Abstract
OBJECTIVE
The American Diabetes Association and the English NHS Diabetic Eye Screening Program recommend annual screening for diabetic retinopathy (DR) with referral to ophthalmology clinics of patients with sight-threatening DR (STDR). Using only longitudinal data from retinal photographs in the population-based NHS Diabetic Eye Screening Program in Gloucestershire, we developed a simple means to estimate risk of STDR.
RESEARCH DESIGN AND METHODS
From 2005, 14,554 patients with no DR or mild nonproliferative DR only at two consecutive annual digital photographic screenings were categorized by the presence of DR in neither, one, or both eyes at each screening and were followed for a further median 2.8 years.
RESULTS
Of 7,246 with no DR at either screening, 120 progressed to STDR, equivalent to an annual rate of 0.7%. Of 1,778 with no DR in either eye at first screening and in one eye at second screening, 80 progressed to STDR, equivalent to an annual rate of 1.9% and to a hazard ratio (HR) of 2.9 (95% CI 2.2–3.8) compared with those with no DR. Of 1,159 with background DR in both eyes at both screenings, 299 progressed to STDR equivalent to an annual rate of 11% and an HR of 18.2 (14.7–22.5) compared with individuals with no DR.
CONCLUSIONS
Combining the results from 2 consecutive years of photographic screening enables estimation of the risk of future development of STDR. In countries with systematic screening programs, these results could inform decisions about screening frequency.
Diabetic retinopathy (DR) is a complication of diabetes that can lead to blindness. The American Diabetes Association recommends (1) annual eye examinations for all people with diabetes mellitus (DM) and the English NHS Diabetic Eye Screening Program (2) recommends annual screening for DR; both sets of recommendations have the goal of referring people with sight-threatening DR (STDR) to ophthalmologists for assessment and treatment. Two recent studies (3,4) have shown that since 1985, people with diabetes have experienced lower rates of progression to proliferative DR and severe visual loss, probably reflecting improvements in diabetes care. Apart from the convenience of annual screening, the rationale behind the recommendations assumes that people should receive annual screening regardless of their actual risk of developing retinopathy. Most annual eye examinations now include digital retinal photographs to monitor the presence or absence of any DR. There has been a debate whether examinations are required yearly for all people with diabetes, with James et al. (5) reporting annual photographic screening to be cost-effective, but Vijan et al. (6) concluding that annual retinal screening for all patients with type 2 diabetes without previously detected retinopathy may not be warranted on the basis of cost-effectiveness and recommended tailoring the frequency of screening to individual circumstances.
At the time that annual digital photographic screening was introduced in England in 2003, the Department of Health reported ∼1.4 million people known to have diabetes; as of December 2011, the prevalence reported (7) by screening services is now 2.5 million. As the publically funded health care system in the U.K. has limited funds, this has led to challenges providing retinal screening, a concern shared by both developed and developing nations as the number of people with diabetes rises (8). In 2004, Kempen et al. (9) reported that, among an estimated 10.2 million U.S. adults ≥40 years known to have DM, the estimated crude prevalence rates for retinopathy and vision-threatening retinopathy were 40.3 and 8.2%, respectively. In November 2011, the International Diabetes Federation released the 5th edition of the Diabetes Atlas (8), which indicated that the number of people living with diabetes in all age groups in the U.S. had risen to 23.7 million, and the number in the world is expected to rise from 366 million in 2011 to 552 million by 2030.
In order to more efficiently use limited funds, extending the screening interval from annually to longer than annually has been proposed. Two sources of data have been considered: 1) a retinal photograph from one-time screening, and 2) a combination of clinical and demographic risk factors. Decisions based on retinal photography from a single visit, although supported by studies (10–12), were rejected by the American Diabetes Association (1) in 2002, which concluded that the annual eye examination is still warranted, citing that the evidence (12,13) was not generalizable to the greater population of people with diabetes and that it might lead to patients not attending screening. Determining screening intervals using a combination of clinical and demographic risk factors combined with recent photographic screening results (14) is equally problematic, as the clinical information may not be available, may be unreliable (e.g., reported duration of diabetes), and in some countries may not be collected (e.g., HbA1c).
In this study, we used longitudinal data from the results from retinal photographic images from two annual screening episodes in the population-based NHS Diabetic Eye Screening Program in Gloucestershire, U.K., to evaluate the incidence and time to recommended referral of potentially sight-threatening retinopathy.
RESEARCH DESIGN AND METHODS
The patient population from which our cohort was taken comprised 31,329 people with diabetes from the Gloucestershire Diabetic Eye Screening Service who were screened for retinopathy between the 4 January 2005 and 20 December 2010. Patients invited to retinal screening include all people with diabetes (type 1 and type 2 or other) aged ≥12 years in Gloucestershire.
Gloucestershire contains a mixed rural and urban, predominantly white Caucasian population (15). The Retinal Screening Program invites eligible patients with diabetes aged ≥12 years to attend a DR screening clinic in one of 86 locations, where specialist staff take a history, measure visual acuity using Early Treatment Diabetic Retinopathy Study (ETDRS) logMAR charts, and take digital color retinal photographs of two standard 45° fields (macula and disc centered) per eye after dilation of the pupils. Trained assessors in a central location grade the presence and severity of DR using a multilevel, internally and externally quality-assured reading process that meets national recommendations. For these analyses, we included patients with at least three annual graded image sets, the first two of which have either no retinopathy or only background (mild nonproliferative DR [NPDR]) in one or both eyes. Background (equivalent to ETDRS mild NPDR) was defined using the R1M0 category in the English NHS Diabetic Eye Screening Program, namely: presence of microaneurysm(s), hemorrhage, or exudate, but having none of the features (16) indicating referral to an ophthalmologist for STDR, such as features of preproliferative (moderate to severe NPDR) or proliferative DR or photographic markers of diabetic maculopathy.
The criteria used for grading in the Gloucestershire Diabetic Eye Screening Program and the relationship to the ETDRS severity scale (17) are described below:
R0 level identifies no detected DR (equivalent to ETDRS level 10).
R1 level (mild NPDR or background DR) identifies a minimum of at least the presence of one microaneurysm and/or retinal hemorrhage, equivalent to ETDRS levels 14–35. This is the definition used by the NHS Diabetic Eye Screening Program, and it is not possible to identify those with ETDRS levels 14 and 15. However, the numbers of patients who would have hemorrhages alone would be very small.
R2 level (moderate to severe NPDR or preproliferative DR) identifies the presence of multiple deep, round, or blot hemorrhages and/or definite intraretinal microvascular abnormality and/or venous beading and/or reduplication, equivalent to levels 43–53 on the ETDRS scale.
R3 level (proliferative DR) indicates the presence of proliferative DR (including fibrous proliferation), equivalent to a minimum of ETDRS level 61.
M1 (maculopathy) identifies the presence of two-dimensional photographic markers of diabetic maculopathy, specifically exudate within 1 disc diameter of the center of the fovea, circinate, or group of exudates within the macula or any microaneurysm or hemorrhage within 1 disc diameter of the center of the fovea, but only if associated with a best visual acuity of worse than 0.3 logMAR (equivalent to Snellen 6/12).
M0 describes the absence of any M1 features.
We report in this study on 14,554 patients with assessable images showing either no DR (R0M0) or only mild NPDR (R1M0) in one or both eyes at two consecutive annual screenings (hereafter referred to as baseline) and in whom there was at least one further follow-up screening episode (Fig. 1).
Figure 1.
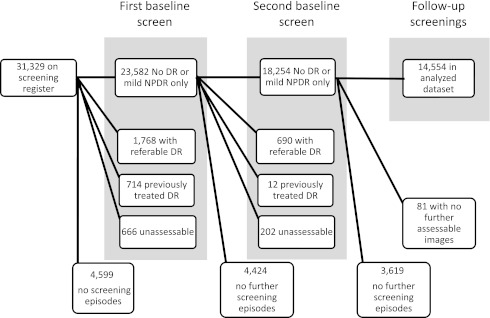
Cohort identification and inclusion/exclusion flow chart.
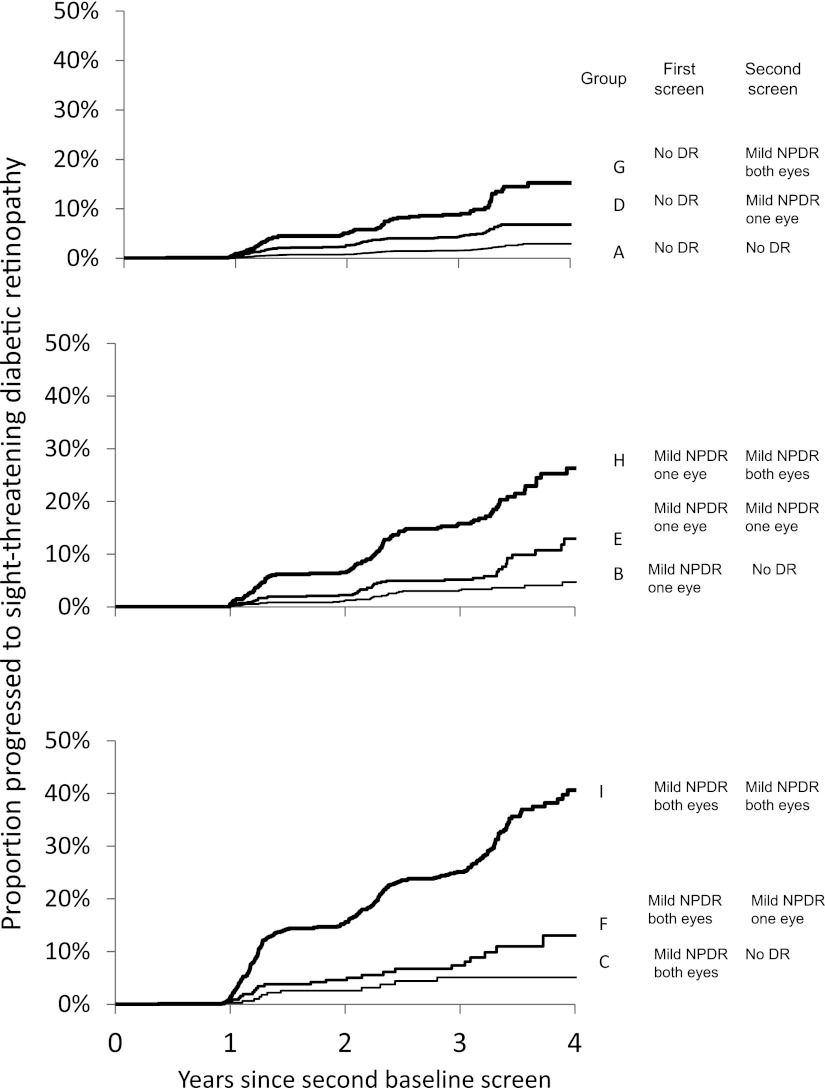
Patients were categorized into groups on the basis of the presence of retinopathy in neither, one, or both eyes at each of the two baseline screening episodes. Patients with unassessable images, with any evidence of previous laser treatment or with features of STDR at either baseline screening event were excluded. STDR was defined by the presence of any R2 (moderate to severe NPDR), R3 (proliferative DR), or M1 (maculopathy) in either eye. Fig. 2 shows the possible grading in the two eyes at the two screening episodes and the resultant categorization groups. Patients were then followed until such time as they developed features of STDR or until the end of the study period.
Figure 2.
Time to detection of STDR from second baseline screening with none or only mild NPDR in one or both eyes. Significant difference across groups (P < 0.0001).
We performed survival analysis, defining the time to event as the time from the second screening to the development of STDR at a subsequent screening episode; hence this was interval-censored. Specifically, for those people who did not develop STDR, the time to event was right censored at the date of the last screening, and for those people who developed STDR, the data were left censored at the date of the last screening at which no STDR was found and with event time at the date of the image set when STDR was found. Data were plotted using Kaplan-Meier estimates to show the cumulative percentage of patients who developed STDR. We fitted Cox proportional hazards models to estimate hazard ratios (HRs). We examined parametric models (γ, Weibull, log logistic, logistic, and exponential) that were used to estimate the proportion of patients expected to have STDR at 1, 2, 3, 5, and 10 years after baseline. The parametric model with the best fit (log logistic) was chosen using Akaike information criterion to optimize the fit of the model. Proportions were compared using χ2 tests and continuous data between groups with ANOVA. We performed analyses with SAS version 9.1.3 (SAS Institute) using Proc LIFEREG for parametric modeling.
RESULTS
There were 31,329 people on the screening register between 2005 and 2010 with a median age of 63 years (interquartile range 52–72). Of these, there were 14,554 people who had two consecutive baseline screening episodes that both demonstrated either no evidence of DR or the presence of only mild NPDR in one or both eyes who had at least one further episode with gradable photographs. The median age of the 14,554 patients included as this cohort was 65 years (56–73). The 16,775 patients who either did not attend for two consecutive screenings or were otherwise excluded were significantly younger (P < 0.0001), median age 60 years (49–72).
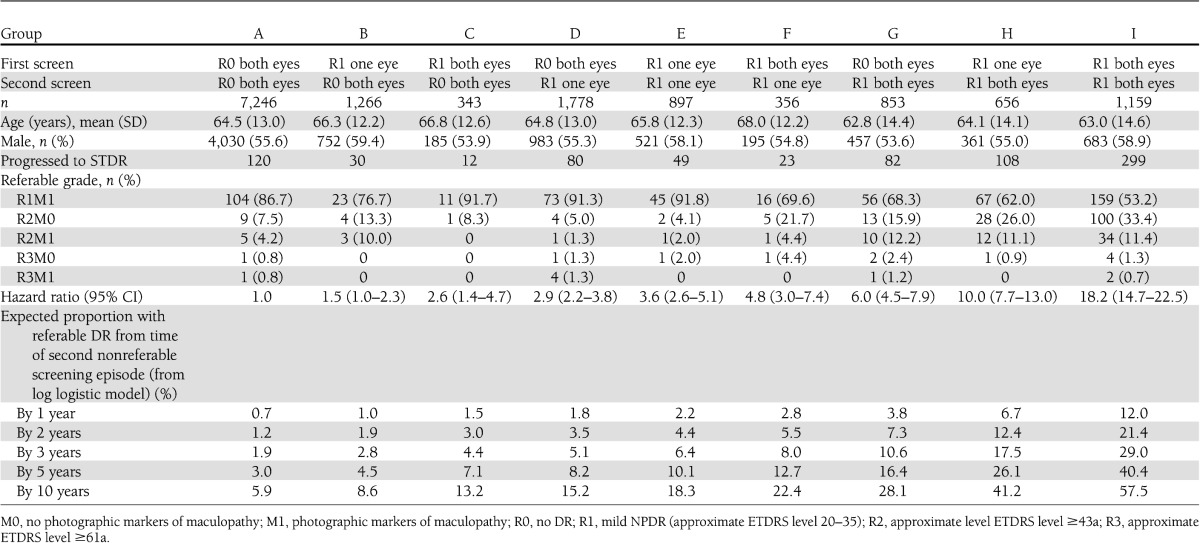
There were 14,554 patients who completed two consecutive annual screenings with gradable photographs showing no DR (R0M0) or mild NPDR (R1M0) who had at least one further episode with gradable photographs. These were characterized into nine possible groups (Table 1). The least severe group comprised 7,246 patients who had no DR (R0M0) on both screening episodes, and the most severe baseline group in this study population were 1,159 patients who had mild NPDR (R1M0) in both eyes at both screenings. Of the remaining patients, 4,240 had mild NPDR (R1M0) in one or both eyes but only at one of these two screening episodes, 897 had mild NPDR (R1M0) in only one eye at each screening episode, and 1,012 had mild NPDR (R1M0) in one eye at one episode but in both eyes at the other screening.
Table 1.
Demographic characteristics and outcomes in the nine baseline categories
The median period between the two baseline screening episodes was 13.5 months (interquartile range 12.0–15.2), and patients were then followed for a median 2.8 years (1.3–3.3). The median time to first follow-up screen in study after baseline was 13.6 months (12.3–15.1), to second screen after baseline was 26.6 months (24.9–28.3), to third 39.2 months (37.4–40.9), and to fourth was 51.6 months (50.8–52.1).
Of those excluded from the study, 2,458 had more severe retinopathy at either first or second baseline screening episodes, 726 had previously treated DR, 949 had at least one unassessable image set, and the rest had not attended three screening episodes (Fig. 1).
There was no significant difference in the proportion of men and women in the categories (P > 0.1). There were differences (P < 0.001) between the age groups in that those with mild NPDR in both eyes at both screenings were younger at 63.0 years (SD 14.6) than the groups who had either one or both eyes without DR at one or both screening episodes. Patients in the three groups that regressed were older.
Table 1 also shows the outcomes in the nine baseline categories. Of 7,246 with no DR at both screenings (group A), 120 subsequently progressed to STDR, equivalent to an annual rate of 0.7%. Of 1,778 with no DR in either eye at first screening but mild NPDR in just one eye at second screening (group D), 80 progressed to STDR, equivalent to an annual rate of 1.9%. This was associated with an HR of 2.9 (95% CI 2.2–3.8) compared with those with no DR at both screenings. Of 1,159 in the most severe category (group I) with mild NPDR in both eyes at both screenings, 299 progressed to STDR, equivalent to 11.0% by 1 year and associated with an HR of 18.2 (14.7–22.5) compared with patients with no DR at both screenings. Those in higher risk groups were more likely to progress to more serious DR (P < 0.001).
Fig. 2 shows the Kaplan-Meier plot of time to detection of STDR from baseline. There are clear differences between the outcome of the groups and obviously increased risk for patients who have mild NPDR in both eyes at both annual screening events (group I) when compared with those with no DR in either eye at both screenings (group A).
CONCLUSIONS
Using data from only two sequential annual photographic screening visits supported by a quality-controlled grading system, we documented significant differences in the time to development of retinopathy requiring referral to an ophthalmologist. As most annual eye examinations in people with diabetes in developed countries now include digital retinal photographs to monitor the presence or absence of DR, these findings suggest that photographs alone could be used to differentiate levels of risk. This implies that the interval for screening could differ by level of risk. We believe that this is the first report identifying a clear differentiation of risk to development of STDR in patients who had either no or only minimal background DR when screened: a group normally considered to be at very low and generally homogenous risk. The difference in risk between groups in this study is large. For example, we demonstrate that a patient who was found to have no retinopathy on the first occasion but mild NPDR in both eyes 1 year later has a risk of subsequently developing STDR approximately six times greater than does a patient who has no DR on both occasions. A patient who has bilateral mild NPDR on both occasions has a risk for subsequent development of STDR that is 18.2 times higher than an individual with no retinopathy at either screening (Table 1).
Whereas the risk of progression in the model by Vijan et al. (6) is based on the Diabetic Retinopathy Study and ETDRS reports, the risk we define in this study is based entirely on two sets of digital images taken ∼1 year apart. With those two seminal studies dating from the 1980s and 1990s, the possibility exists that with the passage of time and the changes in treatment that accompany it, changes will occur in the rate of progression of disease.
This study does not compare risk of referable retinopathy defined by retinal photographs with that defined by clinical criteria (e.g., time since diagnosis of diabetes, HbA1c, blood pressure). It does not estimate risk for those in whom assessment of DR by retinal photography is not possible and who have to be screened using slit-lamp biomicroscopy. It does not test the number of images required nor does it make explicit recommendations on the frequency or cost-effectiveness of screening. Answering these questions requires further modeling that our results could inform by providing transition probabilities from nonreferable to referable retinopathy.
Vijan et al. (18) developed a cost-utility model for screening, with the conclusions that for patients with good glycemic and blood pressure control (that is, a lower risk population), screening every 2 to 3 years would be appropriate while at the same time advocating “close follow-up” for the high-risk patient. They point out that the costs associated with screening low-risk individuals less frequently could be better spent achieving close follow-up of patients at higher risk.
Screening low-risk individuals too frequently implies an inefficient use of limited health care resources. More efficient uses might include spending money on strategies to bring retinal screening to people with diabetes who are poor attendees including, as we have shown in this study, younger patients who tend to be patients at high risk of retinopathy (19), spending to prevent retinopathy in people with diabetes, or spending money elsewhere within the health care system.
Two of the strengths of this study are its size and that the population screened accurately represents the underlying population with diabetes. Over 99% of patients with DM are eligible for screening against the criteria of the English NHS Diabetic Eye Screening Program (20). The eligible population is increasing. In 2005, there were 17,847 patients who were invited for screening, but this figure had risen to 27,520 by December 2010. The average attendance figures following an invitation was 74% in any one year, which compares favorably to other populations within the English NHS Diabetic Eye Screening Program.
A recent report (21) from the Diabetic Retinopathy Screening Service for Wales described the incidence of any and referable DR in people with type 2 diabetes mellitus attending the annual screening service in Wales, whose first screening episode indicated no evidence of retinopathy. Their referable level is higher than in this study, but the results are not directly comparable, as the definition of retinopathy levels are not the same, and their study is based on an initial screening episode, whereas this study population is not restricted to first screening episode.
This study evaluated people with type 1 or type 2 diabetes, most of whom were of white northern European extraction. In addition, the Gloucestershire screening program has a robust quality-control program of the imaging and grading processes. Hence, to be generalizable, the results would need to be validated in other populations.
We propose that estimating risk in this way can be useful in several ways, either in discussions with patients during eye examinations or in countries or institutions with systematic screening programs, to develop screening models to allocate limited monies more efficiently by varying the screening intervals depending on an individual’s identified risk.
The results of this study may also help to design and power clinical trials testing interventions for DR. Many clinical trials require a three-step progression on the ETDRS severity scale. This is equivalent to the progression from R1 mild NPDR to R2 moderate to severe NPDR in this study. If patients were selected who had bilateral mild NPDR on two consecutive screening appointments, we have demonstrated an overall progression to sight threatening DR in 29% after 3 years and 40.4% after 5 years.
This risk estimator essentially helps remove the requirement for complex clinical-based data, as even if none of these are taken into account, we can differentiate risk by 18:1. We believe that this is the first report identifying a clear differentiation of risk to development of STDR in patients who had either no or only minimal background DR when screened: a group normally considered to be at very low and generally homogenous risk.
Acknowledgments
S.J.A. has received consulting fees from AstraZeneca (<5 years ago), Takeda (<5 years ago), Eli Lilly (<5 years ago), and Novartis (<1 year ago); received research support from AstraZeneca and Takeda (both <5 years ago); and is currently receiving research support from Novartis. I.M.S. has received fees for lectures from Novo Nordisk. No other potential conflicts of interest relevant to this article were reported.
I.M.S. raised the research hypothesis, designed and carried out data analyses, and wrote the manuscript. S.J.A. and D.J.T. contributed to the discussion and wrote the manuscript. A.I.A. advised on data analysis procedures and reviewed the manuscript. P.H.S. contributed to the discussion and reviewed and edited the manuscript. I.M.S. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This study was presented in poster form at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank Mark Histed and the team of the Gloucestershire Diabetic Eye Screening Service for high-quality screening and grading and Steve Chave of the same group for data extraction and management.
References
- 1.Diabetic Retinopathy ADA. Diabetes Care 2002;25(Suppl. 1):s90–s93 [Google Scholar]
- 2.Scanlon PH. The English national screening programme for sight-threatening diabetic retinopathy. J Med Screen 2008;15:1–4 [DOI] [PubMed] [Google Scholar]
- 3.Wong TY, Mwamburi M, Klein R, et al. Rates of progression in diabetic retinopathy during different time periods: a systematic review and meta-analysis. Diabetes Care 2009;32:2307–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology 2008;115:1859–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.James M, Turner DA, Broadbent DM, Vora J, Harding SP. Cost effectiveness analysis of screening for sight threatening diabetic eye disease. BMJ 2000;320:1627–1631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijan S, Hofer TP, Hayward RA. Cost-utility analysis of screening intervals for diabetic retinopathy in patients with type 2 diabetes mellitus. JAMA 2000;283:889–896 [DOI] [PubMed] [Google Scholar]
- 7.Department of Health. Integrated Perfomance Measures Monitoring [Internet], 2011. Available at http://www.dh.gov.uk/en/Publicationsandstatistics/Statistics/Performancedataandstatistics/Integratedperfomancemeasuresmonitoring/index.htm. Accessed 3 May 2012
- 8.International Diabetes Federation. One adult in ten will have diabetes by 2030 [Internet], 2011. Available at http://www.idf.org/media-events/press-releases/2011/diabetes-atlas-5th-edition Accessed 27 August 2012
- 9.Kempen JH, O’Colmain BJ, Leske MC, et al. Eye Diseases Prevalence Research Group The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol 2004;122:552–563 [DOI] [PubMed] [Google Scholar]
- 10.Younis N, Broadbent DM, Harding SP, Vora JP. Incidence of sight-threatening retinopathy in Type 1 diabetes in a systematic screening programme. Diabet Med 2003;20:758–765 [DOI] [PubMed] [Google Scholar]
- 11.Younis N, Broadbent DM, Vora JP, Harding SP, Liverpool Diabetic Eye Study Incidence of sight-threatening retinopathy in patients with type 2 diabetes in the Liverpool Diabetic Eye Study: a cohort study. Lancet 2003;361:195–200 [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105:1801–1815 [DOI] [PubMed] [Google Scholar]
- 13.Aiello LP, Gardner TW, King GL, et al. Diabetic retinopathy. Diabetes Care 1998;21:143–156 [DOI] [PubMed] [Google Scholar]
- 14.Mehlsen J, Erlandsen M, Poulsen PL, Bek T. Individualized optimization of the screening interval for diabetic retinopathy: a new model. Acta Ophthalmol 2012;90:109–114 [DOI] [PubMed] [Google Scholar]
- 15.Scanlon PH, Carter SC, Foy C, Husband RF, Abbas J, Bachmann MO. Diabetic retinopathy and socioeconomic deprivation in Gloucestershire. J Med Screen 2008;15:118–121 [DOI] [PubMed] [Google Scholar]
- 16.Harding S, Greenwood R, Aldington S, et al. Diabetic Retinopathy Grading and Disease Management Working Party Grading and disease management in national screening for diabetic retinopathy in England and Wales. Diabet Med 2003;20:965–971 [DOI] [PubMed] [Google Scholar]
- 17.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology 1991;98(Suppl.):823–833 [PubMed] [Google Scholar]
- 18.Vijan S, Hofer TP, Hayward RA. How often should patients be screened for retinopathy. Reply to letter to the Editor. JAMA 2000;284:437–439 [PubMed] [Google Scholar]
- 19.Zoega GM, Gunnarsdóttir T, Björnsdóttir S, Hreietharsson AB, Viggósson G, Stefánsson E. Screening compliance and visual outcome in diabetes. Acta Ophthalmol Scand 2005;83:687–690 [DOI] [PubMed] [Google Scholar]
- 20.National Health Service. Excluding Patients from the NHS Diabetic Retinopathy Screening Programme Temporarily or Permanently: Good Practice Guide [Internet], 2006. Available at http://diabeticeye.screening.nhs.uk/ Accessed 3 May 2012
- 21.Thomas RL, Dunstan F, Luzio SD, et al. Incidence of diabetic retinopathy in people with type 2 diabetes mellitus attending the Diabetic Retinopathy Screening Service for Wales: retrospective analysis. BMJ 2012;344:e874. [DOI] [PMC free article] [PubMed] [Google Scholar]