Abstract
Central nervous system (CNS) axons recover poorly following injury because of the expression of myelin-derived inhibitors of axonal outgrowth such as Nogo, myelin-associated glycoprotein (MAG), and oligodendrocyte-myelin glycoprotein (OMgp), all of which bind to the Nogo-66 receptor 1 (NgR1). Herein we examine the role of NgR1 in the recovery of motor and cognitive function after traumatic brain injury (TBI) using a controlled cortical impact (CCI) model in NgR1 knockout (KO) and wild-type (WT) mice. Four weeks post-injury, scores on the Novel Object Recognition test were significantly increased in NgR1 KO mice compared with WT mice (p<0.05), but motor behavior test scores did not differ significantly between the two groups. Nissl staining showed that NgR1 KO mice had less brain injury volume 2 weeks after CCI (p<0.05). Histological analysis revealed more doublecortin (DCX+) cells (p<0.01) and more Ki-67+ cells in the contralateral dentate gyrus (DG) (p<0.05) 2 weeks after CCI in NgR1 KO mice than in WT. Furthermore, DCX+ cells still retained their longer processes in KO mice (p<0.01) 4 weeks following trauma. The number of bromodeoxyuridine (BrdU)+ cells did not differ between the two groups at 4 weeks post-trauma, but KO mice had higher numbers of cells that co-stained with NeuN, a marker of mature neurons. Increased transcription of growth-associated protein (GAP)-43 in both the injured and contralateral sides of the hippocampus (both p<0.05) was detected in NgR1 KO mice relative to WT. These data suggest that NgR1 negatively influences plasticity and cognitive recovery after TBI.
Key words: cognitive function, locomotor function, Nogo receptors, recovery
Introduction
Traumatic brain injury (TBI) is a leading cause of mortality and morbidity in developed countries,1,2 causing ∼52,000 deaths and also currently causing > 5,300,000 Americans to live with long-term disability.3,4 In addition to physical, emotional, and behavioral dysfunction, TBI leads to substantial disability caused by cognitive impairments in attention, memory, and executive function.4 Although evidence has demonstrated that TBI-associated cognitive impairment is neurophysiologically based,4 no current treatment is able to adequately remediate the anatomical or functional loss caused by TBI.
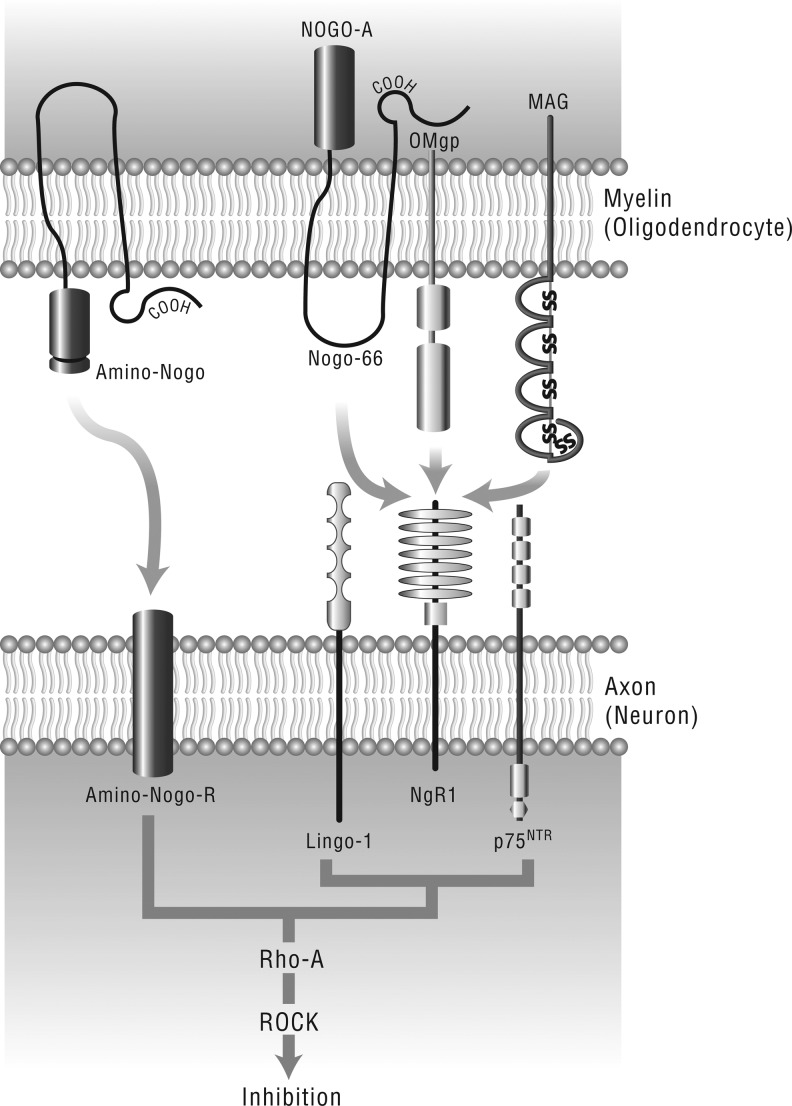
Although portions of the adult brain such as the dentate gyrus (DG) of the hippocampus and the olfactory bulb retain plasticity, most central nervous system (CNS) axons have a limited ability to regenerate following trauma.5 In adults, severed axons in the CNS re-seal and then form a growth cone; however, they do not extend.6 Neuronal regeneration ability is affected by the availability of neurotrophic growth factors such as brain-derived neurotrophic factor (BDNF),7,8 as much as by the limitations imposed by the growth-inhibitive CNS myelin proteins such as myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), and Nogo, all of which bind with high affinity to the Nogo-66 receptor (NgR1)9–13 (Fig. 1) NgR1 is a glycosylphosphatidylinositol-linked cell surface receptor that interacts with p75 neurotrophin receptor and the leucine-rich repeat (LRR) and immunoglobulin (Ig) domain-containing Nogo receptor-interacting protein (LINGO-1) to transduce the growth inhibitory signal from the myelin proteins across the cell membrane.14,15 Furthermore, a 2008 study identified a high affinity association between NgR1 and fibroblast growth factor (FGF) 1 and 2, as well as co-localization of the FGF receptor with NgR1 at postsynaptic sites in the adult hippocampus.16
FIG1.
Myelin-derived neurite growth inhibitors, their receptors, and intracellular signaling pathway. Oligodendrocyte-expressed Nogo, myelin-associated glycoprotein (MAG), and oligodendrocyte-myelin glycoprotein (OMgp) all bind to glycosyl phosphatidylinositol-linked protein NgR1, which recruits co-receptor p75NTR and leucine-rich repeat and immunoglobulin domain-containing Nogo receptor-interacting protein (LINGO)-1 to form a receptor complex to transduce the inhibitory signals in neurons.
Binding of Nogo, MAG, or OMgp to NgR1 inhibits neurite outgrowth from mature neurons.10,17–24 Both enhanced anatomical plasticity and improved functional recovery have been observed in experiments wherein these growth restrictive ligands of NgR1 have been blocked in CNS-injured adult animals.23, 25–34
Genetic studies also suggest that blocking NgR1 signaling can have multiple beneficial effects towards recovering from CNS injury. An ngr-/- mouse knockout (KO) line recovered more motor function than wild type (WT) animals following spinal cord injury, perhaps because of the observed partial axonal regeneration in KO mice.35,36 Similar beneficial outcomes were observed in the ngr-/- mouse line in the setting of ischemic stroke.37 Both improved recovery of motor function, and enhanced axonal crossing from the undamaged cerebral cortex was noted.37 Axonal growth alters the connectivity of a damaged CNS to recover activity to denervated areas by the recruitment of new neurons, or even regions of the brain.
Rebuilding appropriate circuitry between neurons located proximal and distal to the injury site is important to recovering function following brain trauma. Growth-associated protein-43 (GAP-43) is highly expressed in the areas of the adult rat brain with ongoing structural plasticity, such as the hippocampus.38 Furthermore, GAP-43 modulates the formation of new connections between neurons,38 and regulates axonal extensions through a mechanism involving actin cytoskeletal remodeling,39,40 thereby playing a key role in remediation of morphological damage to axons following brain injury.41,42 Synaptophysin (SYP) is a protein that is widely expressed in the brain, particularly by neurons actively undergoing synaptogenesis.43,44 Perhaps the regulation of these proteins with demonstrated activity in processes involving brain plasticity and growth could be directly or indirectly targeted by NgR1 signaling. A preliminary study noted corticospinal fiber sprouting and increased expression of GAP-43 and BDNF in rat spinal cord after pyramidotomy followed by IN-1 antibody treatment.45 Hence, our study focuses on examining the role of NgR1 and probing for potential essential downstream factors such as BDNF, SYP, and GAP-43 in the recovery of motor and cognitive function after TBI using a controlled cortical impact (CCI) model, comparing NgR1 KO and WT mice.
Methods
All protocols involving the use of animals were approved by the University Committee on Animal Resources (UCAR) of the University of Rochester Medical Center and were in compliance with the National Institutes of Health (NIH)'s Guide for the Care and Use of Laboratory Animals.
CCI mouse model
Two groups of mice: NgR1 KO (n=18) and WT (n=18) were given TBI by CCI using previously described methodology.46,47 Mice received induction anesthesia with inhalation of 2.5% isoflurane and a 2:1 N2/O2 mixture followed by 2% isoflurane for maintenance anesthesia. A heating pad (Gaymar Industries, Orchard Park, NY) was used to maintain body temperature at 37.0°C. Mice were placed in a stereotaxic frame adapted for them (Kopf Instruments, Tujunga, CA) and a 5 mm craniotomy was performed over the right parietal cortex leaving the dura mater intact. CCI was applied perpendicular to the brain surface using the following parameters: diameter of the impact tip, 3 mm; impact velocity, 6.7 m/sec; impact duration, 100 ms; and depth, 1 mm. Then the craniotomies were covered with Duragen® Dural Graft Matrix (Integra LifeSciences Corporation, Plainsboro, NJ) and the scalps were sutured immediately. The animals were transferred to an incubator heated to 37°C until recovery of spontaneous motor activity.
Bromodeoxyuridine (BrdU) injection
For immunohistological analyses, bromodeoxyuridine (BrdU; 200 mg/kg; Sigma, B5002) was intraperitoneally injected at days 2, 3, 4 post-surgery to label endogenous cells induced to proliferate by brain injury, and to determine their cell survival rate and fate 2 and 4 weeks after brain trauma (NgR1 KO [n=8], WT [n=8]).
Behavioral function evaluation
Cognitive function evaluation
The Novel Object Recognition (NOR) test was used to assess the cognitive function of the two experimental groups at days 7, 14, 21, and 28 following trauma. This method was used as described previously by other researchers.48–52 Twenty-four hours before testing, mice were allowed to explore the testing box (60 cm×40 cm) for 1 h to reduce neophobia responses and to habituate the animals to the stimuli present in the empty arena. Then, in the first trial two identical objects were placed 30 cm apart at the right and left sides of the box. Animals were placed in the box for 5 min to explore the two objects, and their exploratory activity (i.e., the time spent in object-directed exploration) was recorded by video. After a delay of 4 h, mice were reintroduced for 5 min to the same cage in which one of the objects was replaced by a new one. The cumulative time spent by a mouse at each of the objects was recorded. Two observers blinded to the mice's genotype collected all the data. Exploration of an object was defined as follows: directing the nose to the object at a distance of ≤2 cm and/or touching it with the nose; turning around or sitting on the object was not considered an exploratory behavior. Recognition memory was the percent of the total exploration time that the animal spent investigating the novel object (percent of time spent on the new object/total time). Most healthy rodents spend relatively more time exploring a new object than a familiar one.52,53
Motor function evaluation
The beam walk and ROTOR-ROD tests were used to assess the locomotor function recovery of the two groups at days 1, 3, 7, 14, 21, and 28 after TBI.
Beam walk testing
Beam walk testing is used to discriminate differences in fine motor coordination.54–56 The testing device consisted of a narrow wooden beam 9 mm wide and 300 mm in length that was suspended 300 mm above a 60 mm thick foam rubber pad. Mice were placed on one end of the beam, and the number of foot faults for the left front- and hindlimb (contralateral to the injured hemisphere) was recorded over 30 steps counted. The percentage of the normal front- and hindlimb steps among all steps was calculated as the measure of fine motor coordination. Mice were allowed to walk across the beam two times for training purposes before surgery.
ROTOR-ROD test
All mice were trained on the ROTOR-ROD for five trials/day for 3 days prior to CCI. Initial ROTOR-ROD tests were performed for each animal 24 h prior to TBI to record the baseline time before it fell from the device, hereafter termed “latency.” Each mouse underwent three trials and the average score was taken. Mice were mounted on a motorized rotating rod (ROTOR-ROD, San Diego Diagnostics) turning with an initial velocity of 1–10 rpm for the first 30 sec, increased to 10–20 rpm over the next 30–60 sec, and then increased to 20–50 rpm over the last 60–120 sec. The latencies measured for the mice within each group were averaged and expressed as a percent of their respective baseline values for each time point tested after injury.
Histology
Before immunohistochemical and contusion volume analyses, mice were deeply anesthetized with 100 mg/kg ketamine and 15 mg/kg xylazine, and subsequently transcardially perfused with phosphate-buffered saline (PBS) followed by 4% paraformaldehyde (PFA). Brains were removed and post-fixed in 4% PFA overnight, then cryoprotected by incubation in 15% followed by 30% sucrose/1X PBS solution overnight at 4°C. The brains were frozen in optimal cutting temperature (OCT) embedding media (VWR®), sectioned into 30 μm thickness coronal sections in a cryostat, and mounted on slides in 10 alternating sets. The slides were stored at −80°C until staining.
Brain contusion volume analysis
For the lesion volume analysis, 0.3mm interval brain slices were collected and mounted on slides, and then stained with 1% cresyl violet solution (Sigma, Cat# C1791). The contusion area was measured using imageJ software (NIH) by an observer blinded to the mice's genotype, and then the data were integrated to calculate the corresponding lesion volumes.
Immunohistochemistry
To detect proliferating cells 2–4 days after TBI and to determine the maturational fate of newly generated cells, antibodies against BrdU, doublecortin (DCX), NeuN and glial fibrillary acidic protein (GFAP) were used to fluorescently label dividing cells, immature neurons, mature neurons, and astrocytes, respectively. Briefly, three parallel brain sections were permeabilized with PBS containing 0.1% Triton X-100 (PBST) and then incubated at 37°C in 2N HCl for 30 min followed by three washes with PBST. Then, nonspecific staining was blocked by incubation with 10% donkey serum/PBST for 1 h at room temperature. The brain slices were incubated overnight at 4°C in blocking buffer with primary antibodies against rabbit anti-DCX (1:200; Cell Signaling, Cat#4604)), mouse anti-NeuN (1:400; Millipore, Cat# MAB377), and mouse anti-GFAP (1:400; Sigma, Cat# G3893), each in combination with rat anti-BrdU (1:400; ABcam Cat#Ab6326). After rinsing with PBST on the following day, sections were incubated in the corresponding fluorescently conjugated secondary antibodies (Alexa Fluor 488 or 594; Invitrogen) for 1 h at room temperature. Then the sections were rinsed, and covered with cover-slips using Vectashield mounting medium with DAPI (Vector Laboratories, Cat#H-1200).
To observe cell proliferation at 2 weeks and 4 weeks post-trauma, we stained for another proliferation marker, Ki-67. The protocol for Ki-67 staining was similar to the methods described. Rabbit anti-Ki-67 antibody (1:500, ABcam, Cat# Ab15580) was used.
For observing immature neurons and their processes in the DG of the hippocampus, sections were stained using antibodies to DCX according to standard procedures. Briefly, the sections were washed with PBS. Then, endogenous peroxidase activity was blocked using 3% H2O2, followed by blocking of background staining using 10% normal goat serum in PBST. The sections were incubated with rabbit anti-DCX antibody (1:200, Cell Signaling, Cat#4604) overnight at 4°C. After washing, sections were incubated with biotinylated goat anti-rabbit immunoglobulin G (IgG) (1:200, Vector Laboratories, Cat# BA-1000) for 1 h at room temperature. DCX antibodies were detected using a VECTASTAIN® Elite ABC kit (Vector Laboratories, Cat# PK-6100) and visualized using diaminobenzidine (ImmPACT™ DAB, Vector Laboratories, Cat#SK-4105) allowing the quantification of the length of neuronal processes. Sections were counterstained with hematoxylin, dehydrated in series by increasingly higher percentages of ethanol (75%, 95%, 95%, 100%), washed with xylene, and then cover-slipped with DPX mounting medium.
A streptavidin-Alexa Fluor 594 conjugate (1:10,000; Invitrogen, Cat# S32356) was used to bind and detect biotinylated dextran amine (BDA) via a similar protocol to the one previously described.
Quantification
To quantify the number of DCX+ or Ki67+ cells, five coronal sections (spanning −1.34 to −2.80 mm bregma) per animal in each group were analyzed. All positive cells in the contralateral DG including the subgranular zone (SGZ), granule cell layer (GCL), and hilus were counted using a 40× objective on an Olympus BX-51 light microscope fitted with an Olympus DP70 digital camera for visualization on a computer monitor. Results were expressed as the average number of DCX+ or Ki67+ cells per section and reported as the mean±standard error of the mean (SEM).
The number of BrdU and NeuN, DCX, or GFAP co-labeled cells in brain slices (five sections/brain for each specific marker in each group) were visualized and quantified by confocal microscopy (Olympus IX81 FVF with Fluoview FV00 software). The number of double-labeled cells per the total number BrdU+ cells in the DG was compared among NgR1 WT and KO groups.
Real-time quantitative reverse transcription polymerase chain reaction (RT-PCR)
Eleven mice (NgR1 KO [n=6] and WT [n=5]) were killed at 4 weeks post-trauma. Samples of their ipsilateral or contralateral hippocampus and cortex (hippocampus of trauma side [HT], cortex of trauma side [CT], hippocampus of non-trauma side [HN], and cortex of non-trauma side [CN]) were used to quantify the expression level of BDNF, SYP, and GAP-43 via RT-PCR.
RNA isolation
Animals were killed, then ipsilateral or contralateral hippocampus and cortex tissues were quickly dissected and frozen in dry ice. The tissue samples were homogenized in TRIzol reagent (Cat#15596-026; Invitrogen) at a ratio of 1 mL Trizol per 100 mg tissue. RNA was extracted from the tissues according to the manufacturer's suggested protocol. Total RNA was treated with 10 U of RNase-free DNase I (Cat#74104; Qiagen) to eliminate any possible DNA contamination. The concentration of RNA was measured using a spectrophotometer (optical density at 260 and 280 nm on a NanoDrop ND-1000 Spectrophotometer system; Thermo Fisher Scientific Inc, ND-1000 3.7.1). The ratio of OD260/OD280 in all samples ranged from 1.7 to 2.0.
Reverse transcription (RT)
After evaluating the ratio of RNA to DNA, the High Capacity cDNA Reverse Transcription Kit (with RNase Inhibitor, 4374966, Applied Systems, CA) was used to perform cDNA reverse transcription. Ten microliters of total RNA (1 μg RNA+nuclease-free H2O) plus 10 μL of 2× RT master mix (2.0 μL of 10× RT buffer, 0.8 μL of 25× dNTP mix [100 mM], 2.0 μL of 10× RT random primers, 1.0 μL of MultiScribe™ Reverse Transcriptase, 1.0 μL RNase inhibitor, and 3.2 μL nuclease-free H2O) was used for each 20 μL reaction. For control, MultiScribe™ Reverse Transcriptase was replaced with nuclease-free H2O. The cDNA reverse transcription reaction was conducted at the following thermal cycler conditions: 25°C for 10 min, 37°C for 120 min, 85°C for 4 sec, followed by storage at 4°C (2–6°C).
Real-time RT-PCR
TaqMan PCR reactions were performed with TaqMan Gene Expression Assay Products (Applied Biosystems) for BDNF (Gene ID Mm01334047_m1), SYP (Gene ID Mm00436850 _m1), GAP-43 (Gene ID Mm00500404 _m1), and the endogenous control glyceraldehyde-3-phosphate dehydrogenase (GAPDH Cat#4352932E). Twenty microliter reaction mixtures contained 1×TaqMan Universal PCR Master Mix (cat# 4374966, Applied Biosystems), 1×TaqMan Gene Expression Assay (either BDNF, SYP, or GAP-43, Applied Biosystems), TaqMan Endogenous Control (GADPH) and the cDNA template (20 ng of RNA converted to cDNA). The amplification protocol (TaqMan® Gene Expression Master Mix Protocol) consisted of 2 min at 50°C for optimal uracil-DNA-glycosylase (UDG) enzyme activity and one cycle at 95°C for 10 min to activate the AmpliTag Gold® UP enzyme, followed by 40 cycles at 95°C for 15 sec and 60°C for 1 min. Relative quantity values were analyzed using ABI PRISM® 7000 SDS Software SDS V 1.2 (Applied Biosystems, Inc, CA) according to the 2-ΔΔCt method, which reflects the difference in threshold for each target gene relative to that of a previously validated stable reference gene in the mouse cortex and hippocampus, GAPDH, using a confidence level of 95% (p<0.05).57–59
Statistical analysis
Data were summarized in terms of their means±SEM. Histology staining was presented as cell numbers per section. Analyses of variance (ANOVA) were used to assess the influence of genotype (KO vs. WT) on the outcome variables (e.g., brain injury volume, number of BrDU+ cells, GAP-43 expression level, number of Ki-67+ cells). Whenever possible, these analyses were based on multiple-way ANOVA adjusting for location (cortex of injured side, hippocampus of the injured side, hippocampus of the uninjured side, and cortex of the uninjured side), time post-surgery, and the interaction between these variables and genotype, as appropriate. In situations in which measures were repeatedly collected in each mouse, the statistical analyses relied on mixed effects ANOVA that included the above-listed factors as well as random intercepts to describe the presence of dependencies potentially generated by inter-mouse variability.60 These analyses were also performed after having log-transformed the data. Because both sets of analyses were in close agreement, we report only results based on the original (untransformed) data. Every test was two sided. P values < 0.05 were considered statistically significant. All analyses were conducted using SAS statistical package, version 9.12 (SAS Institute, Cary, NC).
Results
NgR1 KO mice, like WT, have reduced motor function after TBI
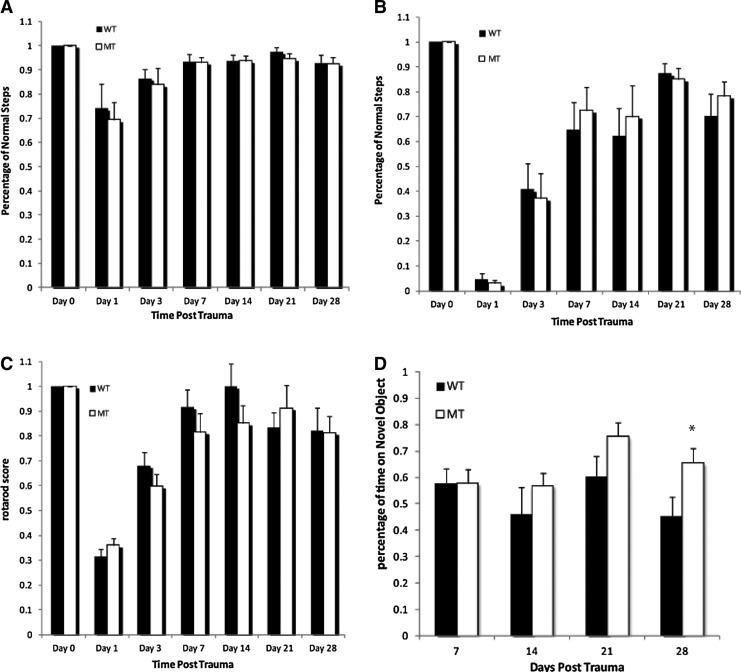
All animals displayed a similar level of significant motor function deficits after CCI. The number of contralateral rear foot faults during the beam walk test was higher in the surgery group and reached a maximum during days 1–3; the mice maintained this high frequency of foot faults until day 28 following CCI. No significant difference in the percentage of successful performances was noted between NgR1 WT (70.2±8.9% [n=8]) and KO mice (78.2±6.0% [n=8]) at day 28 after CCI (p=0.471, Fig. 2A). The number of contralateral front foot faults was also increased in the surgery group at day 1, but returned to almost normal after 7 days. The groups had no significant difference in their front limbs' performance on the beam walk test after CCI (Fig. 2B).
FIG. 2.
Behavioral evaluation of motor and cognitive function recovery following brain trauma. Motor function test scores did not differ significantly between wild type (WT) and knockout (KO) mice in beam walk testing in (A) hindlimb at day 28 after controlled cortical impact (CCI) (p=0.471) or (B) front limb. Rotarod (C) test generated similar result at day 28 after CCI (p=0.94). In the novel object recognition test (D) both groups had their best performance at day 21 after CCI. NgR1 KO mice performed better than WT mice from post-trauma day 14, and were significantly better by post-trauma day 28 (p<0.05) Data presented as mean±SEM, n=8.
In the ROTOR-ROD test, both WT and KO mice showed difficulty in maintaining their balance on the rotating bars for the first 3 days after CCI, but performed well from day 7 until day 28. ROTOR-ROD testing did not show any significant differences between the groups at day 28 following TBI (WT 82.2±9.3%: KO 81.3±6.6%, p=0.94, Fig. 2C).
NgR1 KO mice have reduced cognitive deficits compared with WT after TBI
The NOR test was used to establish whether the absence of NgR1 affected cognitive performance after brain trauma. The NOR test is widely applied to evaluate memory and hippocampus function. Both groups had their best performance at day 21 after CCI. A general trend of better performance from the KO group was observed. NgR1 KO mice performed better than WT mice from post-trauma day 14, and were significantly better by post-trauma day 28 (KO 0.656±0.161; WT 0.455±0.186, p<0.05, Fig. 2D).
NgR1 KO mice have smaller brain injury volumes compared with WT Mice at 2 weeks after CCI
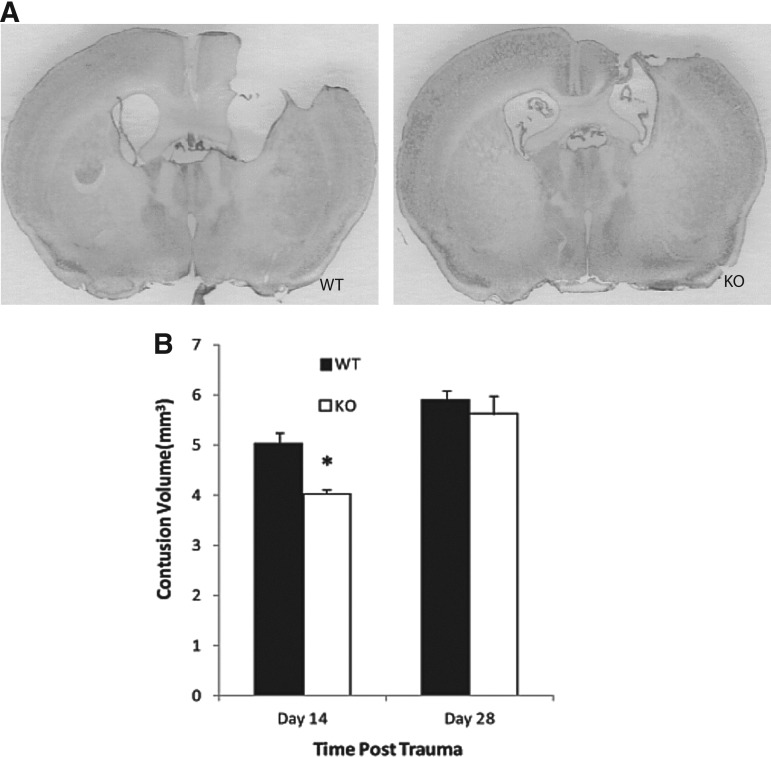
At both 2 and 4 weeks after CCI, mice still had significant cortical contusions in their injured hemispheres compared with their contralateral side. NgR1 KO mice had significantly smaller brain injury volumes (4.021±0.102 mm3) compared with WT mice (5.021±0.207 mm3) at 2 weeks post-injury (p=0.012) (Fig. 3 B). Both groups had contusion volumes at 4 weeks, increased from their 2 week volumes; however, there was no significant difference between the NgR1 KO mice (5.613±0.357 mm3) and WT mice (5.887±0.206 mm3) at the 4 week time point (p=0.525).
FIG. 3.
Brain contusion volume at 2 and 4 weeks following trauma. (A) Nissl staining was used to visualize injured brain areas in knockout (KO) mice and their wild type (WT) littermates. (B) Data analysis shows that contusion volume is significantly less in KO mice at post-trauma week 2 (p=0.012). At post-trauma week 4, a still notable bigger contusion volume was observed in WT mice; however, the difference was not statistically significant (p=0. 25). (Data presented as mean±SEM.)
NgR1 KO mice have more newly matured neurons than WT mice 4 weeks post-trauma
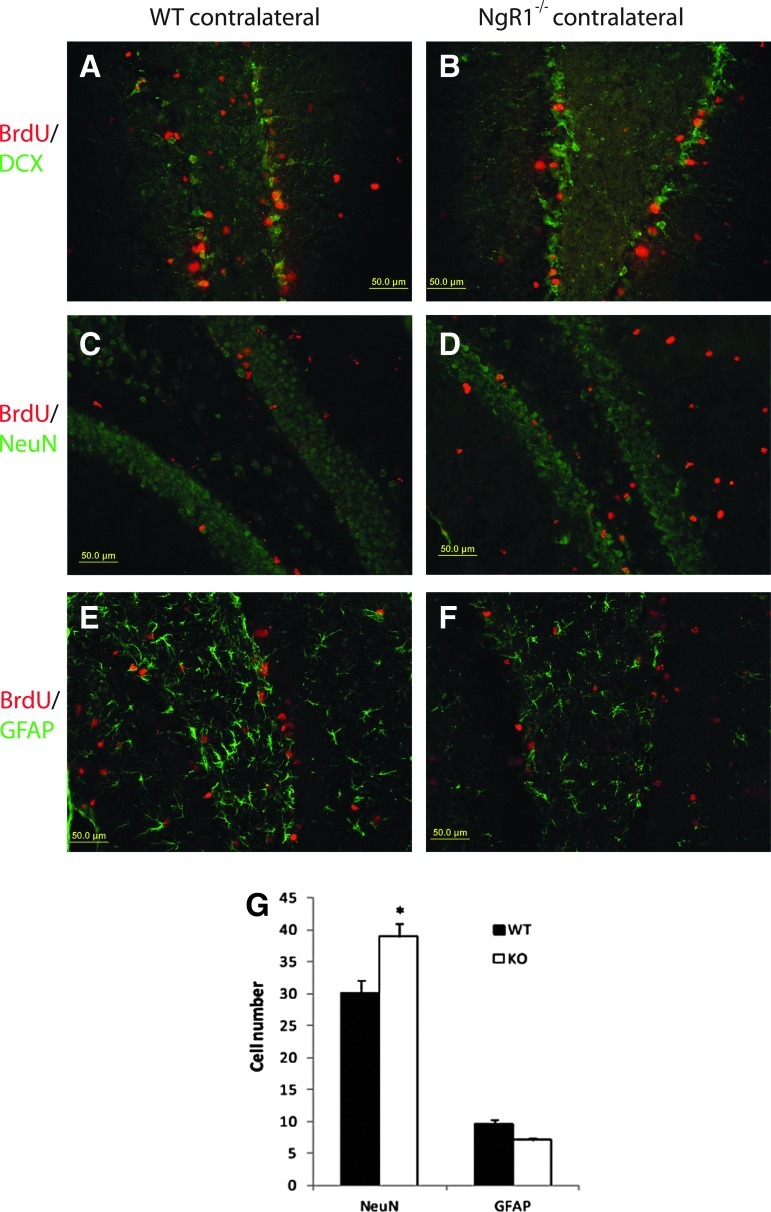
Two to four days after TBI, proliferation was enhanced in the DG of both halves of the brain, especially the injured side, in KO and WT animals. Dividing cells that had incorporated BrdU in their DNA were identified by immunolabeling, and found localized to the SGZ and the hilus of the hippocampus. Because the ipsilateral hippocampus was partly damaged after CCI, the study of histological changes over time is focused on the contralateral hippocampus. The total number of BrdU+ cells in the contralateral DG was similar between NgR1 KO (50.72±5.20 cells) and WT mice (47.29±6.51 cells; p=0.682). Within the contralateral SGZ and hilus, most of the newly generated BrdU+ cells differentiated into either neurons or astrocytes, as indicated by co-staining with NeuN or GFAP 4 weeks following CCI. The percentage of NeuN and BrdU co-labeled cells among all of the BrdU+ cells was elevated at 4 weeks post-trauma in NgR1 KO (76.65±5.41%) relative to WT (63.62±2.33%) mice (p=0.040), suggesting that more proliferating cells differentiated into neurons in the less growth-inhibitive environment (without NgR1). The percentage of new astrocytes, as indicated by the number of GFAP and BrdU co-labeled cells among all of the BrdU+ cells, was lower in NgR1 KO (14.00±4.72%) relative to WT mice (20.11±6.90%), but was not significantly different (p=0.093) (Fig. 4).
FIG. 4.
Injury-induced cell proliferation and cell fate determination. Final fate of proliferative cells in the dentate gyrus after brain trauma was visualized by co-staining for bromodeoxyuridine (BrdU) with either doublecortin (DCX) (A, B), NeuN (C, D), or glial fibrillary acidic protein (GFAP) (E, F). The number of NeuN/BrdU or GFAP/BrdU positive cells was counted 4 weeks following trauma. Cells that double stained by both NeuN and BrdU elevated at 4 weeks post-trauma in NgR1 knockout (KO) relative to wild type (WT) mice (p<0.05) (G). (Data presented as mean±SEM).
NgR1 KO mice have more proliferating cells than WT mice at 2 weeks after CCI
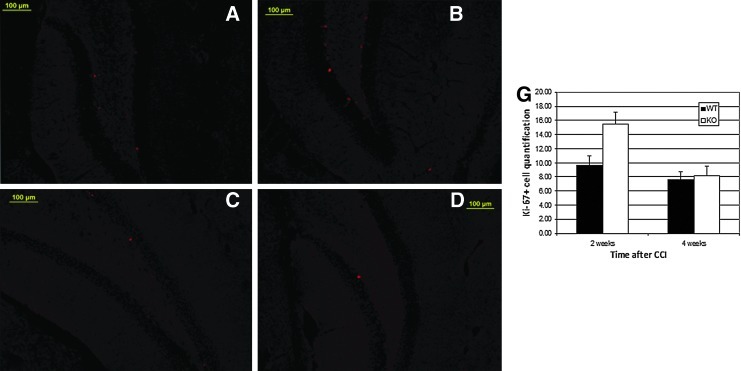
In both mice and humans, Ki-67 protein is exclusively expressed on the cell surface of cells undergoing mitosis.61, 62 An antibody against Ki-67 was employed to visualize the proliferating cells in the subgranular zone (SGZ) and the hilus of the hippocampus at 2 or 4 weeks after CCI. Both groups had more proliferating cells at 2 weeks than at 4 weeks. Whereas NgR1 KO mice had 15.47±1.69 Ki-67+ cells compared with 9.67±1.29 Ki-67+ cells in WT at 2 weeks post-trauma (p=0.011), there was no difference between the 2 groups at 4 weeks post-trauma (KO 8.18±1.29 cells, WT 7.63±1.08 cells, p=0.746) (Fig. 5).
FIG. 5.
Cell Proliferation at 2 and 4 weeks following trauma. Ki-67 was used to visualize the proliferating cells. Both groups had more proliferating cells at 2 weeks than at 4 weeks. NgR1 knockout (KO) mice (B) had more Ki-67+ cells than did wild type (WT) mice (A) at 2 weeks post-trauma (p=0.011). At post-trauma week 4, no difference between KO (D) and WT (C) mice was observed (E) (p=0.746). (Data presented as mean±SEM).
Compared with WT, NgR1 KO mice have more immature neurons at 2 weeks and these neurons have longer processes at 4 weeks after trauma
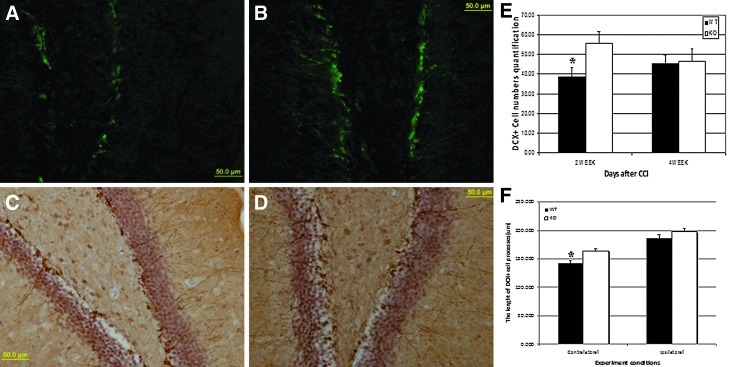
DCX is a microtubule-binding protein that is transiently expressed by immature cells undergoing neurogenesis.5 DCX has been found to reliably indicate the level of neurogenesis occurring in the adult brain,63 predominately the cells born in the previous 12 days before euthanasia.64 Using DCX as a marker for immature neurons, we observed a remarkable difference between NgR1 KO and WT mice. NgR1 KO mice had more DCX+ cells (55.71±5.87) than WT mice (38.79±4.72) in the contralateral SGZ at 2 weeks following CCI (p=0.018). Although the number of DCX+ cells was not significantly different by 4 weeks (KO: 46.40±6.49 cells vs. WT: 45.40±4.39 cells, p=0.900), these cells retained more and longer processes in the SGZ of NgR1 KO mice at 4 weeks, especially on the contralateral side (KO process lengths: 164.29±4.33 μm vs. WT: 142.132±4.53 μm, p=0.0005) (Fig. 6).
FIG. 6.
Doublecortin (DCX) expressing cells at 2 and 4 weeks after trauma. Histological staining with fluorescent secondary antibody revealed a higher number of DCX+ cells in the dentate gyrus of NgR1 knockout (KO) mice (B) compared with wild type (WT) mice (A) at 2 weeks after traumatic brain injury (TBI). Data analyzed in E (p=0.018). Four weeks following trauma, the DCX+ cells still retained their longer processes in NgR1 KO mice (D) than in their WT littermates (C) as shown by DAB staining. Data analyzed in F (p=0.0005). (Data presented as mean±SEM).
Compared with WT, NgR1 KO mice have increased GAP-43 level after TBI
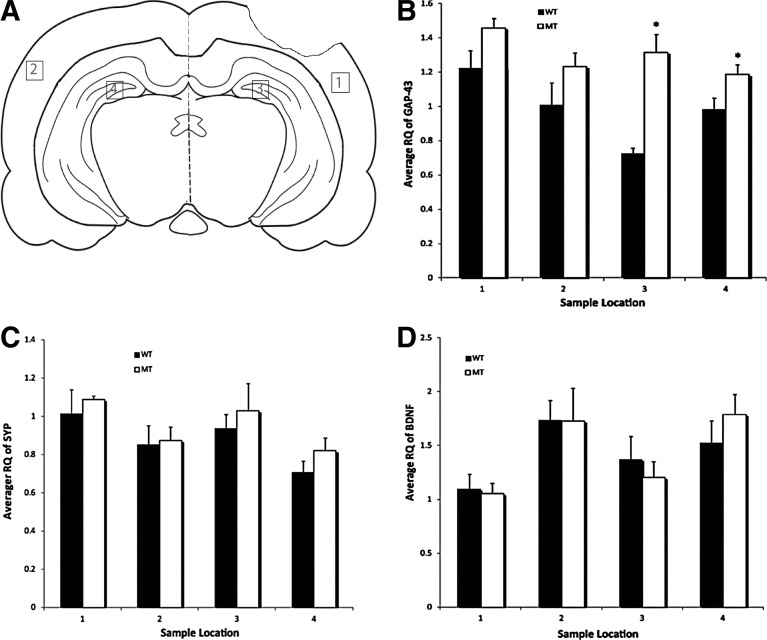
Previous studies have established a role for BDNF, SYP, and growth-associated protein 43 (GAP-43) in neuronal plasticity.7,59, 65 Because of the better recovery of cognitive function in NgR1 KO mice after TBI, we probed the expression level of BDNF, SYP, and GAP-43 via real-time PCR. These assays showed increased expression of GAP-43 in the hippocampus and cortex of both the ipsilateral and contralateral sides of NgR1 KO mice relative to WT, especially in both hippocampus (CT: KO 1.459±0.056, WT 1.224±0.102, p=0.101; CN: KO 1.234±0.080, WT 1.010±0.102, p=0.128; HT: KO 1.317±0.103, WT 0.726±0.031, p=0.042; HN: KO 1.187±0.058, WT 0.983±0.066, \p=0.041) (Figure 6). BDNF and SYP expression levels were not significantly different between NgR1 KO and WT mice (Fig. 7).
FIG. 7.
Real-time polymerase chain reaction (RT-PCR) analysis of growth-associated protein (GAP)-43, synaptophysin (SYN), and brain-derived neurotrophic factor (BDNF). Tissues were collected at regions labeled in A. 1: cortex of injury side; 2: cortex of contralateral side (uninjured side); 3: hippocampus of injury side; 4: hippocampus of contralateral side (uninjured side). (B) RT-PCR showed increased expression of GAP-43 in knockout (KO) mice, especially in the hippocampus of bilateral hemispheres (p=0.042 for the hippocampus of the uninjured side; p=0.041 for the hippocampus of the injury side.). No significant change in BDNF and SYN expression levels were observed (C,D). (Data presented as mean±SEM, n=5 for WT; n=6 for KO.)
Discussion
Many studies have demonstrated that NgR1 signaling plays a critical role in maintaining a growth inhibitory environment in the adult nervous system. Moreover, the specific binding of Nogo, MAG, or OMgp to NgR1 inhibits neurite outgrowth from mature neurons.10,17–24 Also, Nogo also regulates neurite growth in the developing nervous system, as neutralization of the protein's activity results in increased neurite length, decreased branching, and increased fasciculation.66 Modulation of these neural structural properties by the NgR1 pathway can have an important effect on neurogenesis and brain functions; ocular dominance plasticity is extended beyond the usual postnatal period in the NgR1 KO mouse line.67 Based on these data, we speculated that NgR1 could inhibit neurite outgrowth after TBI, and thereby inhibit neuroprotection and/or neurogenesis. Furthermore, as NgR1 plays a regulatory role in the formation of lasting memories,68 we hypothesized that NgR1 might affect cognitive recovery after TBI.
The CCI model is one of the most widely used TBI paradigms in neurotrauma research, commonly employed to represent focal contusion injuries in humans.69–71 A recent study showed that the neuropathology associated with the injury induced by CCI in the mouse model is widespread and diffuse.46 The authors conclude that because of the extent of CCI damage, the neurological deficits associated with the injury would likely include motor dysfunction (e.g., cortex, caudate, thalamus), learning and memory problems (e.g., hippocampus), visual deficits (e.g., occipital cortex and optic tectum), and even endocrine disturbances (e.g., hypothalamus).46 Because the CCI model is an established and well-characterized system, and because most TBI experiments involving mice have been performed using the CCI model, we chose this paradigm for our research into the potential role of NgR1 in recovery from TBI.
In our study, deletion of the NgR1 gene in mice led to improved cognitive functional outcomes after CCI experimental brain injury. The NOR test, which is widely applied to evaluate memory and hippocampus function, was used here to establish whether the absence of NgR1 impacted cognitive performance after brain trauma. NgR1 KO mice performed significantly better on the NOR test than did WT mice by day 28. However, our results conflict with a 2010 study employing two strategies to study the effect of NgR1 ablation on recovery from experimental TBI: pharmacological deletion of NgR1 signaling through specific antibodies, and NgR1 genetic deletion in mice. The authors from the previous study reported reduced cognitive function with the loss of the growth- and plasticity-inhibitive environment fostered by NgR1, partly through a mechanism involving mossy fiber-sprouting in the hippocampus.72 Variance in axonal growth and functional outcomes have been observed previously in genetic studies of other players in the nogo-signaling pathway, such as nogo-abatg/atg.36 Amidst a setting of positive pharmacological studies but varied genetic results, a more complete analysis allowed the researchers ultimately to conclude that in an environment lacking NgR1 signaling, corticospinal tract (CST) axons regenerate following spinal cord hemisection.36 Still other researchers observed variable regenerative abilities in different genetic environments involving NgR1 signaling molecules.73 Different strains of Nogo-A KO mice had different potentials for neuronal outgrowth leading to varying levels of axonal regeneration following spinal cord injury.73 Authors of a review on nerve regeneration suggest that the varying structural plasticity observed in different genetic environments involving members of NgR1 signaling may stem from compensation from other inhibitory pathways;74 perhaps this is also the cause for the differences observed among studies of the role of this pathway in TBI.
The positive cognitive effects associated with deleting NgR1 were potentially, at least in part, the result of enhanced cell proliferation in traumatized brains. Our data showed that the deletion of the NgR1 gene promoted cell proliferation in the dentate gyrus of the hippocampus at an early stage (2 weeks) after TBI. We used an antibody against Ki-67 – a protein in both mice and humans that is exclusively expressed on the surface of cells undergoing mitosis61, 62 – to visualize the proliferating cells in the SGZ and the hilus of the hippocampus at 2 or 4 weeks after CCI. This staining demonstrated that NgR1 KO mice had significantly more Ki-67+ cells than did WT mice at 2 weeks post-trauma, but there was no difference between the two groups at 4 weeks post-trauma. Our results mirror another study that had previously shown that administration of a mitogen following TBI could induce cell proliferation in the subventricular zone and the DG of the hippocampus.75 Similarly, enhanced neural cell proliferation was observed 1 week following injury, but tapered to that of vehicle control levels at 4 weeks. Also, as with our study, these new neurons correlated with improved cognitive performance.75
In this study, we observed the proliferation of cells in the contralateral SGZ and hilus at a very early stage (2, 3, and 4 days) after trauma and determined that many had differentiated to a neuronal cell lineage. BrdU was used to label proliferating cells; there was no difference between the NgR1 KO and WT groups in the total number of BrdU+ cells in the contralateral DG. Within the contralateral SGZ and hilus, most of the newly generated BrdU+ cells differentiated into either neurons or astrocytes 4 weeks following CCI. The percentage of NeuN and BrdU co-labeled cells among all of the BrdU+ cells was elevated in NgR1 KO mice relative to WT mice, indicating that more proliferative cells differentiated into neurons in an environment lacking NgR1.
Furthermore, our study considered the fate of these newly generated cells, using a reliable and specific marker, DCX, which reflects the levels of adult neurogenesis and its modulation.63 In humans, the DCX protein plays an important role in neuronal repair by stabilizing microtubules in neurons; upregulation of DCX has been associated with improved neurological outcomes following TBI.76 Authors of this previous study hypothesized that DCX may be required for proper migration of newly differentiated neuronal cells to enable functional reorganization and establishment of useful connections following brain injury. By staining for this immature neuronal marker, we demonstrated that NgR1 KO mice had more immature neurons at 2 weeks, and by 4 weeks following trauma that these immature neurons had grown longer processes than th neurons of WT mice. Perhaps the presence of DCX also suggests that these new neurons were forming functional connections, enabling the observed positive cognitive outcomes.
Herein, our data show that NgR1 KO mice had better cognitive function recovery, but no improvement in the motor recovery after CCI. The beam walk test and ROTOR-ROD test did not differentiate any significant differences between the KO and WT groups at any time point assessed following TBI.
Mechanisms of neuroprotection can benefit early stages following TBI; reduced contusion volume is considered very important evidence of neuroprotection. Knockout of NgR1 helps to decrease the brain injury volume after TBI. NgR1 KO mice have significantly smaller brain contusion volumes compared with WT mice at 2 weeks after CCI. However, there was no significant difference between the brain contusion volumes of NgR1 KO mice and WT mice at 4 weeks. It is certainly possible that the WY mice caught up to the NgR1 KO mice in terms of the volume of the lesion, but much of the cellularity is astrocyte proliferation. Similar to this later stage result, there was no difference in the infarct volume at 28 days between NgR1+ and NgR1- mice in a model of stroke.37
Rebuilding appropriate circuitry between neurons located proximal and distal to the injury site is important to recovering function following brain trauma. In our study, we observed that improved functional recovery in NgR1 KO mice co-occurred with the upregulation of GAP-43. GAP-43 is considered to be a marker of axonal sprouting. It is a growth-associated, nervous tissue-specific protein, and is synthesized at high levels during axonal growth in neuronal development and axonal regrowth in regenerating the peripheral and central nervous system. Axonal sprouting, an indication of anatomic plasticity, can be identified by the elevated expression of GAP-43.77 Perhaps NgR1-ligand interactions mediate a growth-inhibitory environment through downregulation of proteins, such as GAP-43, which are required for growth cone expansion.6 Notably, Nogo-NgR1 signaling does elicit growth cone collapse.6 Furthermore, GAP-43 is involved in regulating actin cytoskeletal remodeling in growing neurons39, 40 and NgR1 has been implicated in regulating cytoskeletal dynamics, as well.78 Meanwhile a study of the intracerebroventricular infusion of a novel monoclonal antibody to Nogo-A following lateral fluid-percussion brain injury noted a correlation between the treatment and significantly improved cognitive performance along with higher regional GAP-43 expression than in brain-injured, control rats.79
Furthermore, we observed the proper localization of axons to potentially ameliorate the injured part of the brain in NgR1 KO mice. Our data suggests the possibility that more CST fibers crossed to the injured side in NgR1 KO mice than in WT mice, 4 weeks post-trauma. A similar result was observed previously in models of spinal cord injury and stroke using NgR1 strain mice.35,36 Treatment with antagonistic antibodies against a member of the NgR1 signaling pathway following spinal injury caused sensory fibers to grow and project into denervated spinal cords.45 Meanwhile, Li and colleagues noted a correlation between functional improvement after spinal cord injury and treatment with a systemic NgR1 antagonist. Newly regenerated fibers crossed to the site of the lesion in these treated mice, as visualized by BDA tracing.31 Still, no direct evidence from our study proves that the observed axonal sprouting contributed to the cognitive function recovery.
Conclusion
In summary, our study demonstrates that the myelin inhibitor receptor NgR1 plays a role in inhibiting neuronal regeneration and cognitive functional recovery after TBI.
We demonstrated that proliferating cells in the DG of the hippocampus induced by TBI differentiated into neurons, and that there were a higher number of these cells in NgR1 KO mouse brains. Furthermore, we observed that the enhanced functional recovery of NgR1 KO mice was associated with an upregulation of GAP-43. The loss of NgR1 seems to promote the regeneration of CNS neurons after brain trauma in mice and leads to improved cognitive function as evidenced by 1) less brain injury volume in 2 weeks post-trauma, 2) better performance in the NOR test, 3) more neurogenesis in the DG and more contralateral axonal sprouting, and 4) and increased expression of the GAP-43 gene, a protein involved in synaptic function. Ablation of NgR1 function did not benefit motor function recovery. Our results indicate an important role for the myelin inhibitor receptor NgR1 in inhibiting regeneration and cognitive functional recovery after TBI.
Acknowledgments
The authors thank Dr. Roman J. Giger for providing NgR1 KO mice, and Mr. Jiankai Yang, and Drs. Xiaolin Chen and Patricia Smith for help with data collection and analysis. This work was supported, in part, by a University of Rochester institutional grant (JHH), and by NIH-R01-NS-067435 (JHH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Bruns J., Jr. Hauser W.A. The epidemiology of traumatic brain injury: a review. Epilepsia. 2003;44(Suppl. 10):2–10. doi: 10.1046/j.1528-1157.44.s10.3.x. [DOI] [PubMed] [Google Scholar]
- 2.Colantonio A. Croxford R. Farooq S. Laporte A. Coyte P.C. Trends in hospitalization associated with traumatic brain injury in a publicly insured population, 1992–2002. J. Trauma. 2009;66:179–183. doi: 10.1097/TA.0b013e3181715d66. [DOI] [PubMed] [Google Scholar]
- 3.Faul M. Xu L. Wald M.M. Coronado V.G. Centers for Disease Control and Prevention NCfIPaC. Centers for Disease Control and Prevention; Atlanta: 2010. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002–2006. [Google Scholar]
- 4.Arciniegas D.B. Anderson C.A. Topkoff J. McAllister T.W. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr. Dis. Treat. 2005;1:311–327. [PMC free article] [PubMed] [Google Scholar]
- 5.Brown J.P. Couillard–Despres S. Cooper–Kuhn C.M. Winkler J. Aigner L. Kuhn H.G. Transient expression of doublecortin during adult neurogenesis. J. Comp. Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 6.Ng C.E. Tang B.L. Nogos and the Nogo-66 receptor: factors inhibiting CNS neuron regeneration. J. Neurosci. Res. 2002;67:559–565. doi: 10.1002/jnr.10134. [DOI] [PubMed] [Google Scholar]
- 7.Griesbach G.S. Gomez–Pinilla F. Hovda D.A. Time window for voluntary exercise-induced increases in hippocampal neuroplasticity molecules after traumatic brain injury is severity dependent. J. Neurotrauma. 2007;24:1161–1171. doi: 10.1089/neu.2006.0255. [DOI] [PubMed] [Google Scholar]
- 8.Griesbach G.S. Hovda D.A. Gomez–Pinilla F. Sutton R.L. Voluntary exercise or amphetamine treatment, but not the combination, increases hippocampal brain-derived neurotrophic factor and synapsin I following cortical contusion injury in rats. Neuroscience. 2008;154:530–540. doi: 10.1016/j.neuroscience.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong S.T. Henley J.R. Kanning K.C. Huang K.H. Bothwell M. Poo M.M. A p75(NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- 10.Wang K.C. Koprivica V. Kim J.A., et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002;417:941–914. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- 11.Domeniconi M. Cao Z. Spencer T., et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- 12.Liu B.P. Fournier A. GrandPre T. Strittmatter S.M. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- 13.Fournier A.E. GrandPre T. Strittmatter S.M. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- 14.Wang K.C. Kim J.A. Sivasankaran R. Segal R. He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- 15.Mi S. Lee X. Shao Z., et al. LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat. Neurosci. 2004;7:221–228. doi: 10.1038/nn1188. [DOI] [PubMed] [Google Scholar]
- 16.Lee H. Raiker S.J. Venkatesh K., et al. Synaptic function for the Nogo-66 receptor NgR1: regulation of dendritic spine morphology and activity-dependent synaptic strength. J. Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen M.S. Huber A.B. van der Haar M.E., et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- 18.GrandPre T. Li S. Strittmatter S.M. Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature. 2002;417:547–551. doi: 10.1038/417547a. [DOI] [PubMed] [Google Scholar]
- 19.Prinjha R. Moore S.E. Vinson M., et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- 20.Savio T. Schwab M.E. Lesioned corticospinal tract axons regenerate in myelin-free rat spinal cord. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4130–4133. doi: 10.1073/pnas.87.11.4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKerracher L. David S. Jackson D.L. Kottis V. Dunn R.J. Braun P.E. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- 22.Mukhopadhyay G. Doherty P. Walsh F.S. Crocker P.R. Filbin M.T. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 23.Bregman B.S. Kunkel–Bagden E. Schnell L. Dai H.N. Gao D. Schwab M.E. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- 24.Venkatesh K. Chivatakarn O. Sheu S.S. Giger R.J. Molecular dissection of the myelin-associated glycoprotein receptor complex reveals cell type-specific mechanisms for neurite outgrowth inhibition. J. Cell Biol. 2007;177:393–399. doi: 10.1083/jcb.200702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thallmair M. Metz G.A. Z'Graggen W.J. Raineteau O. Kartje G.L. Schwab M.E. Neurite growth inhibitors restrict plasticity and functional recovery following corticospinal tract lesions. Nat. Neurosci. 1998;1:124–131. doi: 10.1038/373. [DOI] [PubMed] [Google Scholar]
- 26.Onose G. Anghelescu A. Muresanu D.F., et al. A review of published reports on neuroprotection in spinal cord injury. Spinal Cord. 2009;47:716–726. doi: 10.1038/sc.2009.52. [DOI] [PubMed] [Google Scholar]
- 27.Li S. Liu B.P. Budel S., et al. Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J. Neurosci. 2004;24:10,511–10,520. doi: 10.1523/JNEUROSCI.2828-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papadopoulos C.M. Tsai S.Y. Alsbiei T. O'Brien T.E. Schwab M.E. Kartje G.L. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann. Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- 29.Seymour A.B. Andrews E.M. Tsai S.Y., et al. Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J. Cereb. Blood Flow Metab. 2005;25:1366–1375. doi: 10.1038/sj.jcbfm.9600134. [DOI] [PubMed] [Google Scholar]
- 30.Yu P. Huang L. Zou J., et al. Immunization with recombinant Nogo-66 receptor (NgR) promotes axonal regeneration and recovery of function after spinal cord injury in rats. Neurobiol. Dis. 2008;32:535–542. doi: 10.1016/j.nbd.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Li S. Strittmatter S.M. Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J. Neurosci. 2003;23:4219–4227. doi: 10.1523/JNEUROSCI.23-10-04219.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cao Y. Shumsky J.S. Sabol M.A., et al. Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat. Neurorehabil. Neural Repair. 2008;22:262–278. doi: 10.1177/1545968307308550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li S. Kim J.E. Budel S. Hampton T.G. Strittmatter S.M. Transgenic inhibition of Nogo-66 receptor function allows axonal sprouting and improved locomotion after spinal injury. Mol. Cell Neurosci. 2005;29:26–39. doi: 10.1016/j.mcn.2004.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harvey P.A. Lee D.H. Qian F. Weinreb P.H. Frank E. Blockade of Nogo receptor ligands promotes functional regeneration of sensory axons after dorsal root crush. J. Neurosci. 2009;29:6285–6295. doi: 10.1523/JNEUROSCI.5885-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J.E. Liu B.P. Park J.H. Strittmatter S.M. Nogo-66 receptor prevents raphespinal and rubrospinal axon regeneration and limits functional recovery from spinal cord injury. Neuron. 2004;44:439–451. doi: 10.1016/j.neuron.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 36.Cafferty W.B. Strittmatter S.M. The Nogo-Nogo receptor pathway limits a spectrum of adult CNS axonal growth. J. Neurosci. 2006;26:12,242–12,250. doi: 10.1523/JNEUROSCI.3827-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee J.K. Kim J.E. Sivula M. Strittmatter S.M. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J. Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Emery D.L. Royo N.C. Fischer I. Saatman K.E. McIntosh T.K. Plasticity following injury to the adult central nervous system: is recapitulation of a developmental state worth promoting? J. Neurotrauma. 2003;20:1271–1292. doi: 10.1089/089771503322686085. [DOI] [PubMed] [Google Scholar]
- 39.Frey D. Laux T. Xu L. Schneider C. Caroni P. Shared and unique roles of CAP23 and GAP43 in actin regulation, neurite outgrowth, and anatomical plasticity. J. Cell Biol. 2000;149:1443–1454. doi: 10.1083/jcb.149.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laux T. Fukami K. Thelen M. Golub T. Frey D. Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell. Biol. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bomze H.M. Bulsara K.R. Iskandar B.J. Caroni P. Skene J.H. Spinal axon regeneration evoked by replacing two growth cone proteins in adult neurons. Nat. Neurosci. 2001;4:38–43. doi: 10.1038/82881. [DOI] [PubMed] [Google Scholar]
- 42.King C.E. Canty A.J. Vickers J.C. Alterations in neurofilaments associated with reactive brain changes and axonal sprouting following acute physical injury to the rat neocortex. Neuropathol. Appl. Neurobiol. 2001;27:115–126. doi: 10.1046/j.1365-2990.2001.00317.x. [DOI] [PubMed] [Google Scholar]
- 43.Tarsa L. Goda Y. Synaptophysin regulates activity-dependent synapse formation in cultured hippocampal neurons. Proc. Natl. Acad. Sci. U. S. A. 2002;99:1012–1016. doi: 10.1073/pnas.022575999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wiedenmann B. Franke W.W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985;41:1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- 45.Bareyre F.M. Haudenschild B. Schwab M.E. Long-lasting sprouting and gene expression changes induced by the monoclonal antibody IN-1 in the adult spinal cord. J. Neurosci. 2002;22:7097–7110. doi: 10.1523/JNEUROSCI.22-16-07097.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hall E.D. Bryant Y.D. Cho W. Sullivan P.G. Evolution of post-traumatic neurodegeneration after controlled cortical impact traumatic brain injury in mice and rats as assessed by the de Olmos silver and fluorojade staining methods. J. Neurotrauma. 2008;25:235–247. doi: 10.1089/neu.2007.0383. [DOI] [PubMed] [Google Scholar]
- 47.Walker C.T. Marky A.H. Petraglia A.L. Ali T. Chow N. Zlokovic B.V. Activated protein C analog with reduced anticoagulant activity improves functional recovery and reduces bleeding risk following controlled cortical impact. Brain Res. 2010;1347:125–131. doi: 10.1016/j.brainres.2010.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ennaceur A. Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 49.Biegon A. Fry P.A. Paden C.M. Alexandrovich A. Tsenter J. Shohami E. Dynamic changes in N-methyl-D-aspartate receptors after closed head injury in mice: Implications for treatment of neurological and cognitive deficits. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5117–5122. doi: 10.1073/pnas.0305741101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlager G.W. Griesmaier E. Wegleiter K., et al. Systemic G-CSF treatment does not improve long-term outcomes after neonatal hypoxic-ischaemic brain injury. Exp. Neurol. 2011;230:67–74. doi: 10.1016/j.expneurol.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 51.Taglialatela G. Hogan D. Zhang W.R. Dineley K.T. Intermediate- and long-term recognition memory deficits in Tg2576 mice are reversed with acute calcineurin inhibition. Behav. Brain. Res. 2009;200:95–99. doi: 10.1016/j.bbr.2008.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bevins R.A. Besheer J. Object recognition in rats and mice: a one-trial non-matching-to-sample learning task to study 'recognition memory'. Nat. Protoc. 2006;1:1306–1311. doi: 10.1038/nprot.2006.205. [DOI] [PubMed] [Google Scholar]
- 53.Ennaceur A. Michalikova S. Bradford A. Ahmed S. Detailed analysis of the behavior of Lister and Wistar rats in anxiety, object recognition and object location tasks. Behav. Brain Res. 2005;159:247–266. doi: 10.1016/j.bbr.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 54.Fox G.B. Faden A.I. Traumatic brain injury causes delayed motor and cognitive impairment in a mutant mouse strain known to exhibit delayed Wallerian degeneration. J. Neurosci. Res. 1998;53:718–727. doi: 10.1002/(SICI)1097-4547(19980915)53:6<718::AID-JNR9>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 55.Fox G.B. LeVasseur R.A. Faden A.I. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: implications for gene targeting approaches to neurotrauma. J. Neurotrauma. 1999;16:377–389. doi: 10.1089/neu.1999.16.377. [DOI] [PubMed] [Google Scholar]
- 56.Faden A.I. Fox G.B. Di X., et al. Neuroprotective and nootropic actions of a novel cyclized dipeptide after controlled cortical impact injury in mice. J. Cereb. Blood Flow Metab. 2003;23:355–363. doi: 10.1097/01.WCB.0000046144.31247.33. [DOI] [PubMed] [Google Scholar]
- 57.Livak K.J. Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 58.Tanic N. Perovic M. Mladenovic A. Ruzdijic S. Kanazir S. Effects of aging, dietary restriction and glucocorticoid treatment on housekeeping gene expression in rat cortex and hippocampus-evaluation by real time RT-PCR. J. Mol. Neurosci. 2007;32:38–46. doi: 10.1007/s12031-007-0006-7. [DOI] [PubMed] [Google Scholar]
- 59.Loncarevic–Vasiljkovic N. Pesic V. Tanic N., et al. Changes in markers of neuronal and glial plasticity after cortical injury induced by food restriction. Exp. Neurol. 2009;220:198–206. doi: 10.1016/j.expneurol.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 60.Searle S.R. Casella G. McCulloch C.E. Variance Components. Wiley-Interscience; New York: 2006. [Google Scholar]
- 61.Winking H. Gerdes J. Traut W. Expression of the proliferation marker Ki-67 during early mouse development. Cytogenet. Genome Res. 2004;105:251–256. doi: 10.1159/000078196. [DOI] [PubMed] [Google Scholar]
- 62.Scholzen T. Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 63.Couillard–Despres S. Winner B. Schaubeck S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 64.Rao M.S. Shetty A.K. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur. J. Neurosci. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- 65.Chytrova G. Ying Z. Gomez–Pinilla F. Exercise normalizes levels of MAG and Nogo-A growth inhibitors after brain trauma. Eur. J. Neurosci. 2008;27:1–11. doi: 10.1111/j.1460-9568.2007.05982.x. [DOI] [PubMed] [Google Scholar]
- 66.Petrinovic M.M. Duncan C.S. Bourikas D., et al. Neuronal Nogo-A regulates neurite fasciculation, branching and extension in the developing nervous system. Development. 2010;137:2539–2550. doi: 10.1242/dev.048371. [DOI] [PubMed] [Google Scholar]
- 67.McGee A.W. Yang Y. Fischer Q.S. Daw N.W. Strittmatter S.M. Experience-driven plasticity of visual cortex limited by myelin and Nogo receptor. Science. 2005;309:2222–2226. doi: 10.1126/science.1114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Karlen A. Karlsson T.E. Mattsson A., et al. Nogo receptor 1 regulates formation of lasting memories. Proc. Natl. Acad. Sci. U. S. A. 2009;106:20,476–20,481. doi: 10.1073/pnas.0905390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Smith D.H. Soares H.D. Pierce J.S., et al. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J. Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- 70.Baldwin S.A. Gibson T. Callihan C.T. Sullivan P.G. Palmer E. Scheff S.W. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical disector method for cell counting. J. Neurotrauma. 1997;14:385–398. doi: 10.1089/neu.1997.14.385. [DOI] [PubMed] [Google Scholar]
- 71.Saatman K.E. Feeko K.J. Pape R.L. Raghupathi R. Differential behavioral and histopathological responses to graded cortical impact injury in mice. J. Neurotrauma. 2006;23:1241–1253. doi: 10.1089/neu.2006.23.1241. [DOI] [PubMed] [Google Scholar]
- 72.Hanell A. Clausen F. Bjork M., et al. Genetic deletion and pharmacological inhibition of Nogo-66 receptor impairs cognitive outcome after traumatic brain injury in mice. J. Neurotrauma. 2010;27:1297–1309. doi: 10.1089/neu.2009.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dimou L. Schnell L. Montani L., et al. Nogo-A-deficient mice reveal strain-dependent differences in axonal regeneration. J. Neurosci. 2006;26:5591–5603. doi: 10.1523/JNEUROSCI.1103-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yiu G. He Z. Glial inhibition of CNS axon regeneration. Nat. Rev. Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun D. Bullock M.R. Altememi N., et al. The effect of epidermal growth factor in the injured brain after trauma in rats. J. Neurotrauma. 2010;27:923–938. doi: 10.1089/neu.2009.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chiaretti A. Barone G. Riccardi R., et al. NGF, DCX, and NSE upregulation correlates with severity and outcome of head trauma in children. Neurology. 2009;72:609–616. doi: 10.1212/01.wnl.0000342462.51073.06. [DOI] [PubMed] [Google Scholar]
- 77.Miyake K. Yamamoto W. Tadokoro M., et al. Alterations in hippocampal GAP-43, BDNF, and L1 following sustained cerebral ischemia. Brain Res. 2002;935:24–31. doi: 10.1016/s0006-8993(02)02420-4. [DOI] [PubMed] [Google Scholar]
- 78.Chivatakarn O. Kaneko S. He Z. Tessier–Lavigne M. Giger RJ. The Nogo-66 receptor NgR1 is required only for the acute growth cone-collapsing but not the chronic growth-inhibitory actions of myelin inhibitors. J Neurosci. 2007 Jul 4;27(27):7117–7124. doi: 10.1523/JNEUROSCI.1541-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Marklund N. Bareyre F.M. Royo N.C., et al. Cognitive outcome following brain injury and treatment with an inhibitor of Nogo-A in association with an attenuated downregulation of hippocampal growth-associated protein-43 expression. J. Neurosurg. 2007;107:844–853. doi: 10.3171/JNS-07/10/0844. [DOI] [PMC free article] [PubMed] [Google Scholar]