Abstract
OBJECTIVE
In patients with long-standing diabetes mellitus (DM), there is increasing evidence for abnormal processing of gastrointestinal sensations in the central nervous system. Using magnetic resonance diffusion tensor imaging, we characterized brain microstructure in areas involved in visceral sensory processing and correlated these findings to clinical parameters.
RESEARCH DESIGN AND METHODS
Twenty-six patients with DM and gastrointestinal symptoms and 23 healthy control subjects were studied in a 3T scanner. The apparent diffusion coefficient (i.e., diffusivity of water) and fractional anisotropy (FA) (i.e., organization of fibers) were assessed in the “sensory matrix” (cingulate cortex, insula, prefrontal and secondary sensory cortex, amygdala, and corona radiata) and in corpus callosum.
RESULTS
Patients had decreased FA values compared with control subjects in 1) all areas (P = 0.025); 2) anterior (P < 0.001), mid- (P = 0.001), and posterior (P < 0.001) cingulate cortex; 3) prefrontal cortex gray matter (P < 0.001); 4) corona radiata (P < 0.001); 5) secondary sensory cortex (P = 0.008); and 6) anterior white matter (P = 0.045), anterior gray matter (P = 0.002), and posterior gray matter (P = 0.002) insula. No difference was found in corpus callosum (P > 0.05). The microstructural changes in some areas correlated with clinical parameters such as bloating (anterior insula), mental well-being (anterior insula, prefrontal cortex, and mid-cingulated and corona radiata), autonomic function based on electrocardiographic results (posterior insula and anterior cingulate), and presence of gastroparesis (anterior insula).
CONCLUSIONS
The findings of this explorative study indicate that microstructural changes of brain areas involved in visceral sensory processing are associated with autonomic dysfunction and therefore may be involved in the pathogenesis of gastrointestinal symptoms in DM patients.
Diabetes mellitus (DM) is a common disease with a worldwide increase in prevalence (1). DM is associated with the risk of severe complications of which especially neuronal dysfunction manifested as peripheral and autonomic neuropathies has great clinical impact (2,3). Evidence of widespread DM-induced nerve damage at peripheral, spinal, and brain levels has been observed (3), but most studies have primarily focused on peripheral neuropathy. The use of magnetic resonance imaging (MRI) with analysis of brain volumetry, spinal cross-section area measurements, spectroscopy, diffusion tensor imaging (DTI), perfusion, and functional MRI has revealed central nervous system changes (3–9).
Gastrointestinal symptoms, such as nausea, vomiting, bloating, postprandial fullness, early satiety, and abdominal pain, are also frequent in DM patients (2,10,11). These symptoms are typically difficult to manage and have a negative impact on health-related quality of life (12). The pathogenesis is complex in nature, multifactorial, and not well understood (2). DM autonomic neuropathy likely plays a central role in the development and progression of the gastrointestinal dysfunction and discomfort (2,13). However, abnormal gut motor dysfunction, glycemic control, and psychological factors, among other factors, are also of relevance for symptom generation in DM (2,14). Neurophysiological changes have been observed in these patients with increased latency and reduced amplitude of esophageal electrically evoked brain potentials and altered sensory brain processing correlating to the gastrointestinal symptoms (15,16). This indicates changes in peripheral nerves as well as changes in the central nervous system with reorganization and neuroplasticity in structures involved in processing of visceral sensations. However, a better understanding of the brains’ processing of afferent information from the gastrointestinal tract is highly needed to explore the mechanisms behind gastrointestinal symptoms in patients with DM.
Previously, we studied the microstructural changes in painful chronic pancreatitis patients using DTI and found abnormal microstructure in areas involved in visceral sensory processing indicating structural reorganization of the sensory neuromatrix (17). DTI allows measurement of the apparent diffusion coefficient (ADC) (i.e., mean diffusivity of water) and fractional anisotropy (FA) (i.e., organization of fibers) of selected brain areas. Even though DTI has been done in DM patients showing altered microstructure in several regions (4–8), DTI-based measurements of areas involved in visceral sensory processing have, to the best of our knowledge, never been conducted.
We hypothesized that patients with long-standing DM and gastrointestinal symptoms have microstructural changes, specifically in brain areas involved in visceral sensory processing, which (as part of a neuropathic-like process) associate with clinical data. Hence, the aims of the study were 1) to assess the brain microstructure described by DTI in brain areas involved in the visceral sensory processing in healthy control subjects and in patients with long-standing DM and gastrointestinal symptoms and 2) to correlate the findings with the clinical parameters in patients.
RESEARCH DESIGN AND METHODS
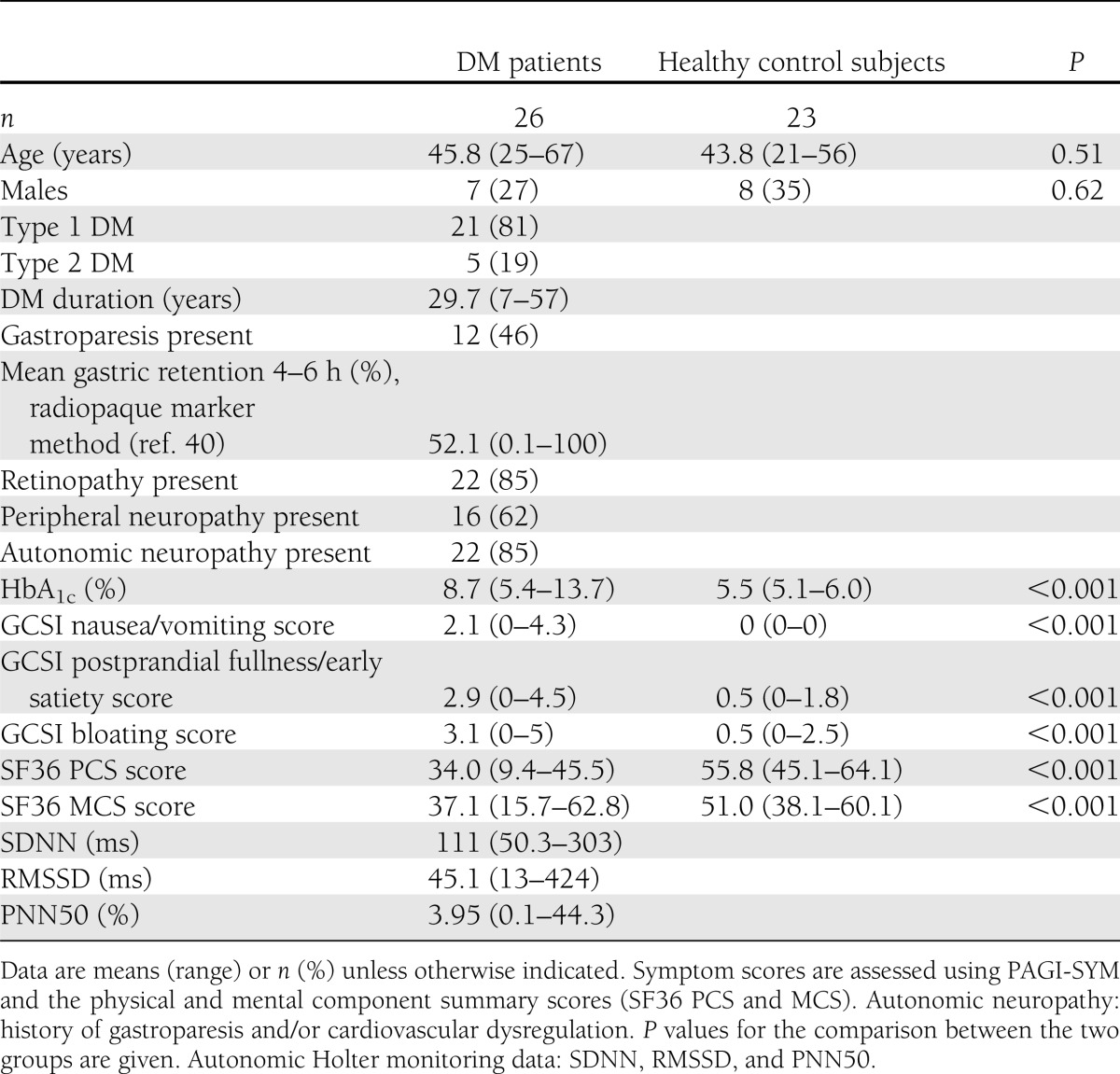
Twenty-six patients with long-standing DM and gastrointestinal symptoms were recruited from the Department of Internal Medicine, Sahlgrenska University Hospital, and the Department of Medicine, Haukeland University Hospital. As healthy control subjects, 23 subjects were recruited among the hospital staff and medical students at Aalborg Hospital and Haukeland University Hospital. Demographic and clinical data are presented in Table 1. The patients underwent examinations adequate for the exclusion of any organic diseases as cause of their symptoms, based on the decision of the treating physician. Furthermore, a standard 24-h ambulatory electrocardiography (Holter monitoring) was performed and nonspectral analysis of heart rate variability was evaluated as a measure of autonomic dysfunction (18). The patients reported their gastrointestinal symptoms covering the preceding 2 weeks using the Patient Assessment of Upper Gastrointestinal Disorder Severity Symptom Index (PAGI-SYM) (19). In its short form, the Gastroparesis Cardinal Symptom Index (GCSI), which consists of the first nine questions of PAGI-SYM, formed into three subgroups (postprandial fullness/early satiety, nausea/vomiting, and bloating), is a reliable and valid tool for measuring symptom severity in patients with gastroparesis (20). The GCSI scale ranges from 0 (no symptoms) to 5 (very severe symptoms). Physical (PCS) and mental (MCS) component summary scores were reported using SF-36, which is a multipurpose, short-form health survey with 36 questions (21). The local ethics committees approved the study protocol, which conforms to the Declaration of Helsinki. Oral and written informed consent was obtained from all subjects.
Table 1.
Demographic and clinical characteristics of patients and healthy volunteers
MRI
All study subjects were imaged on a 3T magnetic resonance scanner (in Aalborg and Bergen: Signa HDxt; General Electric, Milwaukee, WI; in Gothenburg: Philips; Achieva TX, Best, the Netherlands). Both were equipped with eight-channel standard head coils. The image sequences were set up as similarly as possible, and test subjects were scanned at both scanner types showing consistent ADC and FA values between the sites. Axial T1-weighted three-dimensional images with 1.0-mm slice thickness and whole head coverage were obtained for detailed anatomical information. DTI was done by covering the entire cerebrum and was for coregistration purposes acquired axially with an echo planar diffusion-weighted sequence (General Electric and Philips: TR 9,000 ms, TE 69–72 ms, reconstructed matrix 256 × 256, field of view 307 mm, slice thickness 2.4 mm, 40 slices, 32 diffusion directions, and b values 0 and 1,300 s/mm2). Prior to each acquisition, automatic whole-volume first-order shimming was performed to minimize field inhomogeneity. Axial T2-weighted fluid-attenuated inversion recovery–sequence images (field of view 25 × 25 cm, matrix 352 × 224, 5-mm slice thickness, whole brain coverage, repetition time 8,802 ms, echo time 127 ms, inversion time 2,200 ms) were evaluated for atrophy, white matter lesions, and other pathology.
Analysis of DTI data
Analyses of the DTI data were done using a software package (version 2.3; Nordic ICE Diffusion/DTI Module, Nordic Imaging Laboratory, Bergen, Norway) on a voxel-by-voxel basis. From the diffusion-weighted sequence, ADC and FA values in each voxel were calculated. (For definitions, see rev. in 22.) The ADC value represents the mean diffusivity including all directions. The FA value can be interpreted as the magnitude of the diffusion tensor that can be ascribed to anisotropic diffusion; hence, FA values range between 0 (isotropy) and 1 (complete anisotropy), meaning that the higher the value, the more intact the fiber organization (22).
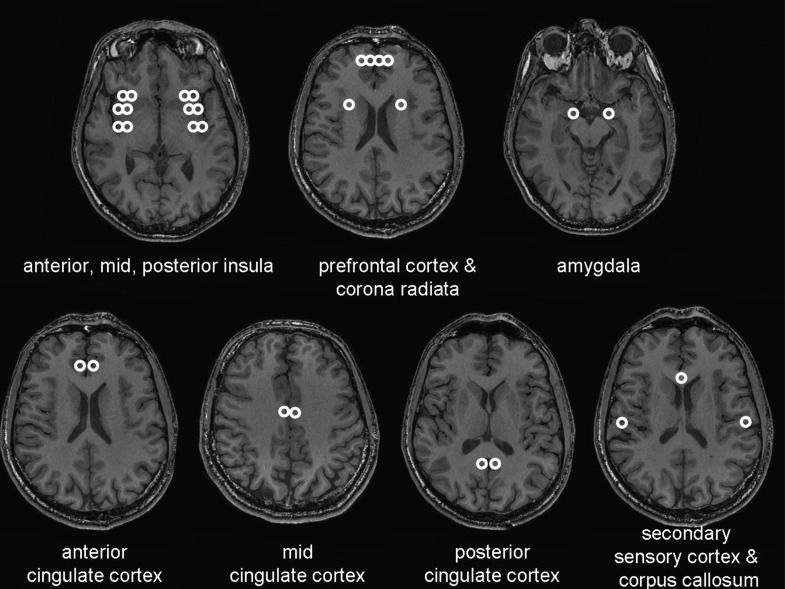
The ADC and FA values were examined in different gray and white matter areas of the brain, and the analysis was done by the same person (L.W.A.). Files were renamed and personal and clinical data hidden to make the analysis blind to the investigator who analyzed the data. The regions of interest (ROIs) are illustrated in Fig. 1 and were as follows: white matter in relation to 1) anterior, mid-, and posterior insula; 2) prefrontal cortex; 3) corpus callosum; and 4) corona radiata and gray matter of 5) amygdala; 6) cingulate cortex (anterior, mid-, and posterior, separately); 7) anterior, mid-, and posterior insula; 8) prefrontal cortex; and 9) secondary sensory cortex (SII). DTI parameters were retrieved from bilateral corresponding (i.e., left and right) brain areas separately, except for corpus callosum.
Figure 1.
Anatomical magnetic resonance images illustrating the analyzed areas involved in the visceral sensory processing and in corpus callosum. White matter substance was analyzed in the anterior, mid-, and posterior insula; prefrontal cortex; and corona radiata. Gray matter substance was analyzed in the amygdala; cingulate cortex (anterior, mid-, and posterior separately); anterior, mid-, and posterior insula; prefrontal cortex; and SII.
The anatomical and DTI data were imported into NordicICE, and the DTI module was used for computing the FA and ADC maps. The FA and ADC maps were coregistered yielding a map where anatomical structures could be identified. Each ROI was identified using a standardized procedure: first, the area was found and drawn on the coregistered ADC and FA map. Second, the retrieved location was checked by viewing the anatomical scan, thus securing the highest accuracy in placing the ROI in the correct anatomical area. The ROI position was then saved for later retrieval. This procedure was repeated for all areas, which were then used to extract appropriate mean values of the individual ROIs from the FA and ADC maps.
Statistics
The data were all normally distributed and had equal variance, and results are expressed as means ± SD. The difference in age and sex distribution between patients and control subjects was analyzed using t test or Fisher exact test as appropriate. For analysis of differences in ADC and FA values, a multivariate ANOVA (MANOVA) was applied with the subject groups (patient vs. control) as fixed factor, the right versus left side as cofactor, and the ADC/FA values of the ROI locations as dependent variables. Correlations between clinical parameters in patients and the ADC/FA values of the ROIs showing a difference between groups were analyzed using Spearman rank correlation test. Correlation analysis in healthy control subjects was not possible, since a high proportion of the subjects had no symptoms at all. ROIs and clinical parameters were selected a priori to limit the likelihood of type II errors. The effect of the presence of gastroparesis, retinopathy, peripheral neuropathy, autonomic neuropathy (noncontinuous variables), and type 1 versus type 2 DM on ADC/FA values of selected ROIs was analyzed using one-way ANOVA. P values <0.05 were considered significant. SPSS Statistics 17.0 (SPSS, Chicago, IL) was used for the analysis.
RESULTS
Age and sex were comparable between groups (age: t = 0.665, P = 0.51; sex: P = 0.62). Clinical characteristics of the patients are displayed in Table 1. All healthy control subjects had a normal structural MRI of the brain. The patients had no white matter lesions or ischemic lesions located in or near the ROIs of the FA and ADC measurements used below.
ADC measurements
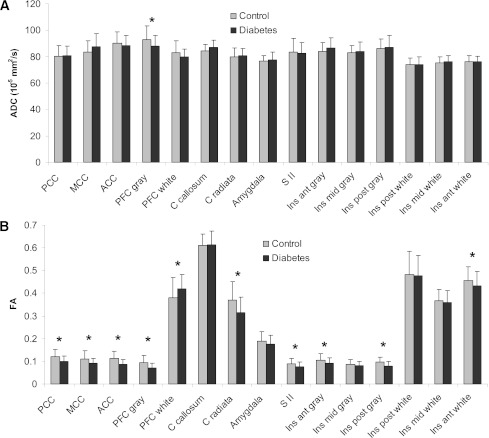
The mean ADC values of all ROIs are shown in Fig. 2A. Overall, no difference in ADC values was found between the patients and control subjects (F = 1.3, P = 0.26). The subsequent univariate tests revealed that the patients had decreased ADC in the prefrontal cortex gray matter (F = 6.5, P = 0.014), while no differences between the two groups were found in other regions (all P values>0.05).
Figure 2.
Degree of water diffusivity (A), expressed as ADC, and degree of fiber organization (B), expressed as FA, in gray and white matter ROIs in DM patients and healthy subjects. The regions are known to be involved in the processing of visceral pain. *P values <0.05. Data are illustrated as means ± SD. ACC, anterior (ant) cingulated cortex; Ins, insula; MCC, mid-cingulate cortex; PCC, posterior (post) cingulated cortex.
FA measurements
The mean FA values of all ROIs are given in Fig. 2B. Overall, the patients had reduced FA values compared with the control subjects (F = 2.0, P = 0.025). The subsequent univariate tests showed that the patients had decreased FA values, i.e., reduced microstructural tissue organization, compared with control subjects in 1) anterior (F = 20, P < 0.001), mid- (F = 10.7, P = 0.001) and posterior (F = 14, P < 0.001) cingulate cortex; 2) prefrontal cortex gray matter (F = 19, P < 0.001); 3) corona radiata (F = 10.9, P < 0.001); 4) SII (F = 7.4, P = 0.008); and 5) anterior white matter (F = 4.1, P = 0.045), anterior gray matter (F = 4.6, P = 0.035), and posterior gray matter (F = 9.9, P = 0.002) insula. The patients had increased FA values compared with control subjects in the prefrontal cortex white matter (F = 4.8, P = 0.032). No differences between the two groups were found in the other regions: corpus callosum, amygdala, mid-insula gray and white matter, and posterior insula white matter (all P values >0.05).
Association between microstructural brain morphometry and clinical data in DM patients
All ROIs showing a difference between DM patients and healthy control subjects were included in the analysis. The following parameters were a priori considered for inclusion in the correlation analysis (see Table 1): 1) DM duration, 2) GSCI scores (nausea/vomiting, postprandial fullness/early satiety, and bloating), 3) SF36 PCS and MCS, and 4) autonomic Holter monitoring data: SD from the mean R-R value (SDNN) as a net effect of the autonomic regulation on cardiovascular function (total variability), root mean square of the SD (RMSSD) as a measure of parasympathetic regulatory function, and percentage of heartbeat intervals differing by >50 ms from previous intervals (PNN50) as a measure of dysregulated sympatico-vagal balance with dominance of the parasympathetic regulatory function.
Cingulate cortex.
A negative association was found between FA and RMSSD in the anterior cingulate cortex (r = −0.32, P = 0.03); i.e., patients with fewer microstructural changes had the most autonomic dysregulation. No other correlations were found (all P > 0.05).
Prefrontal cortex.
A positive association between FA and SF-36 MCS score was found in the prefrontal cortex gray matter (r = 0.33, P = 0.03); i.e., patients with most microstructural changes had reduced mental well-being. No other correlations were found (all P > 0.05).
Corona radiata and SII.
A positive association between FA and SF-36 MCS score was found in corona radiata (r = 0.40, P = 0.008); i.e., patients with most microstructural changes had reduced mental well-being. No other correlations were found (all P > 0.05).
Insula.
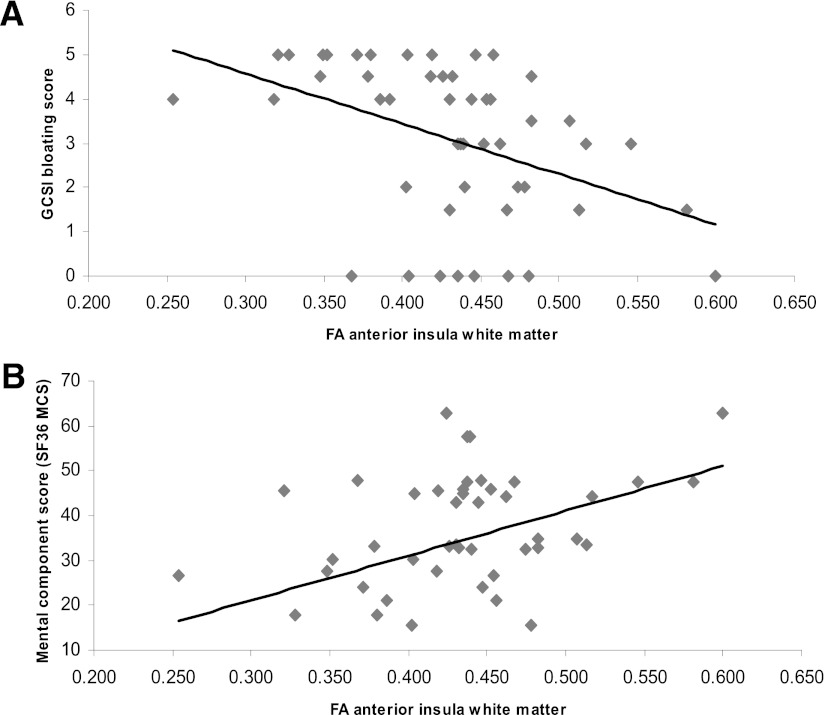
A negative association between FA and GCSI bloating was found in anterior insula white matter (r = −0.47, P = 0.001) (Fig. 3A); i.e., patients with the most microstructural changes had the most symptoms. A positive association between FA and SF-36 MCS score was found in anterior insula white matter (r = 0.31, P = 0.038) (Fig. 3B); i.e., patients with the most microstructural changes had reduced mental well-being. A positive association was found between FA and SDNN (r = 0.30, P = 0.04), RMSSD (r = 0.35, P = 0.02), and PNN50 (r = 0.30, P = 0.04) in the posterior insula gray matter; i.e., patients with most microstructural changes had the most pronounced autonomic dysregulation. A positive correlation between FA and DM duration was found in the anterior insula white matter (r = 0.32, P = 0.024). No other correlations were found (all P > 0.05).
Figure 3.
Correlation between anterior insula white matter microstructure (FA, describing fiber organization) and the bloating score (A) and SF36 MCS score (B) in patients with long-standing DM and gastrointestinal symptoms.
Presence of gastroparesis, retinopathy, peripheral and autonomic neuropathy, and DM type.
Patients with gastroparesis had reduced FA in the anterior insula white matter (mean with, 0.421 ± 0.063, and mean without, 0.445 ± 0.059; F = 6.5, P = 0.039). Patients with retinopathy had reduced FA in the prefrontal cortex gray matter (mean with, 0.066 ± 0.017, and mean without, 0.092 ± 0.025; F = 10, P = 0.007). Patients with peripheral neuropathy had increased FA in the anterior insula white matter (mean with, 0.444 ± 0.069, and mean without, 0.397 ± 0.049; F = 4.2, P = 0.022). Patients with type 2 DM had reduced FA in the posterior insula gray matter (mean type 1, 0.082 ± 0.020, and mean type 2, 0.065 ± 0.011; F = 4.5, P = 0.016), but otherwise no differences were seen between type 1 and 2 DM and data on patients were analyzed together. No other differences were found for the selected parameters.
CONCLUSIONS
In this explorative study, we found that patients with long-standing DM and gastrointestinal symptoms had microstructural changes in several gray and white matter areas known to be involved in visceral sensory processing. This was particularly evident as decreased fractional anisotropy (i.e., reduced fiber organization). The microstructural changes were associated with the presence of gastroparesis and associated with clinical scores such as bloating, reduced mental well-being, and autonomic dysfunction. This suggests that microstructural brain changes could be involved in the pathogenesis and persistence of gastrointestinal symptoms in DM patients.
Methodological considerations
DTI measures the magnitude (described as ADC) and directionality (described as FA) of water diffusion in tissues. The exact nature of the neurostructural changes responsible for increased ADC and reduced FA is not clear, but the most accepted hypothesis is that the integrity of the myelin sheath and axonal membrane is reflected by restriction of diffusion perpendicular to the fibers, whereas the integrity of intra-axonal structures (such as microtubules) is reflected by diffusion parallel to the fibers (22). Decreased FA is a common feature of several diseases associated with neuronal abnormalities, such as schizophrenia, depression, chronic alcohol use, Alzheimer disease, and, also, type 1 DM (4,22,23).
The selection of ROIs in this study was hypothesis driven and based on knowledge of visceral sensory processing (16,24), which was proven to be valid in our previous study of painful chronic pancreatitis (17). Hence, the selected brain areas are involved in the so-called sensory brain matrix including regions such as amygdala, prefrontal cortex, cingulate cortex, and insula that are strongly connected and receive projections from the thalamus and other limbic and subcortical structures, which are also central in the processing of visceral pain (25–32). Corpus callosum is outside the sensory matrix and was included as also in previous DTI studies of DM. It is very sensitive to DTI analysis owing to the dense fiber structure.
Microstructural brain changes in DM
Only few DTI studies have been conducted in DM patients. In type 1 DM, Kodl et al. (4) found reduced FA values in the posterior corona radiata and optic radiation but no significant changes in the corpus callosum. These findings were associated with the neurocognitive performance and DM duration. In a more recent study, they found reduced cortical thickness in areas with high connectivity to the posterior corona radiata and optic radiation, which indicates a relation between white matter tract and cortical pathology (5). In type 2 DM patients, Hsu et al. (6) found reduced mean diffusion in a global analysis and reduced FA in frontal white matter and higher mean diffusion in the cerebellum, temporal white matter, parahippocampal gyrus, fusiform gyrus, and cuneus. Association with the disease duration was seen in several regions. Furthermore, they found that the decrease in FA was related to transverse and not axial diffusivity, which suggests a process of demyelination more than axonal injury (6,33). This was supported by a recent study in rats with streptozotocin-induced DM where disarrangement of myelin sheaths and fragmentation of neurofilaments were detected (34). Hyperglycemia-induced oxidative stress is also known to cause oligodendrocyte death and subsequent demyelination (35). In obese type 2 DM patients, Yau et al. (7,8) also found reduced FA and increased ADC in several temporal, prefrontal, parietal, and cingulate regions.
Even though we did not evaluate the exact same regions as in the above studies, the reduction primarily in FA values (and not ADC) observed in the current study seems consistent with the previous studies. This FA deduction could indicate a demyelination process. Furthermore, the lack of microstructural changes in corpus callosum and reduced FA in corona radiata are identical to the finding by Kodl et al. (4). Also prefrontal and cingulate microstructural changes were observed by Yau et al. (7,8).
Visceral sensory changes in DM
Based on the above, it can be speculated whether the observed microstructural changes are due to generalized DM-induced brain changes or whether the changes are specifically involved in the process of a disordered visceral sensory system or a combination of both. In type 1 DM patients with gastrointestinal symptoms, we previously showed reduced sensitivity to esophageal and duodenal stimulations accompanied by an increase in the somatic referred pain areas indicating central neuronal changes (36). Peripheral neuropathy is likely involved in induction of structural changes at both spinal and brain levels resulting in a dysfunctional sensory system. Neurophysiological changes have been observed in type 1 DM patients with gastrointestinal symptoms associated with characteristics of the altered esophageal electrically induced brain potentials (15). Detailed analysis revealed a posterior shift of the electrical sources in the anterior cingulate cortex in DM patients and additional sources close to the posterior insula and in the medial frontal gyrus (16). Altogether, this indicates an altered central processing of visceral sensation in DM with reorganization and neuropathic-like changes of the visceral afferent system. This is of relevance for the development of future treatment strategies where drugs targeting the central neuropathic dysfunction may be effective. Furthermore, the neural damage and dysfunction in DM have been shown not to be irreversible but, rather, can be halted or reversed by improved metabolic function (9).
The microstructural changes observed in the current study further extend the neurophysiological evidence of changes in the visceral sensory system in patients with long-standing DM and gastrointestinal symptoms. The exact association between functional and microstructural changes cannot be established by the current study, but especially the changes observed in the cingulate cortex and insula correspond to the neurophysiological changes. This was further extended by the negative correlation between FA and bloating in the anterior insula white matter. Also, patients with gastroparesis had reduced FA in the anterior insula white matter, which is an interesting finding, since the insula is involved in both visceral sensory and motor integration. However, the nausea and vomiting scores did not correlate to the microstructural findings, which is likely because these symptoms are mainly controlled by central structures in area postrema rather than visceral nerves. The microstructural changes observed in this study were more consistently in the anterior compared with other parts of insula. These findings are consistent with previous studies where visceral structures were more extensively represented in the anterior insula (37).
The observed association in several brain areas between disordered microstructure and the SF36 MCS could be due to a generalized effect of long-standing DM on the brain, which is also manifested as a reduction of general mental well-being and may not solely be related to dysfunction of the visceral sensory system.
The association between autonomic dysfunction and microstructural changes in the insula and cingulate cortex is consistent with the fact that both autonomic regulation and visceral pain/sensation are processed in similar brain areas (38,39). The conflict between anterior cingulate cortex and posterior insula in the current study (negative versus positive correlation between FA and autonomic parameters) could likely be explained by the different regulatory roles of these two brain areas. In support of this, electrical stimulation of the right insular cortex in animals and humans increases blood pressure and heart rate, whereas stimulation of the cingulate gyrus decreased heart rate and blood pressure (38).
Although the different ROIs and clinical parameters were preselected, we compared several structures with the clinical data. Hence, the validity of some of the correlations such as the positive correlation between FA and DM duration/peripheral neuropathy was difficult to explain from a pathophysiological point of view and should be explored in future studies. Furthermore, additional groups of DM patients without gastrointestinal symptoms as well as alimentary habits and other data relevant for gastrointestinal function should be included in future studies to further evaluate the detailed pathophysiological mechanisms behind gastrointestinal symptoms.
In conclusion, patients with long-standing DM and gastrointestinal symptoms have microstructural changes in brain areas involved in visceral sensory processing. Even though the observed microstructural changes could partly be related to generalized DM-induced brain changes, some of the changes could be of functional significance, as they are associated with clinical gastrointestinal symptoms, autonomic parameters, and mental well-being and are consistent with previous electrophysiological studies. The findings may contribute to our understanding of the pathophysiology underlying gastrointestinal symptoms in patients with long-standing DM.
Acknowledgments
The research leading to these results was partly funded by the European Community’s Seventh Framework Programme FP7/2007-2013 under grant 223630. The Karen Elise Jensen Foundation and the Obelske Family Foundation cofunded this project.
No potential conflicts of interest relevant to this article were reported.
J.B.F. performed the investigations, designed the study, analyzed data, and wrote the manuscript. L.W.A. and Y.Y. analyzed data. C.B. and H.G. designed the study. M.S., E.S., and G.D. recruited patients. M.L. performed the investigations. A.M.D. designed the study and wrote the manuscript. J.B.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were presented in abstract form at the 48th Annual Meeting of the European Association for the Study of Diabetes, Berlin, Germany, 1–5 October 2012, and at the Joint International Neurogastroenterology and Motility Meeting, Bologna, Spain, 6–8 September 2012.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 2004;27:1047–1053 [DOI] [PubMed] [Google Scholar]
- 2.Horowitz M, Samsom M, Ed. Gastrointestinal Function in Diabetes Mellitus Chichester, U.K., John Wiley & Sons Ltd., 2004 [Google Scholar]
- 3.Selvarajah D, Wilkinson ID, Davies J, Gandhi R, Tesfaye S. Central nervous system involvement in diabetic neuropathy. Curr Diab Rep 2011;11:310–322 [DOI] [PubMed] [Google Scholar]
- 4.Kodl CT, Franc DT, Rao JP, et al. Diffusion tensor imaging identifies deficits in white matter microstructure in subjects with type 1 diabetes that correlate with reduced neurocognitive function. Diabetes 2008;57:3083–3089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Franc DT, Kodl CT, Mueller BA, Muetzel RL, Lim KO, Seaquist ER. High connectivity between reduced cortical thickness and disrupted white matter tracts in long-standing type 1 diabetes. Diabetes 2011;60:315–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu JL, Chen YL, Leu JG, et al. Microstructural white matter abnormalities in type 2 diabetes mellitus: a diffusion tensor imaging study. Neuroimage 2012;59:1098–1105 [DOI] [PubMed] [Google Scholar]
- 7.Yau PL, Javier DC, Ryan CM, et al. Preliminary evidence for brain complications in obese adolescents with type 2 diabetes mellitus. Diabetologia 2010;53:2298–2306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yau PL, Javier D, Tsui W, et al. Emotional and neutral declarative memory impairments and associated white matter microstructural abnormalities in adults with type 2 diabetes. Psychiatry Res 2009;174:223–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorina P, Vezzulli P, Bassi R, et al. Near normalization of metabolic and functional features of the central nervous system in type 1 diabetic patients with end-stage renal disease after kidney-pancreas transplantation. Diabetes Care 2012;35:367–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with type 2 diabetes mellitus. Diabet Med 1999;16:670–674 [DOI] [PubMed] [Google Scholar]
- 11.Spångéus A, El-Salhy M, Suhr O, Eriksson J, Lithner F. Prevalence of gastrointestinal symptoms in young and middle-aged diabetic patients. Scand J Gastroenterol 1999;34:1196–1202 [DOI] [PubMed] [Google Scholar]
- 12.Talley NJ, Young L, Bytzer P, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol 2001;96:71–76 [DOI] [PubMed] [Google Scholar]
- 13.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care 2003;26:1553–1579 [DOI] [PubMed] [Google Scholar]
- 14.Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care 2001;24:371–381 [DOI] [PubMed] [Google Scholar]
- 15.Frøkjaer JB, Søfteland E, Graversen C, et al. Central processing of gut pain in diabetic patients with gastrointestinal symptoms. Diabetes Care 2009;32:1274–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frøkjær JB, Egsgaard LL, Graversen C, et al. Gastrointestinal symptoms in type-1 diabetes: is it all about brain plasticity? Eur J Pain 2011;15:249–257 [DOI] [PubMed] [Google Scholar]
- 17.Frøkjær JB, Olesen SS, Gram M, et al. Altered brain microstructure assessed by diffusion tensor imaging in patients with chronic pancreatitis. Gut 2011;60:1554–1562 [DOI] [PubMed] [Google Scholar]
- 18.Takase B, Kitamura H, Noritake M, et al. Assessment of diabetic autonomic neuropathy using twenty-four-hour spectral analysis of heart rate variability: a comparison with the findings of the Ewing battery. Jpn Heart J 2002;43:127–135 [DOI] [PubMed] [Google Scholar]
- 19.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res 2004;13:1737–1749 [DOI] [PubMed] [Google Scholar]
- 20.Revicki DA, Rentz AM, Dubois D, et al. Gastroparesis Cardinal Symptom Index (GCSI): development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual Life Res 2004;13:833–844 [DOI] [PubMed] [Google Scholar]
- 21.Speight J, Reaney MD, Barnard KD. Not all roads lead to Rome-a review of quality of life measurement in adults with diabetes. Diabet Med 2009;26:315–327 [DOI] [PubMed] [Google Scholar]
- 22.Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology 2007;245:367–384 [DOI] [PubMed] [Google Scholar]
- 23.Cullen KR, Klimes-Dougan B, Muetzel R, et al. Altered white matter microstructure in adolescents with major depression: a preliminary study. J Am Acad Child Adolesc Psychiatry 2010;49:173–183, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aziz Q, Schnitzler A, Enck P. Functional neuroimaging of visceral sensation. J Clin Neurophysiol 2000;17:604–612 [DOI] [PubMed] [Google Scholar]
- 25.Drewes AM, Dimcevski G, Sami SA, et al. The “human visceral homunculus” to pain evoked in the oesophagus, stomach, duodenum and sigmoid colon. Exp Brain Res 2006;174:443–452 [DOI] [PubMed] [Google Scholar]
- 26.Hobson AR, Furlong PL, Worthen SF, et al. Real-time imaging of human cortical activity evoked by painful esophageal stimulation. Gastroenterology 2005;128:610–619 [DOI] [PubMed] [Google Scholar]
- 27.Drewes AM, Sami SA, Dimcevski G, et al. Cerebral processing of painful oesophageal stimulation: a study based on independent component analysis of the EEG. Gut 2006;55:619–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Derbyshire SW. Visceral afferent pathways and functional brain imaging. ScientificWorldJournal 2003;3:1065–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunckley P, Wise RG, Aziz Q, et al. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience 2005;133:533–542 [DOI] [PubMed] [Google Scholar]
- 30.Schreckenberger M, Siessmeier T, Viertmann A, et al. The unpleasantness of tonic pain is encoded by the insular cortex. Neurology 2005;64:1175–1183 [DOI] [PubMed] [Google Scholar]
- 31.Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 1997;14:2–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer EA, Aziz Q, Coen S, et al. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterol Motil 2009;21:579–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage 2003;20:1714–1722 [DOI] [PubMed] [Google Scholar]
- 34.Hernández-Fonseca JP, Rincón J, Pedreañez A, et al. Structural and ultrastructural analysis of cerebral cortex, cerebellum, and hypothalamus from diabetic rats. Exp Diabetes Res 2009;2009:329632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith KJ, Kapoor R, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol 1999;9:69–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frøkjaer JB, Andersen SD, Ejskaer N, et al. Gut sensations in diabetic autonomic neuropathy. Pain 2007;131:320–329 [DOI] [PubMed] [Google Scholar]
- 37.Ostrowsky K, Isnard J, Ryvlin P, Guénot M, Fischer C, Mauguière F. Functional mapping of the insular cortex: clinical implication in temporal lobe epilepsy. Epilepsia 2000;41:681–686 [DOI] [PubMed] [Google Scholar]
- 38.Jones SE. Imaging for autonomic dysfunction. Cleve Clin J Med 2011;78(Suppl. 1):S69–S74 [DOI] [PubMed] [Google Scholar]
- 39.Gianaros PJ, Sheu LK. A review of neuroimaging studies of stressor-evoked blood pressure reactivity: emerging evidence for a brain-body pathway to coronary heart disease risk. Neuroimage 2009;47:922–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stotzer PO, Fjälling M, Grétarsdóttir J, Abrahamsson H. Assessment of gastric emptying: comparison of solid scintigraphic emptying and emptying of radiopaque markers in patients and healthy subjects. Dig Dis Sci 1999;44:729–734 [DOI] [PubMed] [Google Scholar]