Abstract
OBJECTIVE
Patients with the metabolic syndrome (MetS) have impaired insulin-induced enhancement of vasodilator responses. The incretin hormone glucagon-like peptide 1 (GLP-1), beyond its effects on blood glucose, has beneficial actions on vascular function. This study, therefore, aimed to assess whether GLP-1 affects insulin-stimulated vasodilator reactivity in patients with the MetS.
RESEARCH DESIGN AND METHODS
Forearm blood flow responses to acetylcholine (ACh) and sodium nitroprusside (SNP) were assessed in MetS patients before and after the addition of GLP-1 to an intra-arterial infusion of saline (n = 5) or insulin (n = 5). The possible involvement of oxidative stress in the vascular effects of GLP-1 in this setting was investigated by infusion of vitamin C (n = 5). The receptor specificity of GLP-1 effect during hyperinsulinemia was assessed by infusing its metabolite GLP-1(9-36) (n = 5). The metabolic actions of GLP-1 were also tested by analyzing forearm glucose disposal during hyperinsulinemia (n = 5).
RESULTS
In MetS patients, GLP-1 enhanced endothelium-dependent and -independent responses to ACh and SNP, respectively, during hyperinsulinemia (P < 0.001 for both), but not during saline (P > 0.05 for both). No changes in vasodilator reactivity to ACh and SNP were seen after GLP-1 was added to insulin and vitamin C (P > 0.05 for both) and after GLP-1(9-36) was given during hyperinsulinemia (P > 0.05 for both). Also, GLP-1 did not affect forearm glucose extraction and uptake during hyperinsulinemia (P > 0.05 for both).
CONCLUSIONS
In patients with the MetS, GLP-1 improves insulin-mediated enhancement of endothelium-dependent and -independent vascular reactivity. This effect may be influenced by vascular oxidative stress and is possibly exerted through a receptor-mediated mechanism.
Insulin resistance is a key pathogenetic factor in the metabolic and cardiovascular abnormalities associated with the metabolic syndrome (MetS) (1,2). Although our understanding of the mechanisms underlying the decreased insulin sensitivity present in this condition is incomplete, an abnormal insulin-stimulated microvascular perfusion at the level of the skeletal muscle is considered to contribute to its pathophysiology (3). In line with this hypothesis, recent data from our group have indicated that insulin physiologically enhances vascular relaxation in response to a variety of endothelium-dependent and -independent stimuli (4,5) and that this effect is impaired in patients with obesity-related MetS (6).
Glucagon-like peptide 1 (GLP-1) is a peptide hormone secreted by the gastrointestinal tract in a nutrient-dependent manner (7). Beyond its effects on glucose homeostasis, growing evidence indicates that GLP-1 may exert multiple cardiovascular actions (8) and improve endothelial function in normal subjects (9) and in patients with type 2 diabetes and coronary artery disease (10). These endothelial effects seem largely mediated by interaction with the GLP-1 receptor, likely in relation to activation of the phosphoinositide 3-kinase/Akt pathway (11). However, in experimental studies, some vasoprotective actions have also been described for the metabolite GLP-1(9-36) (12), which does not activate the GLP-1 receptor, hence suggesting the presence of a dual, yet undefined, signaling pathway.
Whether GLP-1 affects vasoactive and metabolic responses to hyperinsulinemia in insulin-resistant patients with obesity-related MetS has not been investigated. Therefore, the primary aim of the current study was to assess the effects of GLP-1 on insulin-stimulated vasodilator reactivity and insulin-dependent glucose disposal in these patients. Additionally, we assessed whether oxidative stress is involved in the vascular actions of GLP-1 under those conditions and investigated the regional hemodynamic effects of its metabolite GLP-1(9-36).
RESEARCH DESIGN AND METHODS
Study subjects
Patients with obesity-related MetS, defined according to the National Cholesterol Education Program’s Adult Treatment Panel III (2), and metabolically healthy obese control subjects with no history or current evidence of hypertension, hyperlipidemia, diabetes, cardiovascular disease, or any other systemic conditions were recruited for this study. All control subjects were obese and had high waist circumference, but all of them had blood pressure, plasma HDL cholesterol, triglycerides, and glucose below the threshold levels for the MetS. Exclusion criteria were a history or presence of coronary artery disease, peripheral occlusive arterial disease, coagulopathy, vasculitis, or any other systemic conditions. In patients with the MetS taking antihypertensive and/or lipid-lowering drugs, treatment was discontinued for 2 weeks before the vascular function studies. During the time when antihypertensive therapy was stopped, patients monitored their blood pressure daily and reported the values to a study physician if they were above 160/100. Patients were excluded and treated appropriately if repeated measurements showed values persistently above 160/100 mmHg and/or there was definite evidence for accelerated hypertension or target organ damage. Aspirin and vitamin supplements were also stopped 1 week before participation in the study. The local institutional review boards in Rome, Italy, approved the study protocol, and all participants gave written informed consent.
Protocols
All studies were performed in the morning in a quiet room with a temperature of ∼22°C. Each study consisted of infusions of drugs into the brachial artery and measurement of forearm blood flow (FBF) by means of strain-gauge venous occlusion plethysmography. All drugs used in this study were prepared by the local pharmaceutical service following specific procedures to ensure accurate bioavailability and sterility of the solutions. Participants were asked to refrain from smoking and drinking alcohol or beverages containing caffeine for at least 24 h and to fast for at least 8 h before the studies. While participants were supine, a 20-gauge Teflon catheter (Arrow Inc., Limerick, PA) was inserted into the brachial artery of the nondominant arm (left in most cases) for drug infusion. Another 20-gauge catheter (Abbott Laboratories, Abbott Park, IL) was inserted into a deep antecubital vein of the same arm for blood sampling. The extended arm was positioned slightly above the level of the right atrium, and a mercury-filled strain gauge was placed around the widest part of the forearm. The strain gauge was connected to a plethysmograph (model EC-6; Hokanson Inc., Bellevue, WA) calibrated to measure the percent change in volume and connected to a personal computer through an analog-to-digital converter. For each measurement, a cuff placed around the upper arm was inflated to 40 mmHg with a rapid cuff inflator (model E-10; Hokanson Inc.) to occlude venous outflow from the extremity. A wrist cuff was inflated to suprasystolic pressures 1 min before each measurement to exclude the hand circulation. Flow measurements were recorded for ∼7 s every 15 s; seven readings were obtained for each mean value. Blood pressure was recorded with the use of a standard mercury manometer. Throughout all studies, volumes infused were matched by administration of variable amounts of saline.
Protocol 1: Assessment of the effects of GLP-1 on vascular reactivity during hyperinsulinemia in MetS patients and in control subjects
To determine the effects of GLP-1 on forearm vascular responses during hyperinsulinemia, five patients with the MetS and five metabolically healthy obese control subjects were enrolled in this protocol (Supplementary Fig. 1). After the forearm was instrumented, baseline blood samples were collected and normal saline was given intra-arterially for 15 min, at which point an infusion of regular insulin (Humulin; Eli Lilly, Indianapolis, IN) at 0.1 mU/kg/min (1 mL/min infusion rate) was started in the same line. To avoid any confounding effect related to changes in glycemia, during insulin administration plasma glucose levels were determined every 15 min until the steady state was reached (in ∼60 min) and every 30 min thereafter; simultaneously, an infusion of 20% dextrose into a contralateral arm vein was adjusted to maintain glucose levels at values similar to baseline. The doses of glucose needed to maintain glycemic levels were generally very small in all participants. At the end of this period, venous blood samples were again collected from the instrumented arm for insulin measurement. After 45 min, basal FBF was measured and dose-response curves to the endothelium-dependent vasodilator ACh chloride (ACh; Clinalfa, Läufelfingen, Switzerland) and the exogenous nitric oxide (NO) donor sodium nitroprusside (SNP; Malesci, Florence, Italy) were obtained as reported previously (6). The sequence of ACh and SNP infusion was randomized to avoid bias related to the order of these procedures. The baseline FBF was then measured again, and an intra-arterial infusion of GLP-1(7-36) (Bachem AG, Bubendorf, Switzerland) at 20 pmol/min (1 mL/min infusion rate) was started. This dose was aimed to achieve intravascular concentrations of GLP-1 within the infused forearm, similar to those previously demonstrated able to normalize plasma glucose levels in patients with type 2 diabetes (13), and to improve vascular response to vasodilators in the human forearm (9). After 45 min, venous blood samples were again collected from the instrumented arm for GLP-1 measurement, basal FBF was reassessed, and the dose-response curves to ACh and SNP were repeated as before.
Protocol 2: Assessment of the effects of GLP-1 on vascular reactivity of MetS patients in the absence of hyperinsulinemia
To assess whether, in patients with the MetS, GLP-1 affects endothelium-dependent and -independent vascular reactivity in the absence of hyperinsulinemia, five participants were enrolled in the following protocol (Supplementary Fig. 2). After the forearm was instrumented, normal saline was infused intra-arterially for 15 min, basal FBF was measured, and dose-response curves to ACh and SNP were obtained as in protocol 1. After measurement of a new baseline FBF, an intra-arterial infusion of GLP-1(7-36) at 20 pmol/min (1 mL/min infusion rate) was then started. After 30 min, baseline FBF was reassessed and the dose-response curves to ACh and SNP were repeated according to the same protocol and in the same order as detailed above.
Protocol 3: Assessment of the effect of GLP-1 on vasodilator function during hyperinsulinemia and vitamin C infusion in MetS patients
To assess whether the effects of GLP-1 on vascular reactivity in patients with the MetS are influenced by enhanced oxidative stress, further studies were performed by use of the antioxidant vitamin C. To this purpose, five additional patients with the MetS were recruited for participation in the following protocol (Supplementary Fig. 3). After the forearm was instrumented, the intra-arterial infusion of regular insulin, as described in protocol 1, and of vitamin C (Bracco, Milan, Italy) was started. During insulin administration, plasma glucose was determined every 15 min until the steady state was reached (in ∼60 min) and every 30 min thereafter; simultaneously, 20% dextrose was infused at variable rates to maintain glucose levels at values similar to baseline. Vitamin C was administered at a rate of 25 mg/min (1 mL/min infusion rate), a dose intended to produce maximal suppression of oxidative stress and previously proved effective to prevent premature NO deactivation by superoxide anions (14). After 15 min, baseline FBF was measured and dose-response curves to ACh and SNP were obtained as detailed in protocol 1. A 20-min rest period was then allowed, and an intra-arterial infusion of GLP-1 (7-36), at the same dose as in protocol 1, was started. After 45 min, the same dose-response curves to ACh and SNP were repeated.
Protocol 4: Assessment of the effects of the metabolite GLP-1(9-36) on vascular reactivity during hyperinsulinemia in MetS patients
To verify whether the enhancing effects on the vascular responses to ACh and SNP during hyperinsulinemia extend to GLP-1(9-36), the NH2-terminal truncated metabolite of GLP-1(7-36), five additional MetS patients were studied according to the following protocol (Supplementary Fig. 4). After the forearm was instrumented, normal saline was given intra-arterially for 15 min, at which point an infusion of regular insulin at 0.1 mU/kg/min was started in the same line. During insulin administration, plasma glucose was determined every 15 min until the steady state was reached (in ∼60 min) and every 30 min thereafter; simultaneously, 20% dextrose was infused at variable rates to maintain glucose levels at values similar to baseline. The same dose-response curves to ACh and SNP as detailed in protocol 1 were then obtained. Subsequently, a 20-min rest period was allowed and an intra-arterial infusion of GLP-1(9-36) (Bachem AG) at the same dose as in protocol 1 was started. After 45 min, a basal FBF measurement was obtained; the dose-response curves to ACh and SNP were then repeated in the same fashion as before.
Protocol 5: Assessment of the effects of GLP-1 on forearm glucose disposal during hyperinsulinemia in MetS patients
To determine whether GLP-1 affects insulin-induced forearm glucose disposal, we enrolled five additional patients with the MetS in the following protocol (Supplementary Fig. 5). After the forearm was instrumented, normal saline was given intra-arterially for 15 min, followed by the infusion of regular insulin at 0.1 mU/kg/min. Throughout the administration of insulin, venous blood samples for the measurement of glucose and insulin levels were drawn from an ipsilateral deep vein at 15-min intervals until the steady state was reached (in ∼60 min) and every 30 min thereafter, while a simultaneous infusion of 20% dextrose in a contralateral arm vein was adjusted to clamp systemic glucose levels at the baseline value.
Forearm fractional glucose extraction (FGE), calculated as ([Gart] − [Gven] / [Gart] × 100), and forearm glucose uptake (FGU), calculated as ([Gart] − [Gven]) × FBF), where Gart is the arterial concentration of glucose and Gven is the venous concentration of glucose, were determined after 90 min of infusion of insulin alone. GLP-1(7-36) infusion was then superimposed to insulin at the same dose as in protocol 1, and FGE and FGU were assessed again after 60 min of combined infusion.
Analytical procedures
Glucose was determined in duplicate by the glucose oxidase method on a glucose analyzer (Beckman Instruments, Fullerton, CA). Insulin plasma concentrations were determined by electrochemiluminescent immunoassay (Roche Diagnostics, Mannheim, Germany). Plasma GLP-1(7-36) levels were measured by ELISA (Millipore, Billerica, MA), with the lowest level of detection at 2 pmol/L and no cross-reactivity to GLP-1(9-36).
Statistical analysis
Group comparisons were performed by unpaired t test and two-way ANOVA, as appropriate. Within-group analyses were performed by paired t test, one-way ANOVA, and two-way ANOVA for repeated measures, as appropriate. All calculated probability values are two-tailed, and a P value <0.05 was considered statistically significant. All group data are reported as mean ± SEM.
RESULTS
A total of 25 patients with the MetS and five metabolically healthy obese control subjects participated in this investigation. Their baseline anthropometric and biochemical characteristics are reported in the Supplementary Table 1. None of the participants was engaged in a formal exercise program, and the levels of physical activity were comparable between the two groups. Mean arterial pressure and heart rate did not change significantly after infusion of any of the drugs used in the study, thus indicating that the drug effects were limited to the infused forearm and did not extend to the systemic circulation.
In patients with the MetS participating in studies with hyperinsulinemia, forearm insulin plasma levels were 16 ± 3 μU/mL at baseline and rose to 198 ± 39 μU/mL following intra-arterial infusion of insulin (P < 0.001 vs. baseline). Effluent venous forearm GLP-1(7-36) plasma levels in patients with MetS receiving intra-arterial infusion of exogenous GLP-1 were 8.3 ± 0.1 pmol/L at baseline and rose to 22.3 ± 1.4 pmol/L after GLP-1 infusion (P < 0.001 vs. baseline).
Effects of GLP-1 on vasodilator reactivity during hyperinsulinemia in MetS patients and in control subjects
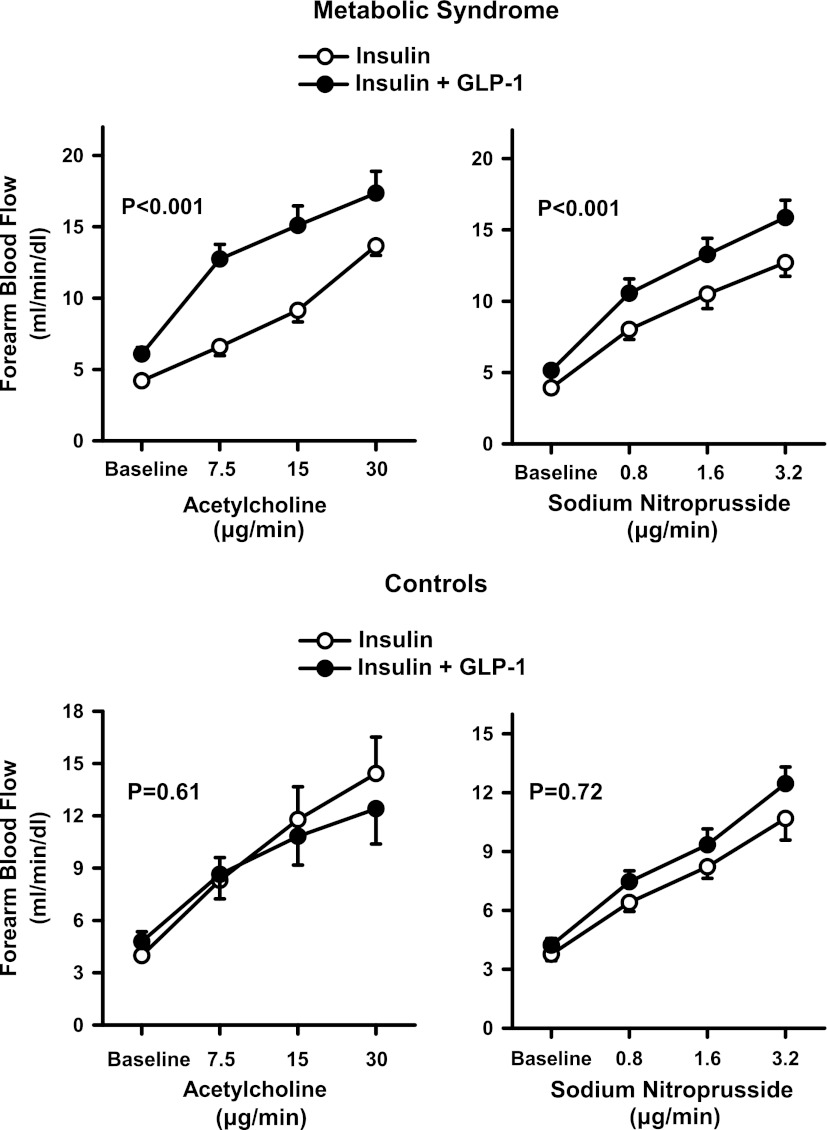
During insulin infusion, the administration of escalating doses of ACh and SNP resulted in a progressive increase in FBF from baseline (P < 0.001 for both). When the ACh and SNP curves were repeated after GLP-1 had been added on top of the insulin infusion, the vasodilator responses to these drugs were enhanced, so that the FBF increases were significantly higher during GLP-1 administration than during insulin infusion alone (Fig. 1, top). In the metabolically healthy obese control subjects, in the absence of GLP-1, FBF response to ACh during hyperinsulinemia tended to be higher than in patients with the MetS (17.2 ± 3.2 mL/min/dL vs. 14.0 ± 2.3 mL/min/dL at the highest dose, respectively; P = 0.09) and vascular response to SNP was significantly higher than in patients with the MetS (15.5 ± 2.1 mL/min/dL vs. 11.7 ± 1.8 mL/min/dL at the highest dose, respectively; P = 0.02). In contrast with the results obtained in patients with the MetS, however, in these subjects the addition of GLP-1 did not significantly affect ACh- and SNP-induced forearm vasodilation when compared with the response observed during insulin infusion alone (Fig. 1, bottom).
Figure 1.
Plots showing FBF responses to intra-arterial infusion of escalating doses of ACh (left) and SNP (right), during the concomitant infusion of insulin alone (○) or insulin and GLP-1 (●) in the MetS patients (top) and metabolically healthy obese control subjects (bottom). The P values refer to the comparisons of vascular responses under different conditions by two-way ANOVA for repeated measures. All values are means ± SEM.
Effects of GLP-1 on baseline vascular reactivity in MetS patients
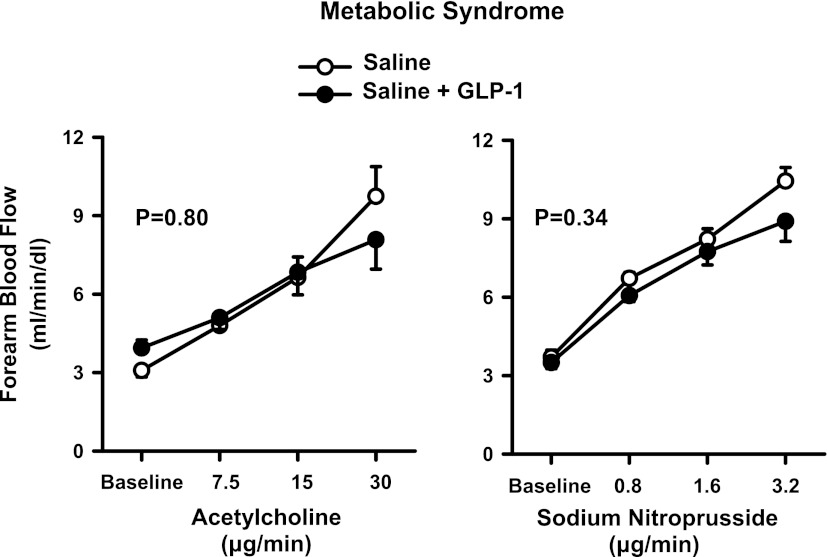
During saline administration, the infusion of escalating doses of ACh and SNP resulted in a progressive increase in FBF from baseline (P < 0.001 for both drugs). No significant changes in the vasodilator responses to ACh and SNP were observed after GLP-1 had been added to the infusion with saline alone (Fig. 2).
Figure 2.
Plots showing FBF responses to intra-arterial infusion of escalating doses of ACh (left) and SNP (right) during the concomitant infusion of saline (○) or GLP-1 (●) in the MetS patients. The P values refer to the comparisons of vascular responses under different conditions by two-way ANOVA for repeated measures. All values are means ± SEM.
Effect of vitamin C on vasodilator function during hyperinsulinemia in MetS patients
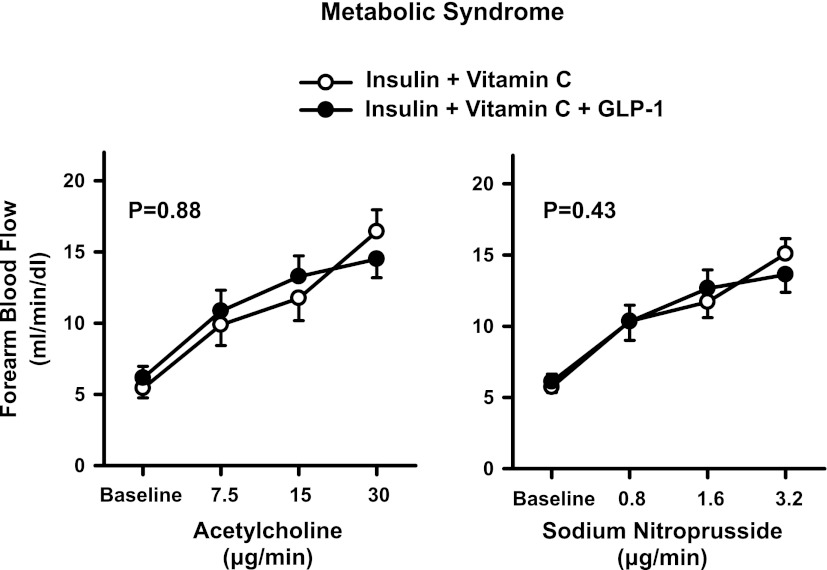
In the MetS patients, during the concomitant infusion of insulin and vitamin C, increasing doses of ACh and SNP resulted in a progressive rise in FBF from baseline (P < 0.001 for both). In this group of patients, no significant changes in FBF responses to ACh and SNP were observed when GLP-1 infusion was added on top of insulin and vitamin C (Fig. 3).
Figure 3.
Plots showing FBF responses to intra-arterial infusion of escalating doses of ACh (left) and SNP (right) during the concomitant infusion of insulin and vitamin C (○) or insulin and vitamin C and GLP-1 (●) in the MetS patients. The P values refer to the comparisons of vascular responses to ACh and SNP under different conditions by two-way ANOVA for repeated measures. All values are means ± SEM.
Effects of the metabolite GLP-1(9-36) on vascular reactivity during hyperinsulinemia in MetS patients
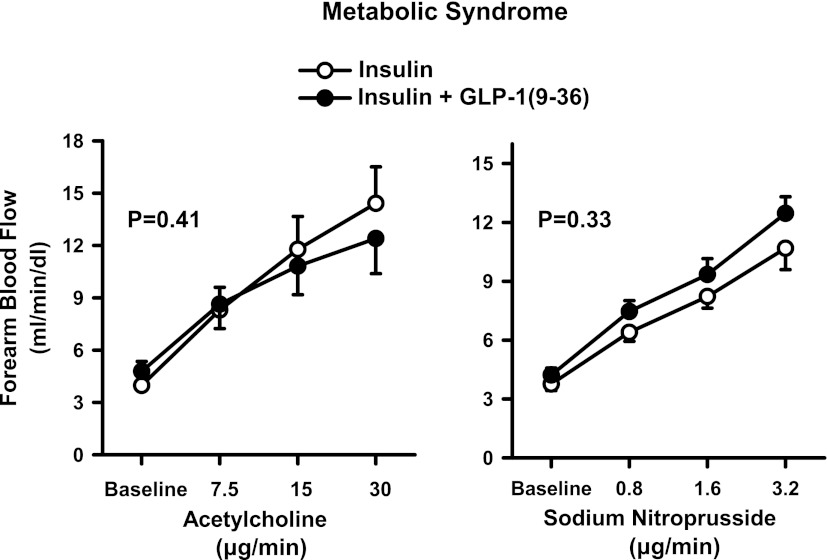
During insulin administration, the infusion of escalating doses of ACh and SNP resulted in a progressive increase in FBF from baseline (P < 0.001 for both drugs). In contrast with the results obtained during the infusion of GLP-1(7-36), no significant changes in the vasodilator responses to ACh and SNP were observed after GLP-1(9-36) was added to the insulin infusion (Fig. 4).
Figure 4.
Plots showing FBF responses to intra-arterial infusion of escalating doses of ACh (left) and SNP (right) during the concomitant infusion of insulin alone (○) or insulin and GLP-1(9-36) (●) in the MetS patients. The P values refer to the comparisons of vascular responses under different conditions by two-way ANOVA for repeated measures. All values are means ± SEM.
Effect of GLP-1 on FGU during hyperinsulinemia in MetS patients
In this protocol, FGE and FGU responses to hyperinsulinemia were measured before and after the addition of exogenous GLP-1. Before infusion of GLP-1, FGE was 20 ± 6% and FGU was 10.2 ± 2.4 mg/min/dL; after infusion of exogenous GLP-1, FGE was 21 ± 5% and FGU was 11.1 ± 2.8 mg/min/dL (P = 0.17 and P = 0.43, respectively, vs. insulin alone).
CONCLUSIONS
The main novel finding of the current study is that GLP-1(7-36) improves both endothelium-dependent and -independent NO-mediated vasodilator responsiveness during hyperinsulinemia in patients with the MetS. It is interesting that this enhancing effect of GLP-1(7-36) on forearm vasodilator reactivity was not observed in the absence of hyperinsulinemia. Furthermore, even in the presence of high circulating insulin levels, GLP-1(7-36) did not modify vasodilator responses to both ACh and SNP in obese control subjects without the MetS, hence supporting the specificity of the effect of GLP-1(7-36) to ameliorate the abnormal vascular responsiveness observed in patients with the MetS.
Even though a number of recent studies have established that GLP-1 exerts beneficial actions on vascular function, the underlying mechanisms still remain controversial. Thus, some investigations have suggested that GLP-1 is provided of vasorelaxing properties mediated by endothelial release of NO (15,16). Other studies, however, have not confirmed these results, indicating that GLP-1 may act by directly relaxing vascular smooth muscle independently of the endothelium (17,18). In the MetS patients participating in our study, GLP-1(7-36) did not improve the responsiveness to ACh in the absence of hyperinsulinemia, hence suggesting that GLP-1–related mechanism does not play a primary role to ameliorate the endothelial dysfunction commonly seen in this population (19). This finding is apparently at odds with previous observation in the forearm microcirculation of healthy subjects (9) and in conductance arteries of diabetic patients with coronary artery disease (10), showing that infusion of exogenous GLP-1 ameliorates endothelium-dependent vasodilator responsiveness. Although the exact reasons for these discrepancies remain unclear, it must be noted that in our study GLP-1 was infused locally, whereas in previous investigations it had been given systemically; this suggests that other potential factors might have been involved in the effects of GLP-1on endothelial function reported by those studies.
Previous work in our laboratory has shown that patients with the MetS have a generalized defect in insulin-stimulated responsiveness to a wide variety of vasodilator stimuli, suggestive of abnormality in insulin signaling in vascular smooth muscle cells (VSMCs) (6). Our current observation that GLP-1 favorably impacts forearm vascular reactivity to both ACh and SNP only in the presence of hyperinsulinemia, therefore, is consistent with an action of the peptide to restore the facilitatory role of insulin on VSMCs vasodilator capacity in patients with the MetS.
Strong evidence suggests that impaired insulin receptor function and/or intracellular signaling coupling are central mechanisms in the altered reactivity of resistance vessels observed in insulin-resistant states and that augmented generation/availability of reactive oxygen species (ROS) has a major causative effect (20). It is important that insulin has been shown to induce ROS production in all the arterial layers of insulin-resistant rat models (21) and treatment with antioxidants has demonstrated to immediately restore vascular function in these models (22,23). In the current study, we tested the possibility that increased oxidative stress during hyperinsulinemia in patients with the MetS could be involved in the vascular effects of GLP-1 by infusion of the antioxidant vitamin C. Our results demonstrate that, during hyperinsulinemia, when GLP-1 is given on top of vitamin C it does not further enhance the vasodilator responsiveness to ACh and SNP. The most likely explanation of this finding is that, after ablating oxidative stress with vitamin C, GLP-1 is no longer effective because insulin-stimulated vasodilation has been restored. This interpretation would have been more straightforward if, in our study, vascular reactivity had been studied in the same patient both in the absence and the presence of vitamin C. Also, assessment of the vascular effects of vitamin C in a control group would have added to the specificity of these results. However, previous data obtained in our laboratory have clearly shown within-patient improvement of vascular responsiveness to vasodilators during hyperinsulinemia after infusion of vitamin C in patients with the MetS (6). Moreover, as part of that study, we also evaluated the effect of vitamin C on insulin-stimulated vascular responsiveness in a control group without the MetS (n = 5) and we did not observe any significant change in the response to both ACh and SNP (unpublished data). Considering these potential limitations in the interpretation of our data, therefore, the possibility that GLP-1 might exert vascular benefits in the MetS by directly reducing oxidative stress cannot be completely ruled out. This view is supported by the results of previous studies in experimental models, showing that GLP-1 reduces lipid peroxidation in streptozotocin-induced diabetic rats (24). Similarly, in Zucker diabetic fatty rats, increased GLP-1 levels after dipeptidyl peptidase-4 inhibition attenuated ROS-induced vascular senescence in a receptor-dependent manner (25). In addition or alternatively to a possible direct antioxidant effect of GLP-1, another putative mechanism to explain our findings relies on the fact that ROS may affect vascular reactivity via direct actions on ion channels. In particular, ROS-dependent mechanisms impair arterial ATP-sensitive potassium K+ channels (26), which are widely expressed on both endothelium and VSMCs. Because GLP-1 has been shown to induce endothelium-independent vasorelaxation through stimulation of the ATP-sensitive potassium K+ channel (17), it is possible that this mechanism might be involved in restoring normal vasodilator responsiveness in the MetS.
Because the active circulating GLP-1(7-36) is rapidly cleaved by DDP-4 into the metabolite GLP-1(9-36), we also evaluated whether the cleavage product could share similar vascular effects in patients with MetS. This aspect may be of clinical relevance, since either DDP-4–resistant GLP-1 analogs or DDP-4 inhibitors to prevent GLP-1(7-36) degradation are used for treatment of type 2 diabetes. Therefore, if GLP-1(9-36) shares the beneficial vascular effects of GLP-1(7-36), then inhibition of DDP-4 may not be the best choice. To this end, experiments were performed to assess vascular responses to vasodilator agents during hyperinsulinemia in patients with the MetS, before and after infusion of exogenous GLP-1(9-36). These studies showed that GLP-1(9-36) does not affect insulin-stimulated vascular reactivity to ACh and SNP in these patients, thereby supporting the notion of a receptor-specific effect of GLP-1(7-36) to ameliorate vasodilator responsiveness in this setting. Our results are in keeping with those of recent studies showing that GLP-1(9-36) is devoid of the cardiovascular actions of GLP-1(7-36) in conscious rats (27). By contrast, these findings are at odds with those studies showing that GLP-1(7-36) and GLP-1(9-36) share comparable NO-dependent vasodilator properties in isolated mouse mesenteric arteries (9). Even though the precise reasons are not entirely clear, these discrepancies might be explained by differences in species and vascular beds; alternatively, and more likely, the concentrations of GLP-1(9-36) to which isolated vessels are exposed are much higher than those that can be achieved in the in vivo circulation.
Previous studies have reported controversial findings with regard to the possible effect of GLP-1 to increase glucose disposal in insulin-sensitive tissues. In our study, infusion of GLP-1 did not enhance insulin-stimulated FGE and FGU by the forearm skeletal muscle of patients with the MetS. These results are at odds with those of previous investigations reporting that long-term treatment with GLP-1 enhances insulin sensitivity in patients with type 2 diabetes (28). In keeping with our findings, however, other investigators have reported that acute infusion of exogenous GLP-1 does not significantly modify insulin sensitivity in patients with type 2 diabetes and coronary artery disease (10). Beyond differences in study methodologies and infused doses of GLP-1, the main reason for these discrepancies seems related to GLP-1 administration times. Thus, prolonged infusion of the peptide or chronic treatments targeting GLP-1 receptors seems necessary to improve glucose disposal in insulin-resistant patients. Therefore, the acute effects of GLP-1 to improve vasodilator reactivity observed in our study were independent of changes in insulin sensitivity and glucose metabolism in insulin-resistant patients with the MetS.
There are some limitations of the current study that should be acknowledged. Because of the demanding nature of the protocols, our study enrolled a limited number of participants for each group, which may have reduced our statistical power. However, despite the small size of the groups, we were able to detect statistically significant effects of GLP-1 infusion during hyperinsulinemia in patients with the MetS. Therefore, the effect that we may have possibly missed in the other experimental groups because of a type II error would be small. Also, because our study was performed in the intact human circulation in vivo, a limit inherent with its methodology is the inability to fully explore the precise molecular mechanisms involved. Finally, enrollment of a proper control group for all the protocols carried out in this study would have certainly strengthened the interpretation of our results.
In conclusion, the results of our study indicate that GLP-1 improves insulin-stimulated vasodilator responsiveness in patients with obesity-related MetS, likely acting through a receptor-mediated mechanism influenced by vascular oxidative stress. Our findings bear potential clinical implications by suggesting that in these patients GLP-1–based treatments provide additional benefits beyond glycemic control and that GLP-1 receptor is an important therapeutic target for vascular prevention.
Supplementary Material
Acknowledgments
This work was supported by a grant from Merck Sharp & Dohme and the Fondazione Roma to M.T. U.C. is supported by National Heart, Lung, and Blood Institute, National Institutes of Health, Grant K12-HL083790. C.C. is supported by Fondi d’Ateneo grants from Università Cattolica del Sacro Cuore. No other potential conflicts of interest relevant to this article were reported.
M.T. wrote the protocol and researched the data. F.S., A.A., and V.R. recruited the patients and researched the data. F.M. researched the data. N.M. prepared the solutions and discussed the data. A.B. researched and discussed the data. D.P. and D.L. recruited the patients and contributed to the discussion. G.G. contributed to the discussion and reviewed the manuscript. U.C. wrote the manuscript. C.C. wrote the protocol and edited and reviewed the manuscript. C.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT00856700, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc12-0763/-/DC1.
A slide set summarizing this article is available online.
References
- 1.Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest 2000;106:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C, American Heart Association. National Heart, Lung, and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109:433–438 [DOI] [PubMed] [Google Scholar]
- 3.Clark MG. Impaired microvascular perfusion: a consequence of vascular dysfunction and a potential cause of insulin resistance in muscle. Am J Physiol Endocrinol Metab 2008;295:E732–E750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taddei S, Virdis A, Mattei P, Natali A, Ferrannini E, Salvetti A. Effect of insulin on acetylcholine-induced vasodilation in normotensive subjects and patients with essential hypertension. Circulation 1995;92:2911–2918 [DOI] [PubMed] [Google Scholar]
- 5.Rask-Madsen C, Domínguez H, Ihlemann N, Hermann T, Køber L, Torp-Pedersen C. Tumor necrosis factor-alpha inhibits insulin’s stimulating effect on glucose uptake and endothelium-dependent vasodilation in humans. Circulation 2003;108:1815–1821 [DOI] [PubMed] [Google Scholar]
- 6.Schinzari F, Tesauro M, Rovella V, et al. Generalized impairment of vasodilator reactivity during hyperinsulinemia in patients with obesity-related metabolic syndrome. Am J Physiol Endocrinol Metab 2010;299:E947–E952 [DOI] [PubMed] [Google Scholar]
- 7.Kim W, Egan JM. The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev 2008;60:470–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yoon JS, Lee HW. Understanding the cardiovascular effects of incretin. Diabetes Metab J 2011;35:437–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basu A, Charkoudian N, Schrage W, Rizza RA, Basu R, Joyner MJ. Beneficial effects of GLP-1 on endothelial function in humans: dampening by glyburide but not by glimepiride. Am J Physiol Endocrinol Metab 2007;293:E1289–E1295 [DOI] [PubMed] [Google Scholar]
- 10.Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 11.Sjöholm A. Impact of glucagon-like peptide-1 on endothelial function. Diabetes Obes Metab 2009;11(Suppl. 3):19–25 [DOI] [PubMed] [Google Scholar]
- 12.Ban K, Noyan-Ashraf MH, Hoefer J, Bolz SS, Drucker DJ, Husain M. Cardioprotective and vasodilatory actions of glucagon-like peptide 1 receptor are mediated through both glucagon-like peptide 1 receptor-dependent and -independent pathways. Circulation 2008;117:2340–2350 [DOI] [PubMed] [Google Scholar]
- 13.Meier JJ, Gallwitz B, Salmen S, et al. Normalization of glucose concentrations and deceleration of gastric emptying after solid meals during intravenous glucagon-like peptide 1 in patients with type 2 diabetes. J Clin Endocrinol Metab 2003;88:2719–2725 [DOI] [PubMed] [Google Scholar]
- 14.Jackson TS, Xu A, Vita JA, Keaney JF., Jr Ascorbate prevents the interaction of superoxide and nitric oxide only at very high physiological concentrations. Circ Res 1998;83:916–922 [DOI] [PubMed] [Google Scholar]
- 15.Richter G, Feddersen O, Wagner U, Barth P, Göke R, Göke B. GLP-1 stimulates secretion of macromolecules from airways and relaxes pulmonary artery. Am J Physiol 1993;265:L374–L381 [DOI] [PubMed] [Google Scholar]
- 16.Golpon HA, Puechner A, Welte T, Wichert PV, Feddersen CO. Vasorelaxant effect of glucagon-like peptide-(7-36)amide and amylin on the pulmonary circulation of the rat. Regul Pept 2001;102:81–86 [DOI] [PubMed] [Google Scholar]
- 17.Green BD, Hand KV, Dougan JE, McDonnell BM, Cassidy RS, Grieve DJ. GLP-1 and related peptides cause concentration-dependent relaxation of rat aorta through a pathway involving KATP and cAMP. Arch Biochem Biophys 2008;478:136–142 [DOI] [PubMed] [Google Scholar]
- 18.Nyström T, Gonon AT, Sjöholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept 2005;125:173–177 [DOI] [PubMed] [Google Scholar]
- 19.Kim JA, Montagnani M, Koh KK, Quon MJ. Reciprocal relationships between insulin resistance and endothelial dysfunction: molecular and pathophysiological mechanisms. Circulation 2006;113:1888–1904 [DOI] [PubMed] [Google Scholar]
- 20.Busija DW, Miller AW, Katakam P, Erdos B. Adverse effects of reactive oxygen species on vascular reactivity in insulin resistance. Antioxid Redox Signal 2006;8:1131–1140 [DOI] [PubMed] [Google Scholar]
- 21.Katakam PV, Tulbert CD, Snipes JA, Erdös B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. Am J Physiol Heart Circ Physiol 2005;288:H854–H860 [DOI] [PubMed] [Google Scholar]
- 22.Erdös B, Simandle SA, Snipes JA, Miller AW, Busija DW. Potassium channel dysfunction in cerebral arteries of insulin-resistant rats is mediated by reactive oxygen species. Stroke 2004;35:964–969 [DOI] [PubMed] [Google Scholar]
- 23.Erdös B, Snipes JA, Miller AW, Busija DW. Cerebrovascular dysfunction in Zucker obese rats is mediated by oxidative stress and protein kinase C. Diabetes 2004;53:1352–1359 [DOI] [PubMed] [Google Scholar]
- 24.Ozyazgan S, Kutluata N, Afşar S, Ozdaş SB, Akkan AG. Effect of glucagon-like peptide-1(7-36) and exendin-4 on the vascular reactivity in streptozotocin/nicotinamide-induced diabetic rats. Pharmacology 2005;74:119–126 [DOI] [PubMed] [Google Scholar]
- 25.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 2010;30:1407–1414 [DOI] [PubMed] [Google Scholar]
- 26.Bari F, Louis TM, Meng W, Busija DW. Global ischemia impairs ATP-sensitive K+ channel function in cerebral arterioles in piglets. Stroke 1996;27:1874-1880 [DOI] [PubMed] [Google Scholar]
- 27.Gardiner SM, March JE, Kemp PA, Bennett T, Baker DJ. Possible involvement of GLP-1(9-36) in the regional haemodynamic effects of GLP-1(7-36) in conscious rats. Br J Pharmacol 2010;161:92–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet 2002;359:824–830 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.