Abstract
OBJECTIVE
This study aims to describe body composition in term infants of mothers with gestational diabetes mellitus (GDM) compared with infants of mothers with normal glucose tolerance (NGT).
RESEARCH DESIGN AND METHODS
This cross-sectional study included 599 term babies born at Royal Prince Alfred Hospital, Sydney, Australia. Neonatal body fat percentage (BF%) was measured within 48 h of birth using air-displacement plethysmography. Glycemic control data were based on third-trimester HbA1c levels and self-monitoring blood glucose levels. Associations between GDM status and BF% were investigated using linear regression adjusted for relevant maternal and neonatal variables.
RESULTS
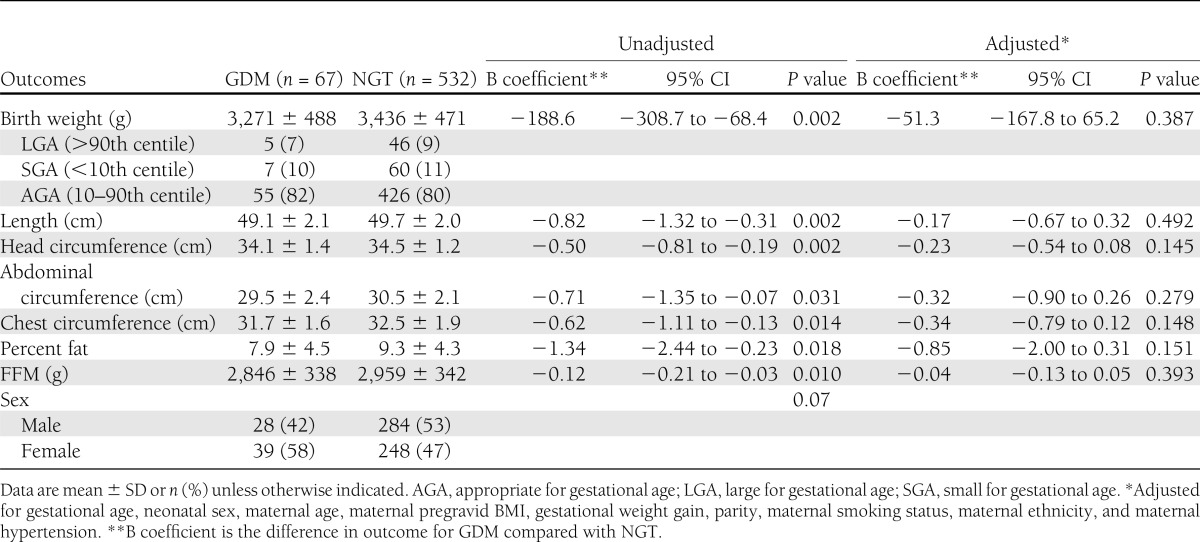
Of 599 babies, 67 (11%) were born to mothers with GDM. Mean ± SD neonatal BF% was 7.9 ± 4.5% in infants with GDM and 9.3 ± 4.3% in infants with NGT, and this difference was not statistically significant after adjustment. Good glycemic control was achieved in 90% of mothers with GDM.
CONCLUSIONS
In this study, neonatal BF% did not differ by maternal GDM status, and this may be attributed to good maternal glycemic control.
Fetal growth and development is affected through the altered intrauterine environment of gestational diabetes mellitus (GDM) (1,2). An accurate method to characterize overgrowth is by estimation of body composition, which includes fat mass (FM) and fat-free mass (FFM) (3,4). Previous studies have shown that increases in FM are present in infants of GDM pregnancies, regardless of their weight for gestational age (1,5). The gold-standard method of measuring body composition changes is air-displacement plethysmography (ADP) (4,6,7). The aim of this study was to describe body composition and anthropometric measurements at birth in term infants of women with GDM compared with infants of mothers with normal glucose tolerance (NGT) levels.
RESEARCH DESIGN AND METHODS
This was a cross-sectional study of singleton, term infants (37–42 weeks gestation) born between September and October 2010 at Royal Prince Alfred Hospital (RPAH), a major public teaching hospital in Sydney, Australia. Eligible infants were singleton, term babies with no congenital anomalies. For practical reasons, we a priori excluded babies who were admitted to the neonatal intensive care unit for >2 days.
The diagnosis of GDM was based on the Australasian Diabetes In Pregnancy Society (ADIPS) criteria at the time of the study (8). Dietary and physical activity advice was given to the mothers with GDM. They were requested to self-monitor their blood glucose levels (BGLs) (Accu-Chek; Roche Diagnostics, Mannheim, Germany) four times daily. Third-trimester means of preprandial BGLs for breakfast, as well as 1-h postprandial BGLs for breakfast, lunch, and dinner, were obtained by reviewing glucose logbooks. If logbooks were not accessible, progress notes were reviewed to see whether BGLs were within targets as assessed by the endocrinologist or diabetes educator. HbA1c levels were obtained from medical records. Insulin therapy was commenced when glycemic targets could not be met on dietary adjustment (9).
Neonatal anthropometric measurements were made within 48 h of birth and have been described previously (A.E. Carberry, C.H. Raynes-Greenow, R.M. Turner, L.M. Askie, H.E. Jeffery, unpublished data) (10). Neonatal body fat percentage (BF%), FM, and FFM were assessed at birth by ADP using the PEA POD (COSMED USA, Inc.) body composition system (4,6,7). The intraobservational precision for BF% using the PEA POD was 0.1%. Anthropometric measurements (birth weight, length, head, abdominal, and chest circumferences) were made to the nearest millimeter (10). Birth weight was measured using the digital scales on the PEA POD to within 0.1 g.
Associations between GDM status and neonatal body composition (BF% and FFM) and other anthropometric measurements were investigated using linear regression both unadjusted and adjusted for potential confounders. The potential confounders were gestational age, neonatal sex, maternal age, maternal pregravid BMI, gestational weight gain, parity, maternal smoking status, maternal ethnicity, and maternal hypertension. The association between neonatal BF% and maternal glycemic indices (mean fasting and postprandial BGLs and HbA1c) in the GDM group was assessed using correlation coefficients.
All analyses were conducted in SPSS (version 20.0.0; IBM, New York, NY). P values <0.05 were considered statistically significant.
The study was approved by the Human Research Ethics Committees of RPAH and the University of Sydney. Informed written parental consent was obtained, and participation was voluntary.
RESULTS
Eight hundred and fifteen mothers and their babies were approached for our study. Thirty-three were ineligible due to >2 days of neonatal intensive care unit admission, of which 2 were in the GDM group; 150 women were ineligible as they were discharged early before measurements could be taken, and 30 refused participation. A further three mothers had pregestational diabetes and were excluded. Thus, our study population consisted of 532 participants in the NGT group and 67 in the GDM group.
There was no significant difference in maternal age between the GDM (33.2 ± 4.7 years) and NGT (32.5 ± 5.1 years, P = 0.22) groups. There was a significant difference (P = 0.001) in the distribution of maternal ethnicity between the GDM and NGT groups: 27 (40%) versus 335 (63%) Caucasians, respectively; 36 (54%) versus 164 (31%) Asians, respectively; and 4 (6%) and 33 (6%) other ethnicities including African, Middle Eastern, and Polynesian, respectively. Mothers with GDM were more likely to be overweight or obese (36 compared with 22%; P = 0.011).
Good glycemic control was achieved in most subjects, with 56 of 62 (90%) women meeting both fasting and postprandial ADIPS targets at the time of study. Mean ± SD third-trimester HbA1c for the whole GDM group was 5.4 ± 0.4 mmol/L. We obtained self-monitoring data for 46 women (mean of 132 readings per patient): mean ± SD BGLs were 4.8 ± 0.5 mmol/L fasting, 6.7 ± 1.1 mmol/L 1-h postbreakfast, 6.4 ± 0.7 mmol/L postlunch, and 6.5 ± 0.7 mmol/L postdinner.
After adjusting for gestational age, neonatal sex, and maternal variables known to influence body composition (Table 1), there was no significant difference in BF% between the GDM and NGT infants (mean difference −0.85 [95% CI −2.00 to 0.31]; P = 0.151) (Table 1). Similarly, after adjustment, there were no significant differences between the GDM and NGT infants in terms of birth weight and other anthropometric measurements.
Table 1.
Neonatal anthropometric data for GDM versus NGT infants
CONCLUSIONS
To our knowledge, this is the first study to demonstrate that normal neonatal body composition can be achieved in infants born to mothers with GDM with good glycemic control. Previous studies have reported increased FM and birth weight, as well as disproportionate anthropometry (decreased head-to-shoulder ratio) in infants of mothers with GDM compared with infants born to nondiabetic mothers (1,11,12). A recent study using ADP found a higher mean BF% in GDM infants of 12.1% (5). It is difficult for us to make comparisons with that study because the proportion of GDM control, as well as maternal characteristics such as pregravid BMI and ethnicity, are different, and this may account for the differences in BF%.
Nevertheless, the degree of glycemic control achieved in our mothers with GDM was consistent with the recent consensus guidelines from ADIPS, which recommend a fasting BGL ≤5.0 mmol/L and 1-h postprandial BGL ≤7.4 mmol/L (13). It was therefore reassuring that neonatal BF% was normalized with good maternal glycemic control and establishes that this is the benchmark for other clinical settings.
This study suggests that fetal adiposity is corrected with the treatment and control of GDM, and thus early detection and treatment of GDM can be a means to prevent neonatal overgrowth, which is strongly related to childhood obesity and diabetes. Our future work includes follow-up of our cohort to evaluate long-term outcomes.
Acknowledgments
The PEA POD was funded by Tenix Pty. Ltd. and an anonymous donor from the Faculty of Medicine at the University of Sydney. C.H.R.-G. is supported by the Australian National Health and Medical Research Council Early Career Fellowship 511481. R.M.T. was supported by National Health and Medical Research Council Grant 633003 to the Screening and Test Evaluation Program.
No potential conflicts of interest relevant to this article were reported.
C.P.A. contributed to the data design and acquisition of data, analyzed the data, and wrote the manuscript. C.H.R.-G. designed the study, contributed to the analysis and interpretation of data and the discussion, and reviewed and edited the manuscript. R.M.T. provided statistical advice and reviewed and edited the manuscript. A.E.C. assisted with the study design and supervised data collection. H.E.J. designed the study; supervised data collection; contributed to data analysis, interpretation, and discussion; and reviewed and edited the manuscript. C.P.A. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
These data were presented as an oral paper at the ADIPS Annual Scientific Meeting, Gold Coast, Australia, 31 August–1 September 2012 and the 16th Annual Congress of the Perinatal Society of Australia and New Zealand and 17th Congress of the Federation of Asia and Oceania Perinatal Societies, Sydney, Australia, 18–21 March 2012 and as a poster at the GP11 conference of the Royal Australian College of General Practitioners, Hobart, Tasmania, 6–8 October 2011 and at GP12, Gold Coast, Australia, 25–27 October 2012. This study was also published in abstract form in The Journal of Paediatrics and Child Health [Au CP, Raynes-Greenow CH, Carberry AE, Jeffery HE. Body composition and neonatal outcomes of infants born to mothers with gestational diabetes mellitus. J Paediatr Child Health 2012;48(Suppl. 1):79].
The authors thank Dr. Glynis Ross, RPAH endocrinologist, for access to patient data and helpful suggestions; Lucia Wang, Erin Donnelley, and Elizabeth Hayles from the University of Sydney and Meredith Li, midwife at RPAH, for assistance in data collection; and the RPAH Midwifery and Neonatal staff and the mothers and babies who participated in the study.
References
- 1.Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol 2003;189:1698–1704 [DOI] [PubMed] [Google Scholar]
- 2.Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: associations with neonatal anthropometrics. Diabetes 2009;58:453–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carberry AE, Colditz PB, Lingwood BE. Body composition from birth to 4.5 months in infants born to nonobese women. Pediatr Res 2010;68:1–5 [DOI] [PubMed] [Google Scholar]
- 4.Ellis KJ, Yao M, Shypailo RJ, Urlando A, Wong WW, Heird WC. Body-composition assessment in infancy: air-displacement plethysmography compared with a reference 4-compartment model. Am J Clin Nutr 2007;85:90–95 [DOI] [PubMed] [Google Scholar]
- 5.Lingwood BE, Henry AM, d’Emden MC, et al. Determinants of body fat in infants of women with gestational diabetes mellitus differ with fetal sex. Diabetes Care 2011;34:2581–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma G, Yao M, Liu Y, et al. Validation of a new pediatric air-displacement plethysmograph for assessing body composition in infants. Am J Clin Nutr 2004;79:653–660 [DOI] [PubMed] [Google Scholar]
- 7.Sainz RD, Urlando A. Evaluation of a new pediatric air-displacement plethysmography for body-composition assessment by means of chemical analysis of bovine tissue phantoms. Am J Clin Nutr 2003;77:364–370 [DOI] [PubMed] [Google Scholar]
- 8.Hoffman L, Nolan C, Wilson JD, Oats JJ, Simmons D, The Australasian Diabetes in Pregnancy Society Gestational diabetes mellitus—management guidelines. Med J Aust 1998;169:93–97 [DOI] [PubMed] [Google Scholar]
- 9.Ross GP. Gestational diabetes. Aust Fam Physician 2006;35:392–396 [PubMed] [Google Scholar]
- 10.Wood AJ, Raynes-Greenow CH, Carberry AE, Jeffery HE. Neonatal length inaccuracies in clinical practice and related percentile discrepancies detected by a simple length-board. J Paediatr Child Health. In press [DOI] [PubMed] [Google Scholar]
- 11.Ahlsson F, Lundgren M, Tuvemo T, Gustafsson J, Haglund B. Gestational diabetes and offspring body disproportion. Acta Paediatr 2010;99:89–93 [DOI] [PubMed] [Google Scholar]
- 12.McFarland MB, Trylovich CG, Langer O. Anthropometric differences in macrosomic infants of diabetic and nondiabetic mothers. J Matern Fetal Med 1998;7:292–295 [DOI] [PubMed] [Google Scholar]
- 13.Nankervis A, McIntyre D, Moses R, Ross GP, Callaway L; The Australasian Diabetes in Pregnancy Society. ADIPS Consensus Guidelines for the Testing and Diagnosis of Gestational Diabetes Mellitus in Australia 2012 [Internet]. Available from http://adips.org/images/stories/documents/2012_gdm_guidelines.pdf Accessed 1 August 2012


