Abstract
OBJECTIVE
To investigate the association between smoking habits and risk of autoimmune diabetes in adults and of type 2 diabetes.
RESEARCH DESIGN AND METHODS
We used data from the three surveys of the Nord-Trøndelag Health Study, spanning 1984–2008 and including a cohort of 90,819 Norwegian men (48%) and women (52%) aged ≥20 years. Incident cases of diabetes were identified by questionnaire and classified as type 2 diabetes (n = 1,860) and autoimmune diabetes (n = 140) based on antibodies to glutamic decarboxylase (GADA) and age at onset of diabetes. Hazard ratios (HRs) adjusted for confounders were estimated by Cox proportional hazards regression models.
RESULTS
The risk of autoimmune diabetes was reduced by 48% (HR 0.52 [95% CI 0.30–0.89]) in current smokers and 58% in heavy smokers (0.42 [0.18–0.98]). The reduced risk was positively associated with number of pack-years. Heavy smoking was associated with lower levels of GADA (P = 0.001) and higher levels of C-peptide (964 vs. 886 pmol/L; P = 0.03). In contrast, smoking was associated with an increased risk of type 2 diabetes, restricted to overweight men (1.33 [1.10–1.61]). Attributable proportion due to an interaction between overweight and heavy smoking was estimated to 0.40 (95% CI 0.23–0.57).
CONCLUSIONS
In this epidemiological study, smoking is associated with a reduced risk of autoimmune diabetes, possibly linked to an inhibitory effect on the autoimmune process. An increased risk of type 2 diabetes was restricted to overweight men.
Data on the influence of smoking on autoimmune diabetes are limited. A protective effect seems plausible because an anti-inflammatory effect of nicotine has been demonstrated both in vitro (1) and in vivo (2). Also, an inhibitory effect of nicotine on autoimmune diabetes has been documented in one animal study (3). In a previous study based on prospective data from the Norwegian Nord-Trøndelag Health Study (HUNT) 1984–1997, we found a reduced risk of latent autoimmune diabetes in adults (LADA) in smokers (4). Confirmatory and extended evidence for such an effect is, however, desirable.
In contrast, smoking, in particular heavy smoking, is clearly associated with an increased risk of type 2 diabetes (5). The increased risk has been attributed to impaired insulin sensitivity (6), increased systematic inflammation (7), greater accumulation of abdominal adipose tissue (8), and/or adverse effects on pancreatic tissue and β-cell function (9). Overweight may modify the influence of smoking on type 2 diabetes; in one Japanese study, the association between smoking and type 2 diabetes was limited to overweight individuals (10), and findings from a Finnish study suggest that smoking is more detrimental in individuals with high BMI (11). Further studies on a possible interaction are, however, needed.
The aim of this study was to extend our previous analyses of smoking in autoimmune diabetes in adults with regard to cases and follow-up time and also to include in-depth analysis of the established association of smoking with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Study population and design
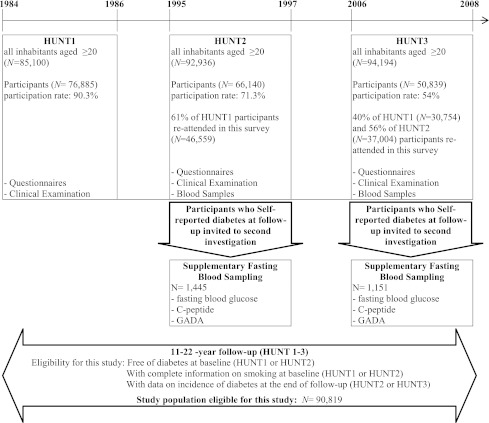
The general design of the HUNT study and the basis for the present analyses are illustrated in Fig. 1. HUNT is a population-based study, conducted in the county of Nord-Trøndelag in Norway. Three surveys were conducted from 1984 to 2008. All inhabitants aged ≥20 years in the county were invited to participate at each survey. The investigations carried out are presented in detail on the HUNT website (http://www.hunt.ntnu.no/index.php?side=english). In brief, in HUNT1, 1984–1986, a total of 76,885 (90.3%) of the eligible individuals participated. Participants completed questionnaires on health and lifestyle and also underwent a clinical examination, including anthropometrical measurements (12). In HUNT2, 1995–1997, a total of 66,140 of 92,936 eligible individuals (71.2%) participated. Data in the third survey (HUNT3) were collected from 2006 to 2008. This survey included 50,839 individuals (representing 54% of the population). A total of 37,004 individuals constituting 56% of HUNT2 participants and ∼40% of participants in HUNT1 (n = 30,754) reattended in HUNT3. The present study is based on individuals who participated in at least two HUNT surveys. Our study population included 90,819 individuals, free of diabetes at baseline (HUNT1 or HUNT2) with complete baseline information on smoking. The HUNT study is approved by the Norwegian Data Inspectorate and Regional Committee for Medical Research Ethics. The participants provided informed consent.
Figure 1.
A chart presentation for the HUNT Study, 1984–2008.
Each participant’s record at HUNT is linked to his or her exclusive 11-digit personal identification number, which allows linkage with other health registries. For the current study, we acquired date of death from the National Mortality Registry (13).
Identification and classification of diabetes
Individuals with diabetes were identified by questionnaire. All with self-reported diabetes at HUNT2 or HUNT3 were given an appointment for fasting blood sampling. Samples were analyzed for glucose, C-peptide, and antibodies to glutamic decarboxylase (GADA). Information on diabetes medication was also collected. This information together with age at onset of diabetes was used to classify diabetes. Patients aged ≥35 years at diagnosis of diabetes were classified as having type 2 diabetes if, in addition, they were GADA-negative (<0.08) (n = 1,860). We further classified patients as having autoimmune diabetes if they were GADA-positive (≥ 0.08) and were ≥35 years old at onset of diabetes (n = 140). As a further criterion for LADA, we used information on insulin treatment (available for 82% of the participants) to separate LADA from classical type 1 diabetes. Individuals were classified as LADA if they were GADA-positive and did not receive insulin treatment during the first year after diagnosis of diabetes (n = 100). Individuals were classified as having classical type 1 diabetes if insulin treatment was started during the first year after onset and GADA was either positive or negative. In the cases of negative GADA, a low level of C-peptide (<150 pmol/L) was required (n = 32).
Biochemical analysis
GADA was analyzed at Aker University Hospital, Oslo, Norway, by a previously validated method (14). GADA was reported as an antibody index value (15). The sensitivity and specificity of the assay at the cutoff level of >0.08 were 0.64 and 1.00, respectively, according to results obtained in the Diabetes Antibody Standardization Program. The cutoff index of 0.08 was equivalent to 43 World Health Organization units/mL (16). C-peptide was measured by radioimmunoassay (Diagnostic System Laboratories, Webster, TX). Fasting serum levels of glucose were measured by Hemocue at the central laboratory of Levanger Hospital (Levanger, Norway) (13). Homeostasis model assessment for insulin resistance (HOMA-IR) and β-cell function (HOMA-%B) were calculated using the updated model (17).
Assessment of smoking habits
Smoking habits at baseline (HUNT1 or HUNT2) were assessed by questionnaire. Current smoking was defined as current daily smoking of at least 1 g of tobacco in the form of cigarettes, cigars, or pipe. Participants were categorized into never smokers, former smokers, and current smokers. Current and former smokers were inquired at age of starting smoking, years of smoking, and average number of cigarettes smoked per day. The intensity of smoking among current and former smokers was assessed by two categories: light smokers (<20 cigarettes/day) and heavy smokers (≥20 cigarettes/day). Pack-years were calculated according to the formula: cigarettes per day/20 × years smoked. Cumulative quantity of active smoking was assessed in three categories (<6, 6–12, and ≥13 pack-years).
Statistical analysis
Characteristics of the participants were expressed as means and SD. P values for means and proportions were calculated using one-way ANOVA and F test. We used Cox proportional hazards models to estimate hazard ratios (HR) of type 2 diabetes and autoimmune diabetes in relation to smoking habits with 95% CIs (SAS 9.2; SAS Institute Inc, Cary, NC). Person-years of follow-up were calculated from the age that the participants entered the study (HUNT1 or HUNT2) until age of onset of diabetes, death, or the end of the follow-up period at HUNT2 or HUNT3 (1997 or 2008, respectively), whichever came first. Confounders controlled for age (in years, as underlying time scale in Cox model), sex, and baseline information (from either HUNT1 or HUNT2 depending on when the participant entered the study), BMI (calculated as weight [kg]/height [m2], continuous), education (primary school, upper secondary school, or university), and physical activity [physically active or inactive, categorization details are described elsewhere (18)], unless otherwise specified. Additional adjustment for alcohol consumption and family history of diabetes did not change the results (change in HR <10%). These factors were therefore not included in the final model. The analyses were time-dependent, which means that for individuals with information on smoking (or any of the covariates) from more than one point in time (i.e., both HUNT1 and HUNT2), information was updated during follow-up. In all analyses, we used never-smokers as a reference group, unless otherwise specified. For evaluating interaction between BMI and smoking habits, participants were categorized as: 1) BMI <25 kg/m2 and never smoking (−) (as reference group in analyses), 2) BMI <25 kg/m2 and heavy smoking (−+), 3) BMI ≥25 kg/m2 and never smoking (+−), and 4) BMI ≥25 kg/m2 and heavy smoking (++). To estimate additive interaction, we calculated the relative excess risk due to the interaction (RERI) using RERI = HR++ − HR+− − HR−+ + 1 and attributable proportion (AP) due to interaction as AP = RERI/HR++ (19). Correlation between smoking (pack-years) and HOMA indices (logHOMA2-IR and logHOMA2-%B, logarithmic transformation was applied due to skewing of variables) were assessed with Pearson correlation coefficient.
RESULTS
Baseline characteristics
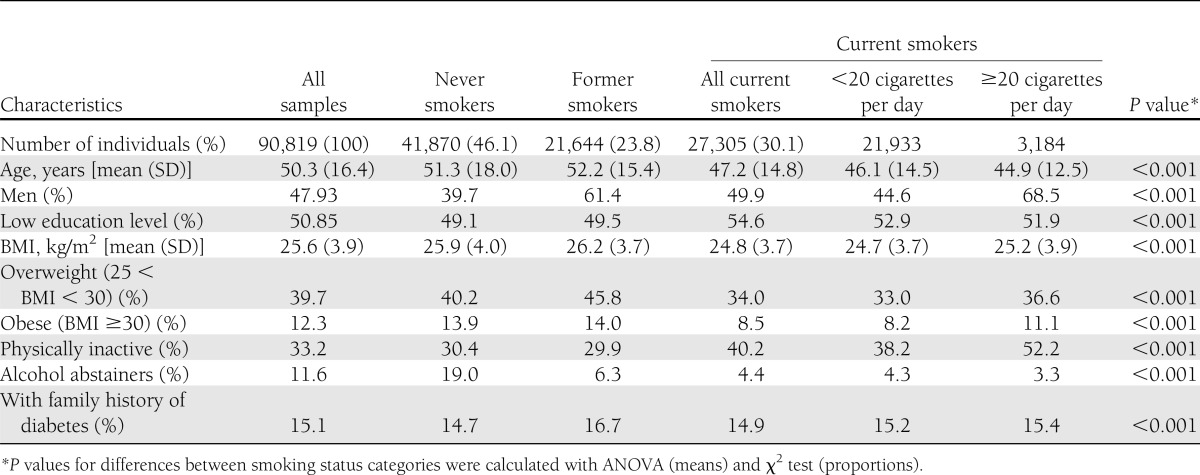
Table 1 shows baseline characteristics of participants by smoking habits. Mean age was ∼50 years. Smokers were younger and less likely to be obese. Seventy percent of heavy smokers were men. More than half of them had low background education. Only 3% were abstainers.
Table 1.
Baseline characteristics of the study population according to smoking status at enrollment in the HUNT study, 1984–1997
By most characteristics, cases of autoimmune and type 2 diabetes were similar (Supplementary Table 1). Compared with individuals without diabetes, those with autoimmune and type 2 diabetes alike tended to be older, heavier, and less physically active. Also, the frequency of family history of diabetes was higher.
Smoking attenuates the incidence of autoimmune diabetes
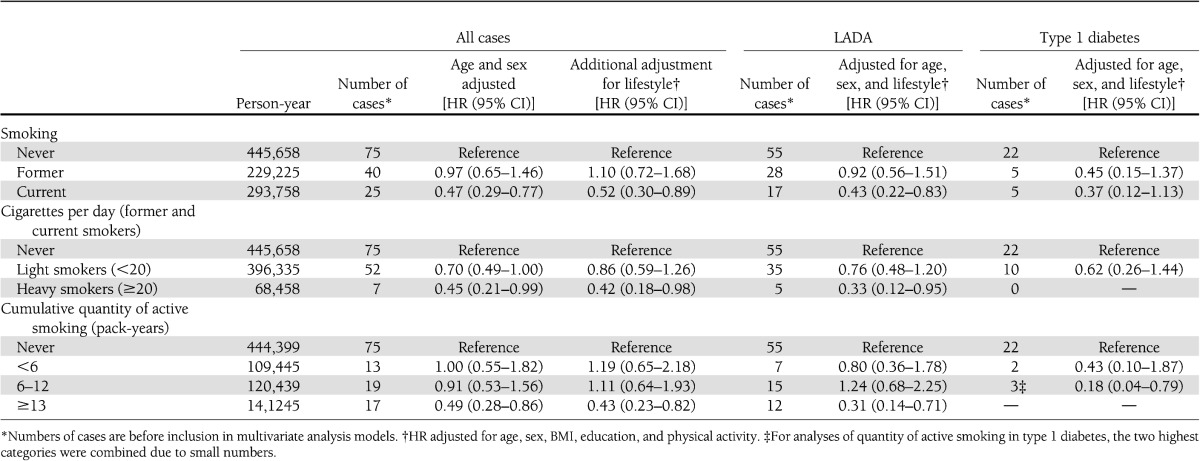
Current smokers displayed a reduced risk of autoimmune diabetes compared with never smokers (HR 0.52 [95% CI 0.30–0.89]; Table 2). This effect was also seen when the analysis was restricted to heavy smokers (0.42 [0.18–0.98]). Stratifying the results by sex did not reveal differences (e.g., in men, HR for ≥13 pack-years was 0.45 [0.21–0.96] and in women, 0.29 [0.07–1.23]). The reduced risk by smoking was upheld when we divided cases with autoimmune diabetes into LADA and classical type 1 diabetes. For cases of LADA, the HR was 0.31 (95% CI 0.14–0.71) for ≥13 pack-years. For cases (few) of classical type 1 diabetes, the HR was 0.18 (CI 0.04–0.79) for ≥6 pack-years. Corresponding estimates for LADA were 0.68 (CI 0.40–1.16).
Table 2.
HR of autoimmune diabetes in adults in relation to smoking: results from the HUNT study, 1984–2008
The reduced risk of autoimmune diabetes in relation to current smoking (all smokers) was not different between normal weight and overweight individuals (HR 0.53 [95% CI 0.19–1.45] for BMI <25 kg/m2 and 0.53 [0.28–0.99] for BMI ≥25 kg/m2). Similar results were seen in relation to ≥13 pack-years.
We analyzed levels of GADA and C-peptide among diabetic patients across categories of smoking. A decrease in levels of GADA was seen across pack-years categories (0.068 [<6 pack-years], 0.065 [6–12 pack-years], 0.022 [≥13 pack-years]; P = 0.01); additional adjustment for diabetes duration did not change the results (P = 0.01). In line with this, heavy smokers (≥20 cigarettes/day) had substantially lower levels of GADA (0.009 vs. 0.056; P = 0.001) compared with never smokers. Current smokers compared with never smokers had higher levels of C-peptide (964 vs. 886; P = 0.03). This same tendency persisted after adjustment for diabetes duration and also after excluding patients with glucose values <7 mmol/L (C-peptide = 942 vs. 856; P = 0.056).
Smoking increases the incidence of type 2 diabetes in overweight men
Current smoking (all smokers) was positively associated with incidence of type 2 diabetes (Table 3). The highest risk was observed in those who smoked ≥20 cigarettes/day (HR 1.32 [95% CI 1.11–1.56] and in those who reported ≥13 pack-years smoking (1.20 [1.05–1.37]).
Table 3.
HR of type 2 diabetes in relation to smoking and BMI: results from the HUNT study, 1984–2008
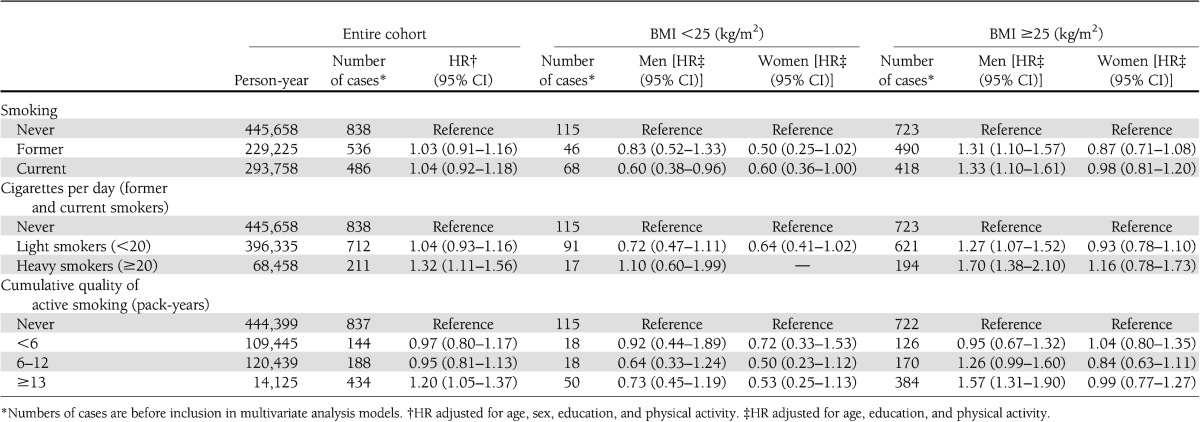
Stratifying the results by BMI and sex indicated that smoking was associated with an increased risk of type 2 diabetes in overweight men (≥25 kg/m2) (HR 1.33 [95% CI 1.10–1.6] in current smokers). In individuals with BMI <25 kg/m2, current smoking was, in contrast, associated with a reduced risk (0.60 [0.38–0.96] in men and 0.60 [0.36–1.00] in women).
Being overweight was associated with an increased risk of type 2 diabetes across all categories of smoking but only in men (demonstrated in Supplementary Fig. 1). Heavy smoking was associated with a fivefold elevated risk of type 2 diabetes (HR 5.62 [95% CI 3.94–8.02]) when combined with overweight and a 12-fold excess risk (12.54 [8.53–18.44]) when combined with obesity (≥30 kg/m2). The relative excess risk due to interaction was estimated at 2.24 (95% CI 1.01–3.46), and the AP was estimated at 0.40 (0.23–0.57), implying that 40% of the cases among heavy smokers with BMI ≥25 kg/m2 were caused by the interaction between these two risk factors.
Increased risk by smoking in type 2 diabetes associates with insulin resistance
There was a positive correlation between number of pack-years of smoking and HOMA2-IR in type 2 diabetes cases with BMI ≥25 kg/m2 (r = 0.11; P = 0.014) There was no clear correlation neither in lean and normal weight type 2 diabetes cases (r = −0.068; P = 0.576) nor in cases with autoimmune diabetes (r = −0.181; P = 0.281).
CONCLUSIONS
We report a strong association between smoking and a reduced risk of autoimmune diabetes. The risk reduction was seen both in men and women across categories of BMI and was related both to development of LADA and classical type 1 diabetes (although the analyses regarding type 1 diabetes were admittedly based on very small numbers). To our knowledge, our previous report (4) of risk reduction by of smoking in relation to autoimmune diabetes is up to now the only one demonstrating in humans this, by its beneficial nature, potentially controversial effect. We note that our observations agree with those in an animal study (3) and are consistent with those from some previous studies, in which a reduced risk of type 1 diabetes was seen in the offspring of smokers (20–22). It seems important that we can now confirm and extend our previous findings by providing a larger sample and a longer time of follow-up of the HUNT cohort.
Our incidence data are in line with an inhibitory effect of smoking on autoimmune activity. This notion is supported by our observation showing lower levels of GADA in long-time heavy smokers than in other LADA patients. In this context, one cannot rule out the possibility that the association with GADA is restricted to alteration of the time dynamics of GADA. GADA levels are known to rise and then fall in pediatric patients with type 1 diabetes (23,24); however, evidence indicates that GADA are more persistent in LADA (25,26), at least in those who display high titers. In a previous study (25), it was shown that a majority of those who developed LADA between HUNT2 and HUNT3 were GADA-positive already at HUNT2 (i.e., during prediabetes) with no significant change in GADA between HUNT2 and 3. Hence, many patients with LADA have GADA persisting from prediabetes and onwards. These findings do not support the possibility of smoking affecting merely the time course of GADA. However, a full investigation into the latter possibility awaits further studies. Which component of smoking is important? In animal and human studies, exposure to nicotine, the major active component of cigarettes, can exert immunosuppressive (3,27) as well as anti-inflammatory effects (1,2,28). Hence, it is likely, but not proven, that nicotine is the component of cigarette smoking behind the risk reduction in autoimmune diabetes.
With regard to type 2 diabetes, our findings confirm a similar increase in risk associated with heavy smoking as in other studies (29–33). In contrast to most previous studies (5), the increased risk of type 2 diabetes was limited to men, and this finding is similar to those in a French cohort study (34). Sex differences in inhalation practice (35) could be a factor. Also, so far unknown risk factors present in men but not in women could be operative. A further distinction in our study was the limitation of risk with smoking to men with BMI ≥25 kg/m2, whereas a reduced risk was actually seen in lean and normal weight smokers. Similar findings were reported in a Japanese study (10). One explanation for the reduced risk in lean smokers could relate to the rise in metabolic rate caused by smoking (36–38). Such a rise would counteract insulin resistance, and this beneficial effect could perhaps, in light smokers with low BMI, more than outweigh any countering influence by smoking-induced insulin resistance (for which the underlying mechanisms are not known).
Previous studies have suggested a possible interaction between high BMI and smoking in the development of type 2 diabetes (11). In confirmation of this, the highest risk of type 2 diabetes was seen in men exposed both to overweight and heavy smoking. Smoking was associated with HOMA-IR and higher levels of C-peptide, findings that are compatible with an insulin resistance effect. As mentioned above, an insulin resistance effect as found in this study is in line with previous observations as mentioned above. One explanation behind the BMI and smoking interaction could be that exposure to both these sources of insulin resistance accelerates exhaustion of β-cells with subsequent inability to maintain glucose homeostasis.
The strengths of this study include an all-population area-based sample, a long follow-up, and data on GADA and C-peptide. Also, it was possible to control for potential sociodemographic, lifestyle, and anthropometric confounders. Information on smoking was collected by several questions and updated during follow-up. However, there are some potential biases to be considered. Diabetes cases were identified by self-reporting. This method has been shown to correctly classify >95% of cases (39), but will miss cases of undiagnosed diabetes. Also, we relied on self-reports of smoking habits. Such socially undesirable behaviors can be afflicted by underreporting (40). Due to prospective nature of this study, any misclassification of smoking can however be assumed to be nondifferential.
In conclusion, smoking was associated with a reduced risk of autoimmune diabetes in adults; an effect is probably exerted by inhibition of the autoimmune process. We further find that the increased risk by smoking in type 2 diabetes is limited to overweight men and that the combination of overweight and smoking is an especially potent combination of risk factors for type 2 diabetes.
Supplementary Material
Acknowledgments
The HUNT study is a collaboration among the HUNT Research Centre (Faculty of Medicine, Norwegian University of Science and Technology), Nord-Trøndelag County Council, and the Norwegian Institute of Public Health. GlaxoSmithKline Norway supported the Diabetes Study at HUNT2 and HUNT3 financially through the Norwegian University of Science and Technology. No other potential conflicts of interest relevant to this article were reported.
B.R. developed the objective of the study, analyzed data, and wrote the manuscript. V.G. and S.C. contributed to developing the objective of the study and interpretation of results and reviewed and revised the manuscript. K.M. researched data and reviewed and revised the manuscript. A.A. contributed to the discussion and reviewed and revised the manuscript. T.A. contributed to data analysis and reviewed and revised the manuscript. All authors read and approved the final version of the manuscript. B.R. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in poster form at the 48th annual meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012.
The authors thank Lisa Olsson, Department of Epidemiology, Institute of Environmental Medicine, Karolinska Institutet, for help with preparing data for analyses. The authors also thank the investigators and the staff of the HUNT data center for valuable contributions.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0913/-/DC1.
References
- 1.Rehani K, Scott DA, Renaud D, et al. Cotinine-induced convergence of the cholinergic and PI3 kinase-dependent anti-inflammatory pathways in innate immune cells. Biochim Biophys Acta 2008;1783:375–382 [DOI] [PubMed] [Google Scholar]
- 2.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol 2007;147:28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mabley JG, Pacher P, Southan GJ, Salzman AL, Szabó C. Nicotine reduces the incidence of type I diabetes in mice. J Pharmacol Exp Ther 2002;300:876–881 [DOI] [PubMed] [Google Scholar]
- 4.Carlsson S, Midthjell K, Grill V, Nord-Trøndelag study Smoking is associated with an increased risk of type 2 diabetes but a decreased risk of autoimmune diabetes in adults: an 11-year follow-up of incidence of diabetes in the Nord-Trøndelag study. Diabetologia 2004;47:1953–1956 [DOI] [PubMed] [Google Scholar]
- 5.Willi C, Bodenmann P, Ghali WA, Faris PD, Cornuz J. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007;298:2654–2664 [DOI] [PubMed] [Google Scholar]
- 6.Frati AC, Iniestra F, Ariza CR. Acute effect of cigarette smoking on glucose tolerance and other cardiovascular risk factors. Diabetes Care 1996;19:112–118 [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Tinker L, Song Y, et al. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Arch Intern Med 2007;167:1676–1685 [DOI] [PubMed] [Google Scholar]
- 8.Berlin I. Smoking-induced metabolic disorders: a review. Diabetes Metab 2008;34:307–314 [DOI] [PubMed] [Google Scholar]
- 9.Ostgren CJ, Lindblad U, Ranstam J, Melander A, Råstam L, Skaraborg Hypertension and Diabetes Project Associations between smoking and beta-cell function in a non-hypertensive and non-diabetic population. Skaraborg Hypertension and Diabetes Project. Diabet Med 2000;17:445–450 [DOI] [PubMed] [Google Scholar]
- 10.Nagaya T, Yoshida H, Takahashi H, Kawai M. Heavy smoking raises risk for type 2 diabetes mellitus in obese men; but, light smoking reduces the risk in lean men: a follow-up study in Japan. Ann Epidemiol 2008;18:113–118 [DOI] [PubMed] [Google Scholar]
- 11.Patja K, Jousilahti P, Hu G, Valle T, Qiao Q, Tuomilehto J. Effects of smoking, obesity and physical activity on the risk of type 2 diabetes in middle-aged Finnish men and women. J Intern Med 2005;258:356–362 [DOI] [PubMed] [Google Scholar]
- 12.Midthjell K, Bjørndal A, Holmen J, Krüger O, Bjartveit K. Prevalence of known and previously unknown diabetes mellitus and impaired glucose tolerance in an adult Norwegian population. Indications of an increasing diabetes prevalence. The Nord-Trøndelag Diabetes Study. Scand J Prim Health Care 1995;13:229–235 [DOI] [PubMed] [Google Scholar]
- 13.Holmen J. The Nord-Trøndelag Health Study 1995–97 (HUNT2): objectives, contents, methods and participation. Norsk Epidemiologi 2003;13:19–32 [Google Scholar]
- 14.Petersen JS, Hejnaes KR, Moody A, et al. Detection of GAD65 antibodies in diabetes and other autoimmune diseases using a simple radioligand assay. Diabetes 1994;43:459–467 [DOI] [PubMed] [Google Scholar]
- 15.Schlosser M, Mueller PW, Törn C, Bonifacio E, Bingley PJ, Participating Laboratories Diabetes Antibody Standardization Program: evaluation of assays for insulin autoantibodies. Diabetologia 2010;53:2611–2620 [DOI] [PubMed] [Google Scholar]
- 16.Davies H, Brophy S, Bain SC, et al. GADA testing: the current state of knowledge. Prim Care Diabetes 2009;3:189–191 [DOI] [PubMed] [Google Scholar]
- 17.Biesma DH, van Iperen CE, Kraaijenhagen RJ, Marx JJ, van de Wiel HB, van de Wiel A. Red blood cell transfusions for total hip replacement in a regional hospital. A six-year analysis. Vox Sang 1994;66:270–275 [DOI] [PubMed] [Google Scholar]
- 18.Stensvold D, Nauman J, Nilsen TI, Wisløff U, Slørdahl SA, Vatten L. Even low level of physical activity is associated with reduced mortality among people with metabolic syndrome, a population based study (the HUNT 2 study, Norway). BMC Med 2011;9:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol 2005;20:575–579 [DOI] [PubMed] [Google Scholar]
- 20.Toschke AM, Ehlin A, Koletzko B, Montgomery SM. Paternal smoking is associated with a decreased prevalence of type 1 diabetes mellitus among offspring in two national British birth cohort studies (NCDS and BCS70). J Perinat Med 2007;35:43–47 [DOI] [PubMed] [Google Scholar]
- 21.Dahlquist G, Bennich SS, Källén B. Intrauterine growth pattern and risk of childhood onset insulin dependent (type I) diabetes: population based case-control study. BMJ 1996;313:1174–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dahlquist G, Källén B. Maternal-child blood group incompatibility and other perinatal events increase the risk for early-onset type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1992;35:671–675 [DOI] [PubMed] [Google Scholar]
- 23.Decochez K, Keymeulen B, Somers G, et al. Belgian Diabetes Registry Use of an islet cell antibody assay to identify type 1 diabetic patients with rapid decrease in C-peptide levels after clinical onset. Diabetes Care 2000;23:1072–1078 [DOI] [PubMed] [Google Scholar]
- 24.Decochez K, Tits J, Coolens JL, et al. High frequency of persisting or increasing islet-specific autoantibody levels after diagnosis of type 1 diabetes presenting before 40 years of age. The Belgian Diabetes Registry. Diabetes Care 2000;23:838–844 [DOI] [PubMed] [Google Scholar]
- 25.Sørgjerd EP, Skorpen F, Kvaløy K, Midthjell K, Grill V. Time dynamics of autoantibodies are coupled to phenotypes and add to the heterogeneity of autoimmune diabetes in adults: the HUNT study, Norway. Diabetologia 2012;55:1310–1318 [DOI] [PubMed] [Google Scholar]
- 26.Borg H, Gottsäter A, Fernlund P, Sundkvist G. A 12-year prospective study of the relationship between islet antibodies and beta-cell function at and after the diagnosis in patients with adult-onset diabetes. Diabetes 2002;51:1754–1762 [DOI] [PubMed] [Google Scholar]
- 27.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol 2002;2:372–377 [DOI] [PubMed] [Google Scholar]
- 28.Guslandi M. Long-term effects of a single course of nicotine treatment in acute ulcerative colitis: remission maintenance in a 12-month follow-up study. Int J Colorectal Dis 1999;14:261–262 [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Curhan GC, Hu FB, Rimm EB, Forman JP. Association between passive and active smoking and incident type 2 diabetes in women. Diabetes Care 2011;34:892–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: a cohort study. Ann Intern Med 2010;152:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowall B, Rathmann W, Strassburger K, et al. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur J Epidemiol 2010;25:393–402 [DOI] [PubMed] [Google Scholar]
- 32.Cho NH, Chan JC, Jang HC, Lim S, Kim HL, Choi SH. Cigarette smoking is an independent risk factor for type 2 diabetes: a four-year community-based prospective study. Clin Endocrinol (Oxf) 2009;71:679–685 [DOI] [PubMed] [Google Scholar]
- 33.Midthjell K. Diabetes in adults in Nord-Trøndelag. Epidemiological and public health aspects of diabetes mellitus in a large, non-selected Norwegian population Trøndheim, The Norwegian University of Science and Technology (NTNU), 2001
- 34.Beziaud F, Halimi JM, Lecomte P, Vol S, Tichet J. Cigarette smoking and diabetes mellitus. Diabetes Metab 2004;30:161–166 [DOI] [PubMed] [Google Scholar]
- 35.Mariner DC, Ashley M, Shepperd CJ, Mullard G, Dixon M. Mouth level smoke exposure using analysis of filters from smoked cigarettes: a study of eight countries. Regul Toxicol Pharmacol 2011;61(Suppl.):S39–S50 [DOI] [PubMed] [Google Scholar]
- 36.Onat A, Ozhan H, Esen AM, et al. Prospective epidemiologic evidence of a “protective” effect of smoking on metabolic syndrome and diabetes among Turkish women—without associated overall health benefit. Atherosclerosis 2007;193:380–388 [DOI] [PubMed] [Google Scholar]
- 37.Onat A, Erginel-Unaltuna N, Coban N, Ciçek G, Yüksel H. APOC3 -482C>T polymorphism, circulating apolipoprotein C-III and smoking: interrelation and roles in predicting type-2 diabetes and coronary disease. Clin Biochem 2011;44:391–396 [DOI] [PubMed] [Google Scholar]
- 38.Olivieri O, Bassi A, Stranieri C, et al. Apolipoprotein C-III, metabolic syndrome, and risk of coronary artery disease. J Lipid Res 2003;44:2374–2381 [DOI] [PubMed] [Google Scholar]
- 39.Midthjell K, Holmen J, Bjørndal A, Lund-Larsen G. Is questionnaire information valid in the study of a chronic disease such as diabetes? The Nord-Trøndelag diabetes study. J Epidemiol Community Health 1992;46:537–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gorber SC, Schofield-Hurwitz S, Hardt J, Levasseur G, Tremblay M. The accuracy of self-reported smoking: a systematic review of the relationship between self-reported and cotinine-assessed smoking status. Nicotine Tob Res 2009;11:12–24 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.