Abstract
OBJECTIVE
Branched-chain and aromatic amino acids are associated with the risk for future type 2 diabetes; however, the underlying mechanisms remain elusive. We tested whether amino acids predict insulin resistance index in healthy young adults.
RESEARCH DESIGN AND METHODS
Circulating isoleucine, leucine, valine, phenylalanine, tyrosine, and six additional amino acids were quantified in 1,680 individuals from the population-based Cardiovascular Risk in Young Finns Study (baseline age 32 ± 5 years; 54% women). Insulin resistance was estimated by homeostasis model assessment (HOMA) at baseline and 6-year follow-up. Amino acid associations with HOMA of insulin resistance (HOMA-IR) and glucose were assessed using regression models adjusted for established risk factors. We further examined whether amino acid profiling could augment risk assessment of insulin resistance (defined as 6-year HOMA-IR >90th percentile) in early adulthood.
RESULTS
Isoleucine, leucine, valine, phenylalanine, and tyrosine were associated with HOMA-IR at baseline and for men at 6-year follow-up, while for women only leucine, valine, and phenylalanine predicted 6-year HOMA-IR (P < 0.05). None of the other amino acids were prospectively associated with HOMA-IR. The sum of branched-chain and aromatic amino acid concentrations was associated with 6-year insulin resistance for men (odds ratio 2.09 [95% CI 1.38–3.17]; P = 0.0005); however, including the amino acid score in prediction models did not improve risk discrimination.
CONCLUSIONS
Branched-chain and aromatic amino acids are markers of the development of insulin resistance in young, normoglycemic adults, with most pronounced associations for men. These findings suggest that the association of branched-chain and aromatic amino acids with the risk for future diabetes is at least partly mediated through insulin resistance.
Insulin resistance is a core defect in the pathogenesis of type 2 diabetes, and the condition is associated with increased risk for diabetes already in early adulthood (1–4). In addition, impaired insulin sensitivity in young adults frequently precedes a dyslipidemic profile and contributes to development of cardiovascular disease (5,6). Studies on the etiology of insulin resistance have predominantly focused on lipid-induced mechanisms (1) and the interplay with obesity (2). Nonetheless, several studies have indicated a prominent amino acid signature of insulin resistance, even in normoglycemic individuals (7–12). Furthermore, the circulating concentrations of branched-chain amino acids (isoleucine, leucine, and valine) and aromatic amino acids (phenylalanine and tyrosine) were recently shown to be associated with the risk of future hyperglycemia and overt diabetes (13–15). However, it remains incompletely understood how these amino acids mediate the risk for development of diabetes. In order to illuminate the role of fasting state amino acid levels in pathogenesis of insulin resistance and type 2 diabetes, we investigated whether amino acids are predictors of insulin resistance index as well as fasting glucose levels cross-sectionally and after 6-year follow-up in young, apparently healthy individuals from a population-based cohort.
RESEARCH DESIGN AND METHODS
The Cardiovascular Risk in Young Finns Study was designed to investigate the contribution of early life risk factors to the risk of cardiovascular diseases (16). The first survey in 1980 included 3,596 children and adolescents aged 3–18 years representatively selected from rural and urban parts of Finland. Participants attending follow-up surveys in both 2001 (baseline) and 2007 were eligible for inclusion in the present longitudinal study (n = 1,809). This study population was representative of the original cohort (17). BMI and blood pressure were measured, and current smoking status and physical activity were obtained from self-reported questionnaires. Physical activity index was computed based on the frequency and intensity of physical activity as previously described (18). Blood samples were drawn after a 12-h fast. Triglycerides and HDL cholesterol were quantified by nuclear magnetic resonance (NMR) spectroscopy (19), and apolipoprotein (apo)A1 and -B and high-sensitivity C-reactive protein were measured by standard assays (16). Fasting plasma insulin was analyzed by microparticle enzyme immunoassay kit (Abbott Laboratories) and glucose enzymatically (Olympus AU400). Insulin resistance index was estimated by homeostasis model assessment (HOMA) of insulin resistance (HOMA-IR) using the HOMA2 calculator (www.dtu.ox.ac.uk/homacalculator/), which applies a structural model used to solve the nonlinear empirical equations describing glucose regulation (20). Individuals missing insulin, glucose, or amino acid quantification (n = 17); pregnant women (n = 80); and study participants on lipid-lowering medications (n = 7) or treatment for diabetes at baseline (n = 18) or follow-up (n = 7) were excluded from analyses, leaving 1,680 individuals for the current study. Of these, 2 individuals had type 2 diabetes at baseline, as did an additional 14 individuals at 6-year follow-up based on fasting glucose >7.0 mmol/L. All participants gave written informed consent, and the study was approved by the local ethics committees and conducted in accordance with the Declaration of Helsinki.
Amino acid quantification
Amino acid concentrations were quantified by high-throughput serum NMR spectroscopy (19). The serum samples were stored at −80°C and thawed overnight in a refrigerator prior to sample preparation. A proton NMR spectrum was acquired where spectral signals from the macromolecules and lipoprotein lipids were suppressed to enhance detection of the amino acid signals. Nine amino acids (isoleucine, leucine, valine, phenylalanine, and tyrosine as well as alanine, glutamine, glycine, and histidine) were quantified in millimoles per liter by NMR. Details of the spectroscopy and amino acid quantification have previously been described (19,21,22). In addition, arginine (n = 1,678) and tryptophan (n = 742) concentrations were determined by isocratic and reverse-phase high-performance liquid chromatography, respectively, as previously described (23,24).
Statistical analyses
Clinical characteristics for men and women were compared using two-tailed t test and Kolmogorov-Smirnov test for normally distributed and skewed variables, respectively. Amino acid concentrations quantified from fasting serum samples collected in 2001 were used to assess associations with HOMA-IR and fasting glucose at baseline as well as at 6-year follow-up. Analyses were stratified by sex, as amino acids are known to exhibit sex-specific associations with insulin resistance (9,12). Amino acids, triglycerides, and HOMA-IR were log transformed prior to linear regression analyses. For each amino acid, a linear regression model was fitted with HOMA-IR or fasting glucose as outcome and the amino acid as explanatory variable with BMI, systolic blood pressure, HDL cholesterol, triglycerides, smoking status, and physical activity index as covariates. Prospective associations with 6-year HOMA-IR and 6-year fasting glucose as outcomes were further adjusted for baseline HOMA-IR and glucose, respectively. To assess the combined effect of the branched-chain and aromatic amino acids, an amino acid score was calculated as the sum of isoleucine, leucine, valine, phenylalanine, and tyrosine concentrations in millimoles per liter. Regression coefficients are reported in standardized units of 1-SD difference in HOMA-IR or fasting glucose (outcome) per 1-SD difference in amino acid concentration (predictor).
For prediction of the highest extent of 6-year insulin resistance evidenced in young adults, a dichotomous score was defined as 6-year HOMA-IR >90th percentile using sex-specific values. This definition of insulin resistance corresponds to fasting insulin ≥17.7 IU/L for men and ≥15.9 IU/L for women in this study population. Odds ratios (ORs) of amino acids with 6-year insulin resistance were assessed using logistic regression models. The incremental value of amino acid profiling for prediction of insulin resistance was further examined. A reference model of established risk factors was derived by backward stepwise selection of the following variables: baseline HOMA-IR, age, BMI, triglycerides, HDL cholesterol, apoA1 and apoB, C-reactive protein, systolic blood pressure, smoking status, and physical activity index (P < 0.05 for retention). The best-fitting model was compared with a model with the amino acid score included. The ability to discriminate risk for 6-year insulin resistance in the two models was compared using area under the receiver operating characteristic curve (AUC), net reclassification improvement (NRI), and integrated discrimination improvement (IDI) (25). For NRI, participants were assigned to four categories (<5, 5–10, 10–20, and >20%) that reflected their 6-year risk for insulin resistance. Log-likelihood ratio χ2 was used to estimate improvement in global model fit. Prediction models were evaluated using 10-fold cross-validation to avoid an individual’s risk assessment being influenced by outcome status (22). Analyses were performed with MATLAB 7.10 (MathWorks, Natick, MA), and statistical significance was inferred as two-tailed P < 0.05.
RESULTS
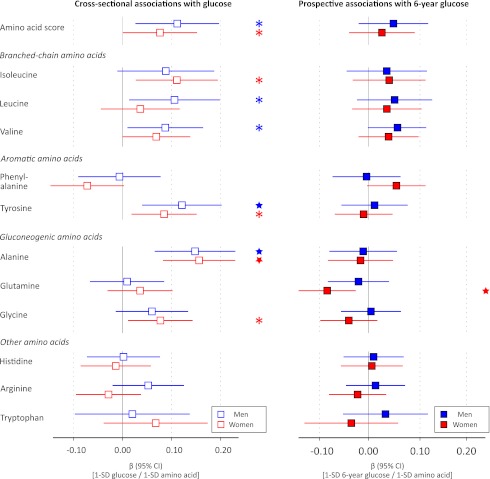
A total of 1,680 individuals free of treatment for diabetes (of whom 911 were women [54%]) were included in this study. Clinical characteristics are provided in Table 1. The median baseline insulin level of 6 IU/L reflects the young age and generally healthy metabolic status of the study participants. Associations of the amino acid score, defined as the sum of branched-chain and aromatic amino acid concentrations, and the 11 assayed amino acids with insulin resistance index at baseline and 6-year follow-up are shown in Fig. 1. Cross-sectionally, branched-chain and aromatic amino acids as well as alanine were associated with HOMA-IR after adjustment for conventional metabolic risk factors. For women, glutamine, glycine, and histidine were also associated with baseline insulin resistance index. The associations of branched-chain and aromatic amino acids were most pronounced for men; the magnitude of association for the branched-chain and aromatic amino acid score was twice as strong for men (β = 0.24) than for women (β = 0.12), where the β-regression coefficient indicates difference in SDs of HOMA-IR per 1-SD difference in amino acid concentration. This corresponds to a difference of 1.2 IU/L fasting insulin for men and 0.65 IU/L for women per 1-SD difference in the amino acid score when adjusting for established risk factors.
Table 1.
Baseline characteristics of the study population
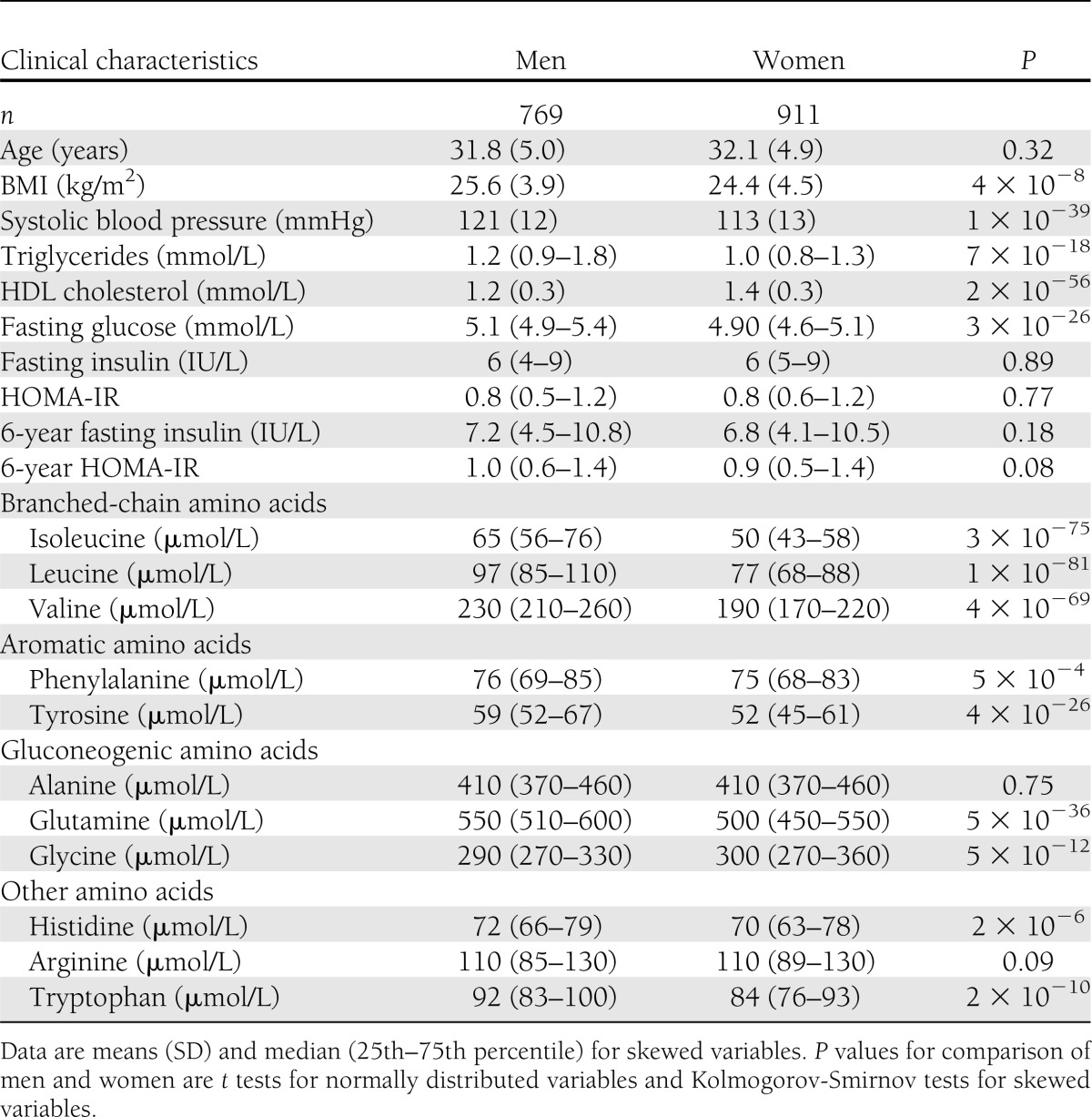
Figure 1.
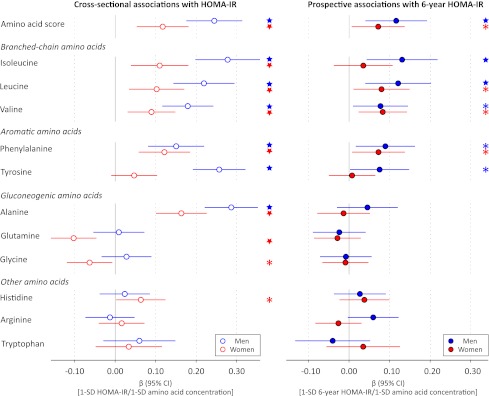
Cross-sectional and prospective associations of amino acids with HOMA-IR in the Cardiovascular Risk in Young Finns Study. Linear regression models were adjusted for baseline age, BMI, systolic blood pressure, HDL cholesterol, triglycerides, smoking status, and physical activity index. Prospective associations (filled circles) for insulin resistance index at 6-year follow-up were further adjusted for baseline HOMA-IR. Association magnitudes are in standardized units of 1 SD HOMA-IR per 1 SD amino acid concentration. Error bars indicate 95% CIs. Amino acid score: sum of isoleucine, leucine, valine, phenylalanine, and tyrosine concentrations. *P < 0.05; ★P < 0.005.
Prospective associations of the amino acids with 6-year HOMA-IR are illustrated in Fig. 1. The longitudinal analyses were adjusted for baseline HOMA-IR in addition to conventional metabolic risk factors. The amino acid score as well as the five branched-chain and aromatic amino acids were predictors of 6-year HOMA-IR for men, whereas only leucine, valine, and phenylalanine displayed significant prospective associations for women (P < 0.05). The magnitudes of association in longitudinal analyses were approximately half of the effect sizes found cross-sectionally when accounting for baseline insulin resistance index. Importantly, none of the other six assayed amino acids were predictors of HOMA-IR at 6-year follow-up (Fig. 1).
Cross-sectional and prospective associations of amino acids with fasting glucose are shown in Fig. 2. Several amino acids were associated with baseline glycemia for both men and women, including the branched-chain and aromatic amino acid score and alanine; yet, the association magnitudes were less prominent than those observed for HOMA-IR. In prospective analyses, however, none of the branched-chain and aromatic amino acids were predictors of 6-year fasting glucose in these young adults (P > 0.05 for all) in contrast to the results observed for insulin resistance index. The only significant association with 6-year glucose levels was the inverse association of glutamine observed for women (P = 0.005).
Figure 2.
Associations of amino acids with baseline and 6-year fasting glucose. Linear regression models were adjusted for baseline age, BMI, systolic blood pressure, HDL cholesterol, triglycerides, smoking status, and physical activity index. Prospective associations (filled squares) were further adjusted for baseline glucose. Association magnitudes are in standardized units of 1 SD glucose per 1 SD difference in amino acid concentration. Error bars indicate 95% CIs. *P < 0.05; ★P < 0.005.
Essentially similar results were found throughout when limiting analyses to normoglycemic subjects (fasting glucose <5.6 mmol/L; n = 1,508) or individuals without the metabolic syndrome (n = 1,486) as defined by the Harmonized definition (26) (data not shown). All results were virtually identical when HOMA-IR was replaced by fasting plasma insulin as outcome.
Prediction of high extent of insulin resistance
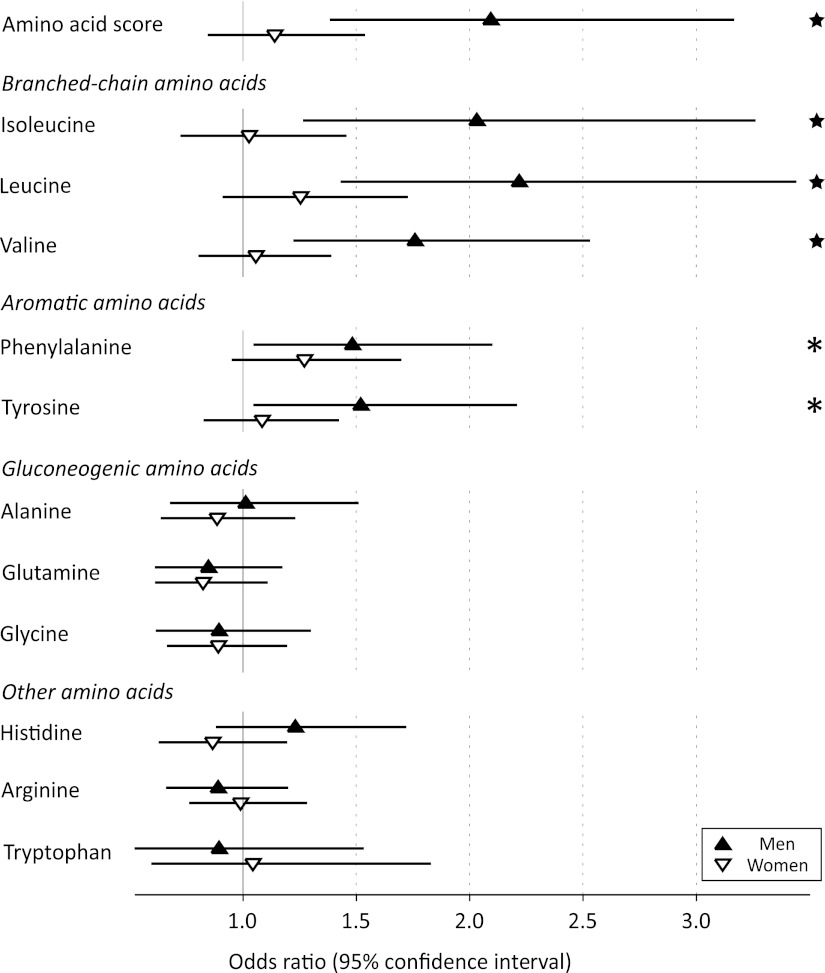
A high extent of insulin resistance in this young study population was defined as 6-year HOMA-IR >90th percentile. With use of this definition, 168 individuals were classified with insulin resistance at follow-up. ORs of each amino acid for 6-year insulin resistance are shown in Fig. 3. For men, the ORs were in line with the results from the continuous analyses, whereas for women no significant associations with a high degree of 6-year insulin resistance were observed (Fig. 3). The multivariate OR for 6-year insulin resistance of the amino acid score for men (OR 2.09 [95% CI 1.38–3.17]; P = 0.0005) was greater than the OR of BMI (1.72 [1.23–2.39]; P = 0.001) and comparable with that of baseline HOMA-IR (2.07 [1.45–2.96]; P = 0.00007). Isoleucine was the most pronounced predictor of high extent of insulin resistance at 6-year follow-up, as in the case of the continuous associations.
Figure 3.
ORs and 95% CIs for insulin resistance at 6-year follow-up (HOMA-IR ≥90th percentile) per 1 SD difference in baseline amino acid concentration. Logistic regression models were adjusted for baseline HOMA-IR, age, BMI, systolic blood pressure, HDL cholesterol, triglycerides, smoking status, and physical activity index. *P < 0.05; ★P < 0.005.
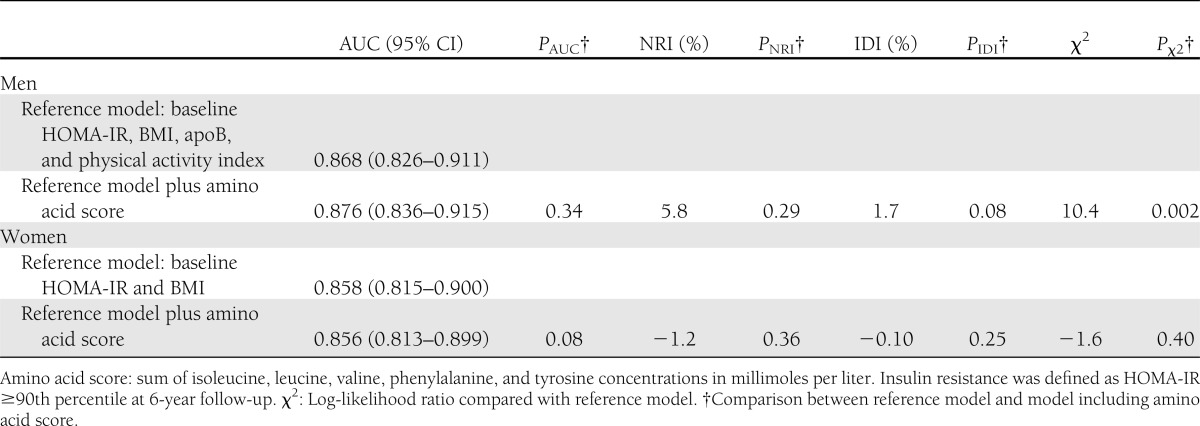
Prediction models for a high degree of 6-year insulin resistance in young adults with and without a score of branched-chain and aromatic amino acids are compared in Table 2. The best-fitted reference model was composed of baseline HOMA-IR and BMI, and for men the model additionally included apoB and physical activity index. The reference model AUCs were relatively high for both men and women, indicating that it is difficult to improve risk assessment. Indeed, inclusion of the amino acid score did not result in a significant change in risk discrimination (AUC) or improved reclassification (NRI and IDI), although in the case of men the model fit was improved (χ2 = 10.4, P = 0.002). Because there is no consensus on what signifies clinically significant insulin resistance in young adults, we further examined the predictive performance of the models using 6-year HOMA-IR ≥85th and 95th sex-specific percentiles to denote insulin resistance, with similar results obtained (data not shown).
Table 2.
Prediction of 6-year insulin resistance with and without branched-chained and aromatic amino acid score
CONCLUSIONS
This study demonstrates that circulating branched-chain and aromatic amino acid levels predict insulin resistance index 6 years later in normoglycemic individuals even when accounting for baseline insulin resistance and conventional risk factors. A set of five branched-chain and aromatic amino acids have recently been linked with the risk for hyperglycemia and diabetes in older populations (13–15); our results suggest that the amino acids mediate the risk for future diabetes because they are markers for the development of insulin resistance. Despite the prospective associations with impaired insulin sensitivity, our findings also indicate limited added value of amino acid profiling for the prediction of insulin resistance in young adults.
Accumulating evidence suggests that branched-chain and aromatic amino acids are associated with the extent of insulin resistance and hyperglycemia at a single time point (8–15,27,28). Already several decades ago, a study found that obese individuals had elevated concentrations of branched-chain and aromatic amino acids and that these amino acids correlated with insulin levels (7). We and others have recently demonstrated pronounced cross-sectional associations of amino acids with HOMA-IR with sex- and obesity-related effects (8–12). Here, our prospective analyses indicate that branched-chain and aromatic amino acids predict future impairment in insulin sensitivity in young adults. Circulating amino acid concentrations have been reported to be lower in overweight adolescents with type 2 diabetes in comparison with normal-weight peers (29). Our population-based results could, however, suggest that elevated branched-chain and aromatic amino acid levels precede the development of insulin resistance already in early adulthood. In a smaller study with similar follow-up time, but consisting of older Finnish individuals, we have shown that branched-chain and aromatic amino acids predict future glycemia in the general population (13). In contrast, no associations were observed with fasting glucose at 6-year follow-up in these young adults. Taken together, these results indicate that altered branched-chain and aromatic amino acid metabolism is associated with impairment of insulin sensitivity prior to effects observed for impaired glucose levels.
Intravenous administration and oral ingestion of multiple amino acids are known to modulate insulin secretion acutely (30,31); however, such effects have not been reported for fasting amino acid levels in prospective settings. For instance, arginine is an effective stimulus for insulin secretion (30); yet, no association was observed between arginine and insulin resistance index. Indeed, none of the other assayed amino acids predicted 6-year insulin resistance (Fig. 1). Although insulin secretion may potentially confound the reported associations with HOMA-IR, we have previously shown that circulating amino acid concentrations in the fasting state are associated primarily with insulin sensitivity rather than insulin secretion (13,14). The majority of the study population (90%) was within normoglycemic range, and exclusion of subjects with impaired fasting glucose did not alter our findings. β-cell dysfunction is therefore unlikely to underpin the associations established in this study (32). Hence, our findings support the notion that the risk for future diabetes induced by the amino acids is at least in part mediated through insulin resistance.
Whether branched-chain and aromatic amino acids are involved in the development of insulin resistance in a functional manner remains to be established. Despite the prospective associations with HOMA-IR, these findings do not imply that the amino acids are mechanistically involved in the pathogenesis. Several physiological and animal studies have suggested direct promotion of insulin resistance by branched-chain amino acids (8,27,28); however, genetic evidence has thus far not provided support for a causal role of branched-chain amino acids in the pathogenesis (12). It is plausible that the prospective associations with insulin resistance occur as a result of indirect effects rather than being caused by altered amino acid levels. Since fasting insulin and glucose are regulated by compensatory mechanisms, elevations in branched-chain and aromatic amino acid concentrations could potentially precede observations of high insulin resistance index and thereby serve as predictors of impaired insulin sensitivity (13). The observational nature of this study, however, precludes assessment of cause and effect, and further functional studies are required to elucidate the mechanisms underlying the links between amino acids and the risk of insulin resistance and overt diabetes.
In addition to branched-chain and aromatic amino acids, alanine and glutamine (inversely) have also recently been associated with future hyperglycemia and the risk for diabetes (11,13,14). While we found significant cross-sectional associations of these amino acids with insulin resistance index, the prospective associations were nonsignificant after adjustment for baseline HOMA-IR (Fig. 1). Nonetheless, glutamine was inversely associated with 6-year glycemia in women (Fig. 2). Insulin resistance has also been suggested to underpin the association with future diabetes in the case of alanine and glutamine (14). Our results could suggest that altered concentrations of these gluconeogenic amino acids are linked to impairment of insulin sensitivity in a later stage of the disease progression than perturbed branched-chain and aromatic amino acid metabolism.
Associations of amino acids with HOMA-IR were markedly different for men and women (Figs. 1 and 3). These findings corroborate prior reports on sex interactions of certain amino acids with insulin resistance (9,12). While the absolute concentrations of branched-chain and aromatic amino acid were significantly higher in men, a similar range of insulin resistance was observed for both sexes (Table 1). We have previously demonstrated that association of branched-chain amino acids with insulin resistance is significant for young women only if they are obese (12); yet, although similar cross-sectional findings were observed here, no such obesity interaction was found in prospective analyses (data not shown). Differences in gestational programming have been suggested to underpin the sex interactions for branched-chain amino acids with insulin resistance (9); however, the molecular underpinnings are still to be determined, and our findings highlight attention to sex differences in the role of amino acids in the pathogenesis of insulin resistance.
Early identification of individuals at high risk for diabetes is of importance for prevention (33). The sum of branched-chain and aromatic amino acid concentrations was recently shown to predict incidence of diabetes independent of baseline metabolic risk factors (15). Further, adding the amino acid score to established risk factors improved risk identification in a study sample of matched high-risk individuals; however, risk discrimination was not augmented in a general population setting (15). In line with these findings, our results indicate that branched-chain and aromatic amino acids are predictors of a high extent of insulin resistance for men (Fig. 2). However, despite an improvement in model fit by inclusion of the amino acid score in the prediction model, our results suggest that the incremental value for prediction may be limited in young adults (Table 2). Nevertheless, the clinical utility of amino acid profiling for the prediction of incident diabetes in older populations remains to be determined (34).
A limitation in this study is the use of HOMA-IR as a surrogate of insulin resistance. However, it has been shown that fasting measures are adequate surrogates of insulin resistance assessed by clamp test in the Finnish population (35). The absence of insulin secretion assessment limits correction of the prediction models to establish independent prospective associations with insulin resistance; yet, we have previously shown that fasting amino acid concentrations are primarily associated with insulin sensitivity rather than insulin secretion (13,14). We acknowledge that there is no established definition of what constitutes clinically significant insulin resistance, in particular for the young age-group studied; however, similar results were obtained using alternative definitions to signify the highest extent of insulin resistance. Due to the apparently healthy status of the young study population, statistical power was inadequate to assess the 6-year risk for type 2 diabetes. Strengths of the study include the young population-based cohort free of medication and prospective data to address associations of amino acids in an early stage of the pathogenesis of insulin resistance.
In summary, we have shown that circulating branched-chain and aromatic amino acids from fasting serum are predictors of insulin resistance index, but not glycemia, at 6-year follow-up in young adults, with the most pronounced associations observed for men. Adding a score of branched-chain and aromatic amino acids to established risk factors for risk assessment of early insulin resistance did not improve discrimination or classification. These results suggest that altered branched-chain and aromatic amino acid metabolism precedes the development of insulin resistance already in early adulthood prior to the occurrence of impaired fasting glucose, and this could at least partly explain how these amino acids are linked with the risk of future type 2 diabetes.
Acknowledgments
This work was supported by the Academy of Finland (grants 250422, 137870, 126925, 121584, and 124282) and the Research Programme of the Academy of Finland Responding to Public Health Challenges (grants 129269, 129429, and 129378), the Instrumentarium Science Foundation, the Paulo Foundation, the Juho Vainio Foundation, the Paavo Nurmi Foundation, the Jenny and Antti Wihuri Foundation, the Social Insurance Institution of Finland, Kuopio Tampere and Turku University Hospital Medical Funds, the Finnish Foundation for Cardiovascular Research, and the Strategic Research Funding from the University of Oulu.
No potential conflicts of interest relevant to this article were reported.
P.W. researched data, wrote the manuscript, and reviewed, commented on, and accepted the manuscript. P.S., A.J.K., and M.A.-K. designed the amino acid profiling platform, analyzed NMR experiments, and reviewed, commented on, and accepted the manuscript. T.R., T.L., M.K., J.S.V., and O.T.R. provided clinical data and interpretations and reviewed, commented on, and accepted the manuscript. P.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev 2007;87:507–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol 2008;9:193–205 [DOI] [PubMed] [Google Scholar]
- 3.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med 1993;329:1988–1992 [DOI] [PubMed] [Google Scholar]
- 4.Warram JH, Martin BC, Krolewski AS, Soeldner JS, Kahn CR. Slow glucose removal rate and hyperinsulinemia precede the development of type II diabetes in the offspring of diabetic parents. Ann Intern Med 1990;113:909–915 [DOI] [PubMed] [Google Scholar]
- 5.Moran A, Jacobs DR, Jr, Steinberger J, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation 2008;117:2361–2368 [DOI] [PubMed] [Google Scholar]
- 6.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci USA 2007;104:12587–12594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felig P, Marliss E, Cahill GF., Jr Plasma amino acid levels and insulin secretion in obesity. N Engl J Med 1969;281:811–816 [DOI] [PubMed] [Google Scholar]
- 8.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 2009;9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huffman KM, Shah SH, Stevens RD, et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009;32:1678–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tai ES, Tan ML, Stevens RD, et al. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 2010;53:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng S, Rhee EP, Larson MG, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation 2012;125:2222–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Würtz P, Mäkinen VP, Soininen P, et al. Metabolic signatures of insulin resistance in 7,098 young adults. Diabetes 2012;61:1372–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Würtz P, Tiainen M, Mäkinen VP, et al. Circulating metabolite predictors of glycemia in middle-aged men and women. Diabetes Care 2012;35:1749–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stančáková A, Civelek M, Saleem NK, et al. Hyperglycemia and a common variant of GCKR are associated with the levels of eight amino acids in 9,369 Finnish men. Diabetes 2012;61:1895–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med 2011;17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raitakari OT, Juonala M, Rönnemaa T, et al. Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol 2008;37:1220–1226 [DOI] [PubMed] [Google Scholar]
- 17.Koskinen J, Magnussen CG, Taittonen L, et al. Arterial structure and function after recovery from the metabolic syndrome: the cardiovascular risk in Young Finns Study. Circulation 2010;121:392–400 [DOI] [PubMed] [Google Scholar]
- 18.Telama R, Yang X, Viikari J, Välimäki I, Wanne O, Raitakari O. Physical activity from childhood to adulthood: a 21-year tracking study. Am J Prev Med 2005;28:267–273 [DOI] [PubMed] [Google Scholar]
- 19.Soininen P, Kangas AJ, Würtz P, et al. High-throughput serum NMR metabonomics for cost-effective holistic studies on systemic metabolism. Analyst (Lond) 2009;134:1781–1785 [DOI] [PubMed] [Google Scholar]
- 20.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care 2004;27:1487–1495 [DOI] [PubMed] [Google Scholar]
- 21.Würtz P, Soininen P, Kangas AJ, et al. Characterization of systemic metabolic phenotypes associated with subclinical atherosclerosis. Mol Biosyst 2011;7:385–393 [DOI] [PubMed] [Google Scholar]
- 22.Würtz P, Raiko JR, Magnussen CG, et al. High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur Heart J 2012;33:2307–2316 [DOI] [PubMed] [Google Scholar]
- 23.Juonala M, Viikari JS, Alfthan G, et al. Brachial artery flow-mediated dilation and asymmetrical dimethylarginine in the cardiovascular risk in young Finns study. Circulation 2007;116:1367–1373 [DOI] [PubMed] [Google Scholar]
- 24.Pertovaara M, Raitala A, Juonala M, et al. Indoleamine 2,3-dioxygenase enzyme activity correlates with risk factors for atherosclerosis: the Cardiovascular Risk in Young Finns Study. Clin Exp Immunol 2007;148:106–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008;27:157–172; discussion 207–212 [DOI] [PubMed] [Google Scholar]
- 26.Alberti KG, Eckel RH, Grundy SM, et al. International Diabetes Federation Task Force on Epidemiology and Prevention. Hational Heart, Lung, and Blood Institute. American Heart Association. World Heart Federation. International Atherosclerosis Society. International Association for the Study of Obesity Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–1645 [DOI] [PubMed] [Google Scholar]
- 27.Krebs M, Krssak M, Bernroider E, et al. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002;51:599–605 [DOI] [PubMed] [Google Scholar]
- 28.Tremblay F, Brûlé S, Hee Um S, et al. Identification of IRS-1 Ser-1101 as a target of S6K1 in nutrient- and obesity-induced insulin resistance. Proc Natl Acad Sci USA 2007;104:14056–14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mihalik SJ, Michaliszyn SF, de las Heras J, et al. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care 2012;35:605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Floyd JC, Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest 1966;45:1487–1502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Loon LJ, Kruijshoop M, Menheere PP, Wagenmakers AJ, Saris WH, Keizer HA. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care 2003;26:625–630 [DOI] [PubMed] [Google Scholar]
- 32.Stančáková A, Javorský M, Kuulasmaa T, Haffner SM, Kuusisto J, Laakso M. Changes in insulin sensitivity and insulin release in relation to glycemia and glucose tolerance in 6,414 Finnish men. Diabetes 2009;58:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM. RB SD. Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med 2007;167:1068–1074 [DOI] [PubMed] [Google Scholar]
- 34.Shah SH, Svetkey LP, Newgard CB. Branching out for detection of type 2 diabetes. Cell Metab 2011;13:491–492 [DOI] [PubMed] [Google Scholar]
- 35.Lorenzo C, Haffner SM, Stančáková A, Laakso M. Relation of direct and surrogate measures of insulin resistance to cardiovascular risk factors in nondiabetic finnish offspring of type 2 diabetic individuals. J Clin Endocrinol Metab 2010;95:5082–5090 [DOI] [PubMed] [Google Scholar]