levels.
Methods
HF-ACTION was a multicenter randomized clinical trial designed to evaluate exercise training plus usual care compared with usual care alone in patients with chronic heart failure.14 Patients with New York Heart Association (NYHA) functional class II–IV heart failure for ≥3 months and left ventricular ejection fraction ≤35% were eligible for participation. Patients were required to be on optimal heart failure therapies at stable doses for ≥6 weeks before enrollment (or have a documented rationale for variation, including patient preference) and had to be sufficiently stable to begin an exercise training program. Patients were excluded if they had recent or planned major cardiovascular events or procedures or had conditions that could interfere with exercise training. The primary end point was all-cause mortality or hospitalization.
A total of 2,331 patients were enrolled at 82 participating centers in the United States (n = 67), Canada (n = 9), and France (n = 6) from April 2003 to February 2007 and were followed for a maximum of 4 years. The trial collected a wide range of information on patient characteristics, heart failure and other clinical measures, laboratory parameters, health care resource use, and health-related quality of life. Baseline hemoglobin and serum creatinine levels were recorded from patients’ medical charts and represented the most recently documented levels up to 1 year before study enrollment.
Medical resource use data were collected quarterly for the first 2 years and yearly thereafter on all patients participating in the trial. These data included all-cause hospitalizations, including procedures and length of stay, urgent/emergent care visits, and nonurgent outpatient visits and procedures. Because all-cause hospitalization was a component of the primary efficacy end point in the trial, information pertaining to inpatient care was confirmed and supplemented with review of patients’ medical records.
Estimated total costs included inpatient care and nonurgent, urgent, and emergent outpatient care through 1 year. As part of the economic evaluation of exercise therapy,13 hospital billing data were obtained for >80% of hospitalizations and emergency department visits that occurred during the follow-up period. Department-level hospital charges were converted to costs with the use of department-specific cost-to-charge ratios derived from each hospital’s annual Medicare cost report. In cases for which bills for emergency department visits were not available, the median cost estimated from collected bills was applied as a proxy. In cases for which bills were not available for inpatient stays, imputed costs were derived by multiplying the length of stay for each visit by estimates of median daily costs that corresponded to 1 of 47 potential reasons for hospitalization, with an additional adjustment to avoid overestimating costs for procedure-based visits typically characterized by high costs and short stays. Medicare fee schedules were used to assign costs for inpatient services provided by physicians, outpatient visits, and inpatient and outpatient procedures. For hospitalizations that continued beyond 1 year, we calculated the daily cost of inpatient care by dividing the total inpatient cost by the length of stay for the admission and summed daily costs incurred up to 1 year beyond randomization. A detailed description of the applied costing methods is available elsewhere.13 Costs were valued in 2008 US dollars.
Statistical Analysis
For baseline characteristics, we report frequencies with percentages for categorical variables and means with SDs for continuous variables. For reporting purposes, we used integer values of hemoglobin levels to categorize patients into 6 groups: ≤11 g/dL, >11–12 g/dL, >12–13 g/dL, >13–14 g/dL, >14–15 g/dL, and >15 g/dL. In our statistical analysis, hemoglobin level was analyzed as a continuous variable. We used analysis of variance to evaluate relationships between hemoglobin level and categorical baseline variables and Pearson correlation tests to evaluate relationships between hemoglobin level and continuous baseline variables.
Distributions of resource use and costs were right-skewed. Therefore, we report medians with interquartile ranges along with means and SDs. When evaluating use of medical care, counts of medical resource use, and costs, we applied generalized linear models. Specifically, we applied logit links and binomial distributions to model the proportions of patients with ≥1 medical event (eg, hospital admission). We applied log links to model exponential functional relationships between hemoglobin level and resource use and between hemoglobin level and costs. We applied negative binomial distributions to model the variance terms for 1-year counts of medical resource use and gamma distributions to model the variance term for total 1-year costs. In the generalized linear models, we also applied an offset variable to adjust for differential follow-up periods across patients. To evaluate the potential influence of cost outliers, we winsorized the top 1% and top 5% of the cost distribution and refitted the model. We also applied a robust regression method developed to analyze data with outliers. Specifically, we used a Huber M estimator that applies reweighted least squares regression across multiple iterations.15
In adjusted analyses, the selection of covariates for each model was based on clinical relevance, use in previous studies, and bivariate associations with hemoglobin level and with measures of resource use and costs. Continuous covariates were centered around their corresponding mean values. Continuous covariates in the models were age, body mass index, resting heart rate, 6-minute walk distance, left ventricular ejection fraction, serum creatinine level, blood urea nitrogen level, peak VO2 during cardiopulmonary exercise testing, and hemoglobin level. Categorical variables in the models were race, hypertension, diabetes mellitus, prior myocardial infarction, prior coronary artery bypass graft, prior percutaneous coronary intervention, NYHA functional class, Canadian Cardiovascular Society angina classification, and variables indicating use of angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, aldosterone antagonists, β-blockers, nitrates, calcium channel blockers, loop diuretics, and digoxin. When developing the models, we evaluated the relative contributions of adding quadratic terms for continuous variables and evaluated the presence of multicollinearity by estimating variance inflation factors for each covariate. Also, instead of parameterizing hemoglobin level as a continuous variable, we evaluated whether the presence of anemia as defined by the World Health Organization (ie, <13 g/dL for men and <12 g/dL for women) was independently associated with total medical costs at 1 year. We used SAS version 9.1 (SAS Institute, Cary, North Carolina) for all analyses.
Results
Of the 2,331 patients enrolled in HF-ACTION, hemoglobin levels were recorded for 1,763 (75.6%). Among patients with baseline hemoglobin values, mean duration of follow-up in the trial was ~2.5 years. At 1 year, 91.2% of patients were alive and participating in the study, 4.7% had died, and 4.1% had discontinued participation. Duration of follow-up was similar across hemoglobin groups at 1 year (log-rank test: P = .49) despite generally higher proportions of patients with lower hemoglobin levels dying within the first year, ranging from 8.8% in the group with hemoglobin levels >11 g/dL through 12 g/dL to 2.6% in the group with hemoglobin levels >14 g/dL through 15 g/dL (P = .001).
Lower hemoglobin level was significantly and positively associated with older age, shorter 6-minute walk distance, higher serum creatinine level, higher blood urea nitrogen level, and lower peak VO2 (Table 1). Patients with lower hemoglobin level were significantly more likely to be women, to be African American, and to have more severe heart failure. In addition, prevalence of hypertension and diabetes were significantly higher among patients with lower hemoglobin level. Use of chronic medications was similar across hemoglobin levels, except for nitrates (P = .04) and loop diuretics (P < .001).
Table 1.
Baseline Characteristics of the Study Population by Hemoglobin Level
| Characteristic | Hemoglobin Level at Baseline, g/dL
|
P Value** | |||||
|---|---|---|---|---|---|---|---|
| ≤11 (n = 129) | >11–12 (n = 215) | >12–13 (n = 347) | >13–14 (n = 414) | >14–15 (n = 348) | >15 (n = 310) | ||
| Age, mean (SD), y | 62 (14) | 60 (13) | 60 (13) | 60 (13) | 58 (12) | 57 (11) | <.001 |
| Female, No. (%) | 47 (36) | 98 (46) | 136 (39) | 112 (27) | 66 (19) | 21 (7) | <.001 |
| Race, n (%) | <.001 | ||||||
| Black/African American | 61 (48) | 102 (48) | 140 (41) | 113 (28) | 92 (27) | 63 (21) | |
| White | 59 (46) | 105 (49) | 184 (53) | 267 (66) | 240 (70) | 214 (71) | |
| Other | 7 (5.5) | 6 (2.8) | 21 (6.1) | 22 (5.5) | 11 (3.2) | 26 (8.6) | |
| NYHA functional class, n (%) | <.001 | ||||||
| II | 66 (51) | 115 (53) | 214 (62) | 276 (67) | 240 (69) | 208 (67) | |
| III | 61 (47) | 95 (44) | 128 (37) | 135 (33) | 107 (31) | 100 (32) | |
| IV | 2 (1.6) | 5 (2.3) | 5 (1.4) | 3 (0.7) | 1 (0.3) | 2 (0.6) | |
| CCS angina class, n (%) | .50 | ||||||
| 4 | 1 (0.8) | 0 (0) | 0 (0) | 3 (0.7) | 0 (0) | 2 (0.6) | |
| 3 | 4 (3.1) | 4 (1.9) | 2 (0.6) | 5 (1.2) | 8 (2.3) | 6 (1.9) | |
| 2 | 7 (5.4) | 17 (7.9) | 22 (6.3) | 25 (6.0) | 21 (6.0) | 13 (4.2) | |
| 1 | 13 (10) | 14 (6.5) | 37 (11) | 45 (11) | 26 (7.4) | 31 (10) | |
| 0 | 104 (81) | 179 (84) | 286 (82) | 335 (81) | 293 (84) | 258 (83) | |
| Six-minute walk distance, median (IQR), m* | 340 (252–418) | 343 (290–402) | 352 (275–418) | 366 (293–441) | 400 (322–446) | 403 (329–457) | <.001 |
| Resting heart rate, median (IQR), bpm† | 69 (62–79) | 70 (64–77) | 69 (62–76) | 70 (62–76) | 70 (62–77) | 72 (64–78) | .77 |
| BMI, median (IQR), kg/m2† | 29 (25–34) | 30 (25–35) | 30 (26–36) | 29 (25–34) | 30 (26–35) | 30 (27–36) | .01 |
| Medical history, n (%) | |||||||
| Hypertension | 96 (74) | 146 (68) | 196 (57) | 239 (58) | 204 (59) | 187 (61) | .01 |
| Diabetes mellitus | 48 (37) | 91 (42) | 114 (33) | 135 (33) | 98 (28) | 83 (27) | <.001 |
| Prior myocardial infarction | 56 (43) | 91 (42) | 156 (45) | 162 (39) | 153 (44) | 128 (41) | .71 |
| Prior CABG | 40 (31) | 61 (28) | 103 (30) | 100 (24) | 83 (24) | 71 (23) | .02 |
| Prior PCI | 34 (26) | 48 (22) | 84 (24) | 100 (24) | 81 (23) | 72 (23) | .64 |
| Chronic medications, n (%) | |||||||
| ACE inhibitor | 100 (78) | 159 (74) | 244 (70) | 304 (73) | 268 (77) | 236 (76) | .49 |
| Angiotensin receptor blocker | 27 (21) | 44 (20) | 93 (27) | 106 (26) | 76 (22) | 71 (23) | .77 |
| β-Blockers | 121 (94) | 199 (93) | 330 (95) | 394 (95) | 326 (94) | 296 (95) | .38 |
| Calcium channel blocker | 13 (10) | 18 (8) | 27 (8) | 24 (6) | 20 (6) | 21 (7) | .09 |
| Digoxin | 53 (41) | 99 (46) | 158 (46) | 190 (46) | 169 (49) | 129 (42) | .89 |
| Loop diuretics | 117 (91) | 188 (87) | 273 (79) | 324 (78) | 266 (76) | 228 (74) | <.001 |
| Nitrates | 47 (36) | 60 (28) | 78 (22) | 106 (26) | 85 (24) | 70 (23) | .04 |
| Spironolactone/eplerenone | 79 (61) | 109 (51) | 200 (58) | 222 (54) | 193 (55) | 162 (52) | .24 |
| Left ventricular ejection fraction, mean (SD)‡ | 26 (8) | 25 (8) | 25 (7) | 25 (8) | 25 (8) | 25 (7) | .09 |
| Laboratory test results, mean (SD) | |||||||
| Creatinine, mg/dL§ | 1.6 (1.0) | 1.4 (0.6) | 1.3 (0.5) | 1.3 (0.6) | 1.3 (0.9) | 1.3 (0.7) | <.001 |
| Blood urea nitrogen, mg/dL|| | 31.6 (20.0) | 26.3 (16.9) | 23.8 (13.5) | 23.9 (17.6) | 22.2 (11.1) | 22.3 (12.0) | <.001 |
| Peak VO2, mL kg−1 min−1¶ | 12.6 (4.2) | 13.3 (3.9) | 14.0 (4.1) | 14.7 (4.8) | 15.9 (4.7) | 16.6 (4.8) | <.001 |
ACE, angiotensin-converting enzyme; BMI, body mass index; CABG, coronary artery bypass graft; CCS, Canadian Cardiovascular Society; IQR, interquartile range; NYHA, New York Heart Association; PCI, percutaneous coronary intervention.
Data missing for 21 patients.
Data missing for 5 patients.
Data missing for 1 patient.
Data missing for 20 patients.
Data missing for 65 patients.
Data missing for 35 patients.
From Pearson correlation tests for continuous variables and t tests and analysis of variance for categoric variables.
Unadjusted Results
Patients with lower hemoglobin level were significantly more likely to be hospitalized at least once for heart failure (P < .001), cardiovascular reasons (P < .001), or any cause (P < .001; Table 2). Similarly, lower hemoglobin level was significantly associated with greater numbers of admissions and inpatient days for heart failure, cardiovascular reasons, and all causes. The mean number of inpatient days incurred over 1 year from randomization ranged from 3.6 days per patient among those with baseline hemoglobin level >15 g/dL to 7.6 days per patient among those with hemoglobin level ≤11 g/dL. Relationships were similar for emergency department and urgent care visits, with estimates ranging from 0.7 visits per patient among those with the highest hemoglobin levels to 1.3 visits per patient among those with the lowest hemoglobin levels. Outpatient visits for nonurgent care were also significantly greater among patients with lower hemoglobin level at baseline.
Table 2.
One-Year Medical Resource Use by Hemoglobin Level
| Resource | Hemoglobin Level at Baseline, g/dL
|
Unadjusted P Value† | Adjusted P Value‡ | |||||
|---|---|---|---|---|---|---|---|---|
| ≤11 (n = 129) | >11–12 (n = 215) | >12–13 (n = 347) | >13–14 (n = 414) | >14–15 (n = 348) | >15 (n = 310) | |||
| Patients with ≥1 events, n (%) | ||||||||
| Heart failure admission | 28 (22) | 42 (20) | 53 (15) | 56 (14) | 42 (12) | 24 (7.7) | <.001 | .15 |
| Cardiovascular admission | 54 (42) | 83 (39) | 114 (33) | 135 (33) | 95 (27) | 85 (27) | <.001 | .25 |
| All-cause admission | 74 (57) | 111 (52) | 150 (43) | 177 (43) | 115 (33) | 111 (36) | <.001 | .002 |
| Emergency or urgent care visit | 73 (57) | 108 (50) | 154 (44) | 183 (44) | 137 (39) | 120 (39) | <.001 | .005 |
| No. of events per patient | ||||||||
| Heart failure admission | ||||||||
| Mean (SD) | 0.4 (0.9) | 0.3 (0.7) | 0.2 (0.7) | 0.2 (0.7) | 0.2 (0.6) | 0.1 (0.7) | <.001 | .12 |
| Median (IQR) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | ||
| Cardiovascular admission | ||||||||
| Mean (SD) | 0.7 (1.1) | 0.7 (1.0) | 0.6 (1.1) | 0.6 (1.0) | 0.5 (1.0) | 0.5 (1.0) | .002 | .19 |
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | ||
| All-cause admission | ||||||||
| Mean (SD) | 1.2 (1.5) | 1.0 (1.3) | 0.9 (1.4) | 0.8 (1.2) | 0.6 (1.2) | 0.7 (1.2) | <.001 | .01 |
| Median (IQR) | 1 (0–2) | 1 (0–2) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | ||
| Inpatient days* | ||||||||
| Mean (SD) | 7.6 (13.0) | 6.5 (13.6) | 5.4 (12.3) | 5.4 (13.0) | 4.2 (15.6) | 3.6 (9.5) | <.001 | .03 |
| Median (IQR) | 2 (0–10) | 2 (0–8) | 0 (0–6) | 0 (0–5) | 0 (0–3) | 0 (0–4) | ||
| Emergency or urgent care visits | ||||||||
| Mean (SD) | 1.3 (2.4) | 0.9 (1.3) | 1.0 (2.1) | 0.9 (1.6) | 0.8 (1.7) | 0.7 (1.2) | <.001 | .006 |
| Median (IQR) | 1 (0–1) | 1 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | ||
| Nonurgent outpatient visits | ||||||||
| Mean (SD) | 15.5 (14.1) | 15.9 (15.6) | 13.6 (14.1) | 12.8 (13.6) | 12.2 (12.0) | 11.5 (13.5) | <.001 | .003 |
| Median (IQR) | 12 (6–18) | 12 (7–21) | 10 (6–16) | 9 (4–17) | 9 (5–16) | 8 (4–14) | ||
| Death, n (%) | 10 (8) | 19 (9) | 15 (4) | 24 (6) | 9 (3) | 10 (3) | .001 | .100 |
IQR, interquartile range.
Inpatient days up to and including 365 days after randomization.
From logit links and binomial distributions for dichotomous outcomes and log links and negative binomial distributions for counts of medical resource use.
Regression models included all variables listed in Table 1.
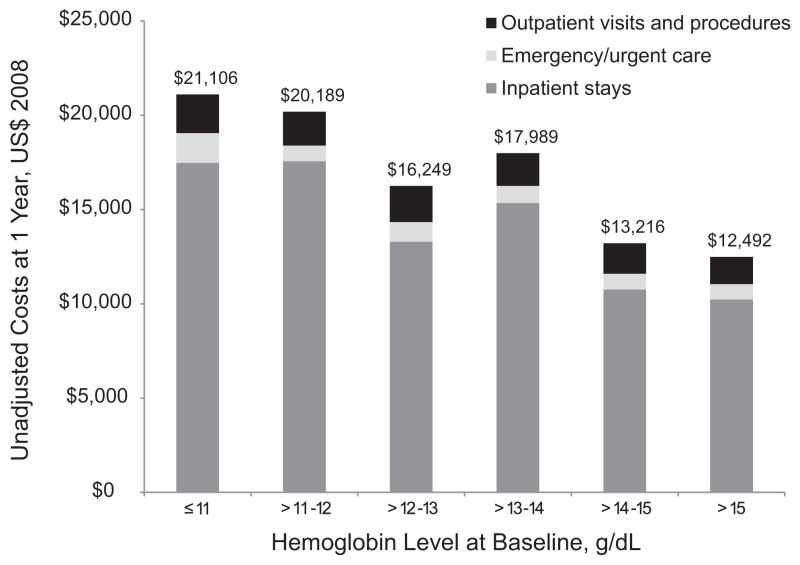
At 1 year of follow-up, cumulative medical costs averaged $16,234 per patient (SD $39,275). The corresponding median was much lower ($2,975, interquartile range $821–$16,392). Lower hemoglobin level was significantly associated with higher costs (P < .001; Table 3). The majority of costs were attributable to inpatient care (Fig. 1). Mean costs were almost 70% higher among patients with the lowest hemoglobin levels (≤11 g/dL) relative to those with the highest hemoglobin levels (>15 g/dL).
Table 3.
One-Year Total Medical Costs by Hemoglobin Level
| Costs | Hemoglobin Level at Baseline, g/dL
|
Unadjusted P Value* | Adjusted P Value† | |||||
|---|---|---|---|---|---|---|---|---|
| ≤11 (n = 129) | >11–12 (n = 215) | >12–13 (n = 347) | >13–14 (n = 414) | >14–15 (n = 348) | >15 (n = 310) | |||
| All observations, mean (SD), $ | 21,106 (41,593) | 20,189 (49,638) | 16,249 (33,392) | 17,989 (44,241) | 13,216 (40,250) | 12,492 (25,289) | <.001 | .003 |
| Top 1%, mean (SD), $‡ | 19,367 (30,728) | 17,865 (29,993) | 15,523 (28,539) | 16,149 (31,228) | 11,937 (27,309) | 12,384 (24,492) | <.001 | .001 |
| Top 5%, mean (SD), $§ | 16,538 (20,010) | 14,872 (18,985) | 13,021 (19,016) | 12,940 (19,104) | 9,437 (16,331) | 10,719 (17,336) | <.001 | .001 |
| Median (IQR), $ | 6,618 (1,630–25,593) | 5,494 (1,453–22,002) | 2,918 (861–17,973) | 3,085 (806–16,994) | 1,805 (722–9353) | 2,228 (567–12,170) | ||
IQR, interquartile range.
From generalized linear models (gamma, log) regression models.
Regression models included all variables listed in Table 1.
Winsorized mean in which the top 1% of the distribution was replaced with a cost corresponding to the 99th percentile value.
Winsorized mean in which the top 5% of the distribution was replaced with a cost corresponding to the 95th percentile value.
Fig. 1.
Components of 1-year total medical costs by hemoglobin level.
Cost outliers were influential across hemoglobin groups, yet the negative association between hemoglobin level and higher costs remained statistically significant after the top 1% and top 5% were winsorized (P < .001; Table 3). When we replaced the top 1% of cost outliers with the 99th percentile value ($179,853), mean costs were $1,257 lower at $14,977. When we replaced the top 5% of cost outliers with the 95th percentile value ($64,748), mean costs were $3,861 lower at $12,373. Among the 89 patients in the top 5th percentile of costs, 20 underwent heart transplant and/or implantation of a left ventricular assist device. Among those 20 patients, the mean hemoglobin level was 13.2 mg/dL (minimum 10.1, maximum 18.2). For the remaining 69 patients with the highest costs, the mean hemoglobin level was 13.4 mg/dL (minimum 10.2, maximum 17.0). These findings demonstrate that patients undergoing heart transplant and left ventricular assist device implantation were not clustered at the lower end of the hemoglobin level distribution. Therefore, neither these patients nor the larger group of patients with the highest costs (ie, outliers) fully accounted for the higher costs associated with lower hemoglobin levels.
When we stratified by the World Health Organization definition of anemia, mean total costs at 1 year were $18,202 (SD $34,101) among 515 patients with anemia compared with $15,422 (SD $41,206) among 1,248 patients without anemia, a difference of $2,780 (P = .05).
Adjusted Results
Results from the multivariable regression models indicated that lower hemoglobin level was significantly associated with more all-cause hospitalizations (means ratio 0.94; P = .01), but independent relationships between hemoglobin level and cause-specific hospitalizations were not statistically significant (Table 4). Each 1-unit decrease in hemoglobin level was associated with a 10% increase in inpatient days (P = .003) and a 7.5% increase in emergency department or urgent care visits across 1 year (P = .006).
Table 4.
Parameter Estimates for Hemoglobin as an Independent Predictor of Medical Resource Use and Total Costs at 1 Year
| Resource Use | Parameter Estimate for Hemoglobin (95% CI)* | P Value |
|---|---|---|
| Heart failure admissions | 0.93 (0.84–1.02) | .12 |
| Cardiovascular admissions | 0.96 (0.91–1.02) | .19 |
| All-cause admissions | 0.94 (0.89–0.99) | .01 |
| Inpatient days | 0.91 (0.84–0.99) | .03 |
| Emergency or urgent care visits | 0.93 (0.88–0.98) | .006 |
| Nonurgent outpatient visits | 0.96 (0.93–0.99) | .003 |
| Total medical costs, all observations | 0.92 (0.87–0.97) | .003 |
| Total medical costs, top 1% winsorized mean | 0.92 (0.87–0.97) | .001 |
| Total medical costs, top 5% winsorized mean | 0.92 (0.88–0.97) | .001 |
Parameter estimates represent the relative impact on expected resource use/costs associated with a 1-unit increase in hemoglobin after adjustment for the variables listed in Table 1.
The significant association between hemoglobin level and medical costs at 1 year remained when we adjusted for baseline clinical characteristics and long-term medication use (Table 4). For each 1-unit decrease in hemoglobin level, expected medical costs at 1 year increased by 8% (P = .003). This relationship was consistent when the top 1% and top 5% of the cost distributions were replaced with the 99th percentile and 95th percentile values, respectively. Results from the robust regression implemented with the Huber M estimator also demonstrated a statistically significant association between lower hemoglobin level and higher costs (P = .01). With this modeling strategy, which down-weights the influence of outliers, each 1-unit decrease in hemoglobin level was independently associated with a $175 increase in 1-year medical costs (95% confidence interval [CI] $25–$291).
Multicollinearity between covariates was not identified across the multivariable models evaluating the independent association between hemoglobin level and medical resource use and costs (variance inflation factor <10). Also, the addition of quadratic terms to model the relationship between hemoglobin level and outcome variables did not significantly improve the fit of the model.
When we dichotomized hemoglobin measures according to the World Health Organization definition of anemia, the results of the multivariable generalized linear models indicated that anemia was not significantly associated with total costs (means ratio 1.10, 95% CI 0.92–1.32; P = .29). However, application of the robust regression approach indicated that the presence of anemia was associated with statistically significant higher medical costs of $616 at 1 year (95% CI $140–$1,092; P = .01).
Discussion
Given the high prevalence of anemia among patients with heart failure, it is important to characterize the relationship between hemoglobin level and medical resource use and costs. These analyses demonstrate progressively greater medical resource use and higher costs among patients with lower baseline hemoglobin levels.
The present study goes beyond simple comparisons of patients with and without anemia by providing greater detail about resource use and costs across a spectrum of hemoglobin levels. Most published studies of patients with heart failure have focused on the relationship between the presence or absence of anemia and downstream medical costs. Allen et al10 evaluated the relationship between anemia and health care costs among patients with heart failure who underwent diagnostic catheterization at a single center. They estimated mean 1-year costs of $27,415 for patients with anemia and $23,162 for patients without anemia (in 2002 US dollars), but the cost difference of $4,253 was not statistically significant. Their estimates were higher than the costs we found: $18,202 for patients with anemia and $15,422 for patients without anemia. The higher costs reported by Allen et al11 may be a result of selecting a sample of patients undergoing cardiac catheterization and higher costs of care at a single tertiary center.
Studies by Nissenson et al1 and Ershler et al9 evaluated the impact of anemia on costs across selected conditions including heart failure. Because those studies relied on diagnosis codes to identify anemia, it is likely that hemoglobin levels in the patients were lower than if the determination had been based on actual hemoglobin levels. Therefore, those studies likely overestimated the impact of anemia on medical costs. However, a study by Nordyke et al8 used actual hemoglobin levels, grouping patients into categories of <10 g/dL, 10.0–11.9 g/dL, and ≥12.0 g/dL. Although those authors analyzed medical charges instead of costs, they also found progressively higher charges among groups with lower hemoglobin levels.
Compared with other published studies evaluating relationships between anemia and medical resource use and costs, we had a greater ability to adjust for numerous baseline characteristics, including several related specifically to severity of heart failure. Although Allen et al11 had access to electronic data on comorbid conditions, laboratory data, and results from diagnostic catheterization, they did not have heart failure–specific variables, such as VO2 from cardiopulmonary testing or 6-minute walk distance. Other studies were more limited, relying on information available in claims data such as basic demographic information, diagnosis codes representing the presence of comorbid conditions, and procedure codes.
We consistently found significant associations between hemoglobin level and medical costs in multivariable regression analyses when we modeled hemoglobin as a continuous variable. However, when we examined the impact of anemia as defined by the World Health Organization, we did not find a statistically significant association. Dichotomizing hemoglobin levels in this way can reduce statistical power and misclassify patients, especially those who are close to the hemoglobin threshold conferring anemia. Although our analyses did not identify a threshold hemoglobin value beyond which medical resource use and costs were notably higher, stratification into 6 groups on the basis of hemoglobin values for descriptive reporting revealed a relatively consistent and progressive relationship between decreasing hemoglobin values and greater medical resource use and costs. These relationships were generally persistent at 2 years, but we chose not to report the findings owing to greater incidence of death, which complicates cost comparisons, and considerable administrative censoring upon completion of the trial.
Simulation studies have demonstrated that modeling cost data is complex, and no single methodologic approach has demonstrated superiority.16,17 Over the past decade, generalized linear models, particularly gamma models with log links, appeared to have gained acceptance and have been widely applied in analyses of medical resource use and cost data.18 More recently, however, regression approaches that allow for a flexible link function, such as extended estimating equations,19 have demonstrated better fit, particularly for the top deciles of distributions representing health care expenditures.18,20 However, successful implementation of regression using extended estimating equations requires sample sizes on the order of several thousand observations.16 Because we could not rely on the validity of a single approach, we applied 2 regression approaches and evaluated the results when limiting the influence of cost outliers and found consistent results between strategies.
Our findings of negative associations between hemoglobin level and medical resource use and costs are compelling, given the lower severity of heart failure among patients enrolled in HF-ACTION. Compared with the general heart failure population, HF-ACTION participants were generally younger (median age 59 years) and were all deemed to be fit enough to participate in an exercise training program. Also, given the high use of evidence-based medical therapy received by HF-ACTION participants, they may have generally received higher-quality care than that provided to patients in other settings.12
Among patients enrolled in HF-ACTION, almost one-fourth were excluded from our analysis because they did not have a hemoglobin value recorded at baseline. Costs associated with hemoglobin testing were not reimbursed as part of the trial. Therefore, documentation relied on whether the patient’s health care provider ordered the test and documented the results within the year before enrollment. The availability of only a single baseline measurement of hemoglobin without an associated date of measurement did not allow us to evaluate the chronicity of anemia. Given the increasing prevalence of anemia at older ages and in more advanced phases of heart failure, we expect that hemoglobin measures may be biased upward, especially given that the “baseline” hemoglobin measurement could have occurred up to 1 year before baseline. The extent to which this may have occurred is uncertain, considering that some patients may have received ESAs, transfusions, or iron supplementation after the hemoglobin measurements used in our analysis.
Our findings provide a detailed picture of medical resource use and costs across a spectrum of hemoglobin levels in an outpatient heart failure population. From an epidemiologic perspective, patients with heart failure are becoming older and are more likely to be female, and these patients tend to have lower hemoglobin levels. The data we examined suggest that hemoglobin is an independent predictor of medical resource use and costs, even after adjustment for age and sex. Therefore, in comparisons of medical resource use or costs across groups defined by other potential risk factors or treatments, it is insufficient to control for differences in patients’ age and sex. Hemoglobin level should be included as a covariate when possible. The estimates we report could also assist researchers undertaking disease modeling studies to evaluate the potential cost-effectiveness of interventions designed to modify hemoglobin levels. However, it is critical to note that we did not present evidence indicating that changing hemoglobin levels would influence medical resource use or costs. The relationships between hemoglobin levels and medical resource use or costs may be attributable to unmeasured confounders. Future studies designed to evaluate serial changes in hemoglobin levels in patients with heart failure and the associated impact on medical cost drivers would help to characterize whether both acute and chronic reductions in hemoglobin levels have similar relationships with medical resource use and costs. High-quality evidence to evaluate the potential cost impact of increasing hemoglobin levels in patients with heart failure could be generated from ongoing clinical trials evaluating the efficacy of ESAs in patients with heart failure and anemia.
In the present large study of well-characterized patients with heart failure, lower hemoglobin levels were associated with higher costs and greater numbers of hospitalizations, emergency department or urgent care visits, and outpatient visits. The significant association between hemoglobin levels and total medical costs at 1 year remained after adjustment for numerous baseline characteristics and use of evidence-based therapies. This study confirmed that patients with lower hemoglobin levels used progressively greater medical resources and incurred higher costs than patients with higher hemoglobin levels.
Acknowledgments
The authors thank the HF-ACTION investigators and participants who made this study possible. They also thank Joelle Y. Friedman, MPA, Duke University, for project management responsibilities associated with this project and Damon M. Seils, MA, Duke University, for assistance with manuscript preparation. Neither Ms Friedman nor Mr Seils received compensation for their assistance apart from their employment at the institution where the study was conducted.
Funding: Research agreement between Duke University and Amgen. HF-ACTION was funded by grants 5U01HL063747, 5U01HL066461, 5U01HL068973, 5U01HL066501, 5U01HL066482, 5U01HL064250, 5U01HL066494, 5U01HL064257, 5U01HL066497, 5U01HL068980, 5U01HL064265, 5U01HL066491, and 5U01HL064264 from the National Heart, Lung, and Blood Institute and grants R37AG018915 and P60AG010484 from the National Institute on Aging. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Footnotes
Trial Registration: ClinicalTrials.gov identifier: NCT00047437.
Disclosures
At the time of the study, Mr Isitt and Dr Cheng were employees of Amgen, which markets epoetin alfa as Epogen and darbepoetin alfa as Aranesp. No other financial disclosures were reported.
References
- 1.Nissenson AR, Wade S, Goodnough T, Knight K, Dubois RW. Economic burden of anemia in an insured population. J Manag Care Pharm. 2005;11:565–74. doi: 10.18553/jmcp.2005.11.7.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart T, Freeman J, Stewart J, Sullivan A, Ward C, Tofler GH. Anaemia in heart failure: a prospective evaluation of clinical outcome in a community population. Heart Lung Circ. 2010;19:730–5. doi: 10.1016/j.hlc.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new onset heart failure. Circulation. 2003;107:223–5. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 4.Felker MG, Adams KF, Jr, Gattis WA, O’Connor CM. Anemia as a risk factor and therapeutic target in heart failure. J Am Coll Cardiol. 2004;44:959–66. doi: 10.1016/j.jacc.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 5.Tehrani F, Dhesi P, Daneshvar D, Phan A, Rafique A, Siegel RJ, et al. Erythropoiesis stimulating agents in heart failure patients with anemia: a meta-analysis. Cardiovasc Drugs Ther. 2009;23:511–8. doi: 10.1007/s10557-009-6203-6. [DOI] [PubMed] [Google Scholar]
- 6.Szczech LA, Barnhart HX, Sapp S, Felker GM, Hernandez A, Reddan D, et al. A secondary analysis of the CHOIR trial shows that comorbid conditions differentially affect outcomes during anemia treatment. Kidney Int. 2010;77:239–46. doi: 10.1038/ki.2009.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McMurray JJ, Anand IS, Diaz R, Maggioni AP, O’Connor C, Pfeffer MA, et al. Design of the Reduction of Events with Darbepoetinalfa in Heart Failure (RED-HF): a phase III, anaemia correction, morbidity-mortality trial. Eur J Heart Fail. 2009;11:795–801. doi: 10.1093/eurjhf/hfp098. [DOI] [PubMed] [Google Scholar]
- 8.Nordyke RJ, Kim JJ, Goldberg GA, Vendiola R, Batra D, McCamish M, et al. Impact of anemia on hospitalization time, charges, and mortality in patients with heart failure. Value Health. 2004;7:464–71. doi: 10.1111/j.1524-4733.2004.74009.x. [DOI] [PubMed] [Google Scholar]
- 9.Ershler WB, Chen K, Reyes EB, Dubois R. Economic burden of patients with anemia in selected diseases. Value Health. 2005;8:629–38. doi: 10.1111/j.1524-4733.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 10.Solid CA, Foley RN, Gilbertson DT, Collins AJ. Anemia and cost in Medicare patients with congestive heart failure. Congest Heart Fail. 2006;12:302–6. doi: 10.1111/j.1527-5299.2006.00127.x. [DOI] [PubMed] [Google Scholar]
- 11.Allen LA, Anstrom KJ, Horton JR, Shaw LK, Eisenstein EL, Felker GM. Relationship between anemia and health care costs in heart failure. J Card Fail. 2009;15:843–9. doi: 10.1016/j.cardfail.2009.06.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reed SD, Whellan DJ, Li Y, Friedman JY, Ellis SJ, Piña IL, et al. Economic evaluation of the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) randomized controlled trial: an exercise training study of patients with chronic heart failure. Circ Cardiovasc Qual Outcomes. 2010;3:374–81. doi: 10.1161/CIRCOUTCOMES.109.907287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whellan DJ, O’Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION): design and rationale. Am Heart J. 2007;153:201–11. doi: 10.1016/j.ahj.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Pires AM, Rodrigues IM. Multiple linear regression with some correlated errors: classical and robust methods. Stat Med. 2007;26:2901–18. doi: 10.1002/sim.2774. [DOI] [PubMed] [Google Scholar]
- 16.Mihaylova B, Briggs A, O’Hagan A, Thompson SG. Review of statistical methods for analysing healthcare resources and costs. Health Econ. 2011;20:897–916. doi: 10.1002/hec.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill SC, Miller GE. Health expenditure estimation and functional form: applications of the generalized gamma and extended estimating equations models. Health Econ. 2010;19:608–27. doi: 10.1002/hec.1498. [DOI] [PubMed] [Google Scholar]
- 18.Manning WG, Basu A, Mullahy J. Generalized modeling approaches to risk adjustment of skewed outcomes data. J Health Econ. 2005;24:465–88. doi: 10.1016/j.jhealeco.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Basu A, Rathouz PJ. Estimating marginal and incremental effects on health outcomes using flexible link and variance function models. Biostatistics. 2005;6:93–109. doi: 10.1093/biostatistics/kxh020. [DOI] [PubMed] [Google Scholar]
- 20.Basu A, Arondekar BV, Rathouz PJ. Scale of interest versus scale of estimation: comparing alternative estimators for the incremental costs of a comorbidity. Health Econ. 2006;15:1091–107. doi: 10.1002/hec.1099. [DOI] [PubMed] [Google Scholar]