Abstract
OBJECTIVE
We estimated the prevalence and incidence of diabetes among specific subgroups of Asians and Pacific Islanders (APIs) in a multiethnic U.S. population with uniform access to care.
RESEARCH DESIGN AND METHODS
This prospective cohort analysis included 2,123,548 adult members of Kaiser Permanente Northern California, including 1,704,363 with known race/ethnicity (white, 56.9%; Latino, 14.9%; African American, 8.0%; Filipino, 4.9%; Chinese, 4.0%; multiracial, 2.8%; Japanese, 0.9%; Native American, 0.6%; Pacific Islander, 0.5%; South Asian, 0.4%; and Southeast Asian, Korean, and Vietnamese, 0.1% each). We calculated age-standardized (to the 2010 U.S. population) and sex-adjusted diabetes prevalence at baseline and incidence (during the 2010 calendar year). Poisson models were used to estimate relative risks.
RESULTS
There were 210,632 subjects with prevalent diabetes as of 1 January 2010 and 15,357 incident cases of diabetes identified during 2010. The crude diabetes prevalence was 9.9% and the incidence was 8.0 cases per 1,000 person-years and, after standardizing by age and sex to the 2010 U.S. Census, 8.9% and 7.7 cases per 1,000 person-years. There was considerable variation among the seven largest API subgroups. Pacific Islanders, South Asians, and Filipinos had the highest prevalence (18.3, 15.9, and 16.1%, respectively) and the highest incidence (19.9, 17.2, and 14.7 cases per 1,000 person-years, respectively) of diabetes among all racial/ethnic groups, including minorities traditionally considered high risk (e.g., African Americans, Latinos, and Native Americans).
CONCLUSIONS
High rates of diabetes among Pacific Islanders, South Asians, and Filipinos are obscured by much lower rates among the large population of Chinese and several smaller Asian subgroups.
Asians and Pacific Islanders (APIs) comprised 5% of the U.S. population in the 2010 Census, a 43% increase compared with the 2000 Census (1). The three largest API subgroups included people of Chinese (3.3 million), South Asian (2.8 million), or Filipino (2.6 million) ancestry. Most national health surveys before 2000 classified Asians as “other race” or, if recognized, combined them with Pacific Islanders; thus, the variation among API subgroups has been neglected.
Epidemiologic studies and U.S. national surveillance report that Asians have a higher prevalence of type 2 diabetes relative to non-Hispanic whites, but lower than that of African Americans and Latinos (2,3). However, aggregation of API subgroups may preclude identifying those at particularly high risk for diabetes (4). A recent report from the U.S. National Health Interview Survey (NHIS) disaggregated API subgroups and found substantive differences in diabetes prevalence (3). Nonetheless, there is a paucity of published data on the prevalence and incidence of diabetes among API subgroups in the U.S (5,6).
Through the Diabetes Study of Northern California (DISTANCE), we estimated racial/ethnic differences in the prevalence and incidence of diabetes in a large, multiethnic cohort of patients receiving care in an integrated health delivery system.
RESEARCH DESIGN AND METHODS
Subjects were adult (≥18 years of age) health plan members of Kaiser Permanente Northern California (KPNC), a large, integrated healthcare delivery system caring for >3 million people who are broadly representative of the local and statewide population (7). We studied the prevalence and incidence of diabetes during the calendar year 2010 (1 January 2010–31 December 2010), while excluding subjects not continuously enrolled during the 12 months (1 January 2009–31 December 2009) prior to baseline to avoid misclassifying new enrollees with pre-existing diabetes as incident cases. These criteria resulted in an eligible adult population of 2,123,548. Those with unknown race/ethnicity (n = 419,185) were included in the denominator for our evaluation of overall prevalence and incidence but excluded from the race-specific analyses. Race/ethnicity was ascertained from self-reported demographic data collected at clinic visits, during health plan enrollment, from member surveys, or on intake for hospitalization. Self-reported race/ethnicity was available for 1,704,363 patients (80%), including white (n = 968,943), Latino (n = 253,821), African American (n = 135,934), Filipino (n = 82,781), Chinese (n = 68,831), Japanese (n = 16,032), Native American/American Indian/Alaska native (n = 9,546), Pacific Islander (Hawaiian, Guamanian, Samoan, or other Pacific Islander; n = 7,732), South Asian (Asian Indian, Pakistani, Bangladeshi, Sri Lankan, or Nepalese; n = 6,768), Southeast Asian (Cambodian, Laotian, Burmese, Thai, Malaysian, Indonesian; n = 1,876), Korean (n = 1,130), Vietnamese (n = 1,671), other and unspecified Asian (n = 101,769), and multiracial (n = 47,529). We also created aggregate categories of Asians (Filipino, Chinese, Japanese, South Asian, Southeast Asian, Korean, Vietnamese, and other/unspecified Asian; n = 280,858) and APIs (n = 288,590) to simulate the current categorization used for presenting demographic data at the national and state level.
The prevalence of clinically recognized diabetes was estimated overall and by race/ethnicity as of 1 January 2010 (baseline), based on available administrative data (algorithm detailed in Table 1). The incidence rate was estimated by incident cases of diabetes identified during the calendar year of 2010 divided by the population at risk (n = 1,912,916), which excluded those with known (prevalent) diabetes at baseline or incomplete membership in the previous year (precluding ability to reliably ascertain if they joined with pre-existing diabetes). We sex and age standardized the 2010 U.S. Census population, yielding rates expected if our population had the identical demographic distribution as the U.S. population. This will facilitate comparisons between our findings and existing and future national surveillance reports. We also estimated relative risk (RR) of incidence and prevalence of diabetes (whites as the reference group) in a series of internally adjusted Poisson regression models with log link functions. The base model was age and sex adjusted, and subsequent models included potential mediators (model 1 added census-based, block-level median income to the base model, and model 2 added BMI and systolic blood pressure to model 1).
Table 1.
Clinical recognition of diabetes
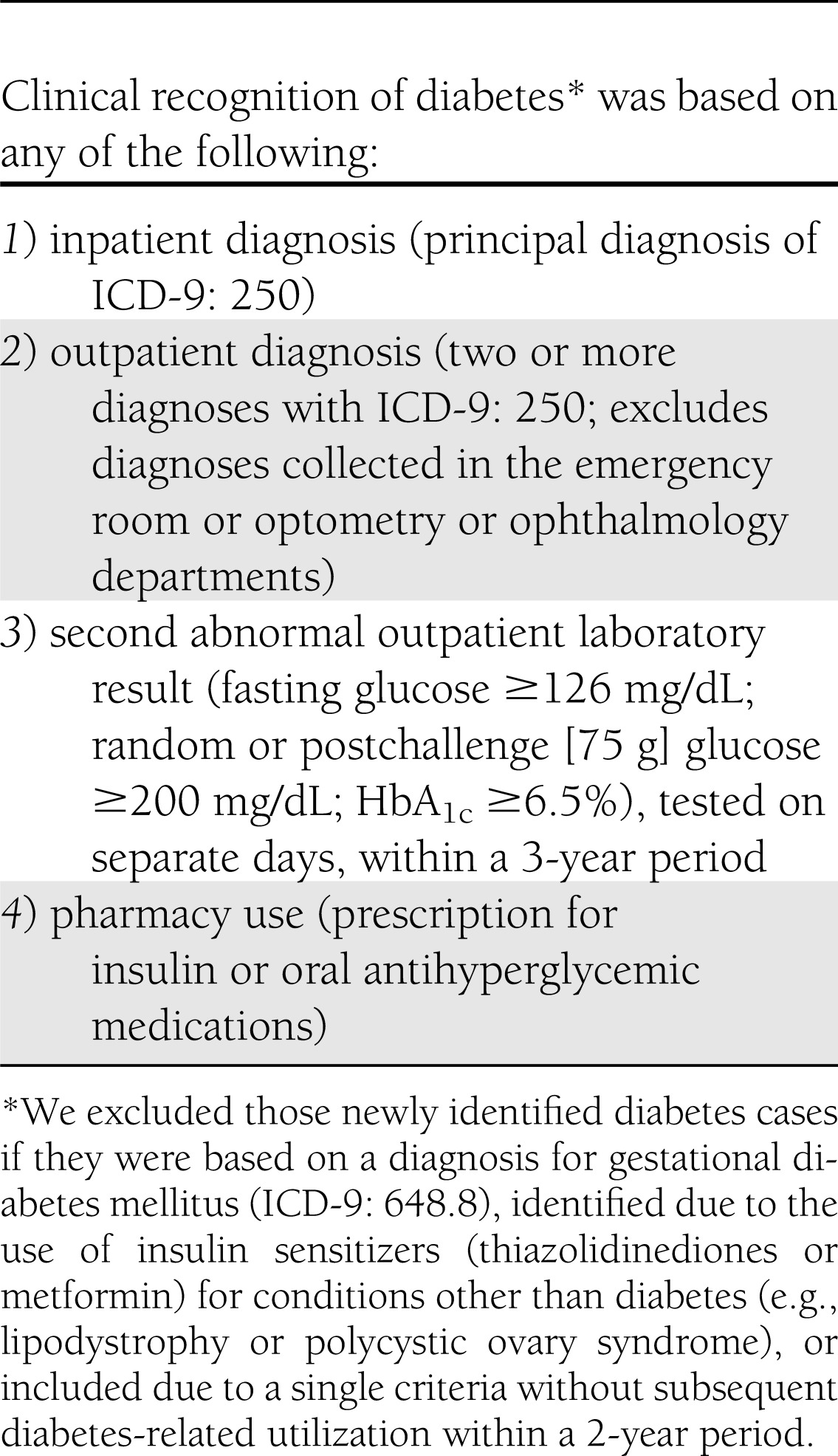
Study methods and description of the KPNC Diabetes Registry (99% sensitivity based on chart review validation) have been published previously (8,9). However, in this study, we also used the new diagnostic criteria of HbA1c ≥6.5% recognized by the American Diabetes Association Clinical Practice Recommendations in 2010 (10). This study was approved by the institutional review boards of the Kaiser Foundation Research Institute and University of California, San Francisco.
RESULTS
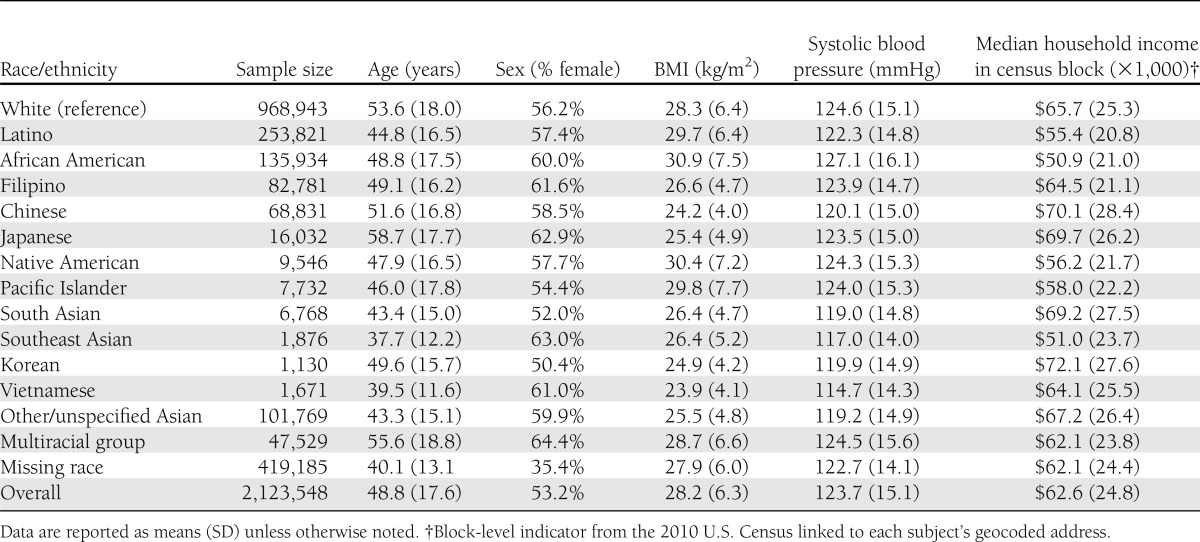
The characteristics for the 2,123,548 adults members of the study population differed substantively by race/ethnicity (Table 2). The average age was 49 years, but Vietnamese and Southeast Asians were younger on average, and Japanese, whites, Chinese, and multiracial members were older. Multiracial, African American, Filipino, and Southeast Asian groups had a greater proportion of women. BMI (kg/m2) was 28.2 on average overall, and highest in African Americans and Native Americans and lowest in Vietnamese, Koreans, Chinese, and Japanese. Systolic blood pressure was 124 mmHg on average but highest in African Americans, whites, and multiracial subjects and lowest in Vietnamese, Southeast Asians, Koreans, and South Asians. The median census block-group level income was $62,600 annually, with the highest salaries associated with Koreans, Chinese, South Asians, and Japanese, and lowest with African Americans, Latinos, and Native Americans.
Table 2.
Race/ethnicity-specific characteristics of our study population (n = 2,123,548); KPNC
We identified 210,632 individuals with prevalent diabetes, yielding an 8.9% prevalence after standardizing to the 2010 U.S. population (Table 3). The prevalence increased with age (P < 0.001); men had higher prevalence than women (10.2% in men vs. 7.8% in women; P < 0.001). Since the age and sex patterns were similar across races/ethnicities, we present age- and sex-standardized prevalence of diabetes for each racial/ethnic category and the age- and sex-adjusted RR (Table 4). Each ethnic minority group had significantly higher diabetes prevalence than whites (reference group) (7.3%). The highest prevalence was observed among Pacific Islanders (18.3%; RR 2.43), followed by Filipinos (16.1%; 2.26), South Asians (15.9%; 2.19), Latinos (14.0%; 1.88), African Americans (13.7%; 1.86), Native Americans (13.4%; 1.86), multiracial patients (12.8%; 1.74), other/unspecified Asians (12.1%: 1.59), Southeast Asians (10.5%; 1.46), Japanese (10.3%; 1.46), Vietnamese (9.9%; 0.98 but NS), Koreans (9.9%; 1.31), and Chinese (8.2%; 1.14). The aggregated categories of Asians and Asians/Pacific Islanders yielded prevalence estimates of 12.2 and 12.3%, respectively (data not shown).
Table 3.
Race/ethnicity-specific diabetes prevalence and incidence sex and age standardized to the 2010 U.S. population; KPNC
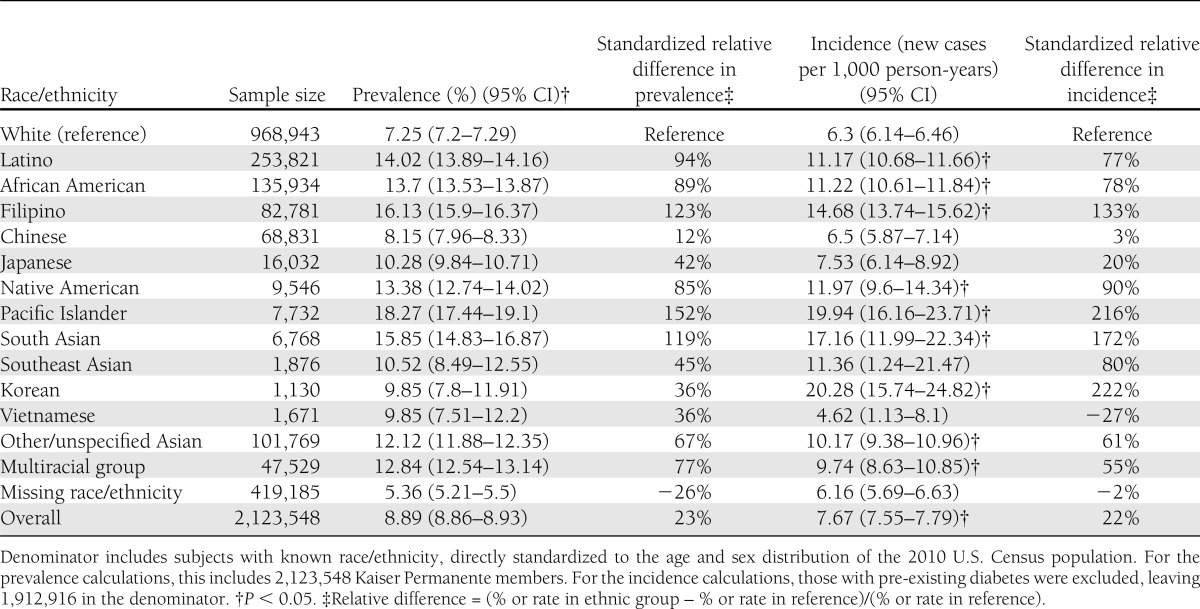
Table 4.
Age- and sex-adjusted, race/ethnicity-specific RR for diabetes prevalence based on a Poisson regression with log link functions; KPNC (n = 2,123,548)
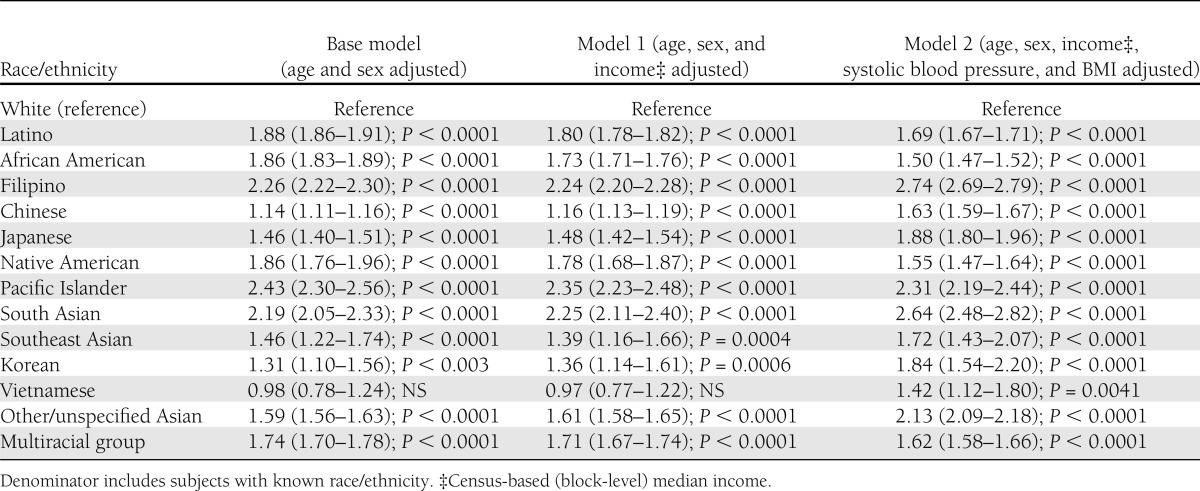
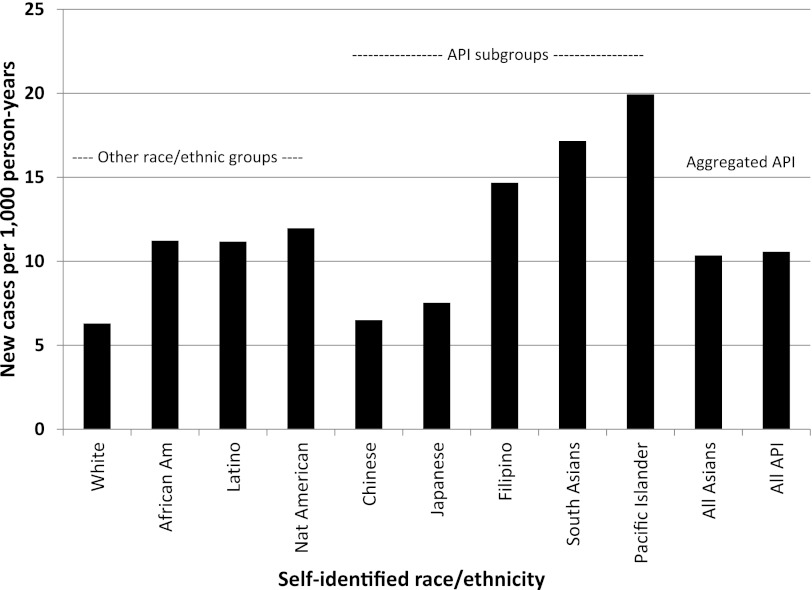
Of the 1,912,916 individuals without prevalent diabetes on 1 January 2010, there were 15,357 incident diabetes cases identified during 2010. After age and sex standardizing, the incidence density was 7.7 incident cases (95% CI 7.55–7.79) per 1,000 person-years. The patterns of age- and sex-standardized incidence (and across races/ethnicities) and internally adjusted RRs (Table 5) were similar to those observed for prevalence. Pacific Islanders (19.9 cases per 1,000 person-years; RR 3.08) had the highest incidence rates, more than triple that of whites, who had 6.3 cases per 1,000 person-years, followed by South Asians (17.2; RR 2.31), Filipinos (14.7; 2.38), Native Americans (12.0; 1.93), Latinos (11.2; 1.76), African Americans (11.2; 1.76), other/unspecified Asians (10.2; 1.55), multiracial patients (9.7; 1.50), Japanese (7.5; 1.26), and Chinese (6.5; 1.02 but NS). We do not report incident rates for Koreans, Vietnamese, and other Southeast Asians due to the low power in these small subgroups, although we do report the RRs. The aggregated categories of all Asians and Asians/Pacific Islanders yielded incidence estimates of 10.4 and 10.6 cases per 1,000 person-years, respectively (Fig. 1).
Table 5.
Age- and sex-adjusted, race/ethnicity-specific RR (95% CI) for diabetes incidence based on a Poisson regression with log link functions; KPNC (n = 1,912,916)
Figure 1.
Standardized diabetes incidence rate (per 1,000 person-years) for each race/ethnic group (2010, KPNC). Identified 15,357 new cases of medically diagnosed diabetes in 2010 among the adults (≥18 years of age) without pre-existing diabetes in KPNC on 1 January 2010. The incidence density was directly standardized to the 2010 U.S. Census. All Asian included Filipino, Chinese, Japanese, South Asian, Southeast Asian, Korean, Vietnamese, unspecified Asian, and multiracial Asian; API included the identical groups as all Asian with the addition of Pacific Islanders.
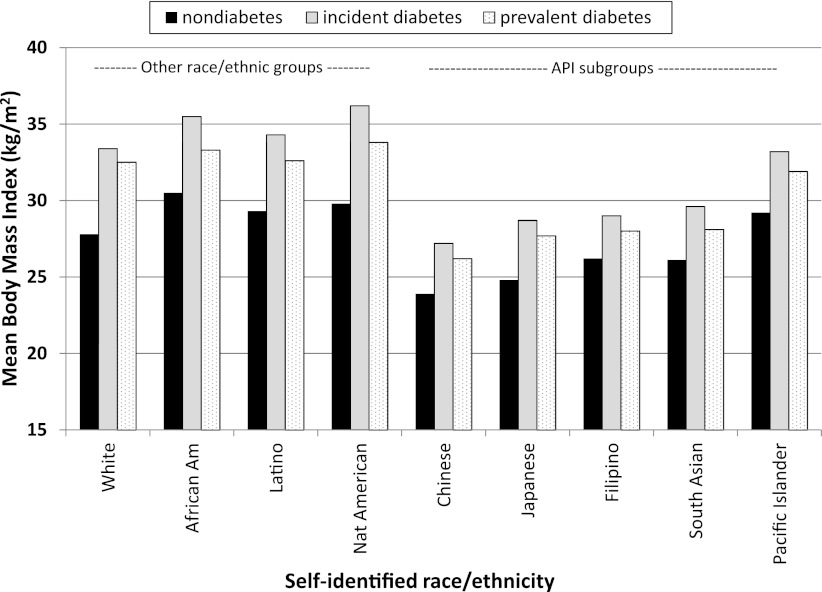
We examined the contribution of several possible explanatory factors. The BMI among the subjects with newly diagnosed diabetes varied widely across race/ethnic groups, ranging from 36.2 kg/m2 in Native Americans to 27.2 kg/m2 in Chinese individuals (Fig. 2). Moreover, the mean BMI among those identified with incident diabetes was consistently higher than in those with prevalent diabetes (by 1–2 points) and in individuals who remained normoglycemic (by 3–6 points). Adjusting for census block-level income in the Poisson regression models for prevalence and incidence (model 2 in Tables 4 and 5) imparted minimal changes to the age- and sex-adjusted point estimates (model 1). After adjustment for the two available clinical factors (BMI and systolic blood pressure in model 2), the point estimate for the RR increased for all of the Asian subgroups but decreased for Pacific Islanders, African Americans, Latinos, Native Americans, and multiracial subjects.
Figure 2.
BMI among adult (≥18 years of age) members of KPNC by race for those with prevalent diabetes (n = 194,614), newly identified diabetes (n = 13,392), and subjects remaining normoglycemic (n = 1,496,357) during calendar year 2010. BMI measured during calendar year 2009 among the 1,704,363 adult members of KPNC with known ethnicity.
CONCLUSIONS
In a large, integrated healthcare delivery system in which participants have uniform access to healthcare, both the diabetes prevalence and incidence rates in the two aggregate API categories (Asians or APIs) were greater than whites but lower than Latinos or African Americans. However, there was substantial variation across the API subgroups. Pacific Islanders, South Asians, and Filipinos had substantially higher prevalence and incidence than all other ethnic groups, including African Americans, Latinos, and Native Americans. Pacific Islanders had more than three times the incidence of diabetes relative to whites, compared with an ∼75% higher diabetes incidence among African Americans and Latinos relative to whites.
Our findings are consistent with previous population-based studies based on the Behavioral Risk Factor Surveillance Study (11) and the NHIS (12), which ranked Asians as intermediate in risk above whites and below African Americans, Latinos, and Native Americans. The use of the aggregated Asian or API categories masks the variation in risk among the subgroups (4). These categories are essentially weighted averages of the component subgroups, with the risk of the larger groups (Chinese and Filipinos) exerting a stronger influence on the overall rate. Thus, the very high risk experienced among Pacific Islanders, South Asians, and Filipinos is masked in the aggregate rate by the much lower risk among Chinese and Japanese.
Our findings are consistent with the NHIS, reporting elevated diabetes prevalence among all Asian ethnic groups combined, but particularly high risk among Filipinos and South Asians (3). In the 2004 New York City Health and Nutrition Examination Survey, the higher diabetes prevalence observed among Asians relative to whites was primarily due to high diabetes prevalence among South Asians (13). Less national data exist for Pacific Islanders, but in smaller epidemiologic studies conducted in Hawaii, diabetes prevalence was similarly high among Pacific Islanders compared with Japanese and even Filipinos (14).
Phenotypic differences by race/ethnicity were also evident among patients newly diagnosed with diabetes. Most noteworthy was that each Asian subgroup other than Pacific Islanders had a substantively lower average BMI at diabetes diagnosis than the remaining groups at diagnosis. The average BMI values of Asian subgroups were in fact below the threshold for obesity based on National Heart, Lung, and Blood Institute standards. The World Health Organization criteria suggested for use in Asian populations (15), with overweight BMI 23–27.5 kg/m2 and obesity ≥27.5 kg/m2, are more useful for helping clinicians identify who to target for behavioral interventions to reduce diabetes risk. Compared with those with incident diabetes, there was a consistent pattern across race/ethnic groups of lower BMI among individuals with prevalent diabetes, and even lower BMI among normoglycemic subjects. Adjusting for BMI actually increased the RR for all the Asian subgroups, while attenuating the RR among the remaining minority groups. This suggests that Asian subgroups have an increased prevalence and incidence of diabetes at comparable levels of BMI (relative to the white reference group).
Few data on diabetes incidence exist for APIs, particularly for clinically recognized rather than self-reported diabetes. The few existing studies again support the somewhat misleading impression that stems from aggregating subgroups into broad Asian or API categories (16). The rate observed in the KPNC cohort adds several additional subgroups for which few current diabetes incidence data exist, notably among Filipinos and South Asians. Moreover, unlike population-based studies, this study population received care in a single integrated healthcare delivery system, and thus the findings are not confounded by differential access to care across the racial/ethnic groups.
These findings may not be representative of other geographical regions, health plans, or population-based samples. Findings are based on a fully insured population from Northern California; caution is needed when generalizing to a wider population. Asian immigrants to the U.S. may differ significantly from the populations from which they originated. Although California has a longer history of immigration from Asia than other parts of the U.S., the timing of immigration varies along with the degree of acculturation to a Western lifestyle. The time of residence and acculturation in the U.S. may alter rates of chronic diseases such as diabetes, and this effect can change over time or in subsequent generations. The stage of life (child, older adults, etc.) when U.S. residence began may also play an important role. Also, it is unclear as to what extent the racial/ethnic differences in screening for diabetes could contribute to our findings. Screening for diabetes has increased in recent years, and, overall, 65% of subjects without pre-existing diabetes completed a fasting, random, or postchallenge glucose test during the 2 years prior to study baseline (2008–2009). Modest differences were noted by race/ethnicity: multiracial subjects (72%), South Asians (71%), Chinese (70%), Japanese (68%), Filipinos (67%), Koreans (66%), whites (65%), African Americans (64%), Native Americans (63%), Vietnamese (63%), Latinos (62%), and Pacific Islanders (60%). However, the race/ethnicity-specific ranking of screening rates was inconsistent with the ranking in diabetes risk, suggesting that these screening differences unlikely accounted for observed differences. The American Diabetes Association introduced HbA1c as a screening test in their 2010 Clinical Practice Recommendations (10), and thus it was only rarely used at the time of the study (only 0.3% of our cohort was screened for diabetes using an HbA1c test alone), diminishing concerns that this may bias ascertainment given the observed differential association between glycemia and HbA1c by race (17). It remains unknown to what extent the rates of undetected diabetes vary across race/ethnicity, and whether the ethnic distribution of diabetes would change substantively if we did not have 19.7% with missing data regarding ethnicity.
These findings represent a departure from the conventional wisdom regarding diabetes burden among APIs as being uniformly lower than African Americans and Latinos but higher than that of whites. Surveillance statistics aggregating all APIs obscure the very high diabetes risk among Pacific Islanders, South Asians, and Filipinos. These groups would benefit from increased diabetes prevention efforts. Subgroup variation in diabetes prevalence is not unique to APIs; variation has been observed among Latino subgroups (e.g., between Cubans, Puerto Ricans, and Mexican Americans) (18,19). Aggregated statistics may be, frankly, misleading.
More work is needed to understand the extent to which subgroup differences in diabetes burden are attributable to behavioral factors, socioeconomic status, education, health literacy, language barriers, or biology. Diabetes is a rapidly growing public health problem for Asian Americans (2), and public health promotion may more effectively target subgroups separately given differences in potential barriers to care (language, culture, education, and socioeconomics). In response to the growing risk of diabetes among Asians and the paucity of surveillance data, the Centers for Disease Control and Prevention’s National Center for Health Statistics has begun oversampling Asians in the National Health and Nutrition Examination Survey (5). Although the causes of differing diabetes rates by race/ethnicity are not well understood, in our efforts to meet our national objective of eliminating health disparities, we must continue to monitor diabetes prevalence and incidence among the API subgroups, as well as across races/ethnicities in general (20).
Acknowledgments
DISTANCE and its investigators were supported by grants from the National Institutes of Health (NIH) (R01-DK-081796, R01-DK-065664, R01-HD046113, and P30-DK-092924). A.M.K. was supported by NIH grants 1RO1-HL-093009-01 and 1R01-AT-004569-01.
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of data; or preparation, review, and approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
A.J.K. conceived the study, researched the data, and wrote the manuscript. D.S., A.S.A., and N.E.A. contributed to the discussion. H.H.M. and J.L. researched the data and reviewed and edited the manuscript. A.M.K. contributed to the discussion and reviewed and edited the manuscript. A.J.K. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Bureau of the Census. Profile of General Population and Housing Characteristics: 2010 Demographic Profile Data Washington DC, U.S. Govt. Printing Office, 2010
- 2.Kanaya A, Karter AJ. Type 2 diabetes in Asian American and Pacific Islander populations: a view from California. California Diabetes Program Issues Brief. May 2010. Available from (http://caldiabetes.org/content_display.cfm?contentID=1248). Accessed 26 September 2012 [Google Scholar]
- 3.Lee JW, Brancati FL, Yeh HC. Trends in the prevalence of type 2 diabetes in Asians versus whites: results from the United States National Health Interview Survey, 1997-2008. Diabetes Care 2011;34:353–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McNeely MJ. Outcomes of diabetes mellitus in Asian Americans. Nat Rev Endocrinol 2011;7:378–379 [DOI] [PubMed] [Google Scholar]
- 5.King GL, McNeely MJ, Thorpe LE, et al. Understanding and addressing unique needs of diabetes in Asian Americans, native Hawaiians, and Pacific Islanders. Diabetes Care 2012;35:1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu WC, Boyko EJ, Fujimoto WY, et al. Pathophysiologic differences among Asians, native Hawaiians, and other Pacific Islanders and treatment implications. Diabetes Care 2012;35:1189–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 1992;82:703–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karter AJ, Ferrara A, Liu JY, Moffet HH, Ackerson LM, Selby JV. Ethnic disparities in diabetic complications in an insured population. JAMA 2002;287:2519–2527 [DOI] [PubMed] [Google Scholar]
- 9.Moffet HH, Adler N, Schillinger D, et al. Cohort profile: The Diabetes Study of Northern California (DISTANCE)—objectives and design of a survey follow-up study of social health disparities in a managed care population. Int J Epidemiol 2009;38:38–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Summary of revisions for the 2010 Clinical Practice Recommendations. Diabetes Care 2010;33(Suppl. 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care 2004;27:66–69 [DOI] [PubMed] [Google Scholar]
- 12.Beckles GL, Zhu J, Moonesinghe R, Centers for Disease Control and Prevention (CDC) Diabetes - United States, 2004 and 2008. MMWR Surveill Summ 2011;60(Suppl.):90–93 [PubMed] [Google Scholar]
- 13.Rajpathak SN, Gupta LS, Waddell EN, et al. Elevated risk of type 2 diabetes and metabolic syndrome among Asians and south Asians: results from the 2004 New York City HANES. Ethn Dis 2010;20:225–230 [PubMed] [Google Scholar]
- 14.Maskarinec G, Erber E, Grandinetti A, et al. Diabetes incidence based on linkages with health plans: the multiethnic cohort. Diabetes 2009;58:1732–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO Expert Consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–163 [DOI] [PubMed] [Google Scholar]
- 16.Nettleton JA, Steffen LM, Ni H, Liu K, Jacobs DR., Jr Dietary patterns and risk of incident type 2 diabetes in the Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Care 2008;31:1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herman WH, Ma Y, Uwaifo G, et al. Differences in A1C by race and ethnicity among patients with impaired glucose tolerance in the Diabetes Prevention Program. Diabetes Care 2007;30:2453–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flegal KM, Ezzati TM, Harris MI, et al. Prevalence of diabetes in Mexican Americans, Cubans, and Puerto Ricans from the Hispanic Health and Nutrition Examination Survey, 1982-1984. Diabetes Care 1991;14:628–638 [DOI] [PubMed] [Google Scholar]
- 19.Allison MA, Budoff MJ, Wong ND, Blumenthal RS, Schreiner PJ, Criqui MH. Prevalence of and risk factors for subclinical cardiovascular disease in selected US Hispanic ethnic groups: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol 2008;167:962–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karter AJ. Race and ethnicity: vital constructs for diabetes research. Diabetes Care 2003;26:2189–2193 [DOI] [PubMed] [Google Scholar]