Abstract
Background and Purpose
The Ca2+-permeable cation channel TRPV4 is activated by mechanical disturbance of the cell membrane and is implicated in mechanical hyperalgesia. Nerve growth factor (NGF) is increased during inflammation and causes mechanical hyperalgesia. 4α-phorbol 12,13-didecanoate (4αPDD) has been described as a selective TRPV4 agonist. We investigated NGF-induced hyperalgesia in TRPV4 wild-type (+/+) and knockout (–/–) mice, and the increases in [Ca2+]i produced by 4αPDD in cultured mouse dorsal root ganglia neurons following exposure to NGF.
Experimental Approach
Withdrawal thresholds to heat, von Frey hairs and pressure were measured in mice before and after systemic administration of NGF. Changes in intracellular Ca2+ concentration were measured by ratiometric imaging with Fura-2 in cultured DRG and trigeminal ganglia (TG) neurons during perfusion of TRPV4 agonists.
Key Results
Administration of NGF caused a significant sensitization to heat and von Frey stimuli in TRPV4 +/+ and –/– mice, but only TRPV4 +/+ mice showed sensitization to noxious pressure. 4αPDD stimulated a dose-dependent increase in [Ca2+]i in neurons from +/+ and –/– mice, with the proportion of responding neurons and magnitude of increase unaffected by the genotype. In contrast, the selective TRPV4 agonist GSK1016790A failed to stimulate an increase in intracellular Ca2+ in cultured neurons. Responses to 4αPDD were unaffected by pretreatment with NGF.
Conclusions and Implications
TRPV4 contributes to mechanosensation in vivo, but there is little evidence for functional TRPV4 in cultured DRG and TG neurons. We conclude that 4αPDD activates these neurons independently of TRPV4, so it is not appropriate to refer to 4αPDD as a selective TRPV4 agonist.
Keywords: TRPV4, sensory neuron, hyperalgesia
Introduction
The Ca2+-permeable cation channel transient receptor potential vanilloid 4 (TRPV4) was first cloned in 2000 (Strotmann et al., 2000) and belongs to the TRP superfamily of channels. TRPV4 expression shows a broad tissue distribution, with expression identified in epithelia of the skin, lung and kidney, in vascular endothelium and smooth muscle and in the brain by a combination of immunohistochemistry, in situ hybridization and functional studies (Strotmann et al., 2000; Jia et al., 2004; Cohen, 2005; Watanabe et al., 2008).
Its physiological roles are equally diverse, with suggested roles in osmoregulation (Liedtke and Friedman, 2003), stretch sensation in the bladder (Gevaert et al., 2007), flow sensation in kidney tubules (Wu et al., 2007) and osteoclast maturation (Masuyama et al., 2008), amongst others. Expression and activation of TRPV4 in the peripheral nervous system is implicated in mechanical nociception, particularly mechanical hypersensitivity following inflammation or neuropathy. It is less clear whether TRPV4 contributes to basal mechanosensation. Sensory deficits in response to noxious pressure (Suzuki et al., 2003) and bladder stretch (Gevaert et al., 2007) have been described in TRPV4 knockout mice. However, other studies have failed to identify basal differences in the response to mechanical stimuli between wild-type and knockout animals (e.g. Grant et al., 2007; Alessandri-Haber et al., 2008).
In common with other TRP channels, TRPV4 is activated by a wide range of physical and chemical stimuli. It was originally identified as a calcium channel responding to extracellular hypo-osmolarity and consequent cell swelling (Liedtke et al., 2000). Application of positive or negative pressure to membranes of TRPV4-expressing HEK293 cells in cell-attached patch clamp experiments did not alter channel activity, which suggested that TRPV4 did not directly respond to swelling-induced membrane stretch (Strotmann et al., 2000). An indirect mechanism of activation was proposed by Watanabe et al. (2003), who suggested swelling activates phospholipase A2-mediated release of arachidonic acid, which is then metabolized to form a TRPV4 agonist 5′,6′-epoxyeicosatrienoic acid (5′,6′-EET). However, the results of two recent studies suggest that direct mechanical gating of TRPV4 by membrane stretch (Loukin et al., 2010) or by force applied to the extracellular matrix (Matthews et al., 2010) can activate the channel. The first synthetic TRPV4 agonist to be identified was 4α-phorbol 12,13-didecanoate (4αPDD), a non-PKC activating phorbol ester with an EC50 around 200 nM at heterologously expressed human and murine TRPV4 (Watanabe et al., 2002a). Direct interaction of 4αPDD with a ligand-binding pocket formed by transmembrane regions 3 and 4 opens the channel (Vriens et al., 2007). The most potent small molecule agonist at TRPV4 identified to date is GSK1016790A, with a structure distinct from that of the phorbol esters. It has an EC50 around 18 nM at heterologously expressed murine TRPV4 (Thorneloe et al., 2008; Willette et al., 2008).
We recently identified TRPV4 expression in rat dorsal root ganglia neurons using RT-PCR and immunohistochemistry. In addition, we demonstrated that TRPV4 was sensitized by protease activated receptor 2 (PAR2) activation in cultured cells, and PAR2 activation in vivo produced a TRPV4-dependent mechanical hypersensitivity in mice (Grant et al., 2007). The increase in intracellular calcium in response to hypotonic challenge was diminished in neurons from TRPV4 knockout mice and was not sensitized by inflammatory ‘soup’, in contrast to the response in wild-type neurons (Alessandri-Haber et al., 2006). Inhibition of tetrodotoxin-resistant sodium currents by hypotonic challenge was reduced in trigeminal ganglia sensory neurons from TRPV4 knockout mice (Chen et al., 2009). Small diameter osmosensitive hepatic neurons were absent in TRPV4 knockout mice. Neurons from these mice only showed a delayed, irreversible increase in intracellular calcium in response to 4αPDD, whereas some wild-type neurons exhibited a rapid, reversible response. The authors interpreted the former response as a non-selective, toxic effect (Lechner et al., 2011). The number of neurons that showed an increase in intracellular calcium after 4αPDD treatment and the size of the increase were diminished in DRG neurons innervating the colon after administration of siRNA directed against TRPV4 (Cenac et al., 2008).
Various laboratories have also carried out studies linking TRPV4 activation to the mechanical hyperalgesia observed in chemotherapeutic and diabetic neuropathies (Alessandri-Haber et al., 2008), colonic inflammation (Sipe et al., 2008), chronic constriction injury of sensory nerves (Zhang et al., 2008) and pancreatitis (Ceppa et al., 2010). Altered nerve growth factor (NGF) signalling is implicated as a causal mechanism of the mechanical hyperalgesia in many neuropathic and inflammatory pain models, via modulation of ion channel expression and activity in sensory neurons (Pezet and McMahon, 2006; Watson et al., 2008). NGF increases the membrane presentation and activity of TRPV1 via phosphorylation at Y200, part of a phosphorylation sequence that is conserved in TRPV4 (Zhang et al., 2005). Thus, it is possible that NGF treatment will similarly enhance TRPV4 activity.
To determine whether sensitization of TRPV4 in DRG neurons is a general mechanism underlying mechanical hyperalgesia, we investigated whether NGF sensitization of mechanosensation is dependent on TRPV4 and studied the TRPV4 agonist-induced changes in [Ca2+]i in neurons from wild-type and TRPV4 knockout mice. We hypothesized that TRPV4 knockout mice would show reduced mechanical hyperalgesia after NGF treatment, and neurons from wild-type animals would show increased responses to TRPV4 activators in the presence of NGF, whilst TRPV4 knockout neurons would not respond to these stimuli.
Methods
Animals
TRPV4 wild-type and knockout C57BL/6 mice (Liedtke and Friedman, 2003) were a gift from Dr W Liedtke, Duke University, and were bred in house from pairs of heterozygote mice. Both male and female mice at least 8 weeks of age (20–30 g) were used in these studies. All mice were maintained on a normal diet and housed in a cage with a maximum of five animals, with access to food and water ad libitum, on a 12 h/12 h light/dark cycle in a climatically controlled environment. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). All procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986 and the rules of the King's College London animal ethics committee.
Collection of mouse dorsal root ganglia neurons, SDS-PAGE and Western blotting
Mice were killed by overdose of sodium pentobarbital (1 mg·g−1), and the spinal column was removed. Thoracic and lumbar dorsal root ganglia (DRG) were removed, combined and snap-frozen in liquid nitrogen. DRG were homogenized in RIPA buffer (50 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM EDTA, pH 7.4) with protease inhibitors (Roche, Welwyn Garden City, UK). Lysates were separated by SDS-PAGE (8% acrylamide), transferred to nitrocellulose membranes and blocked in TBS with 0.1% Tween-20 and 5% powdered milk for 60 min. Membranes were incubated with rabbit anti-TRPV4 (1:250, Abcam, Cambridge, UK; Ab39260 or 1:500, Alomone, Jerusalem, Israel; #ACC-034) and mouse anti-βIII-tubulin (1:3000, Promega, Southampton, UK, #G7121) overnight at 4°C. Membranes were washed and then incubated with donkey anti-rabbit-IRDye 800 (1:15 000, LI-COR, Cambridge, UK) and goat anti-mouse-Alexa Fluor 680 (Molecular Probes, Paisley, UK) for 1 h at room temperature. Immunoreactive proteins were detected using an Odyssey Infrared Imaging System (LI-COR).
Dispersion and culture of mouse dorsal root ganglia and trigeminal ganglia neurons
Mice (both sexes, 3–9 months old) were killed by overdose of sodium pentobarbital, and DRG was collected as above. Trigeminal ganglia (TG) were collected from within the skull. Rather than snap-freezing, DRG and TG were incubated in F12 medium (Gibco, Paisley, UK) with collagenase (1.25%) for 60 min at 37°C. Individual neurons were dispersed by trituration and then pelleted by centrifugation for 6 min at 60× g. Cells were resuspended in F12 basal medium supplemented with 1% penicillin/streptomycin, 30% BSA, 1% N2 neuronal growth supplement (PAA, Yeovil, UK) and plated on to sterile coverslips coated with poly-l-lysine (100 μg·mL−1, Sigma, Dorset, UK) and laminin (10 μg·mL−1; Sigma) in four-well plates.
Mouse NGF (2.5S; 100 ng·mL−1; Alomone) or additional F12 medium (control) was added when required as per experiment protocol, and cells were left in culture overnight prior to calcium imaging experiments.
Immunostaining of cultured dorsal root ganglia neurons
Neurons were collected, dispersed and cultured as described above. After 24 h in culture, neurons were fixed in 4% paraformaldehyde for 30 min at 4°C. Coverslips were washed 3 times in PBS containing 0.1% Triton X-100 (PBS-T). Rabbit anti-TRPV4 (1:200, Abcam) was added to the cells and they were incubated overnight at 4°C. Coverslips were washed three times in PBS-T, then goat anti-rabbit-Alexa 488 (1:1000) was added to the cells, and they were incubated for 2 h at room temperature. Coverslips were washed three times in PBS-T then mounted on microscope slides with Vectashield with DAPI (Vector Labs, Peterborough, UK), and images were collected with an Axioplan 2 microscope (Zeiss, Cambridge, UK).
Behaviour
All behaviour experiments used age-matched male mice. They were acclimatized to the testing environment on 3 days prior to testing and were placed in test chambers 1 h prior to measurements. All measurements were made by experimenters blind to the genotype of the mice. Cutaneous mechanical sensitivity was measured by manual application of von Frey hairs (0.04–4 g) to the plantar surface of both hind paws. Each hair was applied five times, and the frequency of withdrawal was recorded. The 50% withdrawal threshold was considered to be the force applied by the first hair to produce three or more out of five withdrawals. Cutaneous thermal sensitivity was measured by the Hargreaves's test. Infrared radiation was applied to the plantar surface of both hind paws, with intensity calibrated to give an average withdrawal response after 8–10 s. A cut-off time of 28 s was used to avoid any tissue damage. Averages of three applications to each side were recorded, with at least 5 min between applications. Sensitivity to pressure was measured by the Randall–Sellito test. The hind paw was placed on a flat plate, with increasing pressure applied by a probe on the dorsal surface until the paw was withdrawn. A cut-off pressure of 150 g was chosen to avoid tissue damage. Following baseline measurements, mice were injected with NGF (1 μg·g−1 in 100 μL saline, i.p.), and measurements were repeated at 1 and 24 h after injection.
Measurement of intracellular [Ca2+] in mouse DRG neurons
Cultured DRG neurons were used for Ca2+ imaging experiments after 18–24 h in culture. Cells were incubated in buffer (HBSS with 10 mM glucose, 10 mM HEPES, pH 7.4) with Fura-2-AM (2 μM) and probenecid (1 mM) for 60 min at 37°C. Coverslips were washed and mounted in an open chamber. Test compounds were diluted to required concentrations in buffer and applied to cells by continuous perfusion at 37°C. Hypotonic test solutions were generated by addition of deionized water to the standard buffer. The fluorescence of individual cells were measured at 340 and 380 nm excitation, and 510 nm emission using microscope-based imaging system (PTI, Ford, UK). Cells were challenged with KCl (50 mM) at the end of each experiment to activate voltage-gated Ca2+ channels and provide a maximal Ca2+ signal, against which to normalize other cellular responses. Neurons were identified morphologically and were excluded from analysis if they did not respond to KCl.
Measurement of intracellular [Ca2+] in HaCaT cells
The HaCaT human keratinocyte cell line was grown in defined keratinocyte serum-free medium (Invitrogen, Paisley, UK) at 37°C. Cells were plated in black-walled 96-well plates and grown to confluence for Ca2+ imaging experiments. Cells were loaded with Fura-2-AM (2 μM) for 1 h in the presence of probenecid (1 mM) at 30°C in HBSS with 10 mM HEPES. Cells were washed, and the mean fluorescence across each well was measured at 340 and 380 nm excitation, and 520 nm emission using a Flexstation (Molecular Devices, Wokingham, UK). Each experimental run lasted 240 s, with fluorescence readings made at 6 s intervals. GSK1016790A was added at 20 s. In a further series of experiments, cells were pretreated for 15 min with the TRPV4 antagonist HC067047 before the start of Ca2+ imaging, and the antagonist remained in contact with the cells throughout.
Materials
4α-phorbol 12,13-didecanoate, GSK1016790A, HC067047 and probenecid were obtained from Sigma. Mouse NGF (2.5S) was obtained from Alomone. Fura-2-AM was obtained from Molecular Probes (Invitrogen).
Statistical analysis
Comparisons between behaviour results were by one-way repeated-measures anova, followed by Bonferroni's multiple comparisons test. Emission intensity ratios at 340 nm/380 nm excitation in neuronal imaging experiments were calculated using EasyRatioPro software (PTI, Ford, UK). Only neurons where the response to treatment was greater than 20% of the Δ ratio for 50 mM KCl were counted as responders. The response to GSK1016790A in the HaCaT experiments was calculated as maximum Δ ratio in the presence of the agonist minus the mean baseline ratio.
Results
Expression of TRPV4 in dorsal root ganglia
Expression of TRPV4 protein was studied in DRG tissue lysates from wild-type and TRPV4 knockout mice by SDS-PAGE followed by Western blotting. Two different anti-TRPV4 antibodies were studied to allow comparison of their specificities. A strong band at 50 kDa, corresponding to the neuron-specific marker βIII-tubulin, was seen in both sets of samples (Figure 1). A relatively weak double band (98 and 104 kDa) corresponding to TRPV4 was seen in the wild-type sample with both antibodies and in both cases was absent in the lysate from the knockout DRG. Both anti-TRPV4 antibodies also bound non-specifically to other proteins, as indicated by bands of lower molecular weight present in both lysates.
Figure 1.

(A) Expression of TRPV4 in dorsal root ganglia lysate. Lysates (30 μg of total protein) from both TRPV4 +/+ and –/– mice showed expression of βIII-tubulin (50 kDa, left column). Only lysates from TRPV4 +/+ mice showed the double band corresponding to TRPV4 (98 and 104 kDa, right column). (B) Immunostaining of cultured dorsal root ganglion neurons from TRPV4 +/+ and –/– mice with rabbit anti-TRPV4 (1:200) and goat anti-rabbit-Alexa488 (1:1000) revealed no difference in staining intensity between +/+, –/– and +/+ (no primary antibody) cells. Images are representative of data obtained from n = 3 independent experiments.
An attempt was made to immunostain cultured DRG neurons using the more selective of the antibodies (Abcam) tested in the Western blotting experiment. No difference was perceived in immunofluorescence between wild-type neurons, TRPV4 knockout neurons and wild-type neurons only exposed to secondary antibody.
Effect of TRPV4 deletion on thermal and mechanical responses
The latency of paw withdrawal to plantar application of radiant heat was the same at baseline in both TRPV4 +/+ and TRPV4 –/– mice. The latency of withdrawal decreased significantly at 1 and 24 h after the injection of NGF (1 μg·g−1; i.p.) in both TRPV4 +/+ and –/– mice (Figure 2A). The 50% withdrawal threshold to von Frey hairs of increasing thickness was the same at baseline in both TRPV4 +/+ and TRPV4 –/– mice. Injection of NGF (1 μg·g−1, i.p.) produced a significant decrease in withdrawal threshold after 1 and 24 h (Figure 2B). The baseline withdrawal pressure was the same in both TRPV4 +/+ and TRPV4 –/– mice. It was significantly reduced at 1 and 24 h in TRPV4 +/+ mice by systemic injection of NGF (1 μg·g−1, i.p.). However, NGF injection did not affect paw withdrawal to pressure in TRPV4 –/– mice (Figure 2C).
Figure 2.

Effect of systemic NGF (1 μg·g−1; i.p.) treatment on thermal and mechanical nociceptive thresholds in TRPV4 +/+ and –/– mice. (A). Latency of withdrawal to radiant heat, (B) 50% withdrawal threshold to application of von Frey hairs and (C) withdrawal pressure were measured at baseline and 1 and 24 h following NGF administration. *P < 0.05 compared with baseline, n = 10.
Effect of TRPV4 activators on cultured sensory neurons
The normal blood osmolarity in both the wild-type and knockout mice was measured as 295 mOsm (Liedtke and Friedman, 2003). Exposure of cultured DRG neurons from wild-type and TRPV4 knockout mice to hypo-osmotic challenge [decrease from isotonic buffer (292 mOsm) to 264, 234 or 206 mOsm] produced an increase in [Ca2+]i in a subset of neurons (Figure 3). The profile of the response to 206 mOsm buffer was the same in neurons from wild-type and TRPV4 knockout mice (Figure 3A, B). Decreasing the osmolarity increased the proportion of the neurons responding and the size of the change in [Ca2+]i in responding neurons. The proportion of responding neurons and the size of the change in [Ca2+]i in responding neurons was the same in neurons from wild-type and TRPV4 knockout mice (Figure 3C, D).
Figure 3.
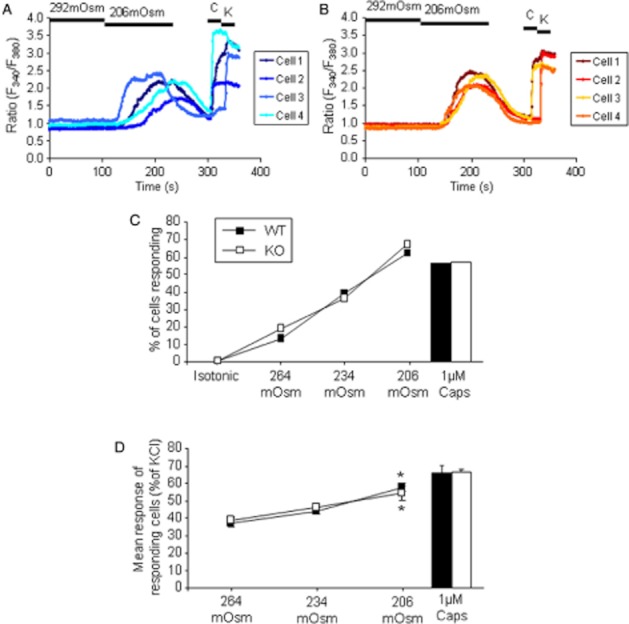
The effect of hypotonic challenge (264, 234 and 206 mOsm) on [Ca2+]i in cultured mouse dorsal root ganglion neurons from TRPV4 +/+ and –/– mice. Example response profiles of (A) TRPV4 +/+ and (B). TRPV4 –/– neurons to 206 mOsm buffer, 1 μM capsaicin (C) and 50 mM KCl (K). (C) The % of total neurons responding and (D) mean response of individual responding neurons relative to the response to KCl are also shown. n = 6–9 coverslips, with a total of at least 200 neurons from three mice of each genotype for each data point. *P < 0.05 compared with 264 mOsm, one-way anova followed by Dunnett's test.
Exposure of cultured DRG neurons from wild-type and TRPV4 knockout mice to the TRPV4 agonist 4α-PDD (1–10 μM) produced an increase in [Ca2+]i in a subset of neurons (Figure 4). The response profiles of these responding neurons can be divided into two main types: some neurons exhibited a transient increase in [Ca2+]i which then returned to baseline, whereas others showed a prolonged elevation in [Ca2+]i even after the removal of 4α-PDD. Neurons of both genotypes showed both types of response (Figure 4A, B). After exposure to 10 μM 4α-PDD, 64 of 145 responding wild-type neurons (44.1%) showed a transient response, whilst 81 of 145 responding neurons (55.9%) showed a sustained response. Similarly, 98 of 222 knockout neurons that responded to 10 μM 4α-PDD (44.1%) had a transient response, whereas 124 of 222 (55.9%) showed a sustained response. Increasing the concentration increased the proportion of the neurons responding and the size of the change in [Ca2+]i in responding neurons. The proportion of responding neurons and the size of the change in [Ca2+]i in responding neurons was the same in neurons from wild-type and TRPV4 knockout mice (Figure 4C, D).
Figure 4.

The effect of 4αPDD (1–10 μM) on [Ca2+]i in cultured mouse dorsal root ganglion neurons from TRPV4 +/+ and –/– mice. Example response profiles of (A) TRPV4 +/+ and (B) TRPV4 –/– neurons to 10 μM 4αPDD, 1 μM capsaicin (C) and 50 mM KCl (K). (C) The % of total neurons responding and (D) mean response of individual responding neurons relative to the response to KCl are also shown. n = 6–9 coverslips, with a total of at least 200 neurons from three mice of each genotype for each data point. *P < 0.05 compared with 1 μM 4αPDD, one-way anova followed by Dunnett's test.
In contrast, exposure of cultured DRG neurons from wild-type mice to the selective TRPV4 agonist GSK1016790A (100 nM–1 μM) failed to increase [Ca2+]i in a greater proportion of neurons than responded to the vehicle control (1% DMSO), with only a small minority of neurons responding (Figure 5A). The size of the change in [Ca2+]i in responding neurons was the same in response to either DMSO or GSK1016790A (Figure 5B). To confirm the ability of GSK1016790A to activate TRPV4, a dose–response curve was generated in HaCaT keratinocyte cells. GSK1016790A (5–500 nM) induced a dose-dependent increase in [Ca2+]i in these cells, with an EC50 of 43 ± 1 nM (Figure 5C). The TRPV4 antagonist HC067047 (5–2000 nM) dose-dependently inhibited the increase in [Ca2+]i to 100 nM GSK1016790A, with an IC50 of 350 ± 1 nM (Figure 5D).
Figure 5.

The effect of GSK1016790A (100 nM–1 μM) on [Ca2+]i in cultured mouse dorsal root ganglion neurons from TRPV4 +/+ mice. (A) The % of total neurons responding and (B) mean response of individual responding neurons relative to the response to KCl are shown. n = 8–9 coverslips, with a total of at least 300 neurons from three mice for each data point. (C) GSK1016790A (5 nM–500 nM) caused a dose-dependent increase in [Ca2+]i in HaCaT keratinocytes. (D) The TRPV4 antagonist HC067047 caused a dose-dependent inhibition of the increase in [Ca2+]i caused by application of 100 nM GSK1016790A to HaCaT keratinocytes.
Few studies have directly examined TRPV4 function in sensory neurons. However, neuronal responses that were absent in cells from TRPV4 knockout animals have been reported in a subset of thoracic DRG neurons (Lechner et al., 2011) and in trigeminal ganglia (TG) neurons (Chen et al., 2009). An increase in [Ca2+]i following exposure to 1 μM GSK1016790A was seen in a small number of neurons cultured from DRG at spinal levels T6-T12. Significantly more wild-type neurons responded to GSK1016790A than knockout neurons (17 of 261 WT vs. 1 of 252 KO; P < 0.001, Chi-squared test). In contrast, 46.3% of wild-type and 44.4% of knockout neurons showed an increase in [Ca2+]i following exposure to 10 μM 4α-PDD (Figure 6A). This was a transient, rather than sustained, increase in 34.7% of wild-type and 32.1% of knockout neurons. There was no significant difference in the size of the change in [Ca2+]i following exposure to either agonist between the genotypes (Figure 6B).
Figure 6.

(A) The % of cultured thoracic DRG and trigeminal ganglion neurons responding to GSK1016790A (1 μM) or 4αPDD (10 μM) with an increase in [Ca2+]i and (B) mean response of individual responding neurons relative to the response to KCl. n = 8–11 coverslips, with a total of at least 250 (thoracic) or 180 (TG) neurons from three mice of each genotype for each data point. (C) The % of total neurons responding to 234 mOsm buffer, 4αPDD (3 μM), AITC (100 μM) and capsaicin (1 μM) on [Ca2+]i in cultured mouse dorsal root ganglion neurons from TRPV4 +/+, TRPV1 –/– and TRPA1 –/– mice. n = 5–6 coverslips, with a total of at least 100 neurons from two mice for each data point.
Similarly, only a small number of cultured TG neurons of either genotype showed an increase in [Ca2+]i following exposure to 1 μM GSK1016790A, whereas greater numbers of both wild-type and TRPV4 knockout neurons responded to 10 μM 4α-PDD (Figure 6A). Again, there was no significant difference between the size of the increase in [Ca2+]i following exposure to these agonists in TG neurons of either genotype (Figure 6B).
Several agonists of TRP channels show poor selectivity and can activate multiple channels. TRPV1 and TRPA1 are both highly expressed in sensory neurons and are activated by numerous different chemical compounds. However, the proportion of neurons exhibiting an increase in [Ca2+]i following exposure to 222 mOsm buffer or 3 μM 4α-PDD was similar in cultures from wild-type, TRPV1 knockout and TRPA1 knockout mice (Figure 6C). In contrast, the increase in [Ca2+]i in response to the TRPV1 agonist capsaicin (1 μM) was abolished in TRPV1 –/– neurons, and the increase in [Ca2+]i in response to the TRPA1 agonist allylisothiocyanate (AITC, 100 μM) was almost entirely absent in TRPA1 –/– neurons (Figure 6C). No increase in [Ca2+]i in response to 234 mOsm buffer or exposure to 3 μM 4α-PDD was observed in neurons from wild-type mice in the absence of external Ca2+ (data not shown).
Addition of NGF (100 ng·mL−1) to the culture medium for the DRG neurons for the entire 18–24 h culture period had no effect on either the proportion of cells responding to submaximally stimulating hypotonic buffer (249 or 222 mOsm) or the mean response of these cells (Figure 7). Similarly, the number of cells showing an increase in [Ca2+]i after exposure to 4α-PDD (1 or 3 μM) and the mean size of this change was unaffected by pre-exposure to NGF (Figure 7).
Figure 7.
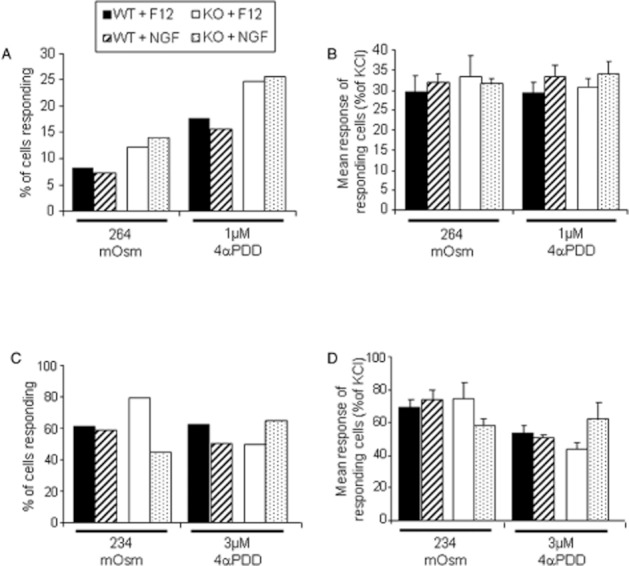
The effect of pre-treatment with NGF (100 ng·mL−1; 24 h) on responses to hypotonic buffer (264 mOsm and 234 mOsm) or 4αPDD (1 and 3 μM) in cultured mouse dorsal root ganglion neurons from TRPV4 +/+ (WT) and TRPV4 –/– (KO) mice. (A and C) The % of total neurons responding and (B and D) mean response of individual responding neurons relative to the response to KCl are shown. n = 8–9 coverslips, with a total of at least 200 neurons from three mice for each data point.
Discussion
These studies suggest that TRPV4 protein is expressed within dorsal root ganglia, and that deletion of the TRPV4 gene can affect mechanosensation in a whole animal. However, our experiments investigating TRPV4 activity in cultured DRG and TG neurons did not identify any differences in the responses of neurons collected from wild-type and TRPV4 knockout mice to various TRPV4 activators. Additionally, these findings do not support previous studies that claim that 4α-PDD is a selective activator of TRPV4.
Our Western blots, carried out with two different TRPV4 antibodies, confirm the presence of TRPV4 protein in whole DRG lysates. A doublet band, with molecular weights of approximately 98 and 104 kDa was observed. It is likely that the doublet is due to glycosylation of the mature TRPV4 protein, as demonstrated by Xu et al. (2006). It was absent in lysates from TRPV4 –/– mice, confirming its identity as TRPV4. However, it is noticeable that both antibodies also recognized bands at lower molecular weights in both wild-type and knockout lysates, suggesting that the antibodies are not truly selective for TRPV4. This has implications for using these antibodies in immunohistochemical study of its localization, as a positive reaction on tissue sections may occur in the absence of TRPV4.
TRPV4 wild-type and knockout mice showed no differences in their baseline sensitivity to radiant heat, and both showed a significant sensitization to a heat stimulus after treatment with NGF. TRPV4 does not play a major role in inflammatory thermal hyperalgesia (Huang et al., 2011), so this result was as expected. NGF promotes increased expression and sensitization of thermosensitive TRPV1 (Ji et al., 2002; Zhang et al., 2005), which probably underlies the observed hyperalgesia. Baseline responses to punctate mechanical stimulation with von Frey hairs and to application of pressure to the paw didn't vary between genotypes. This is in keeping with the majority of previous studies that found no basal difference in somatic sensation in TRPV4 –/– mice (e.g. Grant et al., 2007; Alessandri-Haber et al., 2008). Interestingly, both wild-type and knockout mice showed increased sensitivity to punctate stimuli after NGF, whereas only wild-type mice showed an increase in sensitivity to noxious pressure. This suggests that von Frey hairs and noxious pressure activate different patterns of neuronal and non-neuronal mechanosensory cells in the paw, with TRPV4 activation only required during the detection of pressure. The reason why the sensitization of responses to von Frey hairs by NGF is unaffected by TRPV4 deletion, in contrast to previous studies in chronic pain models where von Frey sensitivity is reduced (e.g. Alessandri-Haber et al., 2008), is unclear. Broadly, TRPV4 activation during mechanosensation seems to be more relevant to pathological conditions of hyperalgesia, such as during inflammation, rather than normal physiological sensation.
The ability to sense and respond to alterations in extracellular osmolarity is a fundamental homeostatic process, so redundancy in these pathways is unsurprising. Hypotonic challenge only produces an increase in [Ca2+]i in HEK cells (Xu et al., 2003; Grant et al., 2007) and CHO cells (Liedtke et al., 2000) after heterologous TRPV4 expression, confirming that decreased extracellular osmolarity can indeed activate the channel. In this study, our finding that hypotonic solutions increase [Ca2+]i as effectively in neurons from TRPV4 knockout mice as in wild-type neurons confirms that mechanisms other than TRPV4 activation can detect extracellular hypotonicity. A recent study by Lechner et al. (2011) identified a subpopulation of osmosensory thoracic DRG neurons in wild-type mice that were absent in TRPV4 –/– animals, suggesting that they require TRPV4 for their osmosensory properties. The vast majority of DRG neurons innervate somatic rather than visceral targets, so these specialized neurons may have also been present in our cultures, but not apparent amongst the far greater number of neurons that can apparently respond to extracellular hypo-osmolarity in the absence of TRPV4. When we focused solely on thoracic neurons, significantly more wild-type (17 of 261) than knockout (1 of 252) neurons responded to the TRPV4 agonist GSK1016790A, which may represent some of these rare TRPV4 expressing neurons. Although some trigeminal ganglia neurons are suggested to respond to hypotonicity via TRPV4 activation (Chen et al., 2009), we were unable to find any difference in the response to GSK1016790A or 4α-PDD in cultured TG neurons to support a functional expression of TRPV4 in these neurons.
The activation of TRPV4 by 4α-PDD was first described by Watanabe et al. (2002a). Exposure to 4α-PDD only produced a significant increase in [Ca2+]i in HEK cells and astrocytoma 1321N1 cells after transfection with TRPV4. We, and others, have also demonstrated a gain in responsiveness to 4α-PDD after transfecting TRPV4 into HEK cells that supports its identification as a TRPV4 agonist (Xu et al., 2003; Grant et al., 2007). However, in this study, we have shown that the increase in [Ca2+]i in cultured murine sensory neurons occurs independently of TRPV4, so it does not seem appropriate to describe 4α-PDD as a selective agonist. Of the many previous studies using 4α-PDD, only a few have confirmed the selectivity of its actions in cells or animals with deletion of TRPV4. Deletion of TRPV4 abolished the increase in cytoplasmic Ca2+ following application of 4α-PDD to murine cochlear hair cells (Shen et al., 2006), urothelial cells (Gevaert et al., 2007), ciliated pulmonary epithelial cells (Lorenzo et al., 2008), macrophages (Hamanaka et al., 2010), chondrocytes (Clark et al., 2010) and oesophageal keratinocytes (Mihara et al., 2011), all of which are non-neuronal. Thus 4α-PDD may selectively activate TRPV4 in certain cell types, but does not do so in DRG or TG neurons. In whole animal studies, the sensitization of viscera motor responses to colorectal distension by 4α-PDD (Cenac et al., 2008) and induction of paw oedema following intraplantar 4α-PDD injection (Vergnolle et al., 2010) were both abolished in TRPV4 knockout mice, and this was taken as evidence for a direct effect on sensory neuron TRPV4. However, an equally valid interpretation of these data is that the 4α-PDD is activating a non-neuronal cell to initiate the biological response.
To our knowledge, only one previous study has studied responses to 4α-PDD in sensory neurons from TRPV4 +/+ and –/– mice. Lechner et al. (2011) identified two distinct responses to 10 μM 4α-PDD: a reversible increase in [Ca2+]i and an irreversible increase, which they suggest is a non-specific toxic effect. The reversible increase was only observed in approximately 5% of TRPV4 +/+ neurons and was never seen in –/– neurons. In contrast to this, we found that approximately 30% (44% of the 70% of neurons that responded) of both +/+ and –/– neurons showed transient increases in [Ca2+]i to 10 μM 4α-PDD. When we focused on thoracic DRG neurons, we identified the transient increase in 34.7% of responding wild-type and 32.1% of responding knockout neurons. Lechner and colleagues may have failed to identify TRPV4 knockout neurons that transiently respond to 4α-PDD because of the relatively small number of neurons considered in their study. Overall, our data suggest that although 4α-PDD is a TRPV4 agonist, it is not selective for TRPV4 on sensory neurons.
Another interesting observation is that not only are responses to hypotonic challenge and 4α-PDD present in neurons from TRPV4 –/– mice, but the proportion of cells that respond and mean size of the response are unchanged. This was surprising, as previous studies have proposed the existence of functional TRPV4 in sensory neurons (e.g. Chen et al., 2009; Lechner et al., 2011). Additionally, the Western blots we performed on whole DRG lysates suggested expression of TRPV4 protein, although we were unable to confirm protein expression in individual cultured neurons by immunochemical staining. It would be expected that, even if hypotonic challenge and 4α-PDD do not selectively activate the channel, TRPV4 would contribute at least partially to the observed increase in [Ca2+]i. If this were the case, then a decrease in the number of responding neurons and/or size of their response would be predicted. To investigate this further, we tested the effects of the recently described synthetic TRPV4 agonist GSK1016790A (Thorneloe et al., 2008), on cultured neurons from wild-type mice. At concentrations that were maximally effective at stimulating an increase in [Ca2+]i in a keratinocyte cell line, no changes in [Ca2+]i greater than those in vehicle-treated neurons were observed. However, when we focused solely on neurons from thoracic DRG, a small group of responding neurons that were not present in knockout cells was observed. This suggests that functional TRPV4 is only present in a restricted subset of our cultured neurons, suggesting that the lack of immunostaining may represent a genuine absence of the protein.
One possible explanation for the TRPV4 expression in whole DRG lysates is that it is actually expression in the vasculature of the DRG or in other non-neuronal cells. TRPV4 expression and function has regularly been demonstrated in murine vascular endothelial cells (e.g. Watanabe et al., 2002b; Hartmannsgruber et al., 2007), so the entirety of the protein seen by Western blotting could be of vascular origin. Alternatively, neuronal TRPV4 expression could be lost under culture conditions. The cells were used for Ca2+ imaging and immunostaining within 24 h of collection to minimize this possibility, well within the culture time used in previous studies (e.g. Lechner et al., 2011). It has been suggested that exposure to NGF can increase cell surface presentation and activity of TRPV1, though phosphorylation of a tyrosine residue conserved in TRPV4 (Zhang et al., 2005). To determine whether TRPV4 required NGF for full functional expression, we supplemented our culture medium with 100 ng·mL−1 NGF. However, this had no significant effect on the responses to either hypotonic challenge or 4α-PDD, further supporting the conclusion that the cultured DRG neurons do not express functional TRPV4. Activating mutations in human TRPV4 have been linked to skeletal dysplasias such as brachyolmia (Rock et al., 2008) and spondylometaphyseal dysplasia (Krakow et al., 2009), and to motor neuropathies such as Charcot-Marie tooth disease type 2C (Landoure et al., 2010). These diseases are not associated with sensory abnormalities, suggesting that human sensory nerves also have little, if any, expression of TRPV4.
The mechanisms by which hypotonic solutions and 4αPDD can induce an elevation in neuronal [Ca2+]i independently of TRPV4 are not clear. The TRPV4 agonist 5′,6-EET has recently been shown to modulate neuronal activity through activation of TRPA1, rather than TRPV4 (Sisignano et al., 2012). However, we found that neurons lacking TRPA1 or TRPV1 still responded to both hypotonic buffer and 4αPDD, suggesting that these channels are not responsible. Neuronal expression of 5-HT3 receptors (Linz and Veelken, 2002), TRPC1 (Staaf et al., 2009) and TRPC5 (Gomis et al., 2008) have all previously been suggested to provide sensitivity to hypo-osmotic challenge. However, identification of the precise mechanisms responsible for hypotonic and 4αPDD-induced neuronal activation is beyond the scope of this study.
In conclusion, our data support the hypothesis that TRPV4 is involved in noxious mechanosensation, but this is not necessarily due to an activation of TRPV4 protein on sensory neurons. Indeed, we have failed to find any evidence for functional TRPV4 expression in the vast majority of DRG and TG neurons in culture. If this is also the case in vivo, the effects of TRPV4 agonists such as 4αPDD on nociceptive behaviours should be interpreted based on a non-neuronal site of TRPV4 activity, such as in the Merkel cells (Boulais et al., 2009) or keratinocytes of the skin (Chung et al., 2003), or as an action on an alternative target.
Acknowledgments
We thank Dr W Liedtke, Duke University, for his generous gift of TRPV4 knockout mice, which were generated originally at the Rockefeller University. These studies were supported by a Capacity Building Award in Integrative Mammalian Biology funded by the BBSRC, BPS, HEFCE, KTN and MRC. Additional funding came from the University of London Central Research Fund and the Royal Society.
Glossary
- 4αPDD
4α-phorbol 12,13-didecanoate
- AITC
allylisothiocyanate
- DRG
dorsal root ganglia
- NGF
nerve growth factor
- TG
trigeminal ganglia
- TRPV4
transient receptor potential vanilloid 4
Conflicts of interest
None.
References
- Alessandri-Haber N, Dina OA, Joseph EK, Reichling D, Levine JD. A transient receptor potential vanilloid 4-dependent mechanism of hyperalgesia is engaged by concerted action of inflammatory mediators. J Neurosci. 2006;26:3864–3874. doi: 10.1523/JNEUROSCI.5385-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessandri-Haber N, Dina OA, Joseph EK, Reichling DB, Levine JD. Interaction of transient receptor potential vanilloid 4, integrin, and SRC tyrosine kinase in mechanical hyperalgesia. J Neurosci. 2008;28:1046–1057. doi: 10.1523/JNEUROSCI.4497-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulais N, Pennec JP, Lebonvallet N, Pereira U, Rougier N, Dorange G, et al. Rat Merkel cells are mechanoreceptors and osmoreceptors. PLoS ONE. 2009;4:e7759. doi: 10.1371/journal.pone.0007759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenac N, Altier C, Chapman K, Liedtke W, Zamponi G, Vergnolle N. Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology. 2008;135:937–946. doi: 10.1053/j.gastro.2008.05.024. 946.e1–e2. [DOI] [PubMed] [Google Scholar]
- Ceppa E, Cattaruzza F, Lyo V, Amadesi S, Pelayo JC, Poole DP, et al. Transient receptor potential ion channels V4 and A1 contribute to pancreatitis pain in mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G556–G571. doi: 10.1152/ajpgi.00433.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Liu C, Liu L. Osmolality-induced tuning of action potentials in trigeminal ganglion neurons. Neurosci Lett. 2009;452:79–83. doi: 10.1016/j.neulet.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MK, Lee H, Caterina MJ. Warm temperatures activate TRPV4 in mouse 308 keratinocytes. J Biol Chem. 2003;278:32037–32046. doi: 10.1074/jbc.M303251200. [DOI] [PubMed] [Google Scholar]
- Clark AL, Votta BJ, Kumar S, Liedtke W, Guilak F. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62:2973–2983. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DM. TRPV4 and the mammalian kidney. Pflugers Arch. 2005;451:168–175. doi: 10.1007/s00424-005-1456-9. [DOI] [PubMed] [Google Scholar]
- Gevaert T, Vriens J, Segal A, Everaerts W, Roskams T, Talavera K, et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J Clin Invest. 2007;117:3453–3462. doi: 10.1172/JCI31766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis A, Soriano S, Belmonte C, Viana F. Hypoosmotic- and pressure-induced membrane stretch activate TRPC5 channels. J Physiol. 2008;586:5633–5649. doi: 10.1113/jphysiol.2008.161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AD, Cottrell GS, Amadesi S, Trevisani M, Nicoletti P, Materazzi S, et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J Physiol. 2007;578:715–733. doi: 10.1113/jphysiol.2006.121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanaka K, Jian MY, Townsley MI, King JA, Liedtke W, Weber DS, et al. TRPV4 channels augment macrophage activation and ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L353–L362. doi: 10.1152/ajplung.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmannsgruber V, Heyken WT, Kacik M, Kaistha A, Grgic I, Harteneck C, et al. Arterial response to shear stress critically depends on endothelial TRPV4 expression. PLoS ONE. 2007;2:e827. doi: 10.1371/journal.pone.0000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Li X, Yu Y, Wang J, Caterina MJ. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Jia Y, Wang X, Varty L, Rizzo CA, Yang R, Correll CC, et al. Functional TRPV4 channels are expressed in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L272–L278. doi: 10.1152/ajplung.00393.2003. [DOI] [PubMed] [Google Scholar]
- Krakow D, Vriens J, Camacho N, Luong P, Deixler H, Funari TL, et al. Mutations in the gene encoding the calcium-permeable ion channel TRPV4 produce spondylometaphyseal dysplasia, Kozlowski type and metatropic dysplasia. Am J Hum Genet. 2009;84:307–315. doi: 10.1016/j.ajhg.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landoure G, Zdebik AA, Martinez TL, Burnett BG, Stanescu HC, Inada H, et al. Mutations in TRPV4 cause Charcot-Marie-Tooth disease type 2C. Nat Genet. 2010;42:170–174. doi: 10.1038/ng.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Markworth S, Poole K, Smith ES, Lapatsina L, Frahm S, et al. The molecular and cellular identity of peripheral osmoreceptors. Neuron. 2011;69:332–344. doi: 10.1016/j.neuron.2010.12.028. [DOI] [PubMed] [Google Scholar]
- Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4-/- mice. Proc Natl Acad Sci U S A. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, et al. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell. 2000;103:525–535. doi: 10.1016/s0092-8674(00)00143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linz P, Veelken R. Serotonin 5-HT(3) receptors on mechanosensitive neurons with cardiac afferents. Am J Physiol Heart Circ Physiol. 2002;282:H1828–H1835. doi: 10.1152/ajpheart.00708.2000. [DOI] [PubMed] [Google Scholar]
- Lorenzo IM, Liedtke W, Sanderson MJ, Valverde MA. TRPV4 channel participates in receptor-operated calcium entry and ciliary beat frequency regulation in mouse airway epithelial cells. Proc Natl Acad Sci U S A. 2008;105:12611–12616. doi: 10.1073/pnas.0803970105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukin S, Zhou X, Su Z, Saimi Y, Kung C. Wild-type and brachyolmia-causing mutant TRPV4 channels respond directly to stretch force. J Biol Chem. 2010;285:27176–27181. doi: 10.1074/jbc.M110.143370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuyama R, Vriens J, Voets T, Karashima Y, Owsianik G, Vennekens R, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008;8:257–265. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Matthews BD, Thodeti CK, Tytell JD, Mammoto A, Overby DR, Ingber DE. Ultra-rapid activation of TRPV4 ion channels by mechanical forces applied to cell surface beta1 integrins. Integr Biol (Camb) 2010;2:435–442. doi: 10.1039/c0ib00034e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reportingexperiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihara H, Boudaka A, Sugiyama T, Moriyama Y, Tominaga M. Transient receptor potential vanilloid 4 (TRPV4)-dependent calcium influx and ATP release in mouse oesophageal keratinocytes. J Physiol. 2011;589:3471–3482. doi: 10.1113/jphysiol.2011.207829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Rock MJ, Prenen J, Funari VA, Funari TL, Merriman B, Nelson SF, et al. Gain-of-function mutations in TRPV4 cause autosomal dominant brachyolmia. Nat Genet. 2008;40:999–1003. doi: 10.1038/ng.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen J, Harada N, Kubo N, Liu B, Mizuno A, Suzuki M, et al. Functional expression of transient receptor potential vanilloid 4 in the mouse cochlea. Neuroreport. 2006;17:135–139. doi: 10.1097/01.wnr.0000199459.16789.75. [DOI] [PubMed] [Google Scholar]
- Sipe WE, Brierley SM, Martin CM, Phillis BD, Cruz FB, Grady EF, et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1288–G1298. doi: 10.1152/ajpgi.00002.2008. [DOI] [PubMed] [Google Scholar]
- Sisignano M, Park CK, Angioni C, Zhang DD, von Hehn C, Cobos EJ, et al. 5,6-EET is released upon neuronal activity and induces mechanical pain hypersensitivity via TRPA1 on central afferent terminals. J Neurosci. 2012;32:6364–6372. doi: 10.1523/JNEUROSCI.5793-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staaf S, Maxvall I, Lind U, Husmark J, Mattsson JP, Ernfors P, et al. Down regulation of TRPC1 by shRNA reduces mechanosensitivity in mouse dorsal root ganglion neurons in vitro. Neurosci Lett. 2009;457:3–7. doi: 10.1016/j.neulet.2009.03.082. [DOI] [PubMed] [Google Scholar]
- Strotmann R, Harteneck C, Nunnenmacher K, Schultz G, Plant TD. OTRPC4, a nonselective cation channel that confers sensitivity to extracellular osmolarity. Nat Cell Biol. 2000;2:695–702. doi: 10.1038/35036318. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K, Imai M. Impaired pressure sensation in mice lacking TRPV4. J Biol Chem. 2003;278:22664–22668. doi: 10.1074/jbc.M302561200. [DOI] [PubMed] [Google Scholar]
- Thorneloe KS, Sulpizio AC, Lin Z, Figueroa DJ, Clouse AK, McCafferty GP, et al. N-((1S)-1-{[4-((2S)-2-{[(2,4-dichlorophenyl)sulfonyl]amino}-3-hydroxypropanoyl)-1 -piperazinyl]carbonyl}-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), a novel and potent transient receptor potential vanilloid 4 channel agonist induces urinary bladder contraction and hyperactivity: part I. J Pharmacol Exp Ther. 2008;326:432–442. doi: 10.1124/jpet.108.139295. [DOI] [PubMed] [Google Scholar]
- Vergnolle N, Cenac N, Altier C, Cellars L, Chapman K, Zamponi GW, et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br J Pharmacol. 2010;159:1161–1173. doi: 10.1111/j.1476-5381.2009.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriens J, Owsianik G, Janssens A, Voets T, Nilius B. Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J Biol Chem. 2007;282:12796–12803. doi: 10.1074/jbc.M610485200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem. 2002a;277:13569–13577. doi: 10.1074/jbc.M200062200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem. 2002b;277:47044–47051. doi: 10.1074/jbc.M208277200. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Murakami M, Ohba T, Takahashi Y, Ito H. TRP channel and cardiovascular disease. Pharmacol Ther. 2008;118:337–351. doi: 10.1016/j.pharmthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Watson JJ, Allen SJ, Dawbarn D. Targeting nerve growth factor in pain: what is the therapeutic potential? BioDrugs. 2008;22:349–359. doi: 10.2165/0063030-200822060-00002. [DOI] [PubMed] [Google Scholar]
- Willette RN, Bao W, Nerurkar S, Yue TL, Doe CP, Stankus G, et al. Systemic activation of the transient receptor potential vanilloid subtype 4 channel causes endothelial failure and circulatory collapse: part 2. J Pharmacol Exp Ther. 2008;326:443–452. doi: 10.1124/jpet.107.134551. [DOI] [PubMed] [Google Scholar]
- Wu L, Gao X, Brown RC, Heller S, O'Neil RG. Dual role of the Trpv4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–F1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- Xu F, Satoh E, Iijima T. Protein kinase C-mediated Ca2+ entry in HEK 293 cells transiently expressing human TRPV4. Br J Pharmacol. 2003;140:413–421. doi: 10.1038/sj.bjp.0705443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Fu Y, Tian W, Cohen DM. Glycosylation of the osmoresponsive transient receptor potential channel TRPV4 on Asn-651 influences membrane trafficking. Am J Physiol Renal Physiol. 2006;290:F1103–F1109. doi: 10.1152/ajprenal.00245.2005. [DOI] [PubMed] [Google Scholar]
- Zhang X, Huang J, McNaughton PA. NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 2005;24:4211–4223. doi: 10.1038/sj.emboj.7600893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang YH, Ge HY, Arendt-Nielsen L, Wang R, Yue SW. A transient receptor potential vanilloid 4 contributes to mechanical allodynia following chronic compression of dorsal root ganglion in rats. Neurosci Lett. 2008;432:222–227. doi: 10.1016/j.neulet.2007.12.028. [DOI] [PubMed] [Google Scholar]


