Abstract
OBJECTIVE
To study the association between ambient air pollutants and serum C-reactive protein (CRP) concentration in 1,392 type 2 diabetic patients in Pune, India.
RESEARCH DESIGN AND METHODS
A cross-sectional study was conducted that linked daily time series of ambient air pollution data (obtained from central monitoring sites) and plasma CRP concentration in type 2 diabetic patients from the Wellcome Trust Genetic (WellGen) Study, recruited between March 2005 and May 2007. Air pollution effects on CRP concentration were investigated with delays (lags) of 0–7 days and multiday averaging spans of 7, 14, and 30 days before blood collection adjusted for age, sex, BMI, hemoglobin, fasting plasma glucose, treatment with agents with anti-inflammatory action, season, air temperature, and relative humidity.
RESULTS
Median CRP concentration was 3.49 mg/L. For 1 SD increase in SO2 and oxides of nitrogen (NOx) concentrations in ambient air, a day before blood collection (lag1), we observed a significant increase in CRP (9.34 and 7.77%, respectively). The effect was higher with lag2 (12.42% for SO2 and 11.60% for NOx) and wore off progressively thereafter. We also found a significant association with multiday averaging times of up to 30 and 7 days for SO2 and NOx, respectively. No significant associations were found between particulate matter with an aerodynamic profile ≤10 µm (PM10) and CRP concentration except in summer. The association was significantly higher among patients with a shorter duration of diabetes, and in those not on statin and thiazolidinedione treatment.
CONCLUSIONS
We demonstrate, for the first time, a possible contribution of ambient air pollution to systemic inflammation in Indian type 2 diabetic patients. This may have implications for vascular complications of diabetes.
Ambient air pollution has been shown to be associated with several acute and chronic adverse effects on human health, especially those involving the respiratory and cardiovascular system (1). The World Health Organization estimated that ∼865,000 premature deaths occur every year due to the adverse health effects of ambient air pollution and that China and India are the most affected countries (2). The underlying biological mechanisms and contribution of specific pollutants that drive these adverse effects are not well understood, but are important for interventional strategies. A growing body of evidence suggests that the harmful effects of ambient air pollution are largely mediated by increased inflammatory responses, both within the local tissues of deposition as well as systemically (3). It has been proposed that inhaled particles and gaseous air pollutants cause the release of reactive oxygen species from cells in the lung and circulating immune cells (4), which in turn trigger the release of inflammatory mediators into the systemic circulation to affect other body organs (5). Such an increase in systemic inflammation has been shown to increase cardiovascular disease (CVD) risk (6). Extremes of age, obesity, and underlying respiratory, cardiovascular, and metabolic disorders (diabetes) seem to increase vulnerability and susceptibility to the harmful effects of air pollution (7), and the systemic inflammatory response may further worsen the disease state (6).
C-reactive protein (CRP) is a nonspecific marker of systemic inflammation that predicts type 2 diabetes (8) and CVD events (9). Epidemiological studies in Europe (10–14) and North America (15–18) have reported that short-term air pollution exposure is associated with increased CRP concentrations, but there is only a little information from Asia (19).
In an earlier study, we reported that urban residents in Pune had significantly higher concentrations of CRP compared with those living in a rural setting (median 0.45 mg/L for urban vs. 0.31 mg/L for rural; P < 0.001) (20). In that study, adiposity explained 35% of the variation of CRP between rural and urban men, and we suggested that higher levels of ambient air pollution in the urban area could be a contributory factor.
In the Wellcome Trust Genetic (WellGen) Study, we have collected extensive phenotypic data on type 2 diabetic patients in Pune, including CRP concentration. We used this database to investigate the relationship between exposure to different ambient air pollutants and concentrations of CRP in the serum. We hypothesized that type 2 diabetic patients will manifest increased CRP concentration in relation to exposure to ambient air pollutants and that this effect may be modified by obesity, treatment, and season.
RESEARCH DESIGN AND METHODS
Subjects
The WellGen Study design has been described in detail elsewhere (21). In brief, type 2 diabetic patients attending the Diabetes Clinic of the King Edward Memorial Hospital in Pune were enrolled since 2005, and the study is currently ongoing. Patients suffering from acute intercurrent illnesses (n = 4) were rescheduled for a blood sample after 4 weeks. Usually, it takes 7–12 days for CRP to return to baseline after an acute illness (22). For the current study, we included patients who were enrolled between March 2005 and May 2007, and who were residing within the Pune Municipal Corporation boundary. The study was approved by the Institutional Ethics Committee, and all patients gave a written informed consent.
Clinical and biochemical measurements
A clinical examination was conducted in all study subjects. In addition, all patients answered a standard questionnaire that gave information about their residential address, age, sex, smoking history, alcohol consumption, and medical history, including current drug treatment. Height and weight were measured, and BMI was calculated. A fasting blood sample was drawn to examine various biochemical measurements, including CRP, glucose, HbA1c, hemoglobin, and other hematological parameters. Blood samples were stored for an average period of 20 months (4–32 months) at 80°C before the measurement of CRP. High-sensitivity CRP concentrations were measured with an ELISA kit (Diagnostic Biochem Canada), and coefficient of variability (CV) for the assay was <16%. All samples were measured over 3 weeks by kits belonging to the same batch.
Air pollutants and meteorological data
Air pollution data for the conventional pollutants sulfur dioxide (SO2), oxides of nitrogen (NOx), and particulate matter with an aerodynamic profile ≤10 µm (PM10) have been measured in Pune under the National Air Quality Monitoring Program since 2004 and are available online in a public domain (http://mpcb.gov.in/). These published data were collected from three locations (Swargate, Nal Stop, and Karve Road) situated around the city center (admissible as per Air Pollution and Health: a European Approach [APHEA] protocol) (23), and the data were accessed in January 2010. The monitoring of pollutants was carried out for 24 h (4-h sampling for gaseous pollutants and 8-h for particulate matter) on 2 consecutive days per week (other than weekends) in Swargate and Nal Stop and 6 days a week in Karve Road (Supplementary Fig. 1). The average concentrations of SO2, NOx, and PM10 from these three sites were used as urban background levels. There was no significant difference in the average 24-h readings from different monitors on a given day.
Missing air pollution data were imputed by linear interpolation technique (24). Ambient air pollutants and meteorological variables for each person’s day of visit (lag0), and up to 7 days before (lag1–7) as a short-term exposure, were then computed. Furthermore, cumulative exposures for more extended periods of time (average of 7, 14, and 30 days before blood collection) were also computed.
Meteorological data for the city of Pune during the period of the study were obtained from the Pune Office of the India Meteorological Department, a national data center. Daily arithmetic mean air temperatures were computed from the daily minimum and maximum temperatures. Apparent temperature value was calculated as −2.653 + (0.994 × DBT) + (0.0153 × DPT2), where DBT and DPT are dry bulb temperature and dew point temperature, respectively (25).
Statistical analysis
Data are presented as mean (±SD) when normally distributed and median (25th–75th percentile) when not normally distributed. CRP and fasting plasma glucose (FPG) concentrations were skewed and were therefore normalized using log10 transformation. Characteristics of the study population were stratified by season (monsoon [June–October], winter [November–February], and summer [March–May]). The differences of variables across season were tested by ANOVA (for normally distributed variables), Kruskal-Wallis (for skewed variables), and χ2 tests (for categorical variables). The differences of CRP concentration between two groups (i.e., men vs. women) were tested using Mann-Whitney test. Associations were tested using Spearman (rs) and Pearson (r) correlation coefficients.
We used multiple linear regression to examine the association between ambient air pollution levels and CRP concentration. The final multiple linear regression model was adjusted for a priori chosen confounders (known or plausible) including age, sex, FPG, hemoglobin, BMI, treatment with anti-inflammatory agents (i.e., statin, aspirin, and thiazolidinedione [TZD]), season, and meteorological variables (relative humidity and air temperature). Model selection was based on minimum values of Akaike’s information criterion (26) to obtain best fit with the minimum number of parameters in the model. The ambient air pollution and corresponding meteorological parameters (relative humidity and air temperature) were entered in the model to capture exposure on the day of the study (lag0) or up to 7 days before (lag1–7) and also as moving averages up to the last 30 days. The autocorrelation plot of the residual for the fully adjusted model was investigated for periodicity in order to avoid bias in the regression coefficient. The results are given as percent changes in geometric mean (GM) of CRP concentration for an SD increment in air pollutant (% change in GM = [10(1SD*b) – 1] × 100), where b is the regression coefficient. Additionally, we checked the effect modification by BMI (below vs. above the median, 25.60 kg/m2), HbA1c (8.80%), FPG (153 mg/dL), current smoking (yes vs. no), intake of anti-inflammatory agent (yes vs. no), and the season (monsoon, winter, and summer).The significance threshold of P = 0.05 was used in all analyses. All statistical analyses were performed using STATA version 11.1 software (STATA Corporation, College Station, TX).
Sensitivity analysis
To explore the robustness of our results, alternative ways of modeling were also performed: 1) possible influence of additional adjustments for meteorological variables, including barometric pressure and apparent temperature, and 2) multipollutant models.
RESULTS
Study population
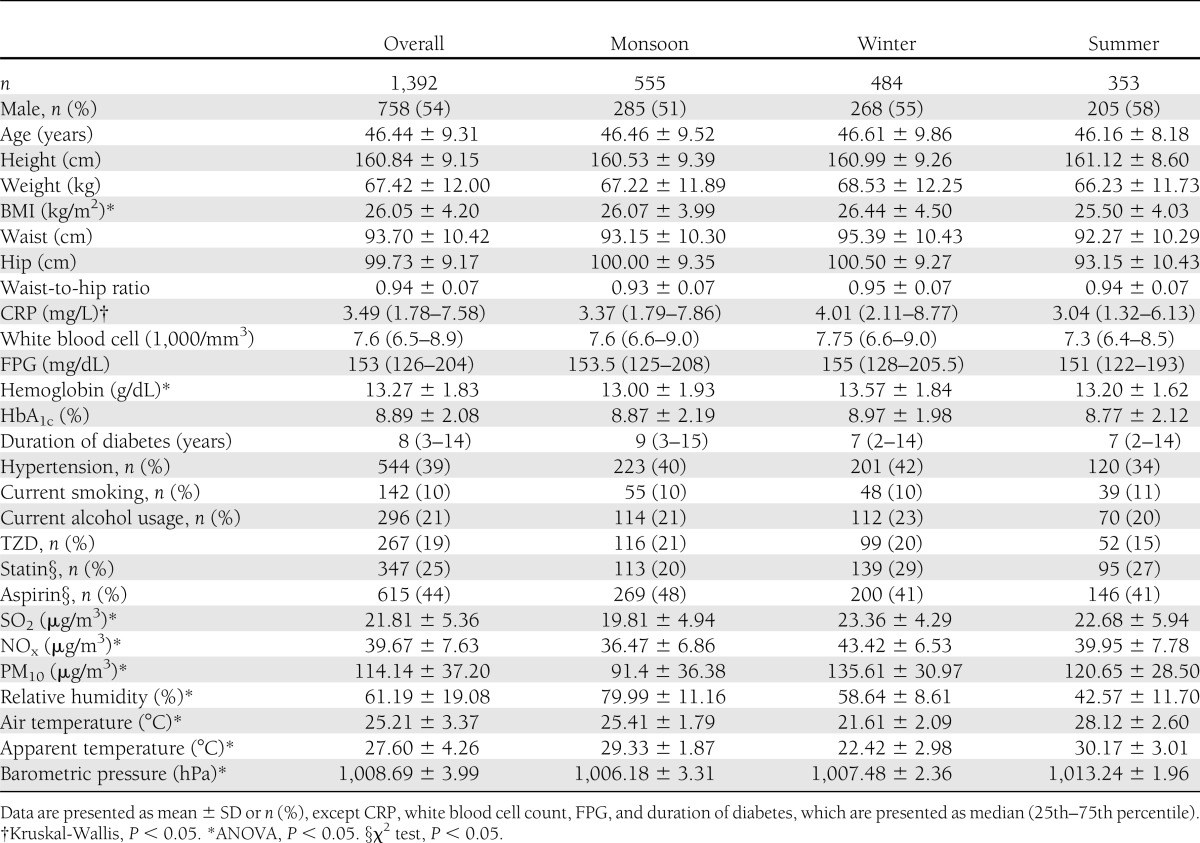
Of the 1,700 patients enrolled in the WellGen Study between 2005 and 2007, 1,392 who lived in Pune City and had a measurement of CRP were included in this study (Table 1). None of the patients were clinically diagnosed as having a chronic infective or inflammatory condition and none were on a steroid treatment.
Table 1.
Characteristics of type 2 diabetic patients and local levels of environmental variables (air pollutants and meteorological measurement)
Inflammatory markers
The median CRP concentration was 3.49 mg/L (1.78–7.58 mg/L). Fifty-seven percent of patients had CRP concentrations in the high coronary risk zone i.e., >3 mg/L, and 19% of patients had abnormally raised CRP concentrations (>10 mg/L). CRP concentration was weakly associated with age (r = −0.06, P = 0.02), and women had a higher concentration compared with men (5.17 mg/L for women vs. 2.76 mg/L for men; P < 0.001). The associations of CRP were therefore adjusted for age and sex. After adjustment, CRP was found to be positively associated with white blood cell count (r = 0.07, P = 0.03), FPG (r = 0.12, P < 0.001), HbA1c (r = 0.09, P = 0.005), and BMI (r = 0.27, P < 0.001) but inversely associated with air temperature (r = −0.10, P < 0.001) and apparent temperature (r = −0.10, P < 0.001). Patients on statins, aspirin, and TZD treatment had lower CRP concentrations compared with those not on these treatments (median CRP for subjects treated with all three agents, 2.30 mg/L; any one agent, 3.45 mg/L; no agent, 3.94 mg/L). CRP concentrations were not associated with hemoglobin concentration, duration of diabetes, smoking, alcohol usage, and relative humidity.
Air pollutants and meteorology
Air pollution data were available for 647 of the 822 days of the study; data were imputed for the remaining days. The air pollutant levels were highest in winter and lowest in monsoon. During the study period, the concentrations of SO2 and NOx never crossed the daily national ambient air quality standard (NAAQS) of 80 µg/m3 (27). For 598 days (62%), the 24-h PM10 levels were found to be above the daily NAAQS of 100 µg/m3. The daily concentrations of ambient SO2, NOx, and PM10 were interrelated in all seasons; rs ranged from 0.27 to 0.75 (P < 0.001), with a lower correlation observed during the winter (Supplementary Fig. 2).
Meteorological measurements were available for all the days during the study period. Air temperature was inversely correlated with air pollutant concentration; rs ranged from −0.13 to −0.22 (P < 0.001) but was positive only with PM10 during monsoon (rs = 0.12, P = 0.002). Relative humidity showed negative correlations with ambient pollutants; rs ranged from −0.28 to −0.45 (P < 0.001).
Regression analysis
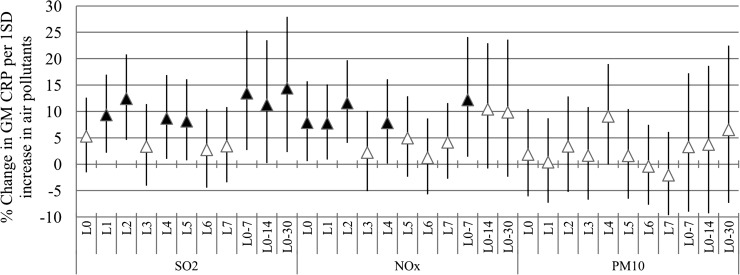
We studied the contribution of ambient air pollution at lag0–7 and different averaging time periods (7, 14, and 30 days) to the concentration of serum CRP. The model and adjustment procedures have been described in the statistical analysis. The final model had no indication of residual serial correlation (autocorrelation). We found that a 1 SD increment in city-wide SO2 was associated with a significant increase in CRP concentrations, ranging from 8.67 to 12.42% for lag periods up to 7 days and of 11.23 to 14.40% for multiday averaging periods of up to 30 days before blood collection. For NOx, the increase was between 7.77 and 11.60% for lag up to 7 days and 12.80% for 7 days average. For PM10, there were no significant associations with CRP concentrations either at different lag days or during averaging times, except during the summer (e.g., for lag4 11.31% [95% CI 3.36–19.88]) (Fig. 1).
Figure 1.
Percent change in GM of CRP per 1 SD increase in air pollutants. Models are adjusted for age, sex, BMI, log10 FPG, hemoglobin, statin, aspirin, and TZD treatment, season, relative humidity, and air temperature. Error bar indicates 95% CI. Black triangle indicates significant association (P < 0.05).
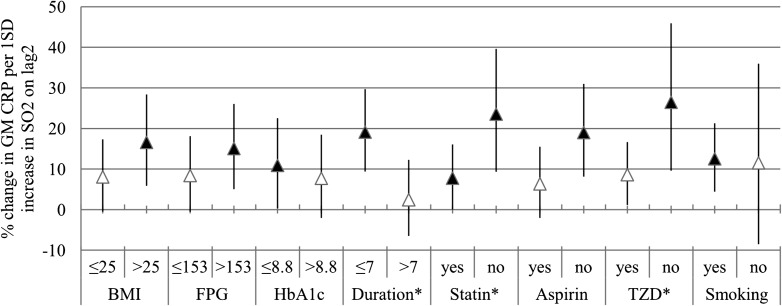
Effect modification analysis
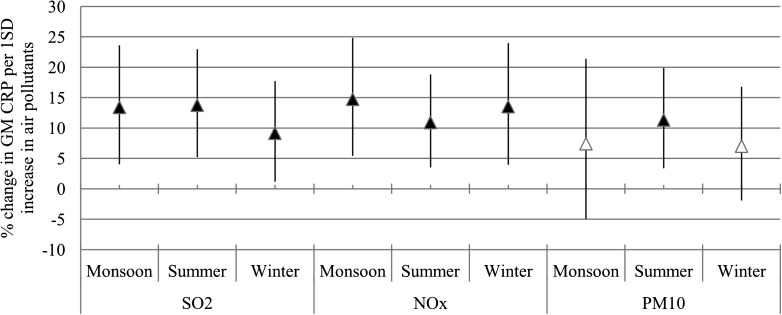
We present the effect modification only on the most influential lag days of SO2 (lag2). Enhanced associations between SO2 and CRP concentrations were observed among patients with a duration of diabetes <7 years and patients who were not treated with statin and TZD medication, reflected in significant interaction terms (Fig. 2). Season did not modify the association between air pollution and CRP concentrations significantly (Fig. 3).
Figure 2.
Effect modification; the estimates are given as percent change in GM of CRP per 1 SD increase of SO2 (lag2). Error bar indicates 95% CI. *Interaction terms are significant at P < 0.05. Black triangle indicates significant association (P < 0.05).
Figure 3.
Effect of the season on the association between air pollutants and CRP concentrations. The estimates are given as percent change in the GM of CRP per 1 SD increase of SO2 (lag2), NOx (lag2), and PM10 (lag4). Error bar indicates 95% CI. Black triangle indicates significant association (P < 0.05).
Sensitivity analysis
Additional adjustment for meteorological variables (i.e., barometric pressure and apparent temperature) did not show any significant effect on the inflammatory response (Supplementary Table 1). Multipollutant models indicated that SO2 was the most important pollutant associated with serum CRP concentration.
CONCLUSIONS
In this study, we report that type 2 diabetic patients residing in Pune, India, show high concentrations of serum CRP concentrations that correlate positively and significantly with ambient levels of SO2 and NOx, even at levels below the NAAQS. Although ambient PM10 levels exceeded NAAQS on 62% of study days, it did not show significant correlation with CRP concentrations. Among the gaseous pollutants, SO2 showed a stronger association compared with NOx. Moreover, the association was particularly stronger in patients with a shorter (<7 years) duration of time since their diabetes diagnosis, and weaker in those who were receiving statins, aspirin, and TZD, drugs with known anti-inflammatory properties. To the best of our knowledge, this is the first study in this region of the world that has shown a significant association between ambient air pollutants and CRP concentrations in type 2 diabetic patients. Inflammation is a powerful risk factor for CVD in type 2 diabetic patients (28), and our results suggest a possible environmental and preventable contribution to this risk.
Temporal characteristics of the associations indicate that circulating CRP concentrations are relatively rapidly affected by exposure to SO2 and NOx (within 1 day) (Fig. 3). The peak association was by 2-day lag, similar to a previous study in patients with coronary heart disease (12), and it wore off progressively thereafter. Multiday averaging analysis suggested an effect up to 30 days for SO2 and 7 days for NOx. For PM10, a peak association was observed on lag4, which was statically insignificant.
The effect of air pollution on CRP was first demonstrated in 112 elderly subjects in the U.K., in whom CRP was significantly associated with city mean concentration of PM10 over the previous 3 days (10). A number of other studies made similar observations in different groups of subjects (11–19), although some studies have failed to show an association (29,30). The difference in the findings in these studies may be attributable to differences in the composition of pollution, underlying medical conditions, and concomitant medication and dietary diversity (31).There is only one previous study in diabetic patients in Belgium (n = 233) (14) that found a significant association between CRP concentrations and exposure to PM10 up to 1 week before blood collection; there was no measurement of SO2 and NOx in this study. In other studies, subgroup analysis reported for diabetic patients suggests that diabetes enhances the effect of pollutants on CRP concentrations (13,16).
CRP is an established risk factor for coronary heart disease (9) and type 2 diabetes (8). It is produced exclusively in the liver during systemic inflammation, and it is synthesized within 24–48 h by hepatocytes (32) in response to cytokines released into circulation by activated leukocytes. Its half-life is ∼19 h (33), and plasma concentrations of CRP reflect its synthesis rate. Inhaled pollutants stimulate the alveolar macrophages and airway epithelial cells to secrete cytokines into circulation, which release leukocytes from bone marrow (34). Preexisting inflammation and oxidative stress enhance cytokine production after exposure to air pollution (7). Enhanced susceptibility of diabetic patients to inhaled particles is likely to be attributed to a heightened state of inflammation, oxidative stress, and the excess adiposity characteristic of this condition (35). It is interesting that in our study, we found that those on statin and TZD treatments (which have anti-inflammatory action) (36) had a lower CRP concentration and a smaller rise in CRP concentrations in relation to ambient pollution compared with those who were not on this treatment. Higher inflammatory response in newly diagnosed diabetic patients could be attributed to their younger age and therefore possibly more exposure to outdoor air pollution, and because many were not yet started on medication that lowers inflammation.
Unlike gaseous pollutants, we did not find an association between PM10 exposure and CRP concentration, even though the average PM10 levels were above NAAQS (27). We have no ready explanation for this finding. The isolated positive association only during summer may be related to the composition of PM10 and meteorological factors (i.e., temperature), which can play an important role in PM toxicity (37,38).
This is a large-scale study reporting an association between air pollution and CRP in urban Indian diabetic patients. The study was possible because we could link the phenotypic data from the WellGen Study with air pollutant data available in the public domain. The availability of extensive information on diabetic patients and meteorological variables allowed adjustments for individual as well as extraneous confounders. Our models and results are robust to additional adjustment to barometric pressure and apparent temperature, and no indication of seasonality was seen in residuals. It is therefore unlikely that our findings are a result of confounding. We believe that the estimated air pollution effect on CRP concentrations in this study represents an underestimate because we used centrally measured air pollution as a surrogate for personal exposure and we did not account for the effect of indoor pollution and time spent outdoors. Frequent prescription of anti-inflammatory medicines among these patients also reduced the effect. In addition, we were not able to account for a dietary pattern that may be associated with CRP concentration (39). Diets rich in saturated fat and refined carbohydrates induce oxidative and inflammatory stress, whereas diets rich in fiber and fruits do not have such an effect (40). In our future research, we will focus on the improvement of the exposure data considering emission inventory, meteorological data, traffic density, time activity, and diet.
In summary, we found that exposure to traffic-related air pollutants is associated with a rapid increase in systemic inflammation (i.e., CRP) in diabetic patients. Given the strong evidence that CRP concentrations are associated with the development and complications of the metabolic syndrome (41), our findings may have implications for enhanced risk of CVD in urban Indian diabetic patients. Our results should promote studies on the effect of air pollution on the risk of noncommunicable disease in India.
Supplementary Material
Acknowledgments
The WellGen Study was supported by the Wellcome Trust (London, U.K.). Air pollutants and meteorological data were provided by the Maharashtra Pollution Control Board and Meteorological Department (Pune Office), respectively.
No potential conflicts of interest relevant to this article were reported.
M.A.K. and C.S.Y. researched, wrote, discussed, and edited the manuscript. S.S.S. and A.O. contributed to the discussion and edited the manuscript. B.K. and S.S.G. contributed to the data analyses and edited the manuscript. C.S.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors acknowledge the contributions of the WellGen Study group and Smita Kulkarni (King Edward Memorial Hospital) in data collection and data management and Dattatray Bhat (King Edward Memorial Hospital) in laboratory measurements.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0388/-/DC1.
References
- 1.Curtis L, Rea W, Smith-Willis P, Fenyves E, Pan Y. Adverse health effects of outdoor air pollutants. Environ Int 2006;32:815–830 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO). Estimated deaths and DALYs attributable to selected environmental risk factors by WHO member state, 2002. Department of Public Health and Environnment [Internet], 2007. Available from http://www.who.int/quantifying_ehimpacts/countryprofilesebd.xls Accessed on 20 December 2011
- 3.Hogg JC, van Eeden S. Pulmonary and systemic response to atmospheric pollution. Respirology 2009;14:336–346 [DOI] [PubMed] [Google Scholar]
- 4.Lodovici M, Bigagli E. Oxidative stress and air pollution exposure. J Toxicol 2011; 2011:487074 [DOI] [PMC free article] [PubMed]
- 5.Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev 2012;15:1–21 [DOI] [PubMed] [Google Scholar]
- 6.Maskrey BH, Megson IL, Whitfield PD, Rossi AG. Mechanisms of resolution of inflammation: a focus on cardiovascular disease. Arterioscler Thromb Vasc Biol 2011;31:1001–1006 [DOI] [PubMed] [Google Scholar]
- 7.Mead MN. Who’s at risk? Gauging susceptibility to air pollutants. Environ Health Perspect 2011;119:A176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee CC, Adler AI, Sandhu MS, et al. Association of C-reactive protein with type 2 diabetes: prospective analysis and meta-analysis. Diabetologia 2009;52:1040–1047 [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM. C-reactive protein and the prediction of cardiovascular events among those at intermediate risk: moving an inflammatory hypothesis toward consensus. J Am Coll Cardiol 2007;49:2129–2138 [DOI] [PubMed] [Google Scholar]
- 10.Seaton A, Soutar A, Crawford V, et al. Particulate air pollution and the blood. Thorax 1999;54:1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters A, Fröhlich M, Döring A, et al. Particulate air pollution is associated with an acute phase response in men; results from the MONICA-Augsburg Study. Eur Heart J 2001;22:1198–1204 [DOI] [PubMed] [Google Scholar]
- 12.Rückerl R, Ibald-Mulli A, Koenig W, et al. Air pollution and markers of inflammation and coagulation in patients with coronary heart disease. Am J Respir Crit Care Med 2006;173:432–441 [DOI] [PubMed] [Google Scholar]
- 13.Hertel S, Viehmann A, Moebus S, et al. Influence of short-term exposure to ultrafine and fine particles on systemic inflammation. Eur J Epidemiol 2010;25:581–592 [DOI] [PubMed] [Google Scholar]
- 14.Emmerechts J, Jacobs L, Van Kerckhoven S, et al. Air pollution-associated procoagulant changes: the role of circulating microvesicles. J Thromb Haemost 2012;10:96–106 [DOI] [PubMed] [Google Scholar]
- 15.Pope CA, 3rd, Hansen ML, Long RW, et al. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect 2004;112:339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubowsky SD, Suh H, Schwartz J, Coull BA, Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect 2006;114:992–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delfino RJ, Staimer N, Tjoa T, et al. Circulating biomarkers of inflammation, antioxidant activity, and platelet activation are associated with primary combustion aerosols in subjects with coronary artery disease. Environ Health Perspect 2008;116:898–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delfino RJ, Staimer N, Tjoa T, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect 2009;117:1232–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chuang KJ, Chan CC, Su TC, Lee CT, Tang CS. The effect of urban air pollution on inflammation, oxidative stress, coagulation, and autonomic dysfunction in young adults. Am J Respir Crit Care Med 2007;176:370–376 [DOI] [PubMed] [Google Scholar]
- 20.Yajnik CS, Joglekar CV, Lubree HG, et al. Adiposity, inflammation and hyperglycaemia in rural and urban Indian men: Coronary Risk of Insulin Sensitivity in Indian Subjects (CRISIS) Study. Diabetologia 2008;51:39–46 [DOI] [PubMed] [Google Scholar]
- 21.Chandak GR, Janipalli CS, Bhaskar S, et al. Common variants in the TCF7L2 gene are strongly associated with type 2 diabetes mellitus in the Indian population. Diabetologia 2007;50:63–67 [DOI] [PubMed] [Google Scholar]
- 22.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 1999;340:448–454 [DOI] [PubMed] [Google Scholar]
- 23.Katsouyanni K, Schwartz J, Spix C, et al. Short term effects of air pollution on health: a European approach using epidemiologic time series data: the APHEA protocol. J Epidemiol Community Health 1996;50(Suppl. 1):S12–S18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed NN, Yahaya AS. Ramli NA, Abdullah MMA. Estimation of missing values in air pollution data using single imputation techniques. Sci Asia 2008;34:341–345 [Google Scholar]
- 25.O’Neill MS, Zanobetti A, Schwartz J. Modifiers of the temperature and mortality association in seven US cities. Am J Epidemiol 2003;157:1074–1082 [DOI] [PubMed] [Google Scholar]
- 26.Akaike H. Information theory and an extension of the maximum likelihood principle. In Proceedings of the Second International Symposium on Information Theory, Budapest, 1973 Academiai Kiado, p. 267–281 [Google Scholar]
- 27.Central Pollution Control Board (CPCB). Air Quality Trends and Action Plan for Control of Air Pollution from Seventeen Cities Delhi, CPCB publication, National Ambient Air Quality Monitoring Series, 2006 (NAAQMS/29/2006-07)
- 28.Soinio M, Marniemi J, Laakso M, Lehto S, Rönnemaa T. High-sensitivity C-reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7-year follow-up study. Diabetes Care 2006;29:329–333 [DOI] [PubMed] [Google Scholar]
- 29.Steinvil A, Kordova-Biezuner L, Shapira I, Berliner S, Rogowski O. Short-term exposure to air pollution and inflammation-sensitive biomarkers. Environ Res 2008;106:51–61 [DOI] [PubMed] [Google Scholar]
- 30.Zuurbier M, Hoek G, Oldenwening M, et al. In-traffic air pollution exposure and CC16, blood coagulation, and inflammation markers in healthy adults. Environ Health Perspect 2011;119:1384–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sacks JD, Stanek LW, Luben TJ, et al. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect 2011;119:446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kushner I, Broder ML, Karp D. Control of the acute phase response. Serum C-reactive protein kinetics after acute myocardial infarction. J Clin Invest 1978;61:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigushin DM, Pepys MB, Hawkins PN. Metabolic and scintigraphic studies of radioiodinated human C-reactive protein in health and disease. J Clin Invest 1993;91:1351–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujii T, Hayashi S, Hogg JC, et al. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am J Respir Cell Mol Biol 2002;27:34–41 [DOI] [PubMed] [Google Scholar]
- 35.Gold DR. Vulnerability to cardiovascular effects of air pollution in people with diabetes. Curr Diab Rep 2008;8:333–335 [DOI] [PubMed] [Google Scholar]
- 36.Dandona P. Effects of antidiabetic and antihyperlipidemic agents on C-reactive protein. Mayo Clin Proc 2008;83:333–342 [DOI] [PubMed] [Google Scholar]
- 37.Nawrot TS, Torfs R, Fierens F, et al. Stronger associations between daily mortality and fine particulate air pollution in summer than in winter: evidence from a heavily polluted region in western Europe. J Epidemiol Community Health 2007;61:146–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosas Pérez I, Serrano J, Alfaro-Moreno E, et al. Relations between PM10 composition and cell toxicity: a multivariate and graphical approach. Chemosphere 2007;67:1218–1228 [DOI] [PubMed] [Google Scholar]
- 39.Galland L. Diet and inflammation. Nutr Clin Pract 2010;25:634–640 [DOI] [PubMed]
- 40.Dandona P, Ghanim H, Chaudhuri A, Dhindsa S, Kim SS. Macronutrient intake induces oxidative and inflammatory stress: potential relevance to atherosclerosis and insulin resistance. Exp Mol Med 2010;42:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridker PM, Buring JE, Cook NR, Rifai N. C-reactive protein, the metabolic syndrome, and risk of incident cardiovascular events: an 8-year follow-up of 14,719 initially healthy American women. Circulation 2003;107:391–397 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.