Abstract
OBJECTIVE
To examine the association between self-reported physical activity (PA) and diabetes-related quantitative traits.
RESEARCH DESIGN AND METHODS
The observational cohort was 1,152 Mexican American adults with dual-energy X-ray absorptiometry, oral and intravenous glucose tolerance tests, and self-reported dietary and PA questionnaires. PA was categorized into three mutually exclusive groups according to the U.S. Department of Health and Human Services PA guidelines for Americans: low (vigorous <75 min/week and moderate <150 min/week), moderate (vigorous ≥75 min/week or moderate ≥150 min/week), and high (vigorous ≥75 min/week and moderate ≥150 min/week). Trends in PA groups were tested for association with metabolic traits in a cross-sectional analysis.
RESULTS
The participants’ mean age was 35 years (range, 18–66 years), mean BMI was 29.6 kg/m2, and 73% were female. Among them, 501 (43%), 448 (39%), and 203 (18%) were classified as having low, moderate, and high PA, respectively. After adjustment for age, a higher PA was significantly associated with lower 2-h glucose, fasting insulin, and 2-h insulin and greater β-cell function (P = 0.001, 0.0003, 0.0001, and 0.004, respectively). The association did not differ significantly by sex. Results were similar after further adjustment for age, sex, BMI, or percent body fat.
CONCLUSIONS
An increasing level of PA is associated with a better glucose and insulin profile and enhanced β-cell function that is not explained by differences in BMI or percent body fat. Our results suggest that PA can be beneficial to β-cell function and glucose regulation independent of obesity.
Clinical trials have demonstrated that lifestyle interventions that include physical activity (PA) can reduce the risk of type 2 diabetes in high-risk individuals (1,2). PA may directly improve insulin sensitivity by enhancing glucose uptake in muscle and the liver (3–5) and may also help restore whole-body glucose disposal, especially nonoxidative glucose disposal (6). Moreover, exercise training has been shown to indirectly augment insulin sensitivity by reducing total body fat as well as visceral fat (7–9).
Intensive exercise training has been shown to improve insulin sensitivity (3–6,9) and β-cell function (10,11). However, the relationship between less intensive exercise training and β-cell function is controversial. Some studies have shown that short-term, moderate aerobic exercise may improve β-cell function in overweight adults (11,12), whereas others reported no significant change in β-cell function after moderate exercise (13). Little is known about the relationship between PA and β-cell function under free-living conditions without the addition of a specific exercise intervention. In this report, we examine this relationship using data from the BetaGene study, a family-based observational study of obesity, insulin resistance, and β-cell function in Mexican Americans.
RESEARCH DESIGN AND METHODS
Study participants
BetaGene participants are Mexican-American adults (both parents and three or more grandparents are Mexican or of Mexican descent) who are 1) women who had gestational diabetes mellitus (GDM) within the previous 5 years, 2) siblings or cousins of those with a history of GDM, or 3) women with normal glucose levels during pregnancy in the past 5 years. Women documented with and without previous GDM were identified from the Los Angeles County/University of Southern California Medical Center, Kaiser Permanente Southern California’s delivery population, and obstetrical/gynecological clinics at local Southern California hospitals. Women without previous GDM were frequency-matched to GDM cases by age, BMI, and parity. Details regarding recruitment have been previously described (14). All protocols for BetaGene were approved by the institutional review boards of participating institutions, and all participants provided written informed consent before participation.
Testing procedures and assays
Research data were collected in two separate visits to the General Clinical Research Center at the University of Southern California. The first visit consisted of a physical examination, dietary and PA questionnaires, a 2-h, 75-g oral glucose tolerance test (OGTT), and fasting blood for lipid measurements (15). Participants with fasting glucose <7.0 mmol/L were invited for a second visit, which consisted of a dual-energy X-ray absorptiometry scan for body composition and an insulin-modified intravenous glucose tolerance test (ivGTT) for measurement of insulin sensitivity and β-cell function (16). Plasma glucose was measured on an autoanalyzer using the glucose oxidase method (YSI model 2300; Yellow Springs Instruments, Yellow Springs, OH). Insulin was measured by a two-site immunoenzymometric assay (TOSOH Biosciences, San Francisco, CA) that has <0.1% cross-reactivity with proinsulin and intermediate split products.
PA and dietary assessment
Trained bilingual (English/Spanish) interviewers administered both PA and dietary questionnaires. The amount and intensity of PA was assessed by questionnaires developed in the Hawaii-Los Angeles Multiethnic Cohort Study (17,18). This questionnaire is comprised of a list of structured questions describing various types of activity (sitting, strenuous sports, vigorous work, and moderate activities including sports and work) during the past year. Responses were then used to estimate the total minutes of moderate and vigorous activity per week. The questionnaire was validated against doubly labeled water and showed a correlation coefficient of 0.3 between hours of total activity and total energy expenditure (unpublished data). The U.S. Department of Health and Human Services (DHHS) recommends at least 75 min/week of vigorous or 150 min/week of moderate activity for Americans (19). Individuals were categorized into three mutually exclusive groups according to how their reported PA matched these DHHS guidelines: low, <75 min/week of vigorous and <150 min/week of moderate activity; moderate, either ≥75 min/week of vigorous or ≥150 min/week of moderate activity; high, reported both ≥75 min/week of vigorous and ≥150 min/week of moderate activity (20). Dietary intake was assessed using the 126-item semiquantitative Harvard food-frequency questionnaire (21). The food-frequency questionnaire consisted of a list of foods with a standardized serving size and a selection of nine frequency categories ranging from never or less than one serving per month to more than six servings per day, during the past year. An open-ended free text section was used to capture food items that did not appear on the standard list and included information on usual serving size and number of servings consumed per week for incorporation in the dietary intake calculation for each subject. Total caloric and nutrient intakes were calculated by the Harvard Channing Laboratory.
Data analysis
Insulin response to oral glucose (∆30-min insulin) was computed as the 30-min OGTT insulin concentration minus the fasting insulin concentration. Total and incremental (above basal) areas under OGTT glucose and insulin curves were calculated by trapezoid method. Insulin sensitivity (SI), glucose effectiveness (SG), and the incremental insulin response to glucose (AIRg) during the first 10 min of the ivGTT were determined using the Millenium version of the Bergman minimal model (22). The disposition index (DI), a measure of β-cell function, was computed as the product of AIRg and SI. It is a measure of β-cell compensation for the degree of insulin resistance.
Characteristics of the study cohort were presented by means, medians, and interquartile ranges. Fasting insulin, postchallenge insulin, insulin response, SI, DI, SG, and total calories were log transformed to approximate normal distribution prior to analysis. Geometric means were presented for these variables, and the associated standard errors were calculated by the Delta method (23). The relationship between diabetes-related metabolic measures and PA was assessed by testing trend association between the metabolic measures and levels of PA in categories (low, moderate, and high) using generalized estimating equations to account for correlations among related individuals within families. Age was included as a covariate to adjust for potential confounding. The impact of sex on the associations was assessed by including sex as a covariate and testing for significant interaction between PA group and sex in the model. If no significant interaction was detected, analyses were conducted using the entire sample with adjustment for age and sex. The impact of body composition on the association was assessed through covariate adjustment for BMI or percent body fat and/or waist-to-hip ratio. Potential confounding by diet was evaluated by the adjustment for total caloric intake. All statistical tests were two sided. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for data analysis.
RESULTS
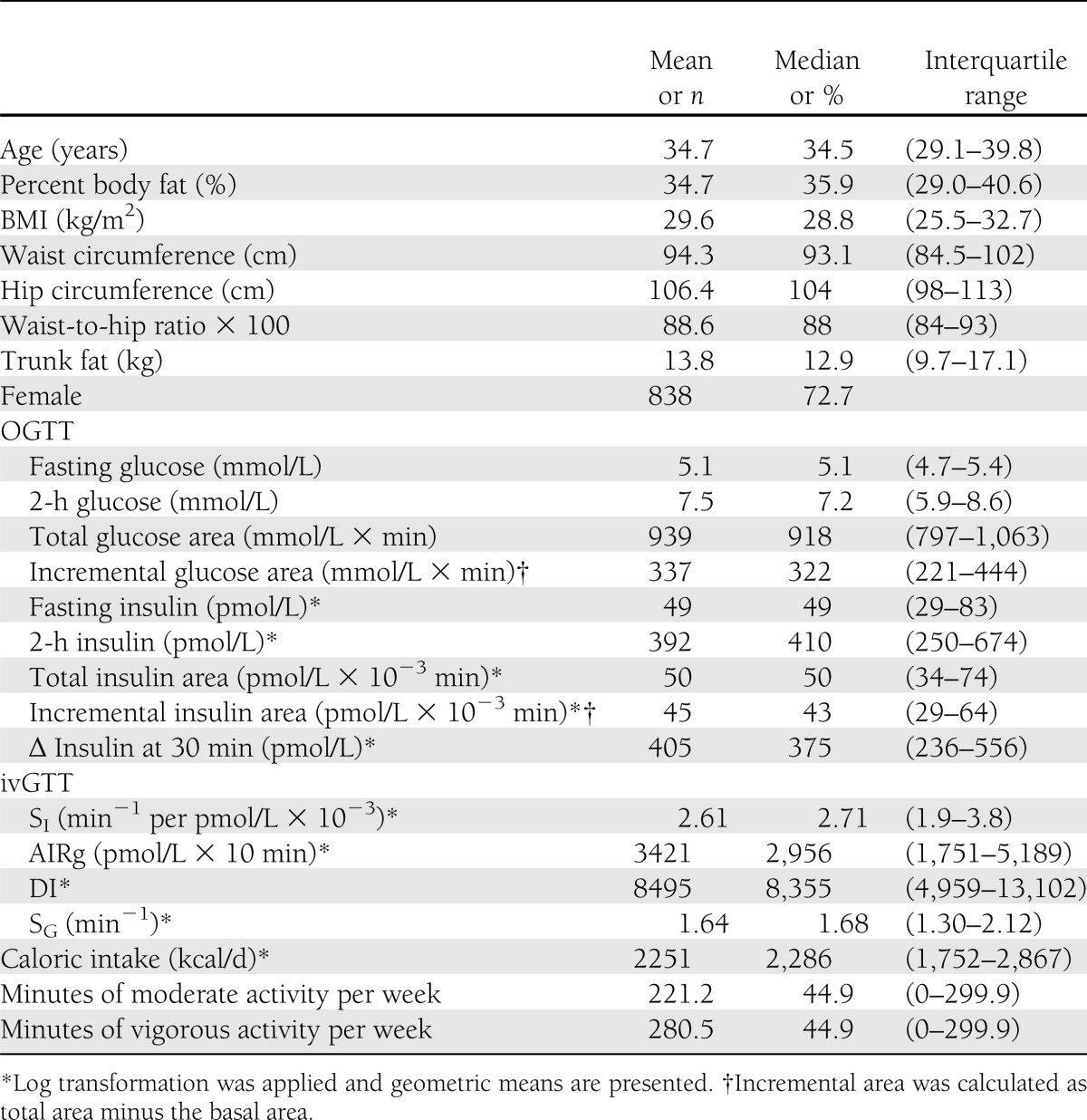
A total of 1,250 participants were recruited into the BetaGene study with completed ivGTTs; of these, 1,152 completed OGTTs and PA questionnaires. Characteristics of the 1,152 participants included in this report are shown in Table 1. The mean age was 34.7 years (range, 17.9–65.6 years), mean BMI was 29.6 kg/m2, mean percent body fat was 34.7%, and 72.3% of the cohort were female. Normal glucose tolerance was present in 707 (61.4%), impaired glucose tolerance in 361 (31.3%), and diabetes by 2-h glucose ≥11.1 mmol/L (24) in 84 (7.3%) participants, respectively. Among females, 29.3% had a history of GDM. The median durations for vigorous and moderate activity were both 45 min/week, considerably below the DHHS recommendation of 150 min/week of moderate activity or 75 min/week of vigorous activity for chronic disease prevention (19).
Table 1.
Cohort characteristics (n = 1,152)
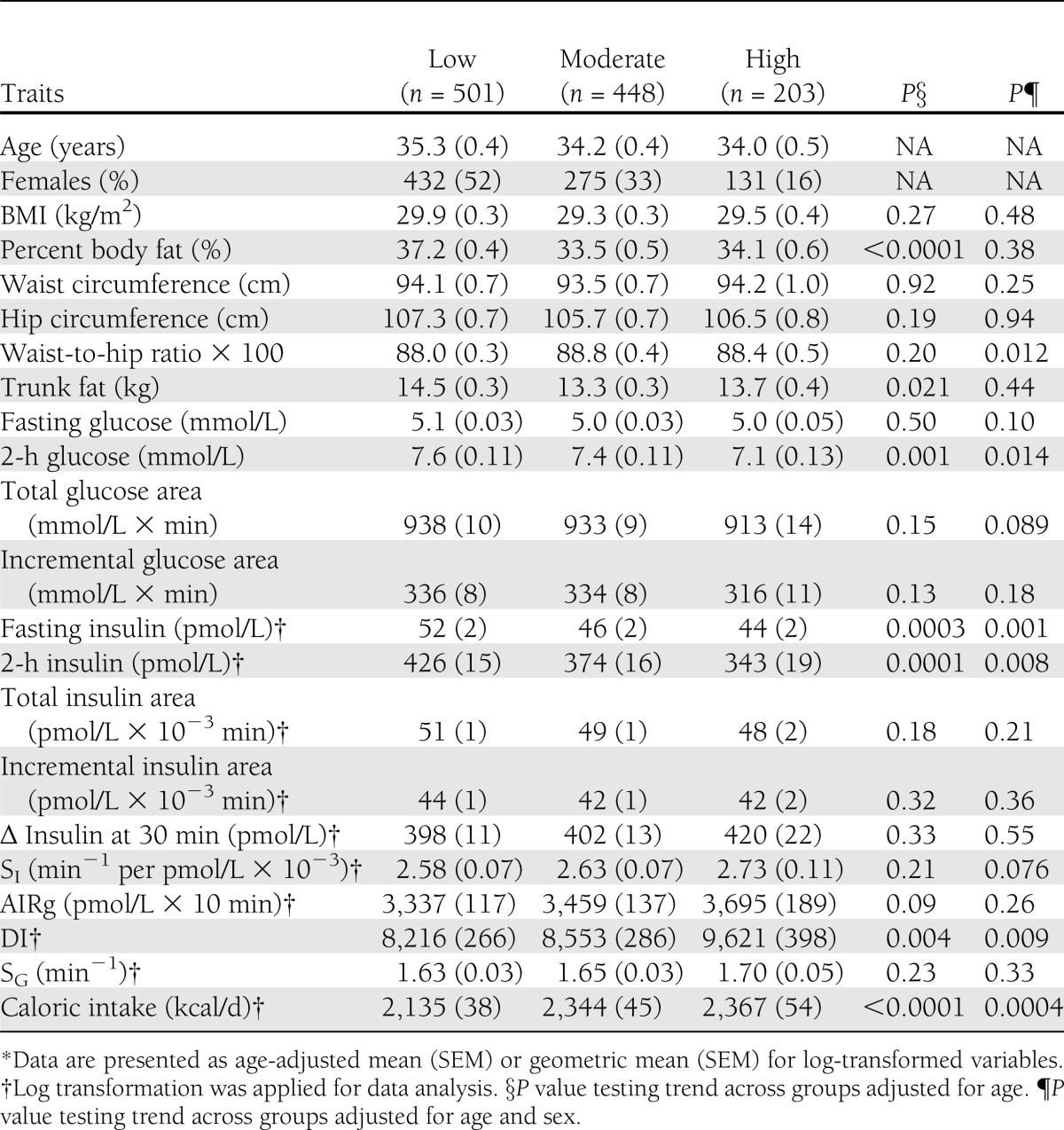
Of the study participants, 501 (43%) were classified as low in PA, 448 (39%) as moderate, and 203 (18%) as high. The higher activity groups were significantly younger (mean age = 35.3, 34.2, 34.0 years in the low, moderate, and high groups, respectively; P = 0.012 for trend). The distribution of females versus males in the three PA groups was significantly different (P < 0.001) and showed a pattern that indicated lower PA among females: low = 52 vs. 22% for females vs. males, moderate = 33 vs. 55%, and high = 16 vs. 23%, respectively.
Table 2 presents the comparison of the age-adjusted means for the metabolic measures among the three PA groups. Although no significant association was observed between PA and BMI (P = 0.28), increasing PA was significantly associated with decreasing percent body fat (P < 0.0001), 2-h glucose (P = 0.001), fasting insulin (P = 0.0003), and 2-h insulin (P = 0.0001) and increasing β-cell function (P = 0.004). An increasing level of PA was marginally associated with an increasing level of AIRg (P = 0.09). The associations between PA and diabetes-related traits appeared to be similar between males and females (interaction test P > 0.28 for each trait after including sex in the model, details by sex analyses) (Supplementary Tables 1 and 2) except OGTT fasting glucose (P = 0.037). Age-adjusted fasting glucose decreased with increasing PA in women (mean ± SEM for low = 5.1 ± 0.04 mmol/L, moderate = 5.0 ± 0.04 mmol/L, and high = 4.9 ± 0.06 mmol/L; P = 0.013) but not in men (low = 5.2 ± 0.08 mmol/L, moderate = 5.1 ± 0.05 mmol/L, and high = 5.2 ± 0.06 mmol/L; P = 0.35). After further adjustment for sex, the association between PA and percent body fat was no longer significant (P = 0.38) (Table 2). The significant associations for 2-h glucose, fasting, and 2-h insulin and DI observed in the age-adjusted analyses remained after further adjustment for sex (Table 2). An increasing level of PA was significantly associated with a lower waist-to-hip ratio (P = 0.012) and marginally associated with decreasing fasting glucose (P = 0.10) and increasing SI (P = 0.076) after adjustment for age and sex.
Table 2.
Comparison of metabolic traits across the three PA groups*
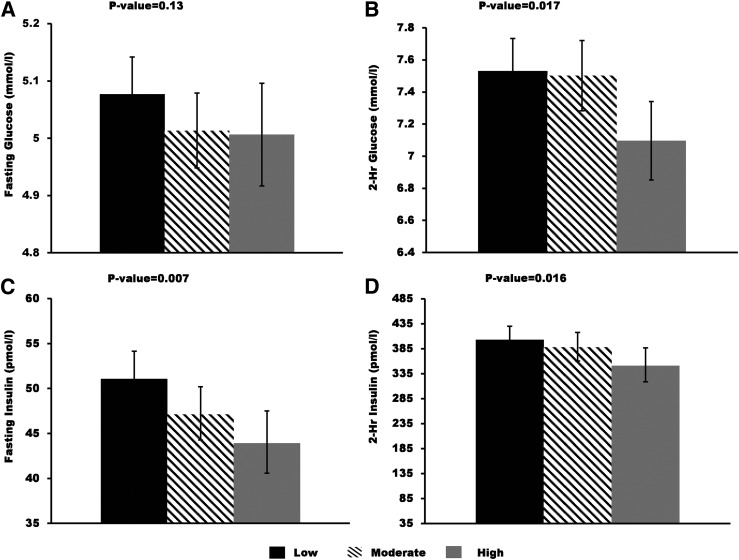
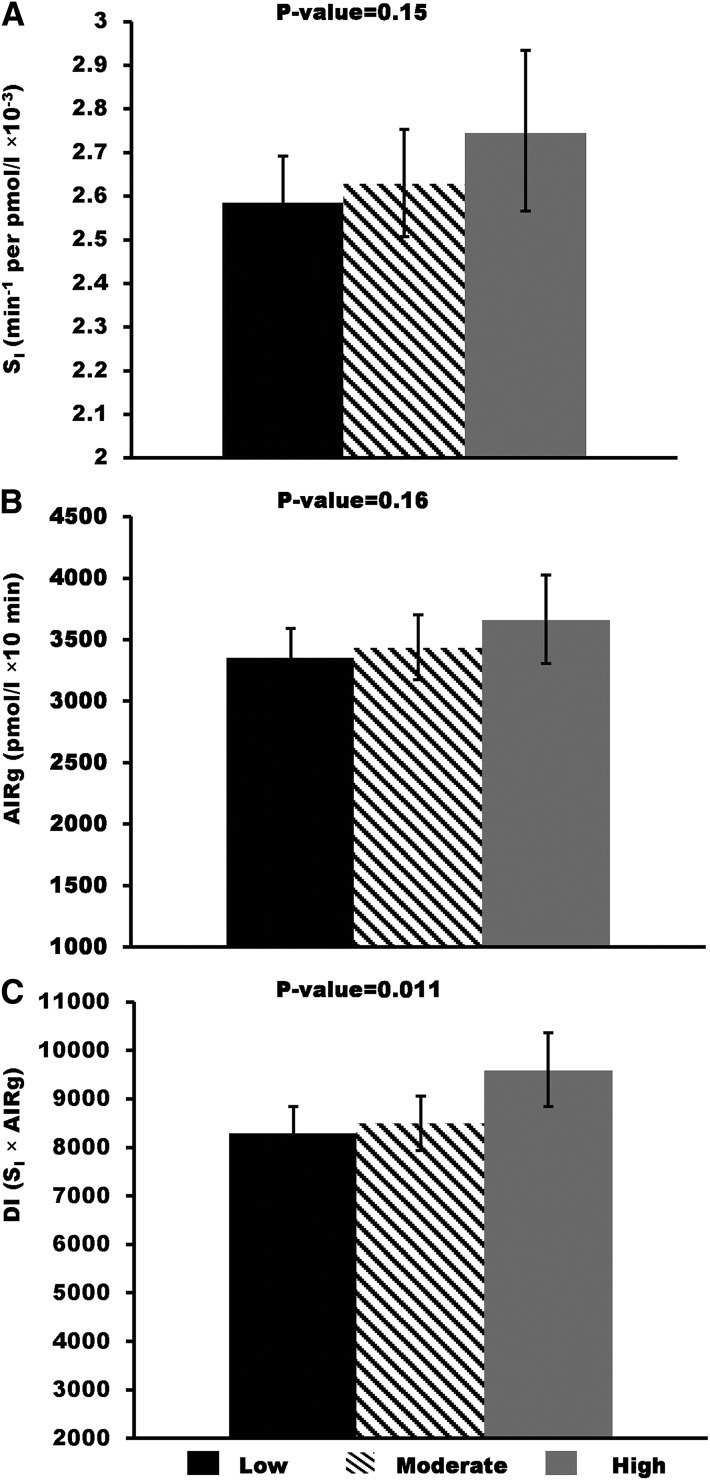
We assessed the relative contribution of body fat to the observed associations between PA and diabetes-related traits by additionally adjusting for percent body fat, BMI, or waist-to-hip ratio. Age-, sex-, and percent body fat–adjusted means for OGTT fasting and 2-h glucose and insulin are shown in Fig. 1. Analogously, adjusted means for SI, AIRg, and DI are shown in Fig. 2. Associations between PA and 2-h glucose, fasting insulin, 2-h insulin, and DI remained significant after further adjusting for percent body fat, and the regression coefficients were only slightly reduced (<15% change). By contrast, adjustment for body fat reduced the association between PA and SI by 26% and increased the association with AIRg by 27%, although these associations remained insignificant (Fig. 2). Results were similar when models were adjusted for BMI or waist-to-hip ratio instead of percent body fat (data not shown). Including waist-to-hip ratio in addition to percent body fat or BMI in the model adjustment did not change the conclusion (data not shown).
Figure 1.
Age-, sex-, and percent body fat–adjusted means and 95% CIs for OGTT fasting and 2-h glucose and insulin by the three PA groups: low, moderate, and high. Geometric means were presented for fasting and 2-h insulin. P values were from the trend association between PA groups and each of the metabolic traits.
Figure 2.
Age-, sex-, and percent body fat–adjusted means and 95% CIs for insulin sensitivity (SI), acute insulin secretion (AIRg), and β-cell compensation for insulin resistance (DI) by the three PA groups: low, moderate, and high. Geometric means were presented for all three traits. P values were from the trend association between PA groups and each of the metabolic traits.
Because higher PA level was typically accompanied by higher caloric intake, we further adjusted for caloric intake. Caloric intake did not significantly alter the age- and sex-adjusted associations between PA and any diabetes-related traits. PA was not significantly associated with BMI (P = 0.66) or percent body fat (P = 0.54) after further adjustment for caloric intake. Further adjustment for caloric intake had little impact on the age-, sex-, and percent body fat–adjusted associations between PA and fasting glucose, 2-h glucose, fasting insulin, 2-h insulin, AIRg, SI, and DI; the additional adjustment resulted in <10% change in the corresponding regression coefficients, with adjusted P values of 0.098, 0.017, 0.0003, 0.016, 0.21, 0.16, and 0.019, respectively.
CONCLUSIONS
In this study, we observed that an increasing level of self-reported PA was significantly associated with decreasing levels of fasting, 2-h insulin, and 2-h glucose and an increasing level of β-cell function assessed as the DI. These associations were not modified significantly by adjustment for percent body fat, BMI, waist-to-hip ratio, or caloric intake. A marginal association was also observed for increasing PA and better insulin sensitivity. These findings suggest that more PA, even in a free-living environment, is beneficial to β-cell function and glucose regulation.
The impact of exercise on glucose and insulin metabolism has been evaluated by several studies. Animal studies have demonstrated that exercise increases glucose uptake by stimulating GLUT4 translocation in muscle cells and increasing glucose uptake by the liver (5). Additionally, an exercise training study among type 2 diabetic patients revealed that exercise enhances the whole-body glucose disposal (6). Although the association between PA and insulin sensitivity did not make the statistical significance based on a P value <0.05 cut point in this cohort, there was a trend that subjects with higher PA levels had better insulin sensitivity (P = 0.076 after adjustment for age and sex). This attenuated relationship was consistent with the lack of association between PA and BMI or percent body fat and could be due to the fact that no exercise training and interventions were applied in this cohort. Thus, our results are consistent with the findings from exercise trainings and support the concept that more PA may contribute to lower OGTT glucose and insulin levels and, to a lesser significant extent, better insulin sensitivity.
The most novel finding in this study was the significant association between PA and β-cell function. Of note, >75% of the participants in this cohort were overweight or obese (25th percentile of BMI was 25.5 kg/m2). Two previous studies evaluated short-term exercise training and changes in β-cell function before and after training. One of the studies included 12 subjects >60 years of age (11). The result showed that moderate exercise training significantly improved insulin sensitivity and β-cell function. The other study included overweight adults and demonstrated that both moderate and vigorous exercise training improved insulin sensitivity and β-cell function, although the improvement of β-cell function was not statistically significant for vigorous activity (12). Although the biological mechanisms of the impact of PA on β-cell function have not been clarified, there has been evidence that exercises expanded β-cell mass by stimulating its proliferations and preventing its apoptosis (25,26). In these rat studies, exercises were shown to enhance the expression of insulin receptor substrate-2, which is crucial for β-cell growth and survival. The beneficial effect of exercises on β-cell function may also be the improvement of adipose tissue biology such as increasing adiponectin and reducing inflammation (27). Adiponectin has been shown to be an important biomarker for metabolic and cardiovascular diseases. Recently, we showed that declining adiponectin was significantly associated with β-cell function deterioration in a longitudinal study independent of weight gain (28). The mechanism for this association may promote β-cell function and survival by increasing ceramidase activity, decreasing intracellular ceramide levels, and increasing antiapoptotic metabolite sphingosine-1 phosphate levels (29,30). Taken together, the results from this report and previous reports suggest that greater amounts of daily living activity might protect β-cell function, even in overweight and obese individuals.
A cross-sectional study in Mexican children suggested that the impact of PA on β-cell function could be mediated by body fat (31). They found that the higher cardiorespiratory fitness, which reflects chronic PA behavior, was significantly correlated with β-cell function as well as insulin resistance. However, the significant correlations disappeared after adjustment for fat mass. We did not observe a significant association between percent body fat or BMI and self-reported PA groups after adjustment for age and sex. In addition, the adjustment for percent body fat and BMI did not significantly reduce the association between PA and β-cell function. The lack of association between PA and BMI or percent body fat in this adult cohort may be due to the fact that our cohort is primarily composed of women with a history of GDM and their family members, the majority of whom are overweight or obese and are presumably at higher than normal risk for diabetes. No exercise training/advice was offered to this cohort. We did not measure fitness levels, and PA was self-reported and included work-related activities such as moving heavy furniture, loading trucks, and gardening, as well as sports activities such as aerobics. Our finding was consistent with the results from several large studies with long-term follow-up, which showed that self-reported PA was associated with a lower incidence of diabetes independent of BMI (32).
In other previous studies, energy intake was shown to confound the effect of exercise on body weight or insulin resistance (33,34). However, in our analysis, the associations between β-cell function, glucose, insulin profiles, and PA were not significantly changed by the adjustment for caloric intake. Therefore, our results indicated a more direct contribution of PA in preserving β-cell function. Moreover, our findings are consistent with the results of a recent study in rats, which demonstrated that voluntary exercise was beneficial for sustaining β-cell compensation without preventing dyslipidemia or obesity (35). One possible mechanism for such an effect would be mitigation of the adverse metabolic effects of obesity. It is also possible that higher PA decreases lipotoxicity, thus improving β-cell survival. Since pancreatic biopsies cannot be performed, a surrogate measure would have been the computed axial tomography scan estimate of peritoneal fat or an ultrasound assessment of hepatic fat content. Detailed mechanisms remain to be investigated.
We acknowledge some limitations of our study. First, we used a self-reported PA questionnaire to collect PA information. Although the questionnaire has been used in previous studies in Mexican Americans (18), it is well known that self-reported PA tends to be overreported on questionnaires (36,37). We elected to categorize participants into three groups based on the U.S. DHHS recommendation for PA instead of using the minutes of PA as a continuous variable to reduce the impact of measurement errors. As evidence of potential overreporting in this cohort, 57% of participants reported meeting or exceeding the DHHS PA guideline, as compared with 36% for Mexican Americans in a national report of participation in aerobic activity (38). However, we also note that our questionnaire included work-related PAs, which might explain the higher than expected percentage. Second, we did not measure physical fitness, which is more objective and a better predictor than self-reported PA for many health outcomes, such as diabetes and cardiovascular diseases (39,40). Third, the cross-sectional and observational design of our study precludes us from examining the dynamic impact of PA on the change in metabolic traits.
The strength of the current study is the unique sample, which includes a large cohort of Mexican Americans with detailed OGTT- and ivGTT-based measures of glucose tolerance, insulin sensitivity, and β-cell function. Unlike other studies that mostly examined the short-term effect of exercise training/intervention on insulin secretion and insulin resistance with small sample sizes, we described the association with PA in a free-living environment to provide a more realistic model than the impact of a specific, short-term exercise intervention. We showed that increasing PA is associated with better β-cell function in a population without overt diabetes. This association is separate from the impact of obesity and energy intake. Our results suggest that an effect on diabetes prevention by lifestyle change (1,2) may be mediated by the improvement of β-cell function. Our findings have implications for a real-world approach to the delay, prevention, and/or early treatment of type 2 diabetes.
In conclusion, our study indicates that a greater level of PA might play a role in improving glucose tolerance and protecting β-cell function in Mexican Americans who are at high risk of developing diabetes. The beneficial impact of PA on β-cell function does not depend on BMI, percent body fat, and energy intakes. Our findings have important public health implications to prevent/slow down the deterioration of β-cell function that leads to type 2 diabetes.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants DK-061628, M01-RR-0043, and UL1-TR-000130, Clinical Research Grant 7-09-CT-09 from the American Diabetes Association Research Award, and by Kaiser Permanente Southern California Direct Community Benefit funds. The sponsors had no role in the conduct and design of the study, collection management and interpretation of the data, or preparation, review, and approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
Z.C., M.H.B., and A.H.X. researched data and wrote the article. R.M.W., J.M.L., and T.A.B. contributed to data collection, edited the article, and contributed to discussion. E.T. contributed to data collection. M.T. contributed to data analysis. All authors reviewed the manuscript. Z.C. and A.H.X. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
This work was presented at the 72nd Scientific Sessions of the American Diabetes Association, Philadelphia, Pennsylvania, 8–12 June 2012.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1485/-/DC1.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lindström J, Louheranta A, Mannelin M, et al. Finnish Diabetes Prevention Study Group The Finnish Diabetes Prevention Study (DPS): lifestyle intervention and 3-year results on diet and physical activity. Diabetes Care 2003;26:3230–3236 [DOI] [PubMed] [Google Scholar]
- 3.Hespel P, Vergauwen L, Vandenberghe K, Richter EA. Important role of insulin and flow in stimulating glucose uptake in contracting skeletal muscle. Diabetes 1995;44:210–215 [DOI] [PubMed] [Google Scholar]
- 4.Galassetti P, Coker RH, Lacy DB, Cherrington AD, Wasserman DH. Prior exercise increases net hepatic glucose uptake during a glucose load. Am J Physiol 1999;276:E1022–E1029 [DOI] [PubMed] [Google Scholar]
- 5.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care 2004;27:2518–2539 [DOI] [PubMed] [Google Scholar]
- 6.Yokoyama H, Mori K, Emoto M, et al. Non-oxidative glucose disposal is reduced in type 2 diabetes, but can be restored by aerobic exercise. Diabetes Obes Metab 2008;10:400–407 [DOI] [PubMed] [Google Scholar]
- 7.Després JP, Pouliot MC, Moorjani S, et al. Loss of abdominal fat and metabolic response to exercise training in obese women. Am J Physiol 1991;261:E159–E167 [DOI] [PubMed] [Google Scholar]
- 8.Borghouts LB, Keizer HA. Exercise and insulin sensitivity: a review. Int J Sports Med 2000;21:1–12 [DOI] [PubMed] [Google Scholar]
- 9.McTiernan A, Sorensen B, Irwin ML, et al. Exercise effect on weight and body fat in men and women. Obesity (Silver Spring) 2007;15:1496–1512 [DOI] [PubMed] [Google Scholar]
- 10.Dela F, von Linstow ME, Mikines KJ, Galbo H. Physical training may enhance beta-cell function in type 2 diabetes. Am J Physiol Endocrinol Metab 2004;287:E1024–E1031 [DOI] [PubMed] [Google Scholar]
- 11.Slentz CA, Tanner CJ, Bateman LA, et al. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care 2009;32:1807–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloem CJ, Chang AM. Short-term exercise improves beta-cell function and insulin resistance in older people with impaired glucose tolerance. J Clin Endocrinol Metab 2008;93:387–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr DB, Utzschneider KM, Boyko EJ, et al. A reduced-fat diet and aerobic exercise in Japanese Americans with impaired glucose tolerance decreases intra-abdominal fat and improves insulin sensitivity but not beta-cell function. Diabetes 2005;54:340–347 [DOI] [PubMed] [Google Scholar]
- 14.Watanabe RM, Allayee H, Xiang AH, et al. Transcription factor 7-like 2 (TCF7L2) is associated with gestational diabetes mellitus and interacts with adiposity to alter insulin secretion in Mexican Americans. Diabetes 2007;56:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Black MH, Fingerlin TE, Allayee H, et al. Evidence of interaction between PPARG2 and HNF4A contributing to variation in insulin sensitivity in Mexican Americans. Diabetes 2008;57:1048–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchanan TA, Xiang AH, Kjos SL, Trigo E, Lee WP, Peters RK. Antepartum predictors of the development of type 2 diabetes in Latino women 11-26 months after pregnancies complicated by gestational diabetes. Diabetes 1999;48:2430–2436 [DOI] [PubMed] [Google Scholar]
- 17.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nöthlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Body mass index and physical activity as risk factors for pancreatic cancer: the Multiethnic Cohort Study. Cancer Causes Control 2007;18:165–175 [DOI] [PubMed] [Google Scholar]
- 19.United States Department of Health and Human Services. Physical activity guidelines for Americans [Internet], 2008. Available from http://www.health.gov/PAGuidelines/guidelines/default.aspx Accessed 1 May 2011
- 20.Plotnikoff R, Johnson S, Loucaides C, Bauman A, Karunamuni N, Pickering M: Population-based estimates of physical activity for adults with type 2 diabetes: a cautionary tale of potential confounding by weight status. J Obes 2011;2011:pii:561432 [DOI] [PMC free article] [PubMed]
- 21.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 22.Boston RC, Stefanovski D, Moate PJ, Sumner AE, Watanabe RM, Bergman RN. MINMOD Millennium: a computer program to calculate glucose effectiveness and insulin sensitivity from the frequently sampled intravenous glucose tolerance test. Diabetes Technol Ther 2003;5:1003–1015 [DOI] [PubMed] [Google Scholar]
- 23.Rosner B. Fundamentals of Biostatistics. 5th ed. Pacific Grove, CA, Duxbury Press, 2000 [Google Scholar]
- 24.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl. 1):S5–S20 [DOI] [PubMed] [Google Scholar]
- 25.Park S, Hong SM, Lee JE, Sung SR. Exercise improves glucose homeostasis that has been impaired by a high-fat diet by potentiating pancreatic beta-cell function and mass through IRS2 in diabetic rats. J Appl Physiol 2007;103:1764–1771 [DOI] [PubMed] [Google Scholar]
- 26.Park S, Hong SM, Sung SR. Exendin-4 and exercise promotes beta-cell function and mass through IRS2 induction in islets of diabetic rats. Life Sci 2008;82:503–511 [DOI] [PubMed] [Google Scholar]
- 27.Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring) 2008;16:241–256 [DOI] [PubMed] [Google Scholar]
- 28.Xiang AH, Kawakubo M, Trigo E, Kjos SL, Buchanan TA. Declining beta-cell compensation for insulin resistance in Hispanic women with recent gestational diabetes mellitus: association with changes in weight, adiponectin, and C-reactive protein. Diabetes Care 2010;33:396–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia 2012;55:2319–2326 [DOI] [PubMed] [Google Scholar]
- 30.Holland WL, Miller RA, Wang ZV, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med 2011;17:55–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ball GDC, Shaibi GQ, Cruz ML, Watkins MP, Weigensberg MJ, Goran MI. Insulin sensitivity, cardiorespiratory fitness, and physical activity in overweight Hispanic youth. Obes Res 2004;12:77–85 [DOI] [PubMed] [Google Scholar]
- 32.LaMonte MJ, Blair SN, Church TS. Physical activity and diabetes prevention. J Appl Physiol 2005;99:1205–1213 [DOI] [PubMed] [Google Scholar]
- 33.Arciero PJ, Vukovich MD, Holloszy JO, Racette SB, Kohrt WM. Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol 1999;86:1930–1935 [DOI] [PubMed] [Google Scholar]
- 34.Gazdag AC, Wetter TJ, Davidson RT, et al. Lower calorie intake enhances muscle insulin action and reduces hexosamine levels. Am J Physiol Regul Integr Comp Physiol 2000;278:R504–R512 [DOI] [PubMed] [Google Scholar]
- 35.Delghingaro-Augusto V, Décary S, Peyot ML, et al. Voluntary running exercise prevents β-cell failure in susceptible islets of the Zucker diabetic fatty rat. Am J Physiol Endocrinol Metab 2012;302:E254–E264 [DOI] [PubMed] [Google Scholar]
- 36.Matthews L, Hankey C, Penpraze V, et al. Agreement of accelerometer and a physical activity questionnaire in adults with intellectual disabilities. Prev Med 2011;52:361–364 [DOI] [PubMed] [Google Scholar]
- 37.Tucker JM, Welk GJ, Beyler NK. Physical activity in U.S.: adult compliance with the Physical Activity Guidelines for Americans. Am J Prev Med 2011;40:454–461 [DOI] [PubMed] [Google Scholar]
- 38.National Center for Health Statistics. Health, United States, 2011: With Special Feature on Socioeconomic Status and Health Hyattsville, MD, Centers for Disease Control and Prevention, 2012 [PubMed] [Google Scholar]
- 39.Wei M, Gibbons LW, Kampert JB, Nichaman MZ, Blair SN. Low cardiorespiratory fitness and physical inactivity as predictors of mortality in men with type 2 diabetes. Ann Intern Med 2000;132:605–611 [DOI] [PubMed] [Google Scholar]
- 40.Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc 2001;33(6 Suppl.):S379–S399; discussion S419–S320 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.