Abstract
OBJECTIVE
A 12-week study assessed the efficacy and safety of a new oral antidiabetic agent, imeglimin, as add-on therapy in type 2 diabetes patients inadequately controlled with metformin alone.
RESEARCH DESIGN AND METHODS
A total of 156 patients were randomized 1:1 to receive imeglimin (1,500 mg twice a day) or placebo added to a stable dose of metformin (1,500–2,000 mg/day). Change in A1C from baseline was the primary efficacy outcome; secondary outcomes included fasting plasma glucose (FPG) and proinsulin/insulin ratio.
RESULTS
After 12 weeks, the placebo-subtracted decrease in A1C with metformin-imeglimin was −0.44% (P < 0.001). Metformin-imeglimin also significantly improved FPG and the proinsulin/insulin ratio from baseline (−0.91 mg/dL and −7.5, respectively) compared with metformin-placebo (0.36 mg/dL and 11.81). Metformin-imeglimin therapy was generally well-tolerated with a comparable safety profile to metformin-placebo.
CONCLUSIONS
Addition of imeglimin to metformin improved glycemic control and offers potential as a new treatment for type 2 diabetes.
Imeglimin is the first in a new tetrahydrotriazine-containing class of oral antidiabetic agents, the glimins. Imeglimin decreases hepatic glucose production, increases muscle glucose uptake, and improves pancreatic glucose-dependent insulin secretion (1).
Previous studies have demonstrated imeglimin to be as effective as metformin in improving glycemia (2). Since metformin is the preferred first-line therapy for type 2 diabetes, the current study examined the efficacy, safety, and tolerability of imeglimin in combination with metformin in patients with type 2 diabetes inadequately controlled with metformin alone.
RESEARCH DESIGN AND METHODS
This was a 12-week, multicenter (20 centers in three countries), randomized, double-blind, placebo-controlled, parallel-group study in subjects with type 2 diabetes inadequately controlled with the maximum tolerated dose of metformin. The average metformin doses at baseline remained the same throughout the study: 1,901 mg in the metformin-imeglimin group and 1,914 mg in the metformin-placebo group.
After screening, eligible subjects were enrolled into a single-blind, placebo, 2-week run-in period under previous metformin treatment and placebo-imeglimin twice a day (BID). Subjects were then randomized 1:1 to receive 1,500 mg BID imeglimin or placebo-imeglimin BID in addition to their lead-in dose of metformin for 12 weeks, followed by a 1-week period with placebo-imeglimin.
Male and female subjects with type 2 diabetes (N = 156), aged ≥18 to ≤70 years, inadequately controlled (A1C ≥7.5%) by metformin alone (1,500–2,000 mg/day, both inclusive) were included. Enrolled subjects had received a stable dose of metformin for at least 10 weeks before randomization and had received no other glucose-lowering medication within 3 months prior to randomization. Most other therapeutic classes of concomitant medication were permitted. Exclusion criteria included impaired hepatic or renal function, inadequately controlled hypertension, and clinically significant microvascular or macrovascular complications.
The study protocol was approved by the institutional review board and conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines (3). All participants provided written informed consent before any study-related activities.
The primary efficacy outcome was change in A1C from baseline to week 12. Secondary end points included changes from baseline in fasting plasma glucose (FPG; mg/dL) and proinsulin (pmol/L)/insulin (μIU/mL) ratio. The percentage of patients achieving an A1C <7% or a decrease from baseline ≥0.5% at week 12 were calculated, and subgroup analyses were performed to determine the effect of baseline A1C and BMI on the change in A1C from baseline to week 12.
Adverse events were specifically assessed into treatment-related and severity.
Statistical analysis
Intention-to-treat efficacy analysis included all randomized subjects who received at least one dose of imeglimin or placebo and provided a baseline and at least one postbaseline A1C value. Data are presented as last observation carried forward (LOCF). Change of A1C from baseline to week 12 or LOCF was assessed with an ANCOVA model. Statistical significance was assessed at the 5% level.
RESULTS
Demographic and baseline characteristics were similar between the two treatment arms. A1C decreased from baseline to week 12 by −0.65% with metformin-imeglimin and −0.21% with metformin-placebo groups (P = 0.001), resulting in a mean treatment difference of −0.44% (95% CI −0.71 to −0.17). Statistically significant reductions in FPG (P < 0.001) and the proinsulin/insulin ratio (P = 0.007) from baseline compared with metformin-placebo were also observed (Table 1).
Table 1.
Efficacy and safety results of the imeglimin add-on to metformin phase II study
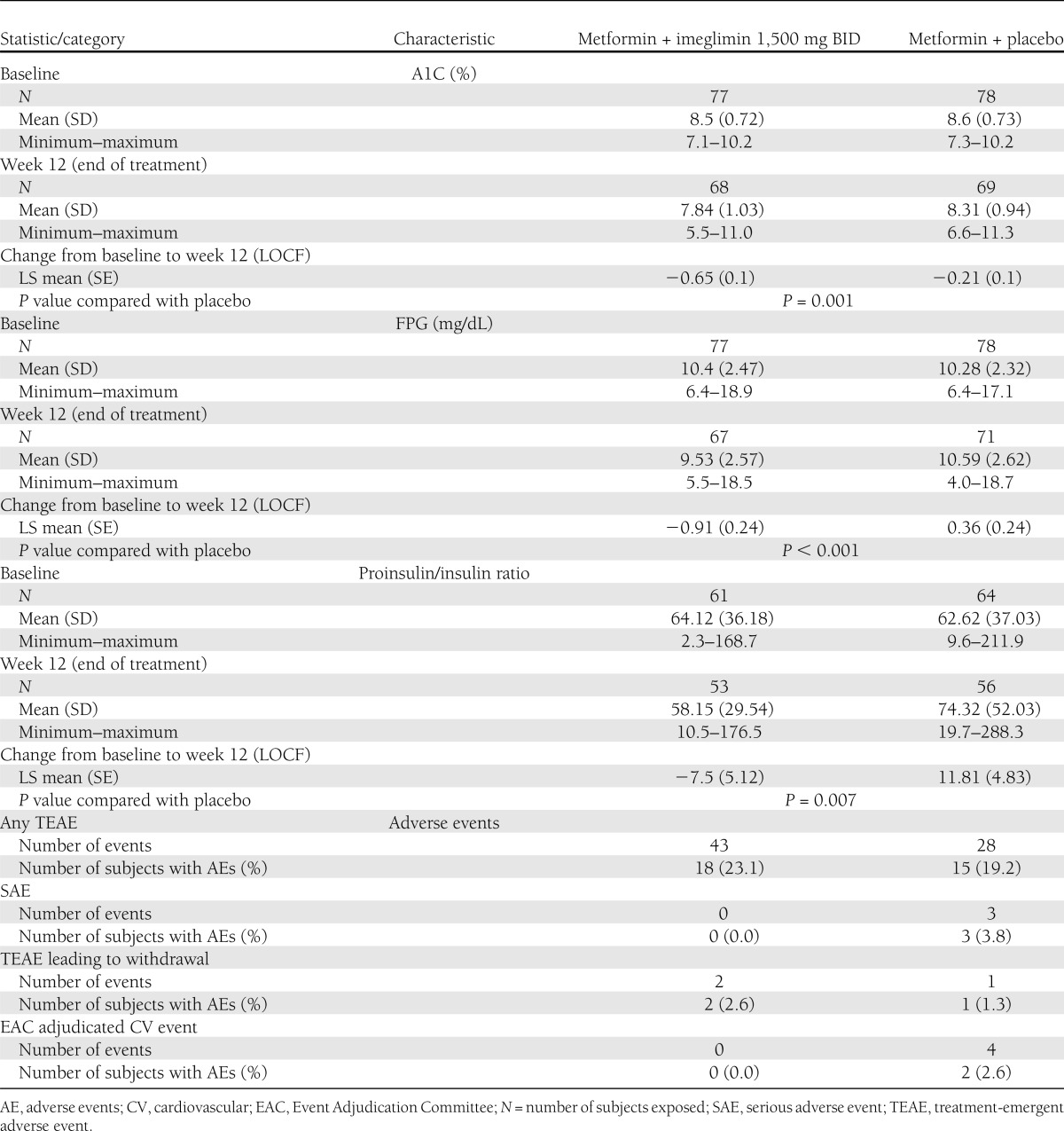
More subjects achieved a decrease in A1C ≥0.5% with metformin-imeglimin (63.6%) than metformin-placebo (36.4%) (P = 0.001). Furthermore, 14.3% of subjects receiving metformin-imeglimin achieved an A1C <7% compared with 3.8% of subjects receiving metformin-placebo (P = 0.04).
Metformin-imeglimin was more effective than placebo in reducing mean A1C from baseline after 12 weeks of treatment in all subgroup analyses. For prespecified baseline A1C subgroup measurements <8.0%, 8.0–9.0%, and >9.0%, reductions in mean (SD) A1C (%) from baseline to week 12 for metformin-imeglimin were −0.41% (0.44), −0.68% (0.86), and −0.78% (0.98), respectively, as compared with −0.09% (0.59), −0.15% (0.96), and −0.43% (0.89) for metformin-placebo. Reductions in A1C based on baseline BMI were similar for subjects with baseline BMI (kg/m2) ≤30 kg/m2 and for subjects with baseline BMI (kg/m2) >30 kg/m2 for both treatment arms of the study.
Metformin-imeglimin therapy was well-tolerated with a safety profile comparable to metformin-placebo. No serious adverse events or cardiovascular events were reported with metformin-imeglimin. Although not statistically significant, a slight decrease in mean values for body weight (P = 0.08) and waist circumference (P = 0.053) was observed for metformin-imeglimin compared with metformin-placebo.
CONCLUSIONS
Current guidelines recommend metformin as the first-line pharmacological treatment for type 2 diabetes (4). However, metformin alone is frequently insufficient to obtain or maintain glycemic goals; therefore, many patients require multiple pharmacotherapies for optimal disease management (5).
The current study shows that imeglimin appeared to complement the actions of metformin by producing modest, but clinically and statistically significant improvements in A1C, FPG, and proinsulin/insulin ratio. Metformin-imeglimin progressively reduced the mean A1C value throughout the 12-week treatment period, implying that further improvements might be expected beyond 12 weeks. This reduction in A1C is comparable to other recently approved antihyperglycemic treatments (6–8). Furthermore, the effect on A1C is related to beneficial effects of imeglimin on both FPG and glucose tolerance, as previously demonstrated (9).
Metformin-imeglimin was effective in reducing the proinsulin/insulin ratio, indicating a potential beneficial effect of imeglimin on β-cell function. Further studies are necessary to determine if this translates to β-cell protection over time, as suggested in previous preclinical studies in which imeglimin reduced β-cell apoptosis induced by cytokine stress and high glucose conditions (2).
Drug–drug interaction studies in healthy subjects have shown that multiple dosing of imeglimin did not affect metformin exposure (unpublished data, Poxel). The novel mechanism of action of imeglimin provides additional benefits to metformin monotherapy in patients with type 2 diabetes.
Metformin-imeglimin was generally well-tolerated compared with metformin-placebo with no serious adverse events or cardiovascular events reported. This phase II study demonstrated that first-in-class imeglimin was well-tolerated and effective when combined with metformin as a potential new treatment for type 2 diabetes.
Acknowledgments
P.F. is an employee of Poxel. V.P. has received a speaker honorarium from Glenmark Pharmaceuticals Ltd. S.E.I. has served as a consultant to Merck Sharp & Dohme, Poxel, Takeda, and Boehringer Ingelheim. C.J.B. has attended advisory board meetings of Bristol-Myers Squibb and AstraZeneca, undertaken ad hoc consultancy for Bristol-Myers Squibb, AstraZeneca, Merck Sharp & Dohme, Novo Nordisk, GlaxoSmithKline, Poxel, and Takeda; received research grants from AstraZeneca and sanofi-aventis; delivered continuing medical educational programs sponsored by Bristol-Myers Squibb, AstraZeneca, GlaxoSmithKline, Merck Serono, and Merck Sharp & Dohme; and received travel/accommodation reimbursement from GlaxoSmithKline and Bristol-Myers Squibb. G.S. has received lecture fees and honoraria for advisory boards from Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Merck, Novartis, Novo Nordisk, Poxel, Roche, sanofi-aventis, Servier, and Takeda. M.D. serves on the advisory board of Abbott, Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, and Poxel; is a consultant for sanofi-aventis; and is a speaker for Eli Lilly, Merck Sharp & Dohme, and Novo Nordisk. Through the VU University Medical Center, M.D. has received research grants from Amylin/Eli Lilly, Merck Sharp & Dohme, Novo Nordisk, sanofi-aventis, and Takeda. M.D. receives no personal payments in connection to the above-mentioned activities, but all payments are directly transferred to the Institutional Research Foundation. H.E.L. is a member of the scientific advisory boards of Amylin Pharmaceuticals, Biocon Pharma, Intarcia Therapeutics, Merck Sharp & Dohme, MetaCure, and Poxel; serves as a consultant for AstraZeneca, Bristol-Myers Squibb, and sanofi-aventis; owns stock in Merck Sharp & Dohme; and is on the Board of Directors of the American Association of Clinical Endocrinologists and MetaCure. No other potential conflicts of interest relevant to this article were reported.
P.F. contributed to the design of the study and researched data. V.P. was the principal investigator of the study. P.F., V.P., S.E.I., C.J.B., G.S., M.D., and H.E.L. all contributed to researching and interpreting the data and reviewed and edited the manuscript. P.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented as a poster at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
The authors thank IMC Healthcare Communication for assistance in preparing the manuscript. This service was funded by Poxel SA.
Footnotes
Clinical trial reg. no. 2010-018580-42, https://eudract.ema.europa.eu/eudract-web/index.faces.
References
- 1.Fouqueray P, Levere X, Fontaine E, et al. Imeglimin–a new oral anti-diabetic that targets the three key defects of type 2 diabetes. J Diabetes Metab 2011;2:4 [Google Scholar]
- 2.Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral antidiabetic, exhibits good glycaemic control in type 2 diabetes mellitus patients. Presented at the 46th Annual Meeting of the European Association for the Study of Diabetes, 20–24 September 2010, Stockholm, Sweden [Google Scholar]
- 3.World Medical Association Declaration of Helsinki Recommendations guiding physicians in biomedical research involving human subjects. JAMA 1997;277:925–926 [PubMed] [Google Scholar]
- 4.American Diabetes Association Standards of medical care in diabetes - 2011. Diabetes Care 2011;34:511–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA) European Association for the Study of Diabetes (EASD) Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2012;35:1364–1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foley JE, Sreenan S. Efficacy and safety comparison between the DPP-4 inhibitor vildagliptin and the sulfonylurea gliclazide after two years of monotherapy in drug-naïve patients with type 2 diabetes. Horm Metab Res 2009;41:905–909 [DOI] [PubMed] [Google Scholar]
- 7.Clinical use of liraglutide in type 2 diabetes and its effects on cardiovascular risk factors [article online], 2011. Available from http://aace.metapress.com/content/p03r8r6026448w8k/fulltext.pdf Accessed 8 November 2011
- 8.Gomis R, Espadero RM, Jones R, Woerle HJ, Dugi KA. Efficacy and safety of initial combination therapy with linagliptin and pioglitazone in patients with inadequately controlled type 2 diabetes: a randomized, double-blind, placebo-controlled study. Diabetes Obes Metab 2011;13:653–661 [DOI] [PubMed] [Google Scholar]
- 9.Pirags V, Lebovitz H, Fouqueray P. Imeglimin, a novel glimin oral anti-diabetic, exhibits a good efficacy and safety profile in type 2 diabetic patients. Diabetes Obes Metab [article online], 2012. Available from http://onlinelibrary.wiley.com/doi/10.1111/j.1463-1326.2012.01611.x/pdf Accessed 24 May 2012 [DOI] [PubMed]


