Abstract
Alzheimer’s disease (AD) involves progressive neurodegeneration in the presence of misfolded proteins and poorly-understood inflammatory changes. However, research has shown that AD is genetically, clinically and pathologically heterogeneous.
In frozen brain samples of frontal cortex (diseased) and cerebellum (non-diseased) from the University of Kentucky Alzheimer’s Disease Center autopsy cohort, we performed gene expression analysis for genes categorizing inflammatory states (termed M1 and M2) from early and late stage AD, and age-matched non-demented controls. We performed analysis of the serum samples for a profile of inflammatory proteins and examined the neuropathological data on these samples.
Striking heterogeneity was found in early AD. Specifically, early-stage AD brain samples indicated apparent polarization toward either the M1 or M2 brain inflammatory states when compared to age-matched non-disease control tissue. This polarization was observed in the frontal cortex and not in cerebellar tissue. We were able to detect both differences in AD neuropathology, and changes in serum proteins that distinguished the individuals apparent M1 versus M2 brain inflammatory polarization.
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized clinically by a progressive loss of cognitive function. Pathologically, AD is defined by the presence of amyloid plaques composed of extracellular aggregated amyloid-β (Aβ) protein, and neurofibrillary tangles comprising of intracellular aggregated, hyperphosphorylated tau protein (Hyman, et al., 2012). Accompanying the primary pathologies of AD is an inflammatory response, mediated partly by the microglial cells in the brain but also involving astrocytes, oligodendrocytes, pericytes and neurons (see review (Colton and Wilcock, 2010)). The upregulation of inflammatory signaling in the brain, termed neuroinflammation, has been shown to occur in all cases of AD to varying degrees (reviewed in (Akiyama, et al., 2000)).
Studies in transgenic mouse models have revealed a diverse role for neuroinflammation in the progression of amyloid plaques and neurofibrillary tangles. Intracranial injection of lipopolysaccharide (LPS), a classic stimulator of inflammation, induces neuroinflammation and results in amyloid plaque reduction in APP+PS1 transgenic mice (DiCarlo, et al., 2001). Additionally, anti-Aβ antibodies injected intracranially or administered systemically in APP transgenic mice results in a neuroinflammatory response and subsequent reductions in amyloid plaques (Wilcock, et al., 2003, Wilcock, et al., 2004). In contrast to the effects of LPS on amyloid plaques injection of LPS into the brains of rTg4510 tau mice results in exacerbation of tau pathology (Lee, et al., 2010) suggesting that tau pathology and amyloid pathology respond differentially to neuroinflammatory stimuli.
Recently, Colton et al showed that inflammatory markers expressed in the brains of amyloid depositing mice and human AD tissue are not restricted to classical inflammatory genes such as IL-1β or TNFα; additional inflammatory markers such as arginase-1, chitinase-3-like proteins and mannose receptor are also highly expressed (Colton, et al., 2006). In contrast to the cytotoxic effects that occur with agents such as IL-1β and TNFα, the additional inflammatory genes are known to be essential for wound healing and repair and may therefore reflect attempts at brain repair in response to the presence of AD pathologies.
We hypothesized that the characteristics of neuroinflammatory signaling in the early stages of clinical AD would associate with clinical and pathological parameters in a biologically informative way. To address this, we performed gene-expression analysis for a profile of neuroinflammatory genes in frozen tissue samples from patients clinically classified as mild AD or late AD and compared these to age-matched non-demented controls. We examined the frontal cortex as our diseased brain region and cerebellum as our non-diseased region. Microglia, the central orchestrators of CNS inflammation, are derived from the same lineage as macrophages so we have chosen to use the same nomenclature for the neuroinflammatory phenotypes as is used in phenotyping macrophages; M1 and M2 (reviewed in (Mantovani, et al., 2004, Mosser and Edwards, 2008)). M1 macrophages are associated with expression of classical inflammatory cytokines such as IL-1β and TNFα while M2 macrophages are associated with expression of wound repair and healing mediators such as arginase-1, chitinase-like proteins and anti-inflammatory cytokines IL-4 and IL-10 (Edwards, et al., 2006, Martinez, et al., 2009, Mosser, 2003).
Materials and methods
Tissue samples
All tissue samples were obtained from the University of Kentucky Alzheimer’s Disease Center (UK-ADC) at the Sanders-Brown Center on Aging. Details of the UK-ADC recruitment have been described previously (Nelson, et al., 2007, Schmitt, et al., 2012, Schmitt, et al., 2001). A series of cognitive tests were performed including the Mini-Mental State Examination (MMSE; (Folstein, et al., 1975)). Pathological assessments were performed at the University of Kentucky as described previously (Nelson, et al., 2009, Nelson, et al., 2007).
Our population of early AD patients was defined as MMSE 20-25 within 6 months of death and was pathologically confirmed. The reason why we used these criteria is that MMSE of 20-25 is usually an inclusion criteria for clinical trials. Our criteria for late stage AD was an MMSE of less than 15 with Braak stage 6 for pathology.
Patient characteristics referent to the samples from the frontal cortex and cerebellum of patients are summarized in table 1. Serum samples for age-matched non-demented controls (N=20) and early AD (N=20) were obtained for protein analysis. The serum was collected from the patients within six months of death and, importantly, was not the postmortem collection. We focused on serum from early AD patients as this was the population to show dichotomy of neuroinflammatory profiles. Tissue- and sera-derived data were correlated with neuropathologic and clinical data including diffuse and neuritic plaque counts, neurofibrillary tangle counts and cerebral amyloid angiopathy (CAA), as well as dichotomous variables related to cerebrovascular risk factors.
Table 1.
Characteristics of Alzheimer’s disease and age-matched, non-demented tissue analyzed. MMSE: mini-mental state exam, PMI: post-mortem interval.
| Group | Age range (years) |
MMSE at last exam |
Time since last exam (mo) |
ApoE4 status |
Braak stage |
PMI range (hours) |
|---|---|---|---|---|---|---|
| Early AD N=23 |
77-100 (mean:87.2) |
20-24 (mean:22.6) |
2-6 (mean:4.2) |
−/4 = 12 4/4 = 3 |
3-5 | 1.0-6.5 (mean:3.6) |
| Late AD N= 16 |
74-88 (mean:85.6) |
0-13 (mean:7.25) |
2-9 (mean:6.4) |
−/4 = 6 4/4 = 4 |
6 | 1.75-11.0 (mean:5.4) |
| Control N=37 |
77-96 (mean:84.3) |
28-30 (mean:29.1) |
4-7 (mean:5.2) |
−/4 = 10 4/4 = 3 |
0-2 | 1.75-8.0 (mean:4.2) |
Quantitative real-time RT-PCR
The frozen brain tissue was pulverized using a mortar and pestle on dry ice with liquid nitrogen and the brain powder was stored at −80°C. RNA was extracted from approximately 80mg frozen pulverized tissue using the Trizol Plus RNA Purification System (Life Technologies, Grand Island NY) according to the manufacturer’s instructions. RNA was quantified using the nanodrop spectrophotometer (Thermo Scientific, Rockford IL) and cDNA was reverse transcribed using the cDNA High Capacity kit (Applied Biosystems, Foster City CA) according to the manufacturer’s instructions. Real-time PCR was performed using the 384-well microfluidic card custom Taqman assays containing the TaqMan Gene Expression probes given in figure 1A (Applied Biosystems, Foster City CA). All gene expression data were normalized to 18S rRNA expression. Fold-change compared was determined using the delta delta Ct) method (Livak and Schmittgen, 2001).
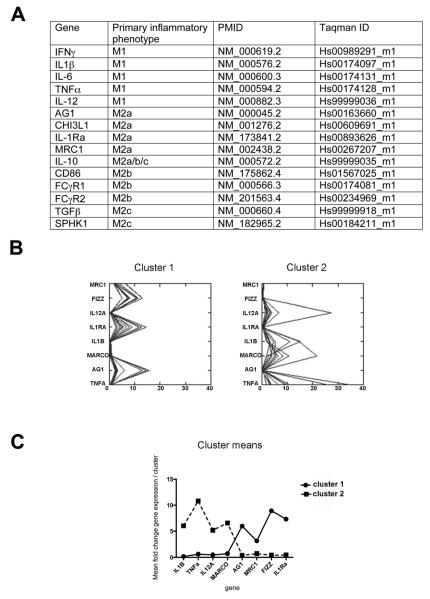
Figure 1.
Early-stage AD brain neuroinflammatory gene expression shows separation into two clusters. Panel A shows the genes that have been measured in the current study. Panel B shows the K-means cluster analysis for the M1 and M2a neuroinflammatory markers that showed significant differences. Panel C shows the cluster means highlighting the pattern of relative gene expression in our two clusters.
Beta-amyloid ELISA
300mg brain powder was first extracted in 500μl PBS containing 1% complete protease/phosphatase inhibitor (Thermo Scientific, Rockford IL). The homogenate was centrifuged at 16,000×g at 4°C for 30 minutes. The supernatant was removed and became the “soluble” extract. The resulting pellet was then homogenized in 100μl 70% formic acid and centrifuged at 16,000×g at 4°C for 30 minutes. The supernatant was removed and diluted 1:20 with 1M Tris-HCl to become the “insoluble” extract. Protein concentration for both the soluble and insoluble extracts was determined using the bicinchonic acid (BCA) protein assay according to manufacturer’s instructions (Thermo Scientific, Rockford IL). We used the Meso-Scale Discovery multiplex ELISA system to measure Aβ38, Aβ40 and Aβ42 (MSD, Gaithersburg MD). ELISA kits were run according to the manufacturer’s instructions.
Serum protein analysis
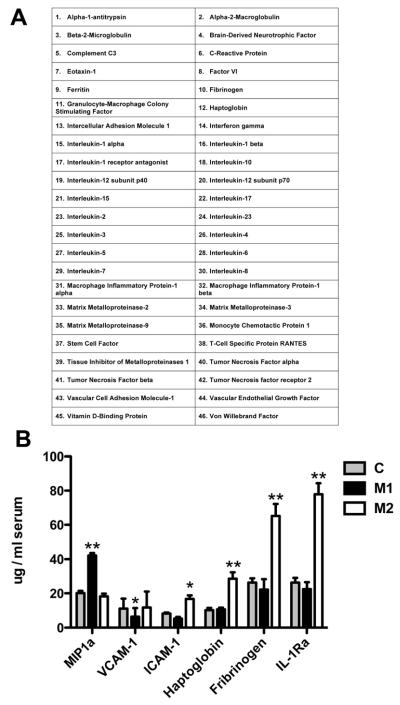
Serum protein analyses were performed by the CLIA-certified laboratory of Myriad-RBM (Austin, TX). Using their Multi-Analyte Profiling (MAP) technology platform we performed the Human InflammationMAP, which analyzes 46 inflammation-related proteins (see list in figure 4A) using Luminex bead technology.
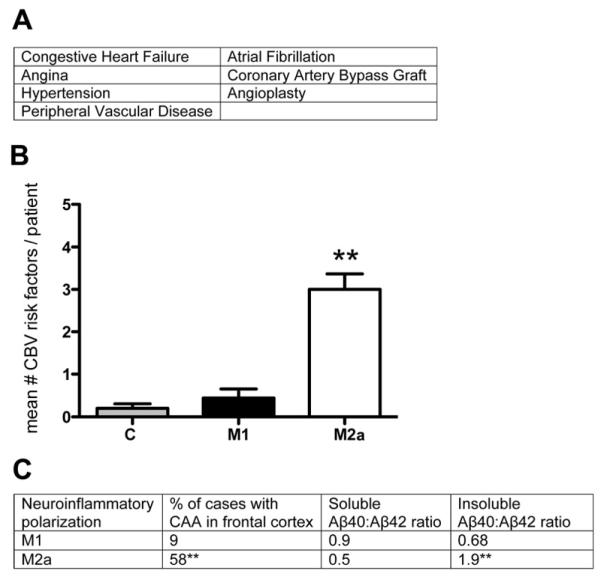
Figure 4.
Cerebrovascular disease is more prevalent in the M2a neuroinflammatory polarized samples than the M1 polarized samples. Panel A shows the cerebrovascular risk factors assessed. Panel B shows the mean number of risk factors per patient. Panel G shows CAA prevalence and Aβ40:Aβ42 ratios for each inflammatory group. ** indicates P<0.01.
Statistics
For gene expression analysis, we first analyzed the age-matched, non-demented controls. After finding little variability within this group we took the mean for this group. Gene expression for individual early AD and late AD samples was then calculated as fold-change relative to the mean of our age-matched non-demented control group. We performed K-means cluster analysis on the 23 samples examining all M1 and M2a markers to determine whether our samples showed patterns of clustering. Once we observed the M1 and M2a polarization, we separated our early AD samples as M1-biased or M2a-biased. The significance of disease and neuroinflammatory phenotype effects were then investigated using unpaired Student’s t tests or two-way ANOVAs. The statistical analysis software JMP (Version 9, SAS, Cary NC) was used for all statistical analyses with p < 0.05 judged as significant. All graphs were made using Graphpad Prism 4 (GraphPad, San Diego CA).
Results
The neuroinflammatory genes analyzed are shown in the table of figure 1A. When we examined the data from the frontal cortex of our early AD tissue we found some highly variable gene expression. We performed K-means cluster analysis on the expression data from all 23 frontal cortex samples to determine whether there were patterns of gene expression. As can be seen from figure 1B we found that our samples separated into two distinct clusters. Cluster 1 showed very high expression levels of FIZZ, IL-1Ra, MRC1 and AG1. Cluster 2 showed very high expression levels of IL-1β, MARCO, TNFα and IL-12A. Figure 1C shows the cluster means highlighting the two groups and their mean expression levels of each gene. K-means cluster analysis for the cerebellum and late-AD samples did not yield distinct clusters and therefore this data is not shown.
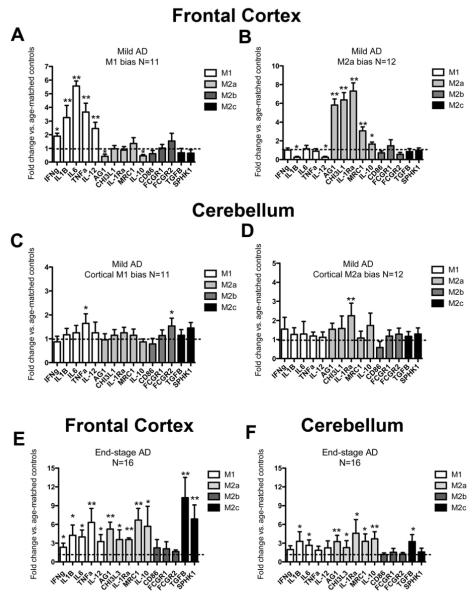
Using the cluster analysis we were able to separate out those samples that showed an M1 neuroinflammatory bias versus an M2a neuroinflammatory bias. We found that eleven of our 23 samples were apparently polarized to the M1 neuroinflammatory phenotype; herein abbreviated as M1-P (figure 2A) while the remaining twelve were apparently polarized to the M2a neuroinflammatory phenotype; herein abbreviated as M2a-P (figure 2B). Importantly, we examined the cerebellum of the same samples, confirmed to lack substantial AD pathology. In the cerebellum we did not observe the polarization of neuroinflammatory phenotype seen in the frontal cortex. While single genes showed some mild elevations, these were not of the same magnitude as the frontal cortex samples (figure 2C and D). We then assessed frontal cortex and cerebellar brain samples from 16 late-stage AD cases. We found that all frontal cortex samples showed elevations in markers of M1, M2a and M2c neuroinflammatory phenotypes (figure 2E). Similarly, in the cerebellum, the same markers were significantly increased, albeit to a lesser extent than the frontal cortex (figure 2F). To determine whether common AD disease risk factors were driving the M1-M2 polarization we performed analyses examining ApoE status, presence of diabetes or osteoarthritis, self-reported non-steroidal anti-inflammatory (NSAID) use and history of stroke or transient ischemic attacks (TIA). As can be seen in table 2, none of these factors accounted for the M1-M2 polarization of our early-AD samples. In addition, we examined cause of death for our samples and did not observe a relationship between cause of death and M1-M2 polarization. We also performed a correlation analysis using age as a factor and found no correlation between age and any of our neuroinflammatory markers.
Figure 2.
Early-stage AD brain shows a heterogeneous inflammatory response with polarization to the M1 or M2a neuroinflammatory states while the late-stage AD brain does not. Panels A-F show relative gene expression for early-stage AD (A-D) or late-stage AD (E-F) frontal cortex (A-B and E) and cerebellum (C-D and F). The dashed line on each graph represents the mean expression of the age-matched, non-demented controls. * indicates P<0.05, ** indicates P<0.01.
Table 2.
Statistical comparisons to determine whether common population covariates account for M1 / M2 polarization (one-way ANOVA).
| Factor | P-value |
|---|---|
| ApoE4 status | 0.60 |
| Diabetes | 0.37 |
| Osteoarthritis | 0.22 |
| Self reported NSAID use | 0.29 |
| Stroke | 0.34 |
| TIA | 0.56 |
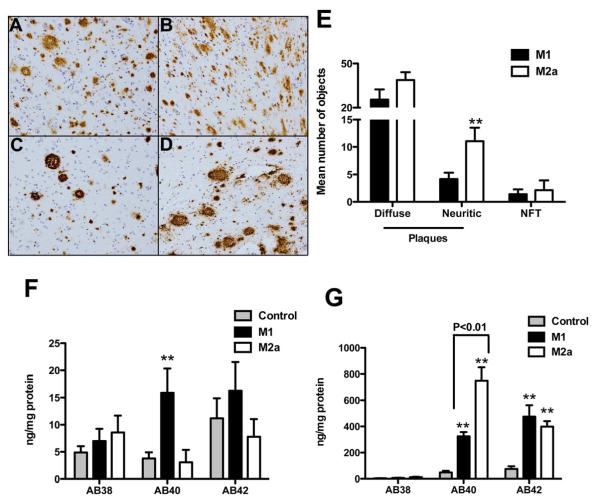
We next sought to determine whether neuroinflammatory state correlates with AD neuropathology and quantified Aβ levels. Amyloid plaques appeared more numerous in representative images from M2a-P early AD samples (figure 3C and D) compared to M1-P early AD samples (figure 3A and B). Quantitative neuropathology data (plaque and tangle counts) were analyzed focusing on the frontal cortex brain sections. We found that there was no difference between the M1-P and M2a-P early-stage AD samples for diffuse amyloid deposits or neurofibrillary tangle counts, but there was a statistically significant increase in the number of neuritic plaques in the M2a-P early-stage AD samples compared to the M1-P samples (figure 3E). Aβ ELISA measurement in the frontal cortex showed that soluble Aβ40 was significantly increased in the M1-P early-AD samples (figure 3F) while insoluble Aβ40 was significantly increased in the M2a-P early-AD samples (figure 3G).
Figure 3.
Early-stage AD neuropathology and amyloid load is different between the M1 and M2a neuroinflammatory polarized samples. Panels A-D show Aβ staining in the frontal cortex of M1 polarized samples (A-B) and M2a polarized samples (C-D). Mag = 100X. E shows numbers of diffuse and neuritic plaques and tangles in the frontal cortex. F and G show soluble (F) and insoluble (G) Aβ levels measured by ELISA. ** indicates P<0.01.
Cerebrovascular disease (CVD) occurs frequently in the aging brain, and is therefore a frequent co-morbidity with AD (Nelson, et al., 2007). We examined our early-AD cohort for CVD. Clinical records based on longitudinal assessments of the patients listed CVD risk factors (figure 4A) as dichotomous variables. We summed the number of risk factors present in each patient of our early AD cohort and our age-matched, non-demented controls and found that the M2a-P, early-stage AD samples had significantly more CVD risk factors present than the M1-P early-stage AD samples or the age-matched non-demented controls (figure 4B). We found that a greater percentage of the M2a-P, early AD samples had CAA present compared to the M1-P, early AD samples (figure 4C). In addition, when we calculated the ratio of Aβ40:Aβ42 we found that the M2a-P, early AD samples showed a significantly increased ratio compared to the M1-P, early AD samples (figure 4C).
Finally, we obtained serum samples from our biorepository for early AD samples (N=10 M1 polarized and M=10 M2a polarized) and age-matched non-demented controls (N=20). Our goal was to establish peripheral markers that would predict the brain neuroinflammatory profile. The serum was analyzed by Myriad-RBM for 46 inflammation-associated proteins (see list in figure 5A). We found that macrophage inflammatory protein 1α (MIP1α) was the only protein significantly increased in the M1-P, early AD samples compared to the M2a-P samples or age-matched, non-demented controls (figure 5B). We found that intercellular adhesion molecule 1 (ICAM1), haptoglobin, fibrinogen and interleukin-1 receptor antagonist were all significantly increased in in the M2a-P, early-stage AD samples compared to either / both the M1-P samples or age-matched, non-demented controls (figure 5B).
Figure 5.
Several serum proteins can be used to distinguish M1 polarization from M2a polarization. Serum was analyzed for 46 inflammation associated proteins listed in panel A. Panel B shows those proteins that were significantly different between M1 and M2a polarized early-stage AD samples. * indicates P<0.05, ** indicates P<0.01.
Discussion
We hypothesized that neuroinflammatory gene expression would provide biologically informative insights into the heterogeneous clinical and pathological context of the AD brain. We chose to categorize our neuroinflammatory genes based on the macrophage phenotypes of M1, M2a, M2b and M2c (reviewed in (Mosser and Edwards, 2008)). We obtained frozen frontal cortex as our diseased brain region and frozen cerebellum as our non-diseased brain region from early AD samples, late AD samples and age-matched non-demented controls. Frontal cortex was selected as our diseased region due to the availability of fresh frozen tissue and also due to the relatively low levels of AD pathology in this region making it ideal to study changes occurring relatively early in the disease process. Cluster analysis revealed that frontal cortex of early AD samples is apparently polarized to either the M1 or M2a neuroinflammatory phenotype. The cerebellum, confirmed to be AD pathology free, did not show polarization of these neuroinflammatory response types / categories. Further, this apparent polarization was not evident in late AD samples, when most of our inflammatory markers were significantly elevated relative to non-diseased controls.
Recent studies in mouse models have shown that the neuroinflammatory response is complex and includes the expression of “alternative inflammatory markers” that are associated with wound healing and repair as well as the expression of “classical inflammatory markers” that are associated with cytotoxicity such as IL-1β and TNFα. Additionally, it has been shown that the AD brain also expresses some of these alternative inflammatory markers. The M1 and M2a inflammatory states have been shown to have opposing actions in the peripheral tissues, where M1 macrophages are involved in recruitment of other immune cells, killing of invading pathogens and clearance of cellular debris (reviewed in (Mosser, 2003, Mosser and Edwards, 2008)). In contrast, the M2a macrophages are known to contribute to the formation of extracellular matrix through the up-regulation of arginase-1, inhibition of phagocytosis and augmentation of adhesion molecule production (Martinez, et al., 2009). One can predict, then, that the presence of one inflammatory state versus another in the AD brain could have different effects on the amyloid plaques, neurofibrillary tangles and neuronal functions. In fact, data in transgenic mouse models suggests that M1 neuroinflammatory phenotypes induced by agents such as LPS may be beneficial to amyloid pathology by lowering amyloid load, but may be detrimental to tau pathology by exacerbating hyperphosphorylation of tau (DiCarlo, et al., 2001, Lee, et al., 2010). In contrast, we recently showed that lithium promotes an M2 neuroinflammatory phenotype and lowers tau hyperphosphorylation but increases amyloid load in a transgenic mouse model (Sudduth, et al., 2012).
Heterogeneity in the neuroinflammatory response could provide an explanation for the dichotomous body of data that has evolved with respect to the role of non-steroidal anti-inflammatory drugs (NSAIDs) in AD. Some studies have found that NSAIDs may not affect AD risk while other studies have shown decreased risk for AD with NSAID use (reviewed (Szekely, et al., 2004)). One recent study showed an increased risk of AD with NSAID use in an elderly community-based study (Breitner, et al., 2009). Also, NSAID use has been associated with elevated neuritic plaque load (Sonnen, et al., 2010). A trial aimed at addressing the potential of NSAIDs to prevent the onset of AD, named the ADAPT trial (Alzheimer’s Disease Anti-Inflammatory Prevention Trial), was an NIH funded, multicenter trial testing the COX2 inhibitor celecoxib and naproxen. Treatment was terminated in 2004 due to concerns of adverse events (Breitner, et al., 2006). Most recently, extended results from the (ADAPT) trial have shown that long-term follow-up reveals those patients who were completely asymptomatic and treated with conventional NSAIDs showed a reduced incidence of AD several years later. However, those who had cognitive impairment (defined as cognitive impairment – no dementia CIND) at the time of NSAID treatment showed accelerated decline (Breitner, et al., 2011). Finally, Leotsakos showed that in the pre-clinical AD population of the ADAPT trial, when patients were separated as slow decliners, fast decliners, and no decline, differential responses to NSAIDs were observed. Fast decliners show a potential benefit of celecoxib whereas slow decliners show a potential benefit of naproxen (Leoutsakos, et al., 2011). It is possible that our data showing dichotomous neuroinflammatory states in the early AD brain can account for some of the positive and negative findings in the NSAID and AD literature.
An additional variable that is often overlooked in AD is the presence of cerebrovascular disease. Estimated to occur in as many as 40% of AD patients, (Bowler, et al., 1998, Kammoun, et al., 2000, Langa, et al., 2004) cerebrovascular disease can independently contribute to the cognitive dysfunction of dementia (Levine and Langa, 2011). While a small number of vascular dementia cases are identified through the history of stroke, transient ischemic attack or clear imaging abnormalities, the majority of cerebrovascular disease is not clinically obvious as it often encompasses microvascular findings at autopsy. We find that our early-stage AD brains apparently polarized to the M2a neuroinflammatory state also have significantly more cerebrovascular disease risk factors and show some histological cerebrovascular abnormalities. This suggests that the altered inflammatory response in this subset of patients may play a role in their course of disease, including their cerebrovascular disease. Importantly, hypertension, one of our cerebrovascular disease risk factors, has been suggested as a risk factor for AD. The Honolulu Asia Aging Study (HAAS) found that midlife hypertension increases risk for late-life dementia (Launer, et al., 2000), and when combined with elevated plasma Aβ, hypertension increases the risk for AD and vascular dementia (Shah, et al., 2012). Additional supporting data for the role of hypertension in dementia comes from the same HAAS study where lowering of midlife blood pressure reduced the risk of late-life dementia (Launer, et al., 2010). The systemic inflammation that arises as a consequence of many of the cerebrovascular risk factors such as hypertension, peripheral vascular disease, congestive heart failure and coronary artery bypass graft most likely contributes to the brain neuroinflammatory state. There is a growing body of data to suggest that systemic inflammation significantly impacts neurodegeneration and neuroinflammation (Moreno, et al., 2011, Perry, 2010). The systemic inflammation factor will be considered in greater detail as we move our studies forward and we will specifically examine these factors where possible.
An alternative, and equally viable, interpretation of our data is that the M1-M2a polarization reflects the progression of AD pathology. We do find a greater number of neuritic plaques in the M2a-polarized group compared to the M1 group, and the M2a group has more severe cerebrovascular pathology. Therefore, the M2a group may represent more pathologically advanced patients. Until further studies are done to expand our sample size and expand our population to examine individual Braak and Braak stages as well as clinical cognitive stages we must consider the possibility that our data implies either two distinct neuroinflammatory populations within the same disease state, or that neuroinflammatory state changes as disease progresses.
From the clinical application of our data, we are especially encouraged that we have identified some plasma markers that appear to classify our two neuroinflammatory populations. MIP1α is elevated in M1-polarized early AD samples while ICAM1, haptoglobin, fibrinogen and IL1-Ra were all elevated in the M2a-polarized early AD samples. Inflammatory biomarkers have been identified in AD populations previously (Dik, et al., 2005, Guerreiro, et al., 2007, Mancinella, et al., 2009, Ray, et al., 2007). In contrast, we are not identifying the presence of AD, but within those diagnosed clinically with early AD we are able to identify those with an M1 polarized brain neuroinflammatory response as opposed to those with an M2a polarized neuroinflammatory response in the brain. This could be important for better targeting of therapeutics to individual populations based on the inflammatory environment of the brain. In addition, it appears that the M2a polarized AD brain has a higher incidence of cerebrovascular disease, thus, identification of this population will permit more aggressive treatment of the cerebrovascular risk factors in this group of patients.
In summary, we have identified the neuroinflammatory state as a source of heterogeneity in the early AD population. We hypothesize that the apparent polarization of the neuroinflammatory state to M1 or M2a in early AD could influence the response to many therapeutics, in particular those targeting the inflammatory pathways such as NSAIDs, or using inflammatory systems such as immunotherapeutic approaches. Most importantly, we have identified several proteins in serum that are predictive of the brain neuroinflammatory state that could be used to stratify groups for clinical trials to determine whether neuroinflammatory phenotype does influence responsiveness to therapies.
Acknowledgements
The project described was supported in part by NIH grant P30 AG028383 and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1RR033173. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors are deeply grateful to all of the study volunteers. The authors would like to thank Sonya Anderson, Ela Patel and Erin Abner for technical support, Greg Cooper MD, Greg Jicha MD, Charles Smith MD, Nancy Stiles MD and Alison Cabat-Holt PhD for the clinical evaluations, Daron Davis MD and Stephen W. Scheff PhD for pathological evaluations and Richard Kryscio PhD for statistical consultation. DMW had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, O’Banion MK, Pachter J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyoma I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Coray T. Inflammation and Alzheimer’s disease. Neurobiology of aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler JV, Munoz DG, Merskey H, Hachinski V. Fallacies in the pathological confirmation of the diagnosis of Alzheimer’s disease. Journal of neurology, neurosurgery, and psychiatry. 1998;64(1):18–24. doi: 10.1136/jnnp.64.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Baker LD, Montine TJ, Meinert CL, Lyketsos CG, Ashe KH, Brandt J, Craft S, Evans DE, Green RC, Ismail MS, Martin BK, Mullan MJ, Sabbagh M, Tariot PN. Extended results of the Alzheimer’s disease anti-inflammatory prevention trial. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011;7(4):402–11. doi: 10.1016/j.jalz.2010.12.014. doi:10.1016/j.jalz.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Haneuse SJ, Walker R, Dublin S, Crane PK, Gray SL, Larson EB. Risk of dementia and AD with prior exposure to NSAIDs in an elderly community-based cohort. Neurology. 2009;72(22):1899–905. doi: 10.1212/WNL.0b013e3181a18691. doi:10.1212/WNL.0b013e3181a18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitner JC, Martin BK, Meinert CL. The suspension of treatments in ADAPT: concerns beyond the cardiovascular safety of celecoxib or naproxen. PLoS clinical trials. 2006;1(8):e41. doi: 10.1371/journal.pctr.0010041. doi:10.1371/journal.pctr.0010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activation genes in AD and in mouse models of AD. J Neuroinflammation. 2006;3:27. doi: 10.1186/1742-2094-3-27. doi:1742-2094-3-27 [pii] 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9(2):174–91. doi: 10.2174/187152710791012053. doi:BSP/CDTCNSND/E-Pub/00025 [pii] [DOI] [PubMed] [Google Scholar]
- DiCarlo G, Wilcock D, Henderson D, Gordon M, Morgan D. Intrahippocampal LPS injections reduce Abeta load in APP+PS1 transgenic mice. Neurobiol Aging. 2001;22(6):1007–12. doi: 10.1016/s0197-4580(01)00292-5. doi:S0197458001002925 [pii] [DOI] [PubMed] [Google Scholar]
- Dik MG, Jonker C, Hack CE, Smit JH, Comijs HC, Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64(8):1371–7. doi: 10.1212/01.WNL.0000158281.08946.68. doi:10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. Journal of leukocyte biology. 2006;80(6):1298–307. doi: 10.1189/jlb.0406249. doi:10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Guerreiro RJ, Santana I, Bras JM, Santiago B, Paiva A, Oliveira C. Peripheral inflammatory cytokines as biomarkers in Alzheimer’s disease and mild cognitive impairment. Neuro-degenerative diseases. 2007;4(6):406–12. doi: 10.1159/000107700. doi:10.1159/000107700. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2012;8(1):1–13. doi: 10.1016/j.jalz.2011.10.007. doi:10.1016/j.jalz.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammoun S, Gold G, Bouras C, Giannakopoulos P, McGee W, Herrmann F, Michel JP. Immediate causes of death of demented and non-demented elderly. Acta neurologica Scandinavica Supplementum. 2000;176:96–9. doi: 10.1034/j.1600-0404.2000.00314.x. [DOI] [PubMed] [Google Scholar]
- Langa KM, Foster NL, Larson EB. Mixed dementia: emerging concepts and therapeutic implications. JAMA : the journal of the American Medical Association. 2004;292(23):2901–8. doi: 10.1001/jama.292.23.2901. doi:10.1001/jama.292.23.2901. [DOI] [PubMed] [Google Scholar]
- Launer LJ, Hughes T, Yu B, Masaki K, Petrovitch H, Ross GW, White LR. Lowering midlife levels of systolic blood pressure as a public health strategy to reduce late-life dementia: perspective from the Honolulu Heart Program/Honolulu Asia Aging Study. Hypertension. 2010;55(6):1352–9. doi: 10.1161/HYPERTENSIONAHA.109.147389. doi:10.1161/HYPERTENSIONAHA.109.147389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launer LJ, Ross GW, Petrovitch H, Masaki K, Foley D, White LR, Havlik RJ. Midlife blood pressure and dementia: the Honolulu-Asia aging study. Neurobiology of aging. 2000;21(1):49–55. doi: 10.1016/s0197-4580(00)00096-8. [DOI] [PubMed] [Google Scholar]
- Lee DC, Rizer J, Selenica ML, Reid P, Kraft C, Johnson A, Blair L, Gordon MN, Dickey CA, Morgan D. LPS-induced inflammation exacerbates phospho-tau pathology in rTg4510 mice. Journal of neuroinflammation. 2010;7:56. doi: 10.1186/1742-2094-7-56. doi:10.1186/1742-2094-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leoutsakos JM, Muthen BO, Breitner JC, Lyketsos CG. Effects of non-steroidal anti-inflammatory drug treatments on cognitive decline vary by phase of pre-clinical Alzheimer disease: findings from the randomized controlled Alzheimer’s Disease Anti-inflammatory Prevention Trial. International journal of geriatric psychiatry. 2011 doi: 10.1002/gps.2723. doi:10.1002/gps.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine DA, Langa KM. Vascular cognitive impairment: disease mechanisms and therapeutic implications. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2011;8(3):361–73. doi: 10.1007/s13311-011-0047-z. doi:10.1007/s13311-011-0047-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. doi:10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Mancinella A, Mancinella M, Carpinteri G, Bellomo A, Fossati C, Gianturco V, Iori A, Ettorre E, Troisi G, Marigliano V. Is there a relationship between high C-reactive protein (CRP) levels and dementia? Archives of gerontology and geriatrics. 2009;49(Suppl 1):185–94. doi: 10.1016/j.archger.2009.09.028. doi:10.1016/j.archger.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends in immunology. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. doi:10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annual review of immunology. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532. doi:10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- Moreno B, Jukes JP, Vergara-Irigaray N, Errea O, Villoslada P, Perry VH, Newman TA. Systemic inflammation induces axon injury during brain inflammation. Annals of neurology. 2011;70(6):932–42. doi: 10.1002/ana.22550. doi:10.1002/ana.22550. [DOI] [PubMed] [Google Scholar]
- Mosser DM. The many faces of macrophage activation. Journal of leukocyte biology. 2003;73(2):209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nature reviews Immunology. 2008;8(12):958–69. doi: 10.1038/nri2448. doi:10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. Journal of neuropathology and experimental neurology. 2009;68(1):1–14. doi: 10.1097/NEN.0b013e3181919a48. doi:10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS, Abner EL, Markesbery WR. Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles "do count" when staging disease severity. Journal of neuropathology and experimental neurology. 2007;66(12):1136–46. doi: 10.1097/nen.0b013e31815c5efb. doi:10.1097/nen.0b013e31815c5efb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta neuropathologica. 2010;120(3):277–86. doi: 10.1007/s00401-010-0722-x. doi:10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- Ray S, Britschgi M, Herbert C, Takeda-Uchimura Y, Boxer A, Blennow K, Friedman LF, Galasko DR, Jutel M, Karydas A, Kaye JA, Leszek J, Miller BL, Minthon L, Quinn JF, Rabinovici GD, Robinson WH, Sabbagh MN, So YT, Sparks DL, Tabaton M, Tinklenberg J, Yesavage JA, Tibshirani R, Wyss-Coray T. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nature medicine. 2007;13(11):1359–62. doi: 10.1038/nm1653. doi:10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Nelson PT, Abner E, Scheff S, Jicha GA, Smith C, Cooper G, Mendiondo M, Danner DD, Van Eldik LJ, Caban-Holt A, Lovell MA, Kryscio RJ. University of Kentucky Sanders-Brown Healthy Brain Aging Volunteers: Donor Characteristics, Procedures, and Neuropathology. Current Alzheimer research. 2012 doi: 10.2174/156720512801322591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt FA, Wetherby MM, Wekstein DR, Dearth CM, Markesbery WR. Brain donation in normal aging: procedures, motivations, and donor characteristics from the Biologically Resilient Adults in Neurological Studies (BRAiNS) Project. The Gerontologist. 2001;41(6):716–22. doi: 10.1093/geront/41.6.716. [DOI] [PubMed] [Google Scholar]
- Shah NS, Vidal JS, Masaki K, Petrovitch H, Ross GW, Tilley C, DeMattos RB, Tracy RP, White LR, Launer LJ. Midlife blood pressure, plasma beta-amyloid, and the risk for Alzheimer disease: the Honolulu Asia Aging Study. Hypertension. 2012;59(4):780–6. doi: 10.1161/HYPERTENSIONAHA.111.178962. doi:10.1161/HYPERTENSIONAHA.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnen JA, Larson EB, Walker RL, Haneuse S, Crane PK, Gray SL, Breitner JC, Montine TJ. Nonsteroidal anti-inflammatory drugs are associated with increased neuritic plaques. Neurology. 2010;75(13):1203–10. doi: 10.1212/WNL.0b013e3181f52db1. doi:10.1212/WNL.0b013e3181f52db1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudduth TL, Wilson JG, Everhart A, Colton CA, Wilcock DM. Lithium Treatment of APPSwDI/NOS2-/- Mice Leads to Reduced Hyperphosphorylated Tau, Increased Amyloid Deposition and Altered Inflammatory Phenotype. PloS one. 2012;7(2):e31993. doi: 10.1371/journal.pone.0031993. doi:10.1371/journal.pone.0031993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekely CA, Thorne JE, Zandi PP, Ek M, Messias E, Breitner JC, Goodman SN. Nonsteroidal anti-inflammatory drugs for the prevention of Alzheimer’s disease: a systematic review. Neuroepidemiology. 2004;23(4):159–69. doi: 10.1159/000078501. doi:10.1159/000078501. [DOI] [PubMed] [Google Scholar]
- Wilcock DM, DiCarlo G, Henderson D, Jackson J, Clarke K, Ugen KE, Gordon MN, Morgan D. Intracranially administered anti-Abeta antibodies reduce beta-amyloid deposition by mechanisms both independent of and associated with microglial activation. J Neurosci. 2003;23(9):3745–51. doi: 10.1523/JNEUROSCI.23-09-03745.2003. doi:23/9/3745 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock DM, Rojiani A, Rosenthal A, Subbarao S, Freeman MJ, Gordon MN, Morgan D. Passive immunotherapy against Abeta in aged APP-transgenic mice reverses cognitive deficits and depletes parenchymal amyloid deposits in spite of increased vascular amyloid and microhemorrhage. J Neuroinflammation. 2004;1(1):24. doi: 10.1186/1742-2094-1-24. doi:1742-2094-1-24 [pii] 10.1186/1742-2094-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]