Abstract
Aims
Food is known to shorten the QTc (QTcI and QTcF) interval and has been proposed as a non-pharmacological method of confirming assay sensitivity in thorough QT (TQT) studies and early phase studies in medicines research. Intake of food leads to a rise in insulin levels together with the release of C-peptide in equimolar amounts. However, it has been reported that euglycaemic hyperinsulinemia can prolong the QTc interval, whilst C-peptide has been reported to shorten the QTc interval. Currently there is limited information on the effects of insulin and C-peptide on the electrocardiogram (ECG). This study was performed to assess the effect of insulin, glucose and C-peptide on the QTc interval under the rigorous conditions of a TQT study.
Methods
Thirty-two healthy male and female, Caucasian and Japanese subjects were randomized to receive six treatments: (1) placebo, (2) insulin euglycaemic clamp, (3) carbohydrate rich ‘continental’ breakfast, (4) calorie reduced ‘American’ FDA breakfast, (5) moxifloxacin without food, and (6) moxifloxacin with food. Measurements of ECG intervals were performed automatically with subsequent adjudication in accordance with the ICH E14 guideline and relevant amendments.
Results
No effect was observed on QTcF during the insulin euglycaemic clamp period (maximal shortening of QTcF by 2.6 ms, not significant). Following ingestion of a carbohydrate rich ‘continental’ breakfast or a calorie reduced ‘American’ FDA standard breakfast, a rapid increase in insulin and C-peptide concentrations were observed. Insulin concentrations showed a peak response after the ‘continental’ breakfast observed at the first measurement time point (0.25 h) followed by a rapid decline. Insulin concentrations observed with the ‘American’ breakfast were approximately half of those seen with the ‘continental’ breakfast and showed a similar pattern. C-peptide concentrations showed a peak response at the first measurement time point (0.25 h) with a steady return to baseline at the 6 h time point. The response to the ‘continental’ breakfast was approximately double that of the ‘American’ FDA breakfast. A rapid onset of the effect on QTcF was observed with the ‘continental’ breakfast with shortening by >5 ms in the time interval from 1 to 4 h. After the ‘American’ FDA breakfast, a similar but smaller effect was seen.
Conclusions
The findings of this study demonstrate that there was no change in QTc during the euglycaemic clamp. Given that insulin was raised to physiological concentrations comparable with those seen after a meal, whilst the release of C-peptide was suppressed, insulin appears to have no effect on the QTc interval in either direction. The results suggest a relationship exists between the shortening of QTc and C-peptide concentrations and indicate that glucose may have a QTc prolonging effect, which will require further research.
Keywords: C-peptide, euglycaemic insulin clamp, clinical trial, meal effects, QTc prolongation, QTc shortening
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
A meal, in particular if rich in carbohydrates alters the QT/RR relationship. This is of interest since this shows that normal physiological changes can alter QTc significantly.
The underlying effects of QTc shortening after a meal are currently unknown. A meal leads to the release of C-peptide and insulin in equimolar amounts.
QTc shortening after administration of C-peptide has been described by other authors and one study has reported that insulin prolongs the QTc.
WHAT THIS STUDY ADDS
This study investigates the effect of insulin on the QT interval under the rigorous conditions of a thorough QT (TQT) study using a single dose of 400 mg moxifloxacin as a positive control.
Insulin concentrations during the euglycaemic clamp were raised to constant concentrations of about 45 μlU ml−1 during which endogenous C-peptide release was successfully suppressed.
During the euglycaemic clamp, no significant change was observed in QTcF. The greatest effect observed was a QTcF shortening of 2.6 ms (95% CI −5.3, 0.2), 1.5 h into the clamp. The observation that insulin does not influence QTcF was confirmed by a concentration−response analysis which revealed that QTc shortening was due to C-peptide and that glucose counteracted this thereby partially offsetting the effect of C-peptide leading to a net QTcF decrease.
Introduction
The effect of food on QT interval has been investigated in numerous studies over the past two decades. These have included reports of prolonged QT in response to low calorie meals and starvation [1–3], reports of post-prandial QT shortening [4, 5] and post-prandial increases in heart rate [6, 7]. The effect of food has recently been re-examined in a study that measured the effect of food on QTc in healthy subjects in the resting state. Heart rate was found to rapidly increase by approximately 9.4 beats min–1 following food and lasted for approximately 4 h. A maximum QTc interval (Fridericia's formula, QTcF) shortening of approximately 8.2 ms (two-sided 95% confidence interval (CI) 6, 10) was observed 2 h post dose [8]. A similar increase in heart rate of 12 beats min–1 has also been reported after food intake which lasted for approximately 3 h [9]. There is good consistency between these more substantive studies and various other reported studies.
Transient endogenous physiological insulinaemia has been associated with meals of high carbohydrate content [6]. A 10 beats min–1 increase in heart rate combined with peak increases in blood glucose concentrations at 40 min and peak insulin concentrations at 1 h 20 min were observed following carbohydrate ingestion [6]. Another study using an insulin euglycaemic clamp demonstrated an effect of insulin on the heart rate and QTcB [10]. However, it has been shown that Bazett's formula artificially prolongs the QTc interval with an increase in heart rate [11]. Therefore, studies reporting prolongation of the QTc (Bazett, QTcB) interval post-prandially or after insulin administration (both of which increase the heart rate) should be interpreted with caution. None of these studies measured the concentrations of C-peptide which is excreted in similar amounts to insulin and has been associated with reductions in QTc [12]. In one study [12], administration of C-peptide to type 1 diabetes patients with autonomic neuropathy shortened the QT interval. It can therefore be surmised that C-peptide may be responsible for the QTc shortening effects observed beyond the immediate post-prandial period.
Based on the available evidence reported in the literature, there is limited information on the effects of insulin and C-peptide on the ECG following food ingestion. The aim of this study was to characterize the effect of insulin/glucose and C-peptide on QT compared with placebo under the rigorous conditions of a TQT study. A single oral dose of 400 mg moxifloxacin was used as a positive control to confirm assay sensitivity.
Methods
Subjects
The study population consisted of 32 healthy non-smoking, Caucasian and Japanese male and female subjects, aged between 20–45 years (inclusive) with a body mass index of between 18 to 25 kg m−2, using an effective contraceptive method (or were abstinent). Subjects were excluded if they had (1) any risk factor for the occurrence of torsades de pointes (marked baseline prolongation of QT/QTc interval, e.g. QTcB > 450 ms, congenital long QT syndrome), (2) any pathology or abnormality with possible clinically significant influence on the ECG (e.g. electrolyte imbalance), (3) impaired drug metabolism/clearance, (4) the use of concomitant medications or foods that impair drug metabolizing capacity, (5) concomitant use of drugs with QT-prolonging effects and (6) impaired glucose tolerance on screening.
Study design
The study was designed as a single centre, randomized, placebo- and positive-controlled, crossover study. Subjects participating in the study attended for screening, two treatment periods (periods 1 and 2) of 4 assessment days each, and a follow-up visit (Table 1). General eligibility of subjects for participation in this study was assessed at screening which took place within 21 days of the first study treatment. Subjects’ eligibility regarding specific ECG criteria was evaluated during screening and confirmed on day −1, prior to the first study treatment.
Table 1.
Summary of study design. This was a randomized, placebo- and positive-controlled, crossover study. Subjects participating in the study attended for screening and two treatment periods (periods 1 and 2) separated by a 3 day wash-out interval
| Period 1 | Washout* | Period 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day −1 | Day 1 | Day 2 | Day 3 | Minimum of 3 days | Day −1 | Day 1 | Day 2 | Day 3 | |||||
| P | P | I | M | P | B | F | M + B | ||||||
| I | B | M | F | P | M + B | ||||||||
| B | F | M | P | I | M + B | ||||||||
| F | P | M | I | B | M + B | ||||||||
| P | I | M + B | B | F | M | ||||||||
| I | B | M + B | F | P | M | ||||||||
| B | F | M + B | P | I | M | ||||||||
| F | P | M + B | I | B | M | ||||||||
P = Placebo, I = Insulin + Glucose (clamp), B = high carbohydrate breakfast (>70% carbohydrates), F = calorie reduced FDA standard breakfast, M = Moxifloxacin.
At least a 3 day wash-out interval between day 3 of period 1 and day −1 of period 2.
Eligible subjects were randomized to one of eight sequences of the treatments, insulin euglycaemic clamp, a carbohydrate rich ‘continental’ breakfast, a calorie reduced ‘American’ FDA standard breakfast, a single dose of moxifloxacin 400 mg, a single dose of moxifloxacin 400 mg with a carbohydrate rich ‘continental’ breakfast and placebo. These sequences provided balance for period and preceding treatment (Williams squares), but always had the treatments including moxifloxacin on day 3 of the two periods to avoid any carry over effects from the treatment with moxifloxacin. Randomization was performed stratified by gender and race. Full nutritional breakdown and calorific content of the carbohydrate rich ‘continental’ breakfast and calorie reduced ‘American’ FDA breakfast is described in Table 2.
Table 2.
Nutritional breakdown and calorific content of meals
| Meal type | Food Iitem | Servings | Calories (kcal) | Protein (g) | Carbohydrates (g) | Fat (g) | Fibre (g) |
|---|---|---|---|---|---|---|---|
| Calorie reduced ‘American’ FDA breakfast | One fried egg in butter (7.9 g) | 1 egg | 90.0 | 6.3 | 0.4 | 6.8 | 0.0 |
| One slice of bacon (grilled) | 1 slice | 44.0 | 2.9 | 0.1 | 3.5 | 0.0 | |
| Hash browns | 54 g | 95.0 | 1.2 | 13.0 | 3.9 | 1.4 | |
| White toast medium | 1 slice | 64.0 | 2.0 | 12.0 | 0.9 | 0.6 | |
| Whole milk | 85 ml | 54.6 | 2.8 | 4.3 | 3.1 | 0.0 | |
| Butter (spreadable) | 7.9 g | 58.0 | 0.0 | 0.0 | 6.5 | 0.0 | |
| Total nutrition of the breakfast | 405.6 | 15.2 | 29.8 | 24.7 | 2.0 | ||
| Carbohydrate rich ‘continental’ breakfast | Cornflakes | 30 g | 112.0 | 2.1 | 25.0 | 0.2 | 0.9 |
| Semi-skimmed milk | 150 ml | 73.2 | 5.1 | 25.0 | 2.6 | 0.0 | |
| Sugar | 10 g | 40.0 | 0.0 | 10.0 | 0.0 | 0.0 | |
| Wholemeal hoagie | 98 g | 235.5 | 11.0 | 39.4 | 2.3 | 6.4 | |
| Jam | 20 ml | 55.6 | 0.1 | 13.8 | 0.0 | 0.2 | |
| Butter | 7.9 g | 58.0 | 0.0 | 0.0 | 6.5 | 0.0 | |
| Apple juice | 200 ml | 88.0 | 0.2 | 20.7 | 0.2 | 0.0 | |
| Total nutrition of the breakfast | 661.8 | 18.5 | 133.9 | 11.8 | 7.5 |
Each period consisted of a baseline ECG day (day −1) and treatment days (day 1, day 2 and day 3). The ECG and samples for PK/PD analysis on the treatment days were taken at the corresponding clock time points as on the baseline day. Each subject received three treatments per period and all the comparisons between treatment effects were intra-individual reducing the anticipated variability and thereby reducing the sample size.
The study was approved by the local NHS Ethics Committee (London Surrey-Borders, UK) and the Medicines and Healthcare products Regulatory Authority (MHRA) and was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki.
ECG assessments and QTc evaluation
Twelve-lead ECGs were recorded using a MAC1200® (500 samples s−1, 4.88 μV amplitude resolution, GE Healthcare, Milwaukee, WI, USA) recorder connected via a fixed network connection to the MUSE® Cardiology Information System (MUSE). All ECGs recorded during the study were stored electronically on the MUSE information system. Only ECGs recorded electronically at a stable heart rate were valid ECGs for any purpose other than safety assessment.
12-lead ECG recordings were made at the following time points, pre-dose (to establish a baseline), 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0 and 6.0 h post-dose/infusion on days −1, 1, 2, and 3 after the subjects had been resting in a supine position for at least 10 min. The subjects avoided postural changes during the ECG recordings and clinical staff ensured that subjects were awake during the ECG recording. The use of a semi-permanent skin marker was to ensure consistent placement of the leads for consecutive study days.
At each time point, the ECGs were recorded in triplicate, to reduce variance and improve the precision of measurement. The triplicates were performed at approximately 1 min intervals. Each ECG recording lasted 10 s. Repeat ECGs were performed until at least three 10 s ECG recordings per scheduled time point met the pre-defined quality criteria to enable reading and analyzing at least five complexes per derivation. Simultaneous 12-lead Holter ECG recordings were taken from pre-dose to 6 h post-dose for later analysis; data not presented.
During treatment days subjects were served lunch (7 h post-dose), dinner (11 h post-dose) and a snack (13.5 h post-dose). When required, the different breakfasts were provided 30 min prior to anticipated dosing and were to be consumed 10 min before anticipated dosing.
Data analysis and statistical methods
Each electronic ECG data file contained the ECG data as well as the result of the automated ECG analysis performed by the Marquette® 12SL™ ECG Analysis Program (MEAP), a programme resident in each of the ECG machines.
All ECGs and their associated automated interval measurements were subsequently reviewed by qualified cardiologists following one of the methods listed in the ICH E14 Guidance for Industry document and ICH E14 Implementation Working Group Questions and Answers document before any of the ECGs were used for the thorough ECG analysis. The manual adjudication process applied in this study is also referred to in the ICH guidance and relevant literature as ‘manual over read’, ‘computer assisted’ or ‘semi automated’ ECG measurements.
The following parameters on each ECG were assessed by a cardiologist using the commercially available MUSE® in its latest version:
QT interval, RR interval, heart rate, PR interval, presence or absence of U-wave, quantitative and qualitative ECG variations
Manual on-screen over-reading using electronic callipers in MUSE® was performed by a small and select group of cardiologists with extensive experience in manual QT measurement (including on-screen measurement with electronic callipers, Interval Editor ECG analysis system, GE healthcare). For all study ECGs, the over-reading cardiologists were blinded to time, date, treatment and any data identifying the subject. All ECGs of a given subject were over-read by the same cardiologist (or cardiologists in case manual adjustments of the automated measurement were necessary).
The primary analysis was based on the change of QTcF from average baseline. A confirmatory analysis was based on the average of QTc over all time points between and including 2 and 4 h, except for the test involving the euglycemic clamp. Descriptive analyses for each time point separately were performed. A linear mixed model with sequence, day, period, gender, race and treatment as fixed effects, and baseline as covariate was adapted, with subject (nested in sequence, gender and race) as random effects. Two-sided 90% CIs for the difference between each treatment and placebo and between the two types of breakfast were derived. All subjects in the safety dataset who had valid ECG data for time points during days 1 to 3 of periods 1 and 2 were included in the primary analysis set. The confirmatory part of the primary analysis followed a hierarchical test procedure testing the following null-hypotheses in the given order: (1) There is no difference between carbohydrate rich breakfast and placebo (two-sided test), (2) there is no difference between calorie reduced FDA breakfast and placebo (two-sided test) and (3) the difference at 1.5 h under the euglycaemic clamp compared with placebo is at least 10 ms (test for non-inferiority). Subsequent predefined confirmatory tests are not relevant for the present investigation. All statistical analyses were performed using R version 2.13.0 [13] or later. The descriptive per-time point analysis followed the same lines.
In addition to the analyses described above, the effect of insulin, C-peptide and glucose on QTcF was investigated using linear mixed effect concentration–response models with the double difference of QTcF (difference to time matched placebo of the change from average baseline) as dependent variable and up to two of the variables change from time matched placebo in insulin, C-peptide and glucose as covariates. In addition, gender and race (Caucasian and Japanese) were entered as fixed effects into the model. Random intercept and slope (with respect to the covariates entered) were included in the model with subject as unit. Model fit was investigated by using normal QQ-plots of the residuals. Models were fitted to the data obtained during the euglycaemic clamp (time points up to and including 1.5 h), and to all data of the two types of breakfast and the euglycaemic clamp arms. Estimates of slopes were given together with their 95% CIs.
Other models based on single differences (no difference to placebo) were also investigated but were found to be inferior.
Sample size was estimated on the average of five adjacent QTc values, which were expected to be highly correlated. Since the baseline was used as a covariate in the analysis, using the SD of the change from baseline in the simple sample size formula was justified. With a correlation between QTc values at different time points of 0.8, the average of five values has about 92% of the SD of the individual components. Assuming a SD of 7 ms for the single differences, sample sizes for the average can therefore work with a SD of 6.5 ms. If the estimate of the SD after the completion of 20 subjects showed a higher SD than 7 ms in a blinded analysis, the sample size could be adapted accordingly up to a maximum of 56 subjects.
Insulin euglycaemic clamp
A euglycaemic/hyperinsulinaemic clamp was used to stop any endogenous C-peptide and insulin production. The clamp acutely raised the plasma insulin concentrations to a steady-state and maintained glucose concentrations at/or slightly lower than the individual subjects baseline reading. For each subject two 18G cannulas were inserted, one in the antecubital fossa for insulin and glucose infusions, and one for pharmacokinetic (PK) and blood glucose sampling. Insulin infusion rates were as follows: 120.6 mU m–2 BSA min−1 (0–2 min), 95.7 mU m–2 BSA min−1 (2–4 min), 75.8 mU m–2 BSA min−1 (4–6 min), 60.2 mU m–2 BSA min−1 (6–8 min), 47.7 mU m–2 BSA min−1 (8–10 min) and 40.0 mU m–2 BSA min−1 (10–120 min). These rates were further adjusted according to individual BSA using individual subject weight and height. For example, a subject with body weight of 65 kg and height of 1.74 m will have a BSA of 1.77 m2. Therefore, the initial insulin infusion rate for 0–2 min would be: 120.6 mU m–2 BSA min−1 × 1.77 m2 = 213.5 mU min−1. Blood glucose concentrations were measured to determine basal concentrations, 4 and 10 min after insulin infusion had started and then every 5 min thereafter until the end of the clamp.
Pharmacodynamic assessments
Venous blood samples for the determination of concentrations of insulin, glucose and C-peptide in plasma were taken during the study up to 6 h post-dose in each study period. The blood samples (4.5 ml) were taken at pre-dose (to establish a baseline), 0.25, 0.5, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0 and 6.0 h post-dose/infusion. All samples were centrifuged and transferred to polypropylene tubes (Nunc Cryotube) and stored at −80°C.
The concentration of C-peptide, insulin and glucose in human plasma was determined by Analytical Services International (ASI, London, UK) using commercially available solid phase, enzyme labelled chemiluminescent immunoassay and enzymatic (hexokinase) assay methods. For C-peptide and insulin, a solid phase (bead), enzyme-labelled chemiluminescent immunometric assay was performed on the Immulite 1000 auto-analyzer using a commercially available kit supplied by Siemens Healthcare Diagnostic Products. C-peptide results falling below 0.1 ng ml−1 and insulin results falling below 2.0 μIU ml−1 were reported as below the limit of quantification (BLQ). For glucose, the enzymatic (hexokinase) assay was performed on the Olympus AU400 auto-analyzer using a commercially available kit supplied by Randox.
Results
Subject demographics and disposition
After 24 subjects had completed the study, the SD for the single differences was estimated to be 8.5 ms assuming a treatment effect of 5 ms, and 7.5 ms for the time 0 values (assuming no treatment effect). Therefore, it was decided to recruit another eight subjects. Thus a total of 32 subjects were included in the study to be randomized to one of the eight treatment sequences. Subject demographics are presented in Table 3.
Table 3.
Subject demographics for the study
| Male (n = 18) | Female (n = 14) | |
|---|---|---|
| Age (years) | 26 ± 3.77 (20–34) | 27.8 ± 4.20 (21–34) |
| Height (cm) | 174 ± 6.52 (164–190) | 163.6 ± 7.44 (154–175) |
| Weight (kg) | 63.0 ± 7.29 (51.3–76.8) | 58.0 ± 6.35 (48.6–69) |
| BMI (kg m−2) | 20.8 ± 1.96 (18.4–24.6) | 21.6 ± 1.35 (18.3–23.1) |
Values are given as mean ± SD with ranges in parentheses.
Plasma concentrations of C-peptide, glucose and insulin at baseline
Average plasma concentrations of C-peptide, glucose and insulin at baseline for the different treatments (carbohydrate rich ‘continental’ breakfast, calorie reduced ‘American’ FDA breakfast and euglycaemic insulin clamp) are presented in Table 4.
Table 4.
Average baseline plasma concentration of C-peptide, glucose and insulin for different treatments
| Treatment | Average pre-dose concentration | ||
|---|---|---|---|
| C-peptide (ng ml−1) | Glucose (mmol l−1) | Insulin (μIU ml−1) | |
| Carbohydrate rich ‘continental’ breakfast | 1.12 | 5.34 | 3.96 |
| Calorie reduced ‘American’ FDA breakfast | 1.24 | 5.3 | 4.77 |
| Euglycaemic insulin clamp | 1.13 | 5.36 | 4.21 |
| Placebo | 1.21 | 5.33 | 4.84 |
Plasma concentrations of insulin, glucose and C-peptide following a carbohydrate rich ‘continental’ breakfast
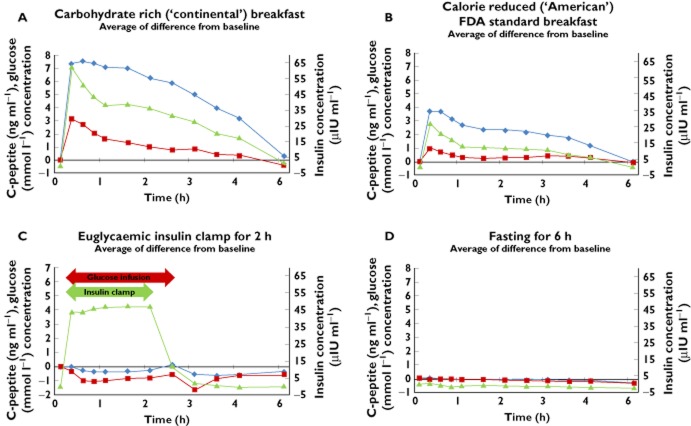
Following consumption of a carbohydrate rich ‘continental’ breakfast, plasma concentrations for insulin, glucose and C-peptide rose rapidly reaching maximum values at approximately 0.25 h post completion of breakfast (62.6 μIU ml−1 [insulin], 3.2 mmol l−1 [glucose], 7.3 ng ml−1 [C-peptide] change from baseline) (Figure 1A). Insulin and glucose plasma concentrations then showed a steady decline up to 1 h post-completion of breakfast, C-peptide plasma concentration was found to be steady up to 2.5 h post-completion of breakfast.
Figure 1.
Average change from baseline of insulin, glucose and C-peptide following carbohydrate rich ‘continental’ breakfast (A), calorie reduced ‘American’ FDA breakfast (B), euglycaemic insulin clamp (C) and placebo (D).  , C-peptide;
, C-peptide;  , Glucose;
, Glucose;  , Insulin
, Insulin
Plasma concentrations of insulin, glucose and C-peptide following a calorie reduced ‘American’ FDA breakfast
A similar pattern was also observed following consumption of a calorie reduced ‘American’ FDA breakfast with plasma concentrations approximately half those seen following a carbohydrate rich ‘continental’ breakfast. Plasma concentrations for insulin, glucose and C-peptide rose rapidly reaching maximum values at approximately 0.25 h post-completion of breakfast (27.2 μIU ml−1 [insulin], 1.0 mmol l−1 [glucose], 3.8 ng ml−1 [C-peptide] change from baseline) (Figure 1B). Insulin, glucose and C-peptide plasma concentrations then showed a steady decline up to 1 h post-completion of breakfast after which plasma concentrations were maintained at a steady level up 2.5 h.
Plasma concentrations of insulin, glucose and C-peptide following an insulin euglycaemic clamp
The glucose target concentration during the clamp was the basal blood glucose minus 0.5 mmol l−1 if the blood glucose was 5.0 mmol l−1 or above or the basal blood glucose minus 0.3 mmol l−1 if the basal blood glucose was below 5.0 mmol l−1. Following administration of the insulin euglycaemic clamp, the plasma concentration of insulin rose rapidly, reaching a value of 43.7 μIU ml−1 within approximately 0.25 h (Figure 1C). This concentration was maintained for the remainder of the clamp time (up to 2 h post-infusion start) due to the continuous infusion of glucose. Plasma glucose concentrations were noted to have decreased during the first 0.5 h (−0.3 mml l−1) which was then maintained until the end of the clamp. Plasma C-peptide concentrtions did not change and endogenous release was suppressed allowing the separation of insulin, glucose and C-peptide effects.
Plasma concentrations of insulin, glucose and C-peptide following placebo
Under placebo conditions there was no change observed from baseline for insulin, glucose and C-peptide concentrations (Figure 1D).
Effects on QTcF: confirmatory analysis
The three relevant confirmatory null hypotheses could all be rejected on the 5% level (one sided), i.e. a difference in QTcF between ‘continental’ breakfast and placebo and between ‘American’ FDA breakfast and placebo could be ascertained. Furthermore, it could be ascertained that the effect of insulin under the euglycaemic clamp at 1.5 h was too small to be of any relevance.
Effect of the euglycaemic clamp on QTcF
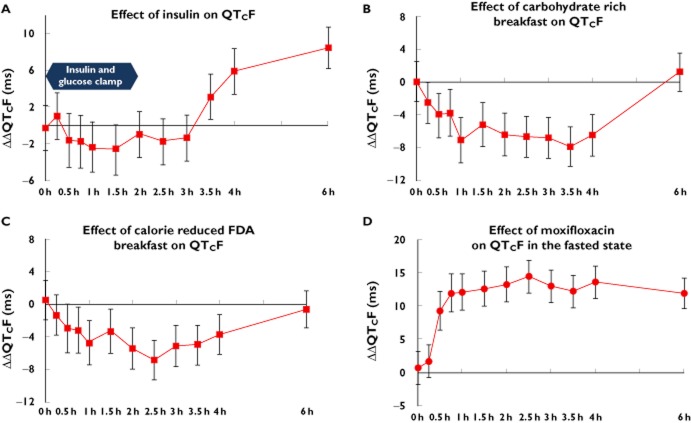
The point estimates of the difference between the euglycaemic clamp compared with placebo on QTcF were small and ranged from −0.3 to −1.4 ms showing a small shortening of QTcF. The maximum QTcF shortening was observed at 1.5 h post-dose with a value of 2.6 ms (two-sided 90% CI −5.3, −0.2) (Figure 2A). The values observed were not significantly different from zero. It should be noted that a slight prolongation was observed at 0.25 h post infusion. The time course of the changes in QTcF did not follow those of insulin, but rather resembled those of glucose. The mean baseline QTcF interval value for euglycaemic clamp was 415.1 ms compared with 416.2 ms for placebo.
Figure 2.
Average change from baseline on QTcF following euglycaemic clamp (A), carbohydrate rich ‘continental’ breakfast (B), calorie reduced ‘American’ FDA breakfast (C) compared with placebo. Effect of 400 mg moxifloxacin in fasted state on QTcF (D)
Effect of carbohydrate rich ‘continental’ breakfast on QTcF
For the continental breakfast the maximum QTcF shortening was observed at 3.5 h post-dose with a value of 7.9 ms (two-sided 90% CI −10.4, −5.5) (Figure 2B). The mean baseline QTcF interval value for the ‘continental’ breakfast was 415.7 ms compared to placebo (416.2 ms).
Effect of calorie reduced ‘American’ FDA breakfast on QTcF
With the carbohydrate reduced ‘American’ style FDA breakfast, the maximum QTcF shortening was observed at 2.5 h post-dose with a value of 6.8 ms (two-sided 90% CI −9.3, −4.3) (Figure 2C). The mean baseline QTcF interval value for the ‘American’ FDA breakfast was 417.1 ms compared with placebo (416.2 ms).
Effect of moxifloxacin 400 mg on QTcF
The assay sensitivity was shown to be adequate. From 0.25 h to 6 h post-dose, the ΔΔQTcF change from baseline following moxifloxacin 400 mg given in fasting condition compared with placebo ranged from 1.7 to 14.4 ms with the lower bound of the 95% CI clearly above 5 ms. The largest QTcF change from baseline was observed at 2.5 h post-dose in the fasted state with a value of 14.4 ms (two-sided 90% CI 11.9, −16.8) (Figure 2D). The mean baseline QTcF interval value for moxifloxacin (fasted state) was 416.7 ms compared with placebo (416.2 ms).
Effects on heart rate
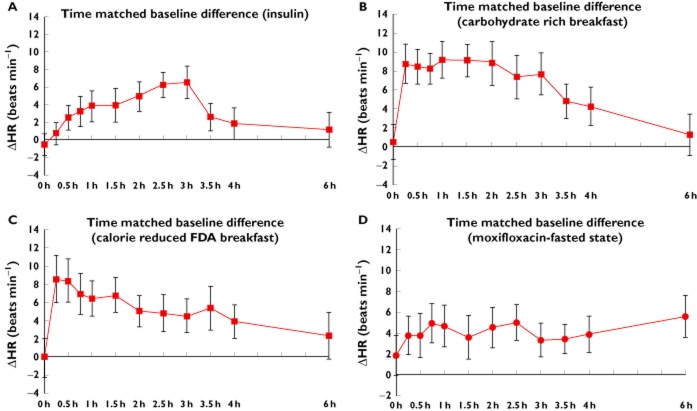
Since QTc and heart rate are closely linked, the same analysis as performed for QTcF was also repeated for heart rate. Results are given in Figure 3. For insulin a gradual increase in heart rate was observed up to 3 h (6.5 beats min–1), returning to near baseline values at the 6 h time point (Figure 3A). A steep increase in heart rate was observed for both the carbohydrate rich ‘continental’ and calorie reduced ‘American’ FDA breakfast. The maximum increase in change of heart rate was observed at 1 h following a carbohydrate rich breakfast (9.1 beats min–1) and 0:25 h following a calorie reduced ‘American’ FDA breakfast (8.5 beats min–1). It should be noted that the observed heart rate effect following a carbohydrate rich breakfast (Figure 3B) was greater in comparison with a calorie reduced ‘American’ FDA breakfast (Figure 3C). With a carbohydrate rich breakfast the change in heart rate stabilized after a steep increase and remained so up to 2 h before gradually returning to near baseline values. A similar trend was observed with a calorie reduced ‘American’ FDA breakfast but the magnitude of the effect was smaller. The magnitude of the observed change in heart rate for 400 mg moxifloxacin (fasted state) is shown in Figure 3D.
Figure 3.
Effect on heart rate with 95% CI on insulin euglycaemic clamp (A), carbohydrate rich ‘continental’ breakfast (B), calorie reduced ‘American’ FDA breakfast (C). Effect of 400 mg moxifloxacin (fasted state) on heart rate (D)
Concentration–response analysis for insulin, glucose and C-peptide
The effect on QTc was investigated using linear mixed effect models with placebo corrected QTcF (change from average baseline) as a dependent variable and insulin, glucose and C-peptide (placebo corrected) as covariates for the data obtained under the euglycaemic clamp as well as for all data obtained under the clamp and the two types of breakfast. The resulting regression coefficients (‘slopes’) are shown in Table 5. Insulin was shown to have no effect on QTc during the euglycaemic clamp, if the model controlled for the effect of glucose. The same holds in a model based on all data, if the model controlled for the influences of both glucose and C-peptide. However, C-peptide and glucose were found to have significant negative and positive effects on QTc, respectively, based on the data from all three treatment arms and all time points. The analysis predicts that an increase of C-peptide apparently causes a decrease in QTcF [−1.2 ms/(ng ml−1)] while an increase of glucose increases QTcF [1.6 ms/(mmol ml−1)) Since both concentrations are positively correlated, their effects antagonize one another. This can be demonstrated with the considerably smaller apparent effect of C-peptide of only 0.7 ms/(ng ml−1) obtained in a model ignoring glucose, i.e. when ascribing the joint effect of C-peptide and glucose to C-peptide only. Likewise, in a model ignoring C-peptide, the apparent effect of glucose appears to be reversed, i.e. the QTc-shortening effect of C-peptide dominates the QTc-prolonging effect of glucose.
Table 5.
Influence of insulin, C-peptide and glucose on QTcF
| Parameter | Units of slope | Data used | Controlling for | Estitmate | Lower | Upper |
|---|---|---|---|---|---|---|
| bound of 95% CI | ||||||
| Insulin | ms/(μIU ml−1) | Clamp | Glucose | −0.01 | −0.06 | 0.05 |
| All | Glucose and C-peptide | 0.01 | −0.02 | 0.04 | ||
| C-peptide | ms/(ng ml−1) | All | Glucose | −1.24 | −1.59 | −0.90 |
| All | None | −0.71 | −0.98 | −0.45 | ||
| Glucose | ms/(mmol ml−1) | Clamp | Insulin | 1.90 | 0.07 | 3.73 |
| All | C-peptide | 1.57 | 0.92 | 2.22 | ||
| All | None | −0.86 | −1.45 | −0.27 | ||
All models use difference from placebo values, QTcF is change from average baseline. Based on linear mixed effects models.
Figure 4 and C illustrates this relationship. The influence of insulin on QTcF during the euglycemic clamp is shown in Figure 4A. In Figure 4B the effect of C-peptide is shown for various concentrations of glucose (aimed at to be kept constant). In addition, the broken line gives the apparent effect of C-peptide if the influence of glucose is disregarded by the model. Figure 4C shows the same for glucose. In addition to the influence of glucose for several fixed concentrations of C-peptide and the apparent effect of glucose estimated in a model that does not control for the influence of C-peptide, the relationship found under the euglycaemic clamp is also added as a dotted line. It should be noted that this relationship, established in a situation where the concentrations of C-peptide were kept constant, in reality, agrees well with those that were established by a model that simultaneously models the influence of both substances. Figure 5 shows a three-dimensional plot of the change in ΔΔQTcF (ms) as a function of glucose (mmol ml−1) and C-peptide (ng ml−1).
Figure 4.
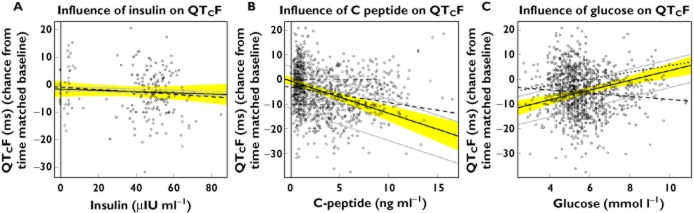
Plot of ΔΔQTcF (ms) against change from time matched placebo of insulin (A), C-peptide (B) and glucose (C) concentration. The solid black lines give the regression lines for insulin after the correction for glucose (A), C-peptide after correction for glucose (B) and for glucose after the correction for C-peptide (C), which are taken as the mean values across the data. The yellow area is the corresponding 95% CI. (A) The grey lines give the predictions for maximum and minimum mean glucose seen over time (change from placebo). The dashed line gives the regression without correction for glucose. (B) The grey lines give the predictions for the glucose concentration seen under placebo and for the maximum mean change of glucose across all time points in the carbohydrate-rich breakfast condition, which occurred at 15 min. The dashed line gives the regression line obtained for C-peptide without correction for glucose. (C) The grey lines give the predictions for the C-peptide level seen under placebo and for the maximum mean change of C-peptide across all time points in the carbohydrate-rich breakfast condition, which occurred at 30 min. The dashed line gives the regression line obtained for glucose without correction for C-peptide. The dotted black line gives the relationship between change of glucose from time matched placebo and ΔΔQTcF established under the euglycaemic clamp. (A) Is based on data obtained under the euglycaemic clamp (time points up to 1.5 h), (B) and (C) are based on all time points of all three regimens (euglycaemic clamp, carbohydrate rich ‘continental’ breakfast and calorie reduced ‘American’ FDA breakfast)
Figure 5.
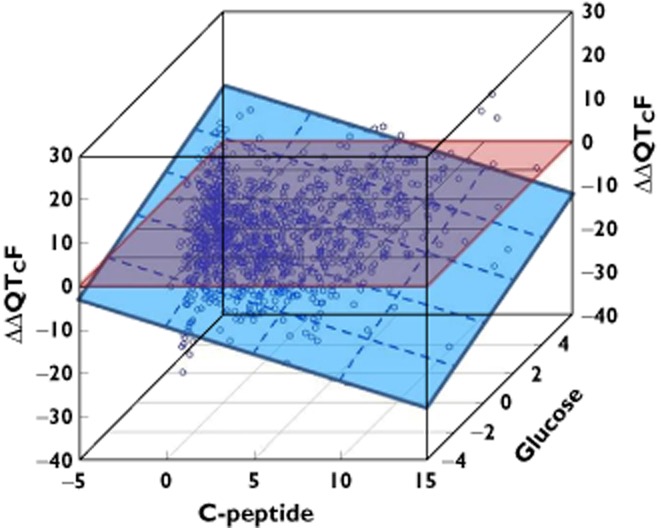
Change in ΔΔQTc (ms) as a function of glucose (mmol ml−1) and C-peptide (ng ml−1). The dashed blue plane shows a concentration dependent shortening with C-peptide and prolongation with glucose
Discussion
As per the ICH E14 guideline [14], the majority of TQT studies use moxifloxacin 400 mg as a positive control to demonstrate assay sensitivity by utilizing its well characterized QTc prolonging effect compared with placebo [8, 15–18]. A positive control is included in these studies to ensure that a TQT study can demonstrate a change in QTc of approximately 5 ms. Moxifloxacin has been shown to produce changes in QTc which are greater and this has led to suggestions of having positive controls which produce smaller effects i.e. closer to that suggested in the guidelines [16]. With the actual effect of moxifloxacin it is considered necessary to ensure that the lower bound of the lower 90% CI excludes 5 ms at least at one time point [16, 19], thereby addressing the concern that a study showing a small moxifloxacin effect of only 5 ms following a 400 mg oral dose would obviously not be indicative of a sufficiently sensitive study able to detect small changes in QTc.
The reported effect of food on QTc provides potential for its use as a positive control in thorough ECG studies and possibly as an alternative to moxifloxacin. One of the first studies to report the relationship between consumption of a standardized meal under the rigorous conditions of a TQT study demonstrated a maximum QTcF shortening of approximately 8.2 ms [8]. The carbohydrate content of the meal appears to be important since a carbohydrate rich meal has been associated with endogenous physiological insulinaemia [6] and two studies have demonstrated increased heart rate and QTcB in response to increased insulin concentrations [6, 20]. In the study by Scott et al. [6], heart rate increased by 17% and was correlated with insulin concentration. However, only heart rate (not QTc) and insulin measurements were provided in that study.
If the expectation that post-prandial insulinaemia has a role in the observed effects of food on ECG is correct, then meals with high levels of carbohydrates would be expected to show a greater effect. Three studies reporting QTc effects have used meals with higher carbohydrate content (68% [8], 57% [9], 53% [3]) compared with, for example, an FDA standard breakfast which has a greater fat (58%) content and a much lower carbohydrate content. This difference in carbohydrate content may lead to a difference in QTc effect observed between the meals but there is currently only limited data to suggest that this is the case.
The exact effects of insulin on the QTc interval have been investigated in this study. C-peptide and insulin are excreted in equimolar amounts [20] following a carbohydrate challenge. It has been demonstrated that administration of C-peptide to type I diabetic patients with neuropathy shortens the QTc [12]. To our knowledge, the data presented in the current study are the first to report the relationship of insulin, glucose and C-peptide following a meal conducted under the conditions of a TQT study. The results show that following ingestion of a carbohydrate rich ‘continental’ breakfast or a calorie reduced ‘American’ FDA standard breakfast, a rapid increase in insulin and C-peptide concentrations occurs. What is apparent is the transient nature of the insulin increase, reaching maximum physiological concentrations almost immediately and then decreasing to half the concentrations by 1 h post ingestion. Of interest is the prolonged steady C-peptide concentration lasting up to 2 h post-ingestion, particularly with the carbohydrate rich ‘continental’ breakfast. Insulin at physiological levels was shown to have no effect on QTcF during the euglycaemic clamp period. This finding contrasts with the finding of Gastaldelli et al. [10] who have reported a QTc prolongation with increased concentrations of insulin. However, in the study by Gastadelli et al. [10], an increase in heart rate of up to 71 beats min–1 was demonstrated. It is known that the use of the Bazett's correction formula (QTcB) leads to false QTc prolongations [11], i.e. these are merely due to changes in heart rate rather than a true change in the QT/RR relationship. As far as the food effect was concerned [8], similar results to those observed by Gastaldelli et al. [10] were observed up to 2 h i.e. increase in the QTcB. However, in the food effect study there was a shortening of QTcB at 4 h post-dose of about 4 ms [8]. This is important because Gastaldelli et al. [10] did not report QTcB after 2 h and also failed to demonstrate a shortening effect on the uncorrected QT. Although, it has been reported that QTcB is unsuitable when dealing with heart rate changes [11], QTcB is still widely used in the clinic and false positives have also been demonstrated for other studies [3, 21]. Our findings showed that shortening of the QTc was correlated to an increase in heart rate. The greatest change in heart rate was observed during a carbohydrate rich ‘continental’ breakfast which correspondingly had the greatest QTcF shortening effect. Notably, for insulin the increase in heart rate followed by a decrease after 3 h to near baseline values corresponded to the changes in QTcF that were observed. As reported previously [8], the effect on QT appears to be greater than proportional and becomes noticeable when applying heart rate correction factors such as QTcF.
In concentration–effect modelling, C-peptide emerges as the most likely candidate to be responsible for the QTc shortening observed following ingestion of a carbohydrate rich ‘continental’ breakfast in this study and where reported previously [8]. The concentration–response analysis has confirmed a QTcF shortening effect with increasing C-peptide concentration. It further appears that the C-peptide (QT shortening) effect couples with the antagonizing (prolongation) effects of glucose. Further investigations are required to assess the direct impact of C-peptide on QTc change under TQT conditions and understanding its role would allow the use of concentration–response models. This finding is of importance because it has been shown in another study with type 1 diabetic patients that increases in glucose can shorten the QTc [22]. However this study did not control for C-peptide. Understanding the role of glucose and C-peptide might also lead to improved prophylaxis in the cardiac complications associated with patients with diabetes. It is well documented that patients with insulin dependent diabetes are prone to sudden arrhythmias that can lead to ‘dead in bed’ syndrome due to QTc prolongation. It could be surmised that because glucose by itself may prolong, with C-peptide antagonizing, the effects on the QTc interval, diabetic patients and particularly those prone to ‘dead in bed’ syndrome may further benefit from tight glucose regulation and may benefit from C-peptide treatment.
There is sufficient evidence in the literature to suggest that the food effect observed in this study is well reproducible both in terms of magnitude of effect and time course. We were able to replicate the same ECG effects in two TQT studies so far. Moreover, it compares favourably with other non-pharmacological methods of experimentally prolonging QTc such as temporary changes in posture as it is not compromised by QT/RR hysteresis and is easier to control than an exercise protocol in an environment that should be free of autonomic effects on the heart [3, 6, 9]. Our findings (Figure 2A) also suggest that late changes after an insulin clamp may lead to significant increases in the QTc interval. Significant increases in the QTc interval were observed at the 4 and 6 h time points, i.e. 2–4 h after the end of the insulin clamp. These changes might be associated with fluctuating serum potassium concentrations. Indeed, the effect of insulin on the QTc as reported by Gastaldelli et al. [10] was postulated to have been mediated by the decrease of serum potassium concentrations with concomitant increase in norepinephrine concentrations. This leads to the notion that insulin augments cellular potassium uptake and hyperpolarization of the cell membrane. However, such changes attributed to serum concentrations of potassium should be treated with caution. For example, in another study [23], it was shown that adjustment for fasting insulin or potassium concentration did not explain the change in QTc. It might be surmised that glucose could itself be implicated in the mechanisms of suboptimal regulation of cardiac ion channels involved in ventricular repolarization and thereby modulate the QT interval. Further work would be necessary to unravel the precise mechanism(s).
In summary, this study demonstrates that there was no change in QTc during the euglycaemic clamp. Given that insulin was raised to physiological concentrations comparable with those seen after a meal, whilst the release of C-peptide was suppressed, insulin appears to have no effect on the QTc interval in either direction. From these data it could be postulated that some type of relationship might exist between shortening of QTc and C-peptide concentrations. There is no doubt that further work would be necessary to tease out the precise mechanism by which this interplay could occur.
Acknowledgments
We would like to thank Dilshat Djumanov for data management, Juleen Gayed for medical work and Carike Coetzee for organizing the trial.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Thwaites BC, Bose M. Very low calorie diets and pre-fasting prolonged QT interval. A hidden potential danger. West Indian Med J. 1992;41:169–171. [PubMed] [Google Scholar]
- 2.Swenne I, Larsson PT. Heart risk associated with weight loss in anorexia nervosa and eating disorders: risk factors for QTc interval prolongation and dispersion. Acta Paediatr. 1999;88:304–309. doi: 10.1080/08035259950170079. [DOI] [PubMed] [Google Scholar]
- 3.Nagy D, DeMeersman R, Gallagher D, Pietrobelli A, Zion AS, Daly D, Heymsfield SB. QTc interval (cardiac repolarization): lengthening after meals. Obes Res. 1997;5:531–537. doi: 10.1002/j.1550-8528.1997.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 4.Hulhoven R, Rosillon D, Bridson WE, Meeus MA, Salas E, Stockis A. Effect of levetiracetam on cardiac repolarization in healthy subjects: a single-dose, randomized, placebo- and active-controlled, four-way crossover study. Clin Ther. 2008;30:260–270. doi: 10.1016/j.clinthera.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Bloomfield D, Kost J, Ghosh K, Hreniuk D, Hickey L, Guitierrez M, Gottesdiener K, Wagner J. The effect of moxifloxacin on QTc and implications for the design of thorough QT studies. Clin Pharmacol Ther. 2008;84:475–480. doi: 10.1038/clpt.2008.33. [DOI] [PubMed] [Google Scholar]
- 6.Scott EM, Greenwood JP, Vacca G, Stoker JB, Gilbey SG, Mary D. Carbohydrate ingestion, with transient endogenous insulinaemia, produces both sympathetic activation and vasodilatation in normal humans. Clin Sci. 2002;102:523–529. [PubMed] [Google Scholar]
- 7.Lu C, Zou X, Orr WC, Chen JDZ. Postprandial changes of sympathovagal balance measured by heart rate variability. Dig Dis Sci. 1999;44:857–861. doi: 10.1023/a:1026698800742. [DOI] [PubMed] [Google Scholar]
- 8.Taubel J, Wong AH, Naseem A, Ferber G, Camm AJ. Shortening of the QT interval after food can be used to demonstrate assay sensitivity in thorough QT studies. J Clin Pharmacol. 2011 doi: 10.1177/0091270011419851. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Widerlov E, Jostell K, Claesson L, Odlind E, Keisu M, Freyschuss U. Influence of food intake on electrocardiograms of healthy male volunteers. Eur J Clin Pharmacol. 1999;55:619–624. doi: 10.1007/s002280050682. [DOI] [PubMed] [Google Scholar]
- 10.Gastaldelli FA, Emdin M, Conforti F, Camastra S, Ferrannini E. Insulin prolongs the QTc interval in humans. Am J Physiol Regul Integr Comp Physiol. 2000;279:R2022–2025. doi: 10.1152/ajpregu.2000.279.6.R2022. [DOI] [PubMed] [Google Scholar]
- 11.Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411–420. doi: 10.1046/j.1540-8167.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 12.Wahren J, Ekbery K, Jornvall H. C-peptide and neuropathy in type 1 diabetes. Immunol Endocr Metab Agents Med Chem. 2007;7:69–77. [Google Scholar]
- 13.R Development Core Team. 2011. R: a language and environment for statistical computing [online]. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, Available at http://www.R-project.org/ (last accessed May 2012)
- 14.ICH Harmonized Tripartite Guideline E14. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs. International Conference on Harmonization, Step 4, Guideline, EMEA, CHMP/ICH/2/04, 2005.
- 15.Dixon R, Job S, Oliver R, Tompson D, Wright JG, Maltby K, Lorch U, Taubel J. Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br J Clin Pharmacol. 2008;66:396–404. doi: 10.1111/j.1365-2125.2008.03250.x. Epub 2008 Jul 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taubel J, Naseem A, Harada T, Wang D, Arezina R, Lorch U, Camm AJ. Levofloxacin can be used effectively as a positive control in thorough QT/QTc studies in healthy volunteers. Br J Clin Pharmacol. 2009;69:391–400. doi: 10.1111/j.1365-2125.2009.03595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demolis JL, Kubitza D, Tenneze L, Funck-Brentano C. Effect of a single oral dose of moxifloxacin (400mg and 800mg) on ventricular repolarization in healthy subjects. Clin Pharmacol Ther. 2000;68:658–666. doi: 10.1067/mcp.2000.111482. [DOI] [PubMed] [Google Scholar]
- 18.Florian J, Tornøe C, Brundage R, Parekh A, Garnett C. Population pharmacokinetic and concentration-QTc models for moxifloxacin: pooled analysis of 20 thorough QT studies. J Clin Pharmacol. 2011;51:1152–1162. doi: 10.1177/0091270010381498. Epub 2011 Jan 12. [DOI] [PubMed] [Google Scholar]
- 19.ICH Harmonized Tripartite Guideline E14. The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. International conference on harmonisation, E14 Implementation and working group, Question and Answers, 2008.
- 20.Johansson BL, Borg K, Fernqvist-Forbes E, Odergren T, Remahl S, Wahren J. C-peptide improves autonomic nerve function in IDDM patients. Diabetologia. 1996;39:687–695. doi: 10.1007/BF00418540. [DOI] [PubMed] [Google Scholar]
- 21.Browne KF, Zipes DP, Heger JJ, Prystowsky EN. Influence of the autonomic nervous system on the Q-T interval in man. Am J Cardiol. 1982;50:1099–1103. doi: 10.1016/0002-9149(82)90425-8. [DOI] [PubMed] [Google Scholar]
- 22.Suys B, Heuten S, De Wolf D, Verherstraeten M, de Beeck LO, Matthys D, Vrints C, Rooman R. Glycemia and corrected QT interval prolongation in young type 1 diabetic patients: what is the relation? Diabetes Care. 2006;2:427–429. doi: 10.2337/diacare.29.02.06.dc05-1450. [DOI] [PubMed] [Google Scholar]
- 23.Dekker JM, Crow RS, Hannan PJ, Schouten EG, AR F, Study ARIC. Heart rate-corrected QT interval prolongation predicts risk of coronary heart disease in Black and White middle-aged men and women: the ARIC study. J Am Coll Cardiol. 2004;18:565–571. doi: 10.1016/j.jacc.2003.09.040. [DOI] [PubMed] [Google Scholar]