Abstract
The proprotein convertases (PCs) furin and PACE4 process numerous substrates involved in tumor growth, invasion, and metastasis. We have previously shown that PCs increase the susceptibility to chemical skin carcinogenesis. Because of the human relevancy of UV radiation in the etiopathogenesis of human skin cancer, we investigated whether or not transgenic mice overexpressing either furin alone or both furin and PACE4 show increased susceptibility to UV carcinogenesis. After backcrossing our previously described furin and PACE4 transgenic lines, targeted to the epidermis, into a SKH-1 background, we exposed both single and double transgenic mice to UV radiation for 34 weeks. The results showed an increase in squamous cell carcinoma (SCC) multiplicity of approximately 70% in the single furin transgenic mouse line SF47 (P < .002) and a 30% increase in the other single transgenic line SF49 when compared to wild-type (WT) SKH-1 mice. Interestingly, there was also an increase in the percentage of high histologic grade SCCs in the transgenic lines compared to the WT mice, i.e., WT = 9%, SF47 = 15%, and SF49 = 26% (P < .02). Targeting both furin and PACE4 to the epidermis in double transgenic mice did not have an additive effect on tumor incidence/multiplicity but did enhance the tumor histopathologic grade, i.e., a significant increase in higher grade SCCs was seen in the bigenic mouse line SPF47 (P < .02). Thus, we observed an increased susceptibility to UV in single furin transgenic mice that was not substantially enhanced in the double furin/PACE4 transgenic mice.
Introduction
Skin carcinogenesis has been studied for almost a century based on very early observations made on chimney sweeps and by later experimentation using coal tar [1]. The use of carcinogenic and cocarcinogenic chemicals lead to the discovery of multiple steps during the process of skin carcinogenesis defined as tumor initiation, tumor promotion, and tumor progression. These operationally defined steps of carcinogenesis have resulted from the use of the two-stage carcinogenesis model or protocol that employs a subcarcinogenic dose of a polycyclic hydrocarbon followed by numerous topical applications of a hyperplasiogenic non-carcinogenic agent, usually a phorbol ester [2–4]. The experiments using this model have been very popular in the last half of the 20th century and resulted in numerous advances in our knowledge of skin carcinogenesis and of carcinogenesis in general. The model of two-stage carcinogenesis is considered a paradigm for the process of human cancer development permitting the dissection of the carcinogenesis process that is applicable to most cancer sites. Nevertheless, when we focus on the etiology of human skin cancer, including non-melanoma cancers, solar UV radiation is the main cause of skin cancer. There is overwhelming evidence that exposure to UV radiation is the principal cause of DNA damage and other molecular events and that UV acts as a complete carcinogen with both initiating and promoting properties [5].
Proprotein convertases (PCs) are a group of serine proteinases implicated in regulating numerous physiological and pathologic processes [6–8] by proteolytic activation of precursor proteins. The following PCs have been identified so far: PC1/3, PC2, furin, PC4, PC5/6, PACE4, PC7, SKI-1/S1P, and PCSK9 [8–11]. Most of these enzymes exert their functions by activating precursor protein substrates, cleaving them at basic residues within the motif (K/R)-(X)-n-(K/R). Many PC protein substrates are growth factors and their cognate receptors, as well as metalloproteinases and adhesion molecules. These substrates have considerable influence on the development of the neoplastic cell phenotype [12–24]. Numerous studies have highlighted the role of PC1, PC2, furin, PC5, PACE4, and PC7 in regulating the biologic behavior of tumors [25–30]. Many focused on furin, a ubiquitously expressed type I membrane-bound proteinase, e.g., our laboratory has reported that this PC is implicated in human squamous epithelial cancer cells of different origins [31–34].
Our laboratory and others determined their significant participation in cutaneous and non-cutaneous tumor development and growth [33–41]. In previous reports, we observed that transgenic expression of either furin or PACE4 in keratinocytes of the epidermal basal layer was more susceptible to chemical carcinogenesis [42,43], evidenced by increased tumor multiplicity, tumor volume, and metastasis. Moreover, inhibition of PCs in this model resulted in decreased cell proliferation and reduced tumor multiplicity and volume, effectively reducing tumor burden [44].
Motivated by the fact that UV exposure of human immortalized keratinocytes is able to induce the expression of one of the most studied PCs, namely, furin [45], and that UV is the principal human skin carcinogen, we decided to evaluate whether furin overexpression would increase the susceptibility of transgenic mice in which the furin transgene is targeted to the epidermis. Moreover, to investigate possible additive or synergistic effects between furin and PACE4, we developed double transgenic mice expressing both PCs targeted to the epidermis.
Materials and Methods
Animals and UV Treatment
Transgenic mice overexpressing furin, under the control of the bovine keratin 5 (K5) promoter, were designed and developed in an FVB background as described in a previous report [43]. Two K5-furin mouse lines (F47 and F49) were backcrossed for at least six generations to wild-type (WT) SKH-1 hairless mice obtained from Charles River Laboratories (Wilmington, MA) for transmission of the trans-gene and hairlessness. Mice were genotyped by polymerase chain reaction (PCR) analysis of tail DNA using primers specific for an internal fragment of human furin and the junction of furin with SV40 polyadenylation signal. The sequence of sense primer was 5′GTGCTGTCTCATCGATTTGGCAA3′ and that of antisense primer was 5′GCGGGCGGTGAGGCGACA3′. After obtaining the backcrossed K5-furin transgenic mouse lines (now called SF47 and SF49), we also obtained by the same backcrossing procedures a K5-PACE4 line on SKH-1 background that was derived from a previously developed mouse line originally in an FVB background [42]. This line, now called SPACE4, was crossed with either SF47 or SF49 to obtain double transgenic mouse lines (SPF47 and SPF49). Table 1 summarizes the genotype of the mice used in this report.
Table 1.
Nomenclature of Mouse Lines Used in the Short-Term and Carcinogenesis Experiments.
| Mouse Line | Furin Transgene | PACE4 Transgene |
| WT (SKH-1) | - | - |
| SF47 | + | - |
| SF49 | + | - |
| SPACE4 | - | + |
| SPF47 | + | + |
| SPF49 | + | + |
Note that all transgenic mouse lines have been backcrossed to an SKH-1 background.
All mice of SKH-1 background were housed in the Laboratory Animal Facility at Fox Chase Cancer Center according to the requirements established by the American Association for Accreditation of Laboratory Animal Care.
Mice were exposed dorsally to gradually increasing doses of ultraviolet B (UVB; starting with a dose of 0.25 kJ/m2 and increasing every 3 weeks by multiples of 0.25 kJ/m2 to reach the full dose of 1 kJ/m2). Philips TS40UVB lamps (American Ultraviolet Company, Murray Hill, NJ), fitted with Kodacel filters (Eastman Kodak, Rochester, NY), were used to deliver emissions in the UVB range of 290 to 320 nm [46]. The animals were irradiated two times weekly for 34 weeks. The mice were placed on a shelf 20 cm below the light tubes for irradiation. The cage order was rotated before each treatment to compensate for uneven lamp output [47,48]. UV radiation dosage was measured using a UVX radiometer (UVX-31) from UVP (Upland, CA).
RNA Extraction and Real-Time PCR
RNA was extracted using the RNAqueous Kit from Ambion, Inc (Austin, TX). Contaminating DNA from RNA preparations was removed using TURBO DNA-free (Ambion, Inc), and the purified RNA was quantified with Agilent 2100 Bioanalyzer in combination with RNA 6000 Nano LabCHip. One hundred twenty nanograms of RNA was reversely transcribed using the M-MLV reverse transcriptase (Ambion, Inc) and a mixture of anchored oligodT and random decamers. Real-time PCR assays for PCs were performed as described previously using similar primers [43] using an ABI 7900 HT instrument.
Tumor Induction and Histopathology
Tumor incidence and multiplicity were observed weekly. The type and number of mice per group in the first experiment involving single transgenic mice were given as follows: WT SKH-1 (n = 18), single furin transgenic line SF47 (n = 19), and single furin transgenic line SF49 (n = 19). In the double transgenic carcinogenesis experiment, we used the following animal groups: WT SKH-1 (n = 20), single transgenic K5 PACE4 [42] backcrossed to SKH-1 background, herein referred to as SPACE4 (n = 23), double transgenic SPF47 (n = 17), and double transgenic SPF49 (n = 19). Papillomas and squamous cell carcinomas (SCCs) were recorded by gross observation twice weekly and confirmed by histologic analysis at 34 weeks.
Autopsies of carcinoma-bearing mice were performed, and metastasis in axial lymph nodes, lung, liver, spleen, and other organs were recorded. All tumors were analyzed histologically. SCCs were classified according to histopathologic grade [49]. Most SCCs were endophytic growths that invaded the dermis and subcutaneous tissue. The differentiation patterns defining the histopathologic grade were given as follows: 1) grade I SCC, very well differentiated with most of the tumors containing keratinizing cells and horny pearls; 2) grade II SCC, moderately differentiated tumors in which up to 50% of the tumor mass is formed by keratinizing cells; 3) grade III SCC, poorly differentiated tumors, containing less than 25% tumor mass showing evidence of keratinization; and 4) grade IV SCCs, very poorly differentiated tumors or spindle cell carcinomas, containing very little or no histologic evidence of keratinization.
Keratinocyte Cultures and Western Blot Analyses
Primary epidermal keratinocytes from newborn mice were used to determine expression levels of furin because of their suitability for in vitro growth and further molecular analyses. Primary epidermal keratinocytes were established in vitro as described [50,51]. Briefly, 1- to 3-day-old mice were killed; the skin was washed in a 1:10 solution of Betadine and rinsed twice in sterile dH2O and twice in 70% alcohol. The skin was removed and floated overnight on 2 ml of dispase (Dispase II, 25 U/ml; BD Biosciences, Bedford, MA). The epidermis was separated from the dermis, minced, and incubated with 2 ml of 0.05% trypsin and 0.01% EDTA for 20 minutes at 37°C. DNase I (100 units) was added, and the cells were mechanically dissociated by vigorous pipetting followed by filtration through a 40-µ m cell strainer (BD Biosciences). The cells were washed in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and plated in a 1:2 mixture of keratinocyte growth medium (KGM) with Ca2+ (catalog number 3101; Cambrex, Waskerville, MD) and Ca2+-free KGM (catalog number 104; Cambrex) plus 4 ml of KGM supplements and growth factors (SingleQuot Kit, catalog number 4131; Cambrex) per 500 ml medium.
The keratinocytes grown in culture dishes were lysed and subjected to furin Western blot analysis according to our previously reported protocol [52]. A monoclonal antibody against human furin that recognizes the transgenic protein but not the endogenous furin (MON-152, ALX-803-017; ALEXIS, Plymouth Meeting, PA) was used as the primary antibody.
The processing of insulin-like growth factor-1 receptor (IGF-1R) in keratinocytes was performed by culturing keratinocytes to 80% to 90% confluency and subsequently treating cells with furin convertase inhibitor, decanoyl-RVKR-chloromethylketone (CMK, ALX-260-022; Enzo Life Sciences, Plymouth Meeting, PA), at different concentrations. Twenty-four hours after CMK treatment, the keratinocytes were lysed and subjected to Western blot analysis with a polyclonal antibody against IGF-1Rβ (C-20, sc-713; Santa Cruz Biotechnology, Santa Cruz, CA). The quantification of Western blot results was performed by using ImageJ developed by the National Institutes of Health.
Primary epidermal keratinocytes from WT and transgenic SKH-1 mice were grown in chamber slides and either sham exposed (0 kJ) or exposed to 0.5 and 1 kJ of UVB as described above to evaluate the acute effect of UV irradiation. Immunohistochemistry (IHC) of furin was evaluated 15 minutes, 5 hours, and 24 hours after irradiation using the IHC technique described below.
Analysis of Epidermal Thickness and Cell Proliferation Following UV Exposure
To evaluate whether furin expression alters the histology and proliferative ability of the epidermis, we compared the proliferative response to an acute exposure of UV radiation. Following the same exposure conditions as described above, we subjected the dorsal skin to UV exposure twice weekly (1.00 kJ/m2) for 2 weeks. Groups of WT or furin transgenic mice (n = 5 per group) were treated, and groups of untreated mice of the same characteristics were used as controls. All mice were killed 48 hours after the last treatment. Paraffin sections were obtained and stained with hematoxylin and eosin, and epidermal thickness was measured with the aid of a morphometry software that permits length measurements using digital imaging (Image Pro-Plus; Media Cybernetics, Silver Spring, MD). For analysis of cell proliferation, paraffin sections were used for detecting the proliferation marker Ki67, using a rat monoclonal antibody (clone TEC-3; Dako, Carpinteria, CA) and a biotinylated goat anti-rat IgG antibody (mouse adsorbed) together with an ABC Detection Kit (Vector Elite; Vector, Burlingame, CA). Slides were mounted and observed with a Nikon Optiphot with a Plan/Apo objective of x20, NA of 0.75, Nikon eyepiece of x10, and a final magnification of x200. Epidermal cell proliferation index was determined in the interfollicular epidermis by scanning the slides using an Aperio CS Scanscope scanner (Aperio, Vista, CA) and counting Ki67-positive and Ki67-negative epidermal basal keratinocytes and determining the percentage of positive cells (three to five mice per group; minimal number of cells counted per mouse was 500 per mouse).
IHC of Furin
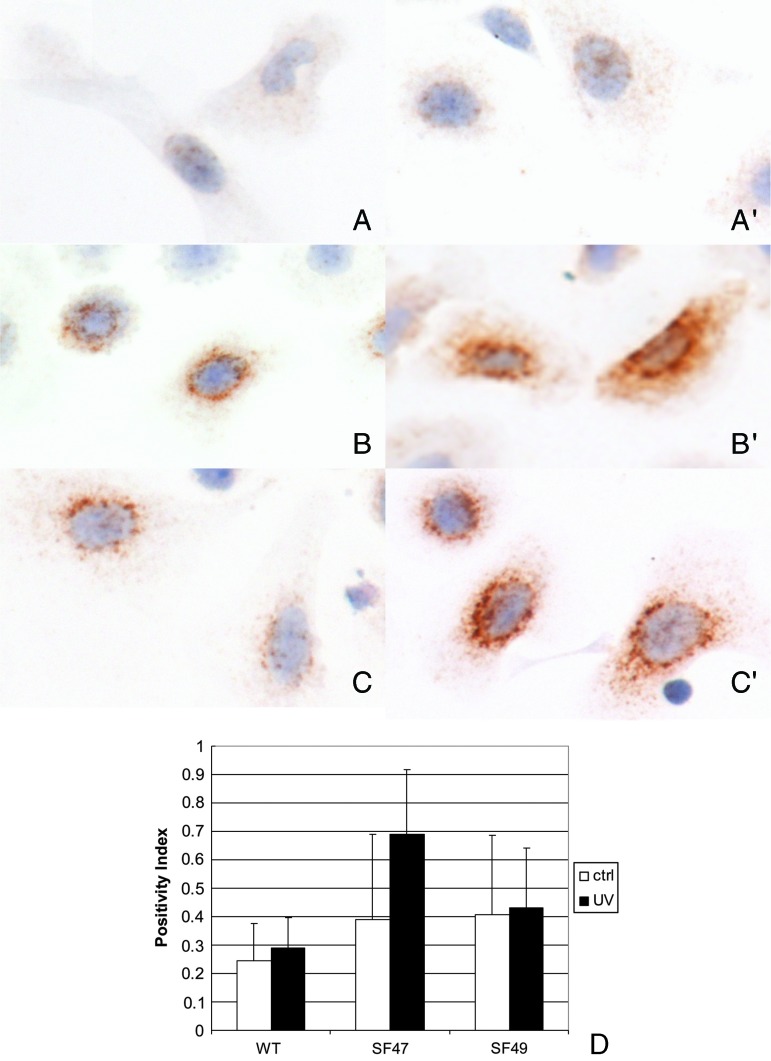
Furin IHC was performed using paraffin-embedded normal skin, normal internal organs, and cutaneous tumors from WT and transgenic mice. All paraffin sections were subjected to a previously published immunostaining protocol [49,53]. A monoclonal antibody against furin (MON-148, ALX-803-015; ALEXIS) was employed as primary antibody at a dilution of 1:200. Furin-positive cells were counted in the interfollicular epidermis and expressed as furin-positive cells per mm of epidermal basement membrane (BM). BM length was determined in furin-stained skin sections and the furin-positive cells of the respective interfollicular epidermal sector was counted using the above-mentioned morphometry software that permits length measurements of digitalized images. BM length was determined in control and UV-treated epidermis (three to five mice per group, minimal BM length per mouse was 2 mm per section). In addition, furin IHC intensity was evaluated in primary keratinocyte cultures counterstained with hematoxylin, which were then scanned with the Aperio ScanScope CS scanner using the Positive Pixel Count V9 algorithm and the ImageScope software built into the scanner. UV-irradiated and control cells were evaluated (n = 223 cells).
Statistical Analysis
Both two-sample t test and Wilcoxon test were used to compare tumor incidence and tumor volume. Wilcoxon test, a non-parametric test, was used when sample size was small. T test was used when the sample size in each comparison group was greater than 30. A P value of less than .05 was considered statistically significant. All statistical analyses were performed using SAS 9.2.
Results
Characterization of Transgenic Mice
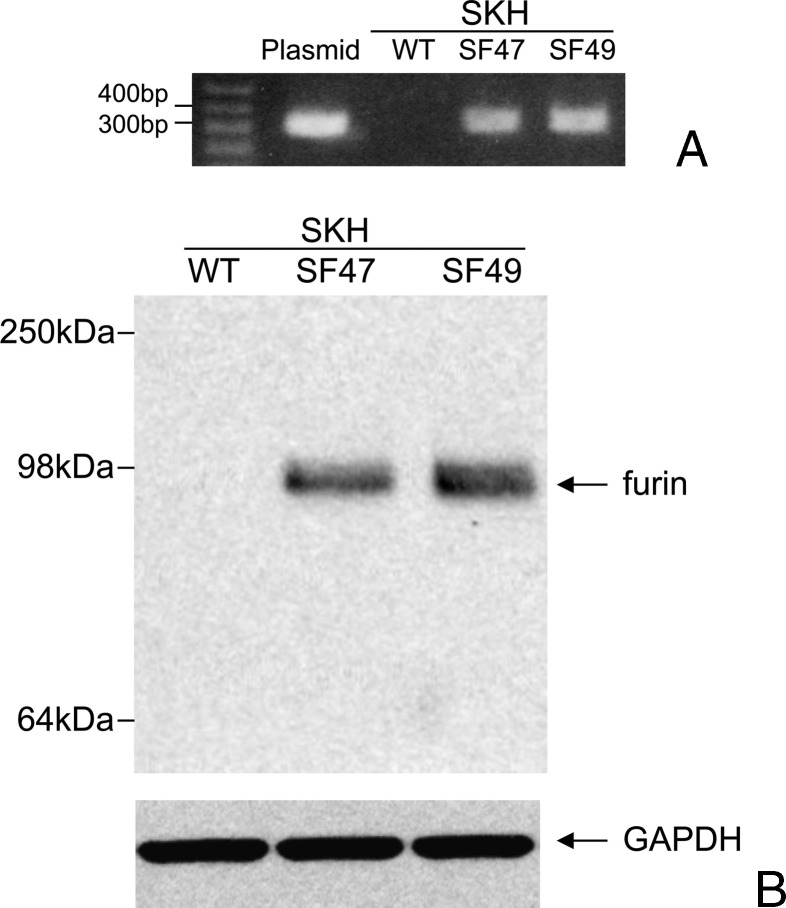
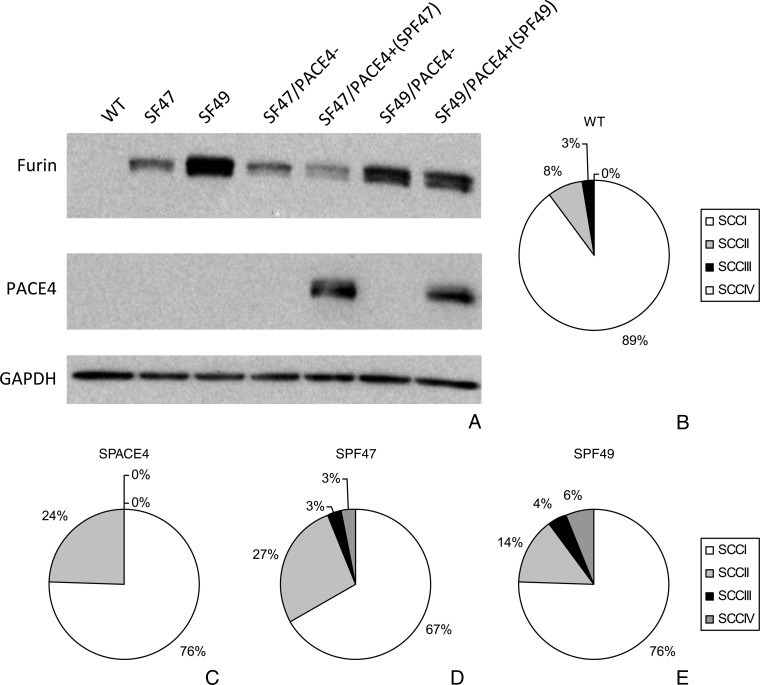
After backcrossing for six generations two K5-furin lines, originally developed in FVB background [43], to SKH-1 background, their transgenic nature was detected by PCR analysis. The two transgenic lines called SF47 and SF49 were genotyped by PCR of genomic DNA using primers amplifying a DNA segment between 300 and 400 bp, and a representative genotyping experiment is shown in Figure 1A. Transgene expression was confirmed by Western blot analysis of furin protein expression. Furin protein was clearly expressed in the keratinocytes from both transgenic mouse lines (molecular weight, ∼100 kDa). WT keratinocytes showed no transgenic furin expression (Figure 1B). Densitometric evaluation of four different Western blots demonstrated that line SF49-derived epidermal keratinocytes expressed approximately twice as much furin as line SF47-derived keratinocytes (ratio SF49/SF47 = 2.5). Double transgenics and SPACE4 mice were similarly characterized for PACE4; for genotyping, we used primers and procedures previously developed [42].
Figure 1.
Furin transgenic mice. (A) PCR from DNA extracted from mouse tails, showing WT and two transgenic animal lines (SF47 and SF49). (B) Western blot showing expression of human furin protein in cultured epidermal cells from transgenic lines.
Furin Expression in the Epidermis and Other Epithelia
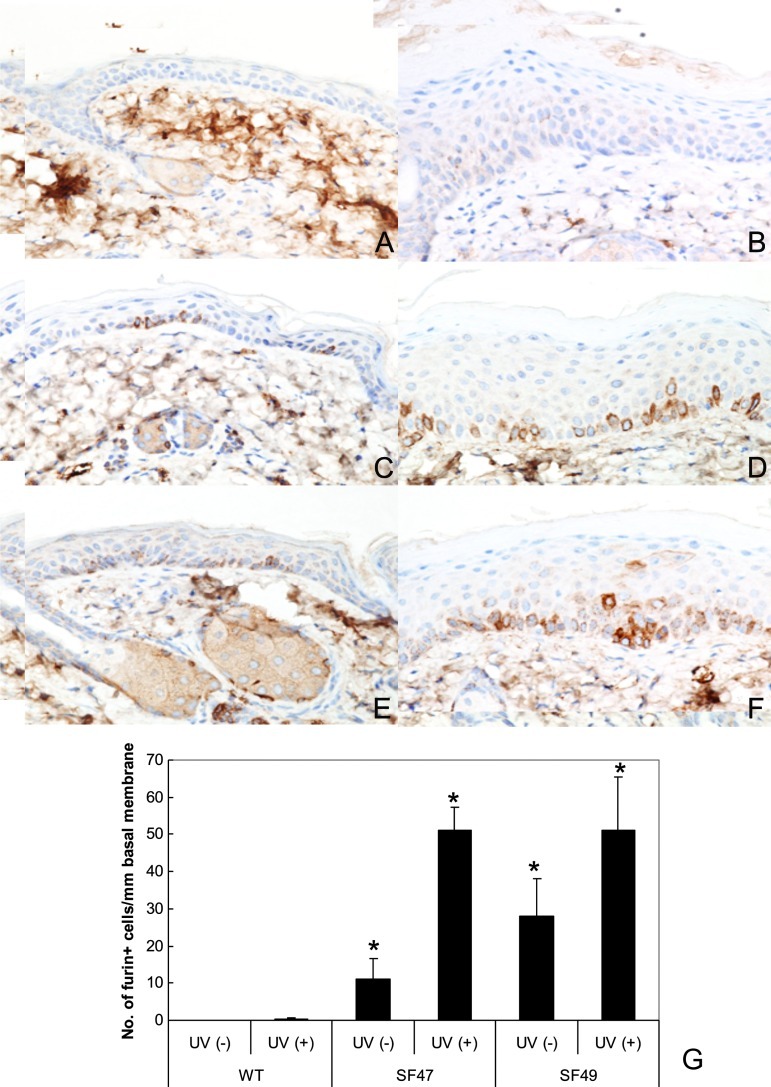
The distribution of furin expression in transgenic mice was further analyzed by IHC staining. In WT mice, furin expression was practically absent, although occasionally it could be observed as minimal punctuate staining in the perinuclear cytoplasm of basal epithelial cells in the epidermis. Conversely, furin was clearly detected by IHC in most squamous epithelia of transgenic mice, including tail epidermis, oral mucosa, genital mucosa, and biliary ducts as has been previously seen in similar transgenics of FVB background [43] (data not shown). The location of furin was cytoplasmic, predominately over-expressed in the epidermal basal layer, mostly undetectable in WT mice (Figure 2A), but clearly observed in both transgenic lines (Figure 2, C and E). Neither gross nor histologic phenotypic alterations were observed by the expression of furin in transgenic mouse lines.
Figure 2.
Immunohistochemistry of furin in dorsal mouse epidermis. Panels A (WT SKH-1), C (F47), and E (F49) depict control unirradiated epidermis. Panels B (WT), D (SF47), and F (SF49) show UV-irradiated epidermis. Both the unirradiated (A) and irradiated WT epidermis (B) show negative or marginal immunostain. Panel C (SF47) exhibits several furin-positive basal keratinocytes that further increased together with occasional positive parabasal keratinocytes after UV irradiation (D). Epidermis from SF49 (E) shows increased expression of furin in basal keratinocytes after UV exposure (F). Furin IHC and hematoxylin counterstain x 150. The histogram (G) shows an increase in the number of positive basal keratinocytes in transgenic epidermis. Asterisks indicate significantly different changes when compared with the respective WT (P < .001).
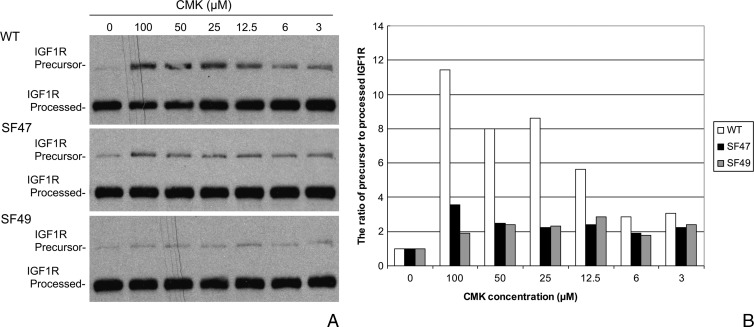
We examined the processing of IGF-1R, one of the most biologically relevant substrates of furin, in mouse-derived epidermal primary keratinocyte cultures. Interestingly, the difference of IGF-1R processing in keratinocytes derived from WT SKH-1 mice and SF47 and SF49 transgenic mice of the same background was marginal, suggesting that the endogenous furin expressed in WT keratinocytes efficiently processed pro-IGF-1R. To observe a difference in the activating ability of transgenic and WT-derived keratinocytes, we challenged the cells in vitro with different doses of the PC inhibitor CMK to investigate the sensitivity of primary keratinocytes to IGF-1R cleavage inhibition by CMK. As shown in Figure 3, a dose as low as 3 µM CMK wasable to inhibit PC-mediated IGF-1R processing in WT keratinocytes, and the inhibitory effect was directly proportional to the CMK dose up to a maximal effect at a dose of 100 µM. The effect was noted by the increasing presence of the IGF-1R proform with increasing concentrations of CMK (Figure 3). Conversely, the CMK treatment, even at the highest dose, failed to increase the levels of the IGF-1R pro-form in SF47- and SF49-derived cells, suggesting that the high level of furin expression in transgenic keratinocytes overcomes the inhibitor and that the PC is able to activate the IGF-1R substrate, leaving little proform behind.
Figure 3.
Western blot analysis of IGF-1R processing in WT and transgenic primary keratinocyte cultures (A). The cells were challenged in vitro with different doses of the PC inhibitor CMK to investigate the sensitivity of primary keratinocytes to IGF-1R cleavage inhibition by CMK; 3 µM CMK, and more clearly after 12.5 µM CMK, was able to inhibit PC-mediated IGF-1R processing in WT keratinocytes. The effect was noted by the presence of IGF-1R precursor or proform with increasing concentrations of CMK. Conversely, the CMK treatment failed to increase significantly the levels of the IGF-1R precursor in SF47- and SF49-derived cells. Panel B shows these changes as depicted by plotting the ratios of densitometric evaluations of IGF-1R precursor to processed bands.
Short-Term In Vitro Experiments
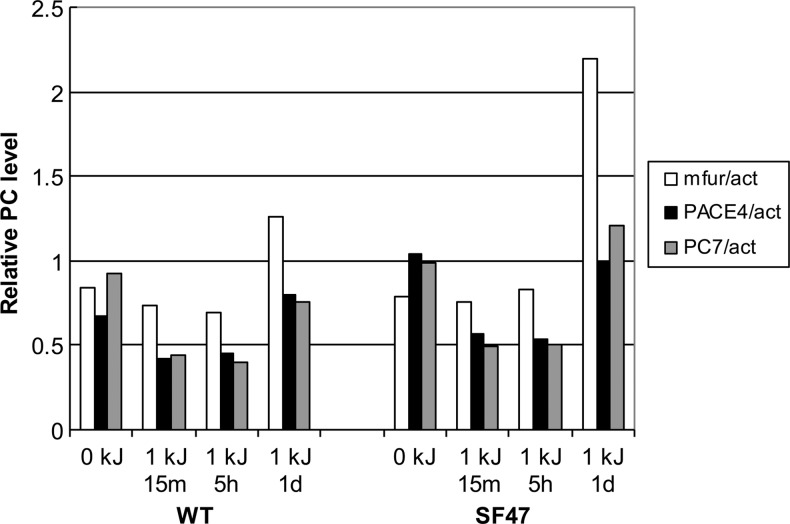
Since a previous report showed that UV exposure of human immortalized keratinocytes can induce up-regulation of furin [45], we exposed WT and transgenic-derived epidermal keratinocytes to UVB radiation. Contrary to the published data, WT mouse cultured keratinocytes showed no or marginal increase in three PCs at most time points studied (furin, PACE4, and PC7). Nevertheless, we found a slight increase in furin RNA 24 hours post-irradiation in keratinocytes derived from WT and SF47 mice (but not in SF49-derived cells, data not shown). Although, at the RNA level, transgenic keratinocytes showed a mild increase in furin transcript expression compared with their WT counterparts (Figure 4), when investigated at the protein level by quantitative image analysis of furin IHC, we observed a statistically significant increase in furin expression in keratinocytes derived from the SF47 mouse line after UV exposure when compared to UV-exposed WT keratinocytes (P < .01; Figure 5).
Figure 4.
Quantitative RT-PCR of PCs using mouse normal keratinocytes in short-term culture. All PC transcript expression values (y axis) are expressed as ratios between the respective PC and actin control expression. Note that the expression level of furin is increased at 24 hours (1 day) after UVB exposure in SF47 transgenic-derived keratinocytes but not at the other time points (0 kJ or untreated, 15 minutes and 5 hours). PCs detected were the endogenous mouse proprotein convertases furin (fur), PACE4, and PC7.
Figure 5.
Furin expression in murine epidermal keratinocytes in primary culture detected with IHC. Panels A, B, and C correspond to unirradiated cultures derived from WT SKH-1, SF47, and SF49 mice, respectively. Similarly A′, B′, and C′ correspond to UVB-irradiated WT (SKH-1)-, SF47-, and SF49-derived cultured keratinocytes. The cells were irradiated with 0.5 kJ as described in the text and evaluated 15 minutes post-exposure. Furin IHC and hematoxylin counterstain x 1000. Panel D shows image analysis values of furin positivity in the cytoplasm using a quantification procedure based on computerized image analysis as described in the Materials and Methods section.
Short-Term In Vivo Experiments
To evaluate differences in epidermal proliferation between WT and transgenic mice, we exposed the skin to two doses of UVB irradiation (0.5 kJ/m2 each, separated by 48 hours) and analyzed furin expression levels, epidermal thickness, and Ki67 index, 48 hours after the last UV administration. When comparing untreated WT, SF47, and SF49 epidermis, it was obvious that the number of strongly expressing furin basal epidermal keratinocytes was higher in SF47 and SF49 mouse epidermis than in WT epidermis (P < .001; Figure 2, A–G). Both SF47 and SF49 epidermis showed an increase in the number of furin-expressing keratinocytes after UV irradiation. This expression was similar in both transgenic mouse lines. Furin-expressing basal keratinocytes in SF47 and SF49 mouse epidermis was significantly enhanced with respect to WT following short-term UVB exposure (P < .001; Figure 2, B, D, and F). As expected, UV exposure increased keratinocyte proliferation as expressed by the variation in epidermal thickness and the number of positively stained nuclei using Ki-67 IHC in the skin samples from all groups (data not shown). This increment was approximately two-fold to three-fold when comparing epidermal thickness increase before and after irradiation in all groups but was more pronounced in SF47 mice (P < .01). Although the proliferation indices were significantly higher in irradiated cells vis-à-vis their unirradiated counterparts in each group studied, the increased proliferation rates after UV irradiation were similar in WT and transgenic mice.
Skin Carcinogenesis Experiments
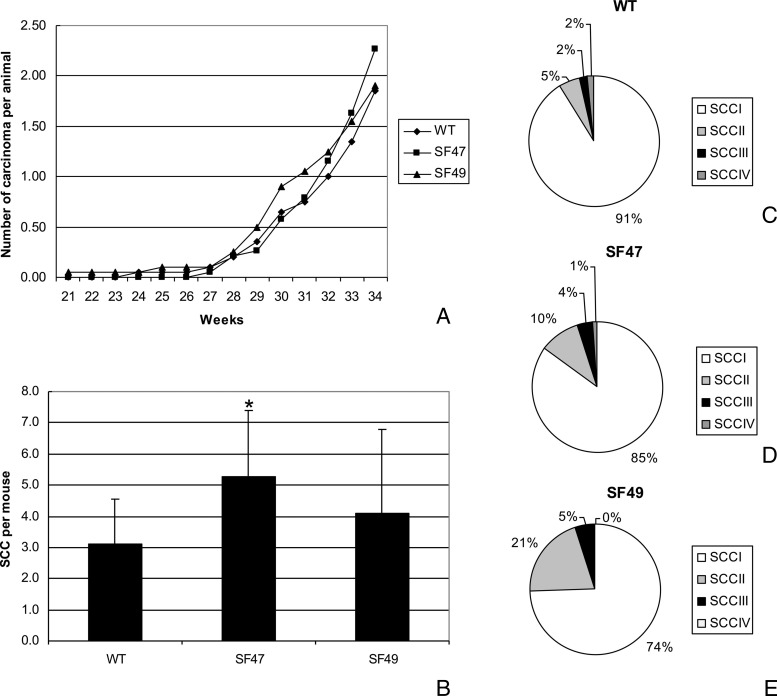
Single furin transgenic mice. To investigate the role of furin during UV carcinogenesis, we treated WT and transgenic animals with twice weekly exposures to UVB. Skin papillomas and carcinomas developed in both transgenic and WT groups at approximately 20 and 25 weeks, respectively. No statistically different incidence and multiplicity of papillomas were noted between WT and transgenic groups (data not shown). The prevalence of SCCs was extremely low in all groups until week 28, and it was in the last 6 weeks (week 28 to week 34) that the number of SCC/mouse increased but remained rather similar at 34 weeks in both transgenic lines and in WT mice (approximately two SSCs per mouse as seen grossly; Figure 6A). Mice from line SF47 developed a slightly higher number of SCC/mouse than did WT mice during the last few weeks of the experiment. However, histologic assessment (Figure 6B) showed that at 34 weeks the SCC multiplicity of SF47 (5.3 SCC/mouse versus the gross count of 2.3 seen in Figure 6A) was higher than that of WT (3.1 SCC/mouse; P < .002), whereas the increased carcinoma/mouse observed histologically in SF49 mice was not statistically significant. Analysis of the histopathologic grade of the SCCs in the different animal lines showed that in WT mice at 34 weeks the majority of SCC were of low-grade tumors, i.e., SCC I was 91% (Figure 6, C–E) and the percentage of high-grade SCC (SCC II to SCC IV) was 9%. Conversely, in the transgenic lines, there was an increase in the percentage of high-grade SCCs, i.e., 15% in SF47 mice and 26% in SF49 mice. Although there was a clear difference with respect to WT animals, only SF49 mice showed a statistically significant difference in percentage of high-grade tumors (P < .02).
Figure 6.
UV carcinogenesis experiments with single transgenic mice: Panel A depicts the tumor multiplicity as expressed by SCC/mouse during the course of the experiment as counted once weekly and determined by the presence of large (>10 mm) infiltrating or ulcerated lesions of the dorsal skin. Panel B shows the number of SCC/mouse at the last time point (34 weeks) as determined by the histopathologic evaluation of all tumors. Pie charts showing the distribution of SCCs of different histopathologic grades in WT SKH-1 mice (C), transgenic SF47 mice (D), and transgenic SF49 mice (E).
Double PACE4/furin transgenic mice. Both double transgenic lines generated (SPF47 and SPF49) showed intense expression of both PCs as detected by Western blot analysis (Figure 7A). To evaluate if the in vivo overexpression of both furin and PACE4 would be more effective in increasing the susceptibility to UV carcinogenesis than any of these PCs alone, we selected both double transgenic SPF47 and SPF49 and compared them with WT SKH-1 and mono-genic transgenic mice expressing PACE4 also on SKH-1 background (SPACE4). Surprisingly, both the single transgenic SPACE4 and the double transgenic mice were not significantly different from the WT SKH-1 mice in their susceptibility to UV carcinogenesis, at least based on number of tumors counted macroscopically during the experiment (data not shown). In addition, the tumor multiplicity curve and its values were also quite similar to those seen in Figure 6A, depicting tumor multiplicity in single furin transgenic mice. Nevertheless, following histopathologic evaluation at 34 weeks, double transgenic SPF47 mice showed higher percentage of high-grade histotypes than WT and single transgenic mice (P < .02) (Figure 7, B–D, also compare with the experiments with single furin transgenic mice depicted in Figure 6, C–E). Although not statistically significant, similar trends were observed in the SPF49 double transgenic mouse line (Figure 7E).
Figure 7.
UV carcinogenesis experiments with double transgenic mice: Western blot of epidermal skin keratinocytes derived from single and double transgenic mice (A). Pie charts showing the distribution of SCCs of different histopathologic grades in the mice groups used to evaluate PACE4 and furin double transgenic mice. WT SKH-1 mice (B), single transgenic SPACE4 (C), double transgenic SPF47 (D), and double transgenic SPF49 (E). Note the increase in the percentage of higher grade SCCs in the double transgenic line.
It is worth mentioning that occasional metastases were observed in lymph nodes and lungs. Because of the low numbers, it was not possible to establish any differences between the mouse groups.
Discussion
Furin is the most studied of all PCs and has been detected in numerous experimental and spontaneous malignancies. Furin is frequently overexpressed in several human cancers [32–34,38,41,54] and in chemically induced murine skin tumor cells [44]. Its role in the development and progression of neoplasia has been directly ascribed to its processing activity that results in the activation of many cancer-related proteins including important growth factors and receptors such as IGF-1 and its receptor IGF-1R, transforming growth factor-β, etc. [14,55–58].
In a previous report [43], we described two transgenic mouse lines in which furin was targeted to the epidermis using the K5 promoter (F47 and F49). Using these two single transgenic lines, we showed that the transgenic overexpression of furin is able to increase the susceptibility to skin carcinogenesis when mice were treated with the classic two-stage carcinogenesis protocol using 7,12-dimethylbenz(a)anthracene (DMBA) as the initiator and the phorbol ester tetradecanoylphorbol acetate (TPA) as the promoter [43]. Although chemical carcinogenesis of the skin and especially the multistage protocols have been extensively used as general models or paradigms of human epithelial carcinogenesis, in particular those of lung and head and neck [59,60], it is obvious that UV radiation is the most important etiological agent of human skin cancers. Because of this immediate relevancy to human melanoma and non-melanoma skin cancer, we backcrossed both lines to an SKH-1 background to be able to evaluate the effect of the epidermal over-expression of furin on the susceptibility of UV skin carcinogenesis.
The furin transgene, now on an SKH-1 background (SF47 and SF49), was expressed in the epidermis and also in several other squamous epithelia as we previously showed in similar mice on an FVB background. Interestingly, the difference in expression patterns between the two animal lines were different, i.e., in the FVB lines, F47 expressed more furin than line F49. Backcrossing of these lines to an SKH-1 background resulted in a relative increase of furin expression in line SF49 when compared to line SF47. IHC analysis of both lines revealed increased number of basal keratinocytes expressing furin. The enhanced furin expression in transgenic mice was reflected in higher levels of activated IGF-1R as demonstrated by Western blot analysis. Except for these differences from WT mice, the animals showed no spontaneous gross phenotype and remained healthy for at least 12 months.
The interesting observation that in human immortalized HaCaT keratinocytes UV irradiation induces an immediate increase in furin transcripts [45] was also noted to some extent in UV-irradiated transgenic keratinocytes derived from the SF47 mouse line. Nevertheless, this increase was mild compared to the 10-fold increase described by Skiba et al. [45]. Although this increment in furin transcripts in SF47 keratinocytes was only two-fold with respect to unirradiated keratinocytes (Figure 4), it does correlate with an increase in the in vivo carcinogenicity expressed as an almost two-fold increase in the tumor multiplicity of this mouse transgenic line (Figure 6B).
The main goal of this project was to demonstrate that the transgenic expression of furin in the epidermis would have an enhancing effect on skin UV carcinogenesis. During the entire experimental period after the appearance of benign tumors or papillomas, the mean number of papillomas/mouse was similar in both transgenic mouse lines and not significantly different from WT mice. Similarly, the mean number of SCC/mouse at 34 weeks of treatment with the UVB carcinogenesis protocol showed no major differences when the SCCs were counted macroscopically in the live animals. In contrast, when the tumors were verified by a detailed histopathologic analysis at 34 weeks, we found that the transgenic lines exhibited a higher incidence of SCCs than the control SKH-1 WT mice. This was caused by the presence of smaller non-ulcerated tumors that were judged clinically to be benign but at closer microscopical examination contained invasive cancers. Moreover, the percentage of well-differentiated tumors (SCC I) dropped significantly from 91% in the WT group to 85% in the SF47 mice and to 74% in the SF49 animals exposed to UVB. This corresponds to the significant increase in higher grade SCCs (SCC II to SCC IV), i.e., from 9% in WT to 15% in SF47 and to 26% in SF49 mice.
We also analyzed double transgenic mice that resulted from crossing either SF47 or SF49 mice expressing furin with the monogenic mouse line expressing PACE4, SPACE4. To our surprise, the incidence of SCCs in double transgenic mice was not different from any of the single transgenic mouse lines subjected to the same UVB carcinogenesis protocol. Nevertheless, single transgenic mice as well as the double transgenic mice had a significantly larger number of high-grade SCCs than the WT mice. Especially, the double transgenic SPF47 had 33% of SCC with grades higher than SCC I, whereas single transgenics had between 15% and 26% of SCC II to SCC IV and the WT SKH-1 had only 9% and 11%. The other double transgenic mouse line (SPF49) exposed to UV radiation did not show differences in tumor incidence and multiplicity when compared to single transgenic mice. The latter results may be explained by an in vivo superposition of functions or redundancy between the two PCs involved in the experiments with double transgenic mice. Several studies have evaluated or speculated on the eventual overlap and/or redundancy of PCs as it relates to their function and downstream effects. The development of knockout mice have permitted the evaluation of PCs during embryogenesis, and such studies have revealed that PCs may not be redundant in function since many of them are totally or partially lethal, as, for example, the furin (totally lethal) and PACE4 knockout mice (25% lethality) [61]. However, in adult organs, redundancy may be more common in some specific tissues [61]. It is possible that, to a certain extent, this also happens in our model of double transgenic mice.
The comparison of tumor data in single transgenic mice exposed either to chemical carcinogens [43] or to UVB radiation showed that furin transgenics are more susceptible to chemical carcinogenesis than to UVB. This comparison may not be straightforward, because mice of different backgrounds were used and only one standardized schedule of administration and dosage protocol for each of these experiments was performed. Nevertheless, it is congruent with the fact that in mice, depending on the strain used, UV is usually a weaker carcinogen than the stronger chemical carcinogens used in our two-stage carcinogenesis protocol [62,63].
In summary, furin expression targeted in vivo to the epidermis resulted in an increased pro-proliferative cancer-related substrate activation resulting in a pro-tumorigenic effect that was manifested in an increased number of UVB-induced higher grade SCCs late during the process of carcinogenesis. Moreover, the expected additive or synergistic effect of the two ubiquitous PCs, furin and PACE4, did not pan out as expected, i.e., double transgenics expressing furin and PACE4 showed no increased tumor incidence or multiplicity and only a moderately higher percentage of more aggressive (higher grade) SCCs than their single transgenic counterparts.
Acknowledgments
We thank the following core facilities for their help: Histopathology Facility, Transgenic Mouse Facility, Laboratory Animal Facility, Genomics Facility, and the Biostatistics and Bioinformatics Facility at Fox Chase Cancer Center.
Abbreviations
- IHC
immunohistochemistry
- PCs
proprotein convertases
- SCCs
squamous cell carcinomas
- WT
wild type
Footnotes
This work was supported by grants from the National Institutes of Health, (R01CA133001 and P30CA06927) and by an appropriation from the Commonwealth of Pennsylvania. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Yamagiwa K, Ishikawa K. Ueber die atypische Epithelwucherung. Verhandl Jap Path Ges. 1914;4:136. [Google Scholar]
- 2.Slaga TJ, DiGiovanni J, Winberg LD, Budunova IV. Skin carcinogenesis: characteristics, mechanisms, and prevention. Prog Clin Biol Res. 1995;391:1–20. [PubMed] [Google Scholar]
- 3.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 4.Yuspa SH, Dlugosz AA, Denning MF, Glick AB. Multistage carcinogenesis in the skin. J Investig Dermatol Symp Proc. 1996;1:147–150. [PubMed] [Google Scholar]
- 5.de Gruijl FR. Photocarcinogenesis: UVA vs. UVB radiation. Skin Pharmacol Appl Skin Physiol. 2002;15:316–320. doi: 10.1159/000064535. [DOI] [PubMed] [Google Scholar]
- 6.Seidah NG. What lies ahead for the proprotein convertases? Ann N Y Acad Sci. 2011;1220:149–161. doi: 10.1111/j.1749-6632.2010.05883.x. [DOI] [PubMed] [Google Scholar]
- 7.Seidah NG. The proprotein convertases, 20 years later. Methods Mol Biol. 2011;768:23–57. doi: 10.1007/978-1-61779-204-5_3. [DOI] [PubMed] [Google Scholar]
- 8.Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- 9.Molloy SS, Anderson ED, Jean F, Thomas G. Bi-cycling the furin pathway: from TGN localization to pathogen activation and embryogenesis. Trends Cell Biol. 1999;9:28–35. doi: 10.1016/s0962-8924(98)01382-8. [DOI] [PubMed] [Google Scholar]
- 10.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. 2003;100:928–933. doi: 10.1073/pnas.0335507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seidah NG, Mowla SJ, Hamelin J, Mamarbachi AM, Benjannet S, Toure BB, Basak A, Munzer JS, Marcinkiewicz J, Zhong M, et al. Mammalian subtilisin/kexin isozyme SKI-1: a widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc Natl Acad Sci USA. 1999;96:1321–1326. doi: 10.1073/pnas.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duguay SJ, Milewski WM, Young BD, Nakayama K, Steiner DF. Processing of wild-type and mutant proinsulin-like growth factor-IA by subtilisin-related proprotein convertases. J Biol Chem. 1997;272:6663–6670. doi: 10.1074/jbc.272.10.6663. [DOI] [PubMed] [Google Scholar]
- 13.Lehmann M, Andre F, Bellan C, Remacle-Bonnet M, Garrouste F, Parat F, Lissitsky JC, Marvaldi J, Pommier G. Deficient processing and activity of type I insulin-like growth factor receptor in the furin-deficient LoVo-C5 cells. Endocrinology. 1998;139:3763–3771. doi: 10.1210/endo.139.9.6184. [DOI] [PubMed] [Google Scholar]
- 14.Khatib AM, Siegfried G, Prat A, Luis J, Chretien M, Metrakos P, Seidah NG. Inhibition of proprotein convertases is associated with loss of growth and tumorigenicity of HT-29 human colon carcinoma cells: importance of insulin-like growth factor-1 (IGF-1) receptor processing in IGF-1-mediated functions. J Biol Chem. 2001;276:30686–30693. doi: 10.1074/jbc.M101725200. [DOI] [PubMed] [Google Scholar]
- 15.Cao J, Rehemtulla A, Pavlaki M, Kozarekar P, Chiarelli C. Furin directly cleaves proMMP-2 in the trans-Golgi network resulting in a nonfunctioning proteinase. J Biol Chem. 2005;280:10974–10980. doi: 10.1074/jbc.M412370200. [DOI] [PubMed] [Google Scholar]
- 16.Stawowy P, Meyborg H, Stibenz D, Borges Pereira Stawowy N, Roser M, Thanabalasingam U, Veinot JP, Chretien M, Seidah NG, Fleck E, et al. Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-pro-matrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation. 2005;111:2820–2827. doi: 10.1161/CIRCULATIONAHA.104.502617. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, Tortorella M, England K, Malfait AM, Thomas G, Arner EC, Pei D. Proprotein convertase furin interacts with and cleaves pro-ADAMTS4 (aggrecanase-1) in the trans-Golgi network. J Biol Chem. 2004;279:15434–15440. doi: 10.1074/jbc.M312797200. [DOI] [PubMed] [Google Scholar]
- 18.Komiyama T, Coppola JM, Larsen MJ, van Dort ME, Ross BD, Day R, Rehemtulla A, Fuller RS. Inhibition of furin/proprotein convertase-catalyzed surface and intracellular processing by small molecules. J Biol Chem. 2009;284:15729–15738. doi: 10.1074/jbc.M901540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yana I, Weiss SJ. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol Biol Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bergeron E, Basak A, Decroly E, Seidah NG. Processing of α4 integrin by the proprotein convertases: histidine at position P6 regulates cleavage. Biochem J. 2003;373:475–484. doi: 10.1042/BJ20021630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer G, Boileau G, Bendayan M. Furin interacts with proMT1-MMP and integrin αV at specialized domains of renal cell plasma membrane. J Cell Sci. 2003;116:1763–1773. doi: 10.1242/jcs.00394. [DOI] [PubMed] [Google Scholar]
- 22.Smirnov SP, McDearmon EL, Li S, Ervasti JM, Tryggvason K, Yurchenco PD. Contributions of the LG modules and furin processing to laminin-2 functions. J Biol Chem. 2002;277:18928–18937. doi: 10.1074/jbc.M201880200. [DOI] [PubMed] [Google Scholar]
- 23.Lissitzky JC, Luis J, Munzer JS, Benjannet S, Parat F, Chretien M, Marvaldi J, Seidah NG. Endoproteolytic processing of integrin pro-α subunits involves the redundant function of furin and proprotein convertase (PC) 5A, but not paired basic amino acid converting enzyme (PACE) 4, PC5B or PC7. Biochem J. 2000;346(pt 1):133–138. [PMC free article] [PubMed] [Google Scholar]
- 24.Lehmann M, Rigot V, Seidah NG, Marvaldi J, Lissitzky JC. Lack of integrin α-chain endoproteolytic cleavage in furin-deficient human colon adenocarcinoma cells LoVo. Biochem J. 1996;317(pt 3):803–809. doi: 10.1042/bj3170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khatib AM, Siegfried G, Chretien M, Metrakos P, Seidah NG. Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am J Pathol. 2002;160:1921–1935. doi: 10.1016/S0002-9440(10)61140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Cicco RL, Bassi DE, Benavides F, Conti CJ, Klein-Szanto AJ. Inhibition of proprotein convertases: approaches to block squamous carcinoma development and progression. Mol Carcinog. 2007;46:654–659. doi: 10.1002/mc.20331. [DOI] [PubMed] [Google Scholar]
- 27.Artenstein AW, Opal SM. Proprotein convertases in health and disease. N Engl J Med. 2011;365:2507–2518. doi: 10.1056/NEJMra1106700. [DOI] [PubMed] [Google Scholar]
- 28.D'Anjou F, Routhier S, Perreault JP, Latil A, Bonnel D, Fournier I, Salzet M, Day R. Molecular validation of PACE4 as a target in prostate cancer. Transl Oncol. 2011;4:157–172. doi: 10.1593/tlo.10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maret D, Sadr MS, Sadr ES, Colman DR, Del Maestro RF, Seidah NG. Opposite roles of furin and PC5A in N-cadherin processing. Neoplasia. 2012;14:880–892. doi: 10.1593/neo.121250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Couture F, D'Anjou F, Desjardins R, Boudreau F, Day R. Role of proprotein convertases in prostate cancer progression. Neoplasia. 2012;14:1032–1042. doi: 10.1593/neo.121368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassi DE, Lopez De Cicco R, Mahloogi H, Zucker S, Thomas G, Klein-Szanto AJ. Furin inhibition results in absent or decreased invasiveness and tumorigenicity of human cancer cells. Proc Natl Acad Sci USA. 2001;98:10326–10331. doi: 10.1073/pnas.191199198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bassi DE, Mahloogi H, Al-Saleem L, Lopez De Cicco R, Ridge JA, Klein-Szanto AJ. Elevated furin expression in aggressive human head and neck tumors and tumor cell lines. Mol Carcinog. 2001;31:224–232. doi: 10.1002/mc.1057. [DOI] [PubMed] [Google Scholar]
- 33.Bassi DE, Mahloogi H, Lopez De Cicco R, Klein-Szanto A. Increased furin activity enhances the malignant phenotype of human head and neck cancer cells. Am J Pathol. 2003;162:439–447. doi: 10.1016/s0002-9440(10)63838-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez de Cicco R, Bassi DE, Page R, Klein-Szanto AJ. Furin expression in squamous cell carcinomas of the oral cavity and other sites evaluated by tissue microarray technology. Acta Odontol Latinoam. 2002;15:29–37. [PubMed] [Google Scholar]
- 35.De Vos L, Declercq J, Rosas GG, Van Damme B, Roebroek A, Vermorken F, Ceuppens J, van de Ven W, Creemers J. MMTV-cre-mediated fur inactivation concomitant with PLAG1 proto-oncogene activation delays salivary gland tumorigenesis in mice. Int J Oncol. 2008;32:1073–1083. [PubMed] [Google Scholar]
- 36.Mahloogi H, Bassi DE, Klein-Szanto AJ. Malignant conversion of non-tumorigenic murine skin keratinocytes overexpressing PACE4. Carcinogenesis. 2002;23:565–572. doi: 10.1093/carcin/23.4.565. [DOI] [PubMed] [Google Scholar]
- 37.Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog. 2005;44:151–161. doi: 10.1002/mc.20134. [DOI] [PubMed] [Google Scholar]
- 38.Page RE, Klein-Szanto AJ, Litwin S, Nicolas E, Al-Jumaily R, Alexander P, Godwin AK, Ross EA, Schilder RJ, Bassi DE. Increased expression of the pro-protein convertase furin predicts decreased survival in ovarian cancer. Cell Oncol. 2007;29:289–299. doi: 10.1155/2007/930321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rounseville MP, Davis TP. Prohormone convertase and autocrine growth factor mRNAs are coexpressed in small cell lung carcinoma. J Mol Endocrinol. 2000;25:121–128. doi: 10.1677/jme.0.0250121. [DOI] [PubMed] [Google Scholar]
- 40.Hagiwara S, Murakumo Y, Mii S, Shigetomi T, Yamamoto N, Furue H, Ueda M, Takahashi M. Processing of CD109 by furin and its role in the regulation of TGF-β signaling. Oncogene. 2010;29:2181–2191. doi: 10.1038/onc.2009.506. [DOI] [PubMed] [Google Scholar]
- 41.Cheng M, Watson PH, Paterson JA, Seidah N, Chretien M, Shiu RP. Pro-protein convertase gene expression in human breast cancer. Int J Cancer. 1997;71:966–971. doi: 10.1002/(sici)1097-0215(19970611)71:6<966::aid-ijc10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 42.Bassi DE, Lopez De Cicco R, Cenna J, Litwin S, Cukierman E, Klein-Szanto AJ. PACE4 expression in mouse basal keratinocytes results in basement membrane disruption and acceleration of tumor progression. Cancer Res. 2005;65:7310–7319. doi: 10.1158/0008-5472.CAN-05-1213. [DOI] [PubMed] [Google Scholar]
- 43.Fu J, Bassi DE, Zhang J, Li T, Nicolas E, Klein-Szanto AJ. Transgenic overexpression of the proprotein convertase furin enhances skin tumor growth. Neoplasia. 2012;14:271–282. doi: 10.1593/neo.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bassi DE, Zhang J, Cenna J, Litwin S, Cukierman E, Klein-Szanto AJ. Proprotein convertase inhibition results in decreased skin cell proliferation, tumorigenesis, and metastasis. Neoplasia. 2010;12:516–526. doi: 10.1593/neo.92030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skiba B, Neill B, Piva TJ. Gene expression profiles of TNF-α, TACE, furin, IL-1β and matrilysin in UVA- and UVB-irradiated HaCat cells. Photodermatol Photoimmunol Photomed. 2005;21:173–182. doi: 10.1111/j.1600-0781.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 46.Wilgus TA, Koki AT, Zweifel BS, Kusewitt DF, Rubal PA, Oberyszyn TM. Inhibition of cutaneous ultraviolet light B-mediated inflammation and tumor formation with topical celecoxib treatment. Mol Carcinog. 2003;38:49–58. doi: 10.1002/mc.10141. [DOI] [PubMed] [Google Scholar]
- 47.Cooper SJ, MacGowan J, Ranger-Moore J, Young MR, Colburn NH, Bowden GT. Expression of dominant negative c-jun inhibits ultraviolet B-induced squamous cell carcinoma number and size in an SKH-1 hairless mouse model. Mol Cancer Res. 2003;1:848–854. [PubMed] [Google Scholar]
- 48.Beissert S, Bluestone JA, Mindt I, Voskort M, Metze D, Mehling A, Luger TA, Schwarz T, Grabbe S. Reduced ultraviolet-induced carcinogenesis in mice with a functional disruption in B7-mediated costimulation. J Immunol. 1999;163:6725–6731. [PubMed] [Google Scholar]
- 49.Ruggeri B, Caamano J, Slaga TJ, Conti CJ, Nelson WJ, Klein-Szanto AJ. Alterations in the expression of uvomorulin and Na+, K+-adenosine triphosphatase during mouse skin tumor progression. Am J Pathol. 1992;140:1179–1185. [PMC free article] [PubMed] [Google Scholar]
- 50.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3:799–810. doi: 10.1038/nprot.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Charpentier E, Lavker RM, Acquista E, Cowin P. Plakoglobin suppresses epithelial proliferation and hair growth in vivo. J Cell Biol. 2000;149:503–520. doi: 10.1083/jcb.149.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fu J, Jin F, Zhang J, Fong K, Bassi DE, Lopez De Cicco R, Ramaraju D, Braunewell KH, Conti C, Benavides F, et al. VILIP-1 expression in vivo results in decreased mouse skin keratinocyte proliferation and tumor development. PLoS One. 2010;5:e10196. doi: 10.1371/journal.pone.0010196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahloogi H, Gonzalez-Guerrico AM, Lopez De Cicco R, Bassi DE, Goodrow T, Braunewell KH, Klein-Szanto AJ. Overexpression of the calcium sensor visinin-like protein-1 leads to a cAMP-mediated decrease of in vivo and in vitro growth and invasiveness of squamous cell carcinoma cells. Cancer Res. 2003;63:4997–5004. [PubMed] [Google Scholar]
- 54.Mbikay M, Sirois F, Yao J, Seidah NG, Chretien M. Comparative analysis of expression of the proprotein convertases furin, PACE4, PC1 and PC2 in human lung tumours. Br J Cancer. 1997;75:1509–1514. doi: 10.1038/bjc.1997.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blanchette F, Day R, Dong W, Laprise MH, Dubois CM. TGFβ1 regulates gene expression of its own converting enzyme furin. J Clin Invest. 1997;99:1974–1983. doi: 10.1172/JCI119365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dubois CM, Blanchette F, Laprise MH, Leduc R, Grondin F, Seidah NG. Evidence that furin is an authentic transforming growth factor-β1-converting enzyme. Am J Pathol. 2001;158:305–316. doi: 10.1016/s0002-9440(10)63970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siegfried G, Khatib AM, Benjannet S, Chretien M, Seidah NG. The proteolytic processing of pro-platelet-derived growth factor-A at RRKR86 by members of the proprotein convertase family is functionally correlated to platelet-derived growth factor-A-induced functions and tumorigenicity. Cancer Res. 2003;63:1458–1463. [PubMed] [Google Scholar]
- 58.Lopez de Cicco R, Watson JC, Bassi DE, Litwin S, Klein-Szanto AJ. Simultaneous expression of furin and vascular endothelial growth factor in human oral tongue squamous cell carcinoma progression. Clin Cancer Res. 2004;10:4480–4488. doi: 10.1158/1078-0432.CCR-03-0670. [DOI] [PubMed] [Google Scholar]
- 59.Bassi DE, Klein-Szanto AJ. Carcinogen-induced animal models of head and neck squamous cell carcinoma. Curr Protoc Pharmacol. 2007 doi: 10.1002/0471141755.ph1402s37. Chapter 14, Unit14.2. [DOI] [PubMed] [Google Scholar]
- 60.Walaszek Z, Hanausek M, Slaga TJ. The role of skin painting in predicting lung cancer. Int J Toxicol. 2007;26:345–351. doi: 10.1080/10915810701490422. [DOI] [PubMed] [Google Scholar]
- 61.Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM. Pro-protein convertases: lessons from knockouts. FASEB J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 62.Steinel HH, Baker RS. Sensitivity of HRA/Skh hairless mice to initiation/promotion of skin tumors by chemical treatment. Cancer Lett. 1988;41:63–68. doi: 10.1016/0304-3835(88)90055-9. [DOI] [PubMed] [Google Scholar]
- 63.Strickland PT, Swartz RP. Inheritance of susceptibility to phototumorigenesis and persistent hyperplasia in F1 hybrids between SENCAR mice and BALB/c or C57BL/6 mice. Cancer Res. 1987;47:6294–6296. [PubMed] [Google Scholar]