Abstract
OBJECTIVE
Interpopulation as well as interindividual variations in response to vitamin D intake commonly observed in subjects with type 2 diabetes may be related to genetic makeup. One of the candidate genes potentially responsible for this diversity is vitamin D receptor (VDR). This study aimed to investigate the interactive effect of VDR Fok-I polymorphism and vitamin D intake on diverse aspects of diabetic host response.
RESEARCH DESIGN AND METHODS
Glycemic status, lipid profiles, inflammatory biomarkers, and VDR Fok-I genotypes were determined in diabetic subjects (n = 140) who participated in a randomized controlled trial. Participants consumed two 250-mL bottles per day of yogurt drink (doogh) fortified with 500 IU vitamin D/250 mL for 12 weeks.
RESULTS
Mean serum 25(OH)D increased by ~30 nmol/L (P < 0.001). The time × intervention effect was significant for 25(OH)D (P = 0.030), HDL (P = 0.011), high-sensitivity C-reactive protein (hsCRP) (P < 0.001), interleukin (IL)-4 (P = 0.008), and IL-6 (P = 0.017) among the genotypic groups. The alleles were defined as ‘‘F’’ or ‘‘f’’ depending on the absence or presence of the restriction site, respectively. The least increment in 25(OH)D was in ff (23.0 ± 3.8 nmol/L) compared with Ff (31.2 ± 3.4 nmol/L) and FF (35.6 ± 2.7 nmol/L) (P for trend = 0.009), but only the difference between ff and FF was significant (P = 0.023). FF group had the largest decrement of both hsCRP and IL-6 compared with Ff (P < 0.001 and P = 0.038) and ff (P = 0.010 and P = 0.048), respectively.
CONCLUSIONS
We concluded that those of VDR ff genotype may be regarded as “low responders” to vitamin D intake in terms of response of circulating 25(OH)D and certain inflammatory biomarkers. A nutrigenetic approach may, therefore, be needed to protect diabetic patients from vitamin D deficiency.
Type 2 diabetes is associated with considerable morbidity and mortality (1). Interaction between genetic and environmental factors, including diet, has an important role in development of this disease. Accumulating evidence indicates that insufficient concentrations of circulating 25(OH)D are associated with glucose intolerance, insulin resistance, metabolic syndrome, and increased risk for diabetes (2). Despite some evidence of ameliorated glycemic control (3) and inflammatory biomarkers (3,4) after improvement in vitamin D status, vitamin D supplementations have not resulted in improvement of glycemic or metabolic status in some populations, and interindividual variations in response to vitamin D intake are generally considerable (5). This heterogeneity in diabetic host responses to vitamin D may partly be explained by variations in the genes affecting the individual response to vitamin D intake. One of the candidate genes for controlling responses to vitamin D is vitamin D receptor (VDR), a member of the nuclear hormone receptor superfamily that modulates the transcription of target genes and mediates vitamin D genomic actions. VDR is expressed in a vast array of tissues, including those involved in the regulation of glucose metabolism, such as pancreatic β-cells (6). Several polymorphisms of the VDR gene have been identified, and their possible significance in diabetes has been inconclusively assessed in epidemiological investigations across multiethnic groups (7). One of such polymorphisms is Fok-I variants, whose restriction site is located on exon 2 in the 5′-coding region (6). This polymorphism results in different translation initiation sites on VDR. A thymine to cytosine conversion in the first translation initiation codon adenine thymine guanine (ATG) (methionine) generates long and short variants of VDR. The alleles were defined as ‘‘F’’ or ‘‘f’’ depending on the absence or presence of the restriction site, respectively. In the ff variant, initiation of translation occurs at the first ATG site, giving a long version of VDR protein comprised 427 amino acids. Conversely, in the FF variant, translation begins at the second ATG site instead of the first, resulting in a protein shortened by three amino acids. This is the only known VDR polymorphism, in contrast to other variants, that is translated in two different VDR protein products. Moreover, it is the only polymorphism that is not linked to any of the other VDR variants, thus giving it a unique role (6).
The Fok-I polymorphism, either singly or in combination with other VDR polymorphisms, has been investigated in a few studies on diabetes risk assessment (8,9). Some studies reported the association between FF genotype (either alone or together with other VDR polymorphisms) and diabetes risk in Indian population (8) and Caucasians (10), whereas one study found the subjects with ff genotype, compared with FF subjects, more susceptible to insulin resistance (10). Another study did not find any association between Fok-I polymorphism and increased risk for type 2 diabetes and its complications in a Polish population (9). These data have mostly been derived from descriptive studies, and there has not been a report of a clinical trial on the nutrigenetic association of VDR Fok-I variants with response to vitamin D intake in diabetes to date. This study aimed to compare the effect of vitamin D intake on glycemic, lipidemic, and inflammatory responses among Fok-I VDR variants of diabetic hosts.
RESEARCH DESIGN AND METHODS
One hundred and forty type 2 diabetic patients aged 29–67 years recruited from the Iranian Diabetes Society and Gabric Diabetes Education Association, both located in Tehran, participated in the study. To be eligible, patients needed to be free of any clinical disease including gastrointestinal, cardiovascular, and other endocrinological disorders (i.e., absence of specific symptoms and history of the diseases); a nonsmoker; nonpregnant; and not using insulin or any dietary supplements at least 3 months prior to the study.
This study was approved by the ethics committees of the National Nutrition and Food Technology Research Institute (NNFTRI), Shahid Beheshti University of Medical Sciences, and Tehran University of Medical Sciences (TUMS). All participants provided written informed consent.
Study design
This study was a part of a larger controlled randomized clinical trial that has previously been described (11). Briefly, after a 2-week run-in period, all subjects were instructed to consume a 250-mL bottle of vitamin D–fortified Persian yogurt drink (doogh), containing 170 mg calcium and 500 IU vitamin D/250 mL per bottle, twice a day with their meals (i.e., lunch and dinner). Two bottles of doogh were replaced for one exchange of dairy product (one glass of milk or yogurt) in the participants' diets so that the total energy intake was not affected by the intervention. The subjects were asked to keep their physical activity unchanged during the intervention. Fortified dooghs were given to the participants in 15-bottle packages. Every two weeks, all subjects were visited for assessment of their compliance and provision to them of a new package. Blood pressure measurements as well as anthropometric, dietary, physical activity, and biochemical evaluations were performed for all of the subjects before and after the intervention. Finally, all participants were divided in three groups according to VDR Fok-I variants, i.e., FF, Ff, and ff. Responses to the intervention were compared among the Fok-I genotypic groups. To ascribe changes of the variables to vitamin D intake, the intervention was performed during fall and winter, when dermal vitamin D synthesis was negligible. Moreover, a control group (n = 50) receiving equal amount of plain doogh was also included in the main study. As the results of the comparisons between fortified and plain groups have previously been reported (3,4), data are not presented here.
Anthropometric measurements and blood pressure
Weight was measured in light clothing without shoes using a digital scale (Seca 808; Seca, Hamburg, Germany). Height was measured by a stadiometer (Seca). Waist circumference and blood pressure were measured as previously described (11). BMI was calculated as weight in kilograms divided by the square of height in meters.
Body composition analysis and dietary assessment
Body fat mass was evaluated by bioelectrical impedance analysis (Quadscan 4000 system; Bodystat, Beaconsfield, U.K.). Subjects were asked to maintain their usual dietary intake throughout the study. Dietary intakes were assessed by three 24-h recalls on 3 days (including 2 working days and 1 weekend day) at the beginning and the end of the intervention period. Trained dietitians completed all questionnaires through face-to-face interviews with the participants using a multipass approach. For better quantification of portion sizes, a food album was used during an interview. This method has been used in a national survey on household food consumption patterns in Iran (12).
Laboratory investigations
Blood sampling and handling.
Blood samples collected from all participants after 12–14 h fasting by phlebotomy were divided in two tubes either with EDTA for genotyping and HbA1c determination or without the anticoagulant for serum chemical analyses as previously described (11).
Glycemic status, lipid profile, and urinary albumin-to-creatinine ratio.
Fasting serum glucose (FSG) and insulin concentrations as well as serum lipid profile and albumin-to-creatinine ratio (ACR) were determined as previously described (3). Insulin resistance was evaluated by quantitative insulin check index (QUICKI) (13).
Circulatory 25(OH)D and intact parathyroid hormone.
Serum 25(OH)D3 concentrations were measured by high-performance liquid chromatography method (14). In this study, vitamin D status was defined based on serum 25(OH)D3 concentration as deficiency, <27.5 nmol/L; insufficiency, >27.5 nmol/L but < 50 nmol/L; and sufficiency, >50 nmol/L. According to the Institute of Medicine, these cutoffs were set based on the fact that the vitamin D requirements of at least 97.5% of the population can be met with circulating 25(OH)D concentrations of 50 nmol/L (15). Serum intact parathyroid hormone (iPTH) was measured by enzyme immunoassay (EIA) (DRG, Marburg, Germany) with the aid of a semiautomatic plate reader (StatFax 3200; Awareness Technology, Palm City, FL).
Systemic and endothelial inflammatory biomarkers.
Systemic inflammatory biomarkers including high-sensitivity C-reactive protein (hsCRP) and serum amyloid A (SAA) were determined as previously described (4). Serum endothelial biomarkers including endothelin 1, E-selectin, and matrix metalloproteinase (MMP)-9 were measured as reported earlier (3).
Cytokine assays.
Peripheral blood mononuclear cells were separated using Ficoll density gradient and cultured in RPMI-1640 (16). After 24 h incubation, cell culture supernatants were used for cytokine measurements using enzyme-linked immunosorbent assay (4).
DNA analysis.
DNA was extracted from anticoagulated blood samples using Genet Bio DNA Isolation kit (PrimePrep, Chungnam, South Korea) according to the manufacturer’s protocol. The genotypes of VDR gene polymorphisms were determined by PCR amplification and enzymatic digestion of the products with Fok-I restriction enzyme. The forward and reverse primers (5′-AGC TGG CCC TGG CAC TGA CTC TGC TCT-3′ vs. 5′-ATG GAA ACA CCT TGC TTC TTC TCC CTC-3′) for amplification of the Fok-I VDR polymorphism were the same as those used elsewhere (17). PCR was performed with a gradient palm-cycler (Corbett Research, Sydney, Australia) for 30 cycles and at 58°C annealing temperature. DNA was digested with Fok-I enzyme (Fermentas; Thermo Scientific, Burlington, Ontario, Canada), and the products were analyzed by electrophoresis on a 1.5% agarose gel containing ethidium bromide and visualized in a gel documentation system (UVIdoc; UVItec, Cambridge, U.K.).
Statistical analysis
Normality of data distribution was evaluated using Kolmogrov-Smirnov. Genotype frequencies of Fok-I were tested for Hardy-Weinberg equilibrium using χ2 test. χ2 for trend was done to compare the proportions of vitamin D status among the groups. The statistical difference in genotype distribution among the groups was assessed by χ2. Two-factor repeated-measures ANOVA was used to evaluated time-group interactions, with time and group as factors. In case of a significant time × group interaction, between-group comparison of changes was done using ANOVA followed by Tukey post hoc analysis and with polynomial contrast analysis for trend when indicated. Within-group comparisons were performed using paired t test. Kruskal-Wallis H test was used to compare cigarettes smoked. Categorical and continuous variables are expressed as n (%) and means ± SD, respectively. In this study, P value <0.05 was considered statistically significant. All statistical analyses were done using SPSS (version 14; SPSS, Chicago, IL).
RESULTS
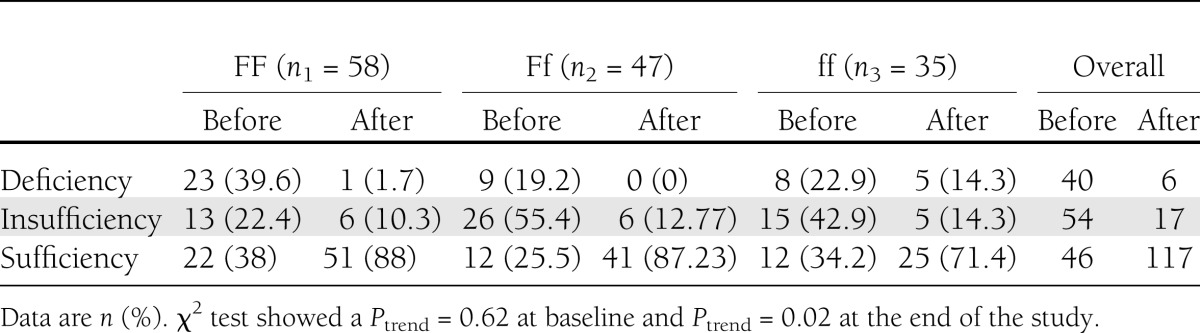
In our study population, the occurrence of VDR variants was in Hardy-Weinberg equilibrium (P = 0.056), with highest frequency of FF genotype, followed by Ff and ff genotypes, respectively. Personal characteristics among the genotypic groups, including age (P = 0.248), sex (P = 0.600), and duration of sun exposure in a day (P = 0.230), did not show any significant difference. In the whole-study population, the supplementation resulted in a considerable decrease in the occurrence of vitamin D insufficiency/deficiency and a remarkable increase in vitamin D sufficiency (from 26 to 92%). However, 4.4 and 14.9% of the subjects still remained vitamin D deficient and insufficient, respectively. Reanalysis of data among Fok-I–variant subgroups revealed no significant trend of distribution of vitamin D deficiency through Fok-I genotypes (χ2 = 0.24; Ptrend = 0.620) at baseline. However, after intervention, trend test was significant (χ2 = 5.3; P for trend = 0.020) showing that 50% of the initially vitamin D–deficient subjects in the ff genotype still remained deficient after 12 weeks’ intervention (Table 1). There was no significant difference in any of the initial variables among the genotypic groups except for FSG, which showed a significant difference (P = 0.011). Post hoc Tukey test revealed that the Ff group had significantly lower FSG compared with FF (P = 0.034) and ff (P = 0.019). However, this difference was not accompanied by a significant difference of other glycemic variables such as HbA1c or QUICKI. Circulating 25(OH)D was expectedly raised in the three genotypic groups.
Table 1.
Vitamin D status among the Fok-I genotypic subgroups before and after the intervention
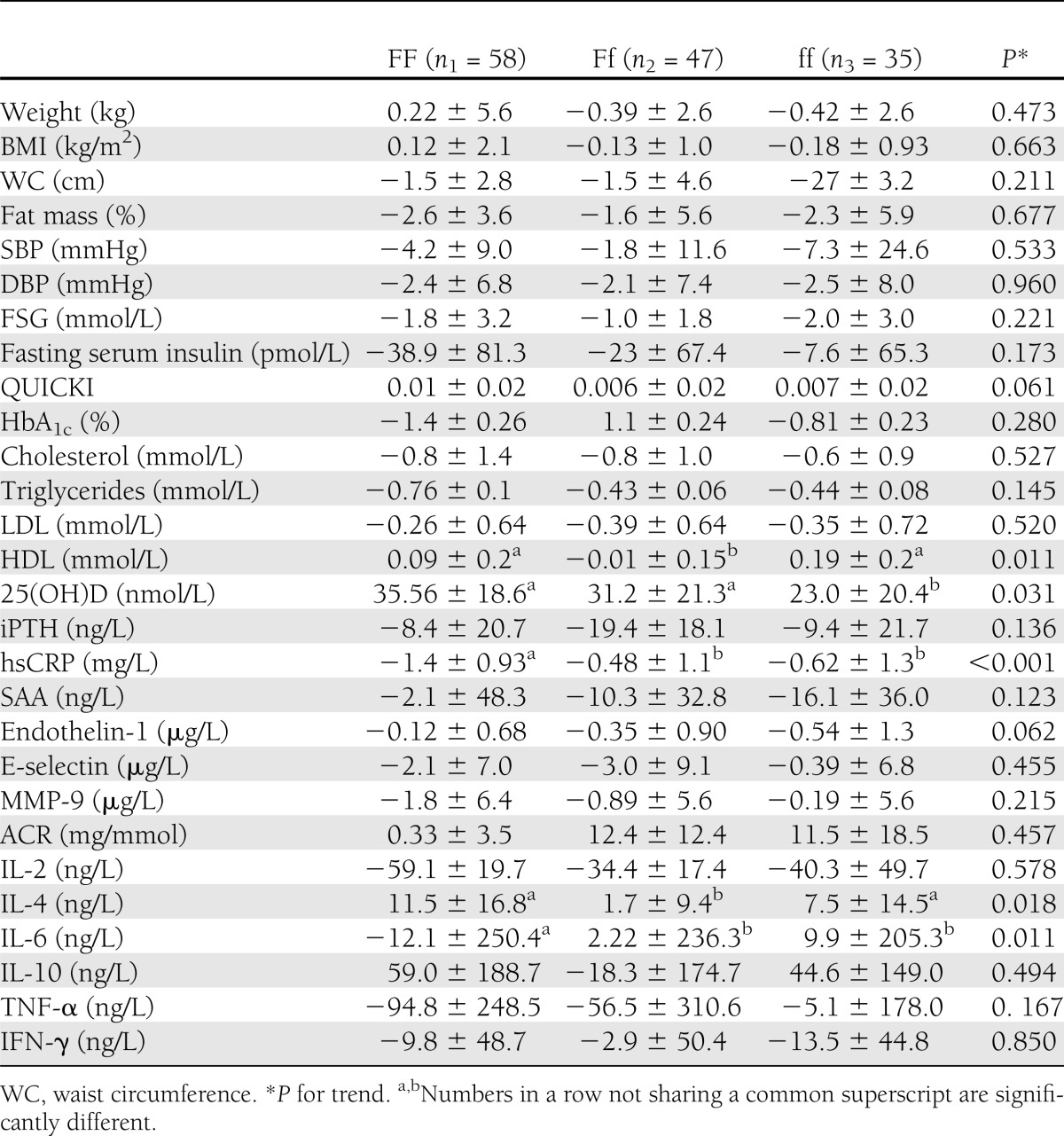
Improvement of vitamin D status was accompanied by amelioration of glycemic, lipidemic, and inflammatory markers in the whole-study population as previously described (3). Repeated-measures ANOVA revealed a significant time effect (i.e., within-subject difference over time) for 25(OH)D (P < 0.001), iPTH (P < 0.001), fat mass (P < 0.001), waist circumference (P = 0.002), systolic blood pressure (SBP) (P = 0.003), diastolic blood pressure (DBP) (P = 0.001), FSG (P < 0.001), HbA1c (P < 0.001), QUICKI (P < 0.001), total cholesterol (P < 0.001), triglycerides (P < 0.001), HDL (P < 0.001), LDL (P < 0.001), hsCRP (P < 0.001), SAA (P = 0.013), E-selectin (P = 0.012), endothelin-1 (P = 0.001), interleukin (IL)-2 (P = 0.006), IL-4 (P < 0.001), and tumor necrosis factor (TNF)-α (P = 0.044). The time effect was insignificant for γ-interferon (IFN-γ) (P = 0.058) (Table 2). However, time × intervention effect was significant only for 25(OH)D (P = 0.031), HDL (P = 0.011), hsCRP (P < 0.001), IL-4 (P = 0.018), and IL-6 (P = 0.011). When changes of the variables were compared using ANOVA followed by Tukey post hoc test, a significant difference in 25(OH)D was observed between FF and ff (P = 0.023), but the differences between FF and Ff (P = 0.573) or Ff and ff (P = 0.23) were not statistically significant. As for hsCRP, FF differed significantly with both Ff (P < 0.001) and ff (P = 0.010), but there was no significant difference between Ff and ff (P = 0.833). The proinflammatory cytokine IL-6 also showed a significant difference between FF and Ff (P = 0.038) as well as between FF and ff (P = 0.048). Again, here, Ff and ff did not differ significantly (P = 0.990). Table 3 demonstrates the results of ANOVA of the changes of the variables among the three VDR Fok-I genotypes.
Table 2.
Comparisons of the variables among the Fok-I genotypic groups with type 2 diabetes before and after 12 weeks’ intervention
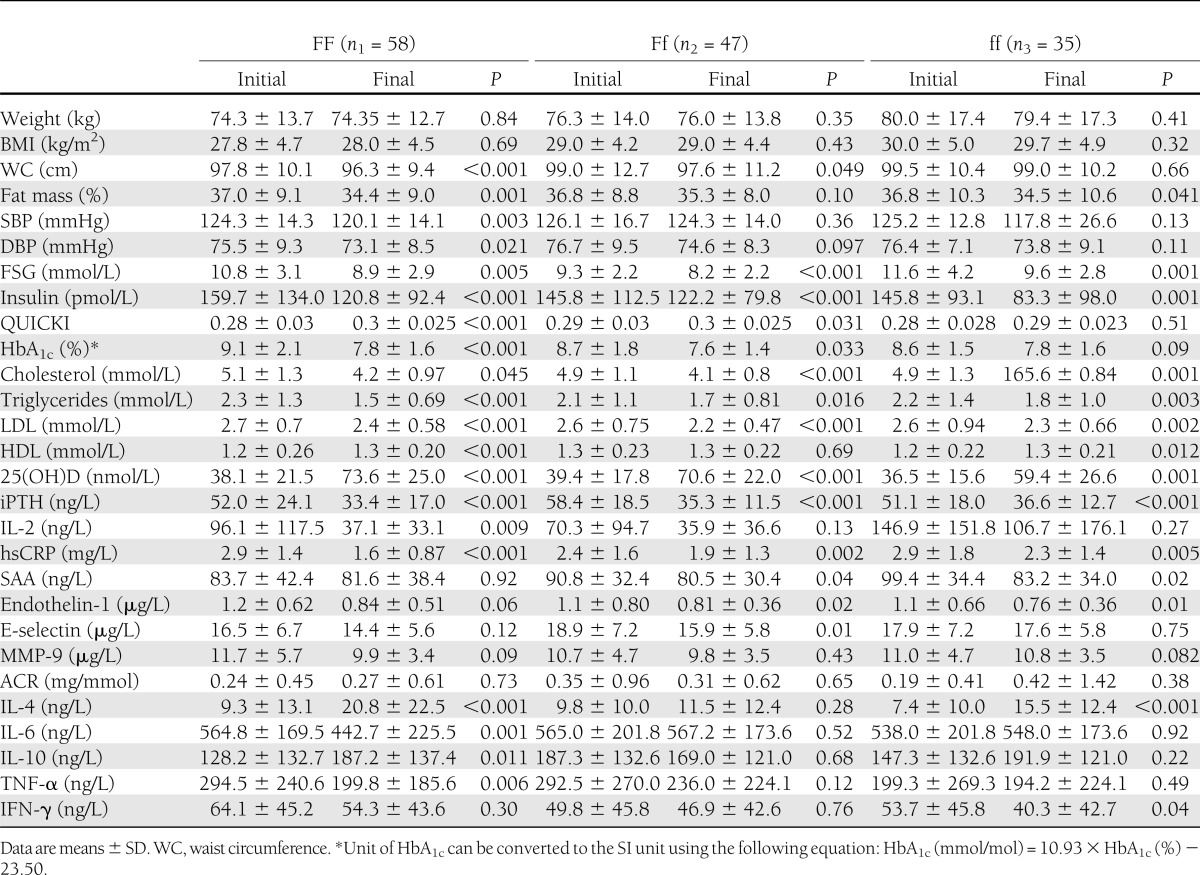
Table 3.
Comparison of the changes of the variables among the Fok-I genotypic groups with type 2 diabetes
CONCLUSIONS
Our data show a significant difference in the diabetic host response to supplemental intake of vitamin D according to Fok-I VDR variants. Specifically, the significantly lowest serum 25(OH)D response to daily intake of vitamin D–fortified doogh in the ff group is considerable. The occurrence of VDR genotypes could, therefore, be pivotal in the outcome of any vitamin D intervention in a healthy as well as a patient population. Some fortification experiences failed to protect the desirable proportion of the study population from vitamin D deficiency (18), which might be, at least partly, due to the genetic variations.
The influence of VDR genotypes on absorption of both calcium and vitamin D has already been reported (19). In a pilot study on the efficacy of vitamin D plus calcium supplementation in 92 U.S. adult subjects, after 6 months’ supplementation with 800 IU vitamin D and 2,000 mg calcium, 25(OH)D reached the desirable levels in only half of the subjects (20). Similarly, supplementation with 800 IU/day vitamin D during the winter resulted in normalization of circulating 25(OH)D in 80% of the postmenopausal women (21). Although no genetic analyses were performed in these studies, genetic variations of VDR among the study populations could be one explanation. In support of this notion, in subjects with low bone density who are heterozygous for allele f, compared with ff homozygotes, an increased tendency for being responsive to supplementation has been reported (22). The inverse association between VDR Fok-I ff genotype and lower vitamin D status has also been observed among the subjects with multiple sclerosis (23) and β-thalassemia (24).
It is noteworthy that despite different 25(OH)D responses, changes of iPTH did not differ significantly among the groups. Contrary to our finding, in an observational study on 95 postmenopausal women, Fok-I variants were related to both secretion and degradation of parathyroid hormone (25). Notwithstanding, our data showed that a parathyroid hormone–suppressing effect of intake of 1,000 IU/day vitamin D could be similar among Fok-I VDR genotypic groups.
The ameliorating effect of vitamin D intake on glycemic status of diabetic subjects was observed in our previous study (3). In the current study, lower 25(OH)D response to vitamin D intake in the ff group had no remarkable effect on either FSG or QUICKI. This might be due to the compensatory increased formation of the active vitamin D metabolite, 1,25(OH)D2 from 25(OH)D by 1-α-hydroxylase, which is also expressed in pancreatic β-cells (26). Although VDR Fok-I polymorphism has been shown to explain some 29% of the variability in insulin sensitivity, it has no influence on β-cell function in healthy Caucasians (10). The association of VDR genotypes with glycemic status has been the focus of several studies. Polymorphisms of VDR Bsm-I have been linked to development of both diabetes and coronary artery disease (CAD) in 293 subjects at high risk of CAD (27). In another study, while the distribution of Apa-I, Bsm-I, and Taq-I variants did not differ between diabetic and nondiabetic subjects, Bsm-I polymorphism was associated with insulin resistance only in nondiabetic Caucasians (28). High prevalence of vitamin D deficiency and its inverse association with Apa-I aa genotype and Fok-I f allele in Caribbean subjects with type 2 diabetes has been described previously (29).
As previously reported (3), daily consumption of vitamin D–fortified doogh resulted in a small but significant increase in serum HDL. However, only in homozygotic subgroups (i.e., FF and ff) were the changes of HDL concentrations statistically significant. Contrary to our findings of increased serum HDL in VDR Fok-I homozygotic groups FF and ff, in a study on 276 nondiabetic overweight men (BMI 28.06 kg/m2) aged 25–65 years, subjects with FF and Ff genotypes, compared with the ff variant, had lower HDL concentrations and higher fasting serum insulin (30). One of the reasons for this inconsistency in observations could be the interactive effects of other genes with VDR, including HLA (31).
Cardiovascular disease (CVD), including CAD, stroke, and peripheral vascular disease, is well-known as the major cause of morbidity and mortality in diabetic subjects (32). However, some studies have shown that intensive treatment of hyperglycemia would lead to only 16 and 25% risk reduction of myocardial infarction and microvascular disease, respectively (33). Therefore, normalization of other risk factors, including inflammatory status, has been proposed to prevent CVD in diabetes (34). The association of inflammation with diabetes and its long-term complications has previously been documented (35). In the current study, although hsCRP decreased in all groups, it did so more in FF than in Ff and ff. Similarly, the decrease of cellular IL-6 in FF was in order of magnitude compared with Ff and ff, which was accompanied by a significant increase in IL-4. These changes suggest the vitamin D–induced immune deviance toward T-helper 2, which was more prominent in FF than in Ff or ff. Considering the predisposing effect of inflammation in long-term diabetes morbidities, notably CVD (36), our findings may indicate different protective effects of vitamin D intake among diabetic subjects carrying various VDR Fok-I genotypes.
Improvement of endothelial (3) as well as systemic (4,37) inflammatory biomarkers after vitamin D intake in the subjects with type 2 diabetes has already been reported. The subsided inflammatory response, however, was not accompanied by a significant change in urinary albumin excretion in either Fok-I genotypic group. The possible synergistic protective effect of both suppressed systemic/endothelial inflammation and raised serum HDL after improvement of vitamin D status in diabetic subjects and their interaction with VDR Fok-I variants could be the focus of further studies.
As vitamin D deficiency adversely affects both glycemic and inflammatory status, it can intuitively be regarded as a predisposing factor for long-term diabetes complications, including atherosclerosis (32). In the current study, 74% of the subjects initially had vitamin D deficiency/insufficiency, similar to 73% occurrence of hypovitaminosis D observed in another study (38). However, not all VDR genotypic groups responded similarly to increased vitamin D intake. Actually, the ff variant showed the lowest response in terms of increments of 25(OH)D and certain inflammatory biomarkers. VDR ff genotype has also been proposed as a contributing factor in aggressive breast cancer, which is less responsive to vitamin D therapy (39). The possible role of VDR variants in diabetes still remains to be clarified by further studies. Notwithstanding, some genotypic groups, notably VDR ff, may be considered “low responders” who may have an increased need for vitamin D. A nutrigenetic approach may, therefore, be needed to protect diabetic subjects from vitamin D deficiency. This possibility warrants further studies with larger sample sizes.
However, some limitations of the current study should be acknowledged. Polymorphisms of other VDR genotypes, i.e., Taq-I, Apa-I, Bsm-I, and Cdx-2, and their possible interactions with Fok-I variants were not evaluated. Measurement of MMP-9 should preferably have been done in plasma instead of serum. Extension of the changes observed after 12 weeks’ intervention to longer periods of time and, above all, their possible protective effect against long-term diabetes complications require well-designed longitudinal controlled studies. Regarding the small amount of 25(OH)D variation explained by genetic determinants, a Mendelian randomization approach could be beneficial to reveal a causal relationship in a large population (40).
As hypovitaminosis D may negatively affect glycemic status (38), and probably the related consequences, the very high prevalence of vitamin D insufficiency/deficiency among the participants of the current study is likely to have a major impact on the results. Also, evaluation of a vast array of variables further allowed us to detect the positive effects of vitamin D supplementation.
To the best of our knowledge, this is the first report of the interactive effects of VDR Fok-I polymorphisms and vitamin D intake on diverse aspects of diabetes host response, including glycemic, lipidemic, and inflammatory as well as immune responses. Our findings indicated that those with VDR Fok-I ff genotype may be regarded as low responders to vitamin D intake in terms of circulating 25(OH)D and certain inflammatory biomarkers. The prevalence of different VDR Fok-I variants among diabetic subjects could explain, at least in part, some discrepancies observed in the effect of vitamin D on various aspects of diabetes host response.
Acknowledgments
This study was funded by NNFTRI, TUMS, and the Iran National Science Foundation (listed in order of their financial contributions). All laboratory bench work was performed at the Laboratory of Nutrition Research, NNFTRI. This study formed a part of the PhD thesis of S.S.-B. at TUMS (grant no. 10533).
The Iran National Science Foundation had no role in the study conception or design. The Iranian Diabetes Society and the Gabric Diabetes Education Association had no role in study design; data collection, synthesis, or interpretation; writing the report; or the decision to submit the manuscript for publication.
Half of the yogurt drinks (both plain and vitamin D fortified) were donated to T.R.N. by Pegah Company, while the remainder was purchased from there. No other potential conflicts of interest relevant to this article were reported.
T.R.N. designed and supervised the study, was involved in laboratory analyses, wrote the finalized manuscript, and supervised the PhD thesis at TUMS by S.S.-B., of which this study formed a part. A.D. helped intellectually in finalizing the study design and supervised the PhD thesis at TUMS by S.S.-B., of which this study formed a part. S.S.-B. helped intellectually in finalizing the study design, performed most of the laboratory analyses, wrote the preliminary manuscript, and was actively involved in the field work. M.R.E. supervised all statistical analyses. A.K., N.S., N.K., M.Z., A.G., and S.A. participated in all laboratory investigations. A.H. provided guidance for dietary assessments. M.C. performed dietary assessments. T.R.N. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the Iranian Diabetes Society and the Gabric Diabetes Education Association for their collaborations. The authors also thank Dr. Simin Vaghefi for refining the manuscript language. Finally, the authors appreciate all the subjects who devotedly participated in this study.
Footnotes
Clinical trial reg. no. NCT01236846, clinicaltrials.gov.
References
- 1.Winer N, Sowers JR. Epidemiology of diabetes. J Clin Pharmacol 2004;44:397–405 [DOI] [PubMed] [Google Scholar]
- 2.Salekzamani S, Neyestani TR, Alavi-Majd H, Houshiarrad A, Kalayi A, Shariatzadeh N, et al. Is vitamin D status a determining factor for metabolic syndrome? A case-control study. Diabetes Metab Syndr Obes 2011;2:205–211 [DOI] [PMC free article] [PubMed]
- 3.Shab-Bidar S, Neyestani TR, Djazayery A, et al. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: a randomized double-blind clinical trial. BMC Med 2011;9:125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shab-Bidar S, Neyestani TR, Djazayery A, et al. Improvement of vitamin D status resulted in amelioration of biomarkers of systemic inflammation in the subjects with type 2 diabetes. Diabetes Metab Res Rev 2012;28:424–430 [DOI] [PubMed] [Google Scholar]
- 5.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr 2011;65:1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palomer X, González-Clemente JM, Blanco-Vaca F, Mauricio D. Role of vitamin D in the pathogenesis of type 2 diabetes mellitus. Diabetes Obes Metab 2008;10:185–197 [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Xi B, Reilly KH, Liu M, Fu M. Quantitative assessment of the associations between four polymorphisms (FokI, ApaI, BsmI, TaqI) of vitamin D receptor gene and risk of diabetes mellitus. Mol Biol Rep 2012;39:9405–9414 [DOI] [PubMed] [Google Scholar]
- 8.Bid HK, Konwar R, Aggarwal CG, et al. Vitamin D receptor (FokI, BsmI and TaqI) gene polymorphisms and type 2 diabetes mellitus: a North Indian study. Indian J Med Sci 2009;63:187–194 [PubMed] [Google Scholar]
- 9.Malecki MT, Frey J, Moczulski D, Klupa T, Kozek E, Sieradzki J. Vitamin D receptor gene polymorphisms and association with type 2 diabetes mellitus in a Polish population. Exp Clin Endocrinol Diabetes 2003;111:505–509 [DOI] [PubMed] [Google Scholar]
- 10.Chiu KC, Chuang LM, Yoon C. The vitamin D receptor polymorphism in the translation initiation codon is a risk factor for insulin resistance in glucose tolerant Caucasians. BMC Med Genet 2001;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shab-Bidar S, Neyestani TR, Djazayery A. Efficacy of vitamin D3-fortified-yogurt drink on anthropometric, metabolic, inflammatory and oxidative stress biomarkers according to vitamin D receptor gene polymorphisms in type 2 diabetic patients: a study protocol for a randomized controlled clinical trial. BMC Endocr Disord 2011;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalantari N, Ghafarpour, M. National Comprehensive Study on Household Food Consumption Pattern and Nutritional Status, IR Iran, 2001–2003. (National Report) 2005
- 13.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 2000;85:2402–2410 [DOI] [PubMed] [Google Scholar]
- 14.Neyestani TR, Gharavi A, Kalayi A. Determination of serum 25-hydroxy cholecalciferol using high-performance liquid chromatography: a reliable tool for assessment of vitamin D status. Int J Vitam Nutr Res 2007;77:341–346 [DOI] [PubMed] [Google Scholar]
- 15.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neyestani TR, Gharavi A, Kalayi A. Selective effects of tea extract and its phenolic compounds on human peripheral blood mononuclear cell cytokine secretions. Int J Food Sci Nutr 2009;60(Suppl. 1):79–88 [DOI] [PubMed] [Google Scholar]
- 17.Deng HW, Shen H, Xu FH, et al. Tests of linkage and/or association of genes for vitamin D receptor, osteocalcin, and parathyroid hormone with bone mineral density. J Bone Miner Res 2002;17:678–686 [DOI] [PubMed] [Google Scholar]
- 18.Välimäki VV, Löyttyniemi E, Välimäki MJ. Vitamin D fortification of milk products does not resolve hypovitaminosis D in young Finnish men. Eur J Clin Nutr 2007;61:493–497 [DOI] [PubMed] [Google Scholar]
- 19.Dawson-Hughes B, Harris SS, Finneran S. Calcium absorption on high and low calcium intakes in relation to vitamin D receptor genotype. J Clin Endocrinol Metab 1995;80:3657–3661 [DOI] [PubMed] [Google Scholar]
- 20.McCullough ML, Bostick RM, Daniel CR, et al. Vitamin D status and impact of vitamin D3 and/or calcium supplementation in a randomized pilot study in the Southeastern United States. J Am Coll Nutr 2009;28:678–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson ML, Blum JM, Hollis BW, Rosen C, Sullivan SS. Supplements of 20 microg/d cholecalciferol optimized serum 25-hydroxyvitamin D concentrations in 80% of premenopausal women in winter. J Nutr 2009;139:540–546 [DOI] [PubMed] [Google Scholar]
- 22.Elnenaei MO, Chandra R, Mangion T, Moniz C. Genomic and metabolomic patterns segregate with responses to calcium and vitamin D supplementation. Br J Nutr 2011;105:71–79 [DOI] [PubMed] [Google Scholar]
- 23.Orton SM, Morris AP, Herrera BM, et al. Evidence for genetic regulation of vitamin D status in twins with multiple sclerosis. Am J Clin Nutr 2008;88:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dimitriadou M, Christoforidis A, Fidani L, et al. Fok-I gene polymorphism of vitamin D receptor in patients with beta-thalassemia major and its effect on vitamin D status. Hematology 2011;16:54–58 [DOI] [PubMed] [Google Scholar]
- 25.Žofková I, Zajíčková K, Hill M. Serum parathyroid hormone levels are associated with FokI polymorphism of the vitamin D receptor gene in untreated postmenopausal women. Eur J Intern Med 2003;14:232–236 [DOI] [PubMed] [Google Scholar]
- 26.Bland R, Markovic D, Hills CE, et al. Expression of 25-hydroxyvitamin D3-1alpha-hydroxylase in pancreatic islets. J Steroid Biochem Mol Biol 2004;89-90:121–125 [DOI] [PubMed] [Google Scholar]
- 27.Ortlepp JR, Lauscher J, Hoffmann R, Hanrath P, Joost HG. The vitamin D receptor gene variant is associated with the prevalence of type 2 diabetes mellitus and coronary artery disease. Diabet Med 2001;18:842–845 [DOI] [PubMed] [Google Scholar]
- 28.Oh JY, Barrett-Connor E. Association between vitamin D receptor polymorphism and type 2 diabetes or metabolic syndrome in community-dwelling older adults: the Rancho Bernardo Study. Metabolism 2002;51:356–359 [DOI] [PubMed] [Google Scholar]
- 29.Vélayoudom-Céphise FL, Larifla L, Donnet JP, et al. Vitamin D deficiency, vitamin D receptor gene polymorphisms and cardiovascular risk factors in Caribbean patients with type 2 diabetes. Diabetes Metab 2011;37:540–545 [DOI] [PubMed] [Google Scholar]
- 30.Filus A, Trzmiel A, Kuliczkowska-Płaksej J, et al. Relationship between vitamin D receptor BsmI and FokI polymorphisms and anthropometric and biochemical parameters describing metabolic syndrome. Aging Male 2008;11:134–139 [DOI] [PubMed] [Google Scholar]
- 31.Al-Daghri NM, Al-Attas O, Alokail MS, et al. Vitamin D receptor gene polymorphisms and HLA DRB1*04 cosegregation in Saudi type 2 diabetes patients. J Immunol 2012;188:1325–1332 [DOI] [PubMed] [Google Scholar]
- 32.Milicevic Z, Raz I, Beattie SD, et al. Natural history of cardiovascular disease in patients with diabetes: role of hyperglycemia. Diabetes Care 2008;31(Suppl. 2):S155–S160 [DOI] [PubMed] [Google Scholar]
- 33.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 34.Laakso M. Hyperglycemia and cardiovascular disease in type 2 diabetes. Diabetes 1999;48:937–942 [DOI] [PubMed] [Google Scholar]
- 35.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813–823 [DOI] [PubMed] [Google Scholar]
- 36.Pickup JC, Mattock MB. Activation of the innate immune system as a predictor of cardiovascular mortality in type 2 diabetes mellitus. Diabet Med 2003;20:723–726 [DOI] [PubMed] [Google Scholar]
- 37.Neyestani TR, Nikooyeh B, Alavi-Majd H, et al. Improvement of vitamin D status via daily intake of fortified yogurt drink either with or without extra calcium ameliorates systemic inflammatory biomarkers, including adipokines, in the subjects with type 2 diabetes. J Clin Endocrinol Metab 2012;97:2005–2011 [DOI] [PubMed] [Google Scholar]
- 38.Nikooyeh B, Neyestani TR, Farvid M, et al. Daily consumption of vitamin D- or vitamin D + calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 2011;93:764–771 [DOI] [PubMed] [Google Scholar]
- 39.Alimirah F, Peng X, Murillo G, Mehta RG. Functional significance of vitamin D receptor FokI polymorphism in human breast cancer cells. PLoS ONE 2011;6:e16024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berry DJ, Vimaleswaran KS, Whittaker JC, Hingorani AD, Hyppönen E. Evaluation of genetic markers as instruments for mendelian randomization studies on vitamin D. PLoS ONE 2012;7:e37465. [DOI] [PMC free article] [PubMed] [Google Scholar]


