Tumour lysis syndrome (TLS) is a life-threatening oncological emergency characterized by hyperuricaemia, hyperkalaemia, hyperphosphataemia, and hypocalcaemia [1, 2] due to the rapid lysis of malignant cells, following the initiation of anticancer therapies [3].
Traditionally, therapy for TLS involved intensive hydration, urinary alkalinization and administration of allopurinol [4–6]. Newer guidelines now include rasburicase, with monitoring of electrolytes, white blood cell counts (WCC) and lactate dehydrogenase (LDH) concentrations [1, 7, 8].
Rasburicase, a recombinant urate oxidase enzyme, effectively decreases existing serum uric acid (UA) by oxidizing it to allantoin which is readily soluble and excretable [3]. Although the recommended dose is 0.2 mg kg−1 day−1 for 5–7 days [9], studies have shown the efficacious use of reduced doses for shorter periods of time and subsequent cost savings [5, 6, 10–17]. Expert guidelines by Coieffer et al. [7] in 2008 and Cairo et al. in 2010 [1] on the management of TLS recommend a rasburicase dose of 0.1–0.2 mg kg−1 on the first day, then repeated for up to 7 days [1] or as necessary [7].
We present an analysis of a fixed 3 mg dose of rasburicase administered to adult patients, treated at a tertiary referral centre. The study was approved by the Alfred Health Human Research Ethics Committee and the Monash University Human Research Ethics Committee.
Demographic data were collected. Biochemical parameters (serum creatinine, serum UA, phosphate and LDH concentrations), at baseline, 24 h and 72 h after initial administration of rasburicase were recorded and compared.
The institution guideline indicates rasburicase to be given before the first dose of chemotherapy in patients considered high risk for TLS. This includes a diagnosis of Burkitt's lymphoma, acute lymhoblastic leukaemia, bulky non-Hodgkin's lymphoma, lymphoblastic lymphoma or acute myeloid leukaemia with one or more of the following: serum UA>0.46 mmol l−1, white cell count (WCC) >50 × 109 l−1 or LDH >two times normal. Patients who were at an ongoing risk of TLS (i.e. elevated UA or LDH or multiple days of aggressive cytoreductive chemotherapy) were allowed a repeat dose of rasburicase 3 mg. Adherence to the guideline was measured.
Forty-one patients received 42 courses of rasburicase over a 40 month period (Figure 1A). Diagnosis, demographic and baseline biochemical data are presented in Table 1.
Figure 1.
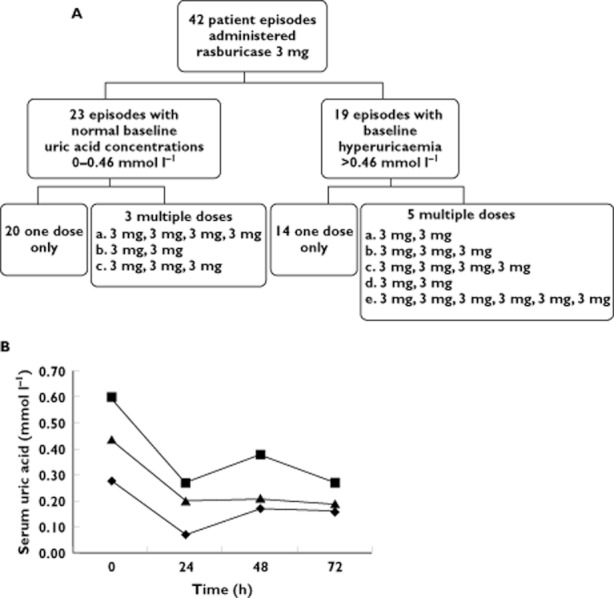
Summaryof rasburicase courses and uric acid concentrations. A) Summary of rasburicase courses administered. B) Median uric acid concentrations over time stratified by presentation a baseline. ♦, normal; ▪, hyperuricaemic; ▴, all patients
Table 1.
Patientcharacteristics
| n | 42 |
| Male gender | 29 (71%) |
| Median (range) | |
|---|---|
| Age (years) | 50 (20–81) |
| Weight (kg) | 72 (35–118) |
| Diagnosis | n (%) |
|---|---|
| Acute lymhocytic leukaemia | 13 (32%) |
| Acute myeloid leukaemia | 12 (29%) |
| Burkitt's lymphoma | 10 (24%) |
| Non-Hodgkin's lymphoma | 3 (7%) |
| Other leukaemia | 2 (4%) |
| Other lymphoma | 2 (4%) |
| Other treatments for TLS | n (%) |
|---|---|
| Allopurinol | 41 (97.5%) |
| Hydration | 42 (100%) |
| Alkalinization | 28 (67.5%) |
| Baseline biochemistry (normal range) | Median (range) |
|---|---|
| Uric acid (0–0.46 mmol l−1) | 0.44 mmol l−1 (0.13–2.0) |
| Creatinine (60–105 μmol l−1) | 88 μmol l−1 (44–657) |
| Phosphate (0.60–1.30 mmol l−1) | 1.26 mmol l−1 (0.22–2.36) |
| Lactic dehydrogenase (125–255 U l−1) | 834 U l−1 (215–10563) |
| White cell count | % patient episodes |
|---|---|
| 50–100 × 109 l−1 | 20% |
| >100 × 109 l−1 | 27% |
Rasburicase was administered as per institution guidelines in 40 (95%) of the patients. Median serum UA concentrations were within normal range at 72 h in all groups; in those who presented with hyperuricaemia, in those who presented with normal baseline serum UA concentrations and overall (Figure 1B).
The majority of patients received one dose of rasburicase 3 mg (Figure 1A). In 34 patient episodes requiring one dose only, there was a decline in the median (range) UA concentration from 0.44 mmol l−1 (0.13–1.15) at baseline to 0.22 mmol l−1 (0.02–0.66) at 24 h. This decrease was maintained at 72 h (P < 0.0001) with a median of 0.21 mmol l−1 (0.02–0.52). Serum creatinine concentrations were within normal range (60–105 μmol l−1) at baseline in 74% of patients, with 82% having a normal creatinine at 72 h. Hyperphosphataemia was present in 29% of patients at baseline and increased to 44% at 72 h.
Eight patient episodes required more than one dose due to the ongoing risk of TLS. In these patients the median (range) baseline UA was 0.50 mmol l−1 (0.02–2.0), 0.33 mmol l−1 (0.02−1.10) at 24 h and 0.24 mmol l−1 (0.02−1.10) at 72 h (P < 0.0001). Of these patients only 52% had a normal creatinine at baseline, increasing to 83% at 72 h. Mean phosphate concentrations decreased over time but all patients remained hyperphosphataemic at 72 h.
No hypersensitivity reactions were noted, no patients required haemodialysis and no deaths were related to the administration of rasburicase.
Our results demonstrate that a single fixed dose of rasburicase 3 mg, repeated if required, should be the standard regimen in the management of TLS.
Recent studies and published guidelines have shown cumulative support for the safe and efficacious use of off-label dosing regimens of rasburicase [1, 5–8, 10, 11, 16, 17]. A quarter of our patients presented with a baseline WCC>100 × 109 l−1 (Table 1), which is considered a high risk for developing TLS [1, 7]. The Product Information recommends rasburicase 0.1–0.2 mg kg−1 day−1 for 1–7 days [9]. We successfully used a fixed 3 mg dose for these patients.
Our data support that presented by Trifilio et al. [11] in a recent study of 287 episodes, the largest published series at this time, of raised UA concentrations successfully treated with a single 3 mg dose of rasburicase, repeated if required. In our cohort, which was smaller in size, a single 3 mg dose was equally effective in both patients who had a normal baseline UA and those with hyperuracaemia. This differed from that published by Trifilio et al., where the single dose was more successful in patients with a lower baseline UA concentration. Our patient cohort also had a higher median LDH.
Suboptimal management of hyperphosphatemia was identified in our cohort. More stringent monitoring of patient phosphate concentrations may be warranted in the future to minimize the risk of renal impairment. Serum creatinine, showing a gradual decrease with time, was used as a surrogate maker to indicate an improvement in renal function. Rasburicase was used in conjunction with allopurinol, urinary alkalinazation and intravenous hydration. This strategy is also supported by recent studies and recommendations [1, 11, 16], although the benefit of administering alkalinization with rasburicase needs further investigation [1, 7].
A single fixed 3 mg dose of rasburicase, in the setting of an institution guideline, was efficacious in the management of TLS.
Competing Interests
There are no competing interests to declare.
References
- 1.Cairo MS, Coiffier B, Reiter A, Younes A. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. Br J Haematol. 2010;149:578–586. doi: 10.1111/j.1365-2141.2010.08143.x. [DOI] [PubMed] [Google Scholar]
- 2.Cheuk DK, Chiang AK, Chan GC, Ha SY. Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer. Cochrane Database Syst Rev. 2010;(6) doi: 10.1002/14651858.CD006945.pub2. CD006945. [DOI] [PubMed] [Google Scholar]
- 3.Mughal TI, Foringer JR, Ejaz A, Coiffier B. An integrated clinical approach for the identification, prevention, and treatment of tumour lysis syndrome. Cancer Treat Rev. 2010;36:164–176. doi: 10.1016/j.ctrv.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Pui C, Jeha S, Irwin D. Recombinant urate oxidase in the prevention and treatment of malignancy-associated hyperuricemia in pediatric and adult patients: results of a compassionate-use trial. Leukemia. 2001;15:1505–1509. doi: 10.1038/sj.leu.2402235. [DOI] [PubMed] [Google Scholar]
- 5.Hummel M, Reiter S, Adam K, Hehlmann R, Buchheidt D. Effective treatment and prophylaxis of hyperuricemia and impaired renal function in tumor lysis syndrome with low doses of rasburicase. Eur J Haematol. 2008;80:331–336. doi: 10.1111/j.1600-0609.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- 6.Giraldez M, Puto K. A single, fixed dose of rasburicase (6 mg maximum) for treatment of tumor lysis syndrome in adults. Eur J Haematol. 2010;85:177–179. doi: 10.1111/j.1600-0609.2010.01457.x. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumours lysis syndrome: an evidence based review. J Clin Oncol. 2008;28:2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 8.Gertz MA. Managing tumour lysis syndrome in 2010. Leuk Lymphoma. 2010;51:179–180. doi: 10.3109/10428190903488788. [DOI] [PubMed] [Google Scholar]
- 9.Sanofi-Aventis. Fasturtec (Rasburicase) Approved Product Information. Macquarie Park: Sanofi-Aventis Australia Pty Ltd; 2010. [Google Scholar]
- 10.Steel S, Coutsouvelis J, McKendrick J. Single dose rasburicase in tumour lysis: one hospitals' experience. Asia Pac J Clin Oncol. 2008;4:18–20. [Google Scholar]
- 11.Trifilio SM, Pi J, Zook J, Golf M, Coyle K, Greenberg D, Newman D, Koslosky M, Mehta J. Effectiveness of a single 3-mg rasburicase dose for the management of hyperuricemia in patients with hematological malignancies. Bone Marrow Transplant. 2011;46:800–805. doi: 10.1038/bmt.2010.212. [DOI] [PubMed] [Google Scholar]
- 12.Trifilio S, Gordon L, Singhal S, Tallman M, Evens A, Rashid K, Fishman M, Masino K, Pi J, Mehta J. Reduced-dose rasburicase (recombinant xanthine oxidase) in adult cancer patients with hyperuricemia. Bone Marrow Transplant. 2006;37:997–1001. doi: 10.1038/sj.bmt.1705379. [DOI] [PubMed] [Google Scholar]
- 13.Hummel M, Buchheidt D, Reiter S, Bergmann J, Adam K, Hehlmann R. Recurrent chemotherapy-induced tumor lysis syndrome (TLS) with renal failure in a patient with chronic lymphocytic leukemia – successful treatment and prevention of TLS with low-dose rasburicase. Eur J Haematol. 2005;75:518–521. doi: 10.1111/j.1600-0609.2005.00550.x. [DOI] [PubMed] [Google Scholar]
- 14.Knoebel RW, Lo M, Crank CW. Evaluation of a low, weight-based dose of rasburicase in adult patients for the treatment or prophylaxis of tumor lysis syndrome. J Oncol Pharm Pract. 2011;17:147–154. doi: 10.1177/1078155210364180. [DOI] [PubMed] [Google Scholar]
- 15.Eaddy M, Seal B, Tangirala M, Davies EH, O'Day K. Economic comparison of rasburicase and allopurinol for treatment of tumour lysis syndrome in pediatric patients. Am J Health Syst Pharm. 2010;67:2110–2114. doi: 10.2146/ajhp100022. [DOI] [PubMed] [Google Scholar]
- 16.Darmon M, Guichard I, Vincent F. Rasburicase and tumor lysis syndrome: lower dosage, consideration of indications, and hyperhydration. J Clin Oncol. 2011;29:67–68. doi: 10.1200/JCO.2010.32.6751. [DOI] [PubMed] [Google Scholar]
- 17.Chiang J, Chan A, Lian T, Tay K, Quek R, Tao M, Lim ST. Management of tumour lysis syndrome with a single fixed dose of rasburicase in Asian lymphoma patients: a case series and literature review. Asia Pac J Clin Oncol. 2011;7:351–356. doi: 10.1111/j.1743-7563.2011.01464.x. [DOI] [PubMed] [Google Scholar]


