Abstract
The complexity of human DNA has been affected by aerobic metabolism, including endurance exercise and oxygen toxicity. Aerobic endurance exercise could play an important role in the evolution of Homo sapiens, and oxygen was not important just for survival, but it was crucial to redox-mediated adaptation. The metabolic challenge during physical exercise results in an elevated generation of reactive oxygen species (ROS) that are important modulators of muscle contraction, antioxidant protection, and oxidative damage repair, which at moderate levels generate physiological responses. Several factors of mitochondrial biogenesis, such as peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), mitogen-activated protein kinase, and SIRT1, are modulated by exercise-associated changes in the redox milieu. PGC-1α activation could result in decreased oxidative challenge, either by upregulation of antioxidant enzymes and/or by an increased number of mitochondria that allows lower levels of respiratory activity for the same degree of ATP generation. Endogenous thiol antioxidants glutathione and thioredoxin are modulated with high oxygen consumption and ROS generation during physical exercise, controlling cellular function through redox-sensitive signaling and protein–protein interactions. Endurance exercise-related angiogenesis, up to a significant degree, is regulated by ROS-mediated activation of hypoxia-inducible factor 1α. Moreover, the exercise-associated ROS production could be important to DNA methylation and post-translation modifications of histone residues, which create heritable adaptive conditions based on epigenetic features of chromosomes. Accumulating data indicate that exercise with moderate intensity has systemic and complex health-promoting effects, which undoubtedly involve regulation of redox homeostasis and signaling. Antioxid. Redox Signal. 18, 1208–1246.
I. Introduction
Oxygen is the final electron acceptor in the mitochondrial electron transport chain during aerobic metabolism. It has been suggested that oxygen played a crucial role in evolution (90), and aerobic metabolism was necessary to develop adaptation to oxygen toxicity, including redox processes (323).
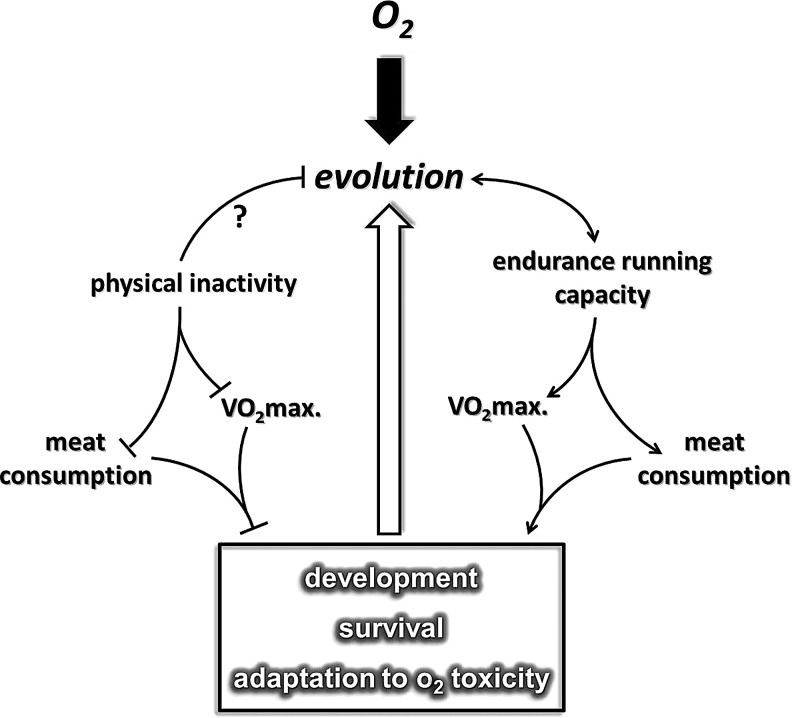
It has been proposed that endurance running played a significant role in the evolution of Homo sapiens (46, 320), which allowed hominem, possibly from the time of Homo erectus (341), to hunt and obtain sufficient amounts of meat for physical development. Based on this hypothesis, early human hunters with extreme endurance capacity were undoubtedly highly successful (320). It can be suggested that on the basis of Darwin's fitness theory, greater capacity at endurance running improved the survival capacity of specific early humans. It appears that endurance capacity or at least one of the most significant markers of aerobic endurance, maximal oxygen uptake (VO2max), even in the XXI century, is important for survival.
There is a great deal of evidence that indicates that higher VO2max is associated with a decreased risk in the incidence of a number of lifestyle-related diseases, including breast, colon, and prostate cancer, cardiovascular diseases, type II diabetes, and Alzheimer's disease (35, 121, 174, 247, 312). In accordance with this, an extremely low level of oxygen uptake could have serious outcomes such as increased incidences of diseases that lead to early death (399). One could suggest that the level of endurance capacity plays an important role in survival (Fig. 1). This phenomenon nicely demonstrates that our dependence on oxygen and oxygen uptake capacity could have started with the evolution of the aerobic organism, through the development of H. sapiens. The question whether an endurance running-dependent hunting lifestyle played a role in the development of the antioxidant system in humans cannot be answered with full certainty. The fact that the specific activity of superoxide dismutase in different organs is higher in humans than in shorter-lived animal species suggests that man has better protection against reactive oxygen species (ROS) (401) than other mammals. Moreover, it is clear that the rate of ROS production is also attenuated in man compared to other species (217). Interestingly, enough regular physical exercise increases the level of antioxidant defense and decreases the rate of ROS production, which seems to happen as the result of the evolution of H. sapiens.
FIG. 1.
The suggested effects of endurance capacity-mediated evolution on the human body. Early human endurance running was important for successful hunting, and high endurance capacity affected metabolism and redox adaptation. On the other hand, modern lifestyle physical inactivity acts against the build-up of endurance capacity. A low level of maximal oxygen uptake is a risk factor for a number of diseases and increases the rate of mortality. VO2max., maximal oxygen uptake.
It has been suggested that the stone-age human spent about 4000 Kcal on physical activity daily, compared to the 400 Kcal utilized by the human male in the present century (40). The effects of physical exercise, or its converse, physical inactivity, are complex and systemic, and strongly affect redox homeostasis and the resistance to oxidative stress (309) (Fig. 2). Indeed, the increase in oxygen flux with exercise could be as high as 100-fold higher than resting values in the contracting skeletal muscle. This oxygen flux naturally results in an increased generation of ROS (77, 309, 356). In turn, this increased ROS production could result in a well-orchestrated adaptive response, which leads to the stimulation of preventive measures against oxidative stress-related diseases (158, 296). Data from human studies indicate that although endurance exercise showed elevated oxidative power of mitochondria, the efficiency of energy transfer (P/O ratio) did not change significantly (402). Moreover, endurance training, which increased the VO2max of subjects by 21% and the lactate threshold by 53%, did not significantly increase the sensitivity of mitochondrial oxidative function to ROS in skinned fibers (424).
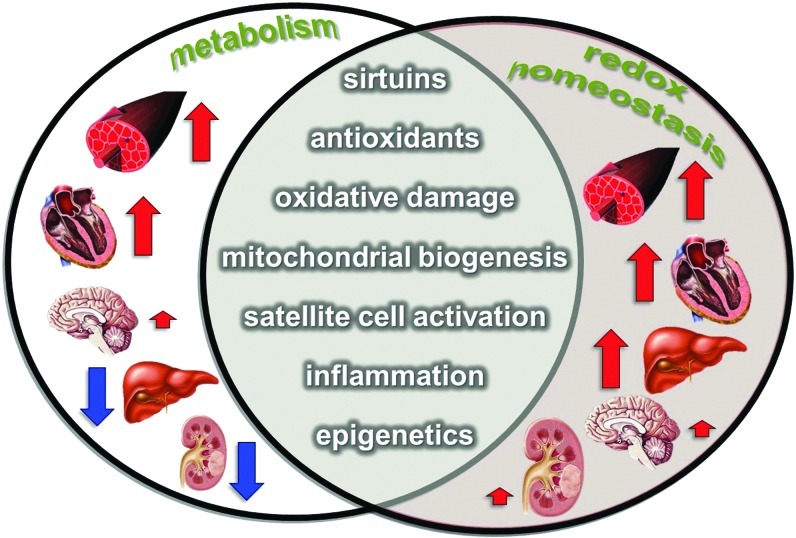
FIG. 2.
There is a strong relationship between the rate of metabolism, ROS formation, and redox homeostasis. In the present review, we are focusing on those regulatory systems that are influenced by both metabolism and exercise. Exercise results in a significant increase in the metabolism of skeletal muscle, and moderate elevation in cerebral metabolism. On the other hand, blood flow and metabolism are significantly decreased in the liver and kidney during exercise. The antioxidant capacity and the resistance against oxidative stress increase in all of these organs. ROS, reactive oxygen species. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Probably, as a result of evolution, humans have evolved to a state where exercise-induced ROS can stimulate elevation of the level of glucose transport to meet the need for increased metabolism, as well as other metabolic adaptations to the high demands of exhaustive exercise (346). Acute bouts of exercise, or exhaustive exercise, can elevate the oxidative damage to lipids, proteins, and DNA and disrupt ROS-modulated signaling, which together could jeopardize cell survival. Therefore, both acute and regular exercise can influence homeostasis, and then adaptation, which involves the enhanced oxygen uptake-related benefits, the risk, and consequences of increased oxygen uptake-dependent modulation of ROS production, and redox homeostasis. The present review aims to focus on the complex effects of physical exercise on oxygen utilization, metabolism, signaling, and redox-dependent adaptations, especially in the skeletal muscle. We will discuss the sources of ROS production, the adaptive response of antioxidant and oxidative damage-repairing systems. Moreover, we will evaluate the hormetic role of oxidative damage, which in a moderate degree could be important to membrane remodeling, protein turnover, specific gene expression, or chromatin opening. Sirtuins are redox-dependent metabolic sensors, and we will highlight the role of these histone deacetylases in exercise-associated adaptation. As well, we will touch upon the enigmatic role of nitric oxide (NO) in repair of skeletal muscle damage and how it could be involved in exercise-induced adaptation. Moreover, it will be demonstrated that ROS are important modulators of satellite cell proliferation in the skeletal muscle, and play a role in exercise-induced neurogenesis as well. Finally, the effects of exercise on hypoxia-inducible factors will be discussed, followed by the role of redox signaling in exercise-related epigenetical modifications.
II. General Adaptations to Exercise
Adaptation is dependent on the modulation of homeostasis. It is clear that homeostasis is a dynamic system with biological and functional/actual endpoints. Biological endpoints are signified by the point at which the system collapses. Exceeding these points is fatal. For example, a 43°C degree fever results in denaturation of proteins and eventually death.
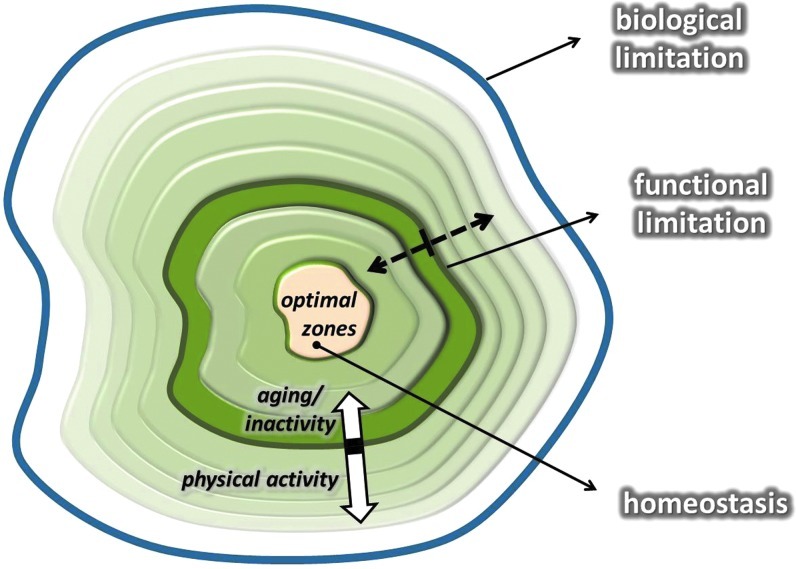
Functional/actual endpoints mean the limit of individual tolerance, which is naturally below the biological endpoint and is a dynamic, variable value. The distance between optimal zone and biological endpoints represents the zone that can be targeted to induce adaptations to extend functional/actual endpoints. In the case of a high degree of adaptation, the distance between the biological endpoints and the functional endpoints can be narrowed. In other words, the distance between optimal zone and functional/actual endpoints can be increased (Fig. 3). It can be assumed that a larger range between optimal zone and functional/actual endpoints represents greater adaptive capability and better tolerance against stressors.
FIG. 3.
This figure shows the hypothetical adaptive range. The middle of the graph represents the optimal zone of the dynamic homeostasis, while the outer line indicates the biological limitations, which cannot be reached without extreme risk of death. The line, called functional limitation, shows the capacity of each individual, and it is a mobile value. The functional/actual limit can be readily altered by exercise training. Aging decreases the rate of adaptive response, and the capacity to maintain homeostasis is decreasing, as demonstrated by the white arrows. Dotted arrows indicate the flexibility of functional limitation. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Two examples will suffice to exemplify this point. The resting heart rate of untrained individuals is around 70 beats/min, while the maximal heart rate is ∼220 minus the age of the individual. Therefore, the adaptive range is 130 beats for a 20-year old and just 50 beats for a centenarian. Well-trained endurance runners, on the other hand, have a significant decrease in resting heart rate, which could be as low as 35 beats per minute. If an individual has the same maximal heart rate as the 20-year old, trained, and untrained, the adaptive range increases from 130 to 165 with the extension of the functional endpoints. Another example is lactic acid tolerance. Both trained and untrained individuals have a resting blood lactic acid level around 1.5 mM/L. If they run until exhaustion on a treadmill, untrained individuals stop when their lactic acid levels reach ∼6–10 mM/L, while elite athletes can still continue until their lactic acid levels are over 20 mM/L.
A similar phenomenon is found for ROS handling. It is known that a single bout of exhaustive exercise results in elevated levels of lipid peroxidation, carbonylation of amino acid residues, and 8-Oxo-7,8 dihydroguanine (8-oxoG) in DNA (318). On the other hand, when a single bout of exhaustive exercise is given to well-trained subjects, the body responds without a large elevation in oxidative damage (318). In addition, regular exercise training-associated adaptation is a precondition against treatment with H2O2, which causes a significant degree of damage for untrained subjects (317). Moreover, when heart attacks or strokes are simulated in untrained and trained animals, the infarct size is significantly smaller in the trained groups (39, 81), showing that regular exercise acts as a preconditioning tool (317) by enhancing the adaptive zone, by narrowing the theoretical distance between functional and biological endpoints. A great deal of evidence exists that suggests that regular exercise-induced adaptations to ROS handling, through redox signaling, including antioxidant and oxidative damage repair systems, significantly contribute to the health-promoting effects of regular exercise. However, this phenomenon can also be interpreted in another way, namely that physical inactivity severely decreases the adaptive capacity, including redox processes, and this increases the incidence of a wide range of diseases, which generally leads to elevated mortality rate and decreases in mean lifespan. This phenomenon could be identified as being in opposition to aerobic endurance-dependent evolution.
III. Exercise, ROS, Antioxidant, and Housekeeping Systems
A. ROS and antioxidants
A great deal of epidemiological data has shown that exercise decreases the incidence of oxidative stress-associated diseases (174, 405). This beneficial effect is due to the fact that exercise-induced ROS production is necessary for oxidative stress-related adaptations. There are a number of ROS generating systems that increase blood flow to skeletal muscle during physical exercise, and more or less maintain blood flow to the brain, and significantly decrease oxygen supply to the liver and kidney (309). The mitochondrial electron transport chain is one main ROS generators found in skeletal muscle (295). As a result of exhaustive exercise, ROS production of complex I and III, with pyruvate/malate and succinate substrates, was increased by 187% and 138%, respectively, compared to the nonexercising group (342). Complex III has been suggested to yield superoxide on the cytoplasmic site of the mitochondrial membrane (47), which could be important for redox signaling. Moreover, mitochondria isolated from skeletal muscle after contraction showed significantly increased levels of H2O2 generation compared to noncontracting values (415). Sahlin et al. have shown that complex I is the major ROS generator in skeletal muscle of ultraendurance runners, as assessed by Amplex red reagents (343). However, it must be noted that the earlier estimation of about 1%–5% of the oxygen that enters mitochondria can be released as ROS (44) could be an overestimation, and the real value could be more than an order of magnitude lower (373). Upon this and related observations, it was suggested that mitochondria are not the main source of ROS production during exercise (298). On the other hand, a recent report suggests that mitochondria might be important sources of ROS, at complex I and III through peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) (19). In this particular study, a positive association was found between ROS production and mitochondrial respiration (19). The effects of the anabolic steroid, stanozolol, were investigated on mitochondrial ROS production, and it was found that complex I- and complex II-linked substrate generated H2O2 after exercise sessions, which was significantly attenuated by stanozolol (342). Therefore, despite the fact that the resting efflux of ROS is a magnitude lower than was suggested, results from the measurements of ROS/H2O2 efflux indicate that mitochondria are generating enhanced levels of ROS during intensive exercise. On the other hand, the release of ROS from each mitochondrial complex could differ significantly.
For example, at complex I, the iron–sulfur clusters, flavoprotein, and oxidoreductase, and at complex III, Q10 semiquinones are suggested to be the main sites of ROS generation (244, 373). Recently, a novel method has been developed, which allows detection of superoxide production in the mitochondrial matrix by the stochastic quantal events of superoxide (mitochondrial superoxide flashes [mSOFs]) (429). It has been shown that during muscle contraction, the activity of mSOF increases in mitochondria and is dependent on the activity of the electron transport chain and, furthermore, has a biphasic nature, showing an increase in brief and a decrease in longer tetanic contractions (429). This finding certainly does not rule out extracellular sources of ROS during contraction, which have been suggested as being important (304, 325, 448). Indeed, 5-lipoxygenase, cyclooxygenase, sarcolemmal NADPH oxidase, and xanthine oxidase (XO) have been implicated in superoxide generation in skeletal muscle (272, 295). NADPH oxidase is one of the major ROS generators during exercise (26, 297), since in the presence of ADP and Fe3+, NADPH oxidase catalyzes electron transfer from NADPH to molecular oxygen to form superoxide (21). XO is especially involved in ROS production during anaerobic exercise, and a linear relationship has been reported between XO and lactic acid levels (304). One of the reasons for this is the fact that XO activity is strongly dependent on the availability of substrate (437). During intense exercise due to the high rate of ATP degradation, AMP generation hypoxanthine and xanthine are formed and serve as substrates for XO, which uses molecular oxygen to generate ROS. Interestingly, we could detect increased XO activity in the liver 1 day after exhaustive acute exercise (305), and the protective effects of administered SOD derivatives showed that endothelium-associated XO is one source of ROS generation during intense exercise (304). Recently, myostatin has emerged as a potential ROS-inducing factor, especially during sarcopenia (372). It has been demonstrated that ablation of the myostatin gene resulted in attenuated loss of muscle mass with aging. Moreover, it turns out that myostation can induce ROS production through tumor necrosis factor-α (TNF-α) and NADPH oxidase (372). The role of exercise on myostation-mediated redox signaling is still unclear, and research is warranted on this topic. Activation of ryanodine receptor 1 (RyR1) in the sarcoplasmic reticulum of skeletal muscle is necessary for Ca2+ release and consequent generation of cross-bridge-related force production. With the aging of skeletal muscle, continuous Ca2+ leak were observed in RyR1 channels, which was associated with decreased tension, exercise capacity, and increased ROS production. Normalization of RyR1 function by pharmacological intervention that stabilized binding of calstabin1 to RyR1 significantly reduced Ca2+ leakage and increased endurance capacity (14).
It has been shown that a single bout of exercise and regular exercise modulate the ROS production in neutrophils differently; namely acute exercise caused apoptosis and reduction of mitochondrial membrane potential, while regular exercise increased the resistance against oxidative challenge (385). Muscle contraction generates heat, which has been shown to enhance ROS production (447). Needless to say, ROS production is an essential physiological process for muscle contraction, and it is estimated that the level of H2O2 can be increased by 100 nM during contractions (155). Indeed, it has been shown that low levels of exogenous H2O2 treatment increase, and the addition of catalase decreases, force production of the diaphragm (324). H2O2 was shown to modulate muscle contraction via Ca2+ channels (15). Administration of N-acetylcysteine (NAC) is often used to appraise the effects of ROS in muscle fatigue (70, 74, 229, 326), and it has been shown to decrease the rate of fatigue at 80% of VO2max, but not at higher intensities (74). NAC has been suggested to act through improved K+ regulation (229). Infusion of NAC appears to interact with some exercise-induced signaling pathways, as shown by attenuation of the exercise-induced activation of JNK. However, phosphorylation of p38 mitogen-activated protein kinase (MAPK), ERK1/2, or p65 subunit of nuclear factor-kappaB (NF-κB) was not affected (283).
Accumulating evidence suggests that ROS are generated during exercise and modulate signaling pathways and the level of muscle contraction, indicating that ROS content and the muscle function–dose relationship can be described by a hormesis curve (310). Low levels of ROS stimulate force production, while high levels do attenuate it.
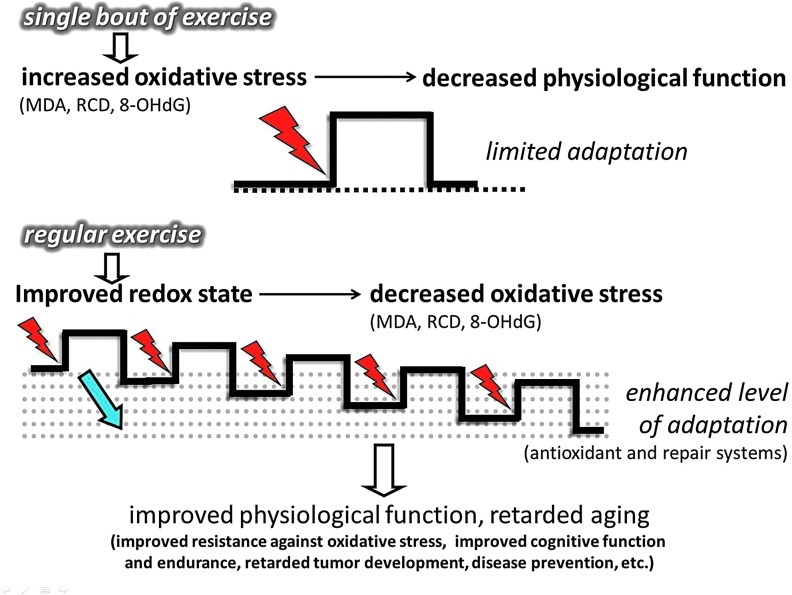
To curb the toxic and deleterious effects of ROS, cells are well equipped with enzymatic and nonenzymatic antioxidant systems. The exercise-induced ROS generation results in increased activity of enzymatic antioxidants, which then results in increased resistance to oxidative challenges, including a wide variety of oxidative stress-related diseases. One of the key issues of this adaptive response is the feature of intermittent exercise. Exercise-induced ROS production, in the recovery period, is mostly overcompensated by the upregulated antioxidant system (111, 112, 160). Chronic exposure to high levels of ROS can exhaust the nonenzymatic antioxidant system and lead to impaired cellular function, macromolecule damage, apoptosis, and necrosis. The intermittent nature of exercise gives a preconditioning role to exercise. After a bout of exercise that gives a metabolic and oxidative challenge, followed by a rest period, the system is allowed to adapt to the challenges caused by this stressor (318). Indeed, during resting periods, the body does not simply store more glycogen in the skeletal muscle, which would allow better performance, but also upregulates antioxidant and oxidative damage repair systems (Fig. 4) (111, 163). Subjects involved in regular exercise, due to an adaptive response, demonstrate higher levels of mitochondrial content, and produce lower levels of ROS at the given intensity than those who are untrained. Therefore, excess physical exercise is detrimental to poorly trained individuals, but progressive training allows the cells to more easily detoxify a larger amount of ROS, partly by the induced activity of the antioxidant systems. Moreover, it has to be mentioned that severe and extreme exercise regimens or intensities may pose serious threat of oxidative damage, even in healthy subjects, and those who are poorly trained and/or not well nourished are in higher risk to suffer oxidative stress.
FIG. 4.
The adaptive response to a single bout of exercise is limited, and oxidative damage often occurs. Moderate levels of oxidative damage could be important to the induction of the oxidative damage repair system. The regular exercise induced adaptation, due to the intermittent feature of exercise and rest periods, allows induction of the antioxidant and damage repair systems, which results in enhanced protection against oxidative stress, attenuates the aging process, and promotes health with increased functional capacities. MDA, malondialdehyde; RCD, reactive carbonyl derivatives; 8-OHdG, 8-hydroxy-2′-deoxyguanosine. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
For example, runners who often suffer from anemia, which could be associated with increased free-iron concentrations in the blood stream, are subjected to enhanced levels of oxidative damage. Moreover, exercise at high altitude also increases the risk of the accumulation of oxidative damage markers (83).
Chronic elevation of ROS is dangerous, as it could easily exhaust the system, while bouts of exposure, like preconditioning ischemia/reperfusion repeats, significantly increase the resistance to oxidative stress (128). Both acute and regular exercise could induce the activity of antioxidant enzymes, including manganese superoxide dismutase (Mn-SOD), copper–zinc superoxide dismutase (Cu,Zn-SOD), extracellular superoxide dismutase (EC-SOD), catalase, and glutathione peroxidase (GPX) (112, 138, 160, 269). The mechanism behind the induction of the antioxidant system complex most probably involves epigenetics, since it was shown that Cu,Zn-SOD knockdown results in decreased methylation of DNA (33). Therefore, it cannot be ruled out that exercise results in changes in DNA methylation and/or histone acetylation, which leads to enhanced activation of antioxidant enzyme genes. The exercise-induced ROS generation is naturally a powerful stimulus to activate the expression of antioxidant enzymes. Mn-SOD could be readily induced by ROS-mediated activation of TNF-α (428) or by NF-kB (440) among others.
B. Thiols and redox signaling in exercise
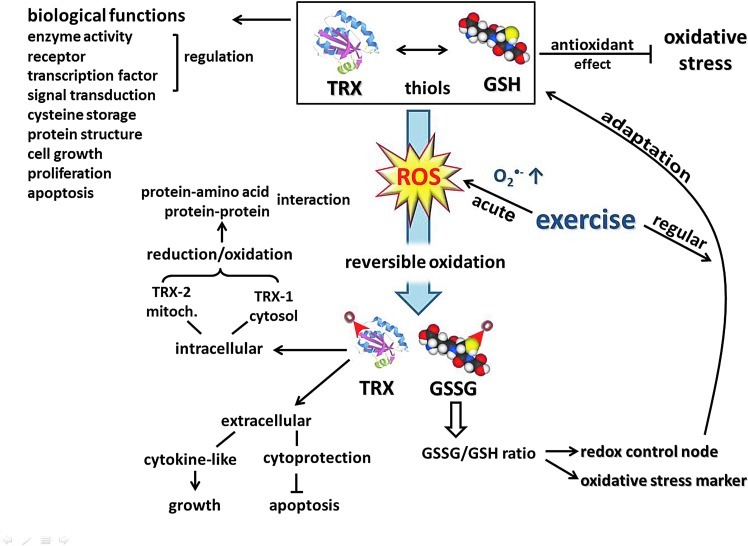
Current evidence indicates that in ROS-mediated signaling, redox-sensitive thiols play a critical role, coupling the intracellular changes in the redox state to regulation of cellular processes (34, 361). Thiols react faster than other amino acids with oxidizing species, and most of their oxidation products can be reversibly reduced. Therefore, reversible redox modification of active-site Cys residues provides a redox switch, an on-off mechanism for control of cellular signaling and function (167). On the other hand, like a rheostat, through allosteric regulation and by S-glutathione-derivatives, thiols can modulate protein function (177). Furthermore, cross-linking of proteins through disulfides yields mechanisms for protein aggregation and trafficking. The endogenous thiols, the glutathione (GSH) and thioredoxin (TRX) systems, play a central role in the antioxidant defenses that control cellular events and regulate protection against oxidative stress (361), a condition that alters normal redox control of cellular signaling, especially by disruption of thiol-redox circuits (166). In addition to their central role in supporting a large network of antioxidant defenses, GSH and TRX have various biological functions such as regulation of enzyme activity, receptors, transcription factors, and ultimately redox-sensitive signal transduction, short-term storage of cysteine, protein structure, cell growth, proliferation, and programmed cell death (358).
GSH (l-gamma-glutamyl-l-cysteinylglycine) is the predominant nonprotein thiol in the cell. GSH plays an essential role in antioxidant protection and provides an appropriate reducing milieu inside the cell. During strenuous physical exercise where oxygen consumption is increased many fold, GSH is oxidized, and as a consequence, the disulfide glutathione (GSSG) accumulates. The balance of oxidized to reduced glutathione (GSSG/GSH) acts as a redox control node to regulate sulfur switches for responses to acute exercise-induced oxidative stress and also to develop adaptations of various defense mechanisms during regular physical training (358).
Oxidation of thiols to disulfides has also been used as a sensitive marker of exercise-induced oxidative stress (203). Since 1985, a growing number of exercise studies have utilized GSSG levels and GSSG/GSH ratios as sensitive markers of oxidative stress, but the results are equivocal (109, 361). Major factors that may affect GSH oxidation response to acute exercise are the type and intensity of the exercise. For example, GSH oxidation is more prominent during exercise performed at higher intensities (362). Another factor may be the training status of the organism. In the trained individual, enhanced antioxidant defenses couple successfully with pro-oxidants to protect the organism against increased oxidative stress. As a result, GSH oxidation, which is a sensitive marker of oxidative stress, occurs less after exercise in trained individuals. Recently, we observed that in the regularly trained horse, with exercise or during recovery, there were no significant changes in the muscle GSSG- or the GSH-redox ratio, although postexercise free-radical amounts correlated positively with postexercise GSSG concentration (184). This can be explained by the well-known fact that regular exercise training of all types enhances GSH levels by inducing GSH synthesis and also by increasing GSH regeneration from GSSG (361).
Data on the role of endogenous GSH on exercise performance, especially endurance capacity, are limited. In one early study, endurance capacity for treadmill running in GSH-deficient rats was found to be reduced by half (360). Although there is a controversial report in the literature regarding the impact of GSH deficiency on prolonged swimming performance in mice (201), one may still postulate that endogenous GSH not only plays a critical role in circumventing exercise-induced oxidative stress but also determines exercise performance.
The TRX system, comprising NADPH, thioredoxin reductase (TrxR), and TRX itself, is critical for cellular redox balance and antioxidant function. TRX is a small multifunctional protein with a dithiol/disulfide active center and is the major cellular protein disulfide reductase. Located extracellularly, TRX shows a cytoprotective activity against oxidative stress-induced apoptosis. TRX has also a cytokine-like function acting as an autocrine growth factor (410). Intracellularly, TRX-1 in the cytosol and TRX-2 in the mitochondria are involved in the regulation of protein–protein or protein–nucleic acid interactions through the reduction/oxidation of protein cysteine residues. In response to a variety of cellular stresses, including oxidative stress, TRX translocates from the cytosol into the nucleus to regulate the expression of various genes. A growing number of transcription factors require TRX reduction for DNA binding (209). TRX and GSH are considered to be functionally and kinetically distinct systems. In both cells and plasma, GSH/GSSG redox is not equilibrated with other major thiol/disulfide couples (TRX and Cys/CySS), providing the basis to consider discrete redox circuits for signaling and control (167). Nevertheless, loss of cellular TRX-1 is known to result in elevated GSH levels (56). Furthermore, after an acute bout of exercise, during recovery, TRX-1 activity correlated negatively with the GSSG/TGSH ratio of oxidized GSH in skeletal muscle of horses (184). These results underline a cross-talk between two major thiol antioxidants in response to exercise-induced oxidative stress (Fig. 5).
FIG. 5.
Thiols are important antioxidants, and the GSH/GSSG ratio modulates vital cellular processes, including antioxidant systems, apoptosis, cellular growth, and signal transduction among others. Thiols are involved in the extracellular and intracellular oxidative defense, including the mitochondrial and nuclear compartments (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.) GSH, glutathione; GSSG, disulfide glutathione; TRX, thioredoxin.
In a setting of thiol antioxidant (alpha-lipoic acid [LA]) supplementation, we observed a negative correlation between the activity of TrxR at rest and postexercise levels of free radicals after LA supplementation (184). Acute exhaustive exercise induced TRX-1 mRNA levels (192), and 8 weeks of endurance training increased TRX-1 protein levels in the rat brain (193). In line with these results, a limited number of studies from other groups also provide evidence that TRX contributes to antioxidant defense against oxidative stress induced by acute exercise of variable intensity and duration. In mouse peripheral blood mononuclear cells, 30 min of swimming exercise induced TRX protein expression 12 and 24 h after exercise (381). Similarly, plasma TRX concentrations increased continuously during an ultramarathon race (228).
Thioredoxin-interacting protein (TXNip, also termed VDUP1 for Vitamin D-upregulated protein or TBP2 for thioredoxin-binding protein) has been identified as an endogenous inhibitor of TRX in a redox-dependent fashion (261). Redox-dependent interaction of TRX with TXNip only exists between the oxidized form of TXNip and the reduced form of TRX (277) and may modulate apoptosis signal kinase (ASK-1), redox factor 1, Forkhead box class O4 (FoxO4), and nod-like receptor proteins, which offers a novel mechanism of redox regulation (442). What is, to our knowledge, the only study in the literature to investigate the role of exercise on TXNip levels found that 8 weeks of exercise training did not influence TXNip levels in the rat brain despite an increase in TRX-1 protein. Interestingly, in the same set of experiments, streptozotocin-induced diabetes increased both TXNip levels and oxidative stress, measured as increased GSSG/TGSH ratio (193), suggesting a link between disturbed redox control and disease.
C. Oxidative damage and housekeeping
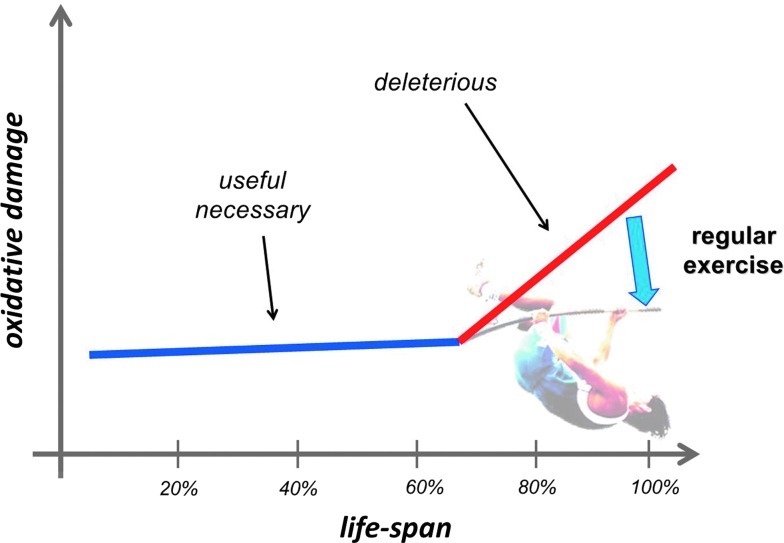
The term oxidative damage is often referred to as a condition where there is a large increase in the stable product of the interaction of ROS with lipids, proteins, and DNA. The deleterious effects of the oxidative damage on cell function are well known and have been reviewed elsewhere (6, 84, 215, 357, 400, 423). The extent of oxidative damage caused by an acute bout of physical exercise, even if it is exhausting, does not cause viability risking increase in oxidative damage, and it can be argued that this might be a necessary factor of adaptation. The fact that the most often measured macromolecule damage markers of oxidative stress, such as lipid peroxidation-derived aldehydes, protein oxidation byproducts, carbonyl groups, or 8-oxoG, are always detectable even with high antioxidant levels raises the possibility that these markers could play a significant physiological role, which, to date, has not been reported. Aging increases the level of oxidative damage, but the elevation could be dangerous after the last quarter of the life span, which can be attenuated by regular exercise (310). On the other hand, the extent of damage is easily measurable at a young age (Fig. 6).
FIG. 6.
The free radical-dependent aging theory is well accepted, and it is clearly demonstrated that the extent of oxidative damage increases in the last quarter of the life span. However, it is also clear that the oxidative modification of lipids, proteins, and DNA is well detectable even at a young age, and this could indicate that a moderate level of oxidative damage is not dangerous to cells; moreover, it even can be necessary. On the other hand, significant elevation of oxidative damage at an advanced age is associated with increased incidence of a wide range of diseases and impaired physiological function. Regular exercise has been shown to attenuate the age-associated increase in oxidative damage, and it also attenuates the deleterious effects of aging on organ function (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
It is clear that ROS are crucial players in aging, although a number of other factors are also involved. In neurons, cardiomyocytes, skeletal myocytes, sensory cells, and other postmitotic cells, the role of ROS could be more pronounced in aging, due to the ROS-mediated damage and accumulation of damage and mutations that are believed to be important in loss of function in these cells. The substitution of aged molecules for mutated ones with loss-of-function molecules leads to a progressive deterioration of such tissues. The increase in oxidative damage in the last quarter of the lifespan is probably the combined effects of the increased level of ROS production (75, 155) and the failure of the oxidative damage repair systems (117, 189).
Lipid peroxidation appears to be an unavoidable process of aerobic life, and despite the well-developed antioxidant systems, certain products of lipid peroxidation are always detectable. Moreover, it has been shown that lipid peroxidation can be generated enzymatically by lipoxygenase. The physiological role of controlled lipid peroxidation is enigmatic, but it could be important in the physiological remodeling of cellular membranes (256). Moreover, targeted, controlled lipid peroxidation by lipoxygenase has been shown to be important for the degradation of mitochondrial membranes during the maturation of erythrocytes (322). Lipoxygenation or lipid peroxidation could modify whole membrane structures, the chemical composition, and the physicochemical properties of phospholipids, resulting in altered permeability, surface charge, and passive electric properties (191).
The phospholipase A2 (PLA2) enzyme family, which consists of more than 20 enzymes, hydrolyzes the sn-2 ester bond of glycerophospholipids and generates free fatty acids and lysophospholipids with physiological activity (349). Ca2+-independent phospholipase A2 (iPLA2-beta) appears to be one enzyme that could repair oxidized cardiolipin, a component of the mitochondrial membrane (185). However, most of the enzymes in the family of PLA2 are generators of oxidative stress and lipid peroxidation during prostaglandin synthesis from arachidonic acid, products of PLA2 reaction, rather than repair of lipid peroxidation (5). The levels of lipid peroxidation in various organs, including skeletal muscle and the brain (290, 384, 398), increase with aging. However, not all the lipid peroxidation products present to the same degree or at the same time. Lipid antioxidants, especially lipid-soluble vitamins and GSH, glutathione-S-transferases, and b-alanyl-l-histidine, which can quench lipid peroxidation-derived aldehydes, including 4-hydroxy-2-nonenal (HNE), are naturally occurring phenomena. Albumin and apolipoproteins and maybe some other proteins with high intracellular concentrations can bind and buffer HNE, resulting in detoxification of the cellular milieu. The platelet-activating factor acetylhydrolase family represents a unique group of PLA2 that exhibits high substrate specificity toward oxidized phospholipids, and has been suggested to act as a Ca2+-independent PLA2 in the repair of lipid peroxidation (256). This enzyme has been shown to be activated in blood samples of well-trained triathletes, to curb the damage caused by lipoproteins (50). Diet and exercise intervention have also been shown to exhibit increased activity of platelet-activating factor acetylhydrolase (332).
A large number of studies have demonstrated increased levels of lipid peroxidation products, including malondialdehyde and F2-isoprostane, after acute exercise bouts (159, 257, 318, 359). However, regular exercise training, in general, results in decreased levels of lipid peroxidation (159, 255, 259, 359). The regular exercise-associated attenuation of lipid peroxidation levels could be due to the increased capacity of the antioxidant systems, but the enhanced activity of lipid repair cannot be ruled out.
All amino acid residues can be modified when attacked by ROS, generating oxidation of amino acid side chains. The level of oxidative damage to proteins is a magnitude higher than that observed in lipids and DNA (319). Oxidized proteins readily generate cross-linkages and aggregate in cells. The accumulation of this oxidative junk can jeopardize the cellular function and fate. However, oxidative damage to proteins renders them susceptible to degradation by increasing the hydrophobicity of the surface of proteins (376), which could serve as a tag for the proteasome system, and lead to degradation (375). This process does not consume energy and is done without ubiquitination (366). It has been shown that proteolytic susceptibility of proteins in relation to oxidative modifications shows a biphasic response, while at moderate oxidant concentrations, proteolytic susceptibility increases, and further oxidation leads to a decline in the proteolytic susceptibility, sometimes even below the basal degradation. Such behavior seems to be a common feature of all globular, soluble proteins with defined secondary and tertiary structures, independent of the origin of the proteins (49, 118, 170, 411).
The feature that carbonylated proteins are targeted for removal by proteasomes suggests that carbonylation may act as a signal ensuring that damaged proteins enter the degradation pathway rather than the chaperone/repair pathway, since carbonylation is an irreversible/unrepairable modification (264). Increased levels of protein carbonyls are linked to a variety of age-associated diseases and often correlate with the progress of disorders and diseases (375). Carbonyl derivatives are formed by a direct metal-catalyzed oxidative attack on the amino acid side chains of arginine, lysine, proline, and threonine. Moreover, carbonyl derivatives on cysteine, histidine, and lysine can be also generated by secondary reactions with reactive carbonyl compounds on sugars, lipids, and advanced glycation/lipoxidation end products (375). Methionine and cysteine residues are even more prone to oxidation than others due to the sulfur content of these residues. The oxidative modifications of these two residues are reversible, indicating a redox-dependent signaling of methionine and cysteine residues. Cysteine residues are converted to disulfide by oxidation and reduced back to cysteine under mild conditions such as in the presence of reducing agents such as NADH without enzyme reaction. Methionine residues are transformed to methionine sulfoxide, which in turn can be repaired by methionine sulfoxidereductases, which catalyze the reduction of methionine sulfoxide back to methionine residues (181). The role of methionine reductase in exercise studies remains to be evaluated. On the other hand, carbonylation, which is an irreversible modification, is very well studied in exercise studies. An acute bout of intensive exercise increases the rate and accumulation of carbonyl derivatives (36, 258, 316), and regular exercise either maintains or decreases carbonyl levels (45, 68, 114, 318). Increased activity of the proteasome system is important for protein remodeling and, of course, to prevent the accumulation of damaged proteins that could impair cell function. The effects of exercise on the proteasome system and carbonyl accumulation could be dependent on whether the exercise is acute or chronic and also the type of exercise (resistance or endurance) (230, 246, 293, 369). Mitochondria, the main site of ROS production, need a very efficient system to prevent the accumulation of oxidized proteins. The main quality control of mitochondrial proteins is mediated by Lon protease and HSP78 (42, 43, 253, 337, 411). Indeed, it has been shown that Lon expression can be readily induced by a moderate level of oxidative stress, which prevents the accumulation of oxidized proteins and protects cell viability (254). Ischemia, for instance, significantly induced the expression of Lon protease, indicating that endoplasmic reticulum-related stress could have an impact on mitochondrial biogenesis and the degradation of mitochondrial proteins (146). Moreover, it has been shown that silencing of Lon protease resulted in hepatic insulin resistance, and the restoration of Lon activity normalized insulin handling in the liver (199). Thus, Lon can be regarded as a stress protein (253).
Lon levels decline with age, which could result in the accumulation of oxidized, or dysfunctional proteins in the mitochondria (42, 43, 196), and perhaps in the loss of mitochondrial function. We have recently observed that regular exercise can prevent the age-related decline in Lon protease (187), and therefore rejuvenate proper quality control of proteins in older cells (411). We have also shown that exercise training induced HSP78 levels in both young and old animals (187), which indicates that regular exercise has beneficial effects on mitochondrial protein quality control.
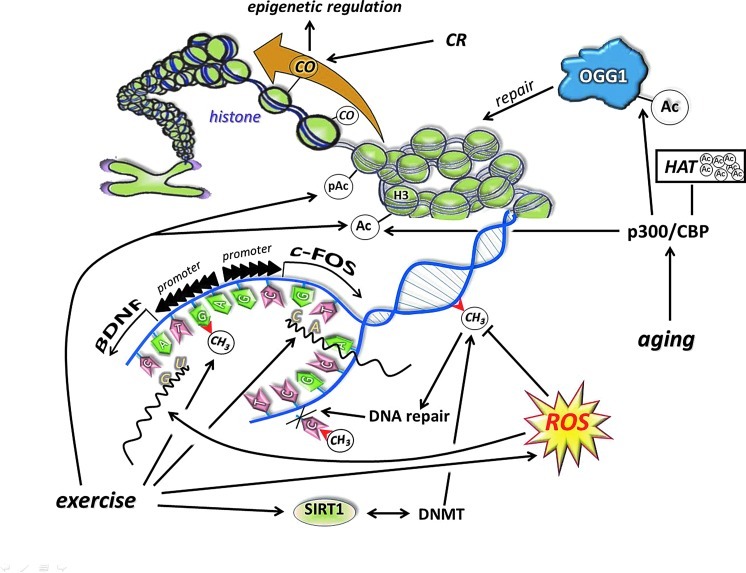
8-oxoG is the most often measured form of oxidative damage to DNA and has been reported to increase with aging, ischemia/reperfusion, radiation, and a wide range of pathological disorders (37, 248, 260, 378). Human 8-oxoguanine-DNA glycosylase (OGG1) plays a major role in the base excision repair pathway by removing 8-oxoguanine base lesions generated by ROS. OGG1 activities are primarily regulated by p300/CBP-mediated acetylation, predominantly on Lys338/Lys341 (32, 148, 388). An increased deacetylase activity of sirtuins may lead to decreases in acetylation levels of proteins, which, in turn, would result in a decline in enzymatic activity of OGG1. We have recently shown that aging increased 8-oxoG content in the hippocampus, with increased levels of OGG1. However, the acetylation of OGG1 was very low in the aged animals (189). Physical activity, on the other hand, increases the acetylation of OGG1 and decreases the age-associated accumulation of OGG1 in human skeletal muscle (319). Indeed, we and others have shown that acute and regular exercise increases the activity of OGG1 (249, 303, 306, 313, 314, 348). Therefore, the upregulated DNA repair could be an important tool by which regular exercise maintains DNA purity and could curb the incidence of certain cancers (333).
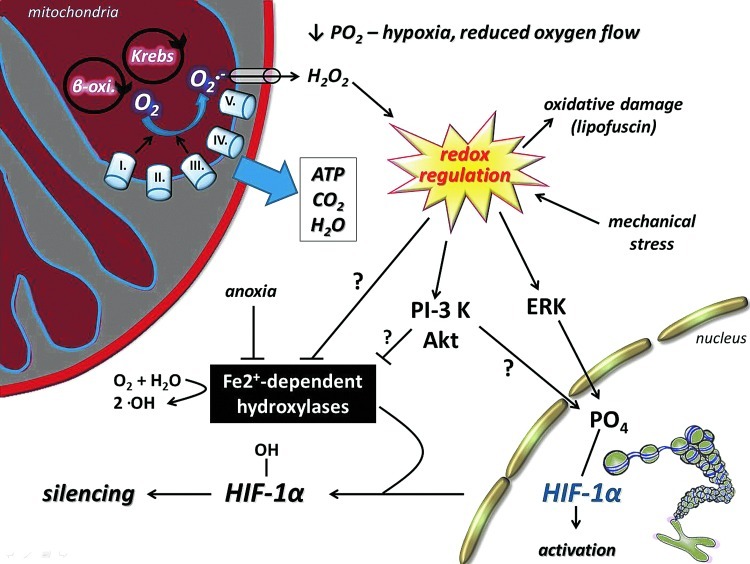
It is highly possible that evolution of the biological system has selected guanine as the potential target of ROS. As a result of low redox potential, attractiveness to ROS is apparent and makes it the most oxidizable nucleic acid base, and this finding suggests that the generation of 8-oxoG could have a significant physiological role (307, 377), as other markers of oxidative damage, such as malondialdyde or protein carbonyls 8-oxoG, are always detectable in cells. An interesting observation made on OGG1 knockout animals is that lipopolysaccharide-induced inflammation was more tolerated than in the wild types (221). The lack of OGG1, hence, resulted in increased levels of 8-oxoG, and reduced removal of this damaged base, which actually provided better protection against inflammation. Thus, the inverse correlation between the activity of OGG1 in this inflammation model suggests that certain amounts of 8-oxoG might be more tolerable to the cells than the excised form that generates inflammation (221). It was recently suggested that oxidation of guanine could be important in the transformation of heterochromatin to euchromatin (307). In addition, it has been demonstrated that exposure of cells to estrogen increases 8-oxoG levels in DNA and recruits OGG1 and topoisomerase IIβ to estrogen-responsive DNA elements in the promoter region of 17β-estradiol (E2)-responsive genes (282). Therefore, 8-oxoG, besides its damaging affect on transversion base pairs, could be important for the generation of signals that are necessary for specific gene transcription. Indeed, it has been shown that the 8-oxoG-OGG1 complex can interact with Ras proteins and work as a guanine nuclear exchange factor (38). In addition, it could be very important to redox signaling that the 8-oxoG-OGG1 complex-mediated Ras activation results in phosphorylation of the mitogen-activated kinases MEK1,2/ERK1,2 and increasing downstream gene expression. This novel information on the interaction of free 8-oxoG, but not other oxidized bases such as 8-oxodG, FapyG, or 8-oxoA, with OGG1 leads to redox signaling and could partly explain why OGG1 knockout mice are more resistant to inflammation than wild types (221).
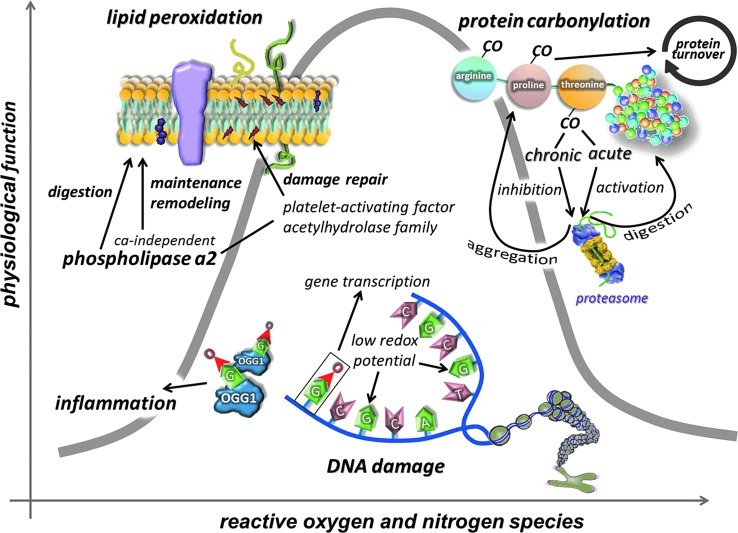
Data from exercise studies suggest that a moderate level of oxidative stress, which could slightly increase the levels of certain oxidative damage markers, might not actually jeopardize cell function, but rather could be necessary to initiate an adaptive response (Fig. 7). Physical exercise is a natural stimulator of ROS production and a mild oxidative stressor, as demonstrated by a moderate level of oxidative damage, which can lead to an enhanced physiological function.
FIG. 7.
Lipid peroxidation byproducts, carbonyl groups, and the 8-oxoG levels are easily detectable, suggesting that these ROS-induced modifications could be necessary for cells. Lipid peroxidation can be induced by enzymatic processes and could be important to membrane remodeling. Carbonylation of amino acid residues could be an important mediator of protein turnover, since carbonylation can serve as a tag for proteolytic degradation. 8-oxoG is necessary for transcription of specific genes and for the opening of chromatin (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.) 8-oxoG, 8-Oxo-7,8 dihydroguanine; OGG1, 8-oxoguanine-DNA glycosylase.
IV. ROS, Metabolism, and Exercise
A. Sirtuins as redox-sensitive energy sensors of exercise
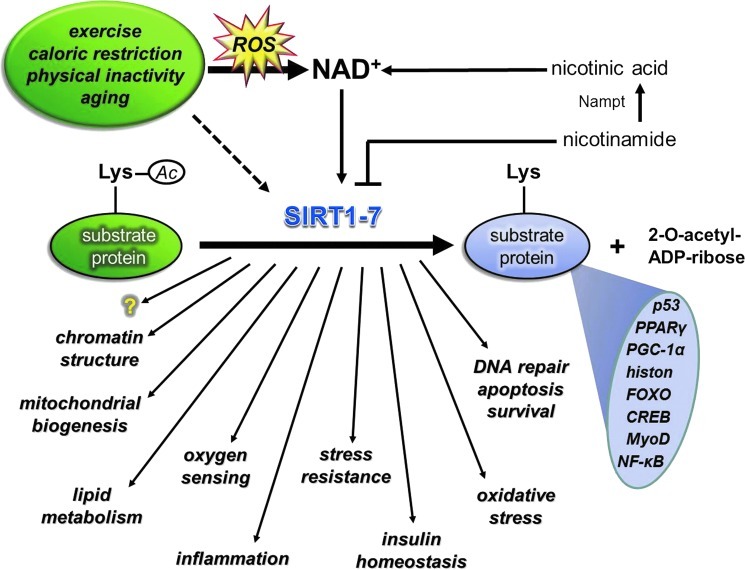
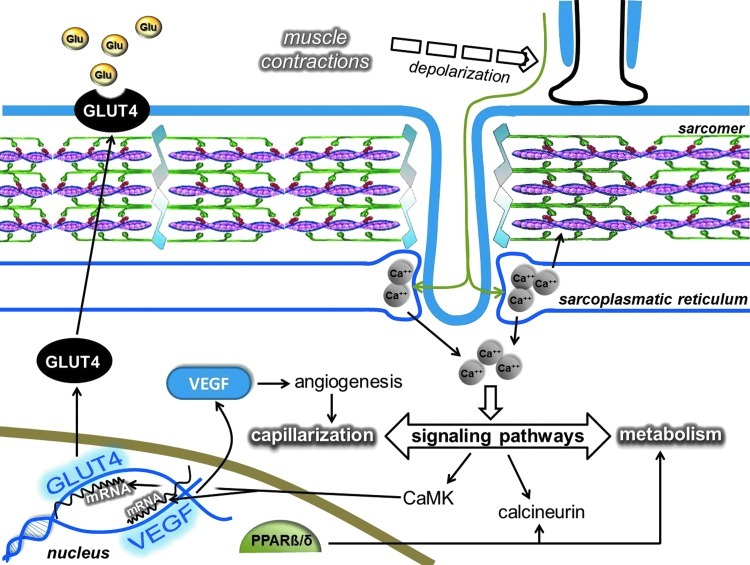
Nicotinamide adenine dinucleotide (NAD), with the ability to carry two electron equivalents, serves as an energy source, and at the same time, the cytoplasmic NAD/NADH ratio is a sensitive marker of the cellular redox state (235, 432, 441). NAD-dependent lysine deacetylases, sirtuins, control various cellular processes, including chromatin structure by the deacetylation of lysine residues of histones (271, 396), apoptosis and cell survival, by the interaction with p53 and FOXO transcription factors (233, 354, 444), mitochondrial biogenesis via deacetylation of PGC1a (252), lipid metabolism by the interaction with SREBP-1c among other proteins (291, 352, 390), insulin homeostasis (98, 205), DNA repair (171, 224, 231), inflammation by modulating the activity of NF-κB (344, 439), and oxygen sensing by deacetylating hypoxia-inducible factor-1a (HIF-1α) and HIF2α (66, 82, 210) among other mechanisms. Sirtuin-mediated deacetylation of these transcription factors would readily effect the expression of pro- and antioxidant genes, such as PUMA, NOXA, PIG3, GADD45, Nrf2, and Mn-SOD, and stress-activated protein kinases etc., which can readily regulate redox signaling. Besides the interaction between SIRT1 and poly(ADP-ribose) polymerase (PARP) to control metabolism (22), the latter is implicated in apoptosis (88), DNA repair (134), ischemia/reperfusion (403), diabetes (387), and inflammation (386).
Sirtuins (silent information regulator 2 [Sir2] proteins) belong to an ancient family of evolutionary-conserved NAD+-dependent enzymes with deacetylase and/or mono-ADP-ribosyltransferase activity. Seven Sir2 homologs, sirtuins (SIRT) 1 to 7, have been identified in mammals. The crystal structure of human SIRT1, which is a homolog of yeast Sir2, revealed a large groove that was intersected by a pocket lined with hydrophobic residues conserved with class-specific protein-binding sites of each Sir2 class (92). Enzymes from the sirtuin family are ubiquitously distributed. However, their level/activity has organ specificity. SIRT 3–5 are predominantly localized in the mitochondria.
The NAD/NADH ratio is changing readily during muscle contraction, due to the reduction of NAD to NADH that is catalyzed by glyceraldehyde 3-phosphate dehydrogenase and the conversion of NADH to NAD by the glycerol phosphate shuttle and lactate dehydrogenase (LDH). LDH facilitates the pyruvate uptake of mitochondria (52) that further influences the NAD/NADH ratio, and one can expect decreased SIRT1 activity right after intensive exercise bouts, due to the increased lactate/pyruvate ratio, which, in turn, decreases the NAD/NADH ratio. However, exercise bouts increase the activity of SIRT1 (188, 308, 383), indicating that within a certain range, SIRT1 activity is independent from the NAD/NADH ratio (9). On the other hand, significant oxidative stress could down-regulate SIRT1 (7).
Sirtuins, especially SIRT1, are implicated in the aging process of different organisms (119, 391), and it has been shown that changes in SIRT1 activity by agents, such as caloric restriction (CR) or resveratrol or SRT501, increase lifespan via a wide range of processes, including suppressed apoptosis, and inflammation, or enhanced DNA repair (2, 292). Although, the beneficial effects of sirtuin activators on mammals are still unclear, some data indicate that sirtuins do play both protective and proaging roles (206). Accumulating evidence suggests that exercise alters the activity, content, and expression of different sirtuins (Fig. 8). Indeed, there are reports that have demonstrated an exercise-associated oxidative challenge that results in increased expression, content, and activity of SIRT1 in humans (73, 225, 308). It seems that besides AMPK, SIRT1 is a sensitive metabolic sensor in skeletal muscle (96), and the fact that histone lysine residues are one of the targets of SIRT1 implies that the redox-sensitive energy sensor SIRT1 links energy metabolism to transcriptional regulation. In addition to this, SIRT1 and p300/CBP have been shown to control myogenic transcription factor, which is associated with dynamic changes to the NAD/NADH ratio (99), and points out the role of redox signaling via SIRT1 in the development of skeletal muscle. SIRT1 is downstream in ROS signaling, but can be importantly upstream in regulating cellular levels such as activation of FOXO3 (164), muscle ring-finger protein 1 (MuRF1) (8), and protein kinase B (Akt) (382). Therefore, these NAD-dependent metabolic sensors are regulating redox signaling in a wide array (222).
FIG. 8.
Sirtuins are NAD+-dependent histone deacetylases and sensitive markers of metabolic and redox processes. SIRT-mediated deacetylation of target proteins is involved in metabolic processes, biogenesis of mitochondria, oxygen sensing, inflammation, apoptosis, and epigenetics, among others. Factors that are modifying redox balance, such as aging, caloric restriction, or physical exercise, readily alter the activity of sirtuins. The exercise-induced adaptive response, which includes metabolic and redox processes, involves the sirtuin protein family. NAD, nicotinamide adenine dinucleotide. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
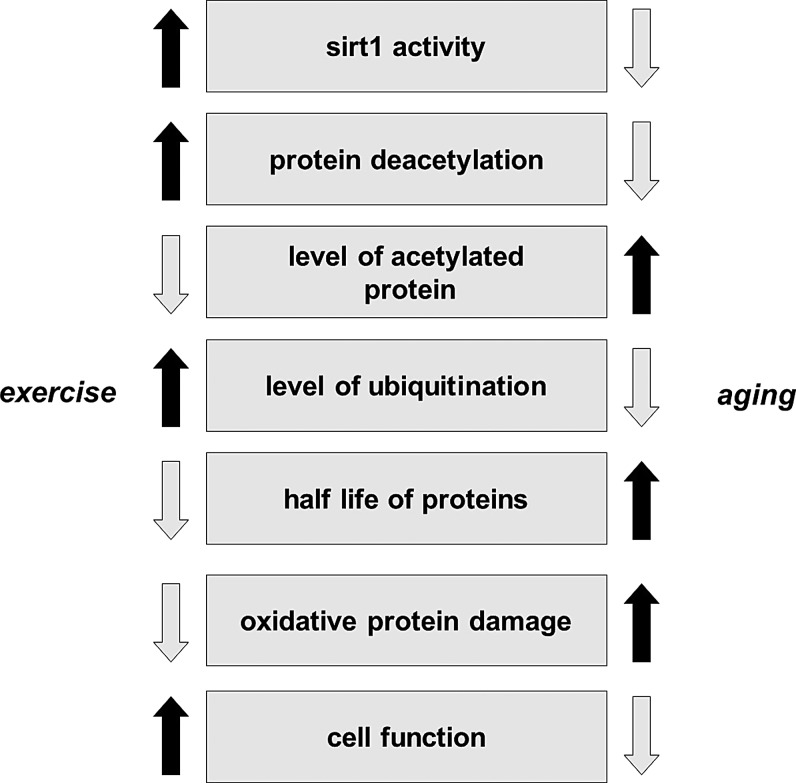
Aging decreases the activity of SIRT1, which results in increased levels of lysine acetylation in the skeletal muscle and brain of rats (188, 189, 227). The effects of acetylation/deacetylation for the function of proteins have been extensively investigated in a number of laboratories. However, the mechanism is still not well understood. It has been shown that acetylation of lysine residues could affect ubiquitination of the same residue, and therefore could impact the stability of proteins. Mechanistically, larger degrees of acetylation, as we have observed with aging, could slow down ubiquitin-mediated degradation resulting in enhanced half-life and then larger accumulation of carbonylation, which could lead to an impaired physiological function. This hypothesis seems to be very attractive and concurs with some well-observed phenomena, such as age-associated increases in the half-life of proteins (116), decreased rates of protein degradation with aging (115), and impaired function as a result of aging. Exercise, depending on the intensity and duration, on the other hand, increases the activity of SIRT1, decreases the level of lysine acetylation, increases the turnover rate of proteins, decreases the accumulation of carbonyl groups, and improves cellular function (Fig. 9). It is important to note that the effects of a single bout of exercise and/or regular exercise could be completely opposite to oxidative stress, ubiquitination, and acetylation. The findings from a recent report indicate that aging does not significantly alter the expression or protein content of MuRF1, while patients with chronic heart failure showed increased expression and content of MuRF1, which was downregulated by 4wk of exercise training (107). Although this study did not examine the levels of sirtuins, it is clear that exercise training on healthy subjects and on patients with diseases would have different effects on the rate of ubiquitination and degradation. The stabilization of HIF-1α, in cardiac muscle during diseases, in the short term could be beneficial, but detrimental in the long term (144). Acetylation on protein stability varies significantly, and HIF-1α, for example, is ubiquitinated at normoxic conditions and degraded by proteasome (200), and acetylation of HIF-1α improves the interaction between ubiquitin ligase, and hence prepare protein for degradation. On the other hand, during hypoxia, SIRT-dependent deacetylation of HIF1α stabilizes the protein (200, 210).The fact that caloric restriction and physical exercise increase the activity of SIRT1 and attenuate the age-associated increase in lysine acetylation is intriguing, and needs further investigation. It is clear that the effects of lysine acetylation/deacetylation, in relation to SIRT1 and protein stability, result in a number of interesting findings.
FIG. 9.
The suggested mechanisms between acetylation, protein stability, aging, and cellular function. Lysine acetylation could prevent the ubiquitination of the same lysine residue, and hence effect protein stability and half-life. Longer half-life results in significantly increased carbonylation of protein residues, which results in impaired cellular function.
SIRT2 is mostly localized in neurons and nerves, and preferentially deacetylates tubulin and histone H4. Moreover, a recent finding suggests that SIRT2 is activating myelin formation in Schwann cells, thus controlling an essential polarity pathway during myelin assembly (25). The effects of exercise on SIRT2 are generally unknown.
Another isoform of the sirtuin family, SIRT6, is closely associated with chromatin and increases the resistance of DNA to oxidative DNA damage and facilitates base-excision repair (239). However, recent data suggest that SIRT6, besides its crucial role in chromatin structure, is a regulator of carbohydrate metabolism. SIRT6-deficient cells show nutrient-replete conditions and shift to lactic acid glycolysis, and transfer metabolism to the survival mode from normal aerobic conditions in the presence of oxygen (445). SIRT6 deficiency activates the gene of pyruvate dehydrogenase kinase and inhibits pyruvate dehydrogenase, resulting in impairment of pyruvate conversion to acetyl-CoA to fuel the TCA cycle (445). In accordance with this, we have observed decreased levels of SIRT6, after a single bout of exercise, in the skeletal muscle of young and old individuals (308). The expression of SIRT6 was not affected by aging in human skeletal muscle, but rat skeletal muscle increased the levels of SIRT6 protein, while regular exercise training attenuated this increase, suggesting that the age-associated changes in metabolism and/or in chromatin structure are normalized by an exercise-driven adaptation (188). SIRT6 appears to be differently regulated by exercise than SIRT1 (188), which could be due to the chromatin tightness, controlling the role of SIRT6. A single bout of exercise results in large metabolic and oxidative challenges to skeletal muscle, which, to regulate an adaptive response, must open chromatin to allow gene expression to cope with the exercise stressor (308). The downregulation of SIRT6 is in accordance with this hypothesis.
SIRT7 is the only mammalian sirtuin that is mainly localized in the nucleoli. When the nucleoli disintegrate, SIRT7 associates with condensed chromosomes (232). The molecular targets of SIRT7 have not been identified, but data obtained from SIRT7 knockout mice reveal that SIRT7 deficiency leads to progressive heart hypertrophy, accompanied by inflammation and decreased stress resistance (412). At least a part of this phenotype may be explained by the fact that SIRT7 deacetylates p53, leading to hyperacetylation of p53 in vivo along with various other changes in the signaling network of cardiomyocytes (412). The effect of exercise on SIRT7 remains to be explored.
Mitochondria, the powerhouse of ATP production, also host sirtuins. One is SIRT3, which could be present in the nuclei and mitochondria (351), and it has been suggested to control metabolism and oxidative stress of mitochondria (29). Overexpression of this enzyme positively regulates the oxidative capacity of mitochondria and attenuates the generation of ROS (190), indicating that SIRT3 plays a key function in redox regulation.
Moreover, it appears that the activation of Mn-SOD is dependent on SIRT3- (302) mediated deacetylation of 122 lysine residues (393). In addition, SIRT3 has a powerful deacetylase activity on histone H4 peptides and is a major regulator of mitochondrial deacetylation (216), affecting fatty acid oxidation (137) via the deacetylation of mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 (364). SIRT3 activates isocitrate dehydrogenase 2 and glutamate dehydrogenase by deacetylation, and these enzymes are involved in the production of mitochondrial NADPH. The elevated NADPH, in turn, is necessary for GSH reductase, which reduces GSSG to GSH, the cofactor necessary for mitochondrial GPX function (29).
We have found that aging increases SIRT3 levels in the rat hippocampus, and is attenuated by exercise training (188). On the other hand, exercise training and diet have been shown to increase the levels of SIRT3 (141, 274) in skeletal muscle and AMP-activated protein kinase (AMPK) as well as cAMP-response element-binding protein (CREB), indicating upstream modulators of SIRT3 (274). These data suggest that SIRT3 is a potent redox-dependent modulator of cellular metabolism and is readily modified by physical exercise.
SIRT4 is also localized in the mitochondria and has been implicated in the regulation of insulin secretion in beta-cells. Knockdown of SIRT4 increased fat oxidation in the liver and skeletal muscle (251), indicating that SIRT4, without significant deacetylase, but with high ADP-ribosyltransferase activity, also modulates cellular metabolism. The effects of exercise on SIRT4 are poorly investigated, but it appears that a single bout of exercise significantly decreases the mRNA expression of this enzymes in peripheral blood mononuclear cells (225).
SIRT5 is hosted by the mitochondria, and recent reports of this poorly studied sirtuin have revealed that SIRT5 is involved in the detoxification of ammonia by deacetylating carbamoyl phosphate synthetase 1, which is one of the key enzymes in the urea cycle, which catalyzes condensation of ammonia with bicarbonate to form carbamoyl phosphate (267). Ammonia is readily elevated during intensive exercise through deamination of AMP and branched-chain amino acids. Therefore, it is very likely that SIRT5 is modulated by exercise, and the adaptive response to regular exercise training involves SIRT5. Moreover, a recent report suggests that SIRT5, besides its deacetylase activity, also shows protein lysine desuccinylase demalonylase activity in the in vitro condition, implying that this post-translational modification might have a physiological role, controlled by redox signaling (86).
The available information strongly suggests that NAD-dependent sirtuins are very sensitive to metabolic challenges, and are modulated by redox signaling. Therefore, sirtuins are readily modified by physical exercise, and data suggest so far that sirtuins could play an important role in exercise-induced adaptations, including physical fitness, health promotion, and the attenuation of the progress of aging.
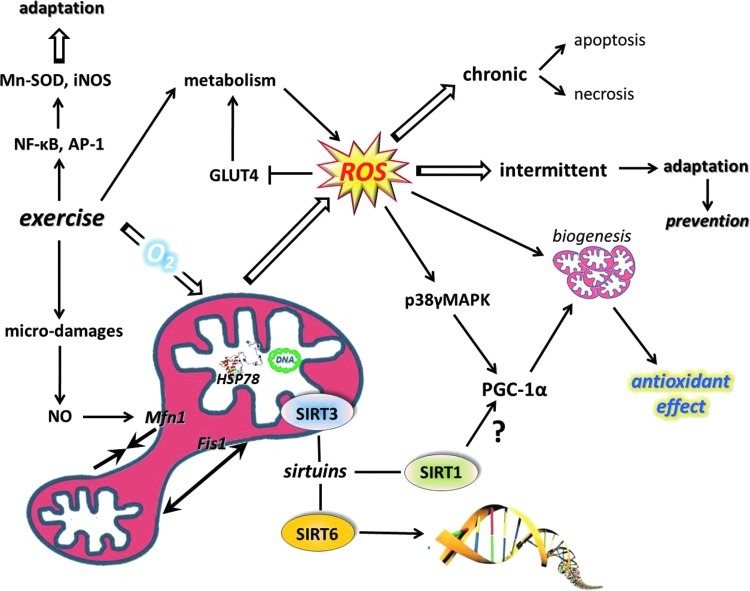
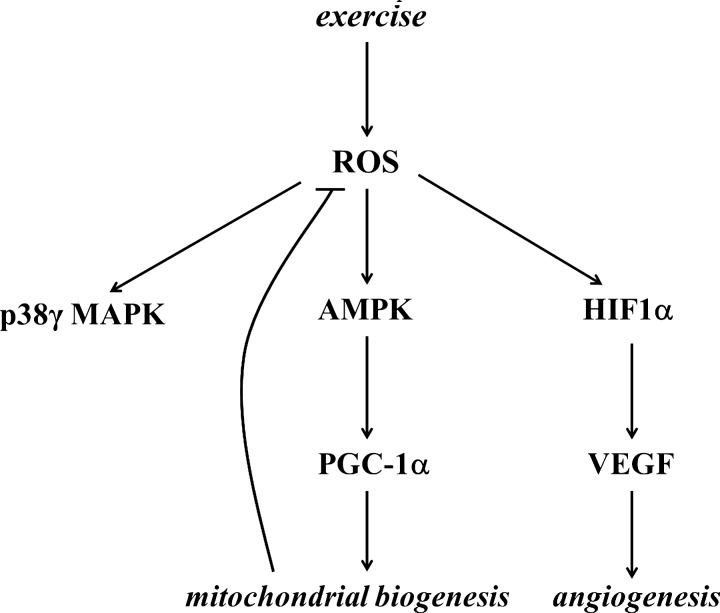
B. Redox signaling and biogenesis of mitochondria
It is clear that metabolic stress (in particular through AMPK/PGC-1α-dependent signaling processes) is one of the well-characterized ways to increase mitochondrial mass in skeletal muscle (123). However, it is also well characterized that increased metabolism is associated with changes in redox regulation (62). Therefore, oxidative stress has been suggested to be one of the causative signals of mitochondrial biogenesis (77), which is an important mechanism to reduce the exercise-induced leak of ROS from the mitochondrial network. For the same ATP production, more mitochondria can work at a lower respiratory capacity; hence, less ROS are produced, and therefore mitochondrial biogenesis can be regarded as part of the antioxidant system. Proteomic analyses yielded over 900 distinct mitochondrial proteins in human muscle and all, except the 13 encoded by mitochondrial DNA, are imprinted on nuclear DNA (273). In skeletal muscle fibers, the mass of mitochondria is dependent on the muscle subtype. The numbers and functions of mitochondria required to maintain fiber homeostasis are determined by regulatory circuits/programs that are under synchronized regulation between the mitochondrial and nuclear genes, to maintain function of respiratory electron transport complexes and proteins that are required for physiological functions of mitochondrial networks, including oxidative phosphorylation, ionic homeostasis, and mitochondrial uncoupling (thermogenesis).
In addition, skeletal muscle is equipped with two types of mitochondria: intermyofibrillar (IMF) and subsarcolemmal (SS). Although the location of mitochondria could be related to the level of ATP production, significant differences were not noted after oxidative stress or exercise training in the subpopulations (64, 65, 169). The findings of a recent study revealed that SIRT3 is present in IMF and SS, and chronic stimulation increased the content of IMF, SS, and SIRT3 in a parallel manner (122).
Mitochondrial gene expression is regulated by mitochondrial transcription factor A (TFAM), while the gene expression of nuclear DNA-encoded mitochondrial proteins is controlled by respiratory factors 1 and 2 (NRF1 and NRF2) (371). The coactivation of the transcription factors to promoter regions is carried out by PGC-1α, which is the master regulator of mitochondrial biogenesis (380). Overexpression of PGC-1α leads to a large increase in mitochondrial biogenesis, cytochrome oxidase II and cytochrome oxidase IV content, ATP synthase, myoglobin, and citrate synthase activity (212). Moreover, PGC-1α appears to have the capacity to increase mitochondrial electron transport activity while stimulating a broad range of anti-ROS mechanisms, including regulation of the expressions of Cu,Zn-SOD, GPX, catalase, uncoupling protein 2 (UCP2), and UCP3 (374). Indeed, knockout of PGC-1α leads to significant decreases in Cu,Zn-SOD, Mn-SOD, and GPX content, compared to those found in wild types of mice (202). Moreover, studies on PGC-1α knockout fibroblasts revealed significantly attenuated responses to H2O2 treatment by Mn-SOD, catalase, and GPX (374). Moreover, transgenic mice with overexpression of PGC-1α showed lower levels of loss in muscle mass as a result of aging, and the rate of oxidative damage was also attenuated, indicating that the exercise-associated upregulation of this transcription factor could cause similar health-promoting effects (431).
These observations confirm that PGC-1α actively is involved in antioxidant defense. Inflammation is associated with increased ROS production and oxidative damage, and the antioxidant role of PGC-1α is further supported by the anti-inflammatory effects of this coactivator. It has been shown that PGC-1α knockout mice, besides reduced endurance activity, exhibit increased levels of inflammation (129). HeLa cells were treated with ethidium bromide to deplete mitochondrial DNA, which resulted in increased oxidative stress and induction of TFAM and NRF-1, suggesting that ROS could be an important signal for mitochondrial biogenesis (236). Homocysteine-induced ROS production, which resulted in an increased expression of NRF-1 and TFAM, was attenuated by antioxidant treatments, again supporting the importance of ROS in mitochondrial biogenesis (281). Aging results in a chronic redox shift toward an oxidized milieu, which, in a significant way, can be attenuated by regular physical activity affecting a number of players in the mitochondrial biogenesis. Indeed, it seems that PGC-1α, TFAM, and NRF1 are all sensitive to redox balance and induced by ROS, leading to increased mitochondrial biogenesis and readily modified by exercise bouts (Fig. 10). Exercise resulted in increased ATP and ROS production by the mitochondria of skeletal muscle of subjects with normal glucose tolerance and impaired glucose tolerance by the induction of TFAM and NRF1 (106). Moreover, it was observed that aging and impaired glucose tolerance resulted in decreased ATP production and maintained levels of ROS (106).
FIG. 10.
Exercise results in large metabolic challenges to skeletal muscle that cause mitochondrial biogenesis and alteration of the mitochondrial network affecting fusion and fission. ROS are important signaling molecules for muscle contractions, PGC-1α, MAPK, as well as for transcription factor NF-κB. NAD+/NADH levels are readily modified by ROS and could affect the activity of sirtuins (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.) AP-1, activator protein-1; iNOS, inducible nitric oxide synthase; MAPK, mitogen-activated protein kinase; Mn-SOD, manganese superoxide dismutase; NF-κB, nuclear factor-kappaB; PGC-1α, peroxisome proliferator-activated receptor-γ coactivator 1α.
Most of the protein components of the mitochondria are encoded by nuclear DNA, including RNAs, and are important to mitochondrial biogenesis. Recent data have revealed that mammalian polynucleotide phosphorylase (PNPase) is localized in the mitochondrial intermembrane space to regulate the translocation of RNA into mitochondria (425). Silencing the PNPase with RNA interference results in decreases in mitochondrial membrane potential, ATP synthesis, and increased production of lactic acid (63). PNPase-deficient cells accumulate 8-oxoG in cellular RNA, indicating that PNPase is involved in the antioxidant/damage repair process (436). Indeed, PNPase is bound in a higher degree to oxidized RNA (436). Overexpression of PNPase could also be harmful to cells, since it can cause increased production of ROS and inflammation (347). Thus, both up- and downregulation of PNPase could impair cell function (133). The available information on the effects of exercise on PNPase is very limited. Our unpublished observations suggest that aging decreases the mRNA levels of PNPase in human skeletal muscle, while exercise decreases it in young and increases the expression in old subjects (41). On the other hand, in skeletal muscle of rats, we have observed increased amounts of PNPase with aging, and this was attenuated with exercise training (187). These data suggest that the relationship between exercise and PNPase is very complex.
SIRT1 has been proposed to be one of the activators of PGC-1α during exercise-induced mitochondrial biogenesis, although a recent report showed no interaction between SIRT1 and PGC-1α (284). Moreover, besides acetylation, other post-translational modifications such as phosphorylation appear to represent an immediate mechanism to activate PGC-1α- and initiate PGC-1α-dependent gene expression (67). The results of a study with human subjects has revealed that NAC supplementation blocked exercise-associated activation of JNK, but the mRNA level of PGC-1α was not affected by the supplementation of this antioxidant (283). Moreover, it is not clear at the moment whether PGC-1α phosphorylation is significantly affected by a redox milieu.
GLUT4, the insulin-dependent carbohydrate transporter, can be modulated by ROS (136), which means a shift in the redox state toward an oxidative milieu suppresses carbohydrate metabolism and increases insulin resistance (48, 430). During exercise, the need for carbohydrate enhances carbohydrate uptake, but during rest, the upregulated antioxidant system provides excellent conditions for the insulin-dependent carbohydrate uptake (213) with increased concentrations of the GLUT4 transporter.
The regulatory role of ROS in mitochondrial biogenesis was suggested many years ago, and a significant body of reference material has accumulated supporting this idea. Moderate levels of ROS appear to stimulate mitochondrial biogenesis via PGC-1α, SIRT1, and TFAM, while the increased levels of mitochondria decrease ROS production, in addition to the antioxidant role of PGC-1α and PNPase.
V. Exercise-Induced Inflammation and ROS
Inflammation is a protective process that is necessary for healing, damage repair, and fighting foreign bodies. Chronic inflammation is generally associated with increased levels of ROS and proinflammatory cytokines. Skeletal muscle is a powerful generator of some myokines, especially interleukin-6 (IL-6). The observation that Vitamin C and E administration decreases the exercise-induced generation of IL-6 suggests that ROS are stimulators of IL-6 production in skeletal muscle (94). Recently, it has been suggested that IL-6 acts as an energy sensor, exhibiting local and endocrine metabolic effects (278, 279). IL-6 and other cytokines such as IL-8, cyclooxygenase2, and TNF-α are significantly activated after unaccustomed muscle contractions, which cause microdamage to sarcomeres (54, 208). The repair of the damage by satellite cells is crucial, as these cells divide and fuse to form myotubes. The differentiation of myotubes to myonuclei then can be inhibited by NF-κB. Indeed, NF-κB has been suggested to be a regulator of muscle differentiation, because the RelA/p65 heterodimer complex can interact with the transcriptional regulator of YinYan, leading to inhibition of myogenesis (426). In addition, TNF-α, which can activate NF-κB, has been shown to cause proliferation of myoblasts, but to inhibit differentiation (95). Indeed, TNF-plus-interferon-gamma-induced activation of NF-κB resulted in significant losses in MyoD mRNA levels in C2C12 myocytes and inhibited differentiation (126). On the other hand, Li and Schwartz (207) demonstrated on C2C12 myoblasts that TNF-α mediated signaling to NF-κB, and serum response factor is important in the early phase of differentiation, indicating that TNF-α- NF-κB signaling on myoblast differentiation and on satellite cell-mediated repair of sarcomeres is dose dependent.
It has been shown that repeated bouts of exercise cause activation of NF-κB and activator protein-1 (AP-1), which might be important for exercise-mediated adaptation (53). The redox-dependent activation of NF-κB and AP-1 has been extensively reviewed (78, 157, 204), and here we briefly focus on the exercise-related mechanism. A single bout of exercise has been shown to activate DNA binding of NF-κB and AP-1, which facilitates the mRNA expression of Mn-SOD (142, 161). Allopurinol-mediated inhibition of XO decreases NF-κB activation, which has been suggested to attenuate the adaptive response to exercise training in skeletal muscle (110). On the other hand, it appears that regular exercise attenuates the age-associated increase in NF-κB activity at least in the liver (311). The findings from human lymphocytes and skeletal muscle of patients with chronic heart failure support the exercise-related activation of NF-κB (3, 76, 420). It seems to be well accepted that activation of the NF-κB-signaling cascade is important to the gene expression Mn-SOD and inducible nitric oxide synthase (iNOS) (162). Table 1 shows the list of some of the key ROS-dependent transcription factors.
Table 1.
Redox-Inducible Transcription Factors
| T | Nuclear factor-κB (NF-κB) |
|---|---|
| B | Redox sensor, regulates genes related with inflammation, cell growth, stress responses, including oxidative stress, and apoptosis |
| E | Activation of NF-κB binding to DNA in rat skeletal muscle in response to acute treadmill running (111, 139, 142, 162) |
| NF-κB activation in human peripheral blood lymphocytes in response to acute spring and endurance exercise (76, 420) | |
| Acute treadmill exercise activates NF-κB in an intensity-dependent manner in human peripheral blood lymphocytes (182) | |
| Acute eccentric exercise induces and submaximal eccentric training decreases NF- kB activation in PBMC (101, 165) | |
| Acute intensive resistance exercise increases NF-κB activity in human skeletal muscle (418) | |
| Acute fatiguing resistance exercise decreases NF-κB binding to DNA in skeletal muscle from healthy humans and mice (87) | |
| NF-κB activation in response to moderate-intensity cycle exercise in muscle from nondiabetic subjects, but not in type II diabetics (392) | |
| Increased basal NF-κB activity in muscle from insulin-resistant and type II diabetic subjects, and in diabetic rat (197, 392) | |
| NF-κB activation for DNA binding by isometric contraction in muscle of adult, but not old mice (416) | |
| Muscle unloading increases NF-κB activity in mice (87, 150) | |
| NF-κB−/− mice is resistant to unloading-induced muscle atrophy (149) | |
| Training increases basal levels, but blunts isometric contraction-induced NF-κB activation in skeletal muscle of mice (53) | |
| Treadmill training increases nuclear levels of NF-κB in skeletal muscle of adult and old rats (105) |
| T | Activator protein-1 (AP-1) |
|---|---|
| B | Senses intracellular redox state and regulates the expression of multiple genes involved in stress response, growth, and differentiation |
| E | Activation of AP-1 for DNA binding in rodent skeletal muscle in response to acute treadmill running (142) |
| AP-1 activation by isometric contraction in muscle of adult, but not old, mice (416) | |
| Training increases AP-1 activation in mouse skeletal muscle (53) |
| T | Nuclear respiratory factors (NRFs) |
|---|---|
| B | Activates expression of genes regulating cellular growth, respiration, heme biosynthesis, and mitochondrial DNA transcription and replication |
| E | Regular physical activity increased expression of NRF1 in young, and a single exercise bout decreased its expression in sketal muscle of both young and old individuals (41) |
| 5-week endurance training increased NRF-1 protein levels in skeletal muscle of young Wistar rats, but not in old ones (80) | |
| Treadmill training increases nuclear levels of NRF-2 in skeletal muscle of adult and old rats (105) | |
| Correlation of VO2peak with NRF-1 mRNA levels in skeletal muscle of healthy human (102) | |
| One bout of 3-h swimming in Wistar rats increases NRF-1- and NRF-2-binding activity (20) | |
| Acute exercise did not change NRF-1 mRNA expression in leg skeletal muscle of trained or untrained human (286) | |
| In healthy trained male cyclist, 10-km cycling increases skeletal muscle NRF-2 mRNA levels (58) | |
| 2 times/day, 3-h running bouts or 2-h swimming↑binding of NRF-1/2 in skeletal muscle of Wistar rats (434) | |
| An acute bout of exercise induced NRF-1 expression in rat muscle (245) |
| Forkhead box class O transcription factors (FOXOs) | |
|---|---|
| T | Control apoptosis, cell cycle arrest, DNA damage repair, detoxification of ROS, cell differentiation, and glucose metabolism |
| B | Marathon running induces PBMC FOXO3A mRNA expression (225) |
| Acute exercise does not alter FOXO expression in human skeletal muscle (72) | |
| A single bout of 60-min cycle exercise did not alter FOXO1/3 mRNA expression in untrained healthy males (72) | |
| High-intensity cycling until exhaustion↑FOXO-1-2 mRNA expression in skeletal muscle of healthy sedentary humans (223) | |
| Exercise training increased FOXO3a protein in the heart tissue of aged rats (91) |
| T | Peroxisome proliferator-activated receptor transcription factors (PPARs) |
|---|---|
| B | Regulate the expression of genes involved in the transport, metabolism, and handling of FFAs |
| E | A single bout of 60-min cycle exercise did not alter PPAR-α/β/γ mRNA expression in untrained healthy males (72) |
| 3-week training did not alter PPAR-α mRNA expression in PBMC of soccer players (299) | |
| High-intensity cycling until exhaustion↑PPAR-γ/δ mRNA expression in skeletal muscle of healthy sedentary humans (223) | |
| One bout of 2-h endurance exercise↑PPAR-β/δ mRNA in skeletal muscle of healthy humans (340) |
| T | Nuclear receptor-binding factor (NRBF) |
|---|---|
| B | Binding partner to PPAR-α and other nuclear receptors |
| E | High-intensity cycling until exhaustion↑NRBF-2 mRNA expression in skeletal muscle of healthy sedentary humans (223) |
| T | Early growth response factor 1(EGR1) |
|---|---|
| B | Stretch-responsive gene activation for ROS detoxification, regulation of Sirt1, inflammation, and immune response regulation |
| E | Mechanical stretch to myotubes increased EGR1 induction (275) |
| Acute exercise decreased EGR-1 expression in younger and increased in older subjects (168) | |
| 2-weeks swim training did not influence EGR-1 protein levels in the rat heart (135) |
| T | Hypoxia- inducible factor 1s (HIF-1s) |
|---|---|
| B | Encodes proteins that help the cellular response to low O2 increased angiogenesis, erythropoiesis, glucose uptake |
| E | Endurance training blunts acute exercise-induced HIF-1/2-α mRNA expression in human skeletal muscle (220) |
| 45 min of one-legged knee-extension exercise elevated protein levels and DNA-binding activity of HIF-1α in human skeletal muscle (11) | |
| 45 min of one-legged knee-extension exercise increased mRNA expression of HIF-1γ, but not HIF-1α (125) | |
| 6 weeks of high-intensity bicycle training under hypoxic, but not normoxic, conditions increased HIF-1α mRNA expression in human skeletal muscle (422) | |
| 6 weeks of endurance training under hypoxic, but not normoxic, conditions increased HIF-1α mRNA expression in human skeletal muscle of male high-level, long-distance runners (446) | |
| 3 weeks of intermittent hypoxic training decreased HIF-1 mRNA expression in skeletal muscle, but not leukocytes, of male endurance athletes (241) | |
| Exercise in acute, but not in chronic, hypoxia increased HIF-1α protein levels in skeletal muscle of CD-1 mice (194) |
| T | Heat-shock factor (HSF) |
|---|---|
| B | Regulates transcriptionally heat-shock protein synthesis |
| E | HSF activation for DNA binding by isometric contraction both in adult and old mouse muscle (416) |
| Endurance training induced the activation and expression of HSF-1 in the skeletal muscle in nondiabetic rats (18) | |
| 2 days of treadmill running exercise increased myocardial HSF-1 protein and HSF-1 activation in both young and old male Fischer-344 rats (79) | |
| Acute intensive exercise induces HSF-1 activation in rat myocardium (214) |
| T | Tumor suppressor protein p53 |
|---|---|
| B | Controls cell cycle arrest, muscle mitochondrial biogenesis, DNA repair, triggers senescence and apoptosis, maintains antioxidant defense, regulates its own levels |
| E | 8 weeks of endurance training decreased p53 protein in skeletal muscle of type II diabetic rats (301) |
| 4 weeks of endurance training decreased p53 mRNA in cardiac muscle of younger and older mice and p53 translocalization to mitochondria in older mice (300) | |
| p53−/− mice have higher aerobic exercise capacity (276) |
| T | Mitochondrial transcription factor A (TFAM) |
|---|---|
| B | Regulates mitochondrial gene transcription, DNA replication, and antioxidant protection |
| E | Physical activity increased the expression of TFAM in muscle of young adults, strong correlation between VO2max and TFAM (41) |
| Elite athletes have a higher level of TFAM expression than moderately trained individuals (263) | |
| Acute exercise increased TFAM mRNA expression in trained or untrained leg skeletal muscle of a healthy human (286) | |
| 4 weeks, 4 days/week, 45 min/day of one-legged training increased TFAM protein levels in skeletal muscle of a healthy human (30) | |
| Correlation of VO2peak with TFAM mRNA expression in skeletal muscle of a healthy human (102) | |
| Endurance training did not influence TFAM in skeletal muscle of healthy subjects and patients with mitochondrial myopathy (4) |
| T | Signal transducer and activator of transcription 3 (STAT3) |
|---|---|
| B | Regulates antiapoptotic signaling and skeletal muscle regeneration, cardiac protection, and myocyte elongation |
| E | STAT3 phosphorylation and translocalization↑in human skeletal muscle after acute resistance exercise (407) |
| Resistance exercise-induced STAT3 phosphorylation, increased with age (408) | |
| 24 and 48 h after single bout of resistance exercise increased STAT3 phosphorylation in skeletal muscle of aged rat (127) | |
| 20 days of eccentric training increase STAT-3 phosphorylation in skeletal muscle of Wistar rats (266) | |
| Acute sprint exercise increased STAT3 and STAT5 phosphorylation in human skeletal muscle (120) | |
| Acute resistance exercise-induced nuclear STAT3 phosphorylation was more prominent in skeletal muscle of old individuals compared to young adults (85) |
T, transcription factor; B, biological function; E, exercise response; ROS, reactive oxygen species; VO2max, maximal oxygen uptake; ↑, increase.
NO, mediated in redox signaling, is out of the scope of the present article, since it deserves an independent review, based on the importance of this signaling molecule. Because the NO-dependent damage repair of skeletal muscle is often overlooked, we will briefly summarize the current understanding of this phenomenon.
Anderson (13) made an interesting observation on the role of NO on satellite cells in skeletal muscle. After mechanical damage of NOS-I knockout or NO inhibited mice, very different repair processes were noted, and NO ablation or inhibition significantly attenuated the repair. It was evident that NO facilitates the activation of satellite cells, which are located in the basal lamina of skeletal muscle and are necessary for repair (13). The beneficial function of NO in damage repair is restricted not only to satellite cell proliferation and differentiation but also to fusion. Moreover, NO and cGMP activate follistatin, which is the antagonist of myostatin (287). NO-driven follistatin activation could be an important anabolic signal that is necessary for remodeling and muscle hypertrophy as well. Interestingly, treadmill running-related overuse of tendon results in increased NO production, which has been suggested to play a role in the repair process (389). The induction of mechanical damage to gastrocnemius muscle resulted in increased NO formation and is believed to initiate the signaling process to damage repair (178).
The muscle contraction-associated increase in IL-6 levels is, at least in part, dependent on ROS concentration, and is a necessary signal for metabolic processes and inflammation. NF-κB and AP-1 are redox-sensitive transcription factors that control inflammation, which in a chronic phase is associated with increased ROS production, but also is involved in antioxidant defense by the regulation of Mn-SOD. Through the activation of satellite cell proliferation, NO is important in damage repair/remodeling in the skeletal muscle, which might be important in delayed muscle soreness (315).
In addition to the role of NO in the repair of skeletal muscle, it should be mentioned that a great deal of exercise-related adaptation is mediated by NO in the vascular system, especially in the endothelium. Due to shear stress, NO seems to be the mechanism responsible for the beneficial impact of exercise training on vascular function and provides a potent physiological stimulus to adaptation in endothelial function and vascular remodeling (183). Exercise stimuli, which result in activation of endothelial nitric oxide synthase (eNOS) and related agonists, such as bradykinin and acetylcholine, significantly increase blood flow causing vasodilation (404). The half-life of NO is relatively short in the circulation, around 0.1 s, (176) and it is rapidly oxidized to nitrate and nitrite. However, alternative pathways can also be present to generate bioactive NO from nitrate and nitrite (10) and are involved in a wide range of processes, such as cellular signaling, regulation of blood flow, cellular metabolism, and hypoxic response (218). Reaction of NO to superoxide generates the highly toxic peroxinitrite that could readily cause DNA damage and impair the activity of DNA repair enzymes, including OGG1 (156). Despite the exercise-induced increase in eNOS and neuronal nitric oxide synthase (nNOS) protein content in skeletal muscle (417), it appears that exercise does not significantly increase the formation of peroxinitrite, since moderate levels of exercise increase OGG1 activity (306).
VI. The Crucial Role of Redox Regulation on New Cell Formation in Skeletal Muscle and Brain
Skeletal muscle cells and neurons are nondividing cells, but it is clear that stem cells after differentiation can lead to new cell formation even in postmitotic tissues.
In skeletal muscle, resident adult myogenic precursor cells referred to as satellite cells are mitotically quiescent, but can be readily activated by a single bout of exercise (59) and a moderate dose of H2O2 supplementation (237). Satellite cells can be divided into low-proliferative and high-proliferative subpopulations (321). It has been shown that the proliferating capacity of these satellite cells is dependent on mitochondrial membrane potential, ATP balance, and ROS generation (336), and high-proliferating capacity favors an oxidized cellular milieu. On the other hand, when satellite cell cultures were challenged by relatively high H2O2 concentrations (1 mM), it turned out that the proliferation reduced significantly, while the viability of the cells did not change to any great extent (327). Aging seems to impair the proliferating capacity of satellite cells and is associated with reduced antioxidant capacity and increased Ca2+ levels (100). The repair of skeletal muscle after injury involves high-mobility group box 1, which is an intracellular protein that translocates to the nucleus to bind to DNA and activates gene expression and stem cells. It appears that a reduced cellular milieu may be important to maintain the bioactivity of high-mobility group box 1, since oxidation abolishes the muscle stem cell migration to the response of high-mobility group box 1 (419). The results of a recent study revealed that angiopoietin-2, which is a powerful factor of angiogenesis, is present in precursor cells of skeletal muscle, and is induced by H2O2 in primary cell culture (237). As described earlier, redox-sensitive TNF-α and NF-κB could impair differentiation of myoblasts to myotubes, and a recent study suggests that IL-1α, along with TNF-α, also has the capability to act upon transforming growth factor-β-activated kinase-1 (406). The signaling pathway of these cytokines is a distinct route that involves the p38α-mediated recruitment of polycomb-repressive complex 2 to the Pax7 promoter in satellite cells (242). The effects of cytokines released upon muscle damage are dependent of the extent of dose, and it is clear that at low concentrations, they stimulate repair, while in large doses, the adverse effects are more pronounced (243).
These data are important to understand why and how precursor cells in the skeletal muscle can be involved in redox-associated angiogenesis, which is important to exercise-induced adaptation and/or damage repair. The available information on satellite cells suggest that redox homeostasis is an important regulatory factor of proliferation; although the results are not unequivocal, it can be suggested that a fine tuning of satellite cells by ROS, depending on the dose, can cause activation or inhibition.
Besides satellite cells, neuronal precursor cells are also sensitive to redox milieu. It is well established that neuronal precursor cells in the dentate gyrus are able to proliferate throughout life and differentiate, and their progenity can lead to neurogenesis (89). Observations suggest that progenitor cells can readily respond to changes in energy homeostasis. Therefore, ischemia/reperfusion, aging, and metabolic pathology or even physical exercise can change the rate of neurogenesis. Indeed, precursor cells exhibit high mitotic and multipotentiality potential, and ROS are an important signals that control their ability to divide and differentiate (211). One of the reasons for this is that precursor cells are very sensitive to oxygen levels that are suggested to be around 2% in the brain (368). Lowering the level of oxygen concentration by transient middle cerebral artery occlusion on the rat brain leads to increases in neurogenesis (17). It has been shown that neuronal precursor cells exhibit about four-times higher ROS levels than other cell types, and the concentration of ROS, which is dependent on the density of precursor cells, is associated with the rate of proliferation (211). The fine redox tuning, therefore, is a necessary modulator of the proliferation of neuronal progenitor cells, and the bell-shaped dose–response represents the relation of ROS and neurogenesis (Fig. 11) (262).
FIG. 11.
The function-promoting effects of regular exercise on different cellular functions include the upregulation of antioxidant, oxidative damage-repairing systems, neurogenesis, and induction of trophic factors. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.) BDNF, brain-derived neurotrophic factor.
The original article that reported that exercise results in neurogenesis has been confirmed by a number of articles that have demonstrated that the newly formed neurons are functional (413, 414). A direct relationship between ROS and exercise-induced neurogenesis has not been reported, but in our recent study, we have observed an age-related decline in neurogenesis, which was associated with increased levels of 8-oxoG (189). The age-related attenuation of neurogenesis has been suggested to be due to blood-borne factors (421), and hence the age-dependent chronic oxidative stress has not been ruled out as a negative regulator. The question whether the exercise-induced neurogenesis is ROS dependent needs to be addressed. Brain-derived neurotrophic factor (BDNF) has been implicated in neurogenesis (27) and shown to be sensitive to redox state (31, 367, 427). Exercise is a known inducer of both, BDNF and neurogenesis. Hence, it cannot be completely ruled out that the exercise-related change in the redox milieu is one of the reasons that exercise stimulates BDNF levels. BDNF expression was elevated after focal ischemia in the cerebral cortex (147), and it has been shown that oxidative damage interplays with BDNF (435). Indeed, oxidative damage has been implicated in brain function.
VII. Exercise, ROS and Oxygen Sensing, HIF, and Vascular Endothelial Growth Factor
Intracellular redox balance, which includes oxidizing and reducing powers, is essential for normal, physiological function. ROS could be produced by both an oxidative and a reduced milieu (83) and physical exercise. However, an abnormal elevation of reducing power could occur with the interruption of electron flow at the electron transport chain, which easily can lead to deleterious effects on cells (151). Indeed, it appears that hypoxia can lead to reductive stress, which also results in increased ROS production by the mitochondrial electron transport system (238). It is believed that ROS are generated at complex I and complex III of the electron transport chain. During hypoxia, less O2 is available to be reduced to H2O at cytochrome oxidase, thus causing accumulation of reducing equivalents within the mitochondrial respiratory sequence. This accumulation is known as reductive stress, and this reaction leads to ROS formation by the auto-oxidation of one or more mitochondrial complexes, such as the ubiquinone–ubiquinol redox couple. Changes in cellular redox state activate one of the most important sensors of oxygen, namely HIF-1α.
A. Hypoxia in skeletal muscle
A gradient of oxygen partial pressure (pO2) exists within the body, due to ∼160 mmHg in inspired gas, ∼115 mmHg in arterial blood, and ∼38 mmHg in capillary blood (328). Within tissues, the pO2 gradient depends on the distance of cells from the closest O2-supplying blood vessels and the metabolic activity and consequent O2 consumption of the resident cells (395). For example, pO2 in intramyocellular cells falls to as low as 3.1 mmHg under normoxia and 2.1 mmHg under hypoxia, respectively (328). Mitochondria house the final biochemical steps in the production of reducing equivalents that react at the terminal oxidases of the respiratory chain, with molecular oxygen provided by the respiratory system from the environment. In mitochondria, O2 finally disappears, and oxygen partial pressure goes to zero (145). Mitochondria thus are an effective oxygen sink, and this allows organisms to use all of the available oxygen partial pressure of the actual environment to drive the respiratory cascade from the lungs through circulation to the mitochondria (394).
The transcription factor, HIF-1α, acts as a master regulator for the expression of genes involved in the hypoxic response of most mammalian cells (355, 365). Namely, HIF-1α is hydroxylated and degraded in normoxia, but is stable in hypoxia and translocates into the nucleus to form an active complex with HIF-1β, which is constitutively expressed (154, 355). Nevertheless, it is clearly expressed in various tissues such as the brain, kidney, liver, heart, and skeletal muscle in mice kept in normoxic conditions (21% O2), whereas it is undetectable in the lungs (379). Further, when mice were kept in extreme hypoxia (6% O2), the expression levels of HIF-1α in the brain, kidney, liver, heart, and skeletal muscle were significantly increased, while expression in the lungs was not affected (379, 443). These findings indicate that there is a clear pO2 gradient between the lungs and other tissues, and that with the exception of relatively well-oxygenated lungs, low pO2 and HIF-1α play physiologically essential roles in homeostasis of various tissues. The proposed explanation might be rather simplistic as factors as oxygen uptake and supply and extraction by individual organs could play an important role in determining the HIF response. It cannot be ruled out that pO2-dependent HIF response after induction is often bell-shaped, and results from both HIF mRNA expression induction and HIF protein stabilization in the absence of oxygen.
Mounier et al. (240) have shown, in untrained human skeletal muscle under normoxic conditions, a higher HIF-1α protein expression in predominantly oxidative muscles than in predominantly glycolytic muscles. The HIF-1α mRNA expression pattern however was not in agreement with the HIF-1α protein level. Interestingly, none of the HIF-1α target genes, such as the most studied angiogenic factor involved in muscle angiogenesis and vascular endothelial growth factor (VEGF), exhibited a muscle fiber-specific related mRNA expression at rest in normoxia. However, soleus presented a significantly higher VEGF protein content than vastus lateralis and muscles of triceps. Taken together, such findings indicate that there are muscle-specific differences in HIF-1α and VEGF expression within human skeletal muscle at rest in normoxic conditions.
Thus, the following section focuses on the significance of various conditions, such as hypoxia, oxidative stress, and physical exercise, in the HIF-1α- and VEGF-signaling pathways, with emphasis on skeletal muscle.
B. HIF-1α-independent regulation of VEGF and angiogenesis
Peroxisome proliferator-activated receptor (PPAR)-γ coactivator 1α (PGC-1α) regulates mitochondrial biogenesis in multiple cell types through coactivation of key transcription factors, including NRF-1 and NRF-2, estrogen-related receptor-α (ERRα), Gabpa/b, PPARs, and the thyroid hormone receptor (16, 265). PGC-1α is a mediator of signaling in response to deprivation of nutrients and oxygen, and it strongly regulates VEGF and other angiogenic factors to elicit neovascularization in vivo. The regulation of VEGF in response to hypoxia is thought to be mediated primarily through the well-known HIF factors (355). Surprisingly, the novel PGC-1α/ERR-α pathway (16) is apparently independent of the HIF pathway. PGC-1α−/− mice are viable, suggesting that PGC-1α is not essential in embryonic vascularization (16). Angiogenesis in the adult occurs in both physiological and pathological contexts (57). The robust induction of vascularization by PGC-1α and its critical function in the response to limb ischemia strongly implicate PGC-1α in the angiogenic response to ischemia, providing protection against further ischemic insults (16).
On the other hand, PGC-1α is coupled to HIF signaling through the regulation of intracellular oxygen availability, allowing cells and tissues to match increased oxygen demand after mitochondrial biogenesis with increased oxygen supply, because of intracellular hypoxia (265). PGC-1α-dependent induction of HIF target genes under physiological oxygen concentrations is not through transcriptional coactivation of HIF or upregulation of HIF-1α mRNA, but through HIF-1α protein stabilization.
Other candidates are mentioned for the HIF-1α-independent regulation of VEGF and angiogenesis. Overexpression of suppressor of cytokine signaling 3 (SOCS3), a well-established negative regulator of signal transducer and activator of transcription 3 (STAT3), a mediator of cytokine signaling, which is a crucial factor for myogenesis, enhances the mRNA expression of downstream targets of STAT3, c-FOS, and VEGF, despite inhibition of STAT3 phosphorylation, and is correlated with enhanced mRNA expression of genes associated with muscle maturation and hypertrophy (55). The mechanism by which SOCS3 may mediate these responses however is unknown and will require further investigation. The decline in the regenerative capacity of skeletal muscle with age may be linked to inefficient STAT3/SOCS3 signaling. Actually, a single bout of maximal leg-extension exercise (resistance exercise) markedly increased the STAT3 signaling, including the SOCS3 gene in human skeletal muscle, accompanied by significant increases in levels of mRNAs for downstream genes of STAT3, c-FOS, JUNB, c-MYC, and VEGF, but probably not via HIF signaling (407). In addition, the levels of HIF-1 mRNA subunits did not change in response to short-term one-legged exercise training, suggesting no change in HIF-1 mRNA transcript levels in the regulation of training-induced VEGF expression (124).
Increased production of ROS, such as H2O2, also induces the expression of VEGF-A, the prototype VEGF ligand, by an HIF-independent and Sp1-dependent mechanism, in which ligation of VEGF-A to VEGF receptor 2 (VEGFR2) results in signal transduction leading to tissue vascularization. Such ligation generates H2O2 via an NADPH oxidase-dependent mechanism (1). That the function of one mitogen, VEGF, largely depends on signaling driven by another mitogen, H2O2, generates a novel paradigm with major therapeutic implications for a variety of angiogenesis-related disorders (338). Furthermore, oxygen–glucose deprivation generates ROS in cerebral endothelial cells, and in turn induces VEGF signaling via its receptor, Flk-1 (VEGFR2), and activates extracellular signal-regulated kinase (ERK) 1/2, thereby suggesting that VEGF acts via ERK 1/2 through oxidative-stress-dependent mechanisms, showing that ERK 1/2 can be considered a molecular target for stroke therapy (250). Interestingly, high glucose blunts VEGF response to hypoxia in immortalized rat proximal tubular cells, this effect being mediated by the oxidative stress-regulated HIF–hypoxia-responsible element pathway (173).
C. p38γ MAPK/PGC-1α signaling regulation by physical exercise and its significance for mitochondrial biogenesis and angiogenesis in skeletal muscle
p38γ MAPK, but not p38α MAPK or p38β, is required for PGC-1α upregulation in mitochondrial biogenesis and angiogenesis in response to voluntary wheel running and nerve stimulation in mice. None of the p38 MAPK isoforms was required for endurance exercise-induced IIb-to-IIa fiber-type transformation in these mice (213). Meanwhile, calcineurin (CnA) nuclear factor of activated T-cell (NFAT) regulatory axis controls fiber-type transformation in endurance exercise-induced skeletal muscle adaptation (288). Collectively, endurance exercise on one hand induces activation of the Ca2+-dependent CnA-NFAT pathway in control of fiber-type transformation, and on the other hand activates p38γ MAPK, which promotes PGC-1α activity and expression in control of mitochondrial biogenesis and angiogenesis (213, 288).
An acute bout of sprinting exercise that increased ROS production, mainly through a nonmitochondrial enzyme, XO, stimulated PGC-1α expression and other key proteins in the mitochondrial biogenic pathway, such as NRF-1 and TFAM. This nuclear-initiated signaling event was accompanied by activation of p38 MAPK and CREB phosphorylation. Inhibition of XO by allopurinol severely attenuated exercise activation of the PGC-1α signaling pathway, thus providing strong evidence that mitochondrial biogenesis in skeletal muscle is controlled, at least in part, by a redox-sensitive mechanism (143, 172). Moreover, stretch-stimulated glucose uptake in skeletal muscle is mediated by an ROS- and p38 MAPK-dependent mechanism that appears to be AMPKα2- and phosphatidylinositol 3-kinase (PI3-K)-independent (60). This novel signaling mechanism is distinct from canonical contraction- and insulin-stimulated signaling that increases glucose uptake, probably providing an alternative pathway for the development of novel therapeutic drugs to overcome insulin resistance. MAPK activation is also involved in the contraction-induced secretion of VEGF protein from skeletal muscle, which is partially mediated via adenosine acting on A2B adenosine receptors (140).
D. Two-faced ROS in HIF activation
ROS are widely believed to cause or aggravate several human pathologies such as neurodegenerative diseases, cancer, stroke, and many ailments. Antioxidants are assumed to counteract the harmful effects of ROS and therefore prevent or treat oxidative stress-related diseases (93). On the other hand, there is growing evidence that ROS have deleterious or beneficial effects; this dual nature of ROS means that ROS act as intracellular signaling molecules as defense mechanisms against microorganisms (108). For instance, the mechanisms by which ROS and cytosolic H2O2 levels are involved in stabilization/activation and eventual degradation/silencing of HIF-1α and other redox-sensitive transcription factors, such as MAPK and PI-3K/Akt, are discussed (179).
Mitochondria and mitochondrion-derived ROS were required for the stabilization of HIF-1α by MAPK and by exposure of hepatoma cells to 6 h of hypoxia (1.5% O2) (61). This hypoxic stabilization of HIF-1α in hepatoma cells was blocked by the overexpression of catalase, which catalyzes dismutation of H2O2 into H2O and O2, and is an inhibitor of the mitochondrion super anion channel. Also, HIF-1α and VEGF overexpression in pulmonary artery smooth muscle was induced by CoCl2, which mimics the hypoxic response, via ROS (28). Such overexpression was inhibited by Vitamin C and by overexpression of GPX and catalase. In contrast, the addition of H2O2 in the absence of a hypoxic stimulus causes stabilization of HIF-1α (61). Anoxia, on the other hand, may stabilize HIF-1α essentially through depletion of the cosubstrate dioxygen of prolyl-hydroxylases, indicating that increases in cytosolic H2O2 in consequence of hypoxia-driven ROS production may cause HIF-1α accumulation in hypoxia through reduction of its hydroxylation (145, 12). Actually, during the acute increase phase of HIF-1α (between 0 and 2 h), 8-hydroxy-2′-deoxyguanosine (8-OHdG) was positively correlated with HIF-1α during sustained hypoxia in humans (285). Moreover, ROS-activated signaling events involving MAPK and PI-3K/Art have been implicated in the modulation of the transcriptional activity of HIF-1α (234). Further, angiotensin II in vivo and in vitro upregulates renal VEGF expression by a mechanism that involves HIF-1 activation and oxidative stress (345).
Thus, it now seems possible that ROS have important roles in the regulation of cell signaling, although these substances are potentially cell damaging. For example, there appears to be a potential feedback loop in which PGC-1α regulates antioxidant mechanisms via regulation of mitochondrial respiration and ROS. In turn, ROS might regulate antioxidant defense genes through activation of redox-sensitive transcription factors, supporting the view that ROS are important signaling molecules in adaptations to exercise (350) (Fig. 12). While increasing evidence suggests that mitochondria play a less-important role in oxidant production in contracting skeletal muscles than was previously predicted, and that the primary site of contraction-induced ROS production in muscle fibers remains unclear (294, 297), PGC-1α is transiently induced by acute exercise (20, 143). This induction has been suggested to occur in response to altered energy demands and because of PGC-1α interacting with the metabolic enzyme AMPK (130). PGC-1α has also been proposed to be a key mediator of long-term adaptations to exercise (130). Indeed, basal levels of PGC-1α gene expression increased approximately twofold after a period of endurance training in humans (339).
FIG. 12.
Exercise results in enhanced ROS production, which activates the p38γ MAPK, AMPK, and HIF1α pathways leading to mitochondrial biogenesis and angiogenesis that are an important part of exercise-induced adaptation. AMPK, AMP-activated protein kinase; HIF-1α, hypoxia-inducible factor-1a; VEGF, vascular endothelial growth factor.
Intriguingly, and in conflict with the free radical theory of aging (131), longevity and health-promoting effects of caloric restriction and specifically reduced glucose metabolism may be due to increased formation of ROS within the mitochondria. This causes an adaptive response that culminates in subsequently increased stress resistance, assumed to ultimately cause a long-term reduction of oxidative stress, which is an adaptive response more specifically named mitochondrial hormesis or mitohormesis (330). Molecular mediators of endogenous ROS defense (Cu,Zn-SOD, Mn-SOD, and GPX) are also induced by exercise, and this effect is blocked by antioxidant supplementation (331). Consistent with the concept of mitohormesis, exercise-induced oxidative stress ameliorates insulin resistance and causes an adaptive response promoting endogenous antioxidant defense capacity. As expectedly or unexpectedly, supplementation with antioxidants may preclude these health-promoting effects of exercise in humans. Taken together, physical exercise induces numerous molecular regulators of insulin sensitivity and antioxidant defense, most of which are almost completely inhibited by antioxidant pretreatment in healthy young men (329–331).
E. Effect of exercise on HIF and VEGF signaling
The study on acute exercise by Ameln et al. (11) was the first to show that several components of the HIF-1 pathway, involving VEGF and erythropoietin, are activated in response to acute changes in oxygen demand in healthy human skeletal muscle, due to a concurrent decrease in von Hippel-Lindau tumor suppressor protein (VHL) levels. This finding suggests that oxygen-sensitive pathways could be relevant for adaptations to physical activity by increasing capillary growth in human skeletal muscle, and supports the general importance of the HIF pathway (11). VEGF mRNA is further increased when blood flow to the exercising leg is restricted.
Several studies have revealed increased levels of the HIF-1 pathway in human skeletal muscle after acute exercise (125, 220). The positive response of mRNAs for HIF-1α and HIF-2α with acute exercise however is blunted with training (220). Moreover, a positive correlation was found between exercise-induced changes in VEGF mRNA on one hand, and those in HIF-1α mRNA, HIF-1β mRNA, and lactate on the other, while no significant differences between the restricted and nonrestricted groups were observed (125), unlike the results found in the study of Ameln et al. (11). Gustafsson et al. (125) noted that blood flow restriction, made possible by application of external pressure 50 mmHg higher than atmospheric pressure, caused about a 20% decrease in blood flow at same work load, creating a condition that allowed the study of the interaction between HIF and VEGF during exercise. This lack of a further increase in VEGF mRNA when flow restriction was added may be explained by the smaller further reduction in oxygen saturation and oxygen tension compared with the rest-to-exercise transition, even if this difference was large enough to induce a greater lactate concentration increase in the restricted condition. Another explanation could be that even if there was no significant VEGF mRNA difference before exercise between the nonrestricted and restricted conditions, a strong influence of the pre-exercise value of VEGF mRNA on the exercise-induced increase makes a difference between the two conditions more difficult to detect.
Exercise induces several pathways that are known to be important for regulating angiogenesis. VEGF is critical for basal and activity-induced regulation of skeletal muscle capillarization, whereas the importance of other growth factor pathways remains to be elucidated. Although several signaling pathways have been identified (104), significant work remains before the intracellular signaling pathways and transcription factors involved in basal and exercise-induced angiogenesis are fully understood.
For example, in addition to AMPK, exercise activates several signaling pathways that may be the links between metabolism and capillarization, including calcium (Ca2+)-regulated pathways (104). Large, but transient, increases in intracellular Ca2+ occur during exercise as a result of neural activation and muscle cell depolarization. Ca2+ is intimately involved in actin–myosin cross-bridge formation and is responsible in part for exercise-induced improvements in insulin sensitivity that occur, in part, through increases in glucose transporter 4. Increasing intracellular Ca2+ leads to the activation of several downstream signaling proteins, including calmodulin-dependent kinase and CnA, possibly resulting in increased expression of VEGF mRNA in primary human myotubes in vitro. This suggests that Ca2+ may be involved in muscle VEGF regulation (104), although much more work needs to be done before these studies are finalized. In addition, PPAR-β, which is also known as PPAR-δ and is involved in muscle development and metabolism, increases VEGF and promotes muscle angiogenesis through a CnA-dependent pathway (103).
Moreover, NO is involved in muscle VEGF regulation (104, 438). nNOS-mediated NO regulates, in part, muscle mitochondrial respiration, whereas endothelial NO is involved in blood flow regulation. Disrupting normal NO production (via L-NAME) eliminates the increase in collateral blood flow induced by training, but does not disturb the increase in muscle capillarity within the active muscle. Similarly, inhibiting VEGFR kinase activity eliminates the increase in collateral-dependent blood flow, and lessens, but does not eliminate, angiogenesis within the calf muscle, illustrating distinctions between the processes influencing angiogenesis and arteriogenesis (Fig. 13) (438).
FIG. 13.
Exercise induces marked release of Ca2+ from the sarcoplasmic reticulum, which binds to troponin to allow the generation of cross bridges between myosin and actin filaments. Moreover, it activates calmodulin-dependent protein kinase (CaMK) that could lead to enhanced expression of GLUT4 glucose transporter. Then, as a result of insulin-mediated signaling, more GLUT4 can translocate to the membranes leading to increased glucose uptake by skeletal muscle. CaMK can also activate increased capillarization via VEGF. PPAR, peroxisome proliferator-activated receptor. (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
In general, the effects of endurance training on the activity of the HIF pathway in human skeletal muscle under hypoxic conditions appear to be definitely higher than those under normoxic conditions (219, 422, 446), although there are several exceptions (97, 241), indicating that combining hypoxia with exercise training appears to improve some aspects of muscle O2 transport and/or metabolism (Fig. 14) (219). Although there were no significant changes in the levels of mRNAs for HIF-1α or VEGF in human skeletal muscle after endurance training under normoxic conditions, the changes in HIF-1α mRNA expression correlated well with those in VEGF mRNA expression (270), suggesting that HIF-1α influences the training-induced VEGF gene expression, or alternatively that HIF-1α and VEGF expression is coregulated at the transcriptional level in human skeletal muscle. Taken together, it is envisioned that cumulative effects of transient changes in transcription during recovery from successive bouts of exercise may represent the underlying kinetic basis for the cellular adaptations associated with endurance training. Furthermore, no plasma VEGF contents in overweight men aged 50–60 years were affected by long-term endurance exercise under normoxic conditions (51). Likewise, low- or high-intensity-resistance exercise did not significantly alter serum VEGF content in humans (334).
FIG. 14.
Oxygen sensing and angiogenesis are dependent on the HIF-1-VEGF axis, which is mediated by hydrogen peroxide-dependent signaling. The figure shows some of the key elements of the suggested signaling pathways (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.) Akt, protein kinase B; ERK, extracellular signal-regulated kinase; PI3-K, phosphatidylinositol 3-kinase.
VIII. Exercise, ROS, and Epigenetics
Environmental and life-style-dependent factors can result in heritable changes in phenotypes through the methylation of DNA and reversible post-translational modification of amino acid residues of histone proteins without altering the basic structure of DNA. Histone modifications and DNA methylation predominantly occur at cytosine/guanine dinucleotide sites (132) by DNA methyltransferases (DNMT). Recently, it was reported that DNMT1, one of the key enzymes of DNA methylation, interacts with SIRT1, which causes deacetylation of DNMT1, which impairs cell cycling at G2/M transition (280). As we stated earlier, SIRT1 activity is redox dependent, and hence it is not surprising that the nature of DNA methylation and post-translational modification of histones are more dynamic than thought before (69).
It has been demonstrated that excessive levels of ROS could lead to genomic instability through an increase in DNA damage and chromosome degradation (24). ROS have been implicated in double-strand breaks that result in chromosome breaks (175). However, we do not intend to further explore the deleterious effects of ROS on chromosomes, rather we would emphasize the regulatory role of ROS in epigenetics.
Histone acetyl transferases play a key role in inserting acetyl groups on lysine residues of histones, and among them, p300/CBP is regulated by the p38/MAPK pathway and coactivates a number of transcription factors, including NF-κB and AP-1 (397). In addition, MAPK/ERK, IGF-1, and other growth factors, as well as cytokines, generate ROS for signaling and show a negative intraspecific relationship with longevity (335). The p38/MAPK-activated p300/CBP acetylates OGG1 (32, 388) and enhances the repair of this enzyme. According to these observations, a low dose of radiation has been shown to facilitate growth (153) via ROS generation, and it can cause DNA hypomethylation, which is related to DNA repair (289), since DNA repair processes incorporate cytosine, but not methylcytosine. This is how radiation-associated DNA repair can cause DNA demethylation and activation of silenced genes.
Interestingly, dietary restriction, which is generally associated with decreased accumulation of oxidative damage, including carbonyl groups, increases the accumulation of histone carbonylation in aged rats (363). Carbonylation of histone proteins increases the charge of basic amino acid residues, resulting in a more compact chromatin structure that would make transcription and repair of DNA less active. This is what happens in aging. Hence, it cannot be ruled out that controlled carbonylation of amino acid residues on histone proteins is involved in epigenic regulation.
Hypomethylation of DNA has been reported immediately after a single bout of exercise in the skeletal muscle samples of healthy subjects at the promoter regions of PGC-1α, pyruvate dehydrogenase kinase isoenzyme 4, and PPAR-δ (23).
It has been reported that exercise results in phosphoacetylation of histone H3 and induction of c-Fos gene in dentate gyrus (71). The excellent work from the laboratory of Gomez-Pinilla has shown that the well-known activating effect of exercise on BDNF includes epigenetic factors. Exercise influences DNA methylation at the BDNF gene promoter region modifying acetylation of H3 (113). It has also been shown that exercise during pregnancy results in increased BDNF expression, enhanced hippocampal cell survival, neurogenesis, and better memory in the offspring (180, 198). These studies did not evaluate the levels of ROS, but it is known that ROS can facilitate BDNF expression and neurogenesis (195, 367).
Regular exercise can increase mean life span, and it was suggested that nearly all models that lead to life extension show resistance against stressors, including ROS (226). One excellent example is from rats bred with intrinsic aerobic endurance running capacity. Low-running-capacity rats suffer from insulin resistance, cardio-vascular problems, and obesity when compared to high-running-capacity rats (HCR) (353, 433), and have a shorter maximal life span (186). Importantly, HCR generate higher levels of ROS, but because of the higher activity of antioxidant and DNA repair enzymes, HCR are more resistant to oxidative stress (409). In addition to this, we observed from the 22nd generation of these selectively bred rats that HCR have significantly higher concentrations of mitochondria and HSP78 content (Hart et al., unpublished observation). We have measured p300/CBP expression levels from the skeletal muscle of young, old, sedentary, and physically active individuals and found age-associated increases (308). These observations fit well with exercise-ROS-dependent changes in epigenetics (Fig. 15).
FIG. 15.
Tight heterochromatin suppresses the rate of transcriptions, while euchromatin allows rapid response to challenges by gene transcription. The tightness of chromatin is dependent on post-translational modification of histone residues. The suggested mechanism by which exercise could play a role in DNA methylation and post-translational modifications of histone residues. Histone acetyl transferases and deacetylases, such as p300/CBP and SIRT1, are readily modified by exercise and aging, which might affect epigenetics. Exercise increases DNA methylation at the BDNF gene promoter region and also increases the acetylation of H3, and these modifications result in enhanced production of BDNF in the hippocampus (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.) CR, caloric restriction; HAT, histone acetyl transferases.
The interaction between genes and their surroundings to produce epigenetics could lead to changes in the phenotype. In the skeletal muscle, the differences in antioxidant and DNA repair capacity between slow- and fast-twitch fibers are strongly dependent on metabolism and ROS production capacity (152, 268, 313, 370). The exercise-associated ROS dependence of epigenetics is still not well studied, but the available limited data suggest that regular exercise could alter DNA methylation and the post-translational modification of histone residues by redox-mediated adaptation.
IX. Conclusion
High oxygen consumption and endurance running-associated evolution have developed a dynamic multifunctional redox homeostasis, with a strong relationship to metabolic function. The regular exercise-associated metabolic challenge is paired with oxidative challenges resulting in a wide range of adaptive responses. On the other hand, modern lifestyle increases in physical inactivity have suppressed the adaptive capacities, both in metabolic and redox homeostasis, leading to increased incidences of ROS-associated diseases and deaths. The increased ROS generation at moderate levels of exercise facilitates muscle contraction and then leads to muscle fatigue, which is an important protective mechanism of the body to avoid cellular damage. ROS are crucial for the activation of the key transcription factors, coactivators to induce adaptive responses to higher metabolic demand, including mitochondrial biogenesis, improved capillarization, enhanced antioxidant, and oxidative damage-repairing systems. We have pointed out in this review that moderate levels of oxidative damage could facilitate remodeling of cell membranes and proteins, while 8-oxoG could be necessary for specific gene activation and the opening of chromosomes. The role of PGC-1a in the antioxidant system is very specific, since moderate ROS could induce this coactivator, which enhances the expression of antioxidant enzymes and could induce the number of mitochondria, which at the same ATP demand would result in decreased levels of ROS. However, other players of the mitochondrial biogenesis fusion and fission team are sensitive to redox balance. Without question, redox homeostasis is crucial for the biogenesis of mitochondria. Sirtuins are NAD+-dependent histone deacetylases and sensitive sensors of metabolic and redox alterations. The target proteins of sirtuin are involved in metabolism, inflammation, DNA repair, apoptosis, and redox regulation. Furthermore, sirtuins are readily modified by exercise-induced changes in metabolism and redox signaling. It has been shown that redox-sensitive inflammatory transcription factors, such as NF-κB and AP-1, are members of the exercise-mediated signaling cascades, by which exercise modulates inflammation, aging, and apoptosis. We have evaluated the possibility that NO is an important mediator of the repair process, after unaccustomed exercise, by the activation of satellite cells in skeletal muscle. Interestingly, ROS could play a similar role in the brain, since neuronal precursor cells are very sensitive to oxygen/ROS availability. HIF is not just an important redox-sensitive oxygen sensor, but an important regulator of exercise-induced adaptation.
Moreover, regular exercise has an impact on the reversible structure of chromosomes, which can transfer adaptive changes to offspring, and redox signaling is involved in this process. Therefore, during compensated physical activity, the level of exercise-induced ROS production does not seriously jeopardize cellular or organ function, but brings about a wide range of health-promoting adaptations to the organism.
In summary, the present review intended to emphasize that (i) aerobic and redox signaling were important for evolution; (ii) regular exercise increases the efficiency of antioxidant and oxidative damage repair systems; (iii) moderate levels of oxidative damage, which can be caused by a single bout of exercise, can be crucial for membrane remodeling, protein turnover, and transcriptional regulation; (iv) sirtuins are NAD+-dependent modulators of a wide range of metabolic and redox processes, and are readily modified by both a single bout and regular exercise; (v) mitochondrial biogenesis is associated with redox signaling and is a key component of antioxidant defense; (vi) exercise-induced activation of stem and precursor cells in skeletal muscle and the brain can be redox dependent; (vii) HIF is an exercise-sensitive oxygen sensor and a redox regulator; and (viii) exercise-induced changes in epigenetics could be redox dependent.
Abbreviations Used
- 8-oxoG
8-oxo-7,8 dihydroguanine
- Akt
protein kinase B
- AMPK
AMP-activated protein kinase
- AP-1
activator protein-1
- ASK-1
apoptosis signal kinase
- BDNF
brain-derived neurotrophic factor
- CaMK
calmodulin-dependent protein kinase
- CnA
calcineurin
- CREB
cAMP response element-binding protein
- Cu,Zn-SOD
copper–zinc superoxide dismutase
- DNMT
DNA methyltransferases
- EC-SOD
extracellular superoxide dismutase
- EGR1
early growth response factor 1
- ERK
extracellular signal-regulated kinase
- ERRα
estrogen-related receptor-α
- Foxo
Forkhead box class O
- GPX
glutathione peroxidase
- GSH
glutathione
- GSSG
disulfide glutathione
- HAT
histone acetyl transferases
- HCR
high-running-capacity rats
- HIF-1α
hypoxia-inducible factor-1α
- HNE
4-hydroxy-2-nonenal
- HSF
heat-shock factor
- IL-6
interleukin-6
- IMF
intermyofibrillar
- LDH
lactate dehydrogenase
- MAPK
mitogen-activated protein kinase
- MDA
malondialdehyde
- Mn-SOD
manganese superoxide dismutase
- mSOFs
mitochondrial superoxide flashes
- MuRF1
muscle ring-finger protein 1
- NAC
N-acetylcysteine
- NAD
nicotinamide adenine dinucleotide
- NFAT
nuclear factor of activated T-cells
- NF-κB
nuclear factor-kappaB
- NO
nitric oxide
- NOS
nitric oxide synthase
- eNOS
endothelial nitric oxide synthase
- iNOS
inducible nitric oxide synthase
- nNOS
neuronal nitric oxide synthase
- NRBF
nuclear receptor-binding factor
- NRF1–2
respiratory factors 1 and 2
- OGG1
8-oxoguanine-DNA glycosylase
- PARP
poly(ADP-ribose) polymerase
- PGC-1α
peroxisome proliferator-activated receptor-γ coactivator 1α
- PI3-K
phosphatidylinositol 3-kinase
- PLA2
phospholipase A2
- PNPase
polynucleotide phosphorylase
- PPAR
peroxisome proliferator-activated receptor
- RCD
reactive carbonyl derivatives
- ROS
reactive oxygen species
- RyR1
ryanodine receptor 1
- Sir2
silent information regulator 2
- SS
subsarcolemmal
- STAT3
signal transducer and activator of transcription 3
- TFAM
mitochondrial transcription factor A
- TNF-α
tumor necrosis factor-α
- TRX
thioredoxin
- TXNip
thioredoxin-interacting protein
- UCP
uncoupling protein
- VEGF
vascular endothelial growth factor
- VO2max
maximal oxygen uptake
- XO
xanthine oxidase
Acknowledgments
The present work was supported by Hungarian grants from ETT 38388, TéT JAP13/02, OTKA (K75702), and TAMOP-4.2.2/B-10/1-2010-0013 awarded to Z. Radák. The authors acknowledge the assistance of Professor AW. Taylor in the preparation of this article.
Finally, we are grateful to the peer reviewers for their constructive comments, which helped us to improve this review.
References
- 1.Abid MR. Tsai JC. Spokes KC. Deshpande SS. Irani K. Aird WC. Vascular endothelial growth factor induces manganese-superoxide dismutase expression in endothelial cells by a Rac1-regulated NADPH oxidase-dependent mechanism. FASEB J. 2001;15:2548–2550. doi: 10.1096/fj.01-0338fje. [DOI] [PubMed] [Google Scholar]
- 2.Acemetacin (Emflex)—yet another NSAID. Drug Ther Bull. 1991;29:78. [PubMed] [Google Scholar]
- 3.Adams V. Spate U. Krankel N. Schulze PC. Linke A. Schuler G. Hambrecht R. Nuclear factor-kappa B activation in skeletal muscle of patients with chronic heart failure: correlation with the expression of inducible nitric oxide synthase. Eur J Cardiovasc Prev Rehabil. 2003;10:273–277. doi: 10.1097/00149831-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Adhihetty PJ. Taivassalo T. Haller RG. Walkinshaw DR. Hood DA. The effect of training on the expression of mitochondrial biogenesis- and apoptosis-related proteins in skeletal muscle of patients with mtDNA defects. Am J Physiol Endocrinol Metab. 2007;293:E672–E680. doi: 10.1152/ajpendo.00043.2007. [DOI] [PubMed] [Google Scholar]
- 5.Adibhatla RM. Hatcher JF. Phospholipase A(2), reactive oxygen species, and lipid peroxidation in CNS pathologies. BMB Rep. 2008;41:560–567. doi: 10.5483/bmbrep.2008.41.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal A. Gupta S. Sharma R. Oxidative stress and its implications in female infertility—a clinician's perspective. Reprod Biomed Online. 2005;11:641–650. doi: 10.1016/s1472-6483(10)61174-1. [DOI] [PubMed] [Google Scholar]
- 7.Aiguo W. Zhe Y. Gomez-Pinilla F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil Neural Repair. 2010;24:290–298. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alamdari N. Aversa Z. Castillero E. Gurav A. Petkova V. Tizio S. Hasselgren PO. Resveratrol prevents dexamethasone-induced expression of the muscle atrophy-related ubiquitin ligases atrogin-1 and MuRF1 in cultured myotubes through a SIRT1-dependent mechanism. Biochem Biophys Res Commun. 2012;417:528–533. doi: 10.1016/j.bbrc.2011.11.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alcendor RR. Gao S. Zhai P. Zablocki D. Holle E. Yu X. Tian B. Wagner T. Vatner SF. Sadoshima J. Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res. 2007;100:1512–1521. doi: 10.1161/01.RES.0000267723.65696.4a. [DOI] [PubMed] [Google Scholar]
- 10.Alzawahra WF. Talukder MA. Liu X. Samouilov A. Zweier JL. Heme proteins mediate the conversion of nitrite to nitric oxide in the vascular wall. Am J Physiol Heart Circ Physiol. 2008;295:H499–H508. doi: 10.1152/ajpheart.00374.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ameln H. Gustafsson T. Sundberg CJ. Okamoto K. Jansson E. Poellinger L. Makino Y. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19:1009–1011. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 12.Anavi S. Harmelin NB. Madar Z. Tirosh O. Oxidative stress impairs HIF1alpha activation: a novel mechanism for increased vulnerability of steatotic hepatocytes to hypoxic stress. Free Radic Biol Med. 2012;52:1531–1542. doi: 10.1016/j.freeradbiomed.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JE. A role for nitric oxide in muscle repair: nitric oxide-mediated activation of muscle satellite cells. Mol Biol Cell. 2000;11:1859–1874. doi: 10.1091/mbc.11.5.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersson DC. Betzenhauser MJ. Reiken S. Meli AC. Umanskaya A. Xie W. Shiomi T. Zalk R. Lacampagne A. Marks AR. Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab. 2011;14:196–207. doi: 10.1016/j.cmet.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andrade FH. Reid MB. Allen DG. Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibers from the mouse. J Physiol. 1998;509(Pt 2):565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arany Z. Foo SY. Ma Y. Ruas JL. Bommi-Reddy A. Girnun G. Cooper M. Laznik D. Chinsomboon J. Rangwala SM. Baek KH. Rosenzweig A. Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 17.Arvidsson A. Collin T. Kirik D. Kokaia Z. Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 18.Atalay M. Oksala NK. Laaksonen DE. Khanna S. Nakao C. Lappalainen J. Roy S. Hanninen O. Sen CK. Exercise training modulates heat shock protein response in diabetic rats. J Appl Physiol. 2004;97:605–611. doi: 10.1152/japplphysiol.01183.2003. [DOI] [PubMed] [Google Scholar]
- 19.Austin S. Klimcakova E. St-Pierre J. Impact of PGC-1alpha on the topology and rate of superoxide production by the mitochondrial electron transport chain. Free Radic Biol Med. 2011;51:2243–2248. doi: 10.1016/j.freeradbiomed.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 20.Baar K. Wende AR. Jones TE. Marison M. Nolte LA. Chen M. Kelly DP. Holloszy JO. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- 21.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 22.Bai P. Canto C. Brunyanszki A. Huber A. Szanto M. Cen Y. Yamamoto H. Houten SM. Kiss B. Oudart H. Gergely P. Menissier-de Murcia J. Schreiber V. Sauve AA. Auwerx J. PARP-2 regulates SIRT1 expression and whole-body energy expenditure. Cell Metab. 2011;13:450–460. doi: 10.1016/j.cmet.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barres R. Yan J. Egan B. Treebak JT. Rasmussen M. Fritz T. Caidahl K. Krook A. O'Gorman DJ. Zierath JR. Acute exercise remodels promoter methylation in human skeletal muscle. Cell Metab. 2012;15:405–411. doi: 10.1016/j.cmet.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Barzilai A. Yamamoto K. DNA damage responses to oxidative stress. DNA Repair. 2004;3:1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Beirowski B. Gustin J. Armour SM. Yamamoto H. Viader A. North BJ. Michan S. Baloh RH. Golden JP. Schmidt RE. Sinclair DA. Auwerx J. Milbrandt J. Sir-two-homolog 2 (Sirt2) modulates peripheral myelination through polarity protein Par-3/atypical protein kinase C (aPKC) signaling. Proc Natl Acad Sci U S A. 2011;108:E952–E961. doi: 10.1073/pnas.1104969108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bejma J. Ji LL. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J Appl Physiol. 1999;87:465–470. doi: 10.1152/jappl.1999.87.1.465. [DOI] [PubMed] [Google Scholar]
- 27.Bekinschtein P. Oomen CA. Saksida LM. Bussey TJ. Effects of environmental enrichment and voluntary exercise on neurogenesis, learning and memory, and pattern separation: BDNF as a critical variable? Semin Cell Dev Biol. 2011;22:536–542. doi: 10.1016/j.semcdb.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 28.BelAiba RS. Djordjevic T. Bonello S. Flugel D. Hess J. Kietzmann T. Gorlach A. Redox-sensitive regulation of the HIF pathway under non-hypoxic conditions in pulmonary artery smooth muscle cells. Biol Chem. 2004;385:249–257. doi: 10.1515/BC.2004.019. [DOI] [PubMed] [Google Scholar]
- 29.Bell EL. Guarente L. The SirT3 divining rod points to oxidative stress. Mol Cell. 2011;42:561–568. doi: 10.1016/j.molcel.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bengtsson J. Gustafsson T. Widegren U. Jansson E. Sundberg CJ. Mitochondrial transcription factor A and respiratory complex IV increase in response to exercise training in humans. Pflugers Arch. 2001;443:61–66. doi: 10.1007/s004240100628. [DOI] [PubMed] [Google Scholar]
- 31.Berry A. Greco A. Giorgio M. Pelicci PG. de Kloet R. Alleva E. Minghetti L. Cirulli F. Deletion of the lifespan determinant p66(Shc) improves performance in a spatial memory task, decreases levels of oxidative stress markers in the hippocampus and increases levels of the neurotrophin BDNF in adult mice. Exp Gerontol. 2008;43:200–208. doi: 10.1016/j.exger.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Bhakat KK. Mokkapati SK. Boldogh I. Hazra TK. Mitra S. Acetylation of human 8-oxoguanine-DNA glycosylase by p300 and its role in 8-oxoguanine repair in vivo. Mol Cell Biol. 2006;26:1654–1665. doi: 10.1128/MCB.26.5.1654-1665.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhusari SS. Dobosy JR. Fu V. Almassi N. Oberley T. Jarrard DF. Superoxide dismutase 1 knockdown induces oxidative stress and DNA methylation loss in the prostate. Epigenetics. 2010;5:402–409. doi: 10.4161/epi.5.5.11853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bindoli A. Fukuto JM. Forman HJ. Thiol chemistry in peroxidase catalysis and redox signaling. Antioxid Redox Signal. 2008;10:1549–1564. doi: 10.1089/ars.2008.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blair SN. Kampert JB. Kohl HW., 3rd Barlow CE. Macera CA. Paffenbarger RS., Jr. Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276:205–210. [PubMed] [Google Scholar]
- 36.Bloomer RJ. Fry AC. Falvo MJ. Moore CA. Protein carbonyls are acutely elevated following single set anaerobic exercise in resistance trained men. J Sci Med Sport. 2007;10:411–417. doi: 10.1016/j.jsams.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Boiteux S. Gellon L. Guibourt N. Repair of 8-oxoguanine in Saccharomyces cerevisiae: interplay of DNA repair and replication mechanisms. Free Radic Biol Med. 2002;32:1244–1253. doi: 10.1016/s0891-5849(02)00822-5. [DOI] [PubMed] [Google Scholar]
- 38.Boldogh I. Hajas G. Aguilera-Aguirre L. Hegde ML. Radak Z. Bacsi A. Sur S. Hazra TK. Mitra S. Activation of ras signaling pathway by 8-oxoguanine DNA glycosylase bound to its excision product, 8-oxoguanine. J Biol Chem. 2012;287:20769–20773. doi: 10.1074/jbc.C112.364620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolli R. Marban E. Molecular and cellular mechanisms of myocardial stunning. Physiol Rev. 1999;79:609–634. doi: 10.1152/physrev.1999.79.2.609. [DOI] [PubMed] [Google Scholar]
- 40.Booth FW. Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics. 2007;28:146–157. doi: 10.1152/physiolgenomics.00174.2006. [DOI] [PubMed] [Google Scholar]
- 41.Bori Z. Zhao Z. Koltai E. Fatouros IG. Jamurtas AZ. Douroudos II. Terzis G. Chatzinikolaou A. Sovatzidis A. Draganidis D. Boldogh I. Radak Z. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp Gerontol. 2012;47:417–424. doi: 10.1016/j.exger.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bota DA. Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nat Cell Biol. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- 43.Bota DA. Van Remmen H. Davies KJ. Modulation of Lon protease activity and aconitase turnover during aging and oxidative stress. FEBS Lett. 2002;532:103–106. doi: 10.1016/s0014-5793(02)03638-4. [DOI] [PubMed] [Google Scholar]
- 44.Boveris A. Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boveris A. Navarro A. Systemic and mitochondrial adaptive responses to moderate exercise in rodents. Free Radic Biol Med. 2008;44:224–229. doi: 10.1016/j.freeradbiomed.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 46.Bramble DM. Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 47.Brand MD. The sites and topology of mitochondrial superoxide production. Exp Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bravard A. Bonnard C. Durand A. Chauvin MA. Favier R. Vidal H. Rieusset J. Inhibition of xanthine oxidase reduces hyperglycemia-induced oxidative stress and improves mitochondrial alterations in skeletal muscle of diabetic mice. Am J Physiol Endocrinol Metab. 2011;300:E581–E591. doi: 10.1152/ajpendo.00455.2010. [DOI] [PubMed] [Google Scholar]
- 49.Bregegere F. Milner Y. Friguet B. The ubiquitin-proteasome system at the crossroads of stress-response and ageing pathways: a handle for skin care? Ageing Res Rev. 2006;5:60–90. doi: 10.1016/j.arr.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Brites F. Zago V. Verona J. Muzzio ML. Wikinski R. Schreier L. HDL capacity to inhibit LDL oxidation in well-trained triathletes. Life Sci. 2006;78:3074–3081. doi: 10.1016/j.lfs.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 51.Brixius K. Schoenberger S. Ladage D. Knigge H. Falkowski G. Hellmich M. Graf C. Latsch J. Montie GL. Prede GL. Bloch W. Long-term endurance exercise decreases antiangiogenic endostatin signalling in overweight men aged 50–60 years. Br J Sports Med. 2008;42:126–129. doi: 10.1136/bjsm.2007.035188. discussion 129. [DOI] [PubMed] [Google Scholar]
- 52.Brooks GA. Are arterial, muscle and working limb lactate exchange data obtained on men at altitude consistent with the hypothesis of an intracellular lactate shuttle? Adv Exp Med Biol. 1999;474:185–204. doi: 10.1007/978-1-4615-4711-2_16. [DOI] [PubMed] [Google Scholar]
- 53.Brooks SV. Vasilaki A. Larkin LM. McArdle A. Jackson MJ. Repeated bouts of aerobic exercise lead to reductions in skeletal muscle free radical generation and nuclear factor kappaB activation. J Physiol. 2008;586:3979–3990. doi: 10.1113/jphysiol.2008.155382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buford TW. Cooke MB. Shelmadine BD. Hudson GM. Redd L. Willoughby DS. Effects of eccentric treadmill exercise on inflammatory gene expression in human skeletal muscle. Appl Physiol Nutr Metab. 2009;34:745–753. doi: 10.1139/H09-067. [DOI] [PubMed] [Google Scholar]
- 55.Caldow MK. Steinberg GR. Cameron-Smith D. Impact of SOCS3 overexpression on human skeletal muscle development in vitro. Cytokine. 2011;55:104–109. doi: 10.1016/j.cyto.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 56.Carmel-Harel O. Storz G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and saccharomyces cerevisiae responses to oxidative stress. Annu Rev Microbiol. 2000;54:439–461. doi: 10.1146/annurev.micro.54.1.439. [DOI] [PubMed] [Google Scholar]
- 57.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 58.Cartoni R. Leger B. Hock MB. Praz M. Crettenand A. Pich S. Ziltener JL. Luthi F. Deriaz O. Zorzano A. Gobelet C. Kralli A. Russell AP. Mitofusins 1/2 and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567:349–358. doi: 10.1113/jphysiol.2005.092031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cassano M. Quattrocelli M. Crippa S. Perini I. Ronzoni F. Sampaolesi M. Cellular mechanisms and local progenitor activation to regulate skeletal muscle mass. J Muscle Res Cell Motil. 2009;30:243–253. doi: 10.1007/s10974-010-9204-y. [DOI] [PubMed] [Google Scholar]
- 60.Chambers MA. Moylan JS. Smith JD. Goodyear LJ. Reid MB. Stretch-stimulated glucose uptake in skeletal muscle is mediated by reactive oxygen species and p38 MAP-kinase. J Physiol. 2009;587:3363–3373. doi: 10.1113/jphysiol.2008.165639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandel NS. McClintock DS. Feliciano CE. Wood TM. Melendez JA. Rodriguez AM. Schumacker PT. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 62.Chau MD. Gao J. Yang Q. Wu Z. Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proc Natl Acad Sci U S A. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen HW. Rainey RN. Balatoni CE. Dawson DW. Troke JJ. Wasiak S. Hong JS. McBride HM. Koehler CM. Teitell MA. French SW. Mammalian polynucleotide phosphorylase is an intermembrane space RNase that maintains mitochondrial homeostasis. Mol Cell Biol. 2006;26:8475–8487. doi: 10.1128/MCB.01002-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Q. Lesnefsky EJ. Depletion of cardiolipin and cytochrome c during ischemia increases hydrogen peroxide production from the electron transport chain. Free Radic Biol Med. 2006;40:976–982. doi: 10.1016/j.freeradbiomed.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 65.Chen Q. Moghaddas S. Hoppel CL. Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008;294:C460–C466. doi: 10.1152/ajpcell.00211.2007. [DOI] [PubMed] [Google Scholar]
- 66.Chen R. Dioum EM. Hogg RT. Gerard RD. Garcia JA. Hypoxia increases sirtuin 1 expression in a hypoxia-inducible factor-dependent manner. J Biol Chem. 2011;286:13869–13878. doi: 10.1074/jbc.M110.175414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen SD. Yang DI. Lin TK. Shaw FZ. Liou CW. Chuang YC. Roles of Oxidative Stress, Apoptosis, PGC-1alpha and Mitochondrial Biogenesis in Cerebral Ischemia. Int J Mol Sci. 2011;12:7199–7215. doi: 10.3390/ijms12107199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chigurupati S. Son TG. Hyun DH. Lathia JD. Mughal MR. Savell J. Li SC. Nagaraju GP. Chan SL. Arumugam TV. Mattson MP. Lifelong running reduces oxidative stress and degenerative changes in the testes of mice. J Endocrinol. 2008;199:333–341. doi: 10.1677/JOE-08-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clayton AL. Hazzalin CA. Mahadevan LC. Enhanced histone acetylation and transcription: a dynamic perspective. Mol Cell. 2006;23:289–296. doi: 10.1016/j.molcel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 70.Cobley JN. McGlory C. Morton JP. Close GL. N-Acetylcysteine's attenuation of fatigue after repeated bouts of intermittent exercise: practical implications for tournament situations. Int J Sport Nutr Exerc Metab. 2011;21:451–461. doi: 10.1123/ijsnem.21.6.451. [DOI] [PubMed] [Google Scholar]
- 71.Collins A. Hill LE. Chandramohan Y. Whitcomb D. Droste SK. Reul JM. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009;4:e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Constantin-Teodosiu D. Constantin D. Stephens F. Laithwaite D. Greenhaff PL. The role of FOXO and PPAR transcription factors in diet-mediated inhibition of PDC activation and carbohydrate oxidation during exercise in humans and the role of pharmacological activation of PDC in overriding these changes. Diabetes. 2012;61:1017–1024. doi: 10.2337/db11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conti V. Corbi G. Russomanno G. Simeon V. Ferrara N. Filippelli W. Limongelli F. Canonico R. Grasso C. Stiuso P. Dicitore A. Filippelli A. Oxidative Stress effects on endothelial cells treated with different athletes' sera. Med Sci Sports Exerc. 2012;44:39–49. doi: 10.1249/MSS.0b013e318227f69c. [DOI] [PubMed] [Google Scholar]
- 74.Corn SD. Barstow TJ. Effects of oral N-acetylcysteine on fatigue, critical power, and W’ in exercising humans. Respir Physiol Neurobiol. 2011;178:261–268. doi: 10.1016/j.resp.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 75.Csiszar A. Podlutsky A. Podlutskaya N. Sonntag WE. Merlin SZ. Philipp EE. Doyle K. Davila A. Recchia FA. Ballabh P. Pinto JT. Ungvari Z. Testing the oxidative stress hypothesis of aging in primate fibroblasts: is there a correlation between species longevity and cellular ROS production? J Gerontol A Biol Sci Med Sci. 2012;67:841–852. doi: 10.1093/gerona/glr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuevas MJ. Almar M. Garcia-Glez JC. Garcia-Lopez D. De Paz JA. Alvear-Ordenes I. Gonzalez-Gallego J. Changes in oxidative stress markers and NF-kappaB activation induced by sprint exercise. Free Radic Res. 2005;39:431–439. doi: 10.1080/10715760500072149. [DOI] [PubMed] [Google Scholar]
- 77.Davies KJ. Quintanilha AT. Brooks GA. Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 78.Demer L. Tintut Y. The roles of lipid oxidation products and receptor activator of nuclear factor-kappaB signaling in atherosclerotic calcification. Circ Res. 2011;108:1482–1493. doi: 10.1161/CIRCRESAHA.110.234245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Demirel HA. Hamilton KL. Shanely RA. Tumer N. Koroly MJ. Powers SK. Age and attenuation of exercise-induced myocardial HSP72 accumulation. Am J Physiol Heart Circ Physiol. 2003;285:H1609–H1615. doi: 10.1152/ajpheart.00982.2002. [DOI] [PubMed] [Google Scholar]
- 80.Derbre F. Gomez-Cabrera MC. Nascimento AL. Sanchis-Gomar F. Martinez-Bello VE. Tresguerres JA. Fuentes T. Gratas-Delamarche A. Monsalve M. Vina J. Age associated low mitochondrial biogenesis may be explained by lack of response of PGC-1alpha to exercise training. Age. 2012;34:669–679. doi: 10.1007/s11357-011-9264-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ding YH. Young CN. Luan X. Li J. Rafols JA. Clark JC. McAllister JP., 2nd Ding Y. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005;109:237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- 82.Dioum EM. Chen R. Alexander MS. Zhang Q. Hogg RT. Gerard RD. Garcia JA. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324:1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 83.Dosek A. Ohno H. Acs Z. Taylor AW. Radak Z. High altitude and oxidative stress. Respir Physiol Neurobiol. 2007;158:128–131. doi: 10.1016/j.resp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 84.Droge W. Oxidative stress and ageing: is ageing a cysteine deficiency syndrome? Philos Trans R Soc Lond B Biol Sci. 2005;360:2355–2372. doi: 10.1098/rstb.2005.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Drummond MJ. Fry CS. Glynn EL. Timmerman KL. Dickinson JM. Walker DK. Gundermann DM. Volpi E. Rasmussen BB. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol. 2011;111:135–142. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du J. Zhou Y. Su X. Yu JJ. Khan S. Jiang H. Kim J. Woo J. Kim JH. Choi BH. He B. Chen W. Zhang S. Cerione RA. Auwerx J. Hao Q. Lin H. Sirt5 is a NAD-dependent protein lysine demalonylase and desuccinylase. Science. 2011;334:806–809. doi: 10.1126/science.1207861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Durham WJ. Li YP. Gerken E. Farid M. Arbogast S. Wolfe RR. Reid MB. Fatiguing exercise reduces DNA binding activity of NF-kappaB in skeletal muscle nuclei. J Appl Physiol. 2004;97:1740–1745. doi: 10.1152/japplphysiol.00088.2004. [DOI] [PubMed] [Google Scholar]
- 88.Erdelyi K. Bai P. Kovacs I. Szabo E. Mocsar G. Kakuk A. Szabo C. Gergely P. Virag L. Dual role of poly(ADP-ribose) glycohydrolase in the regulation of cell death in oxidatively stressed A549 cells. FASEB J. 2009;23:3553–3563. doi: 10.1096/fj.09-133264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Eriksson PS. Perfilieva E. Bjork-Eriksson T. Alborn AM. Nordborg C. Peterson DA. Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 90.Falkowski PG. Evolution. Tracing oxygen's imprint on earth's metabolic evolution. Science. 2006;311:1724–1725. doi: 10.1126/science.1125937. [DOI] [PubMed] [Google Scholar]
- 91.Ferrara N. Rinaldi B. Corbi G. Conti V. Stiuso P. Boccuti S. Rengo G. Rossi F. Filippelli A. Exercise training promotes SIRT1 activity in aged rats. Rejuvenation Res. 2008;11:139–150. doi: 10.1089/rej.2007.0576. [DOI] [PubMed] [Google Scholar]
- 92.Finnin MS. Donigian JR. Pavletich NP. Structure of the histone deacetylase SIRT2. Nat Struct Biol. 2001;8:621–625. doi: 10.1038/89668. [DOI] [PubMed] [Google Scholar]
- 93.Firuzi O. Miri R. Tavakkoli M. Saso L. Antioxidant therapy: current status and future prospects. Curr Med Chem. 2011;18:3871–3888. doi: 10.2174/092986711803414368. [DOI] [PubMed] [Google Scholar]
- 94.Fischer CP. Hiscock NJ. Penkowa M. Basu S. Vessby B. Kallner A. Sjoberg LB. Pedersen BK. Supplementation with vitamins C and E inhibits the release of interleukin-6 from contracting human skeletal muscle. J Physiol. 2004;558:633–645. doi: 10.1113/jphysiol.2004.066779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foulstone EJ. Meadows KA. Holly JM. Stewart CE. Insulin-like growth factors (IGF-I and IGF-II) inhibit C2 skeletal myoblast differentiation and enhance TNF alpha-induced apoptosis. J Cell Physiol. 2001;189:207–215. doi: 10.1002/jcp.10017. [DOI] [PubMed] [Google Scholar]
- 96.Freyssenet D. Energy sensing and regulation of gene expression in skeletal muscle. J Appl Physiol. 2007;102:529–540. doi: 10.1152/japplphysiol.01126.2005. [DOI] [PubMed] [Google Scholar]
- 97.Friedmann B. Kinscherf R. Borisch S. Richter G. Bartsch P. Billeter R. Effects of low-resistance/high-repetition strength training in hypoxia on muscle structure and gene expression. Pflugers Arch. 2003;446:742–751. doi: 10.1007/s00424-003-1133-9. [DOI] [PubMed] [Google Scholar]
- 98.Frojdo S. Durand C. Molin L. Carey AL. El-Osta A. Kingwell BA. Febbraio MA. Solari F. Vidal H. Pirola L. Phosphoinositide 3-kinase as a novel functional target for the regulation of the insulin signaling pathway by SIRT1. Mol Cell Endocrinol. 2011;335:166–176. doi: 10.1016/j.mce.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 99.Fulco M. Schiltz RL. Iezzi S. King MT. Zhao P. Kashiwaya Y. Hoffman E. Veech RL. Sartorelli V. Sir2 regulates skeletal muscle differentiation as a potential sensor of the redox state. Mol Cell. 2003;12:51–62. doi: 10.1016/s1097-2765(03)00226-0. [DOI] [PubMed] [Google Scholar]
- 100.Fulle S. Di Donna S. Puglielli C. Pietrangelo T. Beccafico S. Bellomo R. Protasi F. Fano G. Age-dependent imbalance of the antioxidative system in human satellite cells. Exp Gerontol. 2005;40:189–197. doi: 10.1016/j.exger.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 101.Garcia-Lopez D. Cuevas MJ. Almar M. Lima E. De Paz JA. Gonzalez-Gallego J. Effects of eccentric exercise on NF-kappaB activation in blood mononuclear cells. Med Sci Sports Exerc. 2007;39:653–664. doi: 10.1249/mss.0b013e31802f04f6. [DOI] [PubMed] [Google Scholar]
- 102.Garnier A. Fortin D. Zoll J. N'Guessan B. Mettauer B. Lampert E. Veksler V. Ventura-Clapier R. Coordinated changes in mitochondrial function and biogenesis in healthy and diseased human skeletal muscle. FASEB J. 2005;19:43–52. doi: 10.1096/fj.04-2173com. [DOI] [PubMed] [Google Scholar]
- 103.Gaudel C. Schwartz C. Giordano C. Abumrad NA. Grimaldi PA. Pharmacological activation of PPARbeta promotes rapid and calcineurin-dependent fiber remodeling and angiogenesis in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E297–E304. doi: 10.1152/ajpendo.00581.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gavin TP. Basal and exercise-induced regulation of skeletal muscle capillarization. Exerc Sport Sci Rev. 2009;37:86–92. doi: 10.1097/JES.0b013e31819c2e9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.George L. Lokhandwala MF. Asghar M. Exercise activates redox-sensitive transcription factors and restores renal D1 receptor function in old rats. Am J Physiol Renal Physiol. 2009;297:F1174–F1180. doi: 10.1152/ajprenal.00397.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ghosh S. Lertwattanarak R. Lefort N. Molina-Carrion M. Joya-Galeana J. Bowen BP. Garduno-Garcia Jde J. Abdul-Ghani M. Richardson A. DeFronzo RA. Mandarino L. Van Remmen H. Musi N. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–2060. doi: 10.2337/db11-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gielen S. Sandri M. Kozarez I. Kratzsch J. Teupser D. Thiery J. Erbs S. Mangner N. Lenk K. Hambrecht R. Schuler G. Adams V. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: the randomized leipzig exercise intervention in chronic heart failure and aging catabolism study. Circulation. 2012;125:2716–2727. doi: 10.1161/CIRCULATIONAHA.111.047381. [DOI] [PubMed] [Google Scholar]
- 108.Golbidi S. Laher I. Antioxidant therapy in human endocrine disorders. Med Sci Monit. 2010;16:RA9–RA24. [PubMed] [Google Scholar]
- 109.Goldfarb AH. Bloomer RJ. McKenzie MJ. Combined antioxidant treatment effects on blood oxidative stress after eccentric exercise. Med Sci Sports Exerc. 2005;37:234–239. doi: 10.1249/01.mss.0000152887.87785.be. [DOI] [PubMed] [Google Scholar]
- 110.Gomez-Cabrera MC. Borras C. Pallardo FV. Sastre J. Ji LL. Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol. 2005;567:113–120. doi: 10.1113/jphysiol.2004.080564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gomez-Cabrera MC. Domenech E. Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131. doi: 10.1016/j.freeradbiomed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 112.Gomez-Cabrera MC. Vina J. Ji LL. Interplay of oxidants and antioxidants during exercise: implications for muscle health. Physician Sportsmed. 2009;37:116–123. doi: 10.3810/psm.2009.12.1749. [DOI] [PubMed] [Google Scholar]
- 113.Gomez-Pinilla F. Zhuang Y. Feng J. Ying Z. Fan G. Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur J Neurosci. 2011;33:383–390. doi: 10.1111/j.1460-9568.2010.07508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Goto S. Nakamura A. Radak Z. Nakamoto H. Takahashi R. Yasuda K. Sakurai Y. Ishii N. Carbonylated proteins in aging and exercise: immunoblot approaches. Mech Ageing Dev. 1999;107:245–253. doi: 10.1016/s0047-6374(98)00133-x. [DOI] [PubMed] [Google Scholar]
- 115.Goto S. Takahashi R. Kumiyama AA. Radak Z. Hayashi T. Takenouchi M. Abe R. Implications of protein degradation in aging. Ann N Y Acad Sci. 2001;928:54–64. doi: 10.1111/j.1749-6632.2001.tb05635.x. [DOI] [PubMed] [Google Scholar]
- 116.Goto S. Takahashi R. Radak Z. Sharma R. Beneficial biochemical outcomes of late-onset dietary restriction in rodents. Ann N Y Acad Sci. 2007;1100:431–441. doi: 10.1196/annals.1395.048. [DOI] [PubMed] [Google Scholar]
- 117.Gregg SQ. Gutierrez V. Robinson AR. Woodell T. Nakao A. Ross MA. Michalopoulos GK. Rigatti L. Rothermel CE. Kamileri I. Garinis GA. Stolz DB. Niedernhofer LJ. A mouse model of accelerated liver aging caused by a defect in DNA repair. Hepatology. 2012;55:609–621. doi: 10.1002/hep.24713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grune T. Jung T. Merker K. Davies KJ. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes' during oxidative stress, aging, and disease. Int J Biochem Cell Biol. 2004;36:2519–2530. doi: 10.1016/j.biocel.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 119.Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb Symp Quant Biol. 2011;76:81–90. doi: 10.1101/sqb.2011.76.010629. [DOI] [PubMed] [Google Scholar]
- 120.Guerra B. Olmedillas H. Guadalupe-Grau A. Ponce-Gonzalez JG. Morales-Alamo D. Fuentes T. Chapinal E. Fernandez-Perez L. De Pablos-Velasco P. Santana A. Calbet JA. Is sprint exercise a leptin signaling mimetic in human skeletal muscle? J Appl Physiol. 2011;111:715–725. doi: 10.1152/japplphysiol.00805.2010. [DOI] [PubMed] [Google Scholar]
- 121.Gulati M. Pandey DK. Arnsdorf MF. Lauderdale DS. Thisted RA. Wicklund RH. Al-Hani AJ. Black HR. Exercise capacity and the risk of death in women: the St James women take heart project. Circulation. 2003;108:1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 122.Gurd BJ. Holloway GP. Yoshida Y. Bonen A. In mammalian muscle, SIRT3 is present in mitochondria and not in the nucleus; and SIRT3 is upregulated by chronic muscle contraction in an adenosine monophosphate-activated protein kinase-independent manner. Metabolism. 2012;61:733–741. doi: 10.1016/j.metabol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 123.Gurd BJ. Yoshida Y. McFarlan JT. Holloway GP. Moyes CD. Heigenhauser GJ. Spriet L. Bonen A. Nuclear SIRT1 activity, but not protein content, regulates mitochondrial biogenesis in rat and human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;301:R67–R75. doi: 10.1152/ajpregu.00417.2010. [DOI] [PubMed] [Google Scholar]
- 124.Gustafsson T. Knutsson A. Puntschart A. Kaijser L. Nordqvist AC. Sundberg CJ. Jansson E. Increased expression of vascular endothelial growth factor in human skeletal muscle in response to short-term one-legged exercise training. Pflugers Arch. 2002;444:752–759. doi: 10.1007/s00424-002-0845-6. [DOI] [PubMed] [Google Scholar]
- 125.Gustafsson T. Puntschart A. Kaijser L. Jansson E. Sundberg CJ. Exercise-induced expression of angiogenesis-related transcription and growth factors in human skeletal muscle. Am J Physiol. 1999;276:H679–H685. doi: 10.1152/ajpheart.1999.276.2.H679. [DOI] [PubMed] [Google Scholar]
- 126.Guttridge DC. Mayo MW. Madrid LV. Wang CY. Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- 127.Haddad F. Adams GR. Aging-sensitive cellular and molecular mechanisms associated with skeletal muscle hypertrophy. J Appl Physiol. 2006;100:1188–1203. doi: 10.1152/japplphysiol.01227.2005. [DOI] [PubMed] [Google Scholar]
- 128.Halestrap AP. Clarke SJ. Khaliulin I. The role of mitochondria in protection of the heart by preconditioning. Biochim Biophys Acta. 2007;1767:1007–1031. doi: 10.1016/j.bbabio.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Handschin C. Chin S. Li P. Liu F. Maratos-Flier E. Lebrasseur NK. Yan Z. Spiegelman BM. Skeletal muscle fiber-type switching, exercise intolerance, and myopathy in PGC-1alpha muscle-specific knock-out animals. J Biol Chem. 2007;282:30014–30021. doi: 10.1074/jbc.M704817200. [DOI] [PubMed] [Google Scholar]
- 130.Handschin C. Spiegelman BM. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454:463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 132.Hashimshony T. Zhang J. Keshet I. Bustin M. Cedar H. The role of DNA methylation in setting up chromatin structure during development. Nat Genet. 2003;34:187–192. doi: 10.1038/ng1158. [DOI] [PubMed] [Google Scholar]
- 133.Hayakawa H. Sekiguchi M. Human polynucleotide phosphorylase protein in response to oxidative stress. Biochemistry. 2006;45:6749–6755. doi: 10.1021/bi052585l. [DOI] [PubMed] [Google Scholar]
- 134.Hegedus C. Lakatos P. Olah G. Toth BI. Gergely S. Szabo E. Biro T. Szabo C. Virag L. Protein kinase C protects from DNA damage-induced necrotic cell death by inhibiting poly(ADP-ribose) polymerase-1. FEBS Lett. 2008;582:1672–1678. doi: 10.1016/j.febslet.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heon S. Bernier M. Servant N. Dostanic S. Wang C. Kirby GM. Alpert L. Chalifour LE. Dexrazoxane does not protect against doxorubicin-induced damage in young rats. Am J Physiol Heart Circ Physiol. 2003;285:H499–H506. doi: 10.1152/ajpheart.00047.2003. [DOI] [PubMed] [Google Scholar]
- 136.Hirasaka K. Lago CU. Kenaston MA. Fathe K. Nowinski SM. Nikawa T. Mills EM. Identification of a redox-modulatory interaction between uncoupling protein 3 and thioredoxin 2 in the mitochondrial intermembrane space. Antioxid Redox Signal. 2011;15:2645–2661. doi: 10.1089/ars.2011.3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hirschey MD. Shimazu T. Goetzman E. Jing E. Schwer B. Lombard DB. Grueter CA. Harris C. Biddinger S. Ilkayeva OR. Stevens RD. Li Y. Saha AK. Ruderman NB. Bain JR. Newgard CB. Farese RV., Jr. Alt FW. Kahn CR. Verdin E. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hitomi Y. Watanabe S. Kizaki T. Sakurai T. Takemasa T. Haga S. Ookawara T. Suzuki K. Ohno H. Acute exercise increases expression of extracellular superoxide dismutase in skeletal muscle and the aorta. Redox Rep. 2008;13:213–216. doi: 10.1179/135100008X308894. [DOI] [PubMed] [Google Scholar]
- 139.Ho RC. Hirshman MF. Li Y. Cai D. Farmer JR. Aschenbach WG. Witczak CA. Shoelson SE. Goodyear LJ. Regulation of IkappaB kinase and NF-kappaB in contracting adult rat skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C794–C801. doi: 10.1152/ajpcell.00632.2004. [DOI] [PubMed] [Google Scholar]
- 140.Hoier B. Olsen K. Nyberg M. Bangsbo J. Hellsten Y. Contraction-induced secretion of VEGF from skeletal muscle cells is mediated by adenosine. Am J Physiol Heart Circ Physiol. 2010;299:H857–H862. doi: 10.1152/ajpheart.00082.2010. [DOI] [PubMed] [Google Scholar]
- 141.Hokari F. Kawasaki E. Sakai A. Koshinaka K. Sakuma K. Kawanaka K. Muscle contractile activity regulates Sirt3 protein expression in rat skeletal muscles. J Appl Physiol. 2010;109:332–340. doi: 10.1152/japplphysiol.00335.2009. [DOI] [PubMed] [Google Scholar]
- 142.Hollander J. Fiebig R. Gore M. Ookawara T. Ohno H. Ji LL. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflugers Arch. 2001;442:426–434. doi: 10.1007/s004240100539. [DOI] [PubMed] [Google Scholar]
- 143.Holloszy JO. Regulation by exercise of skeletal muscle content of mitochondria and GLUT4. J Physiol Pharmacol. 2008;59(Suppl 7):5–18. [PubMed] [Google Scholar]
- 144.Holscher M. Schafer K. Krull S. Farhat K. Hesse A. Silter M. Lin Y. Pichler BJ. Thistlethwaite P. El-Armouche A. Maier LS. Katschinski DM. Zieseniss A. Unfavourable consequences of chronic cardiac HIF-1alpha stabilization. Cardiovasc Res. 2012;94:77–86. doi: 10.1093/cvr/cvs014. [DOI] [PubMed] [Google Scholar]
- 145.Hoppeler H. Vogt M. Weibel ER. Fluck M. Response of skeletal muscle mitochondria to hypoxia. Exp Physiol. 2003;88:109–119. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- 146.Hori O. Ichinoda F. Tamatani T. Yamaguchi A. Sato N. Ozawa K. Kitao Y. Miyazaki M. Harding HP. Ron D. Tohyama M. D MS. Ogawa S. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. J Cell Biol. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hsu CY. An G. Liu JS. Xue JJ. He YY. Lin TN. Expression of immediate early gene and growth factor mRNAs in a focal cerebral ischemia model in the rat. Stroke. 1993;24:I78–I81. [PubMed] [Google Scholar]
- 148.Hu J. Imam SZ. Hashiguchi K. de Souza-Pinto NC. Bohr VA. Phosphorylation of human oxoguanine DNA glycosylase (alpha-OGG1) modulates its function. Nucleic Acids Res. 2005;33:3271–3282. doi: 10.1093/nar/gki636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hunter RB. Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hunter RB. Stevenson E. Koncarevic A. Mitchell-Felton H. Essig DA. Kandarian SC. Activation of an alternative NF-kappaB pathway in skeletal muscle during disuse atrophy. FASEB J. 2002;16:529–538. doi: 10.1096/fj.01-0866com. [DOI] [PubMed] [Google Scholar]
- 151.Ido Y. Kilo C. Williamson JR. Cytosolic NADH/NAD+, free radicals, and vascular dysfunction in early diabetes mellitus. Diabetologia. 1997;40(Suppl 2):S115–S117. doi: 10.1007/s001250051422. [DOI] [PubMed] [Google Scholar]
- 152.Isaeva EV. Shkryl VM. Shirokova N. Mitochondrial redox state and Ca2+ sparks in permeabilized mammalian skeletal muscle. J Physiol. 2005;565:855–872. doi: 10.1113/jphysiol.2005.086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Iyer R. Lehnert BE. Svensson R. Factors underlying the cell growth-related bystander responses to alpha particles. Cancer Res. 2000;60:1290–1298. [PubMed] [Google Scholar]
- 154.Jaakkola P. Mole DR. Tian YM. Wilson MI. Gielbert J. Gaskell SJ. Kriegsheim A. Hebestreit HF. Mukherji M. Schofield CJ. Maxwell PH. Pugh CW. Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 155.Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal. 2011;15:2477–2486. doi: 10.1089/ars.2011.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jaiswal M. LaRusso NF. Nishioka N. Nakabeppu Y. Gores GJ. Human Ogg1, a protein involved in the repair of 8-oxoguanine, is inhibited by nitric oxide. Cancer Res. 2001;61:6388–6393. [PubMed] [Google Scholar]
- 157.Jeong W. Jung Y. Kim H. Park SJ. Rhee SG. Thioredoxin-related protein 14, a new member of the thioredoxin family with disulfide reductase activity: implication in the redox regulation of TNF-alpha signaling. Free Radic Biol Med. 2009;47:1294–1303. doi: 10.1016/j.freeradbiomed.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 158.Ji LL. Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med. 1999;222:283–292. doi: 10.1046/j.1525-1373.1999.d01-145.x. [DOI] [PubMed] [Google Scholar]
- 159.Ji LL. Exercise at old age: does it increase or alleviate oxidative stress? Ann N Y Acad Sci. 2001;928:236–247. doi: 10.1111/j.1749-6632.2001.tb05653.x. [DOI] [PubMed] [Google Scholar]
- 160.Ji LL. Modulation of skeletal muscle antioxidant defense by exercise: role of redox signaling. Free Radic Biol Med. 2008;44:142–152. doi: 10.1016/j.freeradbiomed.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 161.Ji LL. Gomez-Cabrera MC. Steinhafel N. Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J. 2004;18:1499–1506. doi: 10.1096/fj.04-1846com. [DOI] [PubMed] [Google Scholar]
- 162.Ji LL. Gomez-Cabrera MC. Vina J. Exercise and hormesis: activation of cellular antioxidant signaling pathway. Ann N Y Acad Sci. 2006;1067:425–435. doi: 10.1196/annals.1354.061. [DOI] [PubMed] [Google Scholar]
- 163.Ji LL. Gomez-Cabrera MC. Vina J. Role of nuclear factor kappaB and mitogen-activated protein kinase signaling in exercise-induced antioxidant enzyme adaptation. Appl Physiol Nutr Metab. 2007;32:930–935. doi: 10.1139/H07-098. [DOI] [PubMed] [Google Scholar]
- 164.Jiang M. Wang J. Fu J. Du L. Jeong H. West T. Xiang L. Peng Q. Hou Z. Cai H. Seredenina T. Arbez N. Zhu S. Sommers K. Qian J. Zhang J. Mori S. Yang XW. Tamashiro KL. Aja S. Moran TH. Luthi-Carter R. Martin B. Maudsley S. Mattson MP. Cichewicz RH. Ross CA. Holtzman DM. Krainc D. Duan W. Neuroprotective role of Sirt1 in mammalian models of Huntington's disease through activation of multiple Sirt1 targets. Nat Med. 2012;18:153–158. doi: 10.1038/nm.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jimenez-Jimenez R. Cuevas MJ. Almar M. Lima E. Garcia-Lopez D. De Paz JA. Gonzalez-Gallego J. Eccentric training impairs NF-kappaB activation and over-expression of inflammation-related genes induced by acute eccentric exercise in the elderly. Mech Ageing Dev. 2008;129:313–321. doi: 10.1016/j.mad.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 166.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–1879. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 167.Jones DP. Radical-free biology of oxidative stress. Am J Physiol Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jozsi AC. Dupont-Versteegden EE. Taylor-Jones JM. Evans WJ. Trappe TA. Campbell WW. Peterson CA. Aged human muscle demonstrates an altered gene expression profile consistent with an impaired response to exercise. Mech Ageing Dev. 2000;120:45–56. doi: 10.1016/s0047-6374(00)00178-0. [DOI] [PubMed] [Google Scholar]
- 169.Judge S. Jang YM. Smith A. Selman C. Phillips T. Speakman JR. Hagen T. Leeuwenburgh C. Exercise by lifelong voluntary wheel running reduces subsarcolemmal and interfibrillar mitochondrial hydrogen peroxide production in the heart. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1564–R1572. doi: 10.1152/ajpregu.00396.2005. [DOI] [PubMed] [Google Scholar]
- 170.Jung T. Catalgol B. Grune T. The proteasomal system. Mol Aspects Med. 2009;30:191–296. doi: 10.1016/j.mam.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 171.Kaidi A. Weinert BT. Choudhary C. Jackson SP. Human SIRT6 promotes DNA end resection through CtIP deacetylation. Science. 2010;329:1348–1353. doi: 10.1126/science.1192049. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 172.Kang C. O'Moore KM. Dickman JR. Ji LL. Exercise activation of muscle peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling is redox sensitive. Free Radic Biol Med. 2009;47:1394–1400. doi: 10.1016/j.freeradbiomed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 173.Katavetin P. Miyata T. Inagi R. Tanaka T. Sassa R. Ingelfinger JR. Fujita T. Nangaku M. High glucose blunts vascular endothelial growth factor response to hypoxia via the oxidative stress-regulated hypoxia-inducible factor/hypoxia-responsible element pathway. J Am Soc Nephrol. 2006;17:1405–1413. doi: 10.1681/ASN.2005090918. [DOI] [PubMed] [Google Scholar]
- 174.Katzmarzyk PT. Janssen I. The economic costs associated with physical inactivity and obesity in Canada: an update. Can J Appl Physiol. 2004;29:90–115. doi: 10.1139/h04-008. [DOI] [PubMed] [Google Scholar]
- 175.Kawahara TL. Michishita E. Adler AS. Damian M. Berber E. Lin M. McCord RA. Ongaigui KC. Boxer LD. Chang HY. Chua KF. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 2009;136:62–74. doi: 10.1016/j.cell.2008.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kelm M. Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990;66:1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- 177.Kemp M. Go YM. Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kerkweg U. Schmitz D. de Groot H. Screening for the formation of reactive oxygen species and of NO in muscle tissue and remote organs upon mechanical trauma to the mouse hind limb. Eur Surg Res. 2006;38:83–89. doi: 10.1159/000092609. [DOI] [PubMed] [Google Scholar]
- 179.Kietzmann T. Fandrey J. Acker H. Oxygen radicals as messengers in oxygen-dependent gene expression. News Physiol Sci. 2000;15:202–208. doi: 10.1152/physiologyonline.2000.15.4.202. [DOI] [PubMed] [Google Scholar]
- 180.Kim H. Lee SH. Kim SS. Yoo JH. Kim CJ. The influence of maternal treadmill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int J Dev Neurosci. 2007;25:243–249. doi: 10.1016/j.ijdevneu.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 181.Kim HY. Gladyshev VN. Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J. 2007;407:321–329. doi: 10.1042/BJ20070929. [DOI] [PubMed] [Google Scholar]
- 182.Kim SY. Jun TW. Lee YS. Na HK. Surh YJ. Song W. Effects of exercise on cyclooxygenase-2 expression and nuclear factor-kappaB DNA binding in human peripheral blood mononuclear cells. Ann N Y Acad Sci. 2009;1171:464–471. doi: 10.1111/j.1749-6632.2009.04915.x. [DOI] [PubMed] [Google Scholar]
- 183.Kingwell BA. Nitric oxide-mediated metabolic regulation during exercise: effects of training in health and cardiovascular disease. FASEB J. 2000;14:1685–1696. doi: 10.1096/fj.99-0896rev. [DOI] [PubMed] [Google Scholar]
- 184.Kinnunen S. Oksala N. Hyyppa S. Sen CK. Radak Z. Laaksonen DE. Szabo B. Jakus J. Atalay M. alpha-Lipoic acid modulates thiol antioxidant defenses and attenuates exercise-induced oxidative stress in standardbred trotters. Free Radic Res. 2009;43:697–705. doi: 10.1080/10715760903037673. [DOI] [PubMed] [Google Scholar]
- 185.Kinsey GR. Blum JL. Covington MD. Cummings BS. McHowat J. Schnellmann RG. Decreased iPLA2gamma expression induces lipid peroxidation and cell death and sensitizes cells to oxidant-induced apoptosis. J Lipid Res. 2008;49:1477–1487. doi: 10.1194/jlr.M800030-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Koch LG. Kemi OJ. Qi N. Leng SX. Bijma P. Gilligan LJ. Wilkinson JE. Wisloff H. Hoydal MA. Rolim N. Abadir PM. van Grevenhof EM. Smith GL. Burant CF. Ellingsen O. Britton SL. Wisloff U. Intrinsic aerobic capacity sets a divide for aging and longevity. Circ Res. 2011;109:1162–1172. doi: 10.1161/CIRCRESAHA.111.253807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Koltai E. Hart N. Taylor AW. Goto S. Ngo JK. Davies KJ. Radak Z. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am J Physiol Regul Integr Comp Physiol. 2012;303:R127–R134. doi: 10.1152/ajpregu.00337.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Koltai E. Szabo Z. Atalay M. Boldogh I. Naito H. Goto S. Nyakas C. Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Koltai E. Zhao Z. Lacza Z. Cselenyak A. Vacz G. Nyakas C. Boldogh I. Ichinoseki-Sekine N. Radak Z. Combined exercise and insulin-like growth factor-1 supplementation induces neurogenesis in old rats, but do not attenuate age-associated DNA damage. Rejuvenation Res. 2011;14:585–596. doi: 10.1089/rej.2011.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Koyama T. Kume S. Koya D. Araki S. Isshiki K. Chin-Kanasaki M. Sugimoto T. Haneda M. Sugaya T. Kashiwagi A. Maegawa H. Uzu T. SIRT3 attenuates palmitate-induced ROS production and inflammation in proximal tubular cells. Free Radic Biol Med. 2011;51:1258–1267. doi: 10.1016/j.freeradbiomed.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 191.Kuhn H. Pliquett F. Wunderlich S. Schewe T. Krause W. Reticulocyte lipoxygenase changes the passive electrical properties of bovine heart submitochondrial particles. Biochim Biophys Acta. 1983;735:283–290. doi: 10.1016/0005-2736(83)90303-6. [DOI] [PubMed] [Google Scholar]
- 192.Lappalainen Z. Lappalainen J. Laaksonen DE. Oksala KJ. Khanna S. Sen CK. Atalay M. Acute exercise and thioredoxin-1 in rat brain, and alpha-lipoic acid and thioredoxin-interacting protein response, in diabetes. Int J Sport Nutr Exerc Metab. 2010;20:206–215. doi: 10.1123/ijsnem.20.3.206. [DOI] [PubMed] [Google Scholar]
- 193.Lappalainen Z. Lappalainen J. Oksala NK. Laaksonen DE. Khanna S. Sen CK. Atalay M. Diabetes impairs exercise training-associated thioredoxin response and glutathione status in rat brain. J Appl Physiol. 2009;106:461–467. doi: 10.1152/japplphysiol.91252.2008. [DOI] [PubMed] [Google Scholar]
- 194.Le Moine CM. Morash AJ. McClelland GB. Changes in HIF-1alpha protein, pyruvate dehydrogenase phosphorylation, and activity with exercise in acute and chronic hypoxia. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1098–R1104. doi: 10.1152/ajpregu.00070.2011. [DOI] [PubMed] [Google Scholar]
- 195.Lee B. Cao R. Choi YS. Cho HY. Rhee AD. Hah CK. Hoyt KR. Obrietan K. The CREB/CRE transcriptional pathway: protection against oxidative stress-mediated neuronal cell death. J Neurochem. 2009;108:1251–1265. doi: 10.1111/j.1471-4159.2008.05864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Lee CK. Klopp RG. Weindruch R. Prolla TA. Gene expression profile of aging and its retardation by caloric restriction. Science. 1999;285:1390–1393. doi: 10.1126/science.285.5432.1390. [DOI] [PubMed] [Google Scholar]
- 197.Lee H. Chang H. Park JY. Kim SY. Choi KM. Song W. Exercise training improves basal blood glucose metabolism with no changes of cytosolic inhibitor B kinase or c-Jun N-terminal kinase activation in skeletal muscle of Otsuka Long-Evans Tokushima fatty rats. Exp Physiol. 2011;96:689–698. doi: 10.1113/expphysiol.2011.057737. [DOI] [PubMed] [Google Scholar]
- 198.Lee HH. Kim H. Lee JW. Kim YS. Yang HY. Chang HK. Lee TH. Shin MC. Lee MH. Shin MS. Park S. Baek S. Kim CJ. Maternal swimming during pregnancy enhances short-term memory and neurogenesis in the hippocampus of rat pups. Brain Dev. 2006;28:147–154. doi: 10.1016/j.braindev.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 199.Lee HJ. Chung K. Lee H. Lee K. Lim JH. Song J. Downregulation of mitochondrial lon protease impairs mitochondrial function and causes hepatic insulin resistance in human liver SK-HEP-1 cells. Diabetologia. 2011;54:1437–1446. doi: 10.1007/s00125-011-2074-z. [DOI] [PubMed] [Google Scholar]
- 200.Lee JW. Bae SH. Jeong JW. Kim SH. Kim KW. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med. 2004;36:1–12. doi: 10.1038/emm.2004.1. [DOI] [PubMed] [Google Scholar]
- 201.Leeuwenburgh C. Ji LL. Glutathione depletion in rested and exercised mice: biochemical consequence and adaptation. Arch Biochem Biophys. 1995;316:941–949. doi: 10.1006/abbi.1995.1125. [DOI] [PubMed] [Google Scholar]
- 202.Leick L. Lyngby SS. Wojtaszewski JF. Pilegaard H. PGC-1alpha is required for training-induced prevention of age-associated decline in mitochondrial enzymes in mouse skeletal muscle. Exp Gerontol. 2010;45:336–342. doi: 10.1016/j.exger.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 203.Lew H. Pyke S. Quintanilha A. Changes in the glutathione status of plasma, liver and muscle following exhaustive exercise in rats. FEBS Lett. 1985;185:262–266. doi: 10.1016/0014-5793(85)80919-4. [DOI] [PubMed] [Google Scholar]
- 204.Li M. Fukagawa NK. Age-related changes in redox signaling and VSMC function. Antioxid Redox Signal. 2010;12:641–655. doi: 10.1089/ars.2009.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li Y. Xu S. Giles A. Nakamura K. Lee JW. Hou X. Donmez G. Li J. Luo Z. Walsh K. Guarente L. Zang M. Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J. 2011;25:1664–1679. doi: 10.1096/fj.10-173492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Li Y. Xu W. McBurney MW. Longo VD. SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 2008;8:38–48. doi: 10.1016/j.cmet.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Li YP. Schwartz RJ. TNF-alpha regulates early differentiation of C2C12 myoblasts in an autocrine fashion. FASEB J. 2001;15:1413–1415. doi: 10.1096/fj.00-0632fje. [DOI] [PubMed] [Google Scholar]
- 208.Liao P. Zhou J. Ji LL. Zhang Y. Eccentric contraction induces inflammatory responses in rat skeletal muscle: role of tumor necrosis factor-alpha. Am J Physiol Regul Integr Comp Physiol. 2010;298:R599–R607. doi: 10.1152/ajpregu.00480.2009. [DOI] [PubMed] [Google Scholar]
- 209.Lillig CH. Holmgren A. Thioredoxin and related molecules—from biology to health and disease. Antioxid Redox Signal. 2007;9:25–47. doi: 10.1089/ars.2007.9.25. [DOI] [PubMed] [Google Scholar]
- 210.Lim JH. Lee YM. Chun YS. Chen J. Kim JE. Park JW. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1alpha. Mol Cell. 2010;38:864–878. doi: 10.1016/j.molcel.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 211.Limoli CL. Rola R. Giedzinski E. Mantha S. Huang TT. Fike JR. Cell-density-dependent regulation of neural precursor cell function. Proc Natl Acad Sci U S A. 2004;101:16052–16057. doi: 10.1073/pnas.0407065101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lin J. Wu H. Tarr PT. Zhang CY. Wu Z. Boss O. Michael LF. Puigserver P. Isotani E. Olson EN. Lowell BB. Bassel-Duby R. Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibers. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 213.Lira VA. Benton CR. Yan Z. Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299:E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Locke M. Noble EG. Tanguay RM. Feild MR. Ianuzzo SE. Ianuzzo CD. Activation of heat-shock transcription factor in rat heart after heat shock and exercise. Am J Physiol. 1995;268:C1387–C1394. doi: 10.1152/ajpcell.1995.268.6.C1387. [DOI] [PubMed] [Google Scholar]
- 215.Loft S. Poulsen HE. Cancer risk and oxidative DNA damage in man. J Mol Med. 1996;74:297–312. doi: 10.1007/BF00207507. [DOI] [PubMed] [Google Scholar]
- 216.Lombard DB. Alt FW. Cheng HL. Bunkenborg J. Streeper RS. Mostoslavsky R. Kim J. Yancopoulos G. Valenzuela D. Murphy A. Yang Y. Chen Y. Hirschey MD. Bronson RT. Haigis M. Guarente LP. Farese RV., Jr. Weissman S. Verdin E. Schwer B. Mammalian Sir2 homolog SIRT3 regulates global mitochondrial lysine acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Lopez-Torres M. Perez-Campo R. Rojas C. Cadenas S. Barja G. Maximum life span in vertebrates: relationship with liver antioxidant enzymes, glutathione system, ascorbate, urate, sensitivity to peroxidation, true malondialdehyde, in vivo H2O2, and basal and maximum aerobic capacity. Mech Ageing Dev. 1993;70:177–199. doi: 10.1016/0047-6374(93)90047-u. [DOI] [PubMed] [Google Scholar]
- 218.Lundberg JO. Weitzberg E. Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 219.Lundby C. Calbet JA. Robach P. The response of human skeletal muscle tissue to hypoxia. Cell Mol Life Sci. 2009;66:3615–3623. doi: 10.1007/s00018-009-0146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Lundby C. Gassmann M. Pilegaard H. Regular endurance training reduces the exercise induced HIF-1alpha and HIF-2alpha mRNA expression in human skeletal muscle in normoxic conditions. Eur J Appl Physiol. 2006;96:363–369. doi: 10.1007/s00421-005-0085-5. [DOI] [PubMed] [Google Scholar]
- 221.Mabley JG. Pacher P. Deb A. Wallace R. Elder RH. Szabo C. Potential role for 8-oxoguanine DNA glycosylase in regulating inflammation. FASEB J. 2005;19:290–292. doi: 10.1096/fj.04-2278fje. [DOI] [PubMed] [Google Scholar]
- 222.Mabley JG. Wallace R. Pacher P. Murphy K. Szabo C. Inhibition of poly(adenosine diphosphate-ribose) polymerase by the active form of vitamin D. Int J Mol Med. 2007;19:947–952. [PMC free article] [PubMed] [Google Scholar]
- 223.Mahoney DJ. Parise G. Melov S. Safdar A. Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19:1498–1500. doi: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- 224.Mao Z. Hine C. Tian X. Van Meter M. Au M. Vaidya A. Seluanov A. Gorbunova V. SIRT6 promotes DNA repair under stress by activating PARP1. Science. 2011;332:1443–1446. doi: 10.1126/science.1202723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Marfe G. Tafani M. Pucci B. Di Stefano C. Indelicato M. Andreoli A. Russo MA. Sinibaldi-Salimei P. Manzi V. The effect of marathon on mRNA expression of anti-apoptotic and pro-apoptotic proteins and sirtuins family in male recreational long-distance runners. BMC Physiol. 2010;10:7. doi: 10.1186/1472-6793-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 226.Martin GM. Austad SN. Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nat Genet. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- 227.Marton O. Koltai E. Nyakas C. Bakonyi T. Zenteno-Savin T. Kumagai S. Goto S. Radak Z. Aging and exercise affect the level of protein acetylation and SIRT1 activity in cerebellum of male rats. Biogerontology. 2010;11:679–686. doi: 10.1007/s10522-010-9279-2. [DOI] [PubMed] [Google Scholar]
- 228.Marumoto M. Suzuki S. Hosono A. Arakawa K. Shibata K. Fuku M. Goto C. Tokudome Y. Hoshino H. Imaeda N. Kobayashi M. Yodoi J. Tokudome S. Changes in thioredoxin concentrations: an observation in an ultra-marathon race. Environ Health Prev Med. 2010;15:129–134. doi: 10.1007/s12199-009-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McKenna MJ. Medved I. Goodman CA. Brown MJ. Bjorksten AR. Murphy KT. Petersen AC. Sostaric S. Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Merker K. Sitte N. Grune T. Hydrogen peroxide-mediated protein oxidation in young and old human MRC-5 fibroblasts. Arch Biochem Biophys. 2000;375:50–54. doi: 10.1006/abbi.1999.1657. [DOI] [PubMed] [Google Scholar]
- 231.Michan S. Li Y. Chou MM. Parrella E. Ge H. Long JM. Allard JS. Lewis K. Miller M. Xu W. Mervis RF. Chen J. Guerin KI. Smith LE. McBurney MW. Sinclair DA. Baudry M. de Cabo R. Longo VD. SIRT1 is essential for normal cognitive function and synaptic plasticity. J Neurosci. 2010;30:9695–9707. doi: 10.1523/JNEUROSCI.0027-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Michishita E. Park JY. Burneskis JM. Barrett JC. Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005;16:4623–4635. doi: 10.1091/mbc.E05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Milner J. Allison SJ. SIRT1, p53 and mitotic chromosomes. Cell Cycle. 2011;10:3049. doi: 10.4161/cc.10.18.16994. [DOI] [PubMed] [Google Scholar]
- 234.Minet E. Michel G. Mottet D. Raes M. Michiels C. Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation. Free Radic Biol Med. 2001;31:847–855. doi: 10.1016/s0891-5849(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 235.Mintz S. Robin ED. Redox state of free nicotinamide-adenine nucleotides in the cytoplasm and mitochondria of alveolar macrophages. J Clin Invest. 1971;50:1181–1186. doi: 10.1172/JCI106595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Miranda S. Foncea R. Guerrero J. Leighton F. Oxidative stress and upregulation of mitochondrial biogenesis genes in mitochondrial DNA-depleted HeLa cells. Biochem Biophys Res Commun. 1999;258:44–49. doi: 10.1006/bbrc.1999.0580. [DOI] [PubMed] [Google Scholar]
- 237.Mofarrahi M. Hussain SN. Expression and functional roles of angiopoietin-2 in skeletal muscles. PloS One. 2011;6:e22882. doi: 10.1371/journal.pone.0022882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mohanraj P. Merola AJ. Wright VP. Clanton TL. Antioxidants protect rat diaphragmatic muscle function under hypoxic conditions. J Appl Physiol. 1998;84:1960–1966. doi: 10.1152/jappl.1998.84.6.1960. [DOI] [PubMed] [Google Scholar]
- 239.Mostoslavsky R. Chua KF. Lombard DB. Pang WW. Fischer MR. Gellon L. Liu P. Mostoslavsky G. Franco S. Murphy MM. Mills KD. Patel P. Hsu JT. Hong AL. Ford E. Cheng HL. Kennedy C. Nunez N. Bronson R. Frendewey D. Auerbach W. Valenzuela D. Karow M. Hottiger MO. Hursting S. Barrett JC. Guarente L. Mulligan R. Demple B. Yancopoulos GD. Alt FW. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124:315–329. doi: 10.1016/j.cell.2005.11.044. [DOI] [PubMed] [Google Scholar]
- 240.Mounier R. Pedersen BK. Plomgaard P. Muscle-specific expression of hypoxia-inducible factor in human skeletal muscle. Exp Physiol. 2010;95:899–907. doi: 10.1113/expphysiol.2010.052928. [DOI] [PubMed] [Google Scholar]
- 241.Mounier R. Pialoux V. Roels B. Thomas C. Millet G. Mercier J. Coudert J. Fellmann N. Clottes E. Effect of intermittent hypoxic training on HIF gene expression in human skeletal muscle and leukocytes. Eur J Appl Physiol. 2009;105:515–524. doi: 10.1007/s00421-008-0928-y. [DOI] [PubMed] [Google Scholar]
- 242.Mozzetta C. Consalvi S. Saccone V. Forcales SV. Puri PL. Palacios D. Selective control of Pax7 expression by TNF-activated p38alpha/polycomb repressive complex 2 (PRC2) signaling during muscle satellite cell differentiation. Cell Cycle. 2011;10:191–198. doi: 10.4161/cc.10.2.14441. [DOI] [PubMed] [Google Scholar]
- 243.Mozzetta C. Minetti G. Puri PL. Regenerative pharmacology in the treatment of genetic diseases: the paradigm of muscular dystrophy. Int J Biochem Cell Biol. 2009;41:701–710. doi: 10.1016/j.biocel.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Muller FL. Liu Y. Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J Biol Chem. 2004;279:49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 245.Murakami T. Shimomura Y. Yoshimura A. Sokabe M. Fujitsuka N. Induction of nuclear respiratory factor-1 expression by an acute bout of exercise in rat muscle. Biochim Biophys Acta. 1998;1381:113–122. doi: 10.1016/s0304-4165(98)00018-x. [DOI] [PubMed] [Google Scholar]
- 246.Murton AJ. Constantin D. Greenhaff PL. The involvement of the ubiquitin proteasome system in human skeletal muscle remodelling and atrophy. Biochim Biophys Acta. 2008;1782:730–743. doi: 10.1016/j.bbadis.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 247.Myers J. Prakash M. Froelicher V. Do D. Partington S. Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 248.Nakabeppu Y. Sakumi K. Sakamoto K. Tsuchimoto D. Tsuzuki T. Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol Chem. 2006;387:373–379. doi: 10.1515/BC.2006.050. [DOI] [PubMed] [Google Scholar]
- 249.Nakamoto H. Kaneko T. Tahara S. Hayashi E. Naito H. Radak Z. Goto S. Regular exercise reduces 8-oxodG in the nuclear and mitochondrial DNA and modulates the DNA repair activity in the liver of old rats. Exp Gerontol. 2007;42:287–295. doi: 10.1016/j.exger.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 250.Narasimhan P. Liu J. Song YS. Massengale JL. Chan PH. VEGF Stimulates the ERK 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke. 2009;40:1467–1473. doi: 10.1161/STROKEAHA.108.534644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Nasrin N. Wu X. Fortier E. Feng Y. Bare OC. Chen S. Ren X. Wu Z. Streeper RS. Bordone L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285:31995–32002. doi: 10.1074/jbc.M110.124164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Nemoto S. Fergusson MM. Finkel T. SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha} J Biol Chem. 2005;280:16456–16460. doi: 10.1074/jbc.M501485200. [DOI] [PubMed] [Google Scholar]
- 253.Ngo JK. Davies KJ. Mitochondrial Lon protease is a human stress protein. Free Radic Biol Med. 2009;46:1042–1048. doi: 10.1016/j.freeradbiomed.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Ngo JK. Pomatto LC. Bota DA. Koop AL. Davies KJ. Impairment of lon-induced protection against the accumulation of oxidized proteins in senescent wi-38 fibroblasts. J Gerontol A Biol Sci Med Sci. 2011;66:1178–1185. doi: 10.1093/gerona/glr145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Niess AM. Simon P. Response and adaptation of skeletal muscle to exercise—the role of reactive oxygen species. Fron Biosci. 2007;12:4826–4838. doi: 10.2741/2431. [DOI] [PubMed] [Google Scholar]
- 256.Nigam S. Schewe T. Phospholipase A(2)s and lipid peroxidation. Biochim Biophys Acta. 2000;1488:167–181. doi: 10.1016/s1388-1981(00)00119-0. [DOI] [PubMed] [Google Scholar]
- 257.Nikolaidis MG. Jamurtas AZ. Paschalis V. Fatouros IG. Koutedakis Y. Kouretas D. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: magnitude and time-course considerations. Sports Med. 2008;38:579–606. doi: 10.2165/00007256-200838070-00005. [DOI] [PubMed] [Google Scholar]
- 258.Nikolaidis MG. Kyparos A. Hadziioannou M. Panou N. Samaras L. Jamurtas AZ. Kouretas D. Acute exercise markedly increases blood oxidative stress in boys and girls. Appl Physiol Nutr Metab. 2007;32:197–205. doi: 10.1139/h06-097. [DOI] [PubMed] [Google Scholar]
- 259.Nikolaidis MG. Kyparos A. Vrabas IS. F-isoprostane formation, measurement and interpretation: the role of exercise. Prog Lipid Res. 2011;50:89–103. doi: 10.1016/j.plipres.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 260.Nishimura S. Involvement of mammalian OGG1(MMH) in excision of the 8-hydroxyguanine residue in DNA. Free Radic Biol Med. 2002;32:813–821. doi: 10.1016/s0891-5849(02)00778-5. [DOI] [PubMed] [Google Scholar]
- 261.Nishiyama A. Matsui M. Iwata S. Hirota K. Masutani H. Nakamura H. Takagi Y. Sono H. Gon Y. Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J Biol Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 262.Noble M. Mayer-Proschel M. Proschel C. Redox regulation of precursor cell function: insights and paradoxes. Antioxid Redox Signal. 2005;7:1456–1467. doi: 10.1089/ars.2005.7.1456. [DOI] [PubMed] [Google Scholar]
- 263.Norrbom J. Wallman SE. Gustafsson T. Rundqvist H. Jansson E. Sundberg CJ. Training response of mitochondrial transcription factors in human skeletal muscle. Acta Physiologica. 2010;198:71–79. doi: 10.1111/j.1748-1716.2009.02030.x. [DOI] [PubMed] [Google Scholar]
- 264.Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. EMBO J. 2005;24:1311–1317. doi: 10.1038/sj.emboj.7600599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.O'Hagan KA. Cocchiglia S. Zhdanov AV. Tambuwala MM. Cummins EP. Monfared M. Agbor TA. Garvey JF. Papkovsky DB. Taylor CT. Allan BB. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A. 2009;106:2188–2193. doi: 10.1073/pnas.0808801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ochi E. Nakazato K. Ishii N. Muscular hypertrophy and changes in cytokine production after eccentric training in the rat skeletal muscle. J Strength Cond Res. 2011;25:2283–2292. doi: 10.1519/JSC.0b013e3181f1592e. [DOI] [PubMed] [Google Scholar]
- 267.Ogura M. Nakamura Y. Tanaka D. Zhuang X. Fujita Y. Obara A. Hamasaki A. Hosokawa M. Inagaki N. Overexpression of SIRT5 confirms its involvement in deacetylation and activation of carbamoyl phosphate synthetase 1. Biochem Biophys Res Commun. 2010;393:73–78. doi: 10.1016/j.bbrc.2010.01.081. [DOI] [PubMed] [Google Scholar]
- 268.Oh-Ishi S. Kizaki T. Yamashita H. Nagata N. Suzuki K. Taniguchi N. Ohno H. Alterations of superoxide dismutase iso-enzyme activity, content, and mRNA expression with aging in rat skeletal muscle. Mech Ageing Dev. 1995;84:65–76. doi: 10.1016/0047-6374(95)01637-f. [DOI] [PubMed] [Google Scholar]
- 269.Ookawara T. Haga S. Ha S. Oh-Ishi S. Toshinai K. Kizaki T. Ji LL. Suzuki K. Ohno H. Effects of endurance training on three superoxide dismutase isoenzymes in human plasma. Free Radic Res. 2003;37:713–719. doi: 10.1080/1071576031000102132. [DOI] [PubMed] [Google Scholar]
- 270.Ookawara T. Suzuk K. Haga S. Ha S. Chung KS. Toshinai K. Hamaoka T. Katsumura T. Takemasa T. Mizuno M. Hitomi Y. Kizaki T. Suzuki K. Ohno H. Transcription regulation of gene expression in human skeletal muscle in response to endurance training. Res Commun Mol Pathol Pharmacol. 2002;111:41–54. [PubMed] [Google Scholar]
- 271.Oppikofer M. Kueng S. Martino F. Soeroes S. Hancock SM. Chin JW. Fischle W. Gasser SM. A dual role of H4K16 acetylation in the establishment of yeast silent chromatin. EMBO J. 2011;30:2610–2621. doi: 10.1038/emboj.2011.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ortenblad N. Young JF. Oksbjerg N. Nielsen JH. Lambert IH. Reactive oxygen species are important mediators of taurine release from skeletal muscle cells. Am J Physiol Cell Physiol. 2003;284:C1362–C1373. doi: 10.1152/ajpcell.00287.2002. [DOI] [PubMed] [Google Scholar]
- 273.Pagliarini DJ. Calvo SE. Chang B. Sheth SA. Vafai SB. Ong SE. Walford GA. Sugiana C. Boneh A. Chen WK. Hill DE. Vidal M. Evans JG. Thorburn DR. Carr SA. Mootha VK. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Palacios OM. Carmona JJ. Michan S. Chen KY. Manabe Y. Ward JL., 3rd Goodyear LJ. Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging. 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Pardo PS. Mohamed JS. Lopez MA. Boriek AM. Induction of Sirt1 by mechanical stretch of skeletal muscle through the early response factor EGR1 triggers an antioxidative response. J Biol Chem. 2011;286:2559–2566. doi: 10.1074/jbc.M110.149153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Park JY. Wang PY. Matsumoto T. Sung HJ. Ma W. Choi JW. Anderson SA. Leary SC. Balaban RS. Kang JG. Hwang PM. p53 improves aerobic exercise capacity and augments skeletal muscle mitochondrial DNA content. Circ Res. 2009;105:705–712. doi: 10.1161/CIRCRESAHA.109.205310. 11 p following 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Patwari P. Higgins LJ. Chutkow WA. Yoshioka J. Lee RT. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J Biol Chem. 2006;281:21884–21891. doi: 10.1074/jbc.M600427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Pedersen BK. Muscular IL-6 and its role as an energy sensor. Med Sci Sports Exerc. 2012;44:392–396. doi: 10.1249/MSS.0b013e31822f94ac. [DOI] [PubMed] [Google Scholar]
- 279.Pedersen BK. Steensberg A. Keller P. Keller C. Fischer C. Hiscock N. van Hall G. Plomgaard P. Febbraio MA. Muscle-derived interleukin-6: lipolytic, anti-inflammatory and immune regulatory effects. Pflugers Arch. 2003;446:9–16. doi: 10.1007/s00424-002-0981-z. [DOI] [PubMed] [Google Scholar]
- 280.Peng L. Yuan Z. Ling H. Fukasawa K. Robertson K. Olashaw N. Koomen J. Chen J. Lane WS. Seto E. SIRT1 deacetylates the DNA methyltransferase 1 (DNMT1) protein and alters its activities. Mol Cell Biol. 2011;31:4720–4734. doi: 10.1128/MCB.06147-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Perez-de-Arce K. Foncea R. Leighton F. Reactive oxygen species mediates homocysteine-induced mitochondrial biogenesis in human endothelial cells: modulation by antioxidants. Biochem Biophys Res Commun. 2005;338:1103–1109. doi: 10.1016/j.bbrc.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 282.Perillo B. Ombra MN. Bertoni A. Cuozzo C. Sacchetti S. Sasso A. Chiariotti L. Malorni A. Abbondanza C. Avvedimento EV. DNA oxidation as triggered by H3K9me2 demethylation drives estrogen-induced gene expression. Science. 2008;319:202–206. doi: 10.1126/science.1147674. [DOI] [PubMed] [Google Scholar]
- 283.Petersen AC. McKenna MJ. Medved I. Murphy KT. Brown MJ. Della Gatta P. Cameron-Smith D. Infusion with the antioxidant N-acetylcysteine attenuates early adaptive responses to exercise in human skeletal muscle. Acta Physiol. 2012;204:382–392. doi: 10.1111/j.1748-1716.2011.02344.x. [DOI] [PubMed] [Google Scholar]
- 284.Philp A. Chen A. Lan D. Meyer GA. Murphy AN. Knapp AE. Olfert IM. McCurdy CE. Marcotte GR. Hogan MC. Baar K. Schenk S. Sirtuin 1 (SIRT1) deacetylase activity is not required for mitochondrial biogenesis or peroxisome proliferator-activated receptor-gamma coactivator-1alpha (PGC-1alpha) deacetylation following endurance exercise. J Biol Chem. 2011;286:30561–30570. doi: 10.1074/jbc.M111.261685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Pialoux V. Mounier R. Brown AD. Steinback CD. Rawling JM. Poulin MJ. Relationship between oxidative stress and HIF-1 alpha mRNA during sustained hypoxia in humans. Free Radic Biol Med. 2009;46:321–326. doi: 10.1016/j.freeradbiomed.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 286.Pilegaard H. Saltin B. Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Pisconti A. Brunelli S. Di Padova M. De Palma C. Deponti D. Baesso S. Sartorelli V. Cossu G. Clementi E. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol. 2006;172:233–244. doi: 10.1083/jcb.200507083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 288.Pogozelski AR. Geng T. Li P. Yin X. Lira VA. Zhang M. Chi JT. Yan Z. p38gamma mitogen-activated protein kinase is a key regulator in skeletal muscle metabolic adaptation in mice. PloS One. 2009;4:e7934. doi: 10.1371/journal.pone.0007934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Pogribny I. Raiche J. Slovack M. Kovalchuk O. Dose-dependence, sex- and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem Biophys Res Commun. 2004;320:1253–1261. doi: 10.1016/j.bbrc.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 290.Poli G. Schaur RJ. Siems WG. Leonarduzzi G. 4-hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med Res Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- 291.Ponugoti B. Kim DH. Xiao Z. Smith Z. Miao J. Zang M. Wu SY. Chiang CM. Veenstra TD. Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Porcu M. Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005;26:94–103. doi: 10.1016/j.tips.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 293.Powell SR. Samuel SM. Wang P. Divald A. Thirunavukkarasu M. Koneru S. Wang X. Maulik N. Upregulation of myocardial 11S-activated proteasome in experimental hyperglycemia. J Mol Cell Cardiol. 2008;44:618–621. doi: 10.1016/j.yjmcc.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 294.Powers SK. Duarte J. Kavazis AN. Talbert EE. Reactive oxygen species are signalling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Powers SK. Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Powers SK. Ji LL. Leeuwenburgh C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: a brief review. Med Sci Sports Exerc. 1999;31:987–997. doi: 10.1097/00005768-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 297.Powers SK. Nelson WB. Hudson MB. Exercise-induced oxidative stress in humans: cause and consequences. Free Radic Biol Med. 2011;51:942–950. doi: 10.1016/j.freeradbiomed.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 298.Powers SK. Talbert EE. Adhihetty PJ. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J Physiol. 2011;589:2129–2138. doi: 10.1113/jphysiol.2010.201327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Proia P. Bianco A. Schiera G. Saladino P. Pomara F. Petrucci M. Traina M. Palma A. The effects of a 3-week training on basal biomarkers in professional soccer players during the preseason preparation period. J Sports Med Phys Fitness. 2012;52:102–106. [PubMed] [Google Scholar]
- 300.Qi Z. He J. Su Y. He Q. Liu J. Yu L. Al-Attas O. Hussain T. Ding S. Ji L. Qian M. Physical exercise regulates p53 activity targeting SCO2 and increases mitochondrial COX biogenesis in cardiac muscle with age. PloS One. 2011;6:e21140. doi: 10.1371/journal.pone.0021140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Qi Z. He J. Zhang Y. Shao Y. Ding S. Exercise training attenuates oxidative stress and decreases p53 protein content in skeletal muscle of type 2 diabetic Goto-Kakizaki rats. Free Radic Biol Med. 2011;50:794–800. doi: 10.1016/j.freeradbiomed.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 302.Qiu X. Brown K. Hirschey MD. Verdin E. Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 303.Radak Z. Apor P. Pucsok J. Berkes I. Ogonovszky H. Pavlik G. Nakamoto H. Goto S. Marathon running alters the DNA base excision repair in human skeletal muscle. Life Sci. 2003;72:1627–1633. doi: 10.1016/s0024-3205(02)02476-1. [DOI] [PubMed] [Google Scholar]
- 304.Radak Z. Asano K. Inoue M. Kizaki T. Oh-Ishi S. Suzuki K. Taniguchi N. Ohno H. Superoxide dismutase derivative reduces oxidative damage in skeletal muscle of rats during exhaustive exercise. J Appl Physiol. 1995;79:129–135. doi: 10.1152/jappl.1995.79.1.129. [DOI] [PubMed] [Google Scholar]
- 305.Radak Z. Asano K. Inoue M. Kizaki T. Oh-Ishi S. Suzuki K. Taniguchi N. Ohno H. Superoxide dismutase derivative prevents oxidative damage in liver and kidney of rats induced by exhausting exercise. Eur J Appl Physiol Occup Physiol. 1996;72:189–194. doi: 10.1007/BF00838637. [DOI] [PubMed] [Google Scholar]
- 306.Radak Z. Atalay M. Jakus J. Boldogh I. Davies K. Goto S. Exercise improves import of 8-oxoguanine DNA glycosylase into the mitochondrial matrix of skeletal muscle and enhances the relative activity. Free Radic Biol Med. 2009;46:238–243. doi: 10.1016/j.freeradbiomed.2008.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Radak Z. Boldogh I. 8-Oxo-7,8-dihydroguanine: links to gene expression, aging, and defense against oxidative stress. Free Radic Biol Med. 2010;49:587–596. doi: 10.1016/j.freeradbiomed.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Radak Z. Bori Z. Koltai E. Fatouros IG. Jamurtas AZ. Douroudos II. Terzis G. Nikolaidis MG. Chatzinikolaou A. Sovatzidis A. Kumagai S. Naito H. Boldogh I. Age-dependent changes in 8-oxoguanine-DNA glycosylase activity are modulated by adaptive responses to physical exercise in human skeletal muscle. Free Radic Biol Med. 2011;51:417–423. doi: 10.1016/j.freeradbiomed.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Radak Z. Chung HY. Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 310.Radak Z. Chung HY. Koltai E. Taylor AW. Goto S. Exercise, oxidative stress and hormesis. Ageing Res Rev. 2008;7:34–42. doi: 10.1016/j.arr.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 311.Radak Z. Chung HY. Naito H. Takahashi R. Jung KJ. Kim HJ. Goto S. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18:749–750. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- 312.Radak Z. Hart N. Sarga L. Koltai E. Atalay M. Ohno H. Boldogh I. Exercise plays a preventive role against Alzheimer's disease. J Alzheimers Dis. 2010;20:777–783. doi: 10.3233/JAD-2010-091531. [DOI] [PubMed] [Google Scholar]
- 313.Radak Z. Kumagai S. Nakamoto H. Goto S. 8-Oxoguanosine and uracil repair of nuclear and mitochondrial DNA in red and white skeletal muscle of exercise-trained old rats. J Appl Physiol. 2007;102:1696–1701. doi: 10.1152/japplphysiol.01051.2006. [DOI] [PubMed] [Google Scholar]
- 314.Radak Z. Naito H. Kaneko T. Tahara S. Nakamoto H. Takahashi R. Cardozo-Pelaez F. Goto S. Exercise training decreases DNA damage and increases DNA repair and resistance against oxidative stress of proteins in aged rat skeletal muscle. Pflugers Arch. 2002;445:273–278. doi: 10.1007/s00424-002-0918-6. [DOI] [PubMed] [Google Scholar]
- 315.Radak Z. Naito H. Taylor AW. Goto S. Nitric oxide: is it the cause of muscle soreness? Nitric Oxide. 2012;26:89–94. doi: 10.1016/j.niox.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 316.Radak Z. Nakamura A. Nakamoto H. Asano K. Ohno H. Goto S. A period of anaerobic exercise increases the accumulation of reactive carbonyl derivatives in the lungs of rats. Pflugers Arch. 1998;435:439–441. doi: 10.1007/s004240050537. [DOI] [PubMed] [Google Scholar]
- 317.Radak Z. Sasvari M. Nyakas C. Pucsok J. Nakamoto H. Goto S. Exercise preconditioning against hydrogen peroxide-induced oxidative damage in proteins of rat myocardium. Arch Biochem Biophys. 2000;376:248–251. doi: 10.1006/abbi.2000.1719. [DOI] [PubMed] [Google Scholar]
- 318.Radak Z. Taylor AW. Ohno H. Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- 319.Radak Z. Zhao Z. Goto S. Koltai E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol Aspects Med. 2011;32:305–315. doi: 10.1016/j.mam.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 320.Raichlen DA. Armstrong H. Lieberman DE. Calcaneus length determines running economy: implications for endurance running performance in modern humans and Neandertals. J Hum Evol. 2011;60:299–308. doi: 10.1016/j.jhevol.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 321.Rantanen J. Hurme T. Lukka R. Heino J. Kalimo H. Satellite cell proliferation and the expression of myogenin and desmin in regenerating skeletal muscle: evidence for two different populations of satellite cells. Lab Invest. 1995;72:341–347. [PubMed] [Google Scholar]
- 322.Rapoport SM. Schewe T. The maturational breakdown of mitochondria in reticulocytes. Biochim Biophys Acta. 1986;864:471–495. doi: 10.1016/0304-4157(86)90006-7. [DOI] [PubMed] [Google Scholar]
- 323.Raymond J. Segre D. The effect of oxygen on biochemical networks and the evolution of complex life. Science. 2006;311:1764–1767. doi: 10.1126/science.1118439. [DOI] [PubMed] [Google Scholar]
- 324.Reid MB. Khawli FA. Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087. doi: 10.1152/jappl.1993.75.3.1081. [DOI] [PubMed] [Google Scholar]
- 325.Reid MB. Shoji T. Moody MR. Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol. 1992;73:1805–1809. doi: 10.1152/jappl.1992.73.5.1805. [DOI] [PubMed] [Google Scholar]
- 326.Reid MB. Stokic DS. Koch SM. Khawli FA. Leis AA. N-acetylcysteine inhibits muscle fatigue in humans. J Clin Invest. 1994;94:2468–2474. doi: 10.1172/JCI117615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Renault V. Thornell LE. Butler-Browne G. Mouly V. Human skeletal muscle satellite cells: aging, oxidative stress and the mitotic clock. Exp Gerontol. 2002;37:1229–1236. doi: 10.1016/s0531-5565(02)00129-8. [DOI] [PubMed] [Google Scholar]
- 328.Richardson RS. Noyszewski EA. Kendrick KF. Leigh JS. Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest. 1995;96:1916–1926. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Ristow M. Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51:327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 330.Ristow M. Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 331.Ristow M. Zarse K. Oberbach A. Kloting N. Birringer M. Kiehntopf M. Stumvoll M. Kahn CR. Bluher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A. 2009;106:8665–8670. doi: 10.1073/pnas.0903485106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Roberts CK. Ng C. Hama S. Eliseo AJ. Barnard RJ. Effect of a short-term diet and exercise intervention on inflammatory/anti-inflammatory properties of HDL in overweight/obese men with cardiovascular risk factors. J Appl Physiol. 2006;101:1727–1732. doi: 10.1152/japplphysiol.00345.2006. [DOI] [PubMed] [Google Scholar]
- 333.Rogers CJ. Colbert LH. Greiner JW. Perkins SN. Hursting SD. Physical activity and cancer prevention: pathways and targets for intervention. Sports Med. 2008;38:271–296. doi: 10.2165/00007256-200838040-00002. [DOI] [PubMed] [Google Scholar]
- 334.Rojas Vega S. Knicker A. Hollmann W. Bloch W. Struder HK. Effect of resistance exercise on serum levels of growth factors in humans. Horm Metab Res. 2010;42:982–986. doi: 10.1055/s-0030-1267950. [DOI] [PubMed] [Google Scholar]
- 335.Rollo CD. Growth negatively impacts the life span of mammals. Evol Dev. 2002;4:55–61. doi: 10.1046/j.1525-142x.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 336.Rossi CA. Pozzobon M. Ditadi A. Archacka K. Gastaldello A. Sanna M. Franzin C. Malerba A. Milan G. Cananzi M. Schiaffino S. Campanella M. Vettor R. De Coppi P. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinsic heterogeneity. PloS One. 2010;5:e8523. doi: 10.1371/journal.pone.0008523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Rottgers K. Zufall N. Guiard B. Voos W. The ClpB homolog Hsp78 is required for the efficient degradation of proteins in the mitochondrial matrix. J Biol Chem. 2002;277:45829–45837. doi: 10.1074/jbc.M207152200. [DOI] [PubMed] [Google Scholar]
- 338.Roy S. Khanna S. Sen CK. Redox regulation of the VEGF signaling path and tissue vascularization: hydrogen peroxide, the common link between physical exercise and cutaneous wound healing. Free Radic Biol Med. 2008;44:180–192. doi: 10.1016/j.freeradbiomed.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 339.Russell AP. Feilchenfeldt J. Schreiber S. Praz M. Crettenand A. Gobelet C. Meier CA. Bell DR. Kralli A. Giacobino JP. Deriaz O. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- 340.Russell AP. Hesselink MK. Lo SK. Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J. 2005;19:986–988. doi: 10.1096/fj.04-3168fje. [DOI] [PubMed] [Google Scholar]
- 341.Ruxton GD. Wilkinson DM. Thermoregulation and endurance running in extinct hominins: Wheeler's models revisited. J Hum Evol. 2011;61:169–175. doi: 10.1016/j.jhevol.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 342.Saborido A. Naudi A. Portero-Otin M. Pamplona R. Megias A. Stanozolol treatment decreases the mitochondrial ROS generation and oxidative stress induced by acute exercise in rat skeletal muscle. J Appl Physiol. 2011;110:661–669. doi: 10.1152/japplphysiol.00790.2010. [DOI] [PubMed] [Google Scholar]
- 343.Sahlin K. Shabalina IG. Mattsson CM. Bakkman L. Fernstrom M. Rozhdestvenskaya Z. Enqvist JK. Nedergaard J. Ekblom B. Tonkonogi M. Ultraendurance exercise increases the production of reactive oxygen species in isolated mitochondria from human skeletal muscle. J Appl Physiol. 2010;108:780–787. doi: 10.1152/japplphysiol.00966.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Salminen A. Kauppinen A. Suuronen T. Kaarniranta K. SIRT1 longevity factor suppresses NF-kappaB -driven immune responses: regulation of aging via NF-kappaB acetylation? BioEssays. 2008;30:939–942. doi: 10.1002/bies.20799. [DOI] [PubMed] [Google Scholar]
- 345.Sanchez-Lopez E. Lopez AF. Esteban V. Yague S. Egido J. Ruiz-Ortega M. Alvarez-Arroyo MV. Angiotensin II regulates vascular endothelial growth factor via hypoxia-inducible factor-1alpha induction and redox mechanisms in the kidney. Antioxid Redox Signal. 2005;7:1275–1284. doi: 10.1089/ars.2005.7.1275. [DOI] [PubMed] [Google Scholar]
- 346.Sandstrom ME. Zhang SJ. Bruton J. Silva JP. Reid MB. Westerblad H. Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol. 2006;575:251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Sarkar D. Lebedeva IV. Emdad L. Kang DC. Baldwin AS., Jr. Fisher PB. Human polynucleotide phosphorylase (hPNPaseold-35): a potential link between aging and inflammation. Cancer Res. 2004;64:7473–7478. doi: 10.1158/0008-5472.CAN-04-1772. [DOI] [PubMed] [Google Scholar]
- 348.Sato Y. Nanri H. Ohta M. Kasai H. Ikeda M. Increase of human MTH1 and decrease of 8-hydroxydeoxyguanosine in leukocyte DNA by acute and chronic exercise in healthy male subjects. Biochem Biophys Res Commun. 2003;305:333–338. doi: 10.1016/s0006-291x(03)00774-5. [DOI] [PubMed] [Google Scholar]
- 349.Schaloske RH. Dennis EA. The phospholipase A2 superfamily and its group numbering system. Biochim Biophys Acta. 2006;1761:1246–1259. doi: 10.1016/j.bbalip.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 350.Scheele C. Nielsen S. Pedersen BK. ROS and myokines promote muscle adaptation to exercise. Trends Endocrinol Metab. 2009;20:95–99. doi: 10.1016/j.tem.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 351.Scher MB. Vaquero A. Reinberg D. SirT3 is a nuclear NAD+-dependent histone deacetylase that translocates to the mitochondria upon cellular stress. Genes Dev. 2007;21:920–928. doi: 10.1101/gad.1527307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Schug TT. Li X. Sirtuin 1 in lipid metabolism and obesity. Ann Med. 2011;43:198–211. doi: 10.3109/07853890.2010.547211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Schwarzer M. Britton SL. Koch LG. Wisloff U. Doenst T. Low intrinsic aerobic exercise capacity and systemic insulin resistance are not associated with changes in myocardial substrate oxidation or insulin sensitivity. Basic Res Cardiol. 2010;105:357–364. doi: 10.1007/s00395-010-0087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Sedding DG. FoxO transcription factors in oxidative stress response and ageing—a new fork on the way to longevity? Biol Chem. 2008;389:279–283. doi: 10.1515/BC.2008.033. [DOI] [PubMed] [Google Scholar]
- 355.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Ann Rev Cell Dev Biol. 1999;15:551–578. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 356.Sen CK. Oxidants and antioxidants in exercise. J Appl Physiol. 1995;79:675–686. doi: 10.1152/jappl.1995.79.3.675. [DOI] [PubMed] [Google Scholar]
- 357.Sen CK. Oxygen toxicity and antioxidants: state of the art. Indian J Physiol Pharmacol. 1995;39:177–196. [PubMed] [Google Scholar]
- 358.Sen CK. Cellular thiols and redox-regulated signal transduction. Curr Top Cell Regul. 2000;36:1–30. doi: 10.1016/s0070-2137(01)80001-7. [DOI] [PubMed] [Google Scholar]
- 359.Sen CK. Antioxidants in exercise nutrition. Sports Med. 2001;31:891–908. doi: 10.2165/00007256-200131130-00001. [DOI] [PubMed] [Google Scholar]
- 360.Sen CK. Atalay M. Hanninen O. Exercise-induced oxidative stress: glutathione supplementation and deficiency. J Appl Physiol. 1994;77:2177–2187. doi: 10.1152/jappl.1994.77.5.2177. [DOI] [PubMed] [Google Scholar]
- 361.Sen CK. Packer L. Thiol homeostasis and supplements in physical exercise. Am J Clin Nutr. 2000;72:653S–669S. doi: 10.1093/ajcn/72.2.653S. [DOI] [PubMed] [Google Scholar]
- 362.Sen CK. Rankinen T. Vaisanen S. Rauramaa R. Oxidative stress after human exercise: effect of N-acetylcysteine supplementation. J Appl Physiol. 1994;76:2570–2577. doi: 10.1152/jappl.1994.76.6.2570. [DOI] [PubMed] [Google Scholar]
- 363.Sharma R. Nakamura A. Takahashi R. Nakamoto H. Goto S. Carbonyl modification in rat liver histones: decrease with age and increase by dietary restriction. Free Radic Biol Med. 2006;40:1179–1184. doi: 10.1016/j.freeradbiomed.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 364.Shimazu T. Hirschey MD. Hua L. Dittenhafer-Reed KE. Schwer B. Lombard DB. Li Y. Bunkenborg J. Alt FW. Denu JM. Jacobson MP. Verdin E. SIRT3 deacetylates mitochondrial 3-hydroxy-3-methylglutaryl CoA synthase 2 and regulates ketone body production. Cell Metab. 2010;12:654–661. doi: 10.1016/j.cmet.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Shirato K. Kizaki T. Sakurai T. Ogasawara JE. Ishibashi Y. Iijima T. Okada C. Noguchi I. Imaizumi K. Taniguchi N. Ohno H. Hypoxia-inducible factor-1alpha suppresses the expression of macrophage scavenger receptor 1. Pflugers Arch. 2009;459:93–103. doi: 10.1007/s00424-009-0702-y. [DOI] [PubMed] [Google Scholar]
- 366.Shringarpure R. Grune T. Mehlhase J. Davies KJ. Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. J Biol Chem. 2003;278:311–318. doi: 10.1074/jbc.M206279200. [DOI] [PubMed] [Google Scholar]
- 367.Siamilis S. Jakus J. Nyakas C. Costa A. Mihalik B. Falus A. Radak Z. The effect of exercise and oxidant-antioxidant intervention on the levels of neurotrophins and free radicals in spinal cord of rats. Spinal Cord. 2009;47:453–457. doi: 10.1038/sc.2008.125. [DOI] [PubMed] [Google Scholar]
- 368.Silver I. Erecinska M. Oxygen and ion concentrations in normoxic and hypoxic brain cells. Adv Exp Med Biol. 1998;454:7–16. doi: 10.1007/978-1-4615-4863-8_2. [DOI] [PubMed] [Google Scholar]
- 369.Sitte N. Merker K. von Zglinicki T. Grune T. Protein oxidation and degradation during proliferative senescence of human MRC-5 fibroblasts. Free Radic Biol Med. 2000;28:701–708. doi: 10.1016/s0891-5849(99)00279-8. [DOI] [PubMed] [Google Scholar]
- 370.Spencer T. Posterino GS. Sequential effects of GSNO and H2O2 on the Ca2+ sensitivity of the contractile apparatus of fast- and slow-twitch skeletal muscle fibers from the rat. Am J Physiol Cell Physiol. 2009;296:C1015–C1023. doi: 10.1152/ajpcell.00251.2008. [DOI] [PubMed] [Google Scholar]
- 371.Spiegelman BM. Heinrich R. Biological control through regulated transcriptional coactivators. Cell. 2004;119:157–167. doi: 10.1016/j.cell.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 372.Sriram S. Subramanian S. Sathiakumar D. Venkatesh R. Salerno MS. McFarlane CD. Kambadur R. Sharma M. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-kappaB. Aging Cell. 2011;10:931–948. doi: 10.1111/j.1474-9726.2011.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.St-Pierre J. Buckingham JA. Roebuck SJ. Brand MD. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J Biol Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 374.St-Pierre J. Drori S. Uldry M. Silvaggi JM. Rhee J. Jager S. Handschin C. Zheng K. Lin J. Yang W. Simon DK. Bachoo R. Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 375.Stadtman ER. Protein oxidation in aging and age-related diseases. Ann N Y Acad Sci. 2001;928:22–38. doi: 10.1111/j.1749-6632.2001.tb05632.x. [DOI] [PubMed] [Google Scholar]
- 376.Stadtman ER. Protein oxidation and aging. Free Radic Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- 377.Steenken S. Jovanovic SV. How easily oxidizable is DNA? One-electron reduction potentials of adenosine and guanosine radicals in aqueous solution. J Am Chem Soc. 1997;119:617–618. [Google Scholar]
- 378.Stevnsner T. Thorslund T. de Souza-Pinto NC. Bohr VA. Mitochondrial repair of 8-oxoguanine and changes with aging. Exp Gerontol. 2002;37:1189–1196. doi: 10.1016/s0531-5565(02)00142-0. [DOI] [PubMed] [Google Scholar]
- 379.Stroka DM. Burkhardt T. Desbaillets I. Wenger RH. Neil DA. Bauer C. Gassmann M. Candinas D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. FASEB J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- 380.Suliman HB. Welty-Wolf KE. Carraway M. Tatro L. Piantadosi CA. Lipopolysaccharide induces oxidative cardiac mitochondrial damage and biogenesis. Cardiovasc Res. 2004;64:279–288. doi: 10.1016/j.cardiores.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 381.Sumida S. Nakamura H. Yodoi J. Thioredoxin induction of peripheral blood mononuclear cells in mice in response to a single bout of swimming exercise. Gen Physiol Biophys. 2004;23:241–249. [PubMed] [Google Scholar]
- 382.Sundaresan NR. Pillai VB. Wolfgeher D. Samant S. Vasudevan P. Parekh V. Raghuraman H. Cunningham JM. Gupta M. Gupta MP. The deacetylase SIRT1 promotes membrane localization and activation of Akt and PDK1 during tumorigenesis and cardiac hypertrophy. Sci Signal. 2011;4:ra46. doi: 10.1126/scisignal.2001465. [DOI] [PubMed] [Google Scholar]
- 383.Suwa M. Nakano H. Radak Z. Kumagai S. Short-term adenosine monophosphate-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside treatment increases the sirtuin 1 protein expression in skeletal muscle. Metabolism. 2011;60:394–403. doi: 10.1016/j.metabol.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 384.Sverko V. Balog T. Sobocanec S. Gavella M. Marotti T. Age-associated alteration of lipid peroxidation and superoxide dismutase activity in CBA and AKR mice. Exp Gerontol. 2002;37:1031–1039. doi: 10.1016/s0531-5565(02)00083-9. [DOI] [PubMed] [Google Scholar]
- 385.Syu GD. Chen HI. Jen CJ. Severe exercise and exercise training exert opposite effects on human neutrophil apoptosis via altering the redox status. PloS One. 2011;6:e24385. doi: 10.1371/journal.pone.0024385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Szabo C. Poly(ADP-ribose) polymerase activation by reactive nitrogen species—relevance for the pathogenesis of inflammation. Nitric Oxide. 2006;14:169–179. doi: 10.1016/j.niox.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 387.Szabo C. Biser A. Benko R. Bottinger E. Susztak K. Poly(ADP-ribose) polymerase inhibitors ameliorate nephropathy of type 2 diabetic Leprdb/db mice. Diabetes. 2006;55:3004–3012. doi: 10.2337/db06-0147. [DOI] [PubMed] [Google Scholar]
- 388.Szczesny B. Bhakat KK. Mitra S. Boldogh I. Age-dependent modulation of DNA repair enzymes by covalent modification and subcellular distribution. Mech Ageing Dev. 2004;125:755–765. doi: 10.1016/j.mad.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 389.Szomor ZL. Appleyard RC. Murrell GA. Overexpression of nitric oxide synthases in tendon overuse. J Orthop Res. 2006;24:80–86. doi: 10.1002/jor.20009. [DOI] [PubMed] [Google Scholar]
- 390.Takemori K. Kimura T. Shirasaka N. Inoue T. Masuno K. Ito H. Food restriction improves glucose and lipid metabolism through Sirt1 expression: a study using a new rat model with obesity and severe hypertension. Life Sci. 2011;88:1088–1094. doi: 10.1016/j.lfs.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 391.Tang BL. Sirt1's systemic protective roles and its promise as a target in antiaging medicine. Transl Res. 2011;157:276–284. doi: 10.1016/j.trsl.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 392.Tantiwong P. Shanmugasundaram K. Monroy A. Ghosh S. Li M. DeFronzo RA. Cersosimo E. Sriwijitkamol A. Mohan S. Musi N. NF-kappaB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am J Physiol Endocrinol Metab. 2010;299:E794–E801. doi: 10.1152/ajpendo.00776.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Tao R. Coleman MC. Pennington JD. Ozden O. Park SH. Jiang H. Kim HS. Flynn CR. Hill S. Hayes McDonald W. Olivier AK. Spitz DR. Gius D. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Taylor CR. Weibel ER. Design of the mammalian respiratory system. I. Problem and strategy. Respir Physiol. 1981;44:1–10. [PubMed] [Google Scholar]
- 395.Taylor CT. Mitochondria and cellular oxygen sensing in the HIF pathway. Biochem J. 2008;409:19–26. doi: 10.1042/BJ20071249. [DOI] [PubMed] [Google Scholar]
- 396.Tennen RI. Berber E. Chua KF. Functional dissection of SIRT6: identification of domains that regulate histone deacetylase activity and chromatin localization. Mech Ageing Dev. 2010;131:185–192. doi: 10.1016/j.mad.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Thomson S. Mahadevan LC. Clayton AL. MAP kinase-mediated signalling to nucleosomes and immediate-early gene induction. Semin Cell Dev Biol. 1999;10:205–214. doi: 10.1006/scdb.1999.0302. [DOI] [PubMed] [Google Scholar]
- 398.Tian L. Cai Q. Wei H. Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. Free Radic Biol Med. 1998;24:1477–1484. doi: 10.1016/s0891-5849(98)00025-2. [DOI] [PubMed] [Google Scholar]
- 399.Timmons JA. Knudsen S. Rankinen T. Koch LG. Sarzynski M. Jensen T. Keller P. Scheele C. Vollaard NB. Nielsen S. Akerstrom T. MacDougald OA. Jansson E. Greenhaff PL. Tarnopolsky MA. van Loon LJ. Pedersen BK. Sundberg CJ. Wahlestedt C. Britton SL. Bouchard C. Using molecular classification to predict gains in maximal aerobic capacity following endurance exercise training in humans. J Appl Physiol. 2010;108:1487–1496. doi: 10.1152/japplphysiol.01295.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Tobon-Velasco JC. Carmona-Aparicio L. Ali SF. Santamaria A. Biomarkers of cell damage induced by oxidative stress in Parkinson's disease and related models. Cent Nerv Syst Agents Med Chem. 2010;10:278–286. doi: 10.2174/187152410793429719. [DOI] [PubMed] [Google Scholar]
- 401.Tolmasoff JM. Ono T. Cutler RG. Superoxide dismutase: correlation with life-span and specific metabolic rate in primate species. Proc Natl Acad Sci U S A. 1980;77:2777–2781. doi: 10.1073/pnas.77.5.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Tonkonogi M. Walsh B. Svensson M. Sahlin K. Mitochondrial function and antioxidative defence in human muscle: effects of endurance training and oxidative stress. J Physiol. 2000;528(Pt 2):379–388. doi: 10.1111/j.1469-7793.2000.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Toth-Zsamboki E. Horvath E. Vargova K. Pankotai E. Murthy K. Zsengeller Z. Barany T. Pek T. Fekete K. Kiss RG. Preda I. Lacza Z. Gero D. Szabo C. Activation of poly(ADP-ribose) polymerase by myocardial ischemia and coronary reperfusion in human circulating leukocytes. Mol Med. 2006;12:221–228. doi: 10.2119/2006-00055.Toth-Zsamboki. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 404.Traverse JH. Wang YL. Du R. Nelson D. Lindstrom P. Archer SL. Gong G. Bache RJ. Coronary nitric oxide production in response to exercise and endothelium-dependent agonists. Circulation. 2000;101:2526–2531. doi: 10.1161/01.cir.101.21.2526. [DOI] [PubMed] [Google Scholar]
- 405.Tremblay MS. Shephard RJ. Brawley LR. Cameron C. Craig CL. Duggan M. Esliger DW. Hearst W. Hicks A. Janssen I. Katzmarzyk PT. Latimer AE. Ginis KA. McGuire A. Paterson DH. Sharratt M. Spence JC. Timmons B. Warburton D. Young TK. Zehr L. Physical activity guidelines and guides for Canadians: facts and future. Can J Public Health. 2007;98(Suppl 2):S218–S224. [PubMed] [Google Scholar]
- 406.Trendelenburg AU. Meyer A. Jacobi C. Feige JN. Glass DJ. TAK-1/p38/nNFkappaB signaling inhibits myoblast differentiation by increasing levels of Activin A. Skelet Muscle. 2012;2:3. doi: 10.1186/2044-5040-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Trenerry MK. Carey KA. Ward AC. Cameron-Smith D. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol. 2007;102:1483–1489. doi: 10.1152/japplphysiol.01147.2006. [DOI] [PubMed] [Google Scholar]
- 408.Trenerry MK. Carey KA. Ward AC. Farnfield MM. Cameron-Smith D. Exercise-induced activation of STAT3 signaling is increased with age. Rejuvenation Res. 2008;11:717–724. doi: 10.1089/rej.2007.0643. [DOI] [PubMed] [Google Scholar]
- 409.Tweedie C. Romestaing C. Burelle Y. Safdar A. Tarnopolsky MA. Seadon S. Britton SL. Koch LG. Hepple RT. Lower oxidative DNA damage despite greater ROS production in muscles from rats selectively bred for high running capacity. Am J Physiol Regul Integr Comp Physiol. 2011;300:R544–R553. doi: 10.1152/ajpregu.00250.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Ueda S. Masutani H. Nakamura H. Tanaka T. Ueno M. Yodoi J. Redox control of cell death. Antioxid Redox Signal. 2002;4:405–414. doi: 10.1089/15230860260196209. [DOI] [PubMed] [Google Scholar]
- 411.Ugarte N. Petropoulos I. Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxid Redox Signal. 2010;13:539–549. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- 412.Vakhrusheva O. Smolka C. Gajawada P. Kostin S. Boettger T. Kubin T. Braun T. Bober E. Sirt7 increases stress resistance of cardiomyocytes and prevents apoptosis and inflammatory cardiomyopathy in mice. Circ Res. 2008;102:703–710. doi: 10.1161/CIRCRESAHA.107.164558. [DOI] [PubMed] [Google Scholar]
- 413.van Praag H. Neurogenesis and exercise: past and future directions. Neuromol Med. 2008;10:128–140. doi: 10.1007/s12017-008-8028-z. [DOI] [PubMed] [Google Scholar]
- 414.van Praag H. Kempermann G. Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 415.Vasilaki A. Mansouri A. Remmen H. van der Meulen JH. Larkin L. Richardson AG. McArdle A. Faulkner JA. Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell. 2006;5:109–117. doi: 10.1111/j.1474-9726.2006.00198.x. [DOI] [PubMed] [Google Scholar]
- 416.Vasilaki A. McArdle F. Iwanejko LM. McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev. 2006;127:830–839. doi: 10.1016/j.mad.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 417.Vassilakopoulos T. Deckman G. Kebbewar M. Rallis G. Harfouche R. Hussain SN. Regulation of nitric oxide production in limb and ventilatory muscles during chronic exercise training. Am J Physiol Lung Cell Mol Physiol. 2003;284:L452–L457. doi: 10.1152/ajplung.00270.2002. [DOI] [PubMed] [Google Scholar]
- 418.Vella L. Caldow MK. Larsen AE. Tassoni D. Della Gatta PA. Gran P. Russell AP. Cameron-Smith D. Resistance exercise increases NF-kappaB activity in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2012;302:R667–R673. doi: 10.1152/ajpregu.00336.2011. [DOI] [PubMed] [Google Scholar]
- 419.Vezzoli M. Castellani P. Corna G. Castiglioni A. Bosurgi L. Monno A. Brunelli S. Manfredi AA. Rubartelli A. Rovere-Querini P. High-mobility group box 1 release and redox regulation accompany regeneration and remodeling of skeletal muscle. Antioxid Redox Signal. 2011;15:2161–2174. doi: 10.1089/ars.2010.3341. [DOI] [PubMed] [Google Scholar]
- 420.Vider J. Laaksonen DE. Kilk A. Atalay M. Lehtmaa J. Zilmer M. Sen CK. Physical exercise induces activation of NF-kappaB in human peripheral blood lymphocytes. Antioxid Redox Signal. 2001;3:1131–1137. doi: 10.1089/152308601317203639. [DOI] [PubMed] [Google Scholar]
- 421.Villeda SA. Luo J. Mosher KI. Zou B. Britschgi M. Bieri G. Stan TM. Fainberg N. Ding Z. Eggel A. Lucin KM. Czirr E. Park JS. Couillard-Despres S. Aigner L. Li G. Peskind ER. Kaye JA. Quinn JF. Galasko DR. Xie XS. Rando TA. Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Vogt M. Puntschart A. Geiser J. Zuleger C. Billeter R. Hoppeler H. Molecular adaptations in human skeletal muscle to endurance training under simulated hypoxic conditions. J Appl Physiol. 2001;91:173–182. doi: 10.1152/jappl.2001.91.1.173. [DOI] [PubMed] [Google Scholar]
- 423.Vollaard NB. Shearman JP. Cooper CE. Exercise-induced oxidative stress:myths, realities and physiological relevance. Sports Med. 2005;35:1045–1062. doi: 10.2165/00007256-200535120-00004. [DOI] [PubMed] [Google Scholar]
- 424.Walsh B. Tonkonogi M. Sahlin K. Effect of endurance training on oxidative and antioxidative function in human permeabilized muscle fibers. Pflugers Arch. 2001;442:420–425. doi: 10.1007/s004240100538. [DOI] [PubMed] [Google Scholar]
- 425.Wang G. Chen HW. Oktay Y. Zhang J. Allen EL. Smith GM. Fan KC. Hong JS. French SW. McCaffery JM. Lightowlers RN. Morse HC., 3rd Koehler CM. Teitell MA. PNPASE regulates RNA import into mitochondria. Cell. 2010;142:456–467. doi: 10.1016/j.cell.2010.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Wang H. Hertlein E. Bakkar N. Sun H. Acharyya S. Wang J. Carathers M. Davuluri R. Guttridge DC. NF-kappaB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes. Mol Cell Biol. 2007;27:4374–4387. doi: 10.1128/MCB.02020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Wang H. Yuan G. Prabhakar NR. Boswell M. Katz DM. Secretion of brain-derived neurotrophic factor from PC12 cells in response to oxidative stress requires autocrine dopamine signaling. J Neurochem. 2006;96:694–705. doi: 10.1111/j.1471-4159.2005.03572.x. [DOI] [PubMed] [Google Scholar]
- 428.Warner BB. Stuart L. Gebb S. Wispe JR. Redox regulation of manganese superoxide dismutase. Am J Physiol. 1996;271:L150–L158. doi: 10.1152/ajplung.1996.271.1.L150. [DOI] [PubMed] [Google Scholar]
- 429.Wei L. Salahura G. Boncompagni S. Kasischke KA. Protasi F. Sheu SS. Dirksen RT. Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease. FASEB J. 2011;25:3068–3078. doi: 10.1096/fj.11-187252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Wei Y. Sowers JR. Nistala R. Gong H. Uptergrove GM. Clark SE. Morris EM. Szary N. Manrique C. Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem. 2006;281:35137–35146. doi: 10.1074/jbc.M601320200. [DOI] [PubMed] [Google Scholar]
- 431.Wenz T. Rossi SG. Rotundo RL. Spiegelman BM. Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 432.Williamson DH. Lund P. Krebs HA. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967;103:514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Wisloff U. Najjar SM. Ellingsen O. Haram PM. Swoap S. Al-Share Q. Fernstrom M. Rezaei K. Lee SJ. Koch LG. Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 434.Wright DC. Han DH. Garcia-Roves PM. Geiger PC. Jones TE. Holloszy JO. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1alpha expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 435.Wu A. Ying Z. Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci. 2004;19:1699–1707. doi: 10.1111/j.1460-9568.2004.03246.x. [DOI] [PubMed] [Google Scholar]
- 436.Wu J. Jiang Z. Liu M. Gong X. Wu S. Burns CM. Li Z. Polynucleotide phosphorylase protects Escherichia coli against oxidative stress. Biochemistry. 2009;48:2012–2020. doi: 10.1021/bi801752p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Xia Y. Zweier JL. Substrate control of free radical generation from xanthine oxidase in the postischemic heart. J Biol Chem. 1995;270:18797–18803. doi: 10.1074/jbc.270.32.18797. [DOI] [PubMed] [Google Scholar]
- 438.Yang HT. Prior BM. Lloyd PG. Taylor JC. Li Z. Laughlin MH. Terjung RL. Training-induced vascular adaptations to ischemic muscle. J Physiol Pharmacol. 2008;59(Suppl 7):57–70. [PMC free article] [PubMed] [Google Scholar]
- 439.Yang SR. Wright J. Bauter M. Seweryniak K. Kode A. Rahman I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-kappaB in macrophages in vitro and in rat lungs in vivo: implications for chronic inflammation and aging. Am J Physiol Lung Cell Mol Physiol. 2007;292:L567–L576. doi: 10.1152/ajplung.00308.2006. [DOI] [PubMed] [Google Scholar]
- 440.Yi MJ. Park SH. Cho HN. Yong Chung H. Kim JI. Cho CK. Lee SJ. Lee YS. Heat-shock protein 25 (Hspb1) regulates manganese superoxide dismutase through activation of Nfkb (NF-kappaB) Radiat Res. 2002;158:641–649. doi: 10.1667/0033-7587(2002)158[0641:hsphrm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 441.Ying W. NAD+/NADH and NADP+/NADPH in cellular functions and cell death: regulation and biological consequences. Antioxid Redox Signal. 2008;10:179–206. doi: 10.1089/ars.2007.1672. [DOI] [PubMed] [Google Scholar]
- 442.Yoshihara E. Chen Z. Matsuo Y. Masutani H. Yodoi J. Thiol redox transitions by thioredoxin and thioredoxin-binding protein-2 in cell signaling. Methods Enzymol. 2010;474:67–82. doi: 10.1016/S0076-6879(10)74005-2. [DOI] [PubMed] [Google Scholar]
- 443.Yu AY. Frid MG. Shimoda LA. Wiener CM. Stenmark K. Semenza GL. Temporal, spatial, and oxygen-regulated expression of hypoxia-inducible factor-1 in the lung. Am J Physiol. 1998;275:L818–L826. doi: 10.1152/ajplung.1998.275.4.L818. [DOI] [PubMed] [Google Scholar]
- 444.Zhao W. Kruse JP. Tang Y. Jung SY. Qin J. Gu W. Negative regulation of the deacetylase SIRT1 by DBC1. Nature. 2008;451:587–590. doi: 10.1038/nature06515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Zhong L. Mostoslavsky R. SIRT6: a master epigenetic gatekeeper of glucose metabolism. Transcription. 2010;1:17–21. doi: 10.4161/trns.1.1.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Zoll J. Ponsot E. Dufour S. Doutreleau S. Ventura-Clapier R. Vogt M. Hoppeler H. Richard R. Fluck M. Exercise training in normobaric hypoxia in endurance runners. III. Muscular adjustments of selected gene transcripts. J Appl Physiol. 2006;100:1258–1266. doi: 10.1152/japplphysiol.00359.2005. [DOI] [PubMed] [Google Scholar]
- 447.Zuo L. Christofi FL. Wright VP. Liu CY. Merola AJ. Berliner LJ. Clanton TL. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279:C1058–C1066. doi: 10.1152/ajpcell.2000.279.4.C1058. [DOI] [PubMed] [Google Scholar]
- 448.Zuo L. Pasniciuc S. Wright VP. Merola AJ. Clanton TL. Sources for superoxide release: lessons from blockade of electron transport, NADPH oxidase, and anion channels in diaphragm. Antioxid Redox Signal. 2003;5:667–675. doi: 10.1089/152308603770310347. [DOI] [PubMed] [Google Scholar]