Abstract
Background
Treatment outcomes for antiretroviral therapy (ART) patients may vary by gender, but estimates from current evidence may be confounded by disease stage and adherence. We investigated the gender differences in treatment response among HIV-positive patients virally suppressed within 6 months of treatment initiation.
Methods
We analyzed data from 7,354 patients initiating ART between April 2004 and April 2010 at Themba Lethu Clinic, a large urban public sector treatment facility in South Africa. We estimated the relations among gender, mortality, and mean CD4 response in HIV-infected adults virally suppressed within 6 months of treatment initiation and used inverse probability of treatment weights to correct estimates for loss to follow-up.
Results
Male patients had a 20% greater risk of death at both 24 months and 36 months of follow-up compared to females. Older patients and those with a low hemoglobin level or low body mass index (BMI) were at increased risk of mortality throughout follow-up. Men gained fewer CD4 cells after treatment initiation than did women. The mean differences in CD4 count gains made by women and men between baseline and 12, 24, and 36 months were 28.2 cells/mm3 (95% confidence interval [CI] 22.2–34.3), 60.8 cells/mm3 (95% CI 71.1-50.5 cells/mm3), and 83.0 cells/mm3 (95% CI 97.1-68.8 cells/mm3), respectively. Additionally, patients with a current detectable viral load (>400 copies/mL) and older patients had a lower mean CD4 increase at the same time points.
Conclusions
In this initially virally suppressed population, women showed consistently better immune response to treatment than did men. Promoting earlier uptake of HIV treatment among men may improve their immunologic outcomes.
Introduction
The widespread use of highly active antiretroviral therapy (HAART) has caused significant reductions in HIV-related mortality worldwide.1,2 Despite limited resources, HIV-infected individuals receiving antiretroviral therapy (ART) in resource-limited settings, including sub-Saharan Africa, have shown improvements in outcomes comparable to results from industrialized countries in terms of immunologic and virologic response.3–9 Even among patients who are adherent to treatment and achieve viral suppression, lack of immunologic response to ART has been noted to be an important predictor of subsequent mortality.10–12 As comprehensive HIV treatment involving ART is still relatively new in some resource-limited settings, however, gender differences in immunologic response to ART among those achieving viral suppression (<400 copies/mL) have not been well described. Differences by gender are critical to understand and address, particularly in a country like South Africa, which has the largest number of HIV-infected adults in the world and where >20% of women aged 20–24 years were estimated to be infected with HIV at the end of 2009.13
Despite the limited information to date, there are reasons to think that men and women may differ with respect to immune recovery even if virally suppressed, as treatment outcomes among those receiving ART (i.e., both suppressed and nonsuppressed patients) have shown differences. Although results from the developed world are conflicting,14–19 results from South Africa20 and India21 showed that women had lower mortality and higher CD4 counts after 1 year on ART. It is not clear from these studies, however, how much of this association relates to differences in immunosuppression at ART initiation, differences in immune recovery over time on ART, biologic differences by sex, or simply different patterns of adherence. If women show increased immunologic responses to ART after achieving viral suppression, this could explain the reduced mortality women have shown in South Africa.20 Additionally, high rates of loss to follow-up in some observational cohorts studied may introduce bias into the results presented.
Therefore, we set out to address the limitations of earlier studies by estimating the relations among gender, mortality, and mean difference in CD4 response at 12, 24, and 36 months on ART in the Themba Lethu Clinic, Johannesburg, South Africa, in a population with good mortality data through matching with the National Vital Registration system and by using inverse probability of censoring weights to correct estimates for loss to follow-up.
Materials and Methods
Cohort description
We conducted a cohort study of patients at the Themba Lethu Clinic. Themba Lethu is one of the largest ART clinics in South Africa, with nearly 32,000 patients enrolled in care since the clinic's inception in April 2004. Over 21,000 of these patients have been initiated on ART. Care was provided by clinic staff during the study period according to the 2004 South African National Department of Health guidelines.22 Use of data for this analysis was approved by the Human Research Ethics Committee of the University of the Witwatersrand. Approval for analysis of de-identified data was granted by the Institutional Review Board of Boston University.
Study population
Our analysis included nonpregnant, ART-naïve, HIV-positive patients >18 years of age, initiated onto standard public sector first-line ART regimens between April 2004 and April 2010. Pubic sector regimens include stavudine (d4T) or zidovudine (AZT) with lamivudine (3TC) and either efavirenz (EFV) or nevirapine (NVP). Patients with a baseline (within 6 months before ART initiation) CD4 count <350 cells/mm3 who suppressed their HIV viral load by 6 months on treatment were included in the analysis. Pregnant women were excluded, as they are initiated on different ART regimens and have more variable CD4 counts than the general treated population because of the hemodilution effect of pregnancy.23
Study variables
Patient-level data at Themba Lethu are captured in an electronic patient management system (Therapy Edge-HIV™). The primary exposure was patient gender. Baseline characteristics were stratified by gender and summarized with descriptive statistics. The primary dependent variables were mortality and mean change in CD4 cell count from baseline to 12, 24, and 36 months of treatment. Deaths are ascertained by either hospital notification, families notifying clinic staff, counselors identifying deaths through active tracing, or linkage with the South African National Vital Registration Infrastructure Initiative.24–26 Loss to follow-up was defined as having not attended a scheduled clinic visit within 4 months. For death, person-time accrued from ART initiation until the earliest of (1) death, (2) loss to follow-up, or (3) close of the dataset (March 31, 2011) was used. Transferred patients were censored at their last visit.
Statistical analysis
Clinical and demographic characteristics of the patients included in the analysis were summarized using simple proportions for categorical variables and medians with interquartile ranges (IQR) for continuous variables. We used a multivariate Cox proportional hazards model to calculate an adjusted association of gender with all-cause mortality. To calculate the association of gender with change in CD4 count from baseline to 12, 24, and 36 months, we used a multivariate linear generalized estimating equation (GEE) model. In both cases, we controlled for potential confounders, including age, time on ART, year of ART initiation, current tuberculosis infection, World Health Organization (WHO) stage, CD4 count, body mass index (BMI), and hemoglobin count.
In both models, we used inverse probability of censoring weights to adjust for selection bias due to loss to follow-up.27–29 Inverse probability of censoring weights reweight individuals who do not become lost to represent those who become lost and, thus, create a weighted pseudopopulation that represents the overall population had there been no censoring, subject to the exchangeability assumption.28 Stabilized weights were obtained by fitting two pooled logistic regression models for becoming lost, one controlling for baseline predictors of loss to follow-up (gender along with baseline same as the baseline covariates), and a second controlling for baseline covariates as well as time-updated and time-dependent predictors of becoming lost (current CD4 count, BMI, and hemoglobin). although similarly constructed weights (inverse probability of treatment weights) can be used to adjust for confounding,27 we did not apply these to this analysis, as treatment in this case is gender, which is time-fixed at baseline, which means that controlling for time-updated covariates in treatment weights may bias our estimates, as these may be affected by gender and not vice versa.
To directly address the issue of whether any differences in immunologic response by gender are actually a reflection of differences in patterns of adherences, our primary analysis was performed on those with a suppressed HIV viral load (<400 copies/mL) within the first 6 months after initiating ART. We did this to determine the relationship between gender and mortality and immunologic response among a population of HIV-infected adults who we could reasonably assume were at least initially adherent to treatment—in other words, where the association between gender and the measured outcomes was not likely to be mediated through adherence. We then also performed a mediation analysis where we adjusted all models for updated viral load status (suppressed or unsuppressed) to determine if the association of gender mediated through viral load (and, by proxy, adherence) differed from those in the main analysis.
Results
Of the 11,692 patients eligible for analysis, 8,119 (69%) had a viral load measurement at 6 months, and 7,354 (91%) of those achieved viral suppression and were included in our analysis. This included 63% (n=4,621) women and 37% (n=2,733) men. The majority of patients were on d4T/3TC/EFV (90.1%). Patients had a median CD4 count of 93 cells/mm3 (IQR 36–159), a median age of 36.7 years (IQR 31.6–43.0), and a median follow-up time on ART of 35.4 months (IQR 20.3–54.7) (Table 1). At ART initiation, men were older (median 37.9 vs. 35.8 years) and had more risk factors for poorer treatment outcomes than women. Men sought treatment with a lower median CD4 cell count (81 vs. 100 cells/mm3) and a lower median BMI (20.4 vs. 22.5 kg/m2) and were more likely to be classified with a WHO clinical stage III/IV condition (46.3% vs. 40.5%) than women.
Table 1.
Baseline Characteristics and Outcomes of ART Patients at Themba Lethu Clinic Stratified by Gender (n=7,354)
| |
Gender |
|
|
|---|---|---|---|
| Characteristic | Male (n=2,733) | Female (n=4,621) | Total (n=7,354) |
| Age, years | |||
| 18–24.9 | 49 (1.8%) | 274 (5.9%) | 323 (4.4%) |
| 25–29.9 | 252 (9.2%) | 747 (16.2%) | 999 (13.6%) |
| 30–39.9 | 1341 (49.1%) | 2090 (45.2%) | 3431 (46.7%) |
| 40–49.9 | 780 (28.5%) | 1129 (24.4%) | 1909 (25.9%) |
| >50 | 311 (11.4%) | 381 (8.3%) | 692 (9.4%) |
| CD4 at ART Initiation (cells/mm3) | |||
| 0–50 | 987 (36.1%) | 1330 (28.8%) | 2317 (31.5%) |
| 51–100 | 586 (21.4%) | 950 (20.6%) | 1536 (20.9%) |
| 101–200 | 887 (32.5%) | 1792 (38.8%) | 2679 (36.4%) |
| 201–350 | 228 (8.3%) | 480 (10.4%) | 708 (9.6%) |
| Missing | 45 (1.7%) | 69 (1.4%) | 114 (1.6%) |
| WHO stage at ART initiation | |||
| I/II | 1468 (53.7%) | 2750 (59.5%) | 4218 (57.4%) |
| III | 1057 (38.7%) | 1590 (34.4%) | 2647 (36.0%) |
| IV | 208 (7.6%) | 281 (6.1%) | 489 (6.6%) |
| First-line ART regimen | |||
| d4T/3TC/EFV | 2484 (90.9%) | 4143 (89.6%) | 6627 (90.1%) |
| d4T/3TC/NVP | 156 (5.7%) | 363 (7.9%) | 519 (7.0%) |
| AZT/3TC/EFV | 87 (3.2%) | 102 (2.2%) | 189 (2.6%) |
| AZT/3TC/NVP | 6 (0.2%) | 13 (0.3%) | 19 (0.3%) |
| CD4 at ART Initiation (cells/mm3) | |||
| Median (IQR) | 81 (29–151) | 100 (41–163) | 93 (36–159) |
| Time on ART (months) | |||
| Median (IQR) | 35.1 (19.5–53.9) | 35.8 (21.0–55.2) | 35.4 (20.3–54.7) |
| Hemoglobin at ART Initiation (μg/dL) | |||
| Median (IQR) | 12.5 (10.7–14.0) | 11.2 (9.8–12.5) | 11.7 (10.1–13.0) |
| BMI at ART Initiation | |||
| Median (IQR) | 20.4 (18.5–22.7) | 22.5 (19.7–25.9) | 21.6 (19.1–24.7) |
| Age at ART Initiation | |||
| Median (IQR) | 37.9 (33.3–43.9) | 35.8 (30.6–42.3) | 36.7 (31.6–43.0) |
| Outcomes | |||
| Death, n (%) | 143 (5.2) | 190 (4.1) | 333 (4.5) |
| Loss to follow-up, n (%) | 576 (21.1) | 806 (17.5) | 1382 (18.8) |
| Transferred, n (%) | 195 (7.1) | 403 (8.7) | 598 (8.1) |
| Alive and in care, n (%) | 1819 (66.6) | 3222 (69.7) | 5041 (68.6) |
ART, antiretroviral therapy; AZT, zidovudine; BMI, body mass index; d4T, stavudine; EFV, efavirenz; IQR, interquartile range; NVP, nevirapine; 3TC, lamivudine; WHO, World Health Organizations.
Mortality
By the end of the study follow-up, 333 (4.5%) patients had died; 1,382 (18.8%) were lost to follow-up, and 598 (8.1%) had transferred to another treatment facility. A higher proportion of men than women (5.2% vs. 4.1%, p=0.026) died during the follow-up period. Similarly, a larger percentage of males were lost than their female counterparts (21.1% vs. 17.4%, p<0.001). Although there was no difference in risk of death at 12 months on treatment, adjusted hazard ratios (HR) showed men had a 20% higher risk of death at both 24 months (HR 1.2, 95% confidence interval [CI] 0.9-1.6) and 36 months (HR 1.2, 95% CI 0.9-1.6) of follow-up compared to women (Table 2). Both these estimates, however, overlapped the null. Additionally, older age and anemia were predictive of death at 12, 24, and 36months of follow-up. Estimates were also adjusted for time on ART, baseline WHO stage, baseline hemoglobin, baseline BMI, tuberculosis status at ART initiation, and year initiated onto ART.
Table 2.
Crude and Adjusted Hazard Ratios of Mortality (n=7,020)
| |
|
12 months |
24 months |
36 months |
|||
|---|---|---|---|---|---|---|---|
| Variable | Crude (95% CI) | Adjusteda(95% CI) | Crude (95% CI) | Adjusteda(95% CI) | Crude (95% CI) | Adjusteda(95% CI) | |
| Gender | Male vs. female | 1.0 (0.7–1.5) | 1.0 (0.7–1.5) | 1.2 (0.9–1.6) | 1.2 (0.9–1.6) | 1.3 (1.0–1.6) | 1.2 (0.9–1.6) |
| Age | ≥40 vs. <40 | 1.3 (0.9–1.9) | 1.4 (1.0–2.0) | 1.4 (1.1–1.8) | 1.5 (1.1–1.9) | 1.3 (1.0–1.7) | 1.4 (1.1–1.8) |
| Baseline CD4 count (cells/mm3) | 100–199 vs. 200–350 | 0.7 (0.4–1.4) | 0.7 (0.4–1.2) | 0.8 (0.5–1.3) | 0.7 (0.4–1.1) | 0.9 (0.5–1.4) | 0.8 (0.5–1.3) |
| 50–99 vs. 200–350 | 1.1 (0.6–2.0) | 0.9 (0.5–1.7) | 1.1 (0.7–1.8) | 0.9 (0.5–1.5) | 1.3 (0.8–2.1) | 1.1 (0.6–1.8) | |
| 0–50 vs. 200–350 | 1.3 (0.7–2.4) | 1.1 (0.6–2.0) | 1.4 (0.9–2.2) | 1.0 (0.6–1.7) | 1.6 (1.0–2.5) | 1.2 (0.8–2.0) | |
| Baseline hemoglobin (μg/dL) | <10.0 vs. ≥10.0 | 1.6 (1.1–2.3) | 1.5 (1.0–2.3) | 1.6 (1.2–2.1) | 1.5 (1.1–2.1) | 1.6 (1.2–2.1) | 1.5 (1.1–2.0) |
| Baseline BMI (kg/m2) | <17.5 vs. ≥17.5 | 1.3 (0.8–2.1) | 1.1 (0.7–1.9) | 1.4 (1.0–2.1) | 1.2 (0.8–1.8) | 1.5 (1.0–2.1) | 1.2 (0.8–1.7) |
Models were also adjusted for time on ART, baseline WHO stage, baseline hemoglobin, baseline BMI, tuberculosis status at ART initiation, and year initiated onto ART.
Difference in CD4 count response
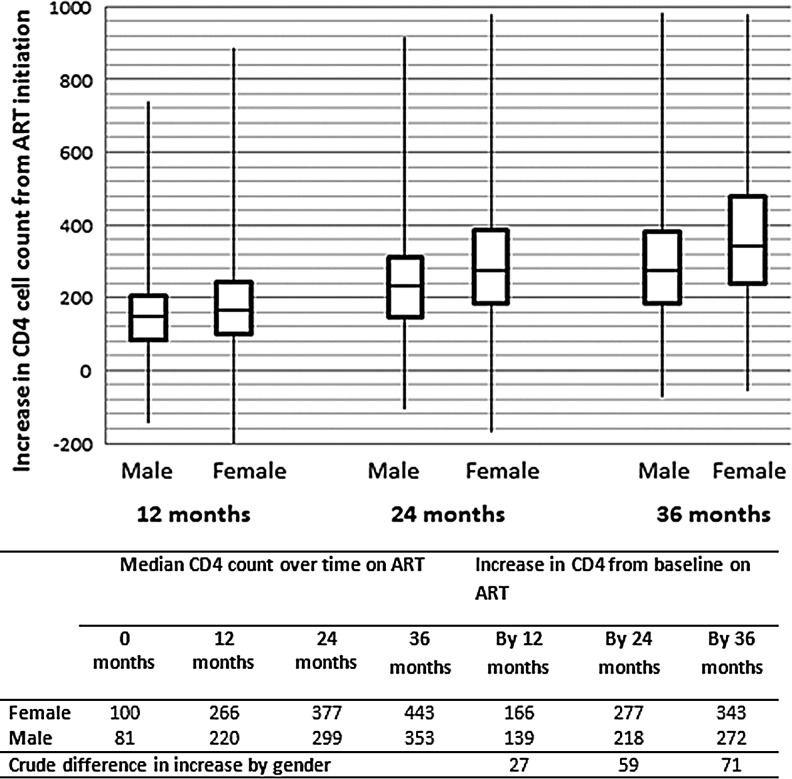
As expected, this population of initially virally suppressed patients showed good immune responses to treatment. Women demonstrated better median CD4 count increases from baseline than men across all time periods after ART initiation (Fig. 1): an increase of 166 cells/mm3 (IQR 99–245) vs. 139 cells/mm3 (IQR 82–204) by 12-months, 277 cells/mm3 (IQR 183–384) vs. 218 cells/mm3 (IQR 148–309) by 24 months, and 343 cells/mm3 (IQR 240–481) vs. 272 cells/mm3 (IQR 184–381) by 36 months on treatment. Estimates from adjusted models also demonstrated disadvantages in immunologic response for men at all time periods, and the differences, although small in the first 12 months on ART, increased with increasing time on treatment (Table 3). The mean differences in CD4 count gains made by women and men between baseline and 12, 24, and 36 months were 28.2 cells/mm3 (95% CI 22.2–34.3), 60.8 cells/mm3 (95% CI 71.1-50.5 cells/mm3), and 83.0 cells/mm3 (95% CI 97.1-68.8 cells/mm3), respectively. Additionally, patients with lower CD4 cell count at ART initiation and more advanced age (>40 vs. <40 years) demonstrated a poorer CD4 cell count increase from baseline to 12, 24, and 36 months of follow-up.
FIG. 1.
Median increase in CD4 cell count at 12, 24, and 36 months of antiretroviral therapy (ART), stratified by gender (n=7,354).
Table 3.
Crude and Adjusted Difference in Mean CD4 Count at 12, 24, and 36 Months of Follow-Up from Baseline (n=7,020)
| |
|
12 months |
24 months |
36 months |
|||
|---|---|---|---|---|---|---|---|
| Variable | Crude (95% CI) | Adjusteda(95% CI) | Crude (95% CI) | Adjusteda(95% CI) | Crude (95% CI) | Adjusteda(95% CI) | |
| Gender | Female vs. male | 29.5 (23.2–35.8) | 28.4 (22.2–34.3) | 56.2 (46.2–66.2) | 60.5 (50.2–70.8) | 76.2 (62.2–90.2) | 83.2 (69.0–97.4) |
| Baseline | 100–199 vs. 200–350 | 13.3 (1.2–25.4) | 8.7 (-3.5–20.9) | 13.5 (-5.8–32.8) | 11.4 (-7.8–30.5) | 26.5 (-2.2–55.2) | 25.0 (-2.3–52.4) |
| CD4 count (cells/mm3) | 50–99 vs. 200–350 | 19.2 (6.7–31.7) | 12.5 (-0.1–25.2) | 26.9 (6.2–47.5) | 23.6 (2.9–44.2) | 53.4 (22.4–84.4) | 49.1 (19.3–78.8) |
| 0–50 vs. 200–350 | 29.2 (17.4–40.9) | 20.7 (8.6–32.9) | 57.0 (37.8–76.1) | 53.4 (34.0–72.8) | 89.6 (60.7–118.6) | 88.2 (60.0–116.5) | |
| Age | <40 vs. ≥40 | 30.3 (24.0–36.6) | 25.5 (19.3–31.7) | 40.4 (29.8–50.9) | 32.9 (22.4–43.4) | 41.6 (27.0–56.3) | 31.9 (17.4–46.3) |
Models were also adjusted for time on ART, baseline WHO stage, baseline hemoglobin, baseline BMI, tuberculosis status at ART initiation, and year initiated onto ART.
Our results demonstrate similar findings to those from previous studies despite accounting for selection bias due to loss to follow-up. This, however, could be because loss to follow-up is, in fact, a mixture of various outcomes, including death,26 so the usefulness of CD4 count in predicting loss to follow-up may be limited. In a sensitivity analysis, we included a second set of weights to adjust for death. These weights were constructed similarly to the censoring weights and made no qualitative difference to our results.
Mediation analysis
We performed mediation analyses estimating the relationships among gender and mortality and increase in CD4 cell count mediated through adherence by adjusting models for current HIV viral load status (suppressed to <400 copies/mL or not). The risk of death for men compared to women was unchanged at 12 months on treatment (HR 1.0, 95% CI 0.9-1.6), and men again had a 20% higher risk of death at both 24 (HR 1.2, 95% CI 0.9-1.6) and 36 months (HR 1.2, 95% CI 1.0-1.6) of follow-up compared to women. The estimates of difference in CD4 count increase also showed very similar results to the primary analysis: men had disadvantages in immunologic response for all time periods, and the differences again increased with increasing time on treatment. The difference in CD4 count gains made by women compared to men between baseline and 12 months was 28.2 cells/mm3 (95% CI 22.0-34.3) greater for women. By 24 months, the difference was 60.8 cells/mm3 (95% CI 71.1-50.5), and by 36 months, this had increased to 83.0 cells/mm3 (95% CI 97.1-68.8) favoring women.
We performed further analyses restricted to those with suppressed viral loads at each time point considered. The proportion missing viral load data was similar at the time points considered (18% at 12 months, 17% at 24 months, and 17% at 36 months), and the proportion missing was similar for women and men (17% vs. 18% at 12 months, 18% vs. 17% at 24 months, and 16% vs. 19% at 36 months). Results were almost identical to the primary analysis for mortality. The estimates of difference in CD4 count increase still demonstrated disadvantages for men for all time periods, although the differences were somewhat smaller than those in the primary analysis. The difference in CD4 count gains made by women compared to men was 23.3 cells/mm3 (95% CI 18.5-28.2) greater for women between baseline and 12 months, 35.5 cells/mm3 (95% CI 30.0-41.1) by 24 months, and 44.5 cells/mm3 (95% CI 35.5-53.9) by 36 months, favoring women.
Discussion
In this analysis of adults initiating treatment at a public sector clinic in Johannesburg, South Africa, we demonstrate gender differences in immunologic response to ART. Controlling for baseline clinical factors (WHO stage and CD4 count) and age at ART initiation, we showed that women demonstrated a small but consistently higher increase in CD4 cell count over 36 months of follow-up than men. Our results are in agreement with previous studies of treatment outcomes in the region, which have shown greater CD4 count increases among women in addition to possible advantages for women in terms of virologic outcomes.5,18,30,31
Gender disparate outcomes are of particular concern in South Africa, which has the largest number of HIV-infected adults in the world and an estimated 20% of all those receiving ART globally.13 Estimates of gender differences in rates of HIV progression and virologic and immunologic responses to ART have varied.32,33 Early studies suggested that clinical disease progression was more rapid in women than men.34–36 These differences were attributed to women being less likely to initiate ART17 or receive treatment for opportunistic infections, more likely to experience violence and discrimination, gynecologic morbidity,37 and pregnancy,38–40 and also more likely to be anemic and younger at the time of ART initiation.41 Conversely, more recent studies have shown that women have a lower risk of morbidity and mortality42 and better virologic and immunologic outcomes than men.5,18,30,43
Although the actual differences in immunologic response to ART in our study were relatively small early after initiating treatment, these differences tended to increase with increasing time on treatment. This result alone may have important public health implications because in sharp contrast to most European and North American cohorts, proportionally more women than men receive ART in Southern African cohorts.41,44 In our own cohort, for example, >60% of those receiving ART were women. Thus, even modest differences in absolute risk may have profound implications for a public health approach to the administration of mass ART in South and sub-Saharan Africa.
Previous studies have shown that baseline CD4 cell count strongly predicts immunologic recovery.12 In our study, men started treatment at lower median CD4 counts, which could account for part of the persistent difference in CD4 gain shown over time. We adjusted for baseline CD4 cell count in all models in an attempt to account for these differences. However, if disproportionately more men than women failed treatment over time, this may further explain any difference in immunologic response. Previous studies that have shown gender differences in clinical and immunologic outcomes have postulated that that is owing to delayed presentation and access to HIV care and also poor adherence once on treatment, and women in this study presented at an earlier disease stage than men. Findings were similar in both sub-Saharan Africa5,41 and the United States,45 where women had higher baseline CD4 counts and were less likely to have an AIDS diagnosis at the time of starting ART. Studies from other African countries suggest that women may seek medical help earlier than men.44,46 If men seek care with more advanced disease and with lower CD4 counts, they are likely to suffer poorer outcomes, even after ART initiation.3,12 Financial constraints have been cited as obstacles to accessing and remaining in care7–9,12,47,48; if this is the case, however, we would expect to see fewer women accessing care, as they have traditionally been overrepresented among the poorest groups in society. In fact, Braitstein et al.41 showed that women in resource limited settings were equally or more likely to access HIV care and initiate ART than men. Somewhat paradoxically, employment may present more difficulties than financial constraints for those attempting to access treatment. As more men (50.5%) were employed than women (40.1%) in this cohort, problems getting time away from work for regular clinic visits or loss of income due to days of work missed may impact men more than women in a setting where clinic visits often involve a significant part of the day spent in queues.
We also considered the effect of gender on mortality and found no difference at 12 months on treatment, but point estimates suggested an increased hazard of death for men at 24 and 36 months on treatment; however, neither of these estimates achieved statistical significance. This may be because of the low event rate at these time points, and longer follow-up may be required to determine if this represents a true effect. Rates of ART adherence have been shown to differ by gender.49,50 It is, therefore, reasonable to suspect that ART adherence may mediate the relationship between gender and ART outcomes. Our analysis attempted to control for adherence by restricting the sample to those who achieved virologic suppression. Although it has been shown that adherence and virologic suppression are closely associated,51 there may be a more subtle level of adherence that is not detected by a dichotomous virologic proxy variable. As we do not collect data directly on patient adherence it is possible that residual confounding is present in the analysis. Despite this, results from our mediation analysis were almost identical to those in the primary analysis. This suggests that different patterns of adherence do not fully explain the differences in CD4 gain by gender and that other explanations should be considered.
Biologic differences between the genders, such as the influence of sex hormones on immunity,52 have been considered as explanations, but recent work among female children demonstrating lower viral loads and higher CD4 counts53 makes this an unlikely explanation of all the effects seen. Differences in the normal range of CD4 counts between men and women are also possible explanations for differences in the rate of CD4 count reconstitution demonstrated in this and other work. Previous research has shown that women have higher CD4 counts than their male counterparts among HIV-uninfected individuals as well as for up to 5 years after HIV infection54,55 but that these differences have little to no impact on rate of disease progression18 in the absence of ART. It is, thus, plausible that the advantages for women in terms of immunologic response are related to the fact that women have a higher normal range to return to after effective treatment is implemented.
Our findings should be considered in light of the limitations of this study. As this is an observational study, we could not fully rule out confounding bias due to systematic differences between the men and women in our study in terms of health-seeking behavior (suggested by the greater proportion of women accessing treatment and the earlier stage at which women present) or other factors. However, we controlled for important predictors of our main outcome, minimizing the potential bias of our main results. Moreover, as gender cannot be randomized, carefully conducted observational studies are the only way to isolate gender-specific effects.
As with any study, results can be significantly biased by high rates of loss to follow-up. This is of particular concern if those who are lost to follow-up are significantly different from those who remain in the study in terms of the exposure or outcome of interest. The rates of loss to follow-up were 4-fold higher among men than women in this cohort, and, thus, the group of men who remained in care were likely to be at less risk of poor treatment outcomes and death than the women who remained in care. We used inverse probability of censoring weights to correct for this effect; but if correction was incomplete, residual bias from differential loss to follow-up may remain. A related issue is that of missing data, of which loss to follow-up is a special case. Here, we were missing viral loads throughout follow-up period. If those viral loads were associated with the true value of the missing viral loads (e.g., if unsuppressed viral loads were more likely to be missing than suppressed viral loads), analysis of the effect of gender on CD4 count or mortality might be biased. Sensitivity analysis among only those with suppressed viral load, however, suggests that any such effect is likely to be small.
Conclusions
Despite the limitations, our results are valuable in understanding the role of gender in response to ART in a resource-limited setting. We used several methodologic techniques in a large cohort of HIV-infected individuals to add to the debate about gender effects. We account for differences in adherence by restricting analysis to initially virally suppressed individuals and corrected for bias as a result of differing rates of loss to follow-up, a problem that can plague cohort analyses. Currently, men are accessing this treatment program with lower CD4 counts and at a later stage in disease progression compared to women. To improve this situation, it is important that counseling and testing programs target men in their publicity campaigns. The establishment of male-friendly clinic practices may facilitate earlier counseling and testing as well as access to treatment among men. This could include after-hours access, weekend clinics, workplace facilities for HIV counseling and testing, and alternative locations for obtaining treatment, such as post office medication collection points. Consideration of interventions such as these may begin to address gender differences due to late presentation. Once access to care is equal, the true biologic differences between genders may become clearer.
Acknowledgments
We express our gratitude to the directors and staff of Themba Lethu Clinic and to Right to Care (RTC), the nongovernmental organization supporting the study site through a partnership with United States Agency for International Development (USAID). We also thank the Gauteng and National Departments of Health for providing for the care of the patients at the Themba Lethu Clinic as part of the Comprehensive Care Management and Treatment plan.
Funding was provided by USAID under the terms of agreement 674-A-00-08-00007-00 with RTC. M.P.F. was also supported by award number K01AI083097 from the National Institute of Allergy and Infectious Diseases (NIAID). D.W. was supported by NIH/NIAID 2P30 AI064518 (Duke Center for AIDS Research) and NIH/NICHD K99-HD-06-3961. The opinions expressed herein are those of the authors and do not necessarily reflect the views of NIH, NIAID, USAID, the Themba Lethu Clinic, or RTC.
Disclosure Statement
The authors declare no other conflicts of interest.
References
- 1.Bendavid E. Bhattacharya J. The President's Emergency Plan for AIDS Relief in Africa, an evaluation of outcomes. Ann Intern Med. 2009;150:688–695. doi: 10.7326/0003-4819-150-10-200905190-00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jensen-Fangel S. Pedersen L. Pedersen C, et al. Low mortality in HIV-infected patients starting highly active antiretroviral therapy, a comparison with the general population. AIDS. 2004;18:89–97. doi: 10.1097/00002030-200401020-00011. [DOI] [PubMed] [Google Scholar]
- 3.Keiser O. Orrell C. Egger M, et al. Public-health and individual approaches to antiretroviral therapy, Township South Africa and Switzerland compared. PLoS Med. 2008;5:e195. doi: 10.1371/journal.pmed.0050148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akileswaran C. Lurie MN. Flanagin TP, et al. Lessons learned from the use of highly active antiretroviral therapy in Africa. Clin Infect Dis. 2005;41:376–385. doi: 10.1086/431482. [DOI] [PubMed] [Google Scholar]
- 5.Nash D. Katyal M. Brinkhof M, et al. Long-term immunologic response to antiretroviral therapy in low-income countries, a collaborative analysis of prospective studies. AIDS. 2008;22:2291–2302. doi: 10.1097/QAD.0b013e3283121ca9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The ART-LINC Collaboration and ART_CC groups. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy, comparison between low-income and high-income countries. Lancet. 2006;367:817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 7.Ivers L. Kendrick D. Doucette K. Efficacy of antiretroviral therapy programs in resource-poor settings, a meta-analysis of the published literature. Clin Infect Dis. 2005;41:217–224. doi: 10.1086/431199. [DOI] [PubMed] [Google Scholar]
- 8.Giordano T. Wright J. Hasan M, et al. Do sex and race/ethnicity influence CD4 cell response in patients who achieve virologic suppression during antiretroviral therapy? Clin Infect Dis. 2003;37:433–437. doi: 10.1086/376638. [DOI] [PubMed] [Google Scholar]
- 9.Maman D. Pujades-Rodriguez M. Subtil F, et al. Gender differences in immune reconstitution: A multicentric cohort analysis in sub-Saharan Africa. PLoS One. 2012;7:e31078. doi: 10.1371/journal.pone.0031078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chêne G. Sterne JA. May M, et al. Prognostic importance of initial response in HIV-1 infected patients starting potent antiretroviral therapy, analysis of prospective studies. Lancet. 2003;362:679–686. doi: 10.1016/s0140-6736(03)14229-8. [DOI] [PubMed] [Google Scholar]
- 11.Carrieri MP. Raffi F. Lewden C, et al. Impact of early versus late adherence to highly active antiretroviral therapy on immuno-virologic response, a 3-year follow-up study. Antivir Ther. 2003;8:585–594. doi: 10.1177/135965350300800606. [DOI] [PubMed] [Google Scholar]
- 12.Lawn S. Harries A. Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNAIDS. Global Report. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS (UNAIDS); 2010. UNAIDS Report on the Global AIDS Epidemic. [Google Scholar]
- 14.Mocoft A. Gill MJ. Davidson W, et al. Are there gender differences in starting protease inhibitors, HAART and disease progression despite equal access to care? J AIDS. 2000;24:475–482. doi: 10.1097/00126334-200008150-00013. [DOI] [PubMed] [Google Scholar]
- 15.Moore AL. Kirk O. Johnson AM, et al. Virologic, immunologic and clinical response to highly active antiretroviral therapy, the gender issue revisited. J AIDS. 2003;32:452–461. doi: 10.1097/00126334-200304010-00017. [DOI] [PubMed] [Google Scholar]
- 16.Moore AL. Sabin CA. Johnson MA, et al. Gender and clinical outcomes after starting highly active antiretroviral treatment, a cohort study. J AIDS. 2002;29:197–202. doi: 10.1097/00042560-200202010-00015. [DOI] [PubMed] [Google Scholar]
- 17.Lemly D. Shepherd B. Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis. 2009;19:991–998. doi: 10.1086/597124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Napravnik S. Poole C. Thomas J, et al. Gender difference in HIV RNA levels,a meta-analysis of published studies. J AIDS. 2002;31:11–19. doi: 10.1097/00126334-200209010-00002. [DOI] [PubMed] [Google Scholar]
- 19.Floris-Moore M. Lo Y. Klein RS, et al. Gender and hospitalisation patterns among HIV-infected drug users before and after the availability of highly active antiretroviral therapy. J AIDS. 2003;34:331–337. doi: 10.1097/00126334-200311010-00012. [DOI] [PubMed] [Google Scholar]
- 20.Cornell M. Myer L. Kaplan R, et al. The impact of gender and income on survival and retention in a South African antiretroviral therapy programme. Trop Med Int Health. 2009;14:722–731. doi: 10.1111/j.1365-3156.2009.02290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumarasamy N. Venkatesh K. Cecelia A, et al. Gender-based differences in treatment and outcome among HIV patients in South India. J Womens Health. 2008;17:1471–1475. doi: 10.1089/jwh.2007.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Department of Health. National antiretroviral treatment guidelines. 1st. 2004. www.doh.gov.za/docs/factsheets/guidelines/artguide04-f.html. [Jul 20;2009 ]. www.doh.gov.za/docs/factsheets/guidelines/artguide04-f.html
- 23.Black V. Hoffman R. Sugar C. Safety and efficacy of initiating highly active antiretroviral therapy in an integrated antenatal and HIV clinic in Johannesburg, South Africa. J AIDS. 2008;49:276–281. doi: 10.1097/QAI.0b013e318189a769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boulle A. Van Cutsem G. Hilderbrand K, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2012;24:563–572. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 25.Fairall LR. Bachmann MO. Louwagie GM, et al. Effectiveness of antiretroviral treatment in a South African program: A cohort study. Arch Intern Med. 2008;168:86–93. doi: 10.1001/archinternmed.2007.10. [DOI] [PubMed] [Google Scholar]
- 26.Fox MP. Brennan AB. Maskew M, et al. Using vital registration data to update mortality among patients lost to follow-up from ART programmes: Evidence from the Themba Lethu Clinic, South Africa. Trop Med Int Health. 2012;15:405–413. doi: 10.1111/j.1365-3156.2010.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herna'n MA. Brumback B. Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 28.Cole SR. Herna'n MA. Constructing inverse probability weights for marginal structural models. Am. J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robins JM. Finkelstein Correcting for noncompliance and dependent censoring in an AIDS clinical trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56:779–788. doi: 10.1111/j.0006-341x.2000.00779.x. [DOI] [PubMed] [Google Scholar]
- 30.Wools-Kaloustian K. Kimaiyo S. Diero L, et al. Viability and effectiveness of large-scale HIV treatment initiatives in sub-Saharan Africa, experience from western Kenya. AIDS. 2006;20:41–48. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- 31.DART Virology Group and Trial Team. Virological response to a triple nucleoside/nucleotide analogue regimen over 48 weeks in HIV-1-infected adults in Africa. AIDS. 2006;20:1391–1399. doi: 10.1097/01.aids.0000233572.59522.45. [DOI] [PubMed] [Google Scholar]
- 32.Poundstone KE. Chaisson RE. Moore RD. Differences in HIV disease progression by injection drug use and by sex in the era of highly active antiretroviral therapy. AIDS. 2001;15:1115–1123. doi: 10.1097/00002030-200106150-00006. [DOI] [PubMed] [Google Scholar]
- 33.Moore J. Schuman P. Schoenbaum E, et al. Severe adverse life events and depressive symptoms among women with, or at risk for, HIV infection in four cities in the United States of America. AIDS. 1999;13:2459–2468. doi: 10.1097/00002030-199912030-00018. [DOI] [PubMed] [Google Scholar]
- 34.Friedland GH. Saltzman B. Vileno J, et al. Survival differences in patients with AIDS. J AIDS. 1991;4:144–153. [PubMed] [Google Scholar]
- 35.Stein MD. Piette J. Mor V, et al. Differences in access to zidovudine (AZT) among symptomatic HIV-infected persons. J Gen Intern Med. 1991;6:35–39. doi: 10.1007/BF02599388. [DOI] [PubMed] [Google Scholar]
- 36.Bozzette SA. Berry SH. Duan N, et al. The care of HIV-infected adults in the United States. N Engl J Med. 1998;339:897–1904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 37.Carpenter CC. Mayer KH. Stein MD, et al. Human immunodeficiency virus infection in North American women: Experience with 200 cases and a review of the literature. Medicine. 1991;60:307–325. doi: 10.1097/00005792-199109000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Minkoff HL. Willoughby A. Mendez H, et al. Serious infections during pregnancy among women with advanced human immunodeficiency virus infection. Am J Obstet Gynecol. 1990;162:30–34. doi: 10.1016/0002-9378(90)90814-n. [DOI] [PubMed] [Google Scholar]
- 39.Justice AC. Feinstein AR. Wells CK. A new prognostic staging system for the acquired immunodeficiency syndrome. N Engl J Med. 1889;320:1388–1393. doi: 10.1056/NEJM198905253202106. [DOI] [PubMed] [Google Scholar]
- 40.Clark RA. Blakley SA. Rice J, et al. Predictors of HIV disease progression in women. J AIDS. 1994;9:43–50. [PubMed] [Google Scholar]
- 41.Braitstein P. Boulle A. Nash D, et al. Gender and use of antiretroviral treatment in resource-constrained settings: Findings from a multicenter collaboration. J Womens Health. 2008;17:47–55. doi: 10.1089/jwh.2007.0353. [DOI] [PubMed] [Google Scholar]
- 42.Nicastri E. Angeletti C. Palmisano L, et al. Gender differences in clinical progression of HIV-1-infected individuals during long-term highly active antiretroviral therapy. AIDS. 2005;19:577. doi: 10.1097/01.aids.0000163934.22273.06. [DOI] [PubMed] [Google Scholar]
- 43.Barth R. van der Meer J. Hoepelman A, et al. Effectiveness of highly active antiretroviral therapy administered by general practitioners in rural South Africa. Eur J Clin Microbiol Infect Dis. 2008;27:977–984. doi: 10.1007/s10096-008-0534-2. [DOI] [PubMed] [Google Scholar]
- 44.Muula AS. Ngulube TJ. Siziya S, et al. Gender distribution of adult patients on highly active antiretroviral therapy (HAART) in Southern Africa, a systematic review. BMC Public Health. 2007;7:1–8. doi: 10.1186/1471-2458-7-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anastos K. Gange SJ. Lau B, et al. Association of race and gender with HIV-1 RNA levels and immunologic progression. J AIDS. 2000;24:218–226. doi: 10.1097/00126334-200007010-00004. [DOI] [PubMed] [Google Scholar]
- 46.Zachariah R. Fitzgerald M. Massaquoi M, et al. Risk factors for high early mortality in patients on antiretroviral treatment in a rural district of Malawi. AIDS. 2006;20:2355–2360. doi: 10.1097/QAD.0b013e32801086b0. [DOI] [PubMed] [Google Scholar]
- 47.Maskew M. Menezes C. MacPhail AP, et al. Lost to follow-up—Contributing factors and challenges in South African patients on antiretroviral therapy. S Afr Med J. 2007;97:853–857. [PubMed] [Google Scholar]
- 48.Weiser S. Wolfe W. Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J AIDS. 2003;34:281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 49.Sayles JN. Wong MD. Cunningham WE. The inability to take medications openly at home: Does it help explain gender disparities in HAART use? J Womens Health. 2006;15:173–181. doi: 10.1089/jwh.2006.15.173. [DOI] [PubMed] [Google Scholar]
- 50.Murri R. Lepri AC. Phillips AN, et al. Access to antiretroviral treatment, incidence of sustained therapy interruptions, and risk of clinical events according to sex. J AIDS. 2003;34:184–190. doi: 10.1097/00126334-200310010-00008. [DOI] [PubMed] [Google Scholar]
- 51.Kuyper L. Wood E. Montaner J, et al. Gender differences in HIV-1 RNA rebound attributed to incomplete antiretroviral adherence among HIV-infected patients in a population-based cohort. J AIDS. 2004;37:1470–1476. doi: 10.1097/01.qai.0000138379.39317.62. [DOI] [PubMed] [Google Scholar]
- 52.Fish EN. The X-files in immunity: Sex-based differences predispose immune responses. Nat Rev Immunol. 2008;8:737–744. doi: 10.1038/nri2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruel TD. Zanoni BC. Ssewanyana I, et al. Sex differences in HIV RNA level and CD4 cell percentage during childhood. Clin Infect Dis. 2001;53:592–599. doi: 10.1093/cid/cir484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maini MK. Cilson RJC. Chavda N, et al. Reference ranges and sources of variability of CD4 counts in HIV seronegative women and men. Genitourin Med. 1996;72:27–31. doi: 10.1136/sti.72.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tollerud D. Clark J. Morris Brown L, et al. The influence of age, race and gender on peripheral blood mononuclear cell subsets in healthy nonsmokers. J Clin Immunol. 1989;9:214–222. doi: 10.1007/BF00916817. [DOI] [PubMed] [Google Scholar]