Abstract
The Wnt/β-catenin pathway is highly regulated to insure the correct temporal and spatial activation of its target genes. In the absence of a Wnt stimulus, the transcriptional coactivator β-catenin is degraded by a multiprotein “destruction complex” that includes the tumor suppressors Axin and adenomatous polyposis coli (APC), the Ser/Thr kinases GSK-3 and CK1, protein phosphatase 2A (PP2A), and the E3-ubiquitin ligase β-TrCP. The complex generates a β-TrCP recognition site by phosphorylation of a conserved Ser/Thr-rich sequence near the β-catenin amino terminus, a process that requires scaffolding of the kinases and β-catenin by Axin. Ubiquitinated β-catenin is degraded by the proteasome. The molecular mechanisms that underlie several aspects of destruction complex function are poorly understood, particularly the role of APC. Here we review the molecular mechanisms of destruction complex function and discuss several potential roles of APC in β-catenin destruction.
β-Catenin is degraded in the absence of a Wnt stimulus. Although the functions of the core components of the multiprotein β-catenin destruction complex are generally known, the role of APC is unclear.
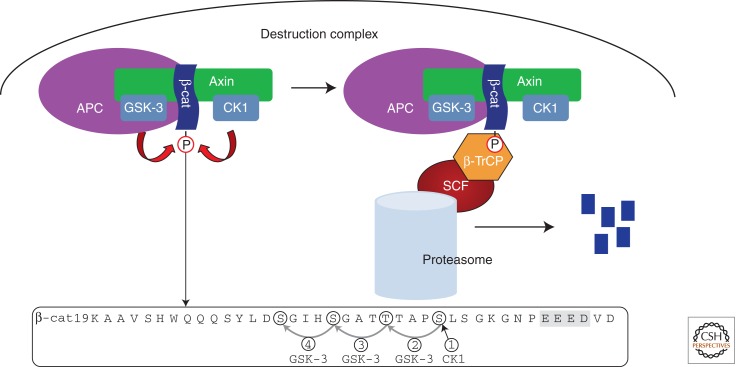
β-Catenin, first discovered as a part of the adherens junction complex with cadherin and α-catenin (Ozawa et al. 1989), also serves as a transcriptional coactivator of Wnt target gene expression. In the absence of an extracellular Wnt stimulus, the nonjunctional pool of cytoplasmic β-catenin is targeted for proteolysis by a large multiprotein assembly termed the β-catenin destruction complex (Fig. 1). Wnt binding to cell–surface receptors turns off β-catenin destruction, and the resulting stabilized β-catenin translocates to the nucleus and binds to TCF/Lef proteins to activate Wnt target gene transcription (Behrens et al. 1996; Molenaar et al. 1996). The importance of β-catenin destruction first came to light with the discovery that mutations of the adenomatous polyposis coli protein associated with familial and sporadic colon cancers produce accumulation of β-catenin and inappropriate activation of target genes (Munemitsu et al. 1995; Korinek et al. 1997; Morin et al. 1997; Rubinfeld et al. 1997a; Clevers 2006). The precise temporal activation or inhibition of Wnt target genes necessary during development may explain the need for the rapid and sensitive responses of β-catenin protein levels.
Figure 1.
Overview of the destruction complex. The phosphorylated sequence in the amino terminus of β-catenin is shown, with CK1 and GSK-3 sites circled. The acidic cluster that primes CK1 phosphorylation is highlighted in gray. SCF, the Skp1/cullin/F-box complex.
The destruction complex is likely a dynamic multiprotein assembly, but its core components include, in addition to β-catenin itself, the Ser/Thr kinases glycogen synthase kinase 3 (GSK-3) and casein kinase 1 (CK1), the scaffolding protein Axin, the adenomatous polyposis coli (APC) protein, and the E3-ubiquitin ligase β-TrCP (Fig. 1). Protein phosphatase 2A (PP2A) also associates with the complex (Hsu et al. 1999; Seeling et al. 1999; Ratcliffe et al. 2000; Yamamoto et al. 2001). Mutations in destruction complex components associated with various cancers result in inappropriate stabilization of β-catenin and Wnt target gene expression in the absence of a Wnt stimulus (Dominguez et al. 1995; Jiang and Struhl 1998; Marikawa and Elinson 1998; Peters et al. 1999; Liu et al. 2000; Satoh et al. 2000; Heisenberg et al. 2001). For example, in the absence of a Wnt signal, the half-life of β-catenin was found to be 50 min in AtT20 cells, but 3 h in the colon cancer cell line SW480, which bears a mutated APC protein (Munemitsu et al. 1996).
Although the core components of the β-catenin destruction complex and their binding interactions are understood in molecular detail, a complete molecular understanding of β-catenin destruction has been elusive. In particular, the essential role of APC in this process, as well as the relationship between the kinase/scaffold complexes and the ubiquitination machinery, remain unclear. Here we review what is known about these core destruction complex functions, focusing on potential molecular mechanisms.
β-CATENIN DESTRUCTION OCCURS VIA PROTEASOMAL DEGRADATION
The actual destruction of β-catenin is accomplished by the proteasome, which proteolytically degrades β-catenin (Aberle et al. 1997; Orford et al. 1997). β-Catenin is presented to the proteasome through its interaction with the F-box containing E3-ligase protein β-TrCP, an adaptor protein that forms a complex with the Skp1/Cullin machinery to attach ubiquitin to its binding partners (Hart et al. 1999; Kitagawa et al. 1999; Latres et al. 1999; Liu et al. 1999; Winston et al. 1999). The binding site for β-TrCP on β-catenin is a short peptide that encompasses two conserved serines, Ser33 and Ser37, which when phosphorylated interact with the β-propeller domain of β-TrCP (Orford et al. 1997; Wu et al. 2003). Cancer cells such as the colon cancer line HCT116 in which the key phosphorylated serines or threonines in β-catenin are mutated or deleted have inappropriately high levels of β-catenin that activate Wnt target genes (Morin et al. 1997).
PHOSPHORYLATION BY THE DESTRUCTION COMPLEX
The phosphorylation of β-catenin Ser33 and Ser37 by GSK-3 is the best-characterized function of the destruction complex. CK1 initially phosphorylates Ser45 of β-catenin (Amit et al. 2002; Liu et al. 2002), and is targeted to this site by a cluster of acidic residues located seven amino acids carboxy-terminally (Fig. 1) (Marin et al. 2003). GSK-3 phosphorylates residues in the sequence S/T-X-X-X-pS/pT, in which the bold residue represents the phosphorylation site, and X is any amino acid (Fiol et al. 1987; Frame et al. 2001). Phosphorylated Ser45 primes GSK-3-mediated phosphorylation of T41, which in turn primes successive phosphorylation of S37 and S33 by GSK-3 to generate the β-TrCP-binding site (Fig. 1) (Yost et al. 1996; Hagen et al. 2002; Liu et al. 2002; Wu and He 2006).
A number of CK1 isoforms can be expressed, including a membrane-tethered version (CK1γ). The α isoform consists almost solely of the catalytic kinase domain, whereas the δ and ε isoforms have carboxy-terminal extensions that can be autophosphorylated, which leads to autoinhibition (Cegielska et al. 1998). The cytoplasmic isoforms α, δ, and ε can bind to Axin, and purified CK1α, δ, or ε can phosphorylate β-catenin at S45 (Amit et al. 2002). RNAi experiments in both mammalian cells and Drosophila and genetic experiments in mice indicate that CK1α is the principal isoform responsible for phosphorylating Ser45 (Liu et al. 2002; Matsubayashi et al. 2004; Elyada et al. 2011).
Mammalian GSK-3 is expressed from two different genes, termed GSK-3α and GSK-3β, which differ principally by the presence of an extended, glycine-rich amino terminus in GSK-3α (Woodgett 1990). The single GSK-3 in Drosophila more closely resembles the shorter GSK-3β. Although isoform-specific roles have been observed in other pathways (e.g., McManus et al. 2005; Patel et al. 2008; Kaidanovich-Beilin et al. 2009), GSK-3α and β appear to function redundantly in β-catenin destruction (Doble et al. 2007). Many studies of the vertebrate Wnt pathway have focused on GSK-3β. Doble et al. (2007) noted that this is likely an historical bias that arose from apparent differences in the ability of the two isoforms to rescue GSK-3 mutants in Drosophila (Siegfried et al. 1992; Ruel et al. 1993), but expression of the two forms was not equal in those studies. GSK-3 can be phosphorylated at a tyrosine on its activation loop (Tyr279/Tyr216 in α/β isoforms), which increases enzymatic activity approximately five-fold, a modest increase compared to the activation of other kinases (Dajani et al. 2003). Mutating this residue to Phe appears to have little (Itoh et al. 1995; Dajani et al. 2003) or moderate (Doble et al. 2007) effects on β-catenin activity.
In addition to its role in the Wnt/β-catenin pathway, GSK-3 has regulatory roles in many other cellular processes, including glycogen biosynthesis, microtubule stability, cell-cycle control, and inflammatory pathways (Frame and Cohen 2001). GSK-3 activity is regulated in several of these pathways by phosphorylation of a serine (Ser21/Ser9, α/β isoforms) present in a conserved sequence near the amino terminus of the protein. When phosphorylated by other serine/threonine kinases, including Akt and p70S6K (Cross et al. 1995; Peyrollier et al. 2000), this phosphopeptide inhibits GSK-3 activity. Although autoinhibition by the phosphorylated amino-terminal peptide is important in non-Wnt GSK-3 functions, mutation of the Ser21/Ser9 does not affect Wnt signaling, and this residue does not become phosphorylated on Wnt stimulation (Ding et al. 2000; McManus et al. 2005). Furthermore, the binding of GSK-3 to Axin, discussed below, probably targets a fraction of cytoplasmic GSK-3 to the destruction complex and insulates GSK-3 from regulatory proteins not involved in Wnt signaling. For example, Ser21/Ser9 of Axin-bound GSK-3 does not become phosphorylated during insulin signaling and activation of Akt (Ding et al. 2000).
AXIN, THE CENTRAL PHOSPHORYLATION SCAFFOLD
Axin was discovered as the product of the mouse Fused locus, whose disruption caused the duplication of the embryonic body axis (Zeng et al. 1997). Drosophila possess only one Axin gene, but vertebrates and nematodes have two, Axin and Axin 2/Conductin. In the mouse, these two isoforms appear to be mechanistically interchangeable, and vary only in their patterns of transcription. Axin is more widely expressed, whereas Axin 2/Conductin has a more restricted expression pattern that is controlled in part by Wnt signaling (Jho et al. 2002; Leung et al. 2002; Chia and Costantini 2005). A study of hepatocellular carcinomas showed that Axin mutations lead to inappropriate β-catenin-mediated transcription (Satoh et al. 2000). Overexpression of Axin in colon cancer cells can rescue the accumulation of β-catenin that results from APC mutation (Behrens et al. 1998; Hart et al. 1998; Kishida et al. 1998; Sakanaka et al. 1998).
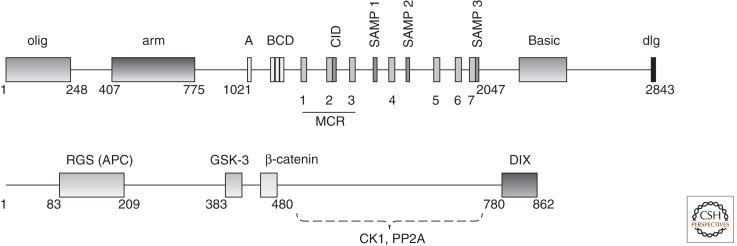
Axin is a largely unstructured, flexible protein (Spink et al. 2000; Noutsou et al. 2011) that contains CK1, GSK-3, and β-catenin-binding sites (Fig. 2) (Ikeda et al. 1998; Yamamoto et al. 1999; Rubinfeld et al. 2001; Liu et al. 2002). The region carboxy-terminal to the β-catenin and GSK-3-binding sites has been reported to bind to the catalytic domain of the PP2A or to the PR61 regulatory subunit of PP2A (Fig. 2) (Hsu et al. 1999; Ikeda et al. 2000; Yamamoto et al. 2001), as well as the phosphatase PP1 (Luo et al. 2007). The simultaneous binding of Axin to a kinase and β-catenin enforces close proximity and thereby increases the effective concentration of enzyme and substrate. This “scaffold effect” is seen with purified proteins; phosphorylation of β-catenin by CK1 and GSK-3β is greatly enhanced by the presence of Axin (Ikeda et al. 1998; Dajani et al. 2003; Ha et al. 2004). Crystal structures have detailed the binding interactions between Axin and β-catenin (Xing et al. 2003), and Axin and GSK-3 (Dajani et al. 2003). The structure of the GSK-3β–Axin complex shows that Axin binds to a region far removed from the catalytic site (Dajani et al. 2003), and we have found that Axin has no effect on the intrinsic catalytic capability of GSK-3 (unpubl.). Likewise, Axin binds to a region of CK1 far from the catalytic site, and a pure scaffolding effect without enhancement of intrinsic catalytic activity has been shown for this interaction (Sobrado et al. 2005).
Figure 2.
Primary structures of human Axin and APC. Key protein–protein interaction regions are indicated. Structured domains are RGS, regulator of G-protein signaling homology; DIX, domain common to Dishevelled and Axin; olig, coiled-coil dimerization domain of human APC; and arm, armadillo repeat domain. 15-mer repeats are marked A–D; 20-mer repeats are marked 1–7. The mutation cluster region (MCR) is the site of the majority of APC truncations found in colorectal cancers. Dashed lines indicate that mapping of the CK1 and PP2A sites on Axin are based on deletion studies and have not been verified with purified proteins.
In addition to the β-catenin, kinase, and PP2A binding sites, Axin also possesses two folded, structured domains. Near the amino terminus, the RGS domain, named for its homology to the regulators of G-protein signaling protein family, binds to the APC protein (Behrens et al. 1998; Kishida et al. 1998; Spink et al. 2000). The interaction with APC is required for efficient phosphorylation of APC (see below) (Fagotto et al. 1999; Ikeda et al. 2000). At the carboxyl terminus, the DIX domain mediates homomeric complex formation, as well as binding to other DIX domain-containing proteins, most notably Dishevelled (Fig. 2) (Fagotto et al. 1999; Kishida et al. 1999; Julius et al. 2000; Capelluto et al. 2002; Schwarz-Romond et al. 2007a; Fiedler et al. 2011; Liu et al. 2011), an interaction crucial for transducing a Wnt signal to turn off β-catenin destruction (MacDonald and He 2012).
Axin is a key control point for β-catenin destruction by virtue of its ability to promote efficient phosphorylation of β-catenin and APC; indeed, the GSK-3 and β-catenin-binding sites are essential for Axin function (Fagotto et al. 1999). In addition, deletion of the Axin RGS domain in both vertebrates and Drosophila compromises β-catenin destruction in vivo (Fagotto et al. 1999; Peterson-Nedry et al. 2008), demonstrating the importance of the Axin–APC interaction. However, the APC interaction mediated by the RGS domain is dispensable when Axin is overexpressed in SW480 colon cancer cells (see below) (Kohler et al. 2009; Roberts et al. 2011). Measurements of destruction complex components in mammalian kidney epithelial cells and Xenopus oocytes gave different absolute and relative concentrations of APC and Axin (Salic et al. 2000; Lee et al. 2003; Tan et al. 2012), suggesting that Wnt signaling operates over a range of component concentrations that vary with cell type. Studies in Drosophila have suggested that precise APC levels are important in modulating Wnt responses; a twofold change in APC2 was shown to profoundly affect signaling in photoreceptor development, indicating that APC levels are likely tuned to optimize responses to morphogen gradients (Benchabane et al. 2008). Thus, although Axin is clearly a key component of the destruction complex, the relative levels of all components appear to be critical for both homeostasis and responsiveness to Wnt.
THE ROLE OF APC IN β-CATENIN DESTRUCTION
APC is an ∼310-kDa protein whose central ∼1000 amino acids contain short peptide motifs that bind to β-catenin or Axin (Fig. 2). The APC protein in most organisms, including Drosophila, expresses in two isoforms, designated APC and APC2. In addition to the central β-catenin- and Axin-binding region, the amino-terminal portion of APC contains a dimerization domain in vertebrates (Day and Alber 2000) and an armadillo repeat (arm) domain. The arm domain interacts with a number of cytoskeletal regulators (Kawasaki et al. 2000; Jimbo et al. 2002; Watanabe et al. 2004; Breitman et al. 2008), and the B56 regulatory subunit of PP2A (Seeling et al. 1999). The carboxy-terminal region of APC1 features a microtubule interaction region (McCartney and Nathke 2008) that is dispensable for β-catenin destruction (Smits et al. 1999). These activities are thought to be important for other aspects of APC biology, including roles in spindle formation, kinetochore attachment, and maintenance of microtubule stability (McCartney and Nathke 2008), but it is not known if interactions with cytoskeletal regulators are important to the function of APC in β-catenin destruction.
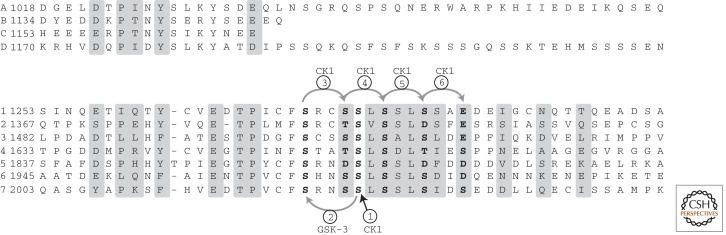
APC proteins contain multiple independent binding sites for β-catenin, comprising homologous repeats of either 15 or 20 amino acids (Fig. 2) (Eklof Spink et al. 2001). Human APC contains four 15-mers (designated 15RA-D) and seven 20-mers (20R1-7) (Fig. 3). The 20-mer repeats can be phosphorylated by GSK-3 and CK1, which enhances affinity for β-catenin up to 1500-fold (Fig. 3) (Rubinfeld et al. 1996; Rubinfeld et al. 1997b; Ha et al. 2004; Liu et al. 2006). Phosphorylation of the 20-mers in vivo likely requires Axin, as a scaffold effect can be shown using purified Axin and the kinases (Ikeda et al. 2000; Su et al. 2008). The level of phosphorylation heterogeneity is not known under physiological conditions, however. The stoichiometry of the APC–β-catenin interaction is also unknown, and may vary depending on the status of Wnt signaling. An early experiment showed that APC protein extracted from cells appears to be bound to only one or two molecules of β-catenin (Rubinfeld et al. 1995).
Figure 3.
Alignment of β-catenin-binding sequences of human APC, showing the 15-mers (top) and 20-mers (bottom). Residues that directly contact β-catenin, as observed in crystal structures of β-catenin bound to 15RA (Spink et al. 2001) and 20R3 (Ha et al. 2004; Xing et al. 2004), are shaded. 20-mer residues phosphorylated by CK1 and GSK-3 are shown in bold, with the phosphorylation order determined by priming rules for each kinase. (From Ha et al. 2004; adapted, with express permission, from the authors.)
Interspersed with the β-catenin-binding 20-mer repeats are Axin-binding sequences of ∼16 amino acids, termed SAMP repeats for the conserved amino acid sequence present in the Axin-binding sites of mammalian APC (Fig. 2) (Behrens et al. 1998; Spink et al. 2000). APCs contain two or three SAMP repeats, depending on species and isoform. Mammalian cell culture studies showed that the APC–Axin interaction contributes to β-catenin destruction (Behrens et al. 1998; Hart et al. 1998), and at least one SAMP repeat in combination with at least two β-catenin-binding repeats were required (Rubinfeld et al. 1997a). In mice, at least one SAMP repeat is needed to regulate Wnt signaling (Smits et al. 1999). Truncations of APC that remove all SAMP repeats are oncogenic, although certain of these truncations may retain some level of β-catenin regulation (Smits et al. 1999; Kohler et al. 2009).
Experiments that rely on APC truncations to remove the SAMP repeats by necessity also remove many of the 20-mer β-catenin-binding sites, which confounds interpretations of the role of the APC–Axin interaction. Peifer and colleagues generated a Drosophila APC2 variant in which the SAMP repeats were deleted cleanly rather than by truncation, so that all β-catenin-binding sites were retained. This mutant APC was nonfunctional in animals, although β-catenin destruction and TCF signaling down-regulation were retained in SW480 cells (Roberts et al. 2011). Curiously, Roberts et al. 2011 found that APC2 lacking SAMP repeats colocalized with Axin, but the mutant APC2 did not coimmunoprecipitate with Axin, suggesting a weak and indirect interaction. It is possible that β-catenin itself bridged Axin and the mutant APC2, because it can interact with Axin and the APC 15-mer or nonphosphorylated 20-mer repeats simultaneously (Ha et al. 2004).
The molecular roles of APC in β-catenin destruction have not been determined decisively. Several distinct and not necessarily mutually exclusive functions for APC in regulating β-catenin have been proposed, which we consider in the following subsections.
Phosphorylation of β-Catenin
The ability of β-catenin, Axin, and APC to interact with one another might suggest a stabilizing role for APC in promoting β-catenin phosphorylation by Axin-bound kinases. Bioinformatic analyses, nuclear magnetic resonance (NMR) and circular dichroism (CD) spectroscopy, and proteolytic sensitivity experiments indicate that, like the central region of Axin (see above), the central portion of APC is intrinsically unstructured (Li and Nathke 2005; Liu et al. 2006). Moreover, biochemical and structural studies have shown that Axin and nonphosphorylated APC repeats can bind simultaneously to β-catenin (Ha et al. 2004). These properties imply that APC can scaffold the Axin–β-catenin interaction. However, addition of purified APC fragments that contain β-catenin and Axin-binding sites to purified β-catenin, Axin, GSK-3β, and CK1 does not enhance β-catenin phosphorylation (Su et al. 2008; JL Stamos and WI Weis, unpubl.).
Measurements of two mammalian kidney cell lines have shown that Axin concentrations are in the range 110–150 nm; total and cytosolic β-catenin concentrations are in the hundreds and tens of nm range, respectively (Tan et al. 2012). APC is found at 12–40× lower concentrations than Axin in these cells. In contrast, in Xenopus oocyte extracts Axin is present at sub-nm concentrations, roughly 2000× lower than β-catenin and 5000× lower than APC (Salic et al. 2000; Lee et al. 2003). Given these concentrations and the weak affinity (μm-range dissociation constants) of the Axin-APC, Axin-β-catenin, and nonphosphorylated APC-β-catenin interactions (Spink et al. 2000; Choi et al. 2006; Liu et al. 2006), it could be argued that the stabilizing effect of a three-way interaction network helps to insure efficient β-catenin phosphorylation in vivo. However, APC truncations that remove the Axin-binding SAMP repeats do not prevent β-catenin phosphorylation (Sadot et al. 2002; Yang et al. 2006), indicating that APC does not enhance Axin-mediated phosphorylation of β-catenin.
Klein and colleagues reported recently that addition of a large fragment of APC encompassing the β-catenin and Axin-binding region enhances phosphorylation of β-catenin in the presence or absence of Axin, and also promotes phosphorylation of other GSK-3 substrates (Valvezan et al. 2012). These findings would seem to contradict those of (Su et al. 2008), and there is at present no evidence for direct APC–GSK-3 interactions required for scaffolding or for allosteric activation. Further studies with purified proteins will be needed to assess the molecular basis of these findings.
Destruction Complex Cycling
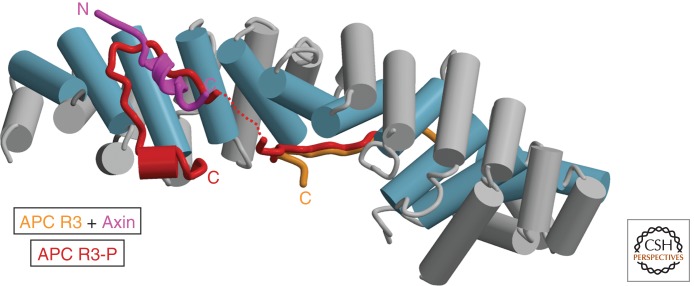
An alternative role for APC in β-catenin destruction was suggested by the observation that, in contrast to the simultaneous binding of nonphosphorylated APC repeats and Axin to β-catenin, phosphorylation of the APC 20-mer repeats enhances their affinity by binding to a surface of β-catenin that overlaps the Axin-binding site (Fig. 4); the higher affinity of the phosphorylated 20-mer versus Axin enables displacement of Axin from β-catenin (Xing et al. 2003; Ha et al. 2004; Xing et al. 2004). Kimelman, Xu, and colleagues (Xing et al. 2003) proposed that CK1 and GSK-3 phosphorylate APC after phosphorylating β-catenin; the phosphorylated APC 20-mer would displace β-catenin from Axin, freeing Axin to interact with another β-catenin molecule. This model also postulates that PP2A, which has been reported to bind to Axin and APC (Hsu et al. 1999; Seeling et al. 1999; Yamamoto et al. 2001) and can modulate APC phosphorylation (Ikeda et al. 2000), dephosphorylates β-catenin-bound APC to reset the system for another round of β-catenin phosphorylation and release.
Figure 4.
Competition between phosphorylated APC and Axin for β-catenin. Crystal structures of nonphosphorylated APC R3 (orange) (Ha et al. 2004), Axin (purple) (Xing et al. 2003), and phosphorylated APC R3-P (red) (Ha et al. 2004; Xing et al. 2004) bound to the armadillo repeat domain of β-catenin (blue and gray) were superimposed to show the relationship between the bound ligands. The portion of APC that becomes ordered on phosphorylation by CK1 and GSK-3 (compare red and orange structures) binds to an overlapping surface with Axin, and biochemical studies show that these two ligands compete for binding. This competition is the basis for models suggesting that Axin is displaced from β-catenin when the Axin-bound kinases phosphorylate APC.
The Kimelman/Xu model is appealing in its explanation of the roles of conserved APC 20-mer phosphorylation sites, the competition between phosphorylated APC and Axin for β-catenin, and the presence of PP2A in the destruction complex, but there are several caveats. First, phosphorylation of APC would have to occur after β-catenin phosphorylation, because phosphorylated APC would prevent Axin from binding to β-catenin. Given the unstructured nature of Axin and APC, it is difficult to envision how such an ordered sequence could be achieved at a molecular level—it seems unlikely that random collisions of these flexible scaffold proteins could produce a strict order of interaction of β-catenin, then APC, with the Axin-bound kinases. A stochastic mechanism would be physically plausible but inefficient. Second, the postulated role of PP2A is not supported by in vitro experiments with purified PP1, whose catalytic domain is very similar to that of PP2A, which showed that phosphorylated APC repeat 3 bound to β-catenin could not be dephosphorylated over 3 h (Ha et al. 2004). This would be inconsistent with the measured half-life of β-catenin (Munemitsu et al. 1996). Equivalent experiments have not been performed with heterotrimeric PP2A, however, and the effect of PP2A interactions with Axin and/or APC have not been assessed in this context. Finally, Peifer and colleagues showed that an APC2 construct in which only the highest affinity 20-mer repeat is retained is unable to promote β-catenin destruction (Roberts et al. 2011), indicating that high-affinity β-catenin binding by phosphorylated APC is not sufficient for destruction complex function.
It is important to note that there is no evidence that phosphorylated β-catenin must dissociate from the destruction complex to be degraded. Indeed, a ubiquitin ligase is part of the destruction complex (Hart et al. 1999; Liu et al. 1999; Major et al. 2007), and there is evidence that other components of the ubiquitination machinery physically associate with the proteasome in other systems (Crosas et al. 2006; Lee et al. 2011). It has been suggested that unfolding of β-catenin by proteasome-associated chaperones provides the energy to remove β-catenin from phosphorylated APC (Ha et al. 2004), which would then allow PP2A associated with the complex to dephosphorylate the APC 20-mers. Indeed, proteasomal activity has been shown to selectively remove and degrade individual components of multiprotein complexes (e.g., see Rape et al. 2001; Shi et al. 2008).
Interfacing to the Ubiquitin/Proteasome Pathway
APC truncations present in several colon cancer cell lines, including SW480, DLD-1, and HT-29, do not prevent β-catenin phosphorylation, but ablate or strongly reduce ubiquitination of β-catenin (Sadot et al. 2002; Yang et al. 2006; Su et al. 2008). Ubiquitination requires the presence of APC 20-mer repeat 2 (20R2), which, importantly, does not bind to β-catenin (Liu et al. 2006; Kohler et al. 2008). Furthermore, transfection of SW480 cells with a construct of APC truncated after 20R3 can still down-regulate β-catenin in a cell culture system (Yang et al. 2006; Kohler et al. 2008), whereas deletion of 20R2 and the strongly conserved sequence that follows it, designated the “catenin inhibitory domain” (CID; also called “sequence B” by Peifer and coworkers), prevents β-catenin destruction (Kohler et al. 2009; Roberts et al. 2011). These data show that the 20R2-CID region of APC has an essential role in promoting β-catenin ubiquitination that is mechanistically downstream from β-catenin phosphorylation, and is independent of β-catenin-binding activity. Su et al. (2008) found that β-TrCP did not coimmunoprecipitate β-catenin from cell lines bearing APCs truncated before 20R2, but coprecipitation was restored by transfection with wild-type APC, suggesting that 20R2-CID mediates interactions with β-TrCP, either directly or through other proteins.
An alternative possibility for the essential role of 20R2-CID in β-catenin ubiquitination comes from the observation that in the absence of APC, Ser33/Ser37 phosphorylated β-catenin was rapidly dephosphorylated in vitro by PP2A, whereas full-length APC or APC fragments containing SAMP repeats could protect β-catenin from PP2A (Su et al. 2008). This suggests that APC prevents PP2A from accessing the amino-terminal phosphorylation sites on β-catenin (Su et al. 2008). Su et al. (2008) proposed that phosphorylated APC spatially orients phospho-β-catenin to prevent its dephosphorylation, although it is unclear how this could work given the unstructured, flexible nature of both APC and the amino-terminal region of β-catenin. The protection occurs only with phosphorylated APC, and the investigators argue APC must bind to β-catenin sufficiently tightly to protect the β-TrCP recognition sequence of β-catenin from PP2A. However, the protective effect of phosphorylated APC was observed even under conditions in which unphosphorylated and phosphorylated APC were equivalently occupied by β-catenin, so enhanced affinity would not appear to produce this result. Instead, binding of the phosphorylated APC 20-mers to β-catenin (Ha et al. 2004; Xing et al. 2004) might create a novel interaction surface for PP2A that inhibits its action on the β-catenin amino terminus.
Sequestration of β-Catenin
Comparison of β-catenin localization in wild-type and APC mutant cells suggested that APC and Axin can retain β-catenin in the cytoplasm (Tolwinski and Wieschaus 2001; Brocardo et al. 2005; Krieghoff et al. 2006; McCartney et al. 2006). Both Axin and APC contain CRM-1-dependent nuclear export signal sequences, and have been reported to mediate nuclear-cytoplasmic shuttling of β-catenin (Rosin-Arbesfeld et al. 2000; Henderson and Fagotto 2002; Cong and Varmus 2004). However, direct observation of intercompartmental movement using fluorescence recovery after photobleaching showed that APC and Axin enriched cytoplasmic β-catenin in a CRM-1 independent manner, and neither increased the rate of nuclear-cytoplasmic shuttling of β-catenin, suggesting that these proteins retain β-catenin in the cytosol (Krieghoff et al. 2006).
Cytoplasmic sequestration of β-catenin would insure that no signaling occurs before its actual destruction. Peifer and colleagues tested the ability of various Drosophila APC2 mutants to rescue β-catenin destruction in SW480 cells, and their effects in flies lacking APC2 only or both APC1 and APC2. Note that APC2 is the major form in fly embryos, but in single APC2 mutants, APC1 provides residual activity such that levels of β-catenin are only slightly elevated, producing effects on cell fate but not leading to complete nonviability (McCartney et al. 1999). By deleting R2 and/or the CID to prevent β-catenin destruction, Roberts et al. (2011) could assess the function of β-catenin-binding activity of the 15-mers and the other 20-mer repeats. An APC2 mutant lacking all 20-mers except for the high-affinity 20R3 was able to reduce nuclear β-catenin enrichment, consistent with a role in cytoplasmic retention. Likewise, Kohler et al. (2009) found that cell lines overexpressing a truncated human APC that included 20R3 displayed down-regulation of β-catenin, whereas removal of 20R3 (but retention of 20R2-CID) produced relatively poor down-regulation. Thus, a lack of high-affinity β-catenin-binding sites impairs down-regulation, consistent with the importance of cytoplasmic retention.
Roberts et al. (2011) found that an APC variant lacking all 20-mers displayed cytoplasmic β-catenin retention activity, presumably owing to the presence of the 15-mers, and could rescue cell-fate defects in single APC2 mutants, although it failed to rescue lethality of double APC1/APC2 mutant flies. Importantly, removal of all 15- and 20-mer repeats destroyed the ability to rescue the single APC2 mutant flies. These data suggest that cytoplasmic retention is an important function of APC, but that no one β-catenin-binding repeat is essential for this purpose. These experiments show that retention activity has at least an important modulatory role, but an APC variant containing only 20R2 and the CID, but none of the other β-catenin-binding repeats, would have to be tested to confirm an absolute requirement for β-catenin binding. The apparent need for cytoplasmic retention is consistent with the observation that colon tumors bearing mutant APCs carry at least one allele encoding a truncated protein. The “just right” model hypothesizes that these mutations are selected to provide sufficient destruction activity to avoid programmed cell death resulting from excessive β-catenin-mediated signaling (Albuquerque et al. 2002); buffering of free β-catenin levels by APC would provide the needed activity.
Ha et al. (2004) proposed that the presence of low- and high-affinity binding sites on APC provides a wide dynamic range for sequestering cytoplasmic β-catenin under different conditions. Binding of APC and Axin via the SAMP-RGS interaction would enable phosphorylation of 20-mer repeats regardless of the presence of β-catenin. High-affinity β-catenin-binding sites produced by APC phosphorylation would sequester β-catenin and prevent its nuclear localization when no Wnt signal is present and the concentration of free β-catenin is low. When β-catenin levels increase following a Wnt stimulus, binding to the lower affinity sites (both 15- and nonphosphorylated 20-mers) would help to reduce free β-catenin levels to turn off the signal. This model, however, does not explain how Axin would be able to access β-catenin bound to high-affinity APC sites to scaffold phosphorylation of β-catenin. It is possible that this is accomplished through the APC–Axin RGS interaction; alternatively, PP2A-mediated dephosphorylation suffices for this purpose, but, as noted above, no measurements of APC dephosphorylation kinetics have been reported.
Destruction Complex Localization
A pool of APC localizes to the basolateral cortex or cell–cell junctions of vertebrate epithelial cells and tissues, and Drosophila APC2 likewise localizes to the cell cortex through interactions with the microtubule and actin cytoskeletons (Näthke et al. 1996; McCartney et al. 1999, 2006; Yu et al. 1999; Rosin-Arbesfeld et al. 2001; Langford et al. 2006a,b; Grohmann et al. 2007; Zhou et al. 2011). In studies of nuclear export by APC, it was found that truncated APCs lacking cytoskeletal interaction regions shuttle between the nucleus and cytoplasm more efficiently than the full-length protein, suggesting that cytoskeletal interactions help to retain APC in the cytosol (Brocardo et al. 2005), consistent with a role in cytoplasmic retention of β-catenin.
Activation of the coreceptors LRP5/6 and Fzd results in recruitment of the destruction complex to the membrane and formation of membrane-associated puncta associated with β-catenin activation (Cliffe et al. 2003; Tolwinski et al. 2003; Bilic et al. 2007; Schwarz-Romond et al. 2007b; Hendriksen et al. 2008; MacDonald and He 2012). The recently described WTX/AMER1 protein binds to the APC arm domain and to PIP2, and appears to be important for targeting the complex to the plasma membrane, although this protein appears to be specific to vertebrates (Grohmann et al. 2007; Tanneberger et al. 2011). In addition, the carboxy-terminal-binding protein (CtBP) binds to the 15-mer repeats of APC and promotes polymerization of truncated APC to form cytoplasmic puncta (Hamada and Bienz 2004; Schneikert et al. 2011), but the relationship between these puncta and the Wnt-stimulated assemblies is unclear.
Collectively, these observations suggest that APC localization might be important in its ability to modulate β-catenin destruction. However, Roberts et al. (2012) recently found that targeting fly APC2 to several different nonphysiological subcellular locations, including mitochondrial membranes and cytoplasmic vesicles, did not significantly alter down-regulation of β-catenin protein levels, TOPFLASH activation, or Drosophila embryo development. Moreover, these APC2 targeting variants do not appear to enter the nucleus at any time (Roberts et al. 2012), indicating that the ability of APC to enter the nucleus has either a relatively minor role in Wnt signaling, or is related to functions of APC apart from the Wnt pathway.
CONCLUDING REMARKS
A great deal is known about elements of the β-catenin destruction complex, but a complete molecular understanding of its function has remained elusive. This is due in part to the complexity of APC, the study of which is complicated by its other roles apart from Wnt signaling (McCartney and Nathke 2008). Outstanding problems include whether β-catenin binding by APC is an essential or modulatory aspect of the destruction complex, how the 20R2-CID region of APC functions in β-catenin ubiquitination, and the mechanism by which amino-terminally phosphorylated and ubiquitinated β-catenin is handed off to the proteasome. The function of PP2A in the complex is another open question. Finally, although the importance of cellular localization of destruction complex components has been challenged recently (Roberts et al. 2012), data from other systems would be useful in assessing potential roles of localization on Wnt signal transduction through LRP5/6, Fzd, and Dvl.
Many of the proteins that are necessary for β-catenin destruction, including GSK-3, CK1, Axin, and APC, also have roles in stabilizing β-catenin on Wnt stimulation (Liu et al. 2002; Cong et al. 2004; Zeng et al. 2005, 2008; Takacs et al. 2008; Tanneberger et al. 2011), thus confounding genetic analyses and cell culture overexpression studies in difficult-to-predict ways. Biochemical studies of purified protein complexes, as well as experiments that carefully control expression levels, will be important in resolving many of the mysteries surrounding the destruction complex.
ACKNOWLEDGMENTS
This work is supported by grant GM56169 from the U.S. National Institutes of Health.
Footnotes
Editors: Roel Nusse, Xi He, and Renee van Amerongen
Additional Perspectives on Wnt Signaling available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Aberle H, Bauer A, Stappert J, Kispert A, Kemler R 1997. β-Catenin is a target for the ubiquitin-proteasome pathway. EMBO J 16: 3797–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque C, Breukel C, van der Luijt R, Fidalgo P, Lage P, Slors FJ, Leitao CN, Fodde R, Smits R 2002. The “just-right” signaling model: APC somatic mutations are selected based on a specific level of activation of the β-catenin signaling cascade. Hum Mol Genet 11: 1549–1560 [DOI] [PubMed] [Google Scholar]
- Amit S, Hatzubai A, Birman Y, Andersen JS, Ben-Shushan E, Mann M, Ben-Neriah Y, Alkalay I 2002. Axin-mediated CKI phosphorylation of β-catenin at Ser 45: A molecular switch for the Wnt pathway. Genes Dev 16: 1066–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kühl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W 1996. Functional interaction of β-catenin with the transcription factor LEF-1. Nature 382: 638–642 [DOI] [PubMed] [Google Scholar]
- Behrens J, Jerchow B-A, Würtele M, Grimm J, Asbrand C, Wirtz R, Kühl M, Wedlich D, Birchmeier W 1998. Functional interaction of an Axin homolog, conductin, with β-catenin, APC, and GSK3β. Science 280: 596–599 [DOI] [PubMed] [Google Scholar]
- Benchabane H, Hughes EG, Takacs CM, Baird JR, Ahmed Y 2008. Adenomatous polyposis coli is present near the minimal level required for accurate graded responses to the Wingless morphogen. Development 135: 963–971 [DOI] [PubMed] [Google Scholar]
- Bilic J, Huang YL, Davidson G, Zimmermann T, Cruciat CM, Bienz M, Niehrs C 2007. Wnt induces LRP6 signalosomes and promotes dishevelled-dependent LRP6 phosphorylation. Science 316: 1619–1622 [DOI] [PubMed] [Google Scholar]
- Breitman M, Zilberberg A, Caspi M, Rosin-Arbesfeld R 2008. The armadillo repeat domain of the APC tumor suppressor protein interacts with Striatin family members. Biochim Biophys Acta 1783: 1792–1802 [DOI] [PubMed] [Google Scholar]
- Brocardo M, Nathke IS, Henderson BR 2005. Redefining the subcellular location and transport of APC: New insights using a panel of antibodies. EMBO Rep 6: 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capelluto DG, Kutateladze TG, Habas R, Finkielstein CV, He X, Overduin M 2002. The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419: 726–729 [DOI] [PubMed] [Google Scholar]
- Cegielska A, Gietzen KF, Rivers A, Virshup DM 1998. Autoinhibition of Casein Kinase Ie (CKIε) is relieved by protein phosphatases and limited proteolysis. J Biol Chem 273: 1357–1364 [DOI] [PubMed] [Google Scholar]
- Chia IV, Costantini F 2005. Mouse axin and axin2/conductin proteins are functionally equivalent in vivo. Mol Cell Biol 25: 4371–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H-J, Huber AH, Weis WI 2006. Thermodynamics of β-catenin–ligand interactions. The roles of the N- and C-terminal tails in modulating binding affinity. J Biol Chem 281: 1027–1038 [DOI] [PubMed] [Google Scholar]
- Clevers H 2006. Wnt/β-catenin signaling in development and disease. Cell 127: 469–480 [DOI] [PubMed] [Google Scholar]
- Cliffe A, Hamada F, Bienz M 2003. A role of Dishevelled in relocating Axin to the plasma membrane during wingless signaling. Curr Biol 13: 960–966 [DOI] [PubMed] [Google Scholar]
- Cong F, Varmus H 2004. Nuclear-cytoplasmic shuttling of Axin regulates subcellular localization of β-catenin. Proc Natl Acad Sci 101: 2882–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong F, Schweizer L, Varmus H 2004. Casein kinase Iε modulates the signaling specificities of dishevelled. Mol Cell Biol 24: 2000–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosas B, Hanna J, Kirkpatrick DS, Zhang DP, Tone Y, Hathaway NA, Buecker C, Leggett DS, Schmidt M, King RW, et al. 2006. Ubiquitin chains are remodeled at the proteasome by opposing ubiquitin ligase and deubiquitinating activities. Cell 127: 1401–1413 [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378: 785–789 [DOI] [PubMed] [Google Scholar]
- Dajani R, Faser E, Roe SM, Yeo M, Good VM, Thompson V, Dale TC, Pearl LH 2003. Structural basis for recruitment of glycogen synthase kinase 3β to the axin-APC scaffold complex. EMBO J 22: 494–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Alber T 2000. Crystal structure of the amino-terminal coiled-coil domain of the APC tumor suppressor. J Mol Biol 301: 147–156 [DOI] [PubMed] [Google Scholar]
- Ding VW, Chen RH, McCormick F 2000. Differential regulation of glycogen synthase kinase 3β by insulin and Wnt signaling. J Biol Chem 275: 32475–32481 [DOI] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR 2007. Functional redundancy of GSK-3α and GSK-3β in Wnt/β-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell 12: 957–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez I, Itoh K, Sokol SY 1995. Role of glycogen synthase kinase 3β as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci 92: 8498–8502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklof Spink K, Fridman SG, Weis WI 2001. Molecular mechanisms of β-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC-β-catenin complex. EMBO J 20: 6203–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyada E, Pribluda A, Goldstein RE, Morgenstern Y, Brachya G, Cojocaru G, Snir-Alkalay I, Burstain I, Haffner-Krausz R, Jung S, et al. 2011. CKIα ablation highlights a critical role for p53 in invasiveness control. Nature 470: 409–413 [DOI] [PubMed] [Google Scholar]
- Fagotto F, Jho E, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F 1999. Domains of axin involved in protein-protein interactions, wnt pathway inhibition, and intracellular localization. J Cell Biol 145: 741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler M, Mendoza-Topaz C, Rutherford TJ, Mieszczanek J, Bienz M 2011. Dishevelled interacts with the DIX domain polymerization interface of Axin to interfere with its function in down-regulating β-catenin. Proc Natl Acad Sci 108: 1937–1942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiol CJ, Mahrenholz AM, Wang Y, Roeske RW, Roach PJ 1987. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem 262: 14042–14048 [PubMed] [Google Scholar]
- Frame S, Cohen P 2001. GSK3 takes centre stage more than 20 years after its discovery. Biochem J 359: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame S, Cohen P, Biondi RM 2001. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol Cell 7: 1321–1327 [DOI] [PubMed] [Google Scholar]
- Grohmann A, Tanneberger K, Alzner A, Schneikert J, Behrens J 2007. AMER1 regulates the distribution of the tumor suppressor APC between microtubules and the plasma membrane. J Cell Sci 120: 3738–3747 [DOI] [PubMed] [Google Scholar]
- Ha N-C, Tonozuka T, Stamos JL, Choi H-J, Weis WI 2004. Mechanism of phosphorylation-dependent binding of APC to β-catenin and its role in β-catenin degradation. Mol Cell 15: 511–521 [DOI] [PubMed] [Google Scholar]
- Hagen T, Daniel ED, Culbert AA, Reith AD 2002. Expression and characterization of GSK-3 mutants and their effect on β-catenin phosphorylation in intact cells. J Biol Chem 277: 23330–23335 [DOI] [PubMed] [Google Scholar]
- Hamada F, Bienz M 2004. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear β-catenin from TCF. Dev Cell 7: 677–685 [DOI] [PubMed] [Google Scholar]
- Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P 1998. Downregulation of β-catenin by human Axin and its association with the APC tumor suppressor, β-catenin, and GSK3β. Curr Biol 8: 573–581 [DOI] [PubMed] [Google Scholar]
- Hart M, Concordet JP, Lassot I, Albert I, del los Santos R, Durand H, Perret C, Rubinfeld B, Margottin F, Benarous R, et al. 1999. The F-box protein β-TrCP associates with phosphorylated β-catenin and regulates its activity in the cell. Curr Biol 9: 207–210 [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, et al. 2001. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev 15: 1427–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BR, Fagotto F 2002. The ins and outs of APC and β-catenin nuclear transport. EMBO Rep 3: 834–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen J, Jansen M, Brown CM, van der Velde H, van Ham M, Galjart N, Offerhaus GJ, Fagotto F, Fornerod M 2008. Plasma membrane recruitment of dephosphorylated β-catenin upon activation of the Wnt pathway. J Cell Sci 121: 1793–1802 [DOI] [PubMed] [Google Scholar]
- Hsu W, Zeng L, Costantini F 1999. Identification of a domain of Axin that binds to the serine/threonine protein phosphatase 2A and a self-binding domain. J Biol Chem 274: 3439–3445 [DOI] [PubMed] [Google Scholar]
- Ikeda S, Kishida S, Yamamoto H, Murai H, Koyama S, Kikuchi A 1998. Axin, a negative regulator of the Wnt signaling pathway, forms a complex with GSK-3β and β-catenin and promotes GSK-3β-depedent phosphorylation of β-catenin. EMBO J 17: 1371–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda S, Kishida M, Matsuura Y, Usui H, Kikuchi A 2000. GSK-3β-dependent phosphorylation of adenomatous polyposis coli gene product can be modulated by β-catenin and protein phosphatase 2A complexed with Axin. Oncogene 19: 537–545 [DOI] [PubMed] [Google Scholar]
- Itoh K, Tang TL, Neel BG, Sokol SY 1995. Specific modulation of ectodermal cell fates in Xenopus embryos by glycogen synthase kinase. Development 121: 3979–3988 [DOI] [PubMed] [Google Scholar]
- Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F 2002. Wnt/β-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol 22: 1172–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Struhl G 1998. Regulation of the Hedgehog and Wingless signalling pathways by the F-box/WD40-repeat protein Slimb. Nature 391: 493–496 [DOI] [PubMed] [Google Scholar]
- Jimbo T, Kawasaki Y, Koyama R, Sato R, Takada S, Haraguchi K, Akiyama T 2002. Identification of a link between the tumour suppressor APC and the kinesin superfamily. Nat Cell Biol 4: 323–327 [DOI] [PubMed] [Google Scholar]
- Julius MA, Schelbert B, Hsu W, Fitzpatrick E, Jho E, Fagotto F, Costantini F, Kitajewski J 2000. Domains of axin and disheveled required for interaction and function in wnt signaling. Biochem Biophys Res Commun 276: 1162–1169 [DOI] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina TV, Takao K, van Eede M, Hattori S, Laliberte C, Khan M, Okamoto K, Chambers JW, Fletcher PJ, et al. 2009. Abnormalities in brain structure and behavior in GSK-3α mutant mice. Mol Brain 2: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Senda T, Ishidate T, Koyama R, Mirishita T, Iwayama Y, Higuchi O, Akiyama T 2000. Asef, a link between the tumor suppressor APC and G-protein signaling. Science 289: 1194–1197 [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Ikeda S, Kishida M, Sakamoto I, Koyama S, Kikuchi A 1998. Axin, a negative regulator of the Wnt signaling pathway, directly interacts with Adenomatous Polyposis Coli and regulates the stabilization of β-catenin. J Biol Chem 273: 10823–10826 [DOI] [PubMed] [Google Scholar]
- Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A 1999. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate β-catenin stability. Mol Cell Biol 19: 4414–4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Hatakeyama S, Shirane M, Matsumoto M, Ishida N, Hattori K, Nakmichi I, Kikuchi A, Nakayama K, Nakayama K 1999. An F-box protein, FWD1, mediates ubiquitin-dependent proteolysis of β-catenin. EMBO J 18: 2401–2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler EM, Derungs A, Daum G, Behrens J, Schneikert J 2008. Functional definition of the mutation cluster region of adenomatous polyposis coli in colorectal tumours. Hum Mol Genet 17: 1978–1987 [DOI] [PubMed] [Google Scholar]
- Kohler EM, Chandra SH, Behrens J, Schneikert J 2009. β-Catenin degradation mediated by the CID domain of APC provides a model for the selection of APC mutations in colorectal, desmoid and duodenal tumours. Hum Mol Genet 18: 213–226 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H 1997. Constitutive transcriptional activation by a β-catenin–Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Krieghoff E, Behrens J, Mayr B 2006. Nucleo-cytoplasmic distribution of β-catenin is regulated by retention. J Cell Sci 119: 1453–1463 [DOI] [PubMed] [Google Scholar]
- Langford KJ, Askham JM, Lee T, Adams M, Morrison EE 2006a. Examination of actin and microtubule dependent APC localisations in living mammalian cells. BMC Cell Biol 7: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford KJ, Lee T, Askham JM, Morrison EE 2006b. Adenomatous polyposis coli localization is both cell type and cell context dependent. Cell Motil Cytoskel 63: 483–492 [DOI] [PubMed] [Google Scholar]
- Latres E, Chiaur DS, Pagano M 1999. Human F box protein β-Trcp associates with the Cul1/Skp1 complex and regulates the stability of β-catenin. Oncogene 18: 849–854 [DOI] [PubMed] [Google Scholar]
- Lee E, Salic A, Krüger R, Heinrich R, Kirschner MW 2003. The roles of APC and axin derived from experimental and theoretical analysis of the Wnt pathway. PLoS Biol 1: 116–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Lee BH, Hanna J, King RW, Finley D 2011. Trimming of ubiquitin chains by proteasome-associated deubiquitinating enzymes. Mol Cell Proteomics 10: R110.003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER 2002. Activation of AXIN2 expression by β-catenin–T cell factor. A feedback repressor pathway regulating Wnt signaling. J Biol Chem 277: 21657–21665 [DOI] [PubMed] [Google Scholar]
- Li Z, Nathke IS 2005. Tumor-associated NH2-terminal fragments are the most stable part of the Adenomatous Polyposis Coli protein and can be regulated by interactions with COOH-terminal domains. Cancer Res 65: 5195–5204 [DOI] [PubMed] [Google Scholar]
- Liu C, Kato Y, Zhang Z, Do VM, Yankner BA, He X 1999. β-Trcp couples β-catenin phosphorylation-degradation and regulates Xenopus axis formation. Proc Natl Acad Sci 96: 6273–6278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, et al. 2000. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating β-catenin/TCF signalling. Nat Genet 26: 146–147 [DOI] [PubMed] [Google Scholar]
- Liu C, Li Y, Semenov M, Han C, Baeg G-H, Tan Y, Zhang Z, Lin X, He X 2002. Control of β-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108: 837–847 [DOI] [PubMed] [Google Scholar]
- Liu J, Xing Y, Hinds TR, Zheng J, Xu W 2006. The third 20 amino acid repeat is the tightest binding site of APC for β-catenin. J Mol Biol 360: 133–144 [DOI] [PubMed] [Google Scholar]
- Liu YT, Dan QJ, Wang J, Feng Y, Chen L, Liang J, Li Q, Lin SC, Wang ZX, Wu JW 2011. Molecular basis of Wnt activation via the DIX domain protein Ccd1. J Biol Chem 286: 8597–8608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo W, Peterson A, Garcia BA, Coombs G, Kofahl B, Heinrich R, Shabanowitz J, Hunt DF, Yost HJ, Virshup DM 2007. Protein phosphatase 1 regulates assembly and function of the β-catenin degradation complex. EMBO J 26: 1511–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.MacDonald BT, He X 2012. Frizzled and LRP5/6 receptors for Wnt/β-catenin signalings. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a007880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major MB, Camp ND, Berndt JD, Yi X, Goldenberg SJ, Hubbert C, Biechele TL, Gingras AC, Zheng N, Maccoss MJ, et al. 2007. Wilms tumor suppressor WTX negatively regulates WNT/β-catenin signaling. Science 316: 1043–1046 [DOI] [PubMed] [Google Scholar]
- Marikawa Y, Elinson RP 1998. β-TrCP is a negative regulator of Wnt/β-catenin signaling pathway and dorsal axis formation in Xenopus embryos. Mech Dev 77: 75–80 [DOI] [PubMed] [Google Scholar]
- Marin O, Bustos VH, Cesaro L, Meggio F, Pagano MA, Antonelli M, Allende CC, Pinna LA, Allende JE 2003. A noncanonical sequence phosphorylated by casein kinase 1 in β-catenin may play a role in casein kinase 1 targeting of important signaling proteins. Proc Natl Acad Sci 100: 10193–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubayashi H, Sese S, Lee J-S, Shirakawa T, Iwatsubo T, Tomita T, Yanagawa S 2004. Biochemical characterization of the Drosophila Wingless signaling pathway based on RNA interference. Mol Cell Biol 24: 2012–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, Nathke IS 2008. Cell regulation by the Apc protein Apc as master regulator of epithelia. Curr Opin Cell Biol 20: 186–193 [DOI] [PubMed] [Google Scholar]
- McCartney BM, Dierick HA, Kirkpatrick C, Moline MM, Baas A, Peifer M, Bejsovec A 1999. Drosophila APC2 is a cytoskeletally-associated protein that regulates wingless signaling in the embryonic epidermis. J Cell Biol 146: 1303–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney BM, Price MH, Webb RL, Hayden MA, Holot LM, Zhou M, Bejsovec A, Peifer M 2006. Testing hypotheses for the functions of APC family proteins using null and truncation alleles in Drosophila. Development 133: 2407–2418 [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR 2005. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. EMBO J 24: 1571–1583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H 1996. XTcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [DOI] [PubMed] [Google Scholar]
- Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW 1997. Activation of β-catenin–Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275: 1787–1790 [DOI] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Souza B, Rubinfeld B, Polakis P 1995. Regulation of intracellular β-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci 92: 3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S, Albert I, Rubinfeld B, Polakis P 1996. Deletion of an amino-terminal sequence stabilizes β-catenin in vivo and promotes hyperphosphorylation of the adenomatous polyposis coli tumor suppressor protein. Mol Cell Biol 16: 4088–4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke IS, Adams CL, Polakis P, Sellin JH, Nelson WJ 1996. The adenomatous polyposis coli tumor suppressor protein localizes to plasma membrane sites involved in active cell migration. J Cell Biol 134: 165–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noutsou M, Duarte AM, Anvarian Z, Didenko T, Minde DP, Kuper I, de Ridder I, Oikonomou C, Friedler A, Boelens R, et al. 2011. Critical scaffolding regions of the tumor suppressor Axin1 are natively unfolded. J Mol Biol 405: 773–786 [DOI] [PubMed] [Google Scholar]
- Orford K, Crockett C, Jensen JP, Weissman AM, Byers SW 1997. Serine phosphorylation-regulated ubiquitination and degradation of β-catenin. J Biol Chem 272: 24735–24738 [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R 1989. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 8: 1711–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR 2008. Tissue-specific role of glycosen synthase kinase 3β in glucose homeostatis and insulin action. Mol Cell Biol 28: 6314–6328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters JM, McKay RM, McKay JP, Graff JM 1999. Casein kinase I transduces Wnt signals. Nature 401: 345–350 [DOI] [PubMed] [Google Scholar]
- Peterson-Nedry W, Erdeniz N, Kremer S, Yu J, Baig-Lewis S, Wehrli M 2008. Unexpectedly robust assembly of the Axin destruction complex regulates Wnt/Wg signaling in Drosophila as revealed by analysis in vivo. Dev Biol 320: 226–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyrollier K, Hajduch E, Blair AS, Hyde R, Hundal HS 2000. L-leucine availability regulates phosphatidylinositol 3-kinase, p70 S6 kinase and glycogen synthase kinase-3 activity in L6 muscle cells: Evidence for the involvement of the mammalian target of rapamycin (mTOR) pathway in the L-leucine-induced up-regulation of system A amino acid transport. Biochem J 350 (Pt 2): 361–368 [PMC free article] [PubMed] [Google Scholar]
- Rape M, Hoppe T, Gorr I, Kalocay M, Richly H, Jentsch S 2001. Mobilization of processed, membrane-tethered SPT23 transcription factor by CDC48(UFD1/NPL4), a ubiquitin-selective chaperone. Cell 107: 667–677 [DOI] [PubMed] [Google Scholar]
- Ratcliffe MJ, Itoh K, Sokol SY 2000. A positive role for the PP2A catalytic subunit in Wnt signal transduction. J Biol Chem 275: 35680–35683 [DOI] [PubMed] [Google Scholar]
- Roberts DM, Pronobis MI, Poulton JS, Waldmann JD, Stephenson EM, Hanna S, Peifer M 2011. Deconstructing the β-catenin destruction complex: Mechanistic roles for the tumor suppressor APC in regulating Wnt signaling. Mol Biol Cell 22: 1845–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Pronobis MI, Poulton JS, Kane EG, Peifer M 2012. Regulation of Wnt signaling by the tumor suppressor APC does not require ability to enter the nucleus nor a particular cytoplasmic location. Mol Biol Cell 10.1091/mbc.E11-11-0965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R, Townsley F, Bienz M 2000. The APC tumour suppressor has a nuclear export function. Nature 406: 1009–1012 [DOI] [PubMed] [Google Scholar]
- Rosin-Arbesfeld R, Ihrke G, Bienz M 2001. Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. EMBO J 20: 5929–5939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P 1995. The APC protein and E-cadherin form similar but independent complexes with α-catenin, β-catenin, and plakoglobin. J Biol Chem 270: 5549–5555 [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Fiol C, Munemitsu S, Polakis P 1996. Binding of GSK3β to the APC-β-catenin complex and regulation of complex assembly. Science 272: 1023–1026 [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Albert I, Porfiri E, Munemitsu S, Polakis P 1997a. Loss of β-catenin regulation by the APC tumor suppressor protein correlates with loss of structure due to common somatic mutations of the gene. Cancer Res 57: 4624–4630 [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P 1997b. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 275: 1790–1792 [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Tice DA, Polakis P 2001. Axin-dependent phosphorylation of the Adenomatous Polyposis Coli protein mediated by casein kinase 1ε. J Biol Chem 276: 39037–39045 [DOI] [PubMed] [Google Scholar]
- Ruel L, Bourouis M, Heitzler P, Pantesco V, Simpson P 1993. Drosophila shaggy kinase and rat glycogen synthase kinase-3 have conserved activities and act downstream of Notch. Nature 362: 557–560 [DOI] [PubMed] [Google Scholar]
- Sadot E, Conacci-Sorrel M, Zhurinsky J, Shnizer D, Lando Z, Zharhary D, Kam Z, Ben-Ze’ev A, Geiger B 2002. Regulation of S33/S37 phosphorylated β-catenin in normal and transformed cells. J Cell Sci 115: 2771–2780 [DOI] [PubMed] [Google Scholar]
- Sakanaka C, Weiss JB, Williams LT 1998. Bridging of β-catenin and glycogen synthase kinase-3β by axin and inhibition of β-catenin-mediated transcription. Proc Natl Acad Sci 95: 3020–3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salic A, Lee E, Mayer L, Kirschner MW 2000. Control of β-catenin stability: Reconstitution of the cytoplasmic steps of the Wnt pathway in Xenopus egg extracts. Mol Cell 5: 523–532 [DOI] [PubMed] [Google Scholar]
- Satoh S, Daigo Y, Furukawa Y, Kato T, Miwa N, Nishiwaki T, Kawasoe T, Ishiguro H, Fujita M, Tokino T, et al. 2000. AXIN1 mutations in hepatocellular carcinomas, and growth suppression in cancer cells by virus-mediated transfer of AXIN1. Nat Genet 24: 245–250 [DOI] [PubMed] [Google Scholar]
- Schneikert J, Brauburger K, Behrens J 2011. APC mutations in colorectal tumours from FAP patients are selected for CtBP-mediated oligomerization of truncated APC. Hum Mol Genet 20: 3554–3564 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Fiedler M, Shibata N, Butler PJ, Kikuchi A, Higuchi Y, Bienz M 2007a. The DIX domain of Dishevelled confers Wnt signaling by dynamic polymerization. Nat Struct Mol Biol 14: 484–492 [DOI] [PubMed] [Google Scholar]
- Schwarz-Romond T, Metcalfe C, Bienz M 2007b. Dynamic recruitment of axin by Dishevelled protein assemblies. J Cell Sci 120: 2402–2412 [DOI] [PubMed] [Google Scholar]
- Seeling JM, Miller JR, Gil R, Moon RT, White R, Virshup DM 1999. Regulation of β-catenin signaling by the B56 subunit of protein phosphatase 2A. Science 283: 2089–2091 [DOI] [PubMed] [Google Scholar]
- Shi W, Ma Z, Willers H, Akhtar K, Scott SP, Zhang J, Powell S 2008. Disassembly of MDC1 foci is controlled by ubiquitin-proteasome-dependent degradation. J Biol Chem 283: 31608–31616 [DOI] [PubMed] [Google Scholar]
- Siegfried E, Chou TB, Perrimon N 1992. Wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell 71: 1167–1179 [DOI] [PubMed] [Google Scholar]
- Smits R, Kielman MF, Breukel C, Zurcher C, Neufeld K, Jagmohan-Changur S, Hofland N, van Dijk J, White R, Edelmann W, et al. 1999. Apc1638T: A mouse model delineating critical domains of the adenomatous polyposis coli protein involved in tumorigenesis and development. Genes Dev 13: 1309–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrado P, Jedlicki A, Bustos VH, Allende CC, Allende JE 2005. Basic region of residues 228–231 of protein kinase CK1α is involved in its interaction with axin: Binding to axin does not affect the kinase activity. J Cell Biochem 94: 217–224 [DOI] [PubMed] [Google Scholar]
- Spink KE, Polakis P, Weis WI 2000. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J 19: 2270–2279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink KE, Fridman SG, Weis WI 2001. Molecular mechanisms of β-catenin recognition by adenomatous polyposis coli revealed by the structure of an APC/β-catenin complex. EMBO J 20: 6203–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Y, Fu C, Ishikawa S, Stella A, Kojima M, Shitoh K, Schreiber EM, Day BW, Liu B 2008. APC is essential for targeting phosphorylated β-catenin to the SCFβ-TrCP ubiquitin ligase. Mol Cell 32: 652–661 [DOI] [PubMed] [Google Scholar]
- Takacs CM, Baird JR, Hughes EG, Kent SS, Benchabane H, Paik R, Ahmed Y 2008. Dual positive and negative regulation of wingless signaling by adenomatous polyposis coli. Science 319: 333–336 [DOI] [PubMed] [Google Scholar]
- Tan CW, Gardiner BS, Hirokawa Y, Layton MJ, Smith DW, Burgess AW 2012. Wnt signalling pathway parameters for mammalian cells. PLoS ONE 7: e31882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanneberger K, Pfister AS, Brauburger K, Schneikert J, Hadjihannas MV, Kriz V, Schulte G, Bryja V, Behrens J 2011. Amer1/WTX couples Wnt-induced formation of PtdIns(4,5)P2 to LRP6 phosphorylation. EMBO J 30: 1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolwinski NS, Wieschaus E 2001. Armadillo nuclear import is regulated by cytoplasmic anchor Axin and nuclear anchor dTCF/Pan. Development 128: 2107–2117 [DOI] [PubMed] [Google Scholar]
- Tolwinski NS, Wehrli M, Rives A, Erdeniz N, DiNardo S, Wieschaus E 2003. Wg/Wnt signal can be transmitted through arrow/LRP5,6 and Axin independently of Zw3/Gsk3β activity. Dev Cell 4: 407–418 [DOI] [PubMed] [Google Scholar]
- Valvezan AJ, Zhang F, Diehl JA, Klein PS 2012. Adenomatous polyposis coli (APC) regulates multiple signaling pathways by enhancing glycogen synthase kinase-3 (GSK-3) activity. J Biol Chem 287: 3823–3832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Wang S, Noritake J, Sato K, Fukata M, Takefuji M, Nakagawa M, Izumi N, Akiyama T, Kaibuchi K 2004. Interaction with IQGAP1 links APC to Rac1, Cdc42, and actin filaments during cell polarization and migration. Dev Cell 7: 871–883 [DOI] [PubMed] [Google Scholar]
- Winston JT, Strack P, Beer-Romero P, Chu CY, Elledge SJ, Harper JW 1999. The SCFβ-TRCP-ubiquitin ligase complex associates specifically with phosphorylated destruction motifs in IκBα and β-catenin and stimulates IκBα ubiquitination in vitro. Genes Dev 13: 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR 1990. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J 9: 2431–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, He X 2006. Threonine 41 in β-catenin serves as a key phosphorylation relay residue in β-catenin degradation. Biochemistry 45: 5319–5323 [DOI] [PubMed] [Google Scholar]
- Wu G, Xu G, Schulman BA, Jeffrey PD, Harper JW, Pavletich NP 2003. Structure of a β-TrCP1-Skp1-β-catenin complex: Destruction motif binding and lysine specificity of the SCF(β-TrCP1) ubiquitin ligase. Mol Cell 11: 1445–1456 [DOI] [PubMed] [Google Scholar]
- Xing Y, Clemens WK, Kimmelman D, Xu W 2003. Crystal structure of a β-catenin/Axin complex suggests a mechanism for the β-catenin destruction complex. Genes Dev 17: 2753–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Clements WK, Le Trong I, Hinde TR, Stenkamp R, Kimelman D, Xu W 2004. Crystal structure of a β-catenin/APC complex reveals a critical role for APC phosphorylation in APC function. Mol Cell 15: 523–533 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Kishida S, Kishida M, Ikeda S, Takada S, Kikuchi A 1999. Phosphorylation of Axin, A Wnt signal negative regulator, by glycogen synthase kinase-3β regulates its stability. J Biol Chem 274: 10681–10684 [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Hinoi T, Michiue T, Fukui A, Usui H, Janssens V, Van Hoof C, Goris J, Asashima M, Kikuchi A 2001. Inhibition of the Wnt signaling pathway by the PR61 subunit of protein phosphatase 2A. J Biol Chem 276: 26875–26882 [DOI] [PubMed] [Google Scholar]
- Yang J, Zhang W, Evans PM, Chen X, He X, Liu C 2006. Adenomatous polyposis coli (APC) differentially regulates β-catenin phosphorylation and ubiquitination in colon cancer cells. J Biol Chem 281: 17751–17757 [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT 1996. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopus embryos by glycogen synthase kinase 3. Genes Dev 10: 1443–1454 [DOI] [PubMed] [Google Scholar]
- Yu X, Waltzer L, Bienz M 1999. A new Drosophila APC homologue associated with adhesive zones of epithelial cells. Nat Cell Biol 1: 144–151 [DOI] [PubMed] [Google Scholar]
- Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek TJ, Perry WL 3rd, Lee JJ, Tilghman SM, Gumbiner BM, Costantini F 1997. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell 90: 181–192 [DOI] [PubMed] [Google Scholar]
- Zeng X, Tamai K, Doble B, Li S, Huang H, Habas R, Okamura H, Woodgett J, He X 2005. A dual-kinase mechanism for Wnt co-receptor phosphorylation and activation. Nature 438: 873–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Huang H, Tamai K, Zhang X, Harada Y, Yokota C, Almeida K, Wang J, Doble B, Woodgett J, et al. 2008. Initiation of Wnt signaling: Control of Wnt coreceptor Lrp6 phosphorylation/activation via frizzled, dishevelled and axin functions. Development 135: 367–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MN, Kunttas-Tatli E, Zimmerman S, Zhouzheng F, McCartney BM 2011. Cortical localization of APC2 plays a role in actin organization but not in Wnt signaling in Drosophila. J Cell Sci 124: 1589–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]