Abstract
OBJECTIVE
C-peptide is a proinsulin cleavage product released from the pancreas in amounts equimolar to insulin, and elevated levels of C-peptide have been found in patients with insulin resistance and early type 2 diabetes mellitus. Recent data suggest that C-peptide could play a causal role in the pathophysiology of vascular disease, but nothing is known about the prognostic value of C-peptide concentrations in the circulation.
RESEARCH DESIGN AND METHODS
We examined whether C-peptide is associated with cardiovascular and total mortality in 2,306 patients from the Ludwigshafen Risk and Cardiovascular Health Study who underwent coronary angiography at baseline (1997–2000).
RESULTS
During a mean follow-up of 7.6 years, 440 deaths (19.1%) occurred, 252 (10.9%) of which were due to cardiovascular causes. Age- and sex-adjusted hazard ratios (HRs) in the third compared with the first tertile of C-peptide were 1.46 (95% CI 1.15–1.85; P = 0.002) for all cause and 1.58 (1.15–2.18; P = 0.005) for cardiovascular mortality. After further adjustment for common risk factors as well as markers of glucose metabolism, these HRs remained significant at 1.46 (1.10–1.93; P = 0.008) and 1.55 (1.07–2.24; P = 0.022), respectively. Moreover, patients in higher tertiles of C-peptide exhibited higher levels of markers of endothelial dysfunction and atherosclerosis as well as a more severe extent of coronary lesions.
CONCLUSIONS
In patients undergoing coronary angiography, C-peptide levels are independently associated with all cause and cardiovascular mortality as well as presence and severity of coronary artery disease. Further studies are needed to examine a potential causal role of C-peptide in atherogenesis in humans.
C-peptide is a proinsulin cleavage product released from the pancreas at amounts equimolar to insulin. Elevated levels of C-peptide have been found in patients with insulin resistance and early type 2 diabetes, reflecting increased insulin secretion, as well as in patients with chronic kidney disease due to impaired renal elimination (1). For a long time, C-peptide has been considered to be biologically inert, but experimental as well as clinical data have recently suggested that it may exhibit biological activity (2). As such, C-peptide treatment of patients with type 1 diabetes, lacking endogenous C-peptide, has been shown to improve nerve conduction velocity as well as microalbuminuria (3–5). In contrast, observational and experimental data suggest that C-peptide may play a causal role in the pathophysiology of arteriosclerosis in patients with early type 2 diabetes. C-peptide colocalizes with monocytes/macrophages and T-cells in early arteriosclerotic lesions of diabetic patients and induces cell migration in vitro, suggesting that C-peptide, once deposited in the subendothelial space, may promote infiltration of inflammatory cells into the vessel wall, thus contributing to early atherogenesis (6,7). In addition, induction of high C-peptide levels leads to increased lesion formation in a mouse model of arteriosclerosis (8). Still, data from other groups suggest potential beneficial effects of C-peptide in vascular cells (9–11).
Several studies examined the predictive role of C-peptide in populations of patients with manifest diabetes or its association with mortality in cancer patients (12,13). Still, to date, it is unknown whether elevated C-peptide levels are associated with cardiovascular events in a patient population referred for coronary angiography. Moreover, we investigated the association of C-peptide with other markers of vascular disease.
RESEARCH DESIGN AND METHODS
Study design, participants, and clinical characterization
A total of 3,316 patients, who were referred for coronary angiography to Ludwigshafen Heart Center in Southwest Germany, were recruited between July 1997 and January 2000. Inclusion criteria were German ancestry, clinical stability except for acute coronary syndromes, and the availability of a coronary angiogram. One of the original purposes of the study also was the identification of genetic components of coronary artery disease (CAD). To avoid population admixture, we aimed at including Caucasians only. For pragmatic reasons, we therefore only included patients whose parents were both native Germans. The indications for angiography in individuals in clinically stable condition were chest pain and/or noninvasive test results consistent with myocardial ischemia. Individuals suffering from any acute illness other than acute coronary syndromes, chronic noncardiac diseases, or malignancy within the 5 past years and those unable to understand the purpose of the study were excluded. People with known diabetes were excluded because C-peptide levels change over the course of diabetes with a high interindividual variability and are in addition altered by different antidiabetic drugs. Moreover, a long duration of type 2 diabetes confers increased cardiovascular risk, thus being an additional potential confounder. Moreover, subjects with missing data on fasting C-peptide were additionally ruled out, resulting in a subgroup of 2,306 (69.5%) of 3,316 Ludwigshafen Risk and Cardiovascular Health (LURIC) study participants for the present analyses. Death certificates were not available for 14 decedents. These subjects were excluded when the associations of C-peptide with cardiovascular mortality were analyzed. The study was approved by the ethics committee at the Ärztekammer Rheinland-Pfalz and was conducted in accordance with the Declaration of Helsinki. Informed written consent was obtained from all participants (14).
Diabetes was diagnosed according to the 2010 guidelines of the American Diabetes Association (15). Newly diagnosed diabetes was defined as diabetes diagnosed at the time of coronary angiogram. Fasting glucose, postchallenge glucose (performed in 1,524 of 2,096 participants with fasting glucose <126 mg/dL), and glycated hemoglobin were used to categorize diabetes. Follow-up data on diabetes development were not available.
Hypertension was diagnosed if the systolic and/or diastolic blood pressure exceeded 140 and/or 90 mmHg or if there was a history of hypertension, evident through the use of antihypertensive drugs.
CAD was diagnosed based on clinical signs and coronary angiography. The severity of CAD was quantified with the Friesinger score (16). Maximum luminal narrowing was estimated by visual analysis as described. Acute myocardial infarction (MI) was defined as an MI that had occurred within the 4 weeks prior to enrollment. A definite ST elevation MI (STEMI) was diagnosed if typical electrocardiogram changes were present along with prolonged chest pain, refractory to sublingual nitrates, and/or enzyme or troponin T elevations (>0.1 µg/L). Non-STEMI was diagnosed if symptoms and/or troponin T criteria, but not the electrocardiogram criteria for STEMI, were met.
Cerebrovascular disease was defined clinically by documented history of a previous cerebrovascular disease event (transient ischemic attack, prolonged ischemic neurologic deficit, and cerebral infarction with or without a remaining neurologic deficit) or by documented carotid plaques (50% luminal obstruction).
Peripheral vascular disease was defined by a history of intermittent claudication, angiographic documentation of atherosclerotic luminal obstruction of the peripheral arteries, or a history of a peripheral arterial intervention for atherosclerotic disease (angioplasty or surgery).
Comorbidity was assessed using the Charlson comorbidity index. We formed three groups: group 0 (0 score points), group 1 (1 score point), and group 2 (≥2 score points) (17).
Physical activity.
A questionnaire with a scoring system ranging from 1, sedentary (avoid walking or exertion), to 11, regular heavy exercise, was used to classify the mean physical activity. The study participants were grouped into the following three categories of physical activity: below average (scores 1–3), average (scores 4–7), and above average (scores 8–11).
Follow-up
There was a follow-up for all-cause and cardiovascular mortality; the latter defined as mortality due to cardiovascular diseases (ICD-9 codes 390–448 and ICD-10 codes I00–I79). The mean (± SD) duration of the follow-up was 7.6 ± 2.1 years. Information on the vital status was obtained from local person registries. Using death certificates, two experienced clinicians independently classified the causes of death. They were blinded to any data of the study participants. In cases of a disagreement or uncertainty concerning the coding of a specific cause of death, classification was made by a principal investigator of the LURIC study (W.M.).
Laboratory analyses
The standard laboratory methods have been described. C-peptide was measured with an enzyme immunoassay (AIA-pack C-peptide) on an AIA 1200 analyzer (Eurogenetics, Eschborn, Germany). Glucose was measured enzymatically on a Hitachi 717 analyzer (Roche, Mannheim, Germany). Insulin was analyzed with an immunoenzymometric assay (AIA pack IRI) on an AIA 1200 analyzer (Eurogenetics). Glycated hemoglobin was measured with an immunoassay (hemoglobin A1c UNIMATE 5; Hoffmann-LaRoche, Grenzach-Whylen, Germany). Lipoproteins were separated using a combined ultracentrifugation–precipitation method and measured on a WAKO 30 R analyzer (WAKO Chemicals GmbH, Neuss, Germany). Triglycerides were quantified with an enzymatic assay on a Hitachi 717 analyzer (Roche). Creatinine was measured with the Jaffé method on a Hitachi 717 analyzer (Roche) (18).
Statistical analysis
We formed tertiles of C-peptide. The baseline characteristics are reported as numbers and percentages in cases of categorical variables and as means with SD or medians with interquartile ranges in cases of continuous variables. Comparisons among the three groups were made with the χ2 test and with ANOVA for categorical and continuous data, respectively. Triglyceride and insulin levels (Shapiro-Wilk W test) were transformed logarithmically before being used in parametric statistical procedures. Kaplan-Meier curves were plotted for the relationships of C-peptide with all-cause and cardiovascular mortality. Unadjusted P values were calculated with the log-rank test. The Cox proportional hazards model was used to perform multivariate analyses for the associations among the tertiles of C-peptide and mortality data. Three predefined models of adjustment (model 1: adjusted for sex and age; model 2: adjusted for sex, age, BMI, hypertension, smoking, glomerular filtration rate [GFR], triglycerides, LDL cholesterol, and HDL cholesterol; and model 3: model 2 with additional adjustment for fasting glucose, fasting insulin, HbA1c, newly diagnosed diabetes, proinsulin, free fatty acids, free glycerol, as well as C-reactive protein) were used. Z-transformation was performed to investigate the increase in risk of all-cause and cardiovascular mortality conferred by SD of C-peptide levels included as a continuous variable. All statistical tests were two-sided, and P values <0.05 were considered significant. The SPSS 15.0 statistical package (SPSS Inc., Chicago, IL) was used.
RESULTS
Baseline characteristics
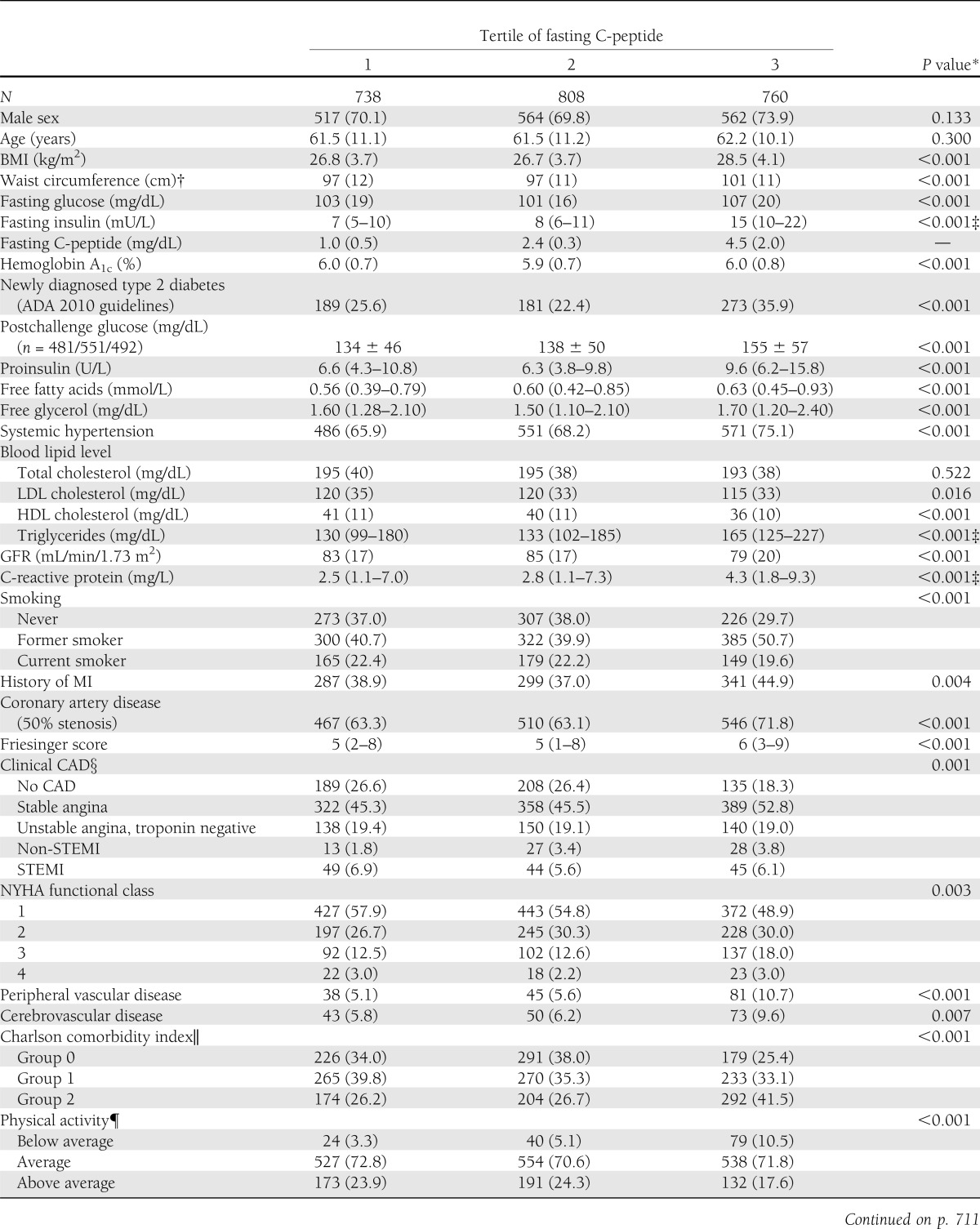
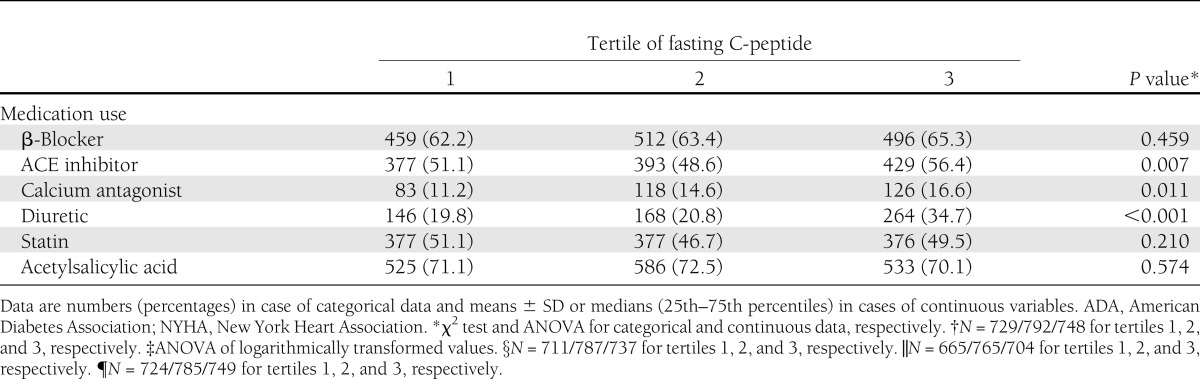
Clinical and laboratory baseline characteristics according to C-peptide tertiles are shown in Table 1. In the overall population analyzed, CAD was ruled out in ∼33% of patients. Persons in the highest C-peptide tertile had a significantly higher prevalence of CAD than subjects in the lower tertiles (71.8 vs. 63.1 vs. 63.3%, respectively). In addition, persons in the highest C-peptide tertile exhibited significantly higher BMI, waist circumference, fasting glucose, fasting insulin, triglycerides, C-reactive protein, and had lower LDL cholesterol levels as well as a lower GFR. Moreover, subjects in the highest tertile had more often newly diagnosed type 2 diabetes, systemic hypertension, high comorbidity, and a lower level of physical activity. The proportion of past and current smokers and the use of ACE inhibitors, calcium antagonists, and diuretics were significantly higher in patients with higher C-peptide levels (Table 1).
Table 1.
Baseline characteristics according to tertile of fasting C-peptide
C-peptide, markers of arteriosclerosis, and vascular disease
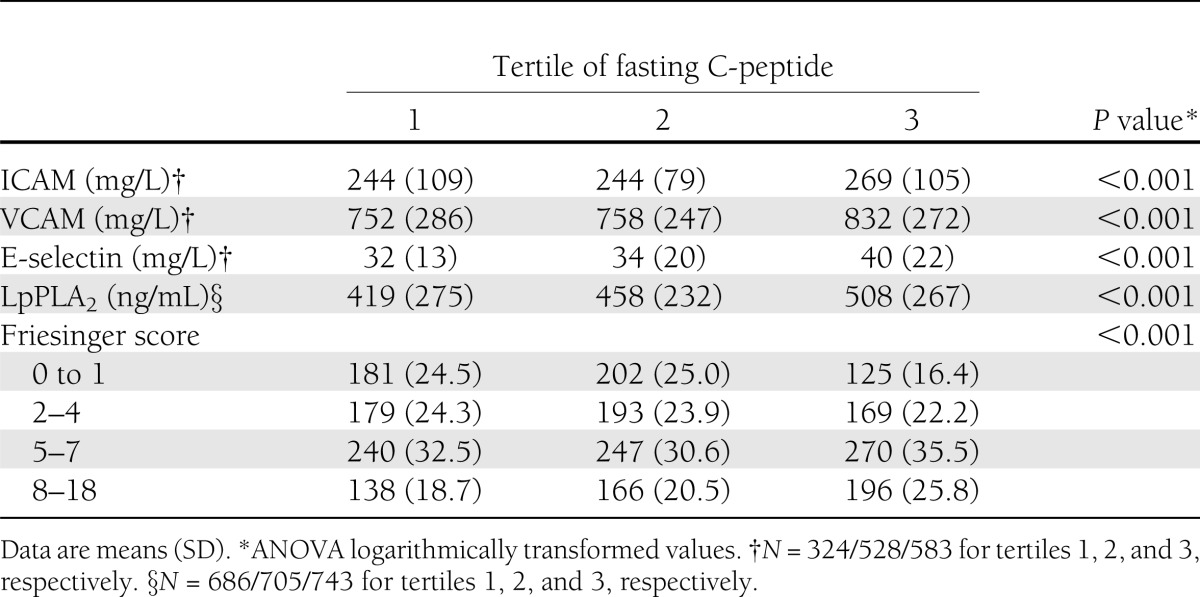
Because experimental data suggest that C-peptide may be causally involved in early atherogenesis, we analyzed the association of C-peptide with biochemical markers of endothelial dysfunction and atherosclerosis. Subjects in the highest C-peptide tertile exhibited significantly higher levels of intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, P-selectin, and E-selectin, as well as lipoprotein-associated phospholipase A2 (LpPLA2) (Table 2). Moreover, history of MI, peripheral vascular disease, and cerebrovascular disease were more frequent in subjects with high C-peptide levels (Table 1). In accordance, the proportion of subjects with prevalent clinical and angiographic CAD (Table 1) as well as lesion extension as assessed by the Friesinger score increased in parallel with the C-peptide tertiles (Table 2). Furthermore, cardiac insufficiency as reflected by high New York Heart Association functional class was more frequent in subjects with high C-peptide levels (Table 1). Taken together, these data underscore the hypothesis that C-peptide could play a direct role in the development of arteriosclerosis.
Table 2.
Markers of atherosclerosis and Friesinger score according to tertile of fasting C-peptide
C-peptide and all-cause mortality
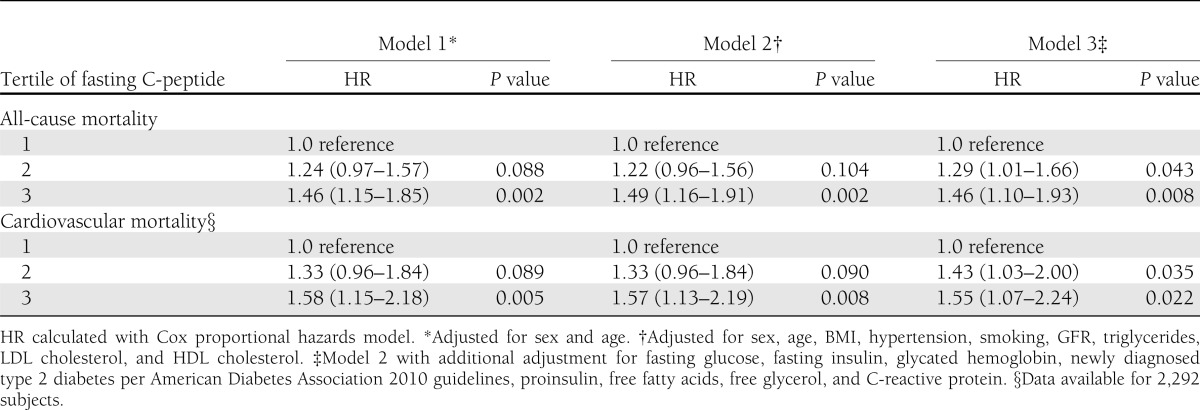
All-cause mortality and causes of death are shown in Supplementary Table 1. In 14 subjects, we did not obtain sufficient information to classify their cause of death and therefore only included them in the analyses of all-cause mortality and excluded them from further analyses. Among the 2,306 patients without known diabetes and available C-peptide levels, 440 deaths (19.1%) occurred during a mean follow-up of 7.6 years. Compared with subjects in the lowest tertile, the age- and sex-adjusted hazard ratio (HR) for death was 1.46 (95% CI 1.15–1.85; P = 0.002) in the highest tertile (Table 3). Further adjustment for BMI, hypertension, smoking, GFR, triglycerides, LDL cholesterol, and HDL cholesterol did not influence the prognostic value of C-peptide in the highest tertile, resulting in an HR of 1.49 (1.16–1.91; P = 0.002) compared with the lowest tertile (Table 3, model 2). Additional adjustment for fasting glucose, fasting insulin, HbA1c, newly diagnosed diabetes, proinsulin, free fatty acids, free glycerol, as well as C-reactive protein had only minor influence on the HR of 1.46 (1.10–1.93; P = 0.008) (Table 3, model 3). C-peptide levels included as continuous variable increased the risk in all-cause mortality per SD by HR 1.12 (1.05–1.20, P = 0.001; model 1). The association remained unchanged after adjustment for cardiovascular risk factors, HR 1.12 (1.04–1.20; P = 0.002; model 2), and after full adjustment in model 3, HR 1.13 (1.04–1.22; P = 0.005).
Table 3.
Mortality according to tertile of fasting C-peptide
C-peptide and cardiovascular mortality
Among the 2,292 subjects with known causes of death, 252 (10.9%) died of cardiovascular causes, 26 (1.1%) of infection, 76 (3.3%) of cancer, and 72 (3.1%) of miscellaneous causes. Compared with the lowest C-peptide tertile, the age- and sex-adjusted HR for cardiovascular death was 1.58 (95% CI 1.15–2.18; P = 0.005) (Table 3). After full adjustment (model 3), the prognostic value of C-peptide in the highest compared with the lowest tertile was still significant, with an HR of 1.55 (1.07–2.24; P = 0.022).
C-peptide levels included as continuous variable increased the risk in cardiovascular mortality per SD by HR 1.16 (95% CI 1.07–1.26; P < 0.001; model 1). The association remained unchanged after adjustment for cardiovascular risk factors, HR 1.15 (1.05–1.25; P = 0.002; model 2), and after full adjustment in model 3, HR 1.16 (1.05–1.28; P = 0.004).
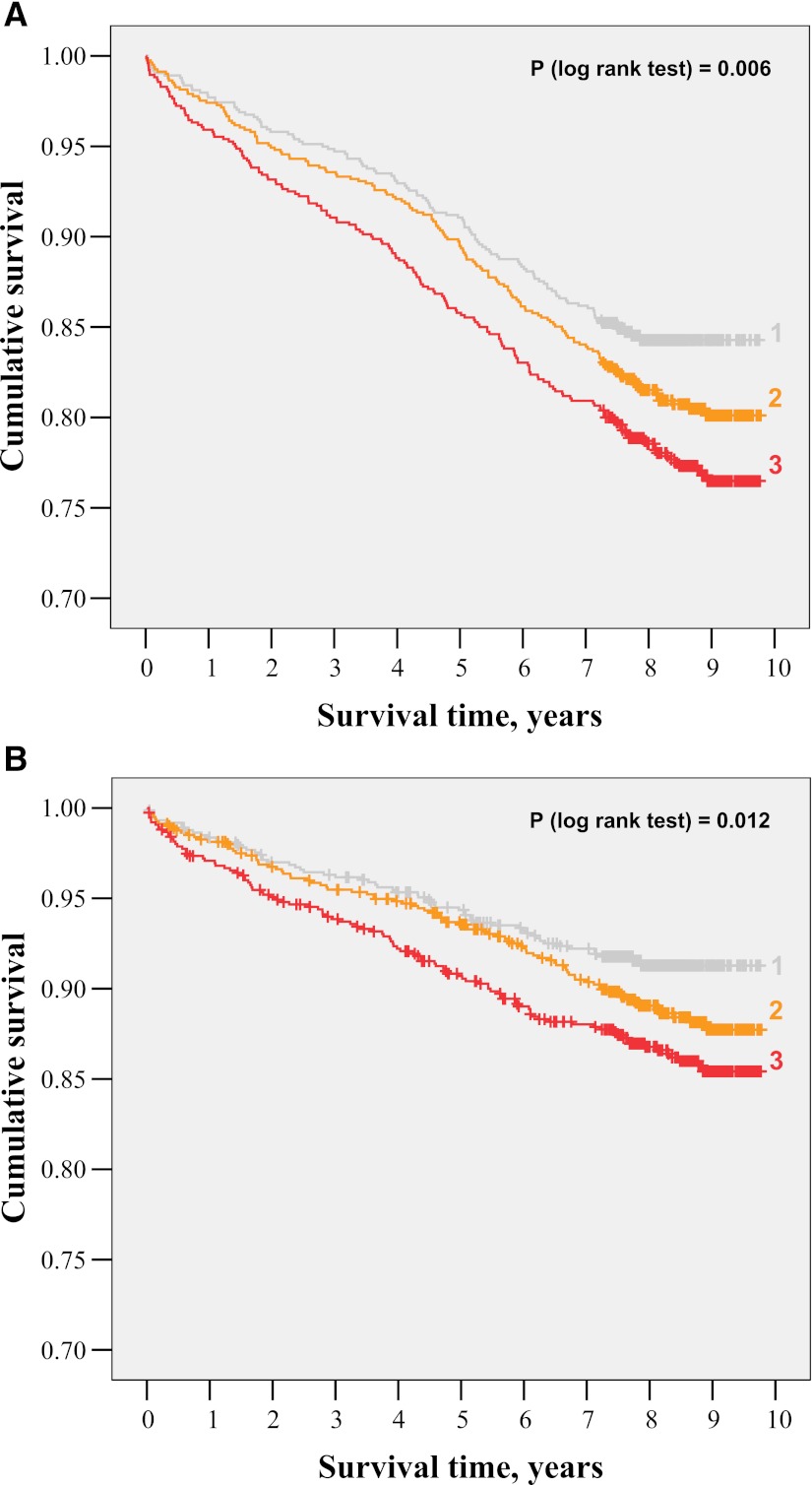
Kaplan-Meier curves followed by a log-rank test showed that all-cause mortality (Fig. 1A) as well as cardiovascular mortality (Fig. 1B) significantly increased in the higher C-peptide tertiles (P = 0.006 and P = 0.012, respectively).
Figure 1.
A: Kaplan-Meier curves for all-cause mortality according to tertile (1–3) of fasting C-peptide. B: Kaplan-Meier curves for cardiovascular mortality according to tertile (1–3) of fasting C-peptide.
CONCLUSIONS
The current study demonstrates that levels of the proinsulin cleavage product C-peptide are independently associated with all-cause mortality and cardiovascular mortality in a large cohort of patients referred to coronary angiography. Moreover, C-peptide was associated with prevalent CAD, and the severity of angiographic changes increased with C-peptide levels. The association of C-peptide with mortality as well as cardiovascular mortality remained significant even after full adjustment including BMI, fasting glucose, fasting insulin, HbA1c, proinsulin, free fatty acids, free glycerol, high-sensitivity C-reactive protein, and GFR, as well as newly diagnosed diabetes.
Elevated levels of the proinsulin cleavage product C-peptide have been found in patients with insulin resistance, early type 2 diabetes, as well as those with chronic kidney disease. Measurement of C-peptide levels has been used as a surrogate for insulin secretion (e.g., in obese patients with insulin resistance) (19). In this context, the association of C-peptide with cardiovascular mortality could simply reflect the increased risk of patients with insulin resistance and early type 2 diabetes in our population. Indeed, subjects in the highest tertile of C-peptide had higher fasting glucose, higher fasting insulin, a higher BMI, as well as a higher prevalence of newly diagnosed diabetes. However, adjustment for all of these factors did basically not change the HR for cardiovascular mortality as well as overall mortality, suggesting that C-peptide is an independent risk factor in our study population.
To the best of our knowledge, this is the first study to demonstrate that C-peptide is associated with mortality and cardiovascular mortality in patients without previously known diabetes. Various studies have shown that elevated C-peptide levels are associated with mortality as well as cardiovascular mortality in patients with diabetes and nondiabetic cancer patients, most likely serving as an indirect marker for obesity, insulin resistance, and undiagnosed diabetes (12,13).
Various data from in vitro as well as animal models have shown proatherogenic effects of C-peptide (1,7,20). Our data, demonstrating an association of C-peptide levels with markers of endothelial activation such as ICAM-1, VCAM-1, and E-selectin, as well as a highly significant association with levels of LpPLA2, a novel marker of arteriosclerosis, raise the question if C-peptide could potentially also directly influence atherogenesis in humans, but our data cannot prove a causal relationship. Furthermore, patients in the highest tertile of C-peptide had the highest incidence of CAD and the most severe lesion extension as assessed by the Friesinger score, underscoring the hypothesis that C-peptide may play a role in the pathophysiology of CAD. Still, our study cannot prove causality, and further research is warranted to address this question. Of note, therapeutic application of C-peptide in patients with type 1 diabetes, lacking endogenous C-peptide, exhibits beneficial effects on microvascular complications such as neuropathy (4), suggesting different mechanisms of action of exogenous C-peptide in C-peptide–deprived patients and those with elevated endogenous C-peptide levels.
Our study has some limitations: the results obtained in this study apply only to a highly selected Caucasian patient population undergoing coronary angiography without other major noncardiac diseases or malignancies. Therefore, the results may not be generalizable to other populations with younger age, other ethnicity, or other underlying diseases. Moreover, each of the study participants’ coronary angiography was clinically indicated, which may have produced referral bias. Finally, despite the fact that we adjusted our results for multiple potential confounders, we cannot rule out additional factors.
Taken together, our study suggests that elevated levels of C-peptide are predictive of both all-cause as well as cardiovascular mortality in patients undergoing coronary angiography and raises the hypothesis that C-peptide could potentially play a causal role in atherogenesis in humans. However, future research has to prove causality and examine the underlying mechanism for the findings of this study.
Supplementary Material
Acknowledgments
This work was supported by the 6th Framework Program (integrated project Bloodomics, Grant LSHM-CT-2004-503485) and 7th of Framework Program (integrated project Atheroremo, Grant Agreement 201668) of the European Union. B.O.B. received grants from the Deutsche Forschungsgemeinschaft GrK 1041 and the Centre of Excellence “Metabolic Diseases” Baden-Wuerttemberg. N.M. received grants from Deutsche Forschungsgemeinschaft (MA 2047/4-1), the European Foundation for the Study of Diabetes, and the Novartis-Stiftung für therapeutische Forschung. No other potential conflicts of interest relevant to this article were reported.
N.M. contributed to the interpretation of the results and wrote the manuscript. G.S. performed the statistical analysis and wrote the manuscript. V.B., M.B., M.E.K., and T.B.G. contributed to the interpretation of the results and reviewed and edited the manuscript. B.R.W., B.O.B., and W.M. designed the study. All authors have read and approved the manuscript as submitted. N.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors thank the participants of the LURIC study, who made the writing of this article possible. The authors also thank the LURIC study team either temporarily or permanently involved in patient recruitment and sample and data handling, the laboratory staff at the Ludwigshafen General Hospital, and the Universities of Freiburg and Ulm.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-1211/-/DC1.
References
- 1.Wahren J, Shafqat J, Johansson J, Chibalin A, Ekberg K, Jörnvall H. Molecular and cellular effects of C-peptide—new perspectives on an old peptide. Exp Diabesity Res 2004;5:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahren J, Ekberg K, Jörnvall H. C-peptide is a bioactive peptide. Diabetologia 2007;50:503–509 [DOI] [PubMed] [Google Scholar]
- 3.Ekberg K, Brismar T, Johansson BL, et al. C-Peptide replacement therapy and sensory nerve function in type 1 diabetic neuropathy. Diabetes Care 2007;30:71–76 [DOI] [PubMed] [Google Scholar]
- 4.Johansson BL, Linde B, Wahren J. Effects of C-peptide on blood flow, capillary diffusion capacity and glucose utilization in the exercising forearm of type 1 (insulin-dependent) diabetic patients. Diabetologia 1992;35:1151–1158 [DOI] [PubMed] [Google Scholar]
- 5.Johansson BL, Sjöberg S, Wahren J. The influence of human C-peptide on renal function and glucose utilization in type 1 (insulin-dependent) diabetic patients. Diabetologia 1992;35:121–128 [DOI] [PubMed] [Google Scholar]
- 6.Marx N, Walcher D, Raichle C, et al. C-peptide colocalizes with macrophages in early arteriosclerotic lesions of diabetic subjects and induces monocyte chemotaxis in vitro. Arterioscler Thromb Vasc Biol 2004;24:540–545 [DOI] [PubMed] [Google Scholar]
- 7.Walcher D, Aleksic M, Jerg V, et al. C-peptide induces chemotaxis of human CD4-positive cells: involvement of pertussis toxin-sensitive G-proteins and phosphoinositide 3-kinase. Diabetes 2004;53:1664–1670 [DOI] [PubMed] [Google Scholar]
- 8.Vasic D, Marx N, Sukhova G, et al. C-peptide promotes lesion development in a mouse model of arteriosclerosis. J Cell Mol Med 2012;16:927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cifarelli V, Geng X, Styche A, Lakomy R, Trucco M, Luppi P. C-peptide reduces high-glucose-induced apoptosis of endothelial cells and decreases NAD(P)H-oxidase reactive oxygen species generation in human aortic endothelial cells. Diabetologia 2011;54:2702–2712 [DOI] [PubMed] [Google Scholar]
- 10.Luppi P, Cifarelli V, Tse H, Piganelli J, Trucco M. Human C-peptide antagonises high glucose-induced endothelial dysfunction through the nuclear factor-kappaB pathway. Diabetologia 2008;51:1534–1543 [DOI] [PubMed] [Google Scholar]
- 11.Mughal RS, Scragg JL, Lister P, et al. Cellular mechanisms by which proinsulin C-peptide prevents insulin-induced neointima formation in human saphenous vein. Diabetologia 2010;53:1761–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irwin ML, Duggan C, Wang CY, et al. Fasting C-peptide levels and death resulting from all causes and breast cancer: the health, eating, activity, and lifestyle study. J Clin Oncol 2011;29:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J, Li H, Giovannucci E, et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 2008;9:1039–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winkelmann BR, März W, Boehm BO, et al. LURIC Study Group (LUdwigshafen RIsk and Cardiovascular Health) Rationale and design of the LURIC study—a resource for functional genomics, pharmacogenomics and long-term prognosis of cardiovascular disease. Pharmacogenomics 2001;2(Suppl. 1):S1–S73 [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2010;33(Suppl. 1):S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friesinger GC, Page EE, Ross RS. Prognostic significance of coronary arteriography. Trans Assoc Am Physicians 1970;83:78–92 [PubMed] [Google Scholar]
- 17.de Groot V, Beckerman H, Lankhorst GJ, Bouter LM. How to measure comorbidity. a critical review of available methods. J Clin Epidemiol 2003;56:221–229 [DOI] [PubMed] [Google Scholar]
- 18.Silbernagel G, Grammer TB, Winkelmann BR, Boehm BO, März W. Glycated hemoglobin predicts all-cause, cardiovascular, and cancer mortality in people without a history of diabetes undergoing coronary angiography. Diabetes Care 2011;34:1355–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hirai FE, Moss SE, Klein BE, Klein R. Relationship of glycemic control, exogenous insulin, and C-peptide levels to ischemic heart disease mortality over a 16-year period in people with older-onset diabetes: the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR). Diabetes Care 2008;31:493–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walcher D, Babiak C, Poletek P, et al. C-Peptide induces vascular smooth muscle cell proliferation: involvement of SRC-kinase, phosphatidylinositol 3-kinase, and extracellular signal-regulated kinase 1/2. Circ Res 2006;99:1181–1187 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.