Abstract
BACKGROUND
Despite the high complete response rates with fludarabine-based regimens, relapse is inevitable in chronic lymphocytic leukemia (CLL). Relapsed patients often acquire 17p deletions [del(17p)], which are closely associated with TP53 mutations. Wild-type p53 upregulates and activates BAX, and it downregulates and inactivates BCL-2. The small-molecule BCL-2 inhibitor ABT-737 induces apoptosis in a BAX- and BAK-dependent manner. The role of p53 in sensitivity of CLL cells to BCL-2 inhibition has not been extensively investigated.
METHODS
We investigated an association of del(17p) with ABT-737 sensitivity in CLL cells from 50 patients. Stable p53 and BAX knockdown cells were used for the mechanistic studies.
RESULTS
CLL cells with del(17p) are less sensitive to ABT-737-induced BAX activation and apoptosis than those without del(17p) (39.0 ± 7.3% versus 63.7 ± 2.9% specific Annexin V induction; P < .01). A positive correlation between the degrees of apoptosis induced by ABT-737 and by the p53-activating MDM2 antagonist Nutlin-3a (r = 0.75; P < .0001) was observed. CLL cells with del(17p) expressed lower levels of BAX than those without (0.67 ± 0.12 versus 1.27 ± 0.10 in relative protein expression levels; P < .01). Knockdown of p53 or BAX in leukemia cells resulted in decreased apoptosis induced by ABT-737.
CONCLUSIONS
Data indicate that p53 dysfunction may lead to decreased apoptosis induction by ABT-737.
Keywords: CLL, BCL-2, BAX, p53, 17p deletion
Introduction
Despite the high complete response rate with fludarabine-based regimens, relapse is inevitable in chronic lymphocytic leukemia (CLL).1–3 Relapsed patients often acquire 17p deletions [del(17p)], which correspond to loss of the tumor suppressor TP53, commonly accompanied by TP53 mutation of the remaining allele.4 Relapsed CLL patients with del(17p) are resistant to most standard therapies and 17p-deleted CLL patients do not even benefit from first-line fludarabine-based regimens.2,3
ABT-737, a small-molecule inhibitor of BCL-2, BCL-XL and BCL-W, induces apoptosis in a BAX- and BAK-dependent manner.5,6 Primary CLL cells express high BCL-2 levels and, importantly, are dependent on BCL-2 for survival.7,8 Studies of sensitivity to ABT-737 have in common two results: (1) cells with BCL-2 primed with high levels of activators like BIM tend to be sensitive to ABT-737; and (2) high expression of MCL-1 or BFL-1/A1 can result in decreased sensitivity to ABT-737.8–13 It has been reported that BAX levels negatively correlate with sensitivity to ABT-737 in diffuse large B cell lymphoma cell lines.9 However, it remains unknown if cellular BAX levels of primary neoplastic cells affect the susceptibility to BCL-2 inhibition.
The balance of anti-apoptotic BCL-2 and pro-apoptotic BAX dictates cellular fate.14 Wild-type p53, but not mutant p53, upregulates and activates BAX, and downregulates and inactivates BCL-2.15–17 The role of p53 in sensitivity of leukemic cells to BCL-2 inhibition is unknown. Here, we investigated the potential therapeutic utility of the BCL-2 antagonists in CLL, focusing on the possible association of defective p53 with sensitivity to ABT-737.
Methods
Reagents
ABT-737 was synthesized at the University of Texas MD Anderson Cancer Center based on the previously published structure.5 Nutlin-3a was kindly provided by Dr. Lyubomir Vassilev (Hoffmann-La Roche, Inc.). Z-VAD-FMK was purchased from Axxora (San Diego, CA).
Clinical data and cell culture
Heparinized peripheral blood samples were obtained from CLL patients after informed consent through the Lymphoma SPORE grant sample core, according to institutional guidelines per Declaration of Helsinki. Lymphocytes were purified and cultured in MEM-α medium supplemented with 10% FBS at a density of 2.5 × 106 cell/mL. We treated cells with 10 nM of ABT-737, based on a previous study that determined the EC50 (effective concentration inducing 50% killing as measured by Annexin V positivity) of 27 nM at 4 hours and 4.5 nM at 48 hours in CLL samples.8 Immunoglobulin VH somatic mutation status was determined as described.18 Somatic hypermutation in the immunoglobulin VH locus was classified as absent when 98% or greater homology to germline was measured. The mutation status was designated as unmutated if fewer than 2.0% mutations (> 98.0% homology to germline sequences) or as mutated if 2.0% or greater mutations (98.0% homology to germline sequences) were detected compared with the germline sequences in VBASE2 (www.vbase2.org). Interphase fluorescence in situ hybridization (FISH) was performed for detection of 13q14, 11q22, 17p13, and trisomy 12. Patient samples were classified as ZAP70 positive when at least 20% of cells were positive and CD38 positive when at least 30% of cells were positive. Human acute lymphoblastic leukemia REH (American Type Culture Collection (ATCC)) and human acute myeloid leukemia MOLM-13 (Deutsche Sammlung von Mikroorganismen und Zellkulturen) cell lines were maintained in RPMI 1640 medium containing 10% FCS. REH and MOLM-13 cells were transduced with retroviruses encoding either p53-specific shRNA (nucleotides 611–629, Genbank NM000546), BAX-specific shRNA (nucleotides 268–286, Genbank NM138761.3) or negative control shRNA and stable shRNA-expressing cells were generated.19
Transfection of BAX siRNA
Five primary CLL samples were transfected with BAX small interfering RNA (siRNA) oligonucleotides (SignalSilence Bax SiRNA; Cell Signaling Technology, Beverly, MA) by the Amaxa electroporator Nucleofector II, using the Human B Cell Nucleofector Kit according to the manufacturer's instructions (Amaxa Biosystem, Cologne, Germany). Nonspecific scrambled siRNA served as negative control. Twenty-four hours post transfection, BAX and β-actin expression were analyzed by Western blot analysis.
Antibodies
The following antibodies were used: mouse monoclonal anti-p53 (Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-BCL-2 (Dako Cytomation, Carpinteria, CA); rabbit polyclonal anti-BAX (BD Biosciences, San Jose, CA); mouse monoclonal anti-BAX (Trevigen, Gaithersburg, MD); mouse monoclonal anti-MCL-1 (BD Biosciences); rabbit polyclonal anti-PUMA (EMD Biosciences, San Diego, CA); mouse monoclonal anti-NOXA (EMD Biosciences); and mouse monoclonal anti-β-actin (Sigma, St. Louis, MO).
Apoptosis analysis
Apoptosis was measured by the Annexin V binding assay. The extent of apoptosis was quantified as percentage of Annexin V-positive cells, and the extent of drug-specific apoptosis was calculated by the formula: % specific apoptosis = (test – control) × 100 / (100 – control). In the formula, the numerator is the actual amount of killing that occurred and the denominator is the potential amount of killing that could occur.
Quantitation of intracellular proteins by flow cytometry
For intracellular p53 detection, cells were fixed with 2% paraformaldehyde, permeabilized with 100% ice-cold methanol and incubated for 1 hour at 4°C with antibody against p53 or its isotypic control (BD Biosciences). Involvement of BAX conformational change was analyzed by means of an antibody directed against the NH2-terminal region of BAX (YTH-6A7; Trevigen).20
Western blot analysis
Equal amounts of protein lysate were separated by SDS-PAGE (12% gel) for 2 hours at 80 V. Proteins were transferred to nitrocellulose membrane, immunoblotted with primary antibodies followed by infrared secondary antibodies (LI-COR Biosciences, Lincoln, NE), and detected by an Odyssey infrared imaging system (LI-COR Biosciences). An anti-β-actin blot was used in parallel as a loading control. Visualized blots were analyzed by the ImageJ 1.44 software (National Institutes of Health, Bethesda, MD).
Real-time quantitative PCR
RNA was prepared from cells using an RNeasy Mini Kit (Qiagen, Valencia, CA), and first-strand cDNA was generated using random hexamers (SuperScript III First-Strand Synthesis SuperMix; Invitrogen, Carlsbad, CA) from 1 μg total RNA. The mRNA expression levels of MCL-1, BAX, PUMA, NOXA, p21, FAS, APAF1, DR5, MDM2 and GAPDH were quantified using TaqMan gene expression assays (MCL-1: Hs03043899_m1, BAX: Hs00180269_m1, PUMA: Hs00248075_m1, NOXA: Hs00382168_m1, p21: Hs00171132_m1, FAS: Hs00163653_m1, APAF1: Hs00185508_m1, DR5: , Hs00366272_m1, MDM2: Hs00242813_m1, GAPDH: Hs99999905_m1, Applied Biosystems, Foster City, CA) on a 7900HT Fast Real-Time PCR System. According to the manufacturer’s guidelines, the level of expression was calculated based upon the PCR cycle number (Ct) at which the exponential growth in fluorescence from the probe passes a certain threshold value. For each sample, relative gene expression level was determined by subtracting the Ct value of the housekeeping gene GAPDH to the Ct value of the target gene (ΔCt = CtTarget gene – Ct18S rRNA). Relative quantification (fold change) between different samples (e.g., treated vs. control) was then determined according to the 2 −ΔΔCt method (ΔΔCt = ΔCttreated sample – Ctcontrol sample).
TP53 mutation analysis
PCR for p53 gene expression followed by direct sequencing was performed as described previously.20
Statistical analysis
Statistical analysis was performed using the 2-tailed Student’s t test, Mann-Whitney U test and Pearson correlation. Results were considered statistically significant at P-values < .01. Unless otherwise indicated, average values were expressed as mean ± SEM.
Results
CLL cells with del(17p) are resistant to Nutlin-3a and less sensitive to ABT-737
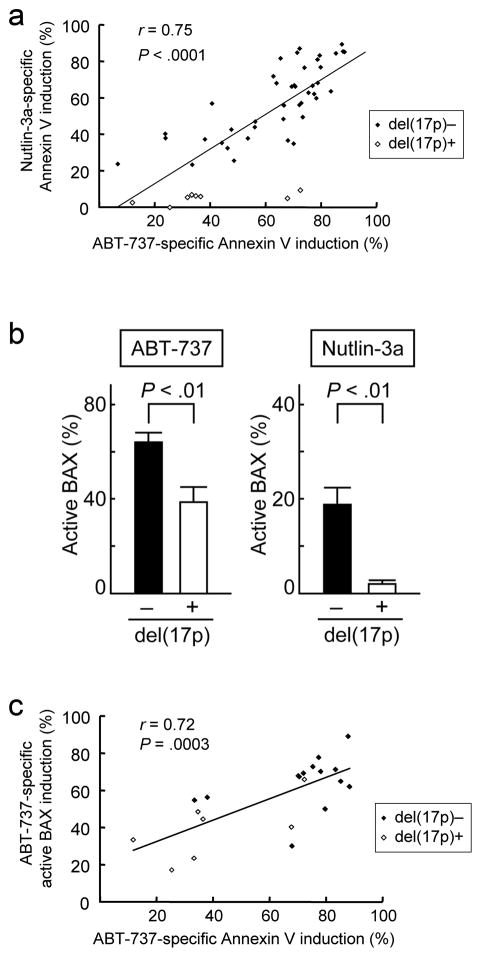
We examined the proapoptotic effects of the MDM2 inhibitor Nutlin-3a and the BCL-2 antagonist ABT-737 on primary cells from 50 patients with CLL. Table 1 summarizes the clinical characteristics of the patients. Cells were exposed to 0.1% DMSO, 10 μM Nutlin-3a or 10 nM ABT-737 for 48 hours. p53 status is the major determinant of response to Nutlin-3a in CLL,20,21 and as expected, CLL cells with del(17p) (n = 8) were resistant to Nutlin-3a compared to those without del(17p) (n = 42) (5.5 ± 1.0% versus 59.0 ± 3.0% specific apoptosis; P < .01). Interestingly, CLL cells with del(17p) were also less sensitive to ABT-737 (39.0 ± 7.3% versus 63.7 ± 2.9% specific apoptosis; P < .01). We also found that the extent of apoptosis induced by ABT-737 correlated with that induced by Nutlin-3a (r = 0.75; P < .0001) (Fig. 1a). Sensitivity to ABT-737 was independent of other prognostic parameters including IgVH status, 11q deletion, previous treatments, CD38 or ZAP-70 positivity (Table 2). We also investigated if these prognostic parameters are associated with sensitivity to Nutlin-3a. The presence of del(17p) was the only factor which affected Nutlin-3a sensitivity (Table 3). Involvement of BAX conformational change was analyzed in 21 primary samples (7 samples with del(17p) and 14 samples without del(17p) as controls). We could not obtain the eighth del(17p) sample, as the patient did not return to the hospital during the study period. 100 μM Z-VAD-FMK was used to inhibit caspase-promoted conformational change of BAX. Increases in the percentage of conformationally active BAX-positive cells were significantly smaller in 17p-deleted samples than those without del(17p) (39.0 ± 6.2% versus 64.6 ± 3.7% specific apoptosis; P < .01) (Fig. 1b). There was a significant positive correlation between the percentage of specific Annexin V induction and that of specific BAX activation (Figure 1c; r = 0.72, P < .01), suggesting a direct role of BAX activation in apoptosis induction by ABT-737.
Table 1.
Characteristics of CLL patients
| Characteristic | Value |
|---|---|
| Sex (n) | |
| Male | 37 |
| Female | 13 |
| Age, yr [median (range)] | 67 (45–88) |
| Rai stage (n) | |
| 0 | 15 |
| 1–2 | 20 |
| 3–4 | 15 |
| Treatment history (n) | |
| Prior treatment | 27 |
| Untreated | 23 |
| FISH abnormality (n)* | |
| Deletion 17p13 | 8 |
| Trisomy 12 | 2 |
| Deletion 11q22 | 11 |
| Deletion 13q14 | 35 |
| None detected | 9 |
| Not assessed | 1 |
| CD38 (n) | |
| < 30% (negative) | 32 |
| ≥ 30% (positive) | 17 |
| Not assessed | 1 |
| IgVH status (n) | |
| Mutated | 19 |
| Not mutated | 18 |
| Not assessed | 13 |
| ZAP70 (n) | |
| < 20% (negative) | 20 |
| ≥ 20% (positive) | 19 |
| Not assessed | 11 |
| β2 microglobulin (mg/ml) [median (range)] † | 3.7 (1.9–15.0) |
FISH indicates fluorescence in situ hybridization;
normal range, 0.7–1.8 mg/ml.
Figure 1. Functional p53 pathway is required for full induction of apoptosis by ABT-737 in CLL.
(a) The extent of apoptosis induced by ABT-737 positively correlates with that induced by Nutlin-3a. Fifty CLL patient samples were treated for 48 hours with either 10 nM ABT-737 or 10 μM Nutlin-3a and then assayed for Annexin V binding by flow cytometry. (b) CLL cells with del(17p) are less sensitive to ABT-737-induced BAX activation. Seven CLL patient samples with and fourteen without del(17p) were treated for 12 hours with 10 nM ABT-737 or 10 μM Nutlin-3a. BAX conformational change was determined by staining with the active conformation-specific anti-BAX antibody YTH-6A7. 100 μM Z-VAD-FMK was used to inhibit caspase-promoted conformational change of BAX. (c) The percentage of specific phosphatidylserine externalization/Annexin V induction positively correlates with that of specific BAX activation in ABT-737-treated CLL cells.
Table 2.
Comparison of clinical characteristics with in vitro sensitivity to ABT-737
| Parameter | Dichotomy | P value |
|---|---|---|
| Sex | Male versus Female | 0.089 |
| Age | 0–65 versus > 65 | 0.749 |
| Rai stage | 0–2 versus 3–4 | 0.197 |
| Prior treatment | Yes versus no | 0.484 |
| Deletion 17p | Yes versus no | 0.006 |
| Deletion 11q | Yes versus no | 0.857 |
| Deletion 13q | Yes versus no | 0.868 |
| CD38 | Positive versus negative | 0.060 |
| IgVH status | Mutated versus not mutated | 0.197 |
| ZAP70 | Positive versus negative | 0.768 |
| β2 microglobulin | 0–4 versus > 4 | 0.115 |
Statistical summary of dichotomized groups. Samples were divided into groups based on their membership in 1 of the 2 classes for each property. Mann-Whitney U test was used to determine whether the 2 groups differed in terms of statistical significance; this is summarized by the P values in the third column.
Table 3.
Comparison of clinical characteristics with in vitro sensitivity to Nutlin-3a
| Parameter | Dichotomy | P value |
|---|---|---|
| Sex | Male versus Female | 0.759 |
| Age | 0–65 versus > 65 | 0.515 |
| Rai stage | 0–2 versus 3–4 | 0.374 |
| Prior treatment | Yes versus no | 0.627 |
| Deletion 17p | Yes versus no | < 0.0001 |
| Deletion 11q | Yes versus no | 0.839 |
| Deletion 13q | Yes versus no | 0.293 |
| CD38 | Positive versus negative | 0.235 |
| IgVH status | Mutated versus not mutated | 0.939 |
| ZAP70 | Positive versus negative | 0.768 |
| β2 microglobulin | 0–4 versus > 4 | 0.181 |
Statistical summary of dichotomized groups. Samples were divided into groups based on their membership in 1 of the 2 classes for each property. Mann-Whitney U test was used to determine whether the 2 groups differed in terms of statistical significance; this is summarized by the P values in the third column.
17p-deleted CLL cells have lower BAX levels than those without del(17p)
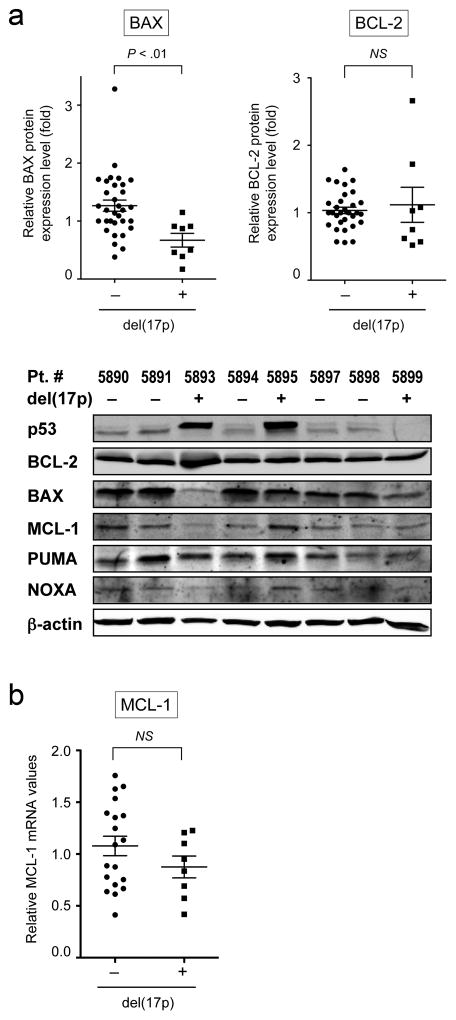
To investigate the contribution of p53-influenced BCL-2 family members to ABT-737-induced apoptosis, we analyzed expression levels of BCL-2, MCL-1, BAX, PUMA and NOXA. BAX levels were lower in 17p-deleted samples than in those without del(17p) (Fig. 2A). Levels of BCL-2, MCL-1, PUMA and NOXA were independent of del(17p). Since MCL-1 protein levels that have been reported to determine cell sensitivity to ABT-737, were low in CLL cells (Fig. 2a),8 real-time quantitative PCR was employed to measure MCL-1 transcription. MCL-1 mRNA levels were not significantly affected by the presence or absence of del(17p) (Fig. 2b).
Figure 2. CLL cells with del(17p) expressed lower levels of Bax than those without del(17p).
(a) BAX expression in CLL cells with del(17p) is low. Expression of BCL-2 family proteins was determined by Western blotting. Protein expression levels relative to a loading control, β-actin, were determined in each sample and then compared to the reference sample from a patient # 5998, which expressed median levels of BAX and BCL-2. Representative Western blots are shown. (b) Real-time PCR measurement of MCL-1. The abundance of MCL-1 mRNA normalized to that of GAPDH is presented.
ABT-737 does not induce p53 responses in CLL cells
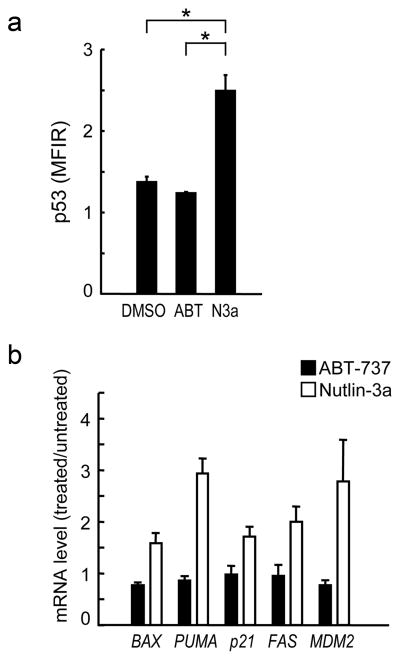
To exclude the possibility that ABT-737 induces p53 responses in CLL cells, three patient samples with known wild-type p53 were treated with 10 nM ABT-737 for six hours and cellular p53 levels and mRNA levels of p53 targets were determined. As shown in Fig. 3a–b, ABT-737 treatment did not increase p53 levels or induce p53-related genes BAX, PUMA, CDKN1A (p21), FAS or MDM2.
Figure 3. ABT-737 itself does not induce p53 response.
(a) Three CLL samples with wild-type p53 were treated for 6 hours with 10 nM ABT-737, 10 μM Nutlin-3a or vehicle (DMSO). p53 expression levels were expressed as mean fluorescence intensity ratio (MFIR) calculated by the formula: MFIR = (MFI for anti-p53 antibody) / (MFI for isotypic control). Samples treated with Nutlin-3a expressed significantly higher levels of p53 (MFIR 2.49 ± 0.35) than untreated (1.37 ± 0.12) or ABT-737-treated (1.23 ± 0.02) cells. (b) p53-target gene activation in response to 10 nM ABT-737 or 10 μM Nutlin-3a in 3 CLL patient samples. Ratios represent ABT-737 or Nutlin-3 values divided by untreated values. Each sample was assayed in duplicate.
p53 knockdown in leukemia cells leads to reduced BAX levels and decreased ABT-737 sensitivity
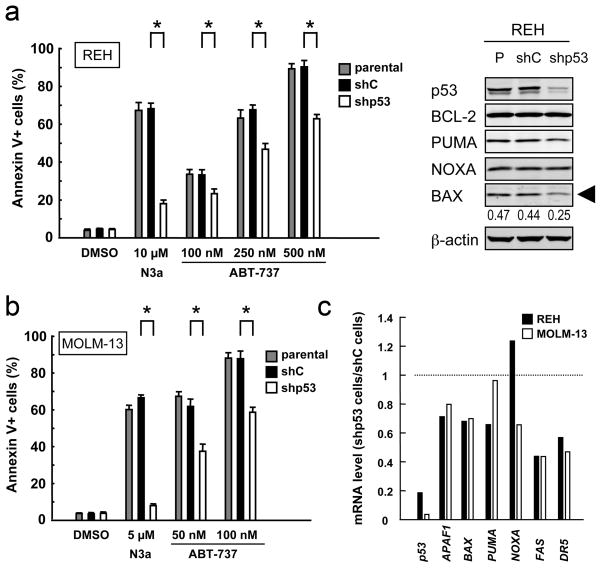
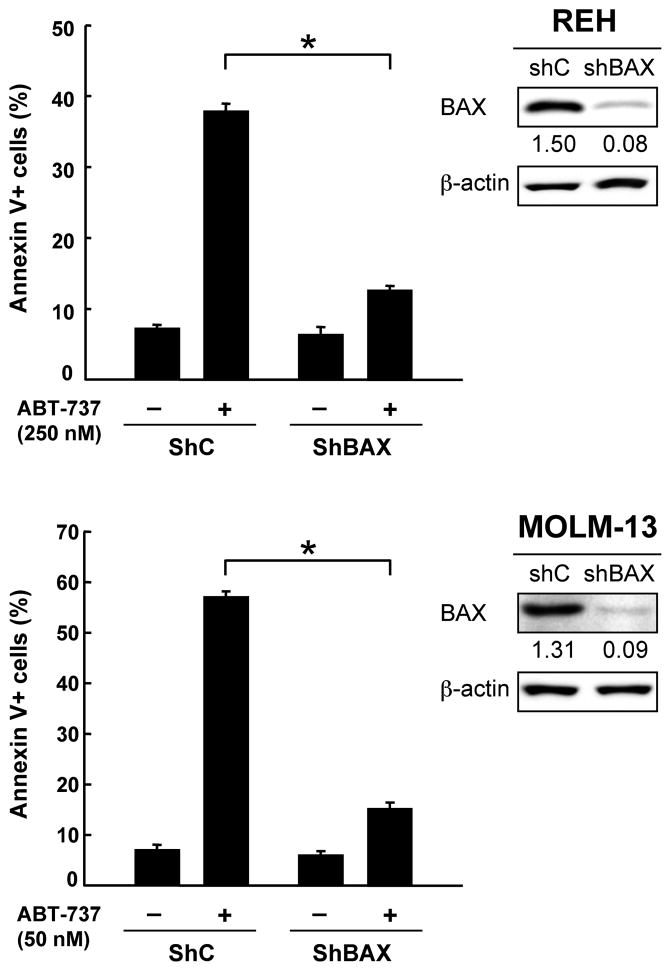
As there are no CLL cell lines available, we used REH and MOLM-13 cell lines for knockdown experiments. p53-knockdown REH cells displayed reduced levels of BAX and were less susceptible to ABT-737-induced apoptosis than parental or cells expressing negative control shRNA (Fig. 4a), suggesting that functional p53 expression may be required for full induction of apoptosis by ABT-737. Desensitization of leukemia cells to ABT-737 was also observed in p53-knockdown MOLM-13 cells, in which steady state Bax mRNA levels were reduced by 30.2% compared to negative control shRNA-expressing cells (Fig. 4b–c). To elucidate if BAX levels affect leukemia cell sensitivity to ABT-737, BAX levels were reduced in REH and MOLM-13 cells. As shown in Fig. 5, BAX knockdown cells were less sensitive to ABT-737-induced apoptosis than cells expressing scrambled shRNA in both cells (P < .01), suggesting that BAX levels affect cell susceptibility to ABT-737 in leukemia cells. Gene silencing by RNA interference could not be accomplished in primary CLL cells because of poor transfection efficiency and high cellular toxicity.
Figure 4. p53 modulates leukemia cell sensitivity to ABT-737.
(a) Parental and transduced REH cells [virus encoding either negative control shRNA (shC) or p53-specific shRNA (shp53)] were incubated with 10 μM Nutlin-3a or the indicated concentrations of ABT-737 for 48 hours, and the Annexin V-positive fractions were measured by flow cytometry. Results are expressed as mean ± SD. Intensity of the immunoblot signals was determined by densitometry using NIH ImageJ 1.44 software, and the intensity of BAX relative to that of β-actin was calculated. *P < .01. (b) Parental and transduced MOLM-13 cells [virus encoding either negative control shRNA (shC) or p53-specific shRNA (shp53)] were incubated with 5 μM Nutlin-3a or the indicated concentrations of ABT-737 for 48 hours, and the Annexin V-positive fractions were measured by flow cytometry. Results are expressed as mean ± SD. (c) Real-time PCR measurement of steady state expression of p53 and p53 target genes. The abundance of mRNA was normalized to that of GAPDH. Ratios represent values of p53-specific shRNA-expressing cells divided by those of negative control shRNA-expressing cells. Each sample was assayed in duplicate. p53-specific shRNA reduced p53 basal levels by 82% in REH and 95% in MOLM-13 cells.
Figure 5. BAX modulates leukemia cell sensitivity to ABT-737.
Transduced REH or MOLM-13 cells [virus encoding either negative control shRNA (shC) or BAX-specific shRNA (shBAX)] were incubated with ABT-737 for 24 hours, and the Annexin V-positive fractions were measured by flow cytometry. Results are expressed as mean ± SD. Intensity of the immunoblot signals was determined by densitometry using NIH ImageJ 1.44 software, and the intensity of BAX relative to that of β-actin was calculated. *P < .01.
Discussion
We here report that CLL cells with del(17p), an adverse prognostic factor closely associated with TP53 mutations,22 are less sensitive to ABT-737 than those without del(17p). We also observed a positive correlation between apoptosis induced by ABT-737 and Nutlin-3a. Considering that sensitivity to Nutlin-3a largely relies on p53 status in CLL, our data suggest that BCL-2 inhibition may require functional p53 signaling to fully induce apoptosis. Other prognostic markers or clinical parameters did not affect ABT-737 sensitivity, in agreement with previous studies.8,13 An association of p53 status with ABT-737 sensitivity has not been extensively investigated in CLL. Mason et al., in a series of 30 CLL cases, reported that 3 del(17p) cases displayed similar sensitivity to ABT-737 as those without del(17p).23 On the other hand, Balakrishnan et al., in a series of 32 cases, found a single case of del(17p), which showed limited apoptosis in response to ABT-737.24 As del(17p) and p53 mutations are closely associated with fludarabine resistance and disease progression,24 the hypothesis that p53 status affects clinical response to ABT-737, could be validated in patients with refractory CLL undergoing clinical trials with ABT-737.
Although the expression of BAX has been found to be up-regulated at the transcriptional level by p53,25 CLL cells with del(17p) expressed BAX to some extent, possibly because non-deleted or non-mutated alleles would maintain low levels of BAX. In addition, it has been reported that BAX can be post-translationally regulated by BCL-2,26 which is overexpressed in CLL.7,8 Furthermore, TA-p73, a p53 family protein, has been found to transcriptionally upregulate BAX in the absence of p53.27 Future studies are required to clarify if p53-independent regulators of BAX contribute to resistance to ABT-737. Bax expression may not be the only determinant for the reduced sensitivity to ABT-737 in CLL cells with del(17p). Since steady state mRNA expression levels of ‘apoptotic’ p53 target genes were modestly but widely repressed in association with stable p53 knockdown (Fig. 4c), cumulative changes in aberrantly expressed apoptosis-regulating proteins might directly or indirectly reduce ABT-737 sensitivity in cases with defective p53.
In conclusion, our data indicate that p53 dysfunction may lead to decreased apoptosis induction by ABT-737 in CLL cells and that BAX levels may dictate sensitivity to BCL-2 inhibition. Recently, Letai et al. has proposed three classes of apoptotic blocks used to maintain cancer survival against BCL-2 inhibition.28 Our case may represent the class B block in which cancer cells eliminate the effector arm of the mitochondrial apoptotic pathway by reducing or eliminating BAX and BAK. As conventional agents used in CLL treatment, including alkylating agents, fludarabine, and rituximab, kill CLL cells via the mitochondrial apoptotic pathway, decreased BAX in del(17p) cells may contribute to resistance to these agents.3 Patients with defective p53 may be considered for alternative treatment approaches including early stem-cell transplantation,29 if BCL-2 inhibitors and other promising agents in clinical trials do not provide sufficient clinical responses in this specific subgroup.
Acknowledgments
This work was supported in part by grants from National Institutes of Health Lymphoma SPORE (CA136411), P01 “The Therapy of AML” (CA55164), Leukemia SPORE (CA100632) and the Paul and Mary Haas Chair in Genetics (to M.A.). The authors would like to thank Teresa McQueen, Duncan Mak and Twee Tsao for valuable technical help. The authors acknowledge Dr. Jeffrey Medeiros, Lymphoma SPORE sample core laboratory, for providing samples.
Footnotes
Disclosure of Potential Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol. 2005;23:4079–4088. doi: 10.1200/JCO.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 2.Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet. 2007;370:230–239. doi: 10.1016/S0140-6736(07)61125-8. [DOI] [PubMed] [Google Scholar]
- 3.Zenz T, Eichhorst B, Busch R, et al. TP53 mutation and survival in chronic lymphocytic leukemia. J Clin Oncol. 2010;28:4473–4479. doi: 10.1200/JCO.2009.27.8762. [DOI] [PubMed] [Google Scholar]
- 4.Zenz T, Kröber A, Scherer K, et al. Monoallelic TP53 inactivation is associated with poor prognosis in chronic lymphocytic leukemia: results from a detailed genetic characterization with long-term follow-up. Blood. 2008;112:3322–3329. doi: 10.1182/blood-2008-04-154070. [DOI] [PubMed] [Google Scholar]
- 5.Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of BCL-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 6.Buron N, Porceddu M, Brabant M, et al. Use of human cancer cell lines mitochondria to explore the mechanisms of BH3 peptides and ABT-737-induced mitochondrial membrane permeabilization. PLoS One. 2010;5:e9924. doi: 10.1371/journal.pone.0009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otake Y, Soundararajan S, Sengupta TK, et al. Overexpression of nucleolin in chronic lymphocytic leukemia cells induces stabilization of bcl2 mRNA. Blood. 2007;109:3069–3075. doi: 10.1182/blood-2006-08-043257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Chen S, Dai Y, Harada H, Dent P, Grant S. MCL-1 down-regulation potentiates ABT-737 lethality by cooperatively inducing BAK activation and BAX translocation. Cancer Res. 2007;67:782–791. doi: 10.1158/0008-5472.CAN-06-3964. [DOI] [PubMed] [Google Scholar]
- 11.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.van Delft MF, Wei AH, Mason KD, et al. The BH3 mimetic ABT-737 targets selective BCL-2 proteins and efficiently induces apoptosis via BAK/BAX if MCL-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogler M, Butterworth M, Majid A, et al. Concurrent up-regulation of BCL-XL and BCL2A1 induces approximately 1000-fold resistance to ABT-737 in chronic lymphocytic leukemia. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]
- 14.Chipuk JE, Green DR. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol. 2008;18:157–164. doi: 10.1016/j.tcb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chipuk JE, Kuwana T, Bouchier-Hayes L, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–4. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 16.Deng X, Gao F, Flagg T, Anderson J, May WS. Bcl2's flexible loop domain regulates p53 binding and survival. Mol Cell Biol. 2006;26:4421–4434. doi: 10.1128/MCB.01647-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kojima K, Konopleva M, Samudio IJ, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin KI, Tam CS, Keating MJ, et al. Relevance of the immunoglobulin VH somatic mutation status in patients with chronic lymphocytic leukemia treated with fludarabine, cyclophosphamide, and rituximab (FCR) or related chemoimmunotherapy regimens. Blood. 2009;113:3168–3171. doi: 10.1182/blood-2008-10-184853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masutomi K, Yu EY, Khurts S, et al. Telomerase maintains telomere structure in normal human cells. Cell. 2003;114:241–253. doi: 10.1016/s0092-8674(03)00550-6. [DOI] [PubMed] [Google Scholar]
- 20.Kojima K, Konopleva M, McQueen T, O'Brien S, Plunkett W, Andreeff M. MDM2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanisms and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108:993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saddler C, Ouillette P, Kujawski L, et al. Comprehensive biomarker and genomic analysis identifies p53 status as the major determinant of response to MDM2 inhibitors in chronic lymphocytic leukemia. Blood. 2008;111:1584–1593. doi: 10.1182/blood-2007-09-112698. [DOI] [PubMed] [Google Scholar]
- 22.Dicker F, Herholz H, Schnittger S, et al. The detection of TP53 mutations in chronic lymphocytic leukemia independently predicts rapid disease progression and is highly correlated with a complex aberrant karyotype. Leukemia. 2009;23:117–124. doi: 10.1038/leu.2008.274. [DOI] [PubMed] [Google Scholar]
- 23.Mason KD, Khaw SL, Rayeroux KC, et al. The BH3 mimetic compound, ABT-737, synergizes with a range of cytotoxic chemotherapy agents in chronic lymphocytic leukemia. Leukemia. 2009;23:2034–41. doi: 10.1038/leu.2009.151. [DOI] [PubMed] [Google Scholar]
- 24.Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell-mediated Mcl-1 induction and drug resistance. Blood. 2009;113:149–53. doi: 10.1182/blood-2008-02-138560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zenz T, Häbe S, Denzel T, et al. Detailed analysis of p53 pathway defects in fludarabine-refractory chronic lymphocytic leukemia (CLL): dissecting the contribution of 17p deletion, TP53 mutation, p53-p21 dysfunction, and miR34a in a prospective clinical trial. Blood. 2009;114:2589–2597. doi: 10.1182/blood-2009-05-224071. [DOI] [PubMed] [Google Scholar]
- 25.Miyashita T, Reed JC. Tumor suppressor p53 is a direct transcriptional activator of the human bax gene. Cell. 1995;80:293–299. doi: 10.1016/0092-8674(95)90412-3. [DOI] [PubMed] [Google Scholar]
- 26.Miyashita T, Kitada S, Krajewski S, Horne WA, Delia D, Reed JC. Overexpression of the Bcl-2 protein increases the half-life of p21Bax. J Biol Chem. 1995;270:26049–26052. doi: 10.1074/jbc.270.44.26049. [DOI] [PubMed] [Google Scholar]
- 27.Soond SM, Carroll C, Townsend PA, et al. STAT1 regulates p73-mediated Bax gene expression. FEBS Lett. 2007;581:1217–1226. doi: 10.1016/j.febslet.2007.02.049. [DOI] [PubMed] [Google Scholar]
- 28.Letai AG. Diagnosing and exploiting cancer's addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 29.Dreger P, Döhner H, Ritgen M, et al. Allogeneic stem cell transplantation provides durable disease control in poor-risk chronic lymphocytic leukemia: long-term clinical and MRD results of the German CLL Study Group CLL3X trial. Blood. 2010;116:2438–2447. doi: 10.1182/blood-2010-03-275420. [DOI] [PubMed] [Google Scholar]