Abstract
Background and Purpose
A series of benzothiazole derivatives were screened for immunosuppressive activity; of these compounds BD750 was found to be the most effective immunosuppressant. The purpose of the current study was to determine the immunosuppressive activity of BD750 on T cell proliferation and its potential mode of action.
Experimental Approach
T cell proliferation, CD25 and CD69 expression and cell cycle distribution were measured in vitro by flow cytometry. Cell viability was determined by CCK-8 assay. Cytokine levels were measured by elisa. The activation of signal-regulated molecules was assessed by Western blot analysis. The effects of BD750 were evaluated in vivo in a mouse model of delayed-type hypersensitivity.
Key Results
BD750 significantly inhibited mouse and human T cell proliferation, stimulated either by anti-CD3/anti-CD28 monoclonal antibodies or by an alloantigen, in a dose-dependent manner in vitro. No obvious cytotoxic effects of BD750 were observed in our experimental conditions. Furthermore, BD750 did not inhibit CD25 and CD69 expression or IL-2 and IL-4 secretion, but induced cell cycle arrest at the G0/G1 phase in activated T cells. In IL-2-stimulated CTLL-2 cells and primary activated T cells, BD750 inhibited cell proliferation and STAT5 phosphorylation, but not Akt or p70S6K phosphorylation. BD750 also reduced the T cell-mediated delayed-type hypersensitivity response in mice in a dose-dependent manner.
Conclusion and Implications
These data indicate that BD750 inhibits IL-2-induced JAK3/STAT5-dependent T cell proliferation. BD750 has the potential to be used as a lead compound for the design and development of new immunosuppressants for preventing graft rejection and treating autoimmune diseases.
Keywords: benzothiazol derivate, immunosuppressants, T cell proliferation, JAK3/STAT5 signal pathway
Introduction
T cells play a pivotal role in immune responses against pathogens, and aberrant T cell responses can also mediate organ transplantation rejection and a variety of autoimmune diseases, including rheumatoid arthritis (RA), systemic lupus erythematosus (SLE), multiple sclerosis (MS) and ulcerative colitis (Ponticelli, 2011). Engagement of T cell receptor (TCR) on T cells by an antigen in the presence of sufficient co-stimulation signalling can induce T cell activation, functional differentiation and proliferation as well as cytokine production. Hence, inhibition of T cell activation and proliferation has been demonstrated to modulate T cell-mediated immunopathogenesis (Halloran, 2004). Currently, there are several immunosuppressants available in the clinic, for the prevention of graft rejection and autoimmune diseases, which inhibit T cell activation and proliferation by different mechanisms (Stucker and Ackermann, 2011). For example, cyclosporin A (CsA) and FK506 inhibit calcineurin activity (Liu et al., 1991). Rapamycin (RAPA) is an inhibitor of mTOR (Brown et al., 1994), and mycophenolic acid (MPA) inhibits inosine monophosphate dehydrogenase and de novo GTP biosynthesis (Senda et al., 1995). However, the use of these immunosuppressants is limited by their toxicity and propensity to induce tolerance (Changelian et al., 2003; Marcen, 2009). Therefore, it is important to keep looking for novel safe and effective immunosuppressants.
TCR-mediated T cell signalling can induce T cell activation through the calcium/calcineurin pathway (Sigal and Dumont, 1992), the MAPK pathway (Franklin et al., 1994) and the PKC/NF-κB pathway(Wang et al., 2004), promoting downstream gene expression. Activated T cells can produce cytokines, such as IL-2 and IL-4. Growth factors, such as IL-2, bind with their receptors to promote activated T cell proliferation with nucleotide synthesis through the JAK3/STAT5 signalling pathway (Pesu et al., 2008), PI3K/AKT pathway (Xie et al., 2007) and mammalian target of rapamycin (mTOR)/p70S6K pathway (Abraham and Wiederrecht, 1996). In recent years, the JAK3/STAT5 signalling pathway has attracted significant attention as a potential target of new immunosuppressants. Mutation of JAK3 in mice and humans results in immunodeficiency, indicating the pivotal role of the JAK3/STAT5 signalling pathway in the immune system (Ghoreschi et al., 2009). Several new immunosuppressants that inhibit the JAK3/STAT5 signalling pathway have been developed and currently evaluated in clinical trials; these include CP690550 (van Gurp et al., 2009; Flanagan et al., 2010; Patel et al., 2011), R348(Deuse et al., 2008; Velotta et al., 2009) and PNU156804 (Xiong et al., 2010).
Benzothiazole is an aromatic heterocyclic compound and many of its derivatives have been shown to have favourable bioactivities, such as inducing tumour cell apoptosis (Chakraborty et al., 2010; Wang et al., 2012), antimicrobial activity (Amirthaganesan et al., 2010), antiviral activity (Abdel-Aziza et al., 2010), anticonvulsant activity (Siddiqui et al., 2009) and have been used to treat chronic diabetic complications (Van Zandt et al., 2009). In addition to the above properties, benzothiazole derivatives have also been shown to exhibit immunosuppressive activity (Mase et al., 1986; 1988; el-Shorbagi et al., 1989). To discover new derivatives of benzothiazole with immunosuppressive activity, BD750 was synthesized and together with its analogues, screened for immunosuppressive activity (Figure 1). We found that, of these compounds, BD750 [2-(2-benzothiazoleyl)-4,5,6,7-tetrahydro-2H-indazol-3-ol, C14H13N3OS, MW: 271.3] was the most effective.
Figure 1.
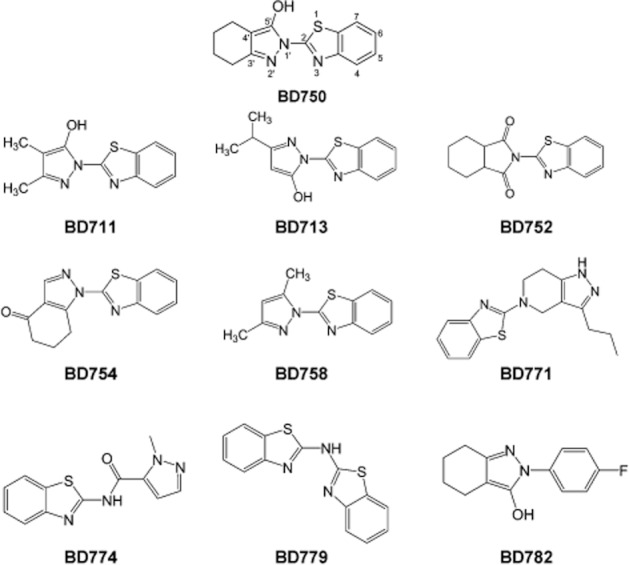
The chemical structures of a series of benzothiazole derivatives.
In this study, we describe a simple method for the synthesis of BD750 and show that BD750 is a potent immunosuppressant; it inhibited T cell proliferation in vitro and attenuated a T cell-mediated delayed-type hypersensitivity (DTH) reaction in vivo. Our findings indicate that the possible mechanism underlying the action of BD750 is inhibition of the JAK3/STAT5 signalling pathway. Potentially, BD750 may be used as a lead compound for the design and development of new immunosuppressants for the prevention of graft rejection and autoimmune diseases.
Methods
Synthesis of BD750
BD750 was synthesized from commercially available 2-hydrazino-benzothiazole and ethyl 2-oxocyclohexanecarboxylate in one step. A mixture of 2-hydrazino-benzothiazole (5.5 g, 33.3 mmol) and ethyl 2-oxocyclohexanecarboxylate (5.0 g, 29.4 mmol) in toluene (70 mL) with a catalylic amount of acetic acid (0.1 mL) was refluxed for 5 h. The reaction was checked by use of TLC (Merck precoated 60F254 plates), and spots were detected by viewing under a UV light, colourising with charring after dipping in 5% sulfuric acid and ethanol solution. After completion of the reaction, the solvent was evaporated under reduced pressure. BD750 (3.6 g, 45% yield) was recrystallized from ethanol as a yellow amorphous powder. Subsequently, the compound was characterized by 1H and 13C NMR spectra on a Bruker Avance 600 spectrometer (Bruker BioSpin, Fällanden, Suisse, Switzerland) for chemical shifting that were expressed in δ (ppm), referring to the tetramethylsilane peak; electrospray ionization mass spectrum (ESIMS) and high-resolution electrospray ionization mass spectrum (HRESIMS) on a BioTOF-Q mass spectrometer; UV spectrum on a Perkin-Elmer Lambda 35 UV/VIS spectrometer (Perkin Elmer, Watham, MA, USA). Reagents were purchased from J&K Chemical Co. (Beijing, China). Solvents were obtained from local suppliers.
Experimental animals
Female BAL b/c and C57BL/6 mice (6–8 weeks) were obtained from Huaxi Laboratory Animal Center of Sichuan University (Chengdu, China). Mice were housed in a specific pathogen-free facility with free access to normal chow and water (32 mice were used in our experiments). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All experiment protocols were established, according to the national guidelines for care and use of laboratory animals and were approved by the Bioethics Committee of Chengdu Medical College.
Cell isolation and culture
Mice were killed by cervical dislocation, and their spleens were dissected out aseptically. Splenic mononuclear cells were prepared and resuspended in RPMI 1640 medium containing 10% FBS referred to as a complete medium. Human peripheral mononuclear cells (PBMC) were isolated from healthy donors by density-gradient centrifugation with Lymphoprep and resuspended in complete medium. CD3+ T cells were negatively selected from spleen cells or PBMC using immunomagnetic beads to deplete non-T cells, according to the manufacturer's instructions (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of the resulting T cell populations was examined by flow cytometry, and a T cell population with purity of 95% was used for the following experiments.
Proliferation assay
The level of cell proliferation was determined by flow cytometry analysis with CFSE labelling. Briefly, the cells (106 cells mL−1) were stained with CFSE (2.5 μM) for 10 min at 37°C, stopped by 20% FBS, washed twice with PBS and resuspended in complete medium. Subsequently, CFSE-labelled mouse or human T cells (106 cells mL−1) were stimulated by plate-bound anti-CD3 (2 μg mL−1) plus soluble anti-CD28 (1 μg mL−1) monoclonal antibodies (mAbs). CFSE-labelled spleen cells from BALB/c mice (106 cells mL−1) were mixed with irradiated spleen cells from C57BL/6 mice at a ratio of 1:1. CFSE-labelled PBMC (106 cells mL−1) were stimulated with equal numbers of irradiated PBMC from another person. CFSE-labelled CTLL-2 cells (106 cells mL−1) were stimulated by addition of IL-2 (50 IU mL−1). The human T cells activated by anti-CD3/anti-CD28 mAbs for 72 h were washed and incubated for 24 h. And these cells were labelled by CFSE and stimulated by IL-2 (50 IU mL−1). Then these activated cells were all incubated for 96 h in the presence of increased concentrations of BD750 dissolved in DMSO that was less than 0.025% or vehicle alone. The proliferation of CFSE-labelled cells was analysed by flow cytometry on a FACSCalibur (Becton Dickinson, San Jose, CA, USA) using CellQuest acquisition and ModFit analysis software (Becton Dickinson).
CCK-8 assay
The effect of BD750 on the survival of human resting naïve T cells, IL-4 treated-activated T cells and fibroblast-like synoviocytes (FLS) was determined using the CCK-8 assay, according to the manufacturer's instruction. Briefly, IL-4-treated, activated T cells were derived from naïve T cells stimulated by anti-CD3/anti-CD28 mAbs for 72 h, then washed and incubated with IL-4. FLS were isolated from the synovial tissues of RA patients. Then human resting naïve T cells, IL-4-treated, activated T cells and FLS were incubated in the presence of different concentrations of BD750 or vehicle alone for 96 h. The viability of cells was evaluated by the CCK-8 assay and detection of absorbance at 450 nm on a SpectraMax M5 microplate reader (Molecular Devices, Sunnyvale, CA, USA).
ELISA detection of cytokines
The purified mouse or human T cells (106 cells mL−1) were stimulated with, or without, plate-bound anti-CD3 (2 μg mL−1) plus soluble anti-CD28 (1 μg mL−1) mAbs in the presence of BD750, MPA, FK506, RAPA or vehicle alone. Their supernatants were harvested, and the concentrations of IL-2 and IL-4 were determined by elisa, by using recombinant cytokines to establish standard curves.
CD25 and CD69 expression assay
The relative levels of CD25 and CD69 expression on T cells were characterized by flow cytometry. Briefly, the purified mouse or human T cells (106 cells mL−1) were stimulated with or without, plate-bound anti-CD3 (2 μg mL−1) plus soluble anti-CD28 (1 μg mL−1) mAbs in the presence of BD750, MPA, FK506, RAPA or vehicle alone for 24 h. The cells were collected, washed and stained with anti-CD25 (APC) or CD69 (APC) for 30 min at 4°C. Subsequently, the cells were washed, fixed and assessed by flow cytometry.
Cell cycle assay
The purified mouse or human T cells (106 cells mL−1) were stimulated with, or without, plate-bound anti-CD3 (2 μg mL−1) plus soluble anti-CD28 (1 μg mL−1) mAbs in the presence of different concentrations of BD750, RAPA or vehicle alone for 72 h. The cells were collected, fixed and permeabilized overnight in 70% ethanol. Subsequently, the fixed cells were washed with PBS and digested with 10 μg mL−1 RNase A, followed by staining with 20 μg mL−1 of PI in the dark at room temperature for 20 min. The DNA contents of different groups of cells were analysed by flow cytometry using CellQuest acquisition and ModFit analysis software.
Western blotting assay
The purified mouse T cells (106 cells mL−1) were stimulated with, or without, plate-bound anti-CD3 (2 μg mL−1) plus soluble anti-CD28 (1 μg mL−1) mAbs in the presence of different concentrations of BD750, RAPA or vehicle alone for 72 h. The cells were washed with cold PBS and lysed in RIPA buffer, followed by centrifugation. After the total amount of protein had been quantified, individual cell lysate samples (20 μg per lane) were separated by electrophoresis on 10% SDS-polyacrylamide gels and transferred onto Immobilon PVDF membrane (Millipore). After being blocked with 5% BSA, the blots were incubated with antibodies against cyclin D3, CDK6 and β-actin respectively. The bound antibodies were detected by HRP-conjugated second antibodies and visualized by enhanced chemiluminescence (Millipore).
Additional Western blot assays were performed in the IL-2-induced CTLL-2 cells, and primary activated T cells. CTLL-2 cells (106 cells mL−1) were washed and treated with BD750, AG-490 or vehicle for 6 h. The purified human T cells (106 cells mL−1) activated by anti-CD3/anti-CD28 mAbs for 72 h were washed and treated with BD750, AG-490, LY294002, RAPA or vehicle for 6 h. Then CTLL-2 cells and T cells were both stimulated with or without IL-2 (50 IU ml−1) for 15 min to characterize the relative levels of STAT5, phospho-STAT5, Akt, phospho-Akt, p70S6K and phospho-p70S6K using specific antibodies respectively.
2,4-Dinitrofluorobenzene (DNFB)-induced delayed type hypersensitivity reaction
Individual BALb/c mice were sensitized topically with 20 μL of 0.5% (v v−1) DNFB in acetone : olive oil (4:1) onto each hind foot of mice on days 0 and 1. These mice were treated i.p. with different doses of BD750, CsA or vehicle alone beginning on day 6 for three consecutive days. The mice were challenged topically with 10 μL of 0.5% (v v−1) DNFB on the inner and outer surfaces of the right ear on day 7. The thickness of both left and right ears and the weight of ear patches (8 mm punches) were measured 48 h post challenge.
Statistical analysis
Data are expressed as mean ± SEM. The inhibitory concentration of the compound that reduced cell proliferation by 50% (IC50) value was determined by using GraphPad Prism 5 (GraphPad, San Diego, CA, USA). The difference among groups of cells was determined by one-way anova with Dunnett's comparisons using SPSS software (SPSS, Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
Drugs, mAbs and reagents
The analogues of BD750 (BD711, BD713, BD752, BD754, BD758, BD771, BD774, BD779 and BD782) were purchased from ChemBridge Corp. (San Diego, CA, USA). RPMI 1640 medium was a product of Invitrogen (Carlsbad, CA, USA). Pan T-cell Isolation Kit II Mouse and Pan T-cell Isolation Kit II Human were products of Miltenyl Biotec. MPA, RAPA, FK506, dimethyl sulfoxide (DMSO), propidium iodide (PI) and DNFB were purchased from Sigma-Aldrich (St Louis, MO, USA). 5-Carboxyfluorescein diacetate succinimide ester (CFSE) was a product of Molecular Probes (Eugene, OR, USA). Counting Kit-8 (CCK-8) was a product of Dojindo (Kumamoto, Japan). Purified NA/LE mAbs against mouse CD3 (145-2C11), mouse CD28 (37.51 clone), human CD3 (HIT3a clone) and human CD28 (CD28.2 clone) and fluorescent-labelled mAbs against mouse CD3 (PE), mouse CD25 (APC), mouse CD69 (APC), human CD3 (PE), human CD25 (APC) and human CD69 (APC) were purchased from BD Biosciences Pharmingen (San Diego, CA, USA). Cytokine elisa detection kits including mouse and human IL-2 and IL-4 were purchased from eBioscience (San Diego, CA, USA). Pharmacological inhibitors AG490 and LY294002 were obtained from Promega (Madison, WI, USA). Polyclonal antibodies against phospho-STAT5, total STAT5, phospho-Akt, total Akt, phospho-p70S6K, total p70S6K, cyclin D3, CDK6, β-actin and species-specific HRP-labelled secondary antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Enhanced chemiluminescence was purchased from Millipore.
Results
BD750 inhibits T-cell proliferation without obvious cytotoxicity in vitro
BD750 was synthesized from commercially available 2-hydrazino-benzothiazole (1) and ethyl 2-oxocyclohexanecarboxylate (2) in one step with a yield of 45%. The synthesis scheme for BD750 is shown in Figure 2. Characterization of BD750 revealed that BD750 had properties of UV (MeOH) λmax = 218 nm; 1H NMR(DMSO-d6, 600 MHz) δ 8.01 (d, 1H, J = 7.8 Hz), 7.78 (d, 1H, J = 7.8 Hz), 7.46 (t, 1H, J = 7.6 Hz), 7.33 (t, 1H, J = 7.5 Hz), 2.50 (m, 2H), 2.20 (m, 2H), 1.73 (m, 2H), 1.67(m, 2H). 13C NMR (DMSO-d6, 150 MHz); δ 162.3, 154.6, 153.3, 148.9, 132.2, 126.9, 124.3, 122.6, 121.0, 102.5, 22.3, 22.2, 21.7, 18.7; ESI-MS: m/z 272 [M + 1]+; HRESIMS m/z calculated for C14H14N3OS [M + 1]+ 272.0852, found 272.0849.
Figure 2.
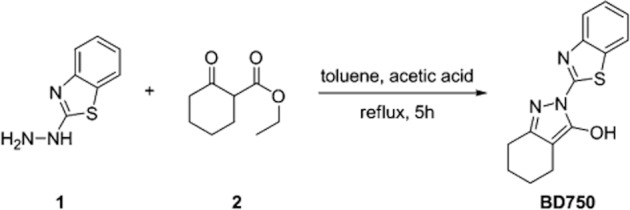
Synthesis of BD750: a mixture of compounds 1 (5.5 g, 33.3 mmol) and 2 (5.0 g, 29.4 mmol) in toluene (70 mL) with a catalylic amount of acetic acid (0.1 mL) was refluxed for 5 h. The reaction was checked by TLC (Merck precoated 60F254 plates), and spots were detected by viewing under a UV light, colourizing with charring after dipping in 5% sulfuric acid and ethanol solution. After completion of the reaction, the solvent was evaporated under reduced pressure. BD750 (3.6 g, 45% yield) was recrystalized from ethanol as a yellow amorphous powder.
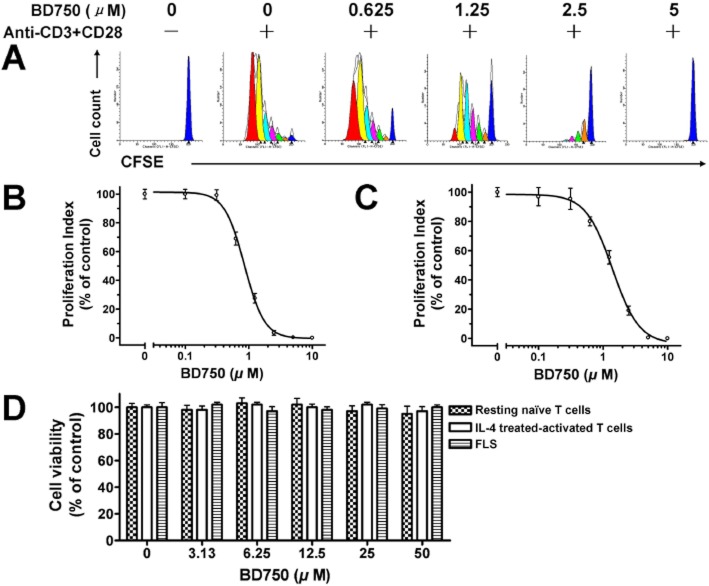
We found that BD750, BD711 and BD713, but not other compounds tested, significantly inhibited mouse and human T cell proliferation stimulated by anti-CD3/anti-CD28 mAbs (Table 1). Of these compounds BD750 was obviously the most potent inhibitor of mouse and human T cell proliferation, hence, we used BD750 for further studies. As shown in Figure 3, BD750 inhibited human T cell proliferation stimulated either by anti-CD3/anti-CD28 mAbs or by alloantigen in a dose-dependent manner with IC50 values of 1.1 ± 0.2 μM (A, B) and 1.3 ± 0.2 μM (C) respectively. In addition, ConA, PMA/ionomycine or alloantigen-induced mouse T cell proliferation and PHA or PMA/ionomycine-induced human T cell proliferation were inhibited by BD750 (data not shown).
Table 1.
Inhibitory effect of benzothiazole derivatives on T cell proliferation
| Compound | IC50 (μM) | |
|---|---|---|
| Inhibition of mouse T-cell proliferation | Inhibition of human T-cell proliferation | |
| BD750 | 1.5 ± 0.2 | 1.1 ± 0.2 |
| BD711 | 14.3 ± 1.2 | 15.7 ± 0.6 |
| BD713 | 32.2 ± 2.8 | 29.6 ± 1.8 |
| BD752 | NAa | NA |
| BD754 | NA | NA |
| BD758 | NA | NA |
| BD771 | NA | NA |
| BD774 | NA | NA |
| BD779 | NA | NA |
| BD782 | NA | NA |
CFSE-labelled T cells (106 cells mL−1) were stimulated with anti-CD3/anti-CD28 mAbs for 96 h in the presence of increasing concentrations of benzothiazole derivatives or vehicle alone. The level of T cell proliferation was determined by flow cytometry and analysed by use of a proliferation index. Results presented are mean ± SEM, n = 3, and are from one experiment, which is representative of two others.
NA, no inhibitory effects on T cell proliferation (the concentration of each compound used to inhibit T cell proliferation had no obvious cytotoxic effects against naïve T cells).
Figure 3.
BD750 inhibits T cell proliferation without obvious cytotoxicity in vitro. The level of T cell proliferation was determined by flow cytometry analysis with CFSE labelling. CFSE-labelled human T cells (106 cells mL−1) were stimulated by anti-CD3/anti-CD28 mAbs, and CFSE-labelled PBMC (106 cells mL−1) were stimulated with equal numbers of irradiated PBMC from another person for 96 h in the presence of increased concentrations of BD750 or vehicle alone. The level of T cell proliferation stimulated by anti-CD3/anti-CD28 mAbs (A, B) and alloantigen (C) were determined by flow cytometry and analysed by proliferation index. The effect of BD750 on the survival of human resting naïve T cells, IL-4 treated-activated T cells and fibroblast-like synoviocytes (FLS) was determined using the CCK-8 assay. IL-4 treated-activated T cells were derived from naïve T cells stimulated by anti-CD3/anti-CD28 mAbs for 72 h, then washed and incubated with IL-4. FLS were isolated from RA patients’ synovial tissues. Then human resting naïve T cells, IL-4 treated-activated T cells and FLS were incubated in the presence of different concentrations of BD750 or vehicle alone for 96 h. The viability of cells was evaluated by the CCK-8 assay and detection of absorbance at 450 nm on a microplate reader. Results presented are mean ± SEM, n = 3. The control group was vehicle-treated activated T cells (A, B and C) or vehicle-treated resting naïve T cells, IL-4 treated-activated T cells and FLS (D). The results presented are from one experiment, which is representative of two others.
Many compounds inhibit T cell proliferation by cytotoxic, but not immunosuppressive activity. To test the cytotoxicity of BD750, the cell viability of BD750-treated human resting naïve T cells, IL-4 treated-activated T cells and FLS was determined by the CCK-8 assay. Resting naïve T cells were not activated and did not proliferate. IL-4-treated activated T cells were derived from naïve T cells stimulated by anti-CD3/anti-CD28 mAbs for 72 h, then washed and incubated with IL-4. IL-4 prevented the deaths of activated T cells and IL-4-treated, activated T cells did not proliferate in vitro (Vella et al., #b1003). FLS were isolated from synovial tissues of RA patients as the non-specific cellular control. FLS can still proliferate for at least 10 generations in vitro in DMEM supplemented with 10% FCS (Noss and Brenner, #b1002; Bartok and Firestein, #b1001). As shown in Figure 3D, there was no significant difference in the relative viability of BD750-treated human resting naïve T cells, IL-4-treated, activated T cells and FLS to control cells among different groups. These results suggested that BD750 had no obvious cytotoxic effects on these cells in our experimental conditions, indicating that BD750 selectively inhibited activated T cell proliferation.
BD750 does not inhibit T cell activation in vitro
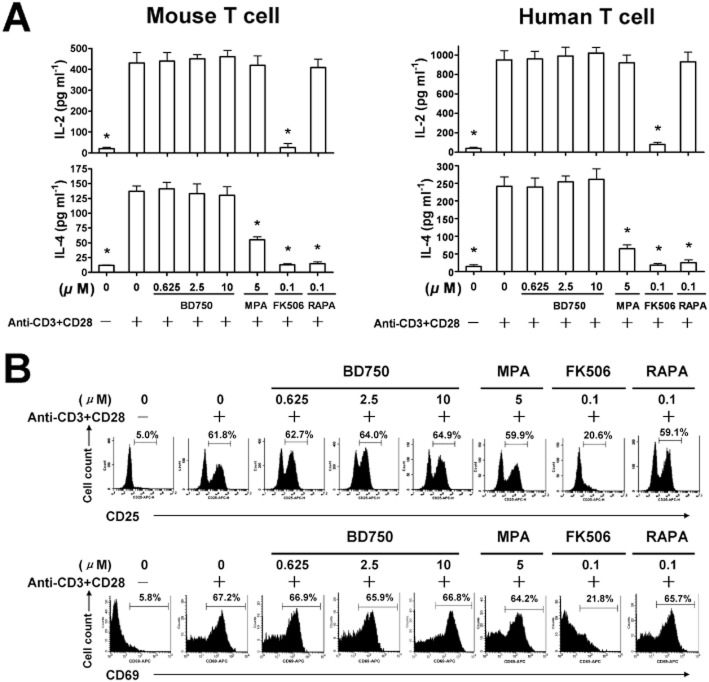
Engagement of TCR on T cells by antigen in the presence of sufficient co-stimulation signalling from CD28 induces CD25 and CD69 expression at the early stages of T cell activation (el-Shorbagi et al., 1989). To determine the effect of BD750 on T cell activation, mouse and human T cells were stimulated with anti-CD3/anti-CD28 mAbs in the presence of different concentrations of BD750, MPA, FK506 and RAPA, respectively, for 24 h; and the relative levels of CD25 and CD69 were characterized by flow cytometry analysis. While treatment with FK506 reduced the frequency of CD25+ and CD69+ T cells, similar to previous findings (Gummert et al., 1999), treatment with any of the concentrations of BD750, MPA or RAPA did not modulate the percentages of CD25+ and CD69+ T cells (Figure 4A).
Figure 4.
BD750 does not inhibit T cell activation in vitro. The purified mouse or human T cells (106 cells mL−1) were stimulated with, or without, anti-CD3/anti-CD28 mAbs in the presence of BD750, MPA, FK506, RAPA or vehicle alone. CD25 and CD69 expression in activated human T cells were assessed by flow cytometry (A). The concentrations of IL-2 and IL-4 were determined by elisa (B). Results are presented are mean ± SEM, n = 3, *P < 0.05 versus control group (activated and treated with vehicle). The results presented are from one experiment, which is representative of two others performed.
Activated T cells produce cytokines. For example, Th1 cells secrete IL-2 (Aversa et al., 1997), while Th2 cells produce IL-4 and other cytokines (Takenaka et al., 1997). To further examine the effect of BD750 on T cell activation, mouse and human T cells were stimulated with anti-CD3/anti-CD28 mAbs in the presence of different concentrations of BD750, MPA, FK506 and RAPA, respectively, and the concentrations of IL-2 and IL-4 in their supernatants were determined by ELISA. While significantly lower concentrations of IL-2 were detected in the supernatants of activated T cells treated with FK506, there was no significant difference in the concentrations of IL-2 in the supernatants of activated T cells treated with, or without, BD750, MPA or RAPA, suggesting that BD750 did not modulate IL-2 production by mouse and human Th1 cells. Similar levels of IL-4 were detected in the supernatants of T cells treated with, or without, BD750, although significantly lower concentrations of IL-4 were detected in the supernatants of MPA, FK506 or RAPA-treated T cells (Figure 4B). Collectively, these data show that BD750 does not inhibit the expression of CD25 and CD69 by activated T cells or the production of IL-2 and IL-4, and indicate that T cell activation is not affected by BD750.
BD750 induces T cell cycle arrest at G0/G1 phase
To understand the mechanisms by which BD750 inhibited T cell proliferation, we characterized the anti-CD3/anti-CD28 mAbs-stimulated T cell cycle. Following stimulation of mouse and human T cells with anti-CD3/anti-CD28 mAbs, the T cells were stained with PI and subjected to flow cytometry analysis. As shown in Figure 5, treatment with increased concentrations of BD750 increased the percentages of T cells at the G0/G1 phase, similar to treatment with RAPA (A, B). A similar pattern of T cells at the G0/G1 phase was observed in human T cells (C).
Figure 5.
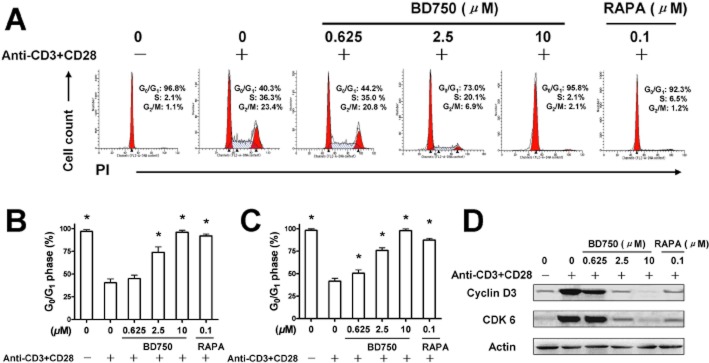
BD750 induces T cell cycling arrest at the G0/G1 phase. Purified mouse or human T cells (106 cells mL−1) were stimulated with, or without, anti-CD3/anti-CD28 mAbs in the presence of different concentrations of BD750, RAPA or vehicle alone for 72 h. The cells were collected, fixed and permeabilized overnight in 70% ethanol. Subsequently, the fixed cells were stained with PI. The DNA contents of different groups of cells were analysed by flow cytometry. Cell cycle analysis of mouse T cells (A, B) and human T cells (C) were determined by assessing the percentage of cells in the G0/G1 phase. The relative levels of cyclin D3 and CDK 6 in mouse T cells were determined by Western blotting assay (D). Results presented are mean ± SEM, n = 3, *P < 0.05 versus control group (activated and treated with vehicle). The results presented are from one experiment, which is representative of two others.
Given that the cell cycle from the G0/G1 phase into the S phase is regulated by specific D-type cyclins (cyclin D1, cyclin D2 and cyclin D3) (Ajchenbaum et al., 1993) and the G1-phase cyclin-dependent kinases CDK4/CDK6 (Lucas et al., 1995), we characterized the relative levels of cyclin D3 and CDK 6 compared to β-actin by Western blot assays. As shown in Figure 5D, treatment with BD750 inhibited cyclin D3 and CDK 6 expression in mouse T cells following stimulation with anti-CD3/anti-CD28 mAbs. These data indicate that BD750 induced T cell cycle arrest at the G0/G1 phase.
BD750 inhibits cell proliferation and STAT5 phosphorylation in IL-2-stimulated CTLL-2 cells and primary activated T cells
From the above studies, we showed that treatment with BD750 inhibits T cell activation but induces T cell cycle arrest at the G0/G1 phase. These findings suggest BD750 inhibits activated T cell proliferation. IL-2 engagement with their receptors can promote cell cycle progression and proliferation of activated T cells through the JAK3/STAT5 (Moriggl et al., 1999), PI3K/AKT and mTOR/p70S6K pathways (Stallone et al., 2005). To further understand the mechanisms underlying the action of BD750, we observed the inhibition of BD750 on cell proliferation by flow cytometry analysis with CFSE labelling and characterized the relative levels of STAT5, Akt and p70S6K expression and phosphorylation by Western blot assays in IL-2-induced CTLL-2 cells (an IL-2-dependent cytotoxic T cell line) and primary activated T cells. As shown in Figure 6, treatment with BD750 inhibited IL-2-induced proliferation of CTLL-2 cells and primary activated T cells in a dose-dependent manner with IC50 values of 1.2 ± 0.1 μM (A) and 1.0 ± 0.1 μM (B). However, treatment with BD750 did not modulate the levels of Akt and p70S6K expression and phosphorylation in IL-2-induced CTLL-2 cells and primary activated T cells. In contrast, even though treatment with BD750 did not modulate the STAT5 expression, it did inhibit the STAT5 phosphorylation in both IL-2 induced CTLL-2 cells and primary activated T cells in a dose-dependent manner. These data suggest BD750 inhibits IL-2-induced JAK3/STAT5-dependent T cell proliferation.
Figure 6.
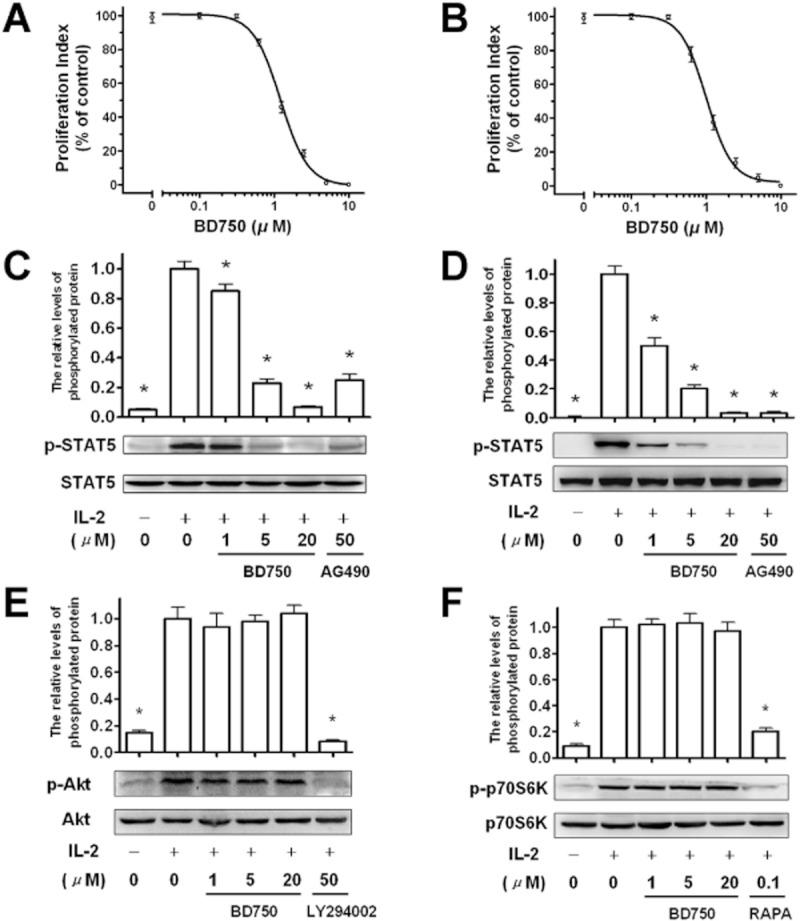
BD750 inhibits cell proliferation and STAT5 phosphorylation in IL-2-stimulated CTLL-2 cells and primary activated T cells. The level of cell proliferation was determined by flow cytometry analysis with CFSE labelling. CFSE-labelled CTLL-2 cells (106 cells mL−1) were stimulated by addition of IL-2 (50 IU mL−1). The human T cells activated by anti-CD3/anti-CD28 mAbs for 72 h were washed and incubated for 24 h. And these cells were labelled by CFSE and stimulated with IL-2 (50 IU mL−1). Then these IL-2-stimulated cells were incubated for 96 h in the presence of increased concentrations of BD750 or vehicle alone. The level of IL-2-induced CTLL-2 cell proliferation (A) and primary activated T cell proliferation (B) were determined by flow cytometry and analysed by use of the proliferation index. STAT5, Akt and p70S6K expression and phosphorylation were measured by Western blot assays. CTLL-2 cells (106 cells mL−1) were washed and treated with BD750, AG-490 or vehicle for 6 h. The purified human T cells (106 cells mL−1) activated by anti-CD3/anti-CD28 mAbs for 72 h were washed and treated with BD750, AG-490, LY294002, RAPA or vehicle for 6 h. Then CTLL-2 cells and T cells were both stimulated with or without IL-2 (50 IU mL−1) for 15 min. The relative levels of STAT5 expression and phosphorylation in IL-2 stimulated CTLL-2 cells (C) and STAT5 (D), Akt (E) and p70S6K (F) expression and phosphorylation in IL-2 stimulated primary activated T cells were measured by Western blot assays. Results presented are mean ± SEM, n = 3, *P < 0.05 versus control group (IL-2 induced cells treated with vehicle). The results presented are from one experiment, which is representative of two others.
BD750 attenuates the DNFB-induced DTH reaction
T cells, particularly Th1 cells, are crucial players in the pathogenesis of DTH (Kobayashi et al., 2001). Finally, we examined the effect of treatment with BD750 on DNFB-induced DTH in BAL b/c mice. Following sensitization, groups of mice were treated with different doses of BD750, and the thickness and the weight of ear patches were measured 48 h post challenge. We did not observe any abnormality in food and water intake and other obvious signs of sickness, and all of mice survived the experimental period. More importantly, we found that treatment with BD750 reduced ear swelling, and its effects were dose-dependent (Figure 7). These findings indicate that BD750 is a safe and effective inhibitor of T cell-mediated inflammation in vivo.
Figure 7.
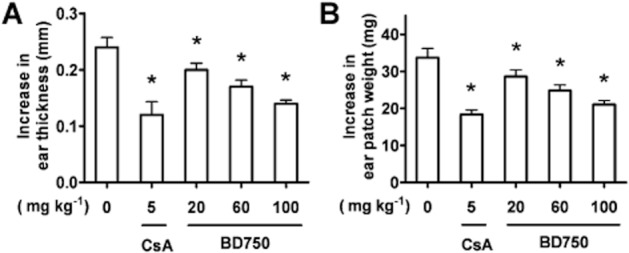
BD750 attenuates the DNFB-induced DTH reaction. Mice were treated i.p. with different doses of BD750, CsA or vehicle alone beginning on day 6 for three consecutive days. Then the mice were challenged with DNFB on day 7. The thickness of both left and right ears and the weight of ear patch were measured 48 h post challenge. Ear swelling was calculated as the increase in ear thickness (A) and ear patch weight (B) between the left (DNFB-untreated) and right (DNFB-treated) ear. Results presented are mean ± SEM, n = 3, *P < 0.05 versus control group (treated with vehicle).
Discussion and conclusions
In this study, a derivative of benzothiazole BD750 was synthesized and together with its analogues, screened for immunosuppressive activity by assessing its inhibitory activity on T cell proliferation. Among the compounds tested, we found that BD750 was the most potent inhibitor of anti-CD3/anti-CD28-stimulated mouse and human T cell proliferation. T cell proliferation stimulated by alloantigen and other T cell stimulators was also inhibited significantly by BD750 in vitro. More importantly, no obvious cytotoxic effects of BD750 against human resting naïve T cells, IL-4-treated, activated T cells and FLS were observed in our experimental conditions, indicating that BD750 selectively inhibited activated T cell proliferation.
BD750 appeared to have no obvious effect on the T cell activation induced by TCR-engagement. This suggests that while engagement of TCR on naïve T cells by anti-CD3 in the presence of anti-CD28 for co-stimulation can induce CD25 and CD69 expression at the early stage of T cell activation (Hulin, 2008), BD750 treatment did not affect the frequency of CD25+ and CD69+ T cells following stimulation with anti-CD3/anti-CD28. Furthermore, activated T cells can differentiate into different functional T cells in an optimal cytokine environment and produce specific cytokines. For example, Th1 cells secrete IL-2 while Th2 cells produce IL-4 and other cytokines (Segoloni and Quaglia, 2006). We tested the effect of BD750 on the levels of cytokine production by anti-CD3/anti-CD28-activated T cells and found that there was no significant difference in the levels of IL-2 and IL-4. These data indicate that BD750 did not modulate the differentiation of ant-CD3/anti-CD28-activated Th1 and Th2 cells. Hence, BD750 does not appear to affect naïve T cell activation and differentiation in vitro.
During proliferation, activated T cells undergo a cell cycling process. To understand the mechanisms underlying the action of BD750, we characterized T cell cycling and found that following stimulation with anti-CD3/anti-CD28 a significantly higher proportion of BD750-treated T cells was at the G0/G1 phase, as compared with vehicle-treated T cells. Furthermore, treatment with BD750 significantly reduced the levels of cyclin D3 and CDK 6 expression in anti-CD3/anti-CD28-stimulated T cells. These data clearly indicate that BD750 induced activated T cell cycling arrest at the G0/G1 phase, which may contribute to the inhibition of T cell proliferation. IL-2 can promote cell cycle progression and proliferation of activated T cells through the JAK3/STAT5 (Moriggl et al., 1999), PI3K/AKT and mTOR/p70S6K pathways (Stallone et al., 2005). To further understand the mechanisms underlying the action of BD750, we demonstrated that BD750 inhibited IL-2-induced CTLL-2 cell and primary activated T cell proliferation. Although treatment with BD750 did not modulate Akt and p70S6K expression and phosphorylation, the STAT5 phosphorylation was significantly reduced by BD750 in both IL-2-induced CTLL-2 cells and primary activated T cells. Given that the JAK3/STAT5 signalling pathway is crucial for activated T cell proliferation (Pesu et al., 2005), the dramatic inhibitory effect of BD750 on STAT5 activation may underlie its inhibitory effect on T cell proliferation. We are interested in further investigating whether BD750 interferes with IL-2 binding to its receptor and/or downstream cytokine receptor phosphorylation, limiting the recruitment of STAT5 and subsequent STAT5 activation in activated T cells.
The in vivo properties of BD750 were illustrated by its inhibitory effect on a T cell-mediated DTH response. T cells, particularly Th1 cells, are crucial players in the pathogenesis of DTH (Shi et al., 2012). Administration of BD750 attenuated the T cell-mediated DTH in mice in a dose-dependent manner in vivo. We did not observe any abnormality in food and water intake and other obvious signs of sickness, and all of mice survived in the experimental period. These data indicate that BD750 is safe and effective at inhibiting T cell-mediated inflammation in vivo.
Analysis of the structure-activity relationship of these benzothiazole derivatives revealed that both pyrazole and benzothiazole groups in these derivatives were essential for their immunosuppressive activity. When one of the two groups in these compounds was destroyed, the inhibitory effect on T cell proliferation was lost. For example, BD752, BD779 and BD782 have no inhibitory effect on T-cell proliferation. The electron-withdrawing groups, such as hydroxyl and carbonyl groups were important for the immunosuppressive activity of these compounds because the active compounds, BD750, BD711 and BD713, had a hydroxyl group at the 5′-position, whereas the compounds that do not have these groups, BD754 and BD758, were inactive. In addition, the 3′-position and 4′-position of compounds with lipophilic groups appeared to be necessary for their immunosuppressive activity. Interestingly, BD771 and BD774 were inactive, suggesting that the connection mode between the pyrazole and benzothiazole groups is also important for immunosuppressive activity. However, how the chain length or ring stiffness of the substituents affects the immunosuppressive activity of these compounds remains to be investigated.
In summary, we described a simple method for the synthesis of BD750 and provided evidence that BD750 has potent immunosuppressive activity mediated through inhibition of T cell proliferation in vitro and attenuation of T cell-mediated inflammation in vivo. Our findings indicate that the possible mechanisms underlying the action of BD750 may be inhibition of the JAK3/STAT5 signalling pathway. Potentially, BD750 could be used as a lead compound for the design and development of new immunosuppressants for the prevention of graft rejection and autoimmune diseases.
Acknowledgments
We thank Professor Z-L Chen and N Fu (Southern Medical University, China) for their considerable advice and Medjaden Bioscience Limited for assisting in the preparation of this manuscript. The authors declare that this work was supported by New Century Excellent Talents in University (no. NCET-09–0888), National Natural Science Foundation of China (no. 21172024, 81072455, 81273530, 81202363), Sichuan Youth Science and Technology Fund (no. 2010JQ0035), Scientific Research Fund of Sichuan Provincial Education Department (nos. 11ZA201, 11ZB169) and Research Fund of Chengdu Medical College (no. CYZ09-019).
Glossary
- BD750
2-(2-benzothiazoleyl)-4,5,6,7-tetrahydro-2H-indazol-3-ol
- CFSE
5-carboxyfluorescein diacetate succinimide ester
- CsA
cyclosporin A
- DTH
delayed-type hypersensitivity
- FLS
fibroblast-like synoviocyte
- MPA
mycophenolic acid
- MS
multiple sclerosis
- mTOR
mammalian target of rapamycin
- RA
rheumatoid arthritis
- RAPA
rapamycin
- SLE
systemic lupus erythematosus
- TCR
T cell receptor
Conflicts of interest
There are no other personal financial holdings of any of the authors that could be perceived as constituting a potential conflict of interest.
References
- Abdel-Aziza HA, Abdel-Wahab BF, Badria FA. Stereoselective synthesis and antiviral activity of (1E,2Z,3E)-1-(piperidin-1-yl)-1-(arylhydrazono)-2-[(benzoyl/benzothiazol-2 -oyl)hydrazono]-4-(aryl(1))but-3-enes. Arch Pharm (Weinheim) 2010;343:152–159. doi: 10.1002/ardp.200900195. [DOI] [PubMed] [Google Scholar]
- Abraham RT, Wiederrecht GJ. Immunopharmacology of rapamycin. Annu Rev Immunol. 1996;14:483–510. doi: 10.1146/annurev.immunol.14.1.483. [DOI] [PubMed] [Google Scholar]
- Ajchenbaum F, Ando K, DeCaprio JA, Griffin JD. Independent regulation of human d-type cyclin gene expression during G1 phase in primary human T lymphocytes. J Biol Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- Amirthaganesan S, Aridoss G, Park KS, Lim KT, Jeong YT. Synthesis, spectral and antimicrobial studies of some N (2)-substituted tetrahydroindazoles. Bull Korean Chem Soc. 2010;31:1135–1142. [Google Scholar]
- Aversa G, Chang CC, Carballido JM, Cocks BG, de Vries JE. Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J Immunol. 1997;158:4036–4044. [PubMed] [Google Scholar]
- Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunological reviews. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, et al. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Chakraborty M, Jin KJ, Glover SA, Novak M. Characterization of the 4-(benzothiazol-2-yl)phenylnitrenium ion from a putative metabolite of a model antitumor drug. J Org Chem. 2010;75:5296–5304. doi: 10.1021/jo101275y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, et al. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science. 2003;302:875–878. doi: 10.1126/science.1087061. [DOI] [PubMed] [Google Scholar]
- Deuse T, Velotta JB, Hoyt G, Govaert JA, Taylor V, Masuda E, et al. Novel immunosuppression: R348, a JAK3- and Syk-inhibitor attenuates acute cardiac allograft rejection. Transplantation. 2008;85:885–892. doi: 10.1097/TP.0b013e318166acc4. [DOI] [PubMed] [Google Scholar]
- Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. 2010;53:8468–8484. doi: 10.1021/jm1004286. [DOI] [PubMed] [Google Scholar]
- Franklin RA, Tordai A, Patel H, Gardner AM, Johnson GL, Gelfand EW. Ligation of the T cell receptor complex results in activation of the Ras/Raf-1/MEK/MAPK cascade in human T lymphocytes. J Clin Invest. 1994;93:2134–2140. doi: 10.1172/JCI117209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gummert JF, Ikonen T, Morris RE. Newer immunosuppressive drugs: a review. J Am Soc Nephrol. 1999;10:1366–1380. doi: 10.1681/ASN.V1061366. [DOI] [PubMed] [Google Scholar]
- van Gurp EA, Schoordijk-Verschoor W, Klepper M, Korevaar SS, Chan G, Weimar W, et al. The effect of the JAK inhibitor CP-690,550 on peripheral immune parameters in stable kidney allograft patients. Transplantation. 2009;87:79–86. doi: 10.1097/TP.0b013e31818bbea7. [DOI] [PubMed] [Google Scholar]
- Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- Hulin A. Today in molecular mechanisms of immunosuppressive drugs actions: roles of pharmacist. Ann Pharm Fr. 2008;66:102–114. doi: 10.1016/j.pharma.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Kaneda K, Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech. 2001;53:241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]
- Liu J, Farmer JD, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991;66:807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Szepesi A, Modiano JF, Domenico J, Gelfand EW. Regulation of synthesis and activity of the PLSTIRE protein (cyclin-dependent kinase 6 (cdk6)), a major cyclin d-associated cdk4 homologue in normal human T lymphocytes. J Immunol. 1995;154:6275–6284. [PubMed] [Google Scholar]
- Marcen R. Immunosuppressive drugs in kidney transplantation: impact on patient survival, and incidence of cardiovascular disease, malignancy and infection. Drugs. 2009;69:2227–2243. doi: 10.2165/11319260-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Mase T, Arima H, Tomioka K, Yamada T, Murase K. Imidazo[2,1-b]benzothiazoles. 2. New immunosuppressive agents. J Med Chem. 1986;29:386–394. doi: 10.1021/jm00153a014. [DOI] [PubMed] [Google Scholar]
- Mase T, Arima H, Tomioka K, Yamada T, Murase K. Imidazo [2, 1-b] benzothiazoles 3: syntheses and immunosuppressive activities of 2-(m-acyloxyphenyl) imidazo [2, 1-b] benzothiazoles. Eur J Med Chem. 1988;23:335–339. doi: 10.1021/jm00153a014. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reportingexperiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriggl R, Topham DJ, Teglund S, Sexl V, McKay C, Wang D, et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. doi: 10.1016/s1074-7613(00)80025-4. [DOI] [PubMed] [Google Scholar]
- Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunological reviews. 2008;223:252–270. doi: 10.1111/j.1600-065X.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- Patel AM, Lupash D, Chew D, Levesque MC, Moreland LW. JAK inhibition in rheumatoid arthritis. Curr Rheumatol Rep. 2011;13:379–380. doi: 10.1007/s11926-011-0196-4. [DOI] [PubMed] [Google Scholar]
- Pesu M, Candotti F, Husa M, Hofmann SR, Notarangelo LD, O'Shea JJ. Jak3, severe combined immunodeficiency, and a new class of immunosuppressive drugs. Immunol Rev. 2005;203:127–142. doi: 10.1111/j.0105-2896.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- Pesu M, Laurence A, Kishore N, Zwillich SH, Chan G, O'Shea JJ. Therapeutic targeting of Janus kinases. Immunol Rev. 2008;223:132–142. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponticelli C. Present and future of immunosuppressive therapy in kidney transplantation. Transplant Proc. 2011;43:2439–2440. doi: 10.1016/j.transproceed.2011.06.025. [DOI] [PubMed] [Google Scholar]
- Segoloni GP, Quaglia M. New immunosuppressive drugs for prevention and treatment of rejection in renal transplant. J Nephrol. 2006;19:578–586. [PubMed] [Google Scholar]
- Senda M, DeLustro B, Eugui E, Natsumeda Y. Mycophenolic acid, an inhibitor of IMP dehydrogenase that is also an immunosuppressive agent, suppresses the cytokine-induced nitric oxide production in mouse and rat vascular endothelial cells. Transplantation. 1995;60:1143–1148. doi: 10.1097/00007890-199511270-00015. [DOI] [PubMed] [Google Scholar]
- Shi L, Wang L, Wang Z, Zhu HL, Song Q. Synthesis of novel cinnamanilides as potential immunosuppressive agents. Eur J Med Chem. 2012;47:585–593. doi: 10.1016/j.ejmech.2011.10.027. [DOI] [PubMed] [Google Scholar]
- el-Shorbagi AN, Sakai S, el-Gendy MA, Omar N, Farag HH. Imidazo[2,1-b]benzothiazoles. II. Synthesis and antiinflammatory activity of some imidazo[2,1-b]benzothiazoles. Chem Pharm Bull (Tokyo) 1989;37:2971–2975. doi: 10.1248/cpb.37.2971. [DOI] [PubMed] [Google Scholar]
- Siddiqui N, Rana A, Khan SA, Haque SE, Arshad MF, Ahmed S, et al. Synthesis and preliminary screening of benzothiazol-2-yl thiadiazole derivatives for anticonvulsant activity. Acta Pharm. 2009;59:441–451. doi: 10.2478/v10007-009-0031-x. [DOI] [PubMed] [Google Scholar]
- Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal transduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Stallone G, Infante B, Gesualdo L. Immunosuppressive drugs and renal transplantation. G Ital Nefrol. 2005;22(Suppl. 33):S76–S79. [PubMed] [Google Scholar]
- Stucker F, Ackermann D. Immunosuppressive drugs – how they work, their side effects and interactions. Ther Umsch. 2011;68:679–686. doi: 10.1024/0040-5930/a000230. [DOI] [PubMed] [Google Scholar]
- Takenaka H, Maruo S, Yamamoto N, Wysocka M, Ono S, Kobayashi M, et al. Regulation of T cell-dependent and -independent IL-12 production by the three Th2-type cytokines IL-10, IL-6, and IL-4. J Leukoc Biol. 1997;61:80–87. doi: 10.1002/jlb.61.1.80. [DOI] [PubMed] [Google Scholar]
- Van Zandt MC, Doan B, Sawicki DR, Sredy J, Podjarny AD. Discovery of [3-(4,5,7-trifluoro-benzothiazol-2-ylmethyl)-pyrrolo[2,3-b]pyridin-1-yl]ac etic acids as highly potent and selective inhibitors of aldose reductase for treatment of chronic diabetic complications. Bioorg Med Chem Lett. 2009;19:2006–2008. doi: 10.1016/j.bmcl.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Vella AT, Dow S, Potter TA, Kappler J, Marrack P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc Natl Acad Sci U S A. 1998;95:3810–3815. doi: 10.1073/pnas.95.7.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velotta JB, Deuse T, Haddad M, Masuda E, Park G, Carroll D, et al. A novel JAK3 inhibitor, R348, attenuates chronic airway allograft rejection. Transplantation. 2009;87:653–659. doi: 10.1097/TP.0b013e318196110f. [DOI] [PubMed] [Google Scholar]
- Wang D, Matsumoto R, You Y, Che T, Lin XY, Gaffen SL, et al. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Mol Cell Biol. 2004;24:164–171. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wang F, Xiao Z, Sheng G, Li Y, Wang Y. Molecular simulation of a series of benzothiazole PI3Kalpha inhibitors: probing the relationship between structural features, anti-tumor potency and selectivity. J Mol Model. 2012;18:2943–2958. doi: 10.1007/s00894-011-1299-6. [DOI] [PubMed] [Google Scholar]
- Xie C, Patel R, Wu T, Zhu J, Henry T, Bhaskarabhatla M, et al. PI3K/AKT/mTOR hypersignaling in autoimmune lymphoproliferative disease engendered by the epistatic interplay of Sle1b and FASlpr. Int Immunol. 2007;19:509–522. doi: 10.1093/intimm/dxm017. [DOI] [PubMed] [Google Scholar]
- Xiong Z, Ma A, Chen H. JAK3 inhibitors in organ transplantation and autoimmune disease. Recent Pat Inflamm Allergy Drug Discov. 2010;4:75–81. doi: 10.2174/187221310789895577. [DOI] [PubMed] [Google Scholar]