Abstract
OBJECTIVE
We aimed to evaluate a selective screening strategy for gestational diabetes mellitus (GDM) based on the presence of risk factors: BMI ≥25 kg/m2, age ≥35 years, family history of diabetes, personal history of GDM, or birth of a child with macrosomia.
RESEARCH DESIGN AND METHODS
Of 20,630 deliveries between 2002 and 2010, we selected 18,775 deliveries in women with no known diabetes and for whom all risk factors were known. GDM was universally screened and defined as fasting plasma glucose level ≥5.3 mmol/L and/or 2-h postload (75 g) glucose level ≥7.8 mmol/L.
RESULTS
The prevalence of at least one risk factor has increased since 2002 (P < 0.001) from 51.7 to 61.5%, with no change in the GDM prevalence (mean 14.4%, intention to screen). At least one risk factor was present in 58.5% of women who represented 65.3% of all those with GDM. The presence of risk factors was significantly associated with GDM (odds ratio 1.4 [95% CI 1.3–1.5], P < 0.001) and with GDM-related events (preeclampsia/large for gestational age/dystocia) (P < 0.001) with the following incidences: no GDM/no risk factor 8.8%, no GDM/risk factor 11.1%, GDM/no risk factor 16.7%, and GDM/risk factor 18.2%.
CONCLUSIONS
The presence of risk factors increased during the last decade. This condition is predictive of GDM and GDM-related events. However, a selective screening would lead to missing one-third of the women with GDM who, even without risk factors, had more events than women without GDM. Therefore, these data stand against the present selective screening currently proposed in the French guidelines.
New international diagnostic criteria for gestational diabetes mellitus (GDM) (1) have been progressively adopted worldwide, leading to a dramatic increase in GDM prevalence. A universal screening has been recommended, but a selective screening, based on the presence of risk factors, has been used as well (2). Using selective screening may lead to missing up to 45% of GDM cases (3). On the other hand, selective screening could help to concentrate medical resources on subjects with the highest risk of complications (2), especially those with a high BMI (4). This is crucial, as the cost-efficacy ratio of GDM screening still needs to be evaluated in women with no risk factors (5). For example, the expert panel for French guidelines stated, as a professional agreement, that there was no sufficient evidence for recommending universal screening (5). Based on the review of literature on GDM risk factors (6), they recommended GDM screening if at least one of the following criteria is present: maternal age ≥35 years, BMI ≥25 kg/m2, history of diabetes in a first-degree relative, personal history of GDM, or birth of a child with macrosomia.
We therefore aimed to retrospectively evaluate this recommendation in a large cohort of women who had delivered in the previous 9 years in the obstetrics department of our university hospital located in an eastern suburb of Paris, France. More specifically, the aims were to evaluate 1) the screening value of risk factors for GDM and 2) the prognostic value of risk factors for GDM-related events. We also took the opportunity of this large cohort to look for changes in the prevalence of GDM and risk factors during the years after 1999.
RESEARCH DESIGN AND METHODS
Participants and GDM screening
A total of 20,653 women delivered in our hospital between January 2002 and December 2010. We did not include the women with known diabetes (n = 204) or those for whom the status for either one of the five risk factors was not known (n = 1674). Therefore, 18,775 pregnancies were analyzed. Data are routinely entered at birth for all women giving birth in our university hospital by the midwife assisting with the delivery and then checked and collected during maternity stay by a single midwife (I.P.) qualified in data management and storage. The definition of parameters did not change over the 9 years of the study. A GDM screening was performed early at 15 weeks’ gestation only for the subjects with a history of GDM or those who had two or more of the following criteria: family history of diabetes; personal history of glucose intolerance; pregravid BMI >27 kg/m2; age >35 years; history of a previous pregnancy with GDM, preeclampsia, or malformation; macrosomia (birth weight >4 kg) and/or intrauterine fetal death; current pregnancy with hypertension; or estimated fetal weight >90th percentile for gestational age on ultrasound scan. Unless GDM had been found in early pregnancy, GDM screening was performed in all women at 24–28 weeks’ gestation or later if it was not possible during this period. In both cases, GDM was diagnosed using a one-step screening and diagnostic test, with a 75-g oral glucose tolerance test (7), and was defined as a fasting plasma glucose value ≥5.3 mmol/L (the fasting plasma glucose target in the previous French recommendations) (8) and/or a 2-h blood glucose value ≥7.8 mmol/L (World Health Organization criteria) (9). This one-step screening was decided to limit the number lost to follow-up in our population characterized by a widespread geographic origin. Screening was precisely prescribed during the hospital routine follow-up visit and then performed out of the hospital. The percentage of women without screening could be considered close to 12.5% (2011 data).
Monitoring and management of the women with GDM
All women with diagnosed GDM were referred to a multidisciplinary team including a diabetologist, an obstetrician, a midwife, a dietician, and a nurse educator. These women received individualized dietary advice and instructions on how to perform self-monitoring of blood glucose levels six times a day. The women with GDM were seen by the diabetologist every 2–4 weeks. They received insulin therapy when fasting, and 2-h postprandial glucose levels were >5.3 and >6.8 mmol/L, respectively, according to the French guidelines (8). Antenatal visits were scheduled every 2–4 weeks up until 34 weeks and weekly thereafter with cardiotocogram and amniotic fluid volume assessment. Detailed fetal anomaly ultrasound scan including a detailed cardiac scan was performed by a referee-accredited practitioner; then, growth scans were performed monthly. The 37-week ultrasound scan was used for fetal weight estimation to discuss the timing and mode of delivery with the patient and obstetric staff. During labor and delivery, continuous electronic fetal heart rate monitoring was routinely used. During the 39th gestational week, labor induction (using prostaglandin E2 or oxytocin infusion) or caesarean section was decided according to obstetric history, maternal condition, and estimated fetal weight. Elective caesarean section was planned if estimated fetal weight was >4,250 g.
Prognosis
The main end point was the occurrence of a GDM-related event. The definition of GDM-related events was based on the classical GDM-related maternal (4), fetal, and neonatal (10) complications as reported in the French guidelines. The composite criterion includes at least one of the following events: 1) preeclampsia (blood pressure ≥140/90 mmHg on two recordings 4 h apart and proteinuria of at least 300 mg/24 h or ≥2+ on dipstick testing in a random urine sample, 2) large for gestational age (LGA) (birth weight >90th percentile for a standard French population [11]), 3) shoulder dystocia defined as the use of obstetric maneuvers (McRoberts, episiotomy after delivery of the fetal head, suprapubic pressure, posterior arm rotation to an oblique angle, rotation of the infant by 180 degrees, and delivery of the posterior arm) (12).
We subsequently considered each of the previous events separately, as well as events that are classically less often associated with GDM: elective and emergency (before or during delivery) caesarean sections, LGA-related caesarean section (caesarean section performed in LGA babies), preterm delivery (delivery before 37 completed weeks), admission to a neonatal intensive care unit, respiratory distress syndrome (based on the clinical course, chest X-ray finding, and blood gas and acid-base values), and finally, intrauterine fetal or neonatal death (in the first 24 h of life).
Statistical analyses
Continuous variables were expressed as means ± SD and compared by one-way ANOVA or Mann-Whitney U test as appropriate. The significance of differences in proportions was tested with the χ2 test. Logistic regression was used for the analyses of GDM effect, risk factor effect, and the analyses of the interaction between GDM and risk factor effect on GDM-related events and each classical event. Statistical analyses were carried out using SPSS software (SPSS, Chicago, IL). The 0.05 probability level was considered for statistical significance.
RESULTS
Characteristics of the study population
The subjects were 29.7 ± 5.8 years of age, their pregravid BMI was 24.1 ± 4.9 kg/m2, and 2.1% of them reported chronic hypertension, while 13.7% reported smoking before pregnancy. The cohort was multiethnic, most of the subjects being from Europe (29.1%), North Africa (27.8%) and sub-Saharian Africa (20.8%), and Pakistan, India, and Sri Lanka (5.1%). The prevalence of each risk factor is reported in Table 1.
Table 1.
Presence of risk factors in the total cohort and in women with or without GDM
Prevalence of GDM and risk factors over 9 years
GDM was diagnosed in 2,710 (14.4%) women with an unchanged prevalence since 2002 (Fig. 1). A total of 10,975 (58.5%) women had at least one risk factor, with a significant progression (P < 0.001) over time. This increase in risk factor prevalence involved especially overweight, which increased from 30.8% in 2002 to 37.6% in 2010 (P < 0.0001) (Fig. 1). During this period of time, there was no obvious change in the population served by our hospital, particularly with regard to ethnicity, age, and parity.
Figure 1.
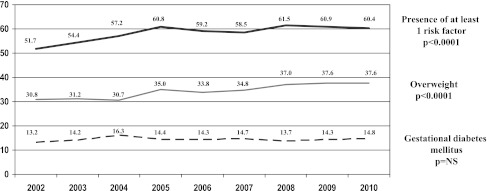
Prevalence over the years of GDM, overweight, and the presence of at least one risk factor according to French guidelines. NS, nonsignificant.
Diagnostic value of GDM risk factors
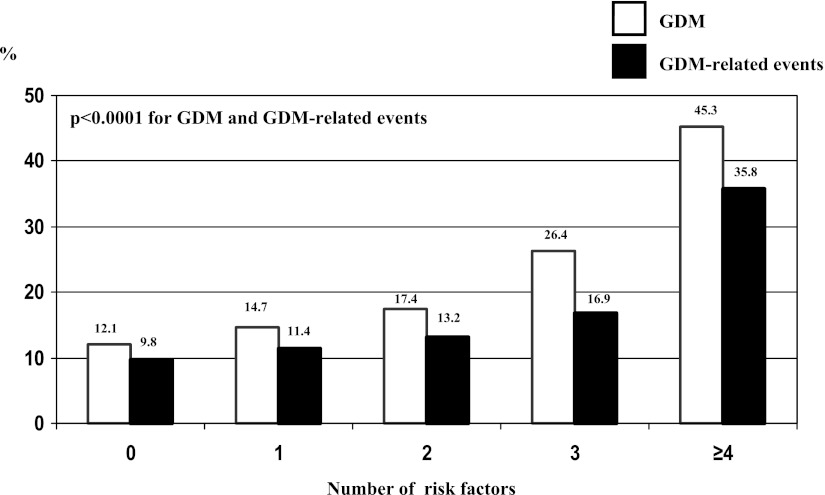
Table 1 shows that the presence of at least one risk factor (odds ratio 1.4 [95% CI 1.3–1.5]) and the presence of either one of the risk factors were significantly associated with a higher rate of GDM. The results were similar over years (data not shown). The performances of the presence of at least one risk factor were as follows: sensitivity 65.3%, specificity 42.7%, and positive and negative predictive values 16.1 and 87.9%, respectively. The higher the number of risk factors, the higher the prevalence of GDM (Fig. 2). Furthermore, compared with Caucasian origin, North African ethnicity (1.5 [1.4–1.7]) and Pakistan, India, and Sri Lanka origins (2.9 [2.5–3.4]) were associated with a higher GDM prevalence.
Figure 2.
Prevalence of GDM and GDM-related events according to the number of risk factors.
Prognostic value of risk factors for GDM-related complications
The women with GDM (GDM effect, P < 0.0001) and those with at least one risk factor (risk factor effect, P < 0.001) had a higher rate of hospitalization before delivery, with no interaction between GDM and risk factor: no GDM/no risk factors, 24.9% of hospitalization; no GDM/risk factor, 26.8%; GDM/no risk factors, 32.4%; and GDM/risk factor, 34.0%. Weight gain was lower (P < 0.05) in women with GDM (no risk factors 8.5 ± 5.5; risk factor 8.5 ± 5.5 kg) than in those without GDM (9.0 ± 5.6 kg whatever the risk factor status). In women with GDM, insulin therapy was initiated in 9.7% of those without risk factors and in 12.1% of those with risk factors (P = 0.056).
Table 2 shows that GDM was associated with the occurrence of GDM-related events (preeclampsia, LGA, and shoulder dystocia) and also with the occurrence of preeclampsia, LGA, dystocia, caesarean section, and LGA-related caesarean section taken separately. The presence of at least one risk factor was independently associated with the occurrence of a GDM-related event, of LGA, and of LGA-related caesarean section. There was no interaction between GDM effect and risk factor effect. The higher the number of risk factors, the higher the incidence of GDM-related events (Fig. 2).
Table 2.
Outcomes according to the presence or absence of GDM and/or at least one risk factor
CONCLUSIONS
We show in the current study that a GDM screening strategy based on risk factors was able to select both the subjects with a higher risk of GDM and those who experienced more GDM-related events, with no interaction between GDM effect and risk factor effect. However, from a clinical perspective, these diagnostic and prognostic factors are not appropriate, since 34.7% of the women without risk factors actually had GDM and since those women with GDM but no risk factors, although treated, experienced more GDM-related events than women without GDM. They would probably have experienced even more complications had they been undiagnosed and thus untreated.
Considering that the new diagnostic criteria (13) were not yet used in 2002–2010, we report here a high prevalence of GDM. This is probably due not only to the diagnostic criteria we used but also to the characteristics of our population: multiethnic, with a low socioeconomic status (7). Trends in GDM prevalence over the last years had never been explored in Europe before the new diagnostic criteria. A recent extensive review of the literature showed an increase in the prevalence of GDM in North America, which was often less marked in Caucasians (6). Here we show a stability in GDM prevalence, possibly because of our diagnostic criteria. The stability in GDM prevalence was nonconcordant with the increase of risk factors in our series. This increase was essentially a result of the progression of overweight, in line with the increase of overweight and obesity in women of reproductive age in France (14).
We confirmed that age is a traditional risk factor for GDM (6). We previously reported that women ≥35 years old compared with those <25 years old had a twofold increased risk of GDM (15). Age is classically used in risk scores for GDM (16–19). It is considered that nearly half the cases of GDM might be a consequence of overweight or obesity (20). A recent meta-analysis has shown that in a comparison with subjects with a normal BMI, the pooled and adjusted odds ratios of GDM were 1.8 for overweight, 3.2 for obesity grade 1, and 4.7 for obesity grades 2 and 3 (21). For each increasing kilogram per meter squared of BMI, the prevalence of GDM rose by 0.92% (21). In the current study, the odds ratios for age and BMI criteria were lower, as in a recent study (19). This might result from other factors such as the vulnerability or ethnicity, which were considered confounders in the French guidelines, whereas they might have been crucial in our current population. In particular, we show in the current study that North Africa and Pakistan, India, and Sri Lanka origins are predictive of GDM, although they are not taken into account in the French recommendations for a selective screening. Thresholds for age and BMI might be lowered. For example, Ogonowski et al. (19) reported that the best cutoff values were 28 years for age and 23 kg/m2 for BMI. Finally, the GDM diagnostic criteria in our series differed from those of the other studies, as we used low thresholds for plasma glucose values. The influence of age and BMI might be more important when glucose thresholds are higher. Family history of diabetes has been reported to multiply by 1.6–3.0 the risk of developing GDM (6,22). In our study population, the odds ratio related to family history of diabetes was lower (1.3 [95% CI 1.1–1.4]), which could be due to some missing reports of family history of diabetes, since many women did not speak French and some others did not know the medical history of their family living abroad. Personal histories of GDM and of a child with macrosomia were associated with GDM with the highest odds ratio (5.9 [5.0–6.9] (P < 0.0001). GDM is well-known to be recurrent in 30–84% of cases (18,19,22–24). History of macrosomia in a previous pregnancy was associated with a 1.6- to 6.2-fold increase of GDM (17,19).
Overall, we showed that the risk factors proposed in the French guidelines significantly predicted GDM, although the clinical relevance appears to be low, since 34.7% of the women with GDM would have been missed without universal screening, in line with previous reports (2). Of note, Jensen et al. (22) had reported excellent screening performances of criteria very close to ours. However, the prevalence of GDM was very low and extrapolated in their study. Finally, and as shown in Fig. 2, the prevalence of GDM was particularly high when the number of risk factors was greater than three. Therefore, three or more risk factors would be very specific for GDM screening, although this condition concerned only 3% of the cohort. It was hypothesized that women with GDM but no risk factors would have a good prognosis and therefore that missing their diagnosis would be of little consequence. For example, in women with GDM and BMI <25 kg/m2, the incidences of macrosomia and shoulder dystocia were reported to be similar regardless of whether GDM was known and treated (25). Conflicting data about the prognosis of GDM detected by selective or universal GDM screening were also reported, with no (26) or beneficial (7) effects of universal screening in retrospective studies. Finally, a prospective study showed a better prognosis associated with universal than with selective screening, but an earlier screening in the universal strategy was confounding (27). In all of these studies, the diagnostic and selective criteria differed. Here, GDM and risk factors were independently associated with a higher incidence of overall GDM-related events (preeclampsia, LGA, and shoulder dystocia) and of LGA and LGA-related caesarean section considered separately. In the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study, higher BMI was associated with more preeclampsia, macrosomia, caesarean section, and shoulder dystocia (28). However, there was in our series no interaction between GDM effect and risk factor effect. Furthermore, from a clinical perspective, women with GDM but no risk factors, although treated, had more GDM-related events than those without GDM, which argues for universal screening. Finally, the number of risk factors seems important to consider, as the incidence of GDM-related events is very high when at or above four. However, this is restricted to multiparous women, as two risk factors (macrosomia and GDM) depend on a previous pregnancy.
Our study has some limitations. The public hospital recruitment probably included a higher proportion of women living with vulnerable conditions and was multiethnic, precluding a generalization of our results. This could also have impacted our results, as some women could not speak French and therefore may have wrongly declared their personal and family medical histories. However, this could also explain some missing reports of family history of diabetes and further argue in favor of universal screening. Although the screening policy was locally universal, some women were not screened. Therefore, we considered the presence of GDM in the intention-to-screen population. The proportion of unscreened women, however, was 12.5% in 2011 and was likely to be similar over the last decade. Considering the prognosis issues, this study was of course not randomized, since all women with diagnosed GDM were treated. Furthermore, GDM-related events could not be weighed according to the glycemic profile of the women during pregnancy. Finally, the results should be confirmed with the new diagnostic criteria of GDM and in other hospitals, as this retrospective study was monocentric.
To conclude, the selective screening of GDM is appealing, as it reduces the burden of unnecessary screening tests if this screening selects the women who have the highest risk of GDM-related complications. We showed, however, that in our population, such a strategy would lead to missing approximately one-third of the women with GDM. Furthermore, despite appropriate treatment, women with GDM had a worse prognosis than women without GDM, even if they were free of risk factors. Therefore, universal screening appears to be beneficial, at least compared with the selective screening proposed in the French guidelines.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
E.C. directed research and wrote the manuscript. A.B., A.R., B.L., D.S.-B., N.A., and C.P. contributed to discussion. I.P. researched data. M.T.N. researched data and assembled statistics. P.V. contributed to discussion and reviewed and edited the manuscript. L.C. directed research, contributed to discussion, and reviewed and edited the manuscript. L.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
The authors thank Dr. Florence Galtier, Centre Hospitalier Regional Universitaire de Montpellier, France, for helpful reading of the manuscript.
References
- 1.Metzger BE, Gabbe SG, Persson B, et al.; International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hiéronimus S, Le Meaux JP. Relevance of gestational diabetes mellitus screening and comparison of selective with universal strategies. Diabetes Metab 2010;36:575–586 [DOI] [PubMed] [Google Scholar]
- 3.Ostlund I, Hanson U. Occurrence of gestational diabetes mellitus and the value of different screening indicators for the oral glucose tolerance test. Acta Obstet Gynecol Scand 2003;82:103–108 [DOI] [PubMed] [Google Scholar]
- 4.Beucher G, Viaris de Lesegno B, Dreyfus M. Maternal outcome of gestational diabetes mellitus. Diabetes Metab 2010;36:522–537 [DOI] [PubMed] [Google Scholar]
- 5.Expert consensus on gestational diabetes mellitus. Summary of expert consensus. Diabetes Metab 2010;36:695–699 [DOI] [PubMed] [Google Scholar]
- 6.Galtier F. Definition, epidemiology, risk factors. Diabetes Metab 2010;36:628–651 [DOI] [PubMed] [Google Scholar]
- 7.Cosson E, Benchimol M, Carbillon L, et al. Universal rather than selective screening for gestational diabetes mellitus may improve fetal outcomes. Diabetes Metab 2006;32:140–146 [DOI] [PubMed] [Google Scholar]
- 8.Lassmann-Vague V, Basdevant A, Cathelineau G, et al. Pregnancy and contraception in the diabetic woman. Gestational diabetes. Recommendations of ALFEDIAM (French Language Association for the Study of Diabetes and Metabolic Diseases). Diabetes Metab 1996;22:459–469 [In French] [PubMed] [Google Scholar]
- 9.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1. Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539–553 [DOI] [PubMed] [Google Scholar]
- 10.Mitanchez D. Foetal and neonatal complications in gestational diabetes: perinatal mortality, congenital malformations, macrosomia, shoulder dystocia, birth injuries, neonatal complications. Diabetes Metab 2010;36:617–627 [DOI] [PubMed] [Google Scholar]
- 11.Leroy B, Lefort F. The weight and size of newborn infants at birth. Rev Fr Gynecol Obstet 1971;66:391–396 [In French] [PubMed] [Google Scholar]
- 12.Spong CY, Beall M, Rodrigues D, Ross MG. An objective definition of shoulder dystocia: prolonged head-to-body delivery intervals and/or the use of ancillary obstetric maneuvers. Obstet Gynecol 1995;86:433–436 [DOI] [PubMed] [Google Scholar]
- 13.Cosson E. Diagnostic criteria for gestational diabetes mellitus. Diabetes Metab 2010;36:538–548 [DOI] [PubMed] [Google Scholar]
- 14.Charles MA, Eschwège E, Basdevant A. Monitoring the obesity epidemic in France: the Obepi surveys 1997-2006. Obesity (Silver Spring) 2008;16:2182–2186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benchimol M, Cosson E, Faure C, Carbillon L, Attali R, Uzan M. Comparison of two routine screening strategies for gestational diabetes mellitus: the experience of Jean-Verdier Hospital. Gynecol Obstet Fertil 2006;34:107–114 [In French] [DOI] [PubMed] [Google Scholar]
- 16.Naylor CD, Sermer M, Chen E, Farine D, Toronto Trihospital Gestational Diabetes Project Investigators Selective screening for gestational diabetes mellitus. N Engl J Med 1997;337:1591–1596 [DOI] [PubMed] [Google Scholar]
- 17.Phaloprakarn C, Tangjitgamol S, Manusirivithaya S. A risk score for selective screening for gestational diabetes mellitus. Eur J Obstet Gynecol Reprod Biol 2009;145:71–75 [DOI] [PubMed] [Google Scholar]
- 18.Teh WT, Teede HJ, Paul E, Harrison CL, Wallace EM, Allan C. Risk factors for gestational diabetes mellitus: implications for the application of screening guidelines. Aust N Z J Obstet Gynaecol 2011;51:26–30 [DOI] [PubMed] [Google Scholar]
- 19.Ogonowski J, Miazgowski T, Homa K, Celewicz Z, Kuczyńska M. Low predictive value of traditional risk factors in identifying women at risk for gestational diabetes. Acta Obstet Gynecol Scand 2007;86:1165–1170 [DOI] [PubMed] [Google Scholar]
- 20.Kim SY, England L, Wilson HG, Bish C, Satten GA, Dietz P. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health 2010;100:1047–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev 2009;10:194–203 [DOI] [PubMed] [Google Scholar]
- 22.Jensen DM, Mølsted-Pedersen L, Beck-Nielsen H, Westergaard JG, Ovesen P, Damm P. Screening for gestational diabetes mellitus by a model based on risk indicators: a prospective study. Am J Obstet Gynecol 2003;189:1383–1388 [DOI] [PubMed] [Google Scholar]
- 23.Kim C, McEwen LN, Kieffer EC, Herman WH, Piette JD. Self efficacy, social support, and association with physical activity and body mass index among women with recent gestational diabetes. Diabetes Educ 2006;34:719–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verier-Mine O. Outcomes in women with a history of gestational diabetes. Screening and prevention of type 2 diabetes. Literature review. Diabetes Metab 2010;36:595–616 [DOI] [PubMed] [Google Scholar]
- 25.Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol 2005;192:989–997 [DOI] [PubMed] [Google Scholar]
- 26.Wen SW, Liu S, Kramer MS, et al. Impact of prenatal glucose screening on the diagnosis of gestational diabetes and on pregnancy outcomes. Am J Epidemiol 2000;152:1009–1014 [DOI] [PubMed] [Google Scholar]
- 27.Griffin ME, Coffey M, Johnson H, et al. Universal vs. risk factor-based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabet Med 2000;17:26–32 [DOI] [PubMed] [Google Scholar]
- 28.HAPO Study Cooperative Research Group Hyperglycaemia and Adverse Pregnancy Outcome (HAPO) Study: associations with maternal body mass index. BJOG 2010;117:575–584 [DOI] [PubMed] [Google Scholar]