Abstract
We have previously reported that mild fluid percussion injury (FPI) is associated with a heightening of the hypothalamic-pituitary-adrenal axis response during the first post-injury weeks. This is the same time period when rehabilitative exercise has been strongly suggested to be ineffective. Here, we explored whether cardiac and temperature autonomic function may also be compromised during this early post-injury period. Following an FPI or sham injury, rats were exercised with forced (fRW) or voluntary (vRW) running wheels on post-injury days 0–4 and 7–11. Results indicated that overall activity levels were decreased and circadian rhythm was affected after FPI. Autonomic disruptions became evident when exercise was introduced, and these disruptions were dependent upon the characteristics of exercise. Elevations in heart rate (HR) and core body temperature (CBT) were observed as a response to vRW and fRW. FPI animals had more pronounced increases in HR as a result of vRW. Likewise, increases in HR were observed with fRW in all animals. A strong stress response has recently been associated with fRW exercise. FPI rats exposed to fRW were more responsive to experimental manipulations and had higher a CBT after the FRW session. The results suggest that subacute exercise, particularly if linked to a strong stress response, may be counterproductive. Here we show that cardiac and temperature autonomic function are compromised during the subacute period following a mild TBI.
Key words: adult brain injury, CBT, circadian, corticosterone, exercise, fatigue, HR
Introduction
The majority of traumatic brain injuries (TBI) can be categorized as either a mild or a concussive injury. It is estimated that 1,400,000–3,800,000 concussions occur in the United States alone.1 Therefore, it is becoming increasingly known that the consequences of a mild TBI can significantly affect quality of life.2,3 Although many of the post-concussive symptoms are resolved without treatment, the consequences of mild TBI can be long lasting. Persisting cognitive impairments and affective disturbances are not uncommon.4,5
Injury-induced alterations in neural activation and metabolic responses are observed during the subacute post-injury period.6,7 It is during this time that the injured brain is more vulnerable to sustaining further damage.8 With an animal model for mild TBI, we have shown that voluntary exercise during the early post-injury period exacerbates cognitive dysfunction.9 The injured brain responds differentially to exercise. For example, increases of key molecular markers of neuroplasticity, such as brain derived neurotrophic factor (BDNF), which normally occur with exercise, are absent during the subacute period. It is also during this post-injury period that there is a heightening of the hypothalamic-pituitary-adrenal (HPA) axis response.10 Moreover, we have recently shown that exercise regimens associated with a strong stress response do not lead to increases of BDNF.11 These findings are of particular concern because of the high incidence of concussions in athletic and military settings.12,13 In both of these settings, concerns have rightfully emerged regarding the return to normal physical activities.
Given the abovementioned effects of TBI on HPA regulation, it is reasonable to suspect that cardiac and temperature autonomic function may also be compromised during this early post-injury period. Changes in heart rate (HR) and core body temperature (CBT) may be detrimental to TBI recovery. Subtle elevations in CBT during the first post-injury week have been associated with axonal damage.14 More recently, the negative effects of hyperthermia have also been observed in rats that endured a mild TBI.15 To our knowledge, however, they have not been described following mild TBI within the context of exercise. Here we describe the HR, CBT, and ambulatory activity during exercise regimens that elicit a low or high stress response.
The data for these studies were obtained from the animals of a previous study analyzing adrenocorticotropic hormone (ACTH) and corticosterone (CORT) responses to post-injury exercise.11
Methods
Subjects
A total of 63 male Sprague–Dawley rats (mean weight: 289 g±3.37 SEM) from Charles River Breeding Labs (Hollister, CA) were utilized in these experiments. Rats underwent surgery to induce either sham injury (n=19) or fluid percussion injury (FPI) (n=22). Additional control rats were only exposed to anesthesia in order to control for surgical stress effects associated with the craniotomy procedure (n=22).16 Animals were handled daily and habituated to a reversed lighting schedule (lights off: 09:30– 21:30 h). During the experiments, rats were single-housed in opaque plastic bins (50.8×25.4×25.4 cm), which were lined with bedding material. Rats had ad libitum access to water and rat chow. All procedures were performed in accordance with the United States National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals, and were approved by the UCLA Chancellor's Animal Research Committee.
Transmitter implantation
One week prior to brain injury, rats were implanted with telemetry devices (Respironics Mini Mitter, Bend, OR) to monitor HR, CBT, and gross motor activity (results to be reported separately). Rats were anesthetized with isofluorane (4% for induction and 2.0% for maintenance in 100% O2) via a nose mask. When the animal was fully anesthetized, the ventral surface of the abdomen was shaved and cleansed with ethanol and betadine. A midline, abdominal skin incision was made followed by an incision along the linea abla. The transmitter was then positioned in the abdominal cavity along the sagittal plane, in front of the caudal arteries and veins. Positive and negative leads from the transmitter were pushed through the abdominal wall, which was then sutured with absorbable material. A small incision of the skin was made near the right clavicle, and a trochar was inserted subcutanously to allow for the end of the negative lead to be pushed and sutured to the pectoralis superficialis. A small skin incision was then made to the left of the xiphoid process and cranial to the last rib. The positive lead was then pushed subcutanously and sutured to a chest muscle. Skin was sutured, bupivacaine (0.25 mg) was injected at the wound site, and triple antibiotic ointment was applied over the incision.
Lateral FPI
Animals were randomly assigned to the surgery conditions described subsequently. As previously described,11 rats were anesthetized with isofluorane via a nose mask. The level of anesthesia was monitored by level of respiration, muscular relaxation, and pedal reflexes. After loss of pedal reflexes, the scalp and scapular regions were shaved, the animal was secured in a stereotaxic head frame, and the scalp was cleansed with ethanol and Betadine. Rectal temperature was monitored and maintained between 36.5 and 38.0°C with a thermostatically controlled heating pad (Braintree Scientific Inc., Braintree, MA). The scalp and temporal muscle were reflected and a 3 mm diameter circular craniotomy was made over the left parietal cortex, centered at 3 mm posterior to bregma and 6 mm lateral to the midline. The bone flap was removed and the dura left intact in all animals to receive FPI. The dura was inspected with the aid of a microscope (Wild, Heerburg, Switzerland) in order to assure that it was intact. A plastic injury cap was placed over the craniotomy with silicone adhesive, cyanoacrylate, and dental cement. When the dental cement hardened, the cap was filled with 0.9% NaCl solution. Anesthesia was discontinued and the animal was removed from the stereotaxic device. The injury cap was attached to the fluid percussion device. At the first sign of hindlimb withdrawal to a paw pinch, a mild/moderate fluid percussion pulse (1.5 atm) was administered. Apnea times were determined as the time from injury to the return of spontaneous breathing. Time of unconsciousness was determined from the time of injury until the return of the hindlimb withdrawal reflex. Immediately upon responding to a paw pinch, anesthesia was restored, the injury cap removed, and the scalp was sutured. Control sham animals underwent an identical preparation with the exception of the FPI. Anesthesia controls were placed under anesthesia for a period of time similar to that of the rats that underwent FPI or sham surgery. A skin incision was also made, as described, to assure that there would be experimenter blindness for the following procedures. After suturing, bupivacaine (0.25 mg) was injected into the margins of the scalp incision and triple antibiotic ointment was applied over the incision. The rat was placed in a recovery chamber for ∼1 h before being returned to its cage. All injuries were performed before 12:00 h.
Forced and voluntary wheel exercise
Animals were randomly assigned to either voluntary exercise (vRW), forced exercise (fRW) or sedentary (Sed) conditions. Exposure to exercise commenced 3–5 h after the FPI. Rats in the vRW condition were placed in cages equipped with a running wheel (RW, diameter=31.8 cm, width=10 cm; Nalge Nunc International, Rochester, NY) that rotated against a resistance of 100g. These animals were allowed to exercise from post-injury days (PIDs) 0–4 and PIDs 7–11. Exercise was quantified by recording the number of wheel revolutions per minute using VitalView Data Acquisition System software (Respironics Mini Mitter Company Inc., Bend, OR). Rats under the fRW condition were exposed to similar wheels as those utilized for the vRW, with the exception that these had a motor attached (Pittman, Harleysville, PA) that allowed for speed to be individually controlled. Rats under the fRW condition received two daily 20 min exercise sessions (at 10:00 h and 14:00 h) on PIDs 0–4 and PIDs 7–11.
Blood was collected by tail venipuncture at PIDs 0, 4, 7, and 11. All blood samples were obtained between 14:00 and 16:00 h during the active dark phase. After the last blood collection on PID 11, rats were killed and the thymus and adrenal glands were removed and weighed. Blood samples were analyzed for CORT and ACTH. These findings have been recently described in a separate manuscript.11
Data collection and statistical analysis
Telemetry data were collected every minute for the duration of the experiment. This allowed us to determine changes in CBT and HR as a response to exercise. This form of data collection was particularly useful for the animals under the vRW condition, because the number of wheel revolutions is variable. In order to obtain values for CBT and HR as a response to vRW, only periods with a minimum of 4 consecutive min of exercise were utilized. Amount of vRW was detected as wheel revolutions. CBT and HR were then normalized for the duration of continuous exercise. In contrast, because the fRW was for a fixed duration, we were able to compare variables during fRW as well as recovery, by analyzing data collected from the 20 min immediately after the fRW session. Post-vRW values were not obtained, because of the lack of a prolonged continuous rest period immediately following a bout of vRW. Average values for each daily 12 h light/dark cycle were also calculated for overall analysis of HR, CBT, and activity. This allowed us to detect increases of measured variables at particular hours (i.e., peaks).
Telemetry and the above-described peak data were analyzed by utilizing a mixed model repeated measures analysis where injury (FPI and control) and exercise (vRW, fRW, Sed) differences were evaluated within time (i.e., PIDs). Telemetry data, as a response to exercise, were also analyzed with a mixed model repeated measures analysis where injury (FPI and Control) differences were evaluated within time. Differences in individual group means were detected with Bonferroni corrected comparisons (SPSS Inc., NY).
Results
Subjects
Injured rats had a mean (±SEM) unconsciousness duration of 145±10 sec and a mean apnea duration of 41±6 sec. No gross motor impairments of ambulatory ability were observed in any of the injured rats. No significant differences were observed between anesthesia controls and sham-injured rats in any of the assessment measures. Therefore, these groups were pooled and will be referred to as control (Ctrl).
Forced and voluntary exercise
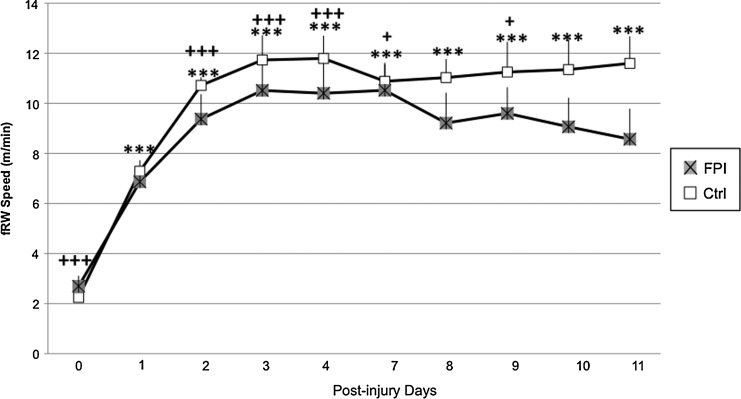
No differences were observed between FPI and Ctrl groups when fRW speed was analyzed. A significant time effect was observed across all groups (F[1,9]=3.93, p<0.0005). Further analysis indicated significantly lower running speeds for all animals at PID 0 compared with the other PIDs (p<0.0005). Significant differences were also observed at PID 1 compared with all other PIDs except days 8, 10, and 11 (p<0.05) (Fig. 1).
FIG. 1.
Running speed of control (Ctrl) and fluid percussion injured (FPI) rats exposed to the forced running wheel. Each value represents the daily mean meters per min±SEM. achieved. Running speed was lower on post-injury days (PIDs) 0 and 1 than on other days. ***p<0.0005 compared with PID 0. +p<0.005, +++p<0.0005 compared with PID 1.
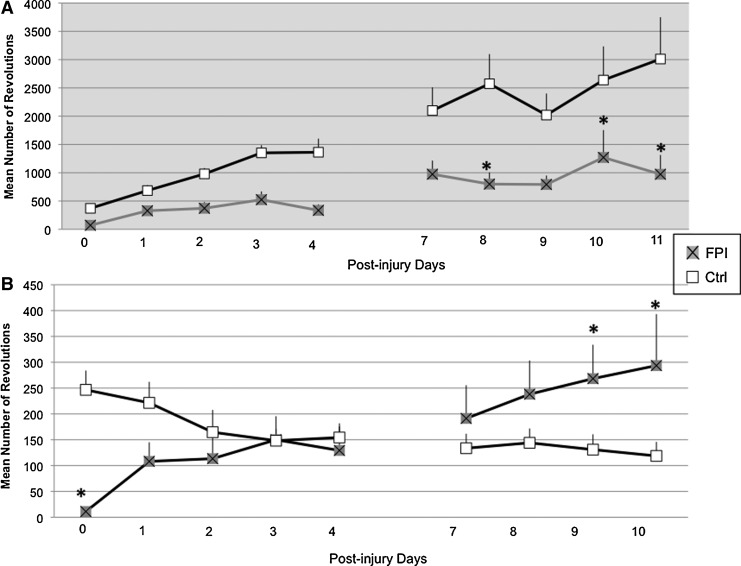
The amount of voluntary exercise was influenced by the corresponding dark/light cycle. Analysis of the number of wheel revolutions during the 12 h dark phase indicated a significant injury×time interaction (F[1,9]=2.53, p<0.05). FPI-vRW rats ran significantly less compared with the Ctrl-vRW at PIDs 8, 10, and 11 (p<0.05). At PIDs 7 and 9, significance was approached (p=0.08 and p=0.06 respectively). The amount of running increased over time across groups, as indicated by a significant time effect (F[1,9]=8.21, p<0.0005) (Fig. 2A).
FIG. 2.
Mean number of wheel revolutions during the 12 h dark (A) and light (B) cycle of control (Ctrl) and fluid percussion injured (FPI) rats exposed to voluntary exercise. Each value represents the mean±SEM. *p<0.05 compared with Ctrl across days.
Analysis of the number of wheel revolutions during the light phase also indicated a significant injury×time interaction (F[1,8]=3.38, p<0.005). Although FPI-vRW rats ran significantly less than the Ctrl-vRW at PID 0 (p<0.05), the amount of exercise of the FPI-vRW rats increased over time. In contrast, the amount of exercise decreased over time in the Ctrl-vRW rats. During the second week, the FPI-vRW group exercised significantly more than the Ctrl-vRW group on PIDs 9 and 10 (p<0.05) (Fig. 2B).
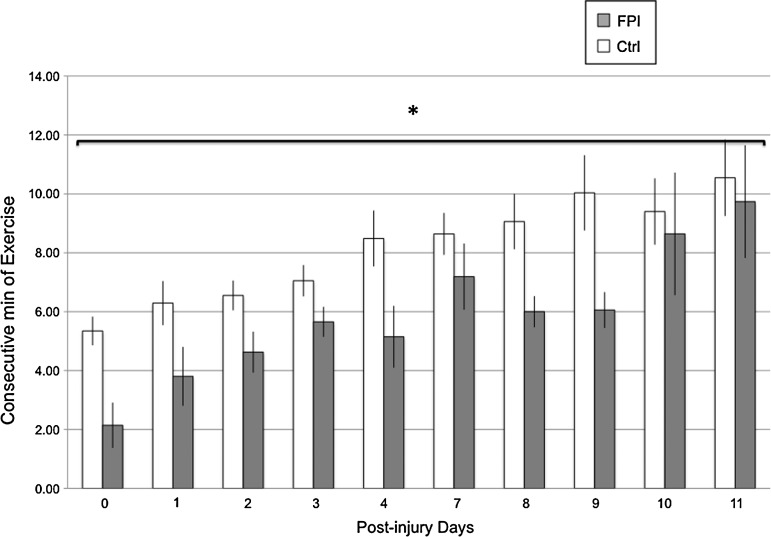
Endurance was analyzed as consecutive minutes of exercise when exposed to the vRW. A significant main injury effect (F[1,9]=5.3 p<0.05) indicated that the FPI-vRW group exercised for fewer consecutive minutes than the Ctrl-vRW group. Endurance increased over time as indicated by a significant time effect (F[1,9]=4.61 p<0.0005) (Fig. 3).
FIG. 3.
Consecutive minutes of voluntary exercise of control (Ctrl) and fluid percussion injured (FPI) rats. Each value represents the mean±SEM. *p<0.05 compared with Ctrl across days.
Activity levels during periods of non-exercise
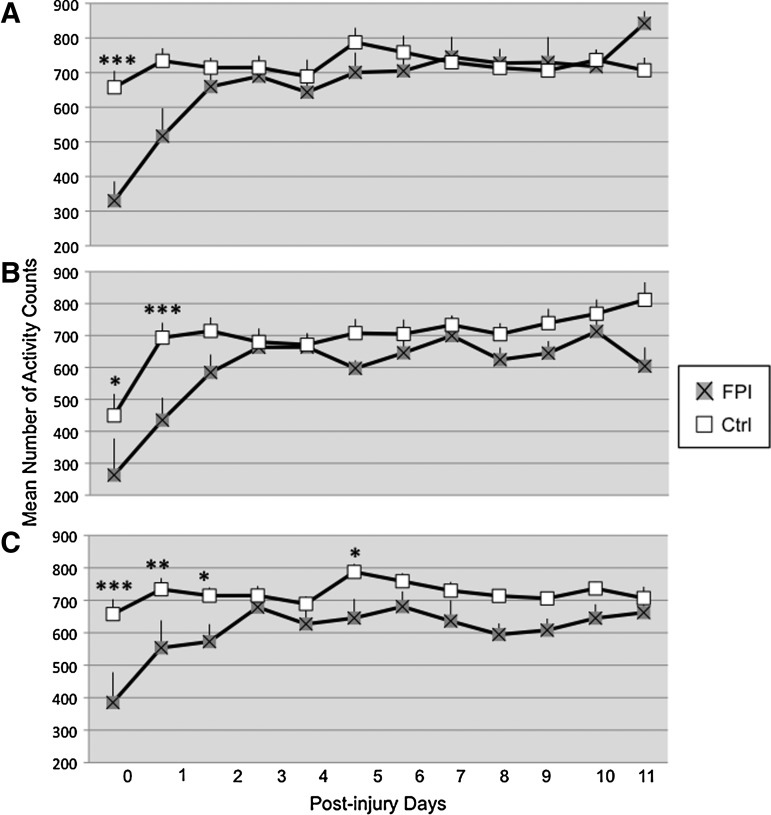
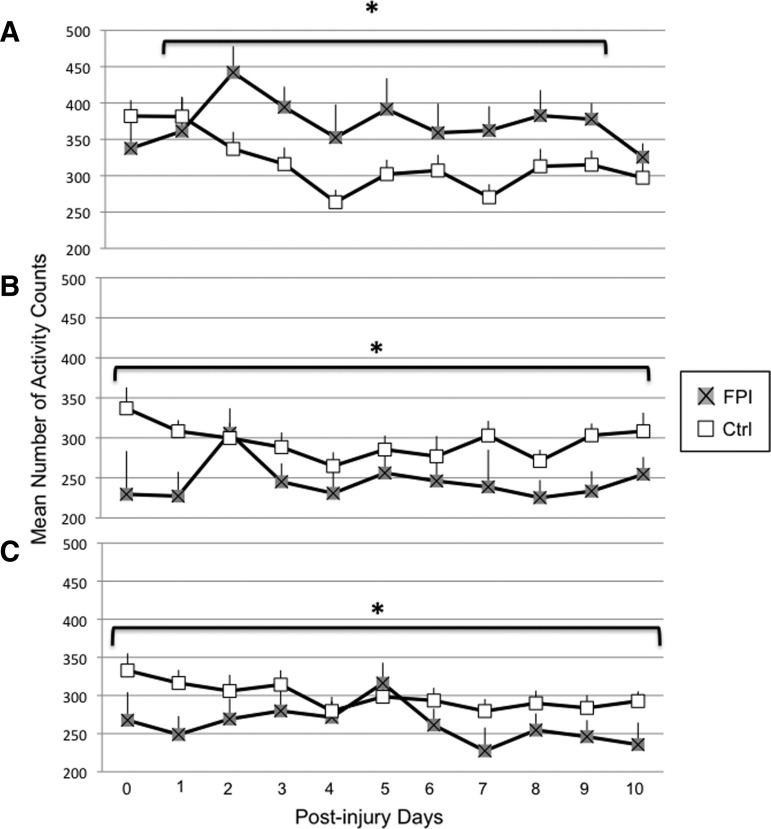
A significant injury×exercise×time interaction was observed for overall activity during the dark 12 h period (F[22,474]=1.53 p<0.05). Within the vRW condition, activity levels were lower in the FPI rats during the non-exercise periods at PID 0 (p<0.0005) and approached significance at PID 1 (p=0.08) (Fig. 4A). Within the Sed groups, activity counts were lower for the FPI animals at PID 0 (p<0.05) and PID 1 (p<0.0005) and approached significance at PID 2 (p=0.07) (Fig. 4B). Likewise, activity counts were lower for the FPI-fRW rats than for Ctrl-fRW during the non-exercise periods. This was particularly more pronounced at PID 0 (p<0.0005), PID 1 (p<0.005), PID 2 (p<0.05), and PID 5 (p<0.05) (Fig. 4C). These findings were supported by a significant injury×time interaction (F[11,469]=3.15, p<0.0005) and a significant main injury effect (F[1,79]=12.21, p<0.005).
FIG. 4.
Overall activity during the dark 12 h period of control (Ctrl) and fluid percussion injured (FPI) rats under voluntary exercise (A), sedentary (B), or forced exercise (C) conditions. Each value represents the mean number of activity counts in a 1 h sampling interval±SEM. *p<0.05 compared with FPI.
Again, the corresponding dark/light cycle had an influence on the analyzed measure. A significant injury×exercise effect was observed for ambulatory activity during the light 12 h period (F[2,98]=8.59, p<0.0005). Activity counts during periods of non-exercise were higher for the FPI-vRW group than for the Ctrl-vRW group (p<0.01) (Fig. 5A). In contrast, activity levels were lower for the FPI-Sed and FPI-fRW (p<0.05) groups than for the corresponding Ctrl-Sed (Fig. 5B) and Ctrl-fRW groups (Fig. 5C), respectively. A significant injury×time interaction (F[10,415]=2.6, p<0.005) was also observed. This was because of significantly lower activity counts in the FPI animals at PID 0 and PID1 (p<0.05). These findings were reflected as a main effect for exercise (F[2,98]=17.13, p<0.0005).
FIG. 5.
Overall activity during the light 12 h period of control (Ctrl) and fluid percussion injured (FPI) rats under voluntary exercise (A), sedentary (B), or forced exercise (C) conditions. Each value represents the mean number of activity counts in a 1 h sampling interval±SEM. *p<0.05 compared with FPI.
Overall HR
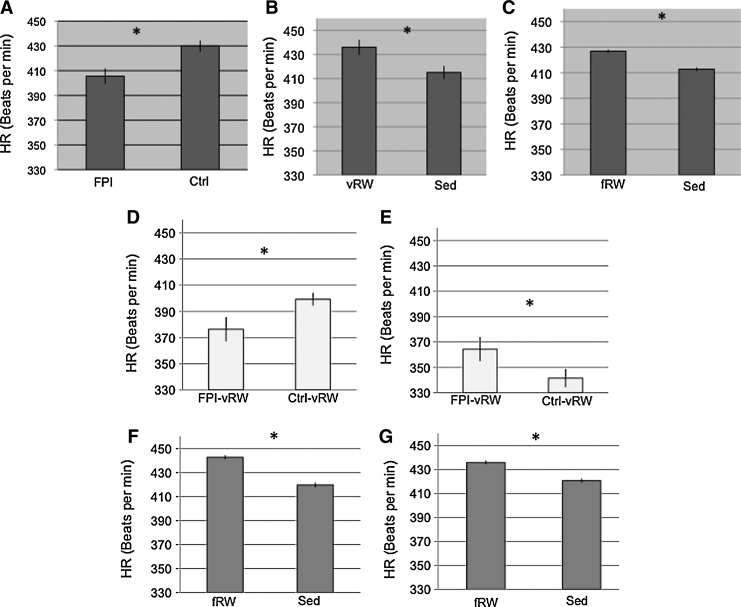
HR was analyzed during the 12 h dark cycle across all injury and exercise groups. Analysis of HR during the dark cycle revealed that on PID 0, HR was lower in the FPI animals than in Ctrl (p<0.05) (Fig. 6A). This was supported by a significant injury×time interaction (F[11,548]=4.6 p<0.0005). HR was higher on PID 11 for animals under the vRW condition than for the Sed groups, irrespective of injury (Fig. 6B). This was supported by a significant exercise×time interaction (F[22,548]=4.6 p<0.05). Across all days, the HR for fRW were significantly higher than those of Sed animals (p<0.05) (Fig 6C).
FIG. 6.
Heart rate (HR) of control (Ctrl) and fluid percussion injured (FPI) rats under voluntary exercise (vRW), sedentary (Sed), or forced exercise (fRW) conditions. An injury effect was found on post-injury day (PID) 0 during the dark period (A). Exercise effects, during the dark period, were found on PID 11 (B) and across all days (C). HR differences for the FPI-vRW group were found, during the light period, at PID 0 (D) and PID 10 (E). Increases in HR were more pronounced in animals exposed to the fRW at 14:00 h (F) and at 19:00 h (G). Each value represents the mean HR (beats per min)±SEM. *p<0.05.
Analysis of HR during the 12 h light period revealed a significant exercise×time×injury interaction (F[20,512]=1.6. p<0.05). Further analysis indicated that at PID 0, HR was lower in the FPI-vRW group than in the Ctrl-vRW group (p<0.05) (Fig. 6D). An opposite effect was observed at PID 10. Here, HR was higher in the FPI-vRW group than in the Ctrl-vRW group (p<0.05) (Fig. 6E). Analysis of HR during the 12 h light cycle across all injury and exercise groups showed that HR tended to decrease over time for all injury groups. A significant exercise×time interaction (F[20,512]=1.92 p<0.05).
Three consistent peaks for HR were detected when average telemetry values for each hour were calculated. These occurred at 10:00, 14:00, and 19:00 h within the dark 12 h cycle. Peaks occurred at the same time across the experimental days. The first two peaks coincided with the initiation time of the fRW sessions. The third peak coincided with the time when the lights were automatically switched on for the commencement of the 12 h light cycle. No significant findings were observed when the 10:00 h HR peak was analyzed. Only a trend suggesting that HR was higher in the fRW groups than in the Sed groups (p=0.06) was observed. Analysis of the second peak indicated a significant main effect for exercise (F[2,88]=6.8, p<0.005). This was attributed to higher HR values in the fRW groups than in the Sed groups (p<0.05) (Fig. 6F). A similar effect was observed for the third peak, in that animals under the fRW condition had a higher HR than those in the Sed condition (p<0.05) (Fig 6G). This was reflected as a significant main effect for exercise (F[2,86]=3.8, p<0.05).
HR as a response to exercise
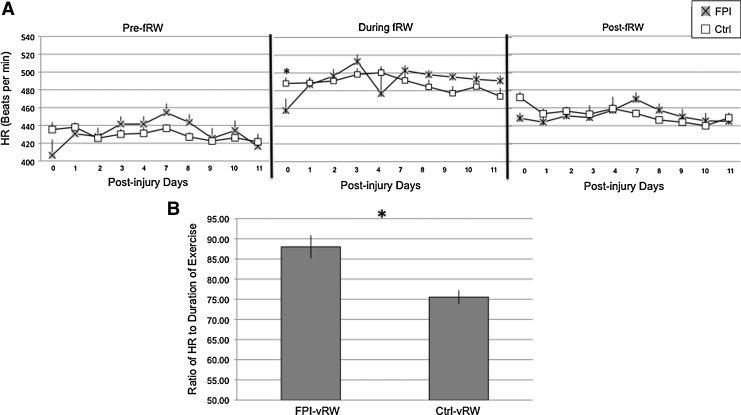
No significant differences in HR between FPI and Ctrl animals were observed when analyzing the 20 min prior to fRW. Analysis of the HR during fRW indicated a significant group×time interaction (F[1,9]=2.05, p<0.05); this was mostly attributed to a lower HR in the FPI-fRW group than in the Ctrl-fRW group at PID 0 (p<0.05). There were no significant group differences in HR during the 20 min period following fRW (Fig 7A).
FIG. 7.
Heart Rate (HR) of control (Ctrl) and fluid percussion injured (FPI) rats in 20 min periods before, during, and after forced exercise (fRW) (A). HR of Ctrl and FPI rats during voluntary exercise (vRW) (B). Each value represents the mean±SEM. *p<0.05.
Analysis of HR during vRW did not include PID 0, because the FPI-vRW group did not achieve the minimum amount of exercise required. Elevations in HR were more pronounced, across PID 1 through PID 11, in the FPI-vRW group than in the Ctrl-vRW group. This was indicated by a significant main effect for group (F[1,8]=6.04 p<0.05) (Fig. 7B).
Overall CBT
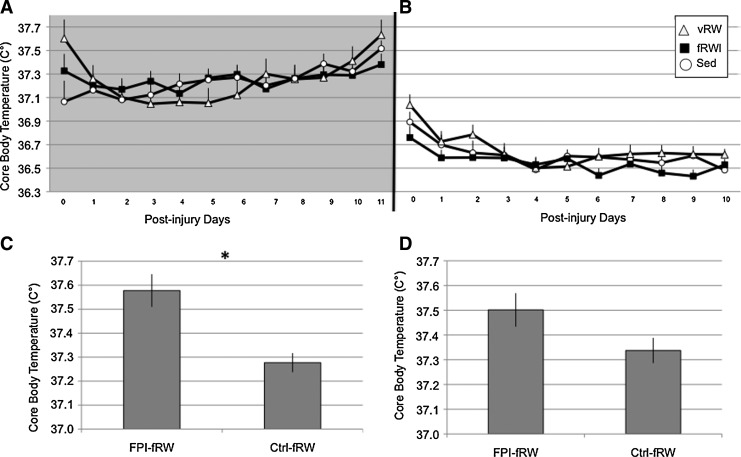
Analysis of overall CBT during the 12 h dark phase revealed a significant injury×time interaction (F[11,501]=2.06, p<0.05). A significant exercise × time effect was observed for CBT (F[22,503]=1.7, p<0.05) (Fig. 8A). No significant individual comparisons were observed. Analysis of overall CBT during the 12 h light phase indicated a significant exercise×time interaction (F[20,468]=1.78, p<0.05) (Fig 8B).
FIG. 8.
Core body temperature (CBT) of Control (Ctrl) and fluid percussion injured (FPI) rats under voluntary exercise (vRW), sedentary (Sed), or forced exercise (fRW) conditions. CBT values varied across time and exercise groups during the dark (A) and light (B) cycles. An increase in CBT was more pronounced in animals exposed to the fRW at 14:00 h (C). A trend was found at 19:00 h (D). Each value represents the mean±SEM. *p<0.05.
Three consistent peaks for CBT were detected when average telemetry values for each hour were calculated. These coincide with the HR peaks at 10:00, 14:00, and 19:00 hrs within the dark 12-hr cycle. No significant effects were found for the 10:00 h. However, a significant injury×exercise interaction (F[9,379]=1.04, p<0.05) was observed for the peak at 14:00 h. This was attributed to significantly higher CBT values for the FPI-fRW group than for the Ctrl-fRW group (p<0.05) (Fig. 8C). Analysis of the 19:00 peak indicated a significant injury×time interaction (F[9,387]=2.48, p<0.01). Although FPI-fRW values tended to be higher than those of Ctrl-fRW, significance was not reached (Fig. 8D).
CBT as a response to exercise
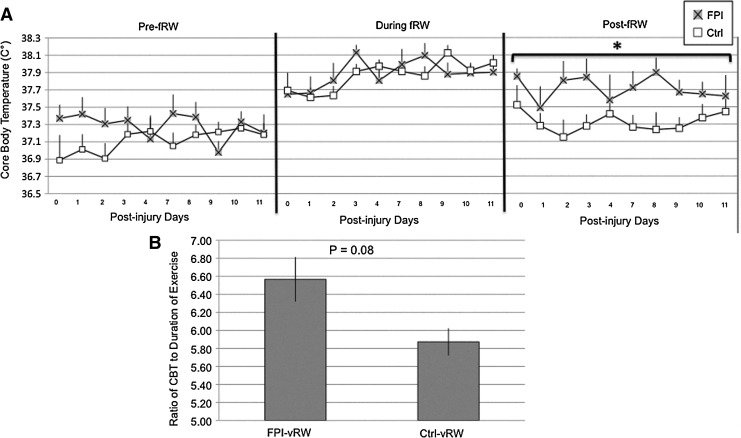
No significant differences in CBT between FPI and Ctrl animals were observed when analyzing the 20 min prior to fRW. Overall CBT increased over days during fRW. This was indicated by a significant tIme effect (F[1,9]=1.92, p<0.05). Although CBT elevations caused by fRW were equivalent for FPI and Ctrl rats, the recovery period was not. FPI-fRW had a higher CBT across days for the 20 min period following fRW (F[1,9]=8.09, p<0.05) (Fig. 9A).
FIG. 9.
Core body temperature (CBT) of control (Ctrl) and fluid percussion injured (FPI) rats in 20 min periods before, during, and after forced exercise (fRW) (A). CBT of Ctrl and FPI rats during voluntary exercise (vRW) (B). Each value represents the mean±SEM. *p<0.05.
Analysis of CBT during vRW did not include PID 0, because the FPI-vRW group did not achieve the minimum amount of exercise required. Although CBT tended to be more pronounced across PID 1 through PID 11, in the FPI-vRW group compared with the Ctrl-vRW group, it did not reach significance (p=0.08) (Fig. 9B).
Discussion
Here we show that cardiac and temperature autonomic function are compromised during the subacute period following a mild TBI. These disruptions become evident when exercise is introduced. Moreover, these physiological responses are dependent on the characteristics of exercise. These findings also indicate that overall activity levels and circadian rhythm are affected after FPI. These findings are likely to be related to the dysregularization of the HPA axis that has been observed in these animals.
Activity levels decrease after FPI
Fatigue is a common complaint in patients following a mild TBI.17 The activity data obtained in this study are in accordance with clinical findings regarding a lack of energy and inertia after TBI. General activity was decreased after FPI, as demonstrated by lower number of activity counts. Decreased activity levels were also evident when evaluating the amount of vRW exercise. In addition, FPI rats exercised for a lesser number of consecutive minutes than did controls, which is indicative of decreased endurance.
A neuroendocrine component may contribute to the diminished activity. Decreases in activity were most noticeable during the first post-injury week; particularly during the first 2 post-injury days. It is during this time window that basal levels of CORT are low.10 This is in accord with clinical findings correlating fatigue with decreased cortisol levels.18,19 After the first post-injury days, activity levels appear to recover, as suggested by activity counts. Clinically, fatigue is most prevalent during the first post-injury week,17 whereas persistence of fatigue is likely to be influenced by other factors such as depression and anxiety.19,20
Circadian rhythm disruption after FPI
Clinically, neuroendocrine alterations following TBI are also observed as HPA circadian rhythm disruptions.21 These may manifest as sleep disturbances that are frequently reported after TBI.22 Likewise, HPA axis abnormalities are likely to have an impact on the schedule for voluntary exercise. This is particularly feasible given that CORT influences running wheel behavior in a dose–response manner.23 CORT levels follow circadian patterns of secretion, with basal levels being lower in the inactive/light period.24 Although it remains to be determined how circadian regulation of CORT is disrupted following a FPI, group comparisons of the amount of vRW exercise, during the dark and light periods, suggest that there is circadian rhythm disruption.
During the second post-injury week, the FPI-vRW rats exercised less during the active/dark period and exercised more during the inactive/light period than did rats under the Ctrl-vRW condition. This increase of vRW exercise, in the FPI rats during the inactive/light period, coincided with the recovery of non-RW activity levels observed during the dark/active period. Paradoxically, when non-RW activity was observed, the activity levels during the light cycle were lower in the FPI rats that did not have access to an RW. Given that non-RW activity counts during the dark period did not differ from those of the Ctrl animals, it is unlikely that this effect was caused by persistence of injury-induced fatigue. This effect may be, instead, attributed to a disruption of neuronal circuits regulating sleep quality and arousal, thus making circadian disruption most noticeable when animals are presented with a rewarding stimulus such as access to a vRW. Sleepdisorders after TBI, are frequently manifested as increased wakefulness and arousal.25–27
CBT and HR as responses to exercise
No alterations in CBT were detected in the unexercised FPI group. The same would have applied for HR, except for an acute decrease on the day of the FPI. This transient decrease in HR continued, only in the FPI-vRW group, for the following light period. Clinically irregularities in HR are reported during the subacute period.28,29 However, variability in HR is primarily found following severe TBI, in which intracranial hypertension is observed.30 Therefore, given that the FPI was more within the mild range, we would not expect HR effects beyond the acute period. Intriguingly, FPI- dependent changes in HR were observed as a response to vRW. Even though Ctrl rats exercised for more consecutive minutes, the FPI animals had more pronounced increases in HR as a result of vRW. Likewise, higher HRs have been reported in concussed athletes as a response to exercise, than in matched controls.31,32
Increases in HR were not observed in the fRW condition, in which the pattern of exercise consisted of two 20 min daily sessions, In contrast to fRW, vRW was ad libitum mostly during the dark phase of PIDs 0–4 and PIDs 7–11. These increases in HR, observed in the FPI-vRW group, are more likely to be reflective of aerobic capacity than of exercise intensity. This conclusion is purely speculative, and further studies evaluating oxygen uptake during exercise are necessary.
A distinction between FPI and Ctrl animals was also observed when evaluating CBT as a response to exercise, particularly following fRW. Although temperature elevations during the fRW session were similar in the FPI and Ctrl groups, the CBT of FPI rats remained elevated in the 20 min period immediately following the fRW session. Elevations in CBT after TBI are of concern. It is well known that fever worsens TBI outcomes in humans and animals.14,33 Among other types of stimulation, hyperthermia has been found to increase extracellular levels of glutamate34,35 and to increase inflammatory processes.36 Cerebrovascular changes such as increases in cerebral blood flow are also observed with hyperthermia.37,38 Moreover, even mild temperature variations can negatively affect outcome. A recent study utilizing an experimental mild TBI demonstrated that peritraumatic mild hyperthermia increased cortical contusion size.15
Injury-induced hyper-responsiveness
We have recently shown that fRW markedly stimulates the HPA axis irrespective of injury. However, whereas Ctrl rats habituate to the fRW, the FPI-fRW rats have a delay of habituation in that the ACTH response remains elevated during the first 11 days.11 Here, we show that these FPI-FRW rats are also more responsive to experimental manipulations. CBT was higher in these FPI rats during the second session of fRW. Given that an increase of CBT was not found during the morning session of fRW, elevations in CBT during the afternoon session can only be partially attributed to impaired thermoregulation following fRW, Moreover, CBT tended to be higher in the FPI-fRW animals when the lights were automatically turned on to change the dark/light cycle, therefore suggesting that these animals are more reactive to environmental changes. This is in accord with previous findings indicating increased sensitivity to stressful events during the first post-injury weeks, as determined by analyzing the HPA axis regulation following restraint-induced stress.11,39
As indicated, fRW has been proven to be stress producing. Here we also find that there is an overall increase in HR during the dark cycle for Ctrl and FPI animals exposed to fRW. In addition, increases in HR were also higher in the fRW groups during the second session of fRW and when the lights were automatically turned on to change the dark/light cycle. It should be noted that both FPI and Ctrl rats under the fRW condition presented sustained elevations of CORT that may have contributed to the observed HR increases.
Conclusion
Exercise has been shown to enhance recovery after TBI if it does not occur during the subacute period. We have previously shown that exercise during the first post-injury week decreases molecular markers of plasticity and worsens cognitive outcome. Here we report that early post-traumatic exercise results in a heightened stress response that is accompanied by a mild increase in body temperature. Acute exercise–induced increases in CBT may be a contributing factor to the negative cognitive effects of early exercise. Prolonged exercise in healthy individuals normally increases CBT.40 However, these normal increases are of significant concern if thermoregulation is impaired and there is a hypersensitivity to stressors as suggested by these studies. These findings are particularly relevant in the military setting where not only is there a strong likelihood of stress-producing events, but also temperature increases are more likely, because of the effects of exercising with combat uniform; which in itself can reduce body ventilation and raise CBT.41
Acknowledgments
We thank Ramin Rajaii and Parastoo Modirshahla for their assistance. This research was supported by NIH grant NS06190 to GSG and the UCLA Brain Injury Research Center.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Laker S.R. Epidemiology of concussion and mild traumatic brain injury. P.M. R. 2011;3:S354–358. doi: 10.1016/j.pmrj.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 2.Bazarian J.J. Blyth B. Cimpello L. Bench to bedside: evidence for brain injury after concussion – looking beyond the computed tomography scan. Acad. Emerg. Med. 2006;13:199–214. doi: 10.1197/j.aem.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 3.McAllister T.W. Flashman L.A. McDonald B.C. Saykin A.J. Mechanisms of working memory dysfunction after mild and moderate TBI: evidence from functional MRI and neurogenetics. J. Neurotrauma. 2006;23:1450–1467. doi: 10.1089/neu.2006.23.1450. [DOI] [PubMed] [Google Scholar]
- 4.Ashman T.A. Gordon W.A. Cantor J.B. Hibbard M.R. Neurobehavioral consequences of traumatic brain injury. Mt. Sinai J. Med. 2006;73:999–1005. [PubMed] [Google Scholar]
- 5.Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Sarkar R. Lee H. Meeker M. Zimmerman R.D. Manley G.T. McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: A 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am. J. Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Avramescu S. Timofeev I. Synaptic strength modulation after cortical trauma: a role in epileptogenesis. J. Neurosci. 2008;28:6760–6772. doi: 10.1523/JNEUROSCI.0643-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vagnozzi R. Signoretti S. Cristofori L. Alessandrini F. Floris R. Isgro E. Ria A. Marziale S. Zoccatelli G. Tavazzi B. Del Bolgia F. Sorge R. Broglio S.P. McIntosh T.K. Lazzarino G. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- 8.Tavazzi B. Vagnozzi R. Signoretti S. Amorini A.M. Belli A. Cimatti M. Delfini R. Di Pietro V. Finocchiaro A. Lazzarino G. Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses––part II. Neurosurgery. 2007;61:390–396. doi: 10.1227/01.neu.0000255525.34956.3f. [DOI] [PubMed] [Google Scholar]
- 9.Griesbach G.S. Hovda D.A. Molteni R. Wu A. Gomez–Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Griesbach G.S. Hovda D.A. Tio D.L. Taylor A.N. Heightening of the stress response during the first weeks after a mild traumatic brain injury. Neuroscience. 2011;178:147–158. doi: 10.1016/j.neuroscience.2011.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griesbach G.S. Tio D.L. Vincelli J. McArthur D.L. Taylor A.N. Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury. J. Neurotrauma. 2012;29:1426–1433. doi: 10.1089/neu.2011.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terrio H. Brenner L.A. Ivins B.J. Cho J.M. Helmick K. Schwab K. Scally K. Bretthauer R. Warden D. Traumatic brain injury screening: preliminary findings in a US Army Brigade Combat Team. J. Head Trauma Rehabil. 2009;24:14–23. doi: 10.1097/HTR.0b013e31819581d8. [DOI] [PubMed] [Google Scholar]
- 13.Thurman D.J. Branche C.M. Sniezek J.E. The epidemiology of sports-related traumatic brain injuries in the United States: recent developments. J. Head Trauma Rehabil. 1998;13:1–8. doi: 10.1097/00001199-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich W.D. Alonso O. Halley M. Busto R. Delayed posttraumatic brain hyperthermia worsens outcome after fluid percussion brain injury: a light and electron microscopic study in rats. Neurosurgery. 1996;38:533–541. doi: 10.1097/00006123-199603000-00023. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai A. Atkins C.M. Alonso O.F. Bramlett H.M. Dietrich W.D. Mild hyperthermia worsens the neuropathological damage associated with mild traumatic brain injury in rats. J. Neurotrauma. 2012;29:313–321. doi: 10.1089/neu.2011.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeKeyser F.G. Leker R.R. Weidenfeld J. Activation of the adrenocortical axis by surgical stress: involvement of central norepinephrine and interleukin-1. Neuroimmunomodulation. 2000;7:182–188. doi: 10.1159/000026437. [DOI] [PubMed] [Google Scholar]
- 17.Norrie J. Heitger M. Leathem J. Anderson T. Jones R. Flett R. Mild traumatic brain injury and fatigue: a prospective longitudinal study. Brain Inj. 2010;24:1528–1538. doi: 10.3109/02699052.2010.531687. [DOI] [PubMed] [Google Scholar]
- 18.Bay E. Xie Y. Psychological and biological correlates of fatigue after mild-to-moderate traumatic brain injury. West. J. Nurs. Res. 2009;31:731–747. doi: 10.1177/0193945909334856. [DOI] [PubMed] [Google Scholar]
- 19.Englander J. Bushnik T. Oggins J. Katznelson L. Fatigue after traumatic brain injury: Association with neuroendocrine, sleep, depression and other factors. Brain Inj. 2010;24:1379–1388. doi: 10.3109/02699052.2010.523041. [DOI] [PubMed] [Google Scholar]
- 20.Bushnik T. Englander J. Wright J. Patterns of fatigue and its correlates over the first 2 years after traumatic brain injury. J. Head Trauma Rehabil. 2008;23:25–32. doi: 10.1097/01.HTR.0000308718.88214.bb. [DOI] [PubMed] [Google Scholar]
- 21.Llompart–Pou J.A. Perez G. Raurich J.M. Riesco M. Brell M. Ibanez J. Perez–Barcena J. Abadal J.M. Homar J. Burguera B. Loss of cortisol circadian rhythm in patients with traumatic brain injury: a microdialysis evaluation. Neurocrit. Care. 2010;13:211–216. doi: 10.1007/s12028-010-9399-1. [DOI] [PubMed] [Google Scholar]
- 22.Castriotta R.J. Murthy J.N. Sleep disorders in patients with traumatic brain injury: a review. CNS Drugs. 2011;25:175–185. doi: 10.2165/11584870-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 23.Lin W.J. Singer G. Funder J. The action of corticosterone on schedule-induced wheelrunning. Eur. J. Pharmacol. 1989;171:9–15. doi: 10.1016/0014-2999(89)90424-x. [DOI] [PubMed] [Google Scholar]
- 24.Nader N. Chrousos G.P. Kino T. Interactions of the circadian CLOCK system and the HPA axis. Trends Endocrinol. Metab. 2010;21:277–286. doi: 10.1016/j.tem.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parcell D.L. Ponsford J.L. Redman J.R. Rajaratnam S.M. Poor sleep quality and changes in objectively recorded sleep after traumatic brain injury: a preliminary study. Arch. Phys. Med. Rehabil. 2008;89:843–850. doi: 10.1016/j.apmr.2007.09.057. [DOI] [PubMed] [Google Scholar]
- 26.Steele D.L. Rajaratnam S.M. Redman J.R. Ponsford J.L. The effect of traumatic brain injury on the timing of sleep. Chronobiol. Int. 2005;22:89–105. doi: 10.1081/cbi-200042428. [DOI] [PubMed] [Google Scholar]
- 27.Wallace D.M. Shafazand S. Ramos A.R. Carvalho D.Z. Gardener H. Lorenzo D. Wohlgemuth W.K. Insomnia characteristics and clinical correlates in Operation Enduring Freedom/Operation Iraqi Freedom veterans with post-traumatic stress disorder and mild traumatic brain injury: an exploratory study. Sleep Med. 2011;12:850–859. doi: 10.1016/j.sleep.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Biswas A.K. Scott W.A. Sommerauer J.F. Luckett P.M. Heart rate variability after acute traumatic brain injury in children. Crit. Care Med. 2000;28:3907–3912. doi: 10.1097/00003246-200012000-00030. [DOI] [PubMed] [Google Scholar]
- 29.Keren O. Yupatov S. Radai M.M. Elad–Yarum R. Faraggi D. Abboud S. Ring H. Groswasser Z. Heart rate variability (HRV) of patients with traumatic brain injury (TBI) during the post-insult sub-acute period. Brain Inj. 2005;19:605–611. doi: 10.1080/02699050400024946. [DOI] [PubMed] [Google Scholar]
- 30.Kahraman S. Dutton R.P. Hu P. Stansbury L. Xiao Y. Stein D.M. Scalea T.M. Heart rate and pulse pressure variability are associated with intractable intracranial hypertension after severe traumatic brain injury. J. Neurosurg. Anesthesiol. 2010;22:296–302. doi: 10.1097/ANA.0b013e3181e25fc3. [DOI] [PubMed] [Google Scholar]
- 31.Gall B. Parkhouse W. Goodman D. Heart rate variability of recently concussed athletes at rest and exercise. Med. Sci. Sports Exerc. 2004;36:1269–1274. doi: 10.1249/01.mss.0000135787.73757.4d. [DOI] [PubMed] [Google Scholar]
- 32.Gall B. Parkhouse W.S. Goodman D. Exercise following a sport induced concussion. Br. J. Sports Med. 2004b;38:773–777. doi: 10.1136/bjsm.2003.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilpatrick M.M. Lowry D.W. Firlik A.D. Yonas H. Marion D.W. Hyperthermia in the neurosurgical intensive care unit. Neurosurgery. 2000;47:850–856. doi: 10.1097/00006123-200010000-00011. [DOI] [PubMed] [Google Scholar]
- 34.Sharma H.S. Hyperthermia influences excitatory and inhibitory amino acid neurotransmitters in the central nervous system. An experimental study in the rat using behavioural, biochemical, pharmacological, and morphological approaches. J. Neural Transm. 2006;113:497–519. doi: 10.1007/s00702-005-0406-1. [DOI] [PubMed] [Google Scholar]
- 35.Suehiro E. Fujisawa H. Ito H. Ishikawa T. Maekawa T. Brain temperature modifies glutamate neurotoxicity in vivo. J. Neurotrauma. 1999;16:285–297. doi: 10.1089/neu.1999.16.285. [DOI] [PubMed] [Google Scholar]
- 36.Chatzipanteli K. Alonso O.F. Kraydieh S. Dietrich W.D. Importance of posttraumatic hypothermia and hyperthermia on the inflammatory response after fluid percussion brain injury: biochemical and immunocytochemical studies. J. Cereb. Blood Flow Metab. 2000;20:531–542. doi: 10.1097/00004647-200003000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Katsumura H. Kabuto M. Hosotani K. Handa Y. Kobayashi H. Kubota T. The influence of total body hyperthermia on brain haemodynamics and blood-brain barrier in dogs. Acta Neurochir. (Wien). 1995;135:62–69. doi: 10.1007/BF02307416. [DOI] [PubMed] [Google Scholar]
- 38.Ohmoto Y. Fujisawa H. Ishikawa T. Koizumi H. Matsuda T. Ito H. Sequential changes in cerebral blood flow, early neuropathological consequences and blood–brain barrier disruption following radiofrequency-induced localized hyperthermia in the rat. Int. J. Hyperthermia. 1996;12:321–334. doi: 10.3109/02656739609022521. [DOI] [PubMed] [Google Scholar]
- 39.Griesbach G.S. Vincelli J. Tio D.L. Hovda D.A. Effects of acute restraint-induced stress on glucocorticoid receptors and brain-derived neurotrophic factor after mild traumatic brain injury. Neuroscience. 2012;210:393–402. doi: 10.1016/j.neuroscience.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hayashi K. Honda Y. Ogawa T. Kondo N. Nishiyasu T. Relationship between ventilatory response and body temperature during prolonged submaximal exercise. J. Appl. Physiol. 2006;100:414–420. doi: 10.1152/japplphysiol.00541.2005. [DOI] [PubMed] [Google Scholar]
- 41.Chinevere T.D. Cadarette B.S. Goodman D.A. Ely B.R. Cheuvront S.N. Sawka M.N. Efficacy of body ventilation system for reducing strain in warm and hot climates. Eur. J. Appl. Physiol. 2008;103:307–314. doi: 10.1007/s00421-008-0707-9. [DOI] [PubMed] [Google Scholar]