Abstract
Breast cancer can recur even decades after the primary therapy. Markers are needed to predict cancer progression and the risk of late recurrence. The estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), proliferation marker Ki-67, and cytokeratin CK5 were studied to find out whether their expression or occurrence in subgroups of breast cancers correlated with the time of recurrence. The expression of HER2, ER, PR, Ki-67, and CK5 was studied by IHC in 72 primary breast cancers and their corresponding recurrent/metastatic lesions. The patients were divided into three groups according to the time of the recurrence/metastasis: before two years, after 5 years, and after 10 years. Based on their IHC profiles, the tumors were divided into surrogates of the genetically defined subgroups of breast cancers and the subtype definitions were as follows: luminal A (ER or PR+HER2–), luminal B (ER or PR+HER2+), HER2 overexpressing (ER–PR–HER2+), triple-negative (ER–PR–HER2–), basal-like (ER–PR–HER2–CK5+), non-classified (ER–PR–HER2–CK5–) and luminobasal (ER or PR+CK5+). In multivariate analysis, tumor size and HER2 positivity were a significant risk of early cancer relapse. The metastases showed a significantly lower CK5 expression. CK5 positivity distinguished triple negative tumors into rapidly and slowly recurring cancers. The IHC subtype ER or PR+HER2– luminal A presented a significantly lower risk of early tumor recurrence. Ki-67 expression denoted early-relapsing tumors and correlated linearly with tumor progression, since Ki-67 positivity declined gradually from early-relapsing toward late-recurring cancers.
Keywords: early and late relapsing breast cancers, CK5, immunohistochemistry
Introduction
Breast cancer is prone to recur even decades after initial treatment. The biology behind the extended survival of cancer cells, known as tumor dormancy, is still poorly understood.1 A small number of single biomarkers, including estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER2), and proliferation marker Ki-67 have been used for several years to predict the prognosis of breast cancer and to guide its therapy. The biological importance of these established markers has been reinforced over the past decade by the results from genomic classification. DNA microarray profiling studies of breast tumors has identified distinct subtypes of breast carcinomas that are associated with different clinical outcomes.2 Using an intrinsic set of 534 genes, Sørlie et al3 analyzed the expression profiles of 115 independent breast tumor samples and categorized the breast tumors into five groups: luminal A (ER+); luminal B (ER+); HER2 overexpressing; normal breast-like, and basal-like.
Based on gene expression studies, the expression of the basal-like breast cancer markers, ie, cytokeratin 5/6 and cytokeratin 17, have been shown to predict poor outcome in breast cancer patients.2–5
The advent of new genetic tests has also emphasized the role of proliferative genes, including Ki-67, as prognostic and predictive markers. Cheang and colleagues described an immunopanel of ER, PR, HER2, and Ki-67 that can segregate the luminal A and B subtypes in a similar manner to that defined by the expression profile of 50 genes.6 Luminal B breast cancers with Ki-67 levels of at least 14% had a worse prognosis for both breast cancer recurrence and survival compared with luminal A tumors with Ki-67 levels of less than 14%.6
HER2 is a member of the Erb family that plays an important role in promoting oncogenic transformation and tumor growth.7,8 The tumors of approximately 25%–30% of the patients with breast cancer over express HER2 protein, and this overexpression is correlated with a poor clinical outcome.9–11 The HER2 receptor has become important as a target for antibody-based therapy with trastuzumab. In addition to the treatment of the metastatic disease, adjuvant treatment of primary HER2-positive breast cancers with trastuzumab has been shown to markedly improve the outcome of the patients.12 In patients with metastatic disease, selection for therapy with trastuzumab has traditionally been based on the HER2 status of the primary tumor.
Ki-67 is a nuclear non-histone protein and an antigen associated with cell proliferation. It was identified after immunization of mice with Hodgkin’s lymphoma.13,14 The murine monoclonal antibody Ki-67 reacts with a human nuclear antigen that is expressed in G1, S, G2, and mitosis, but not in G0.15 Numerous studies have shown that Ki-67 is of prognostic value in many types of malignant tumors. In breast cancer, a strong correlation has been found between the percentage of cells positive for Ki-67 and nuclear grade, age, and mitotic rate.16,17
In normal breast, both luminal epithelial, and the myoepithelial cells exhibit different and distinctive keratin phenotypes. CK 7, 8, 18 and 19 are expressed in the luminal cells, while smooth muscle actin (α) and cytokeratins (CKs) 5, 14 and 17 are found in the myoepithelial/basal cells.18
In a tissue microarray study of a large cohort of breast cancers, the expression of luminal markers CKs 7, 8, 18 and 19 was associated with good prognostic tumor characteristics, in contrast to expression of basal markers.19 The expression of the luminal markers was shown to be related to good overall survival. The opposite was observed in tumors that labeled with the basal markers, and positive cases were associated with poor outcome, particularly with CK5 expression.19
Our aim was to clarify the differences in the expression of the established prognostic and predictive markers ER, PR, HER2 and Ki-67 with the basal/myoepithelial cytokeratin CK5 by IHC, and HER2 also by chromogenic in situ hybridization (CISH), in both early and late relapsing breast cancers. The markers were analyzed individually and by a modified subtype definition used by Cheang et al20 and Carey et al21 who defined the subtypes by biomarkers ER, PR, HER2, EFGR and CK5.
Materials and Methods
Patients and tumors
We collected paraffin-embedded tissue blocks from 72 primary breast cancers and their respective recurrent/metastatic lesions from the archives of the Department of Pathology, Helsinki University Hospital, as previously described.22 The patients had undergone breast cancer surgery between 1974–2006. Recurrence/metastasis was defined as any local or regional recurrence or any distant metastatic disease. The cases were divided into three groups according to the time of recurrence/metastasis: Group 1 (n = 19) tumors with recurrent/metastatic lesions within two years after primary surgery; Group 2 (n = 34) with recurrences/metastases after 5–10 years; and Group 3 (n = 19) with recurrences/metastases after more than 10 (range > 10 to 23) years. The histological tumor type and grade were assigned according to the criteria of Elston and Ellis.23 The clinico-pathological characteristics of the patients and their cancers are summarized in Table 1. The Ethics Committee of the Helsinki University Central Hospital approved the study protocol.
Table 1.
Summary of clinicopathological features of 72 breast cancer patients.
| Group 1 n = 19 | Group 2 n = 34 | Group 3 n = 19 | |
|---|---|---|---|
| Age at surgery of primary tumor | |||
| <50 years | 10 | 14 | 12 |
| ≥50 years | 9 | 20 | 7 |
| Tumor size | |||
| ≥20 mm | 3 | 16 | 11 |
| <20 mm | 16 | 18 | 8 |
| Lymph node | |||
| Negative | 3 | 20 | 12 |
| Positive | 16 | 14 | 8 |
| Grade | |||
| 1 | 0 | 5 | 3 |
| 2 | 8 | 19 | 15 |
| 3 | 11 | 10 | 1 |
| Histological type | |||
| Ductal | 16 | 28 | 12 |
| Lobular | 3 | 5 | 7 |
| Mucinous | 0 | 1 | 0 |
| Tissue site of recurrence/metastasis | |||
| Skin | 5 | 8 | 11 |
| Soft tissue | 6 | 11 | 5 |
| Subcutaneous tissue | 0 | 6 | 2 |
| Lung | 0 | 4 | 0 |
| Lymph node | 1 | 0 | 0 |
| Liver | 5 | 1 | 0 |
| Brain | 2 | 2 | 0 |
| Bone | 0 | 1 | 1 |
| Ovary | 0 | 1 | 0 |
Notes: The patients are divided into Groups 1, 2 and 3 according to the time of relapse after the primary diagnosis. Group 1 includes patients with recurrence/metastasis detected within 2 years of diagnosis. Groups 2 and 3 include patients with recurrence or metastasis detected between 5–10 years, and after 10 years of follow-up, respectively.
Immunohistochemistry
Four μm thick sections were deparaffinized in xylene and rehydrated. Antigen retrieval was done by microwaving in 10 mM citric acid monohydrate for 1 × 5 min at 900 W and for 3 × 5 min at 600 W. Endogenous peroxidase activity was blocked by treatment with 0.5% H2O2. The slides were incubated overnight in a refrigerator at +4 °C with appropriate dilutions of the primary antibodies. The same procedure was used for negative controls, except that the incubation overnight took place in PBS diluent without antibody. The reaction was visualized by the Elite ABC Kit (Vectastain, Vector Laboratories, Burlingame, CA, USA) for ER and by the Envision kit (Dako, Copenhagen, Denmark) for HER2. For PR and CK5, the sections were subjected to dual colorimetric IHC (Envision G/2 Doublestain; Dako).24
The result was quantified as the proportion of positively stained tumor cells (range 0%–100%). For the analyses, the tissue samples were classified as positive for ER and PR when ≥1% of the tumor cells showed positive nuclear staining.25 In PR and CK5 dual staining tumors with ≥1% Diaminobenzidine (DAB)-stained nuclei or ≥1% Perm Red-stained cytoplasm were considered positive.
HER2 were scored based on the intensity and percentage of positive cells on a scale of 0 to 3+. Cases were reported 0 (negative) if no staining or membrane staining in less than 10% of invasive tumor cells was seen, 1+ (negative) if faint/barely perceptive membrane staining was detected in more than 10% of invasive tumor cells, 2+ (positive) if weak to moderate complete membrane staining in more than 10% tumor cells or <30% with strong complete membrane staining, or 3+ (positive) if strong complete membrane staining in more than 30% invasive tumor cells was seen.26 For Ki-67 the tumor was considered positive, if ≥14% of the tumor cells showed positive stained nuclei.6 We evaluated the entire tumor area from one representative section of the primary tumor and metastasis. The results were scored independently by two pathologists (KJ, PH) for ER, HER2 and Ki-67, and by three pathologists/investigators (KBH, KJ, PH) for PR/CK5 dual staining. The antibody clones and the laboratories manufacturing them, as well as the antibody dilutions that were used for ER, HER2 and Ki-67, are presented in Table 2.
Table 2.
List of the antibodies, the laboratories manufacturing them, and the dilutions used in immunohistochemical staining.
| Antigen | Antibody | Clone | Laboratory | Dilutions |
|---|---|---|---|---|
| HER2 | Mouse monoclonal | CB11 | Novo Castra, UK | 1:700 |
| ER alpha | Mouse monoclonal | 6F11 | Novo Castra, Newcastle, UK | 1:50 |
| PR alpha | Rabbit monoclonal | SP2 | Neomarkers, USA | 1:100 |
| CK5 | Mouse monoclonal | XM26 | Leica, UK | 1:100 |
| Ki67 | Mouse monoclonal | MIB-1 | Daco Cytomation, Denmark | 1:75 |
Chromogenic in situ hybridization (CISH)
CISH was performed on all tumors with protein over expression of HER2 (2+ and 3+) by immunohistochemistry. The 4μm paraffin sections were preheated at about +55 °C–58 °C for 2–6 h and dried overnight at 37 °C to avoid detachment, then deparaffined in xylene, dehydrated, and incubated in TRIS-EDTA Buffer (pH 9.0) for 25 min at 98 °C. After digestion in 0.1% trypsin for 40 sec, the slides were post-fixated in 10% formalin for 10 min and dehydrated in a series of increasing alcohol concentrations. Subsequently, 0.4 mL digoxin-labeled ZytoDotSPECHER2 probe was applied, sealed, and denatured on a heat plate at 95 °C for 4 min. Finally the slides were incubated at 37 °C overnight for hybridization. On the second day, the slides were opened and washed in 78 °C standard saline citrate 2 × 2 min, to remove the unspecifically bound probe. The digoxin-labeled HER2 probe was recognized with the primary mouse monoclonal antibody (clone 1.71.256, cat no. 11333062910, Roche) while the amplified signals were visualized with DAB. The immunodetection was performed in a LabVision autostainer by using a PowerVision Poly-HRP IHC Detection kit (DPVB+110DAB/ImmunoVision Technologies CO).
The stainings were examined under a light microscope (40×), where small dots (signals) in the nuclei present the gene copies. No amplification was stated if no more than 5 signals were obtained. A low level of amplification was stated from 6–10 signals, and a high amplificationifover10signalsorclusterswereverified. Stromal cells with normal 2 gene copies/nuclei served as a negative control. The amplified cells should represent at least 10% of the entire tumor. In case of low amplification, the chromosome 17 centromere probe was used to determine whether the extra copies were caused by chromosomal aneuploidy. In these cases, HER2 status was set as the ratio of the average number of HER2 gene copies to the average number of copies of chromosome 17. If the average HER2/Chr17 is ≥2, the result is positive for HER2 gene amplification. Stromal cells with normal 2 gene copies/nuclei served as a negative control.
Statistical methods
All statistical analyses were performed using SPSS 13.0 for Windows (SPSS Incorporation, Chicago, IL, USA). The differences between the staining of primary tumors and their corresponding metastases within the groups were tested using the paired sample t-test.
For analyzing differences in staining between the groups, and also the association between the clinical parameters and the staining results, the categorical two-tailed Pearson’s Chi-squared test was used. Probability values of P < 0.05 were considered significant in all analyses.
The multivariate ordinal regression test was used for analyzing the risk of the speed of tumor recurrence.
Results
Clinicopathological parameters
In univariate analysis, axillary node positivity (P = 0.006), high tumor grade (P = 0.008) and tumor size > 20 mm (P = 0.021) were associated with early tumor recurrence (Table 4). In multivariate analysis, increasing tumor size significantly enhanced the risk of early tumor relapse (OR 1.07, CI 1.01–1.12, Table 6), so a 1 mm increase in tumor size increased the risk of early breast cancer relapse by 7%.
Table 4.
Relationship of clinicopathological parameters, ER, PR, HER2, Ki-67 and CK5 protein expression in primary tumors of 72 breast cancer patients.
| Group | Node negative n (%) | Node positive n (%) | P | |
|---|---|---|---|---|
| 1 | 3 (16) | 18 (84) | ||
| 2 | 20 (59) | 14 (41) | ||
| 3 | 11 (58) | 8 (42) | 0.006* | |
| Grade 1 | Grade 2 | Grade 3 | ||
| 1 | 0 | 8 (42) | 11 (58) | |
| 2 | 5 (15) | 19 (56) | 10 (29) | |
| 3 | 3 (16) | 15 (79) | 1 (5) | 0.008* |
| Size < 20 mm | Size ≥ 20 mm | |||
| 1 | 3 (16) | 16 (84) | ||
| 2 | 16 (47) | 18 (53) | ||
| 3 | 11 (58) | 8 (42) | 0.021* | |
| ER negative | ER positive | |||
| 1 | 10 (53) | 9 (47) | ||
| 2 | 10 (29) | 24 (71) | ||
| 3 | 3 (16) | 16 (84) | 0.047* | |
| PR negative | PR positive | |||
| 1 | 13 (68) | 6 (32) | ||
| 2 | 15 (44) | 19 (56) | ||
| 3 | 6 (32) | 13 (68) | 0.066 | |
| HER2 negative | HER2 positive | |||
| 1 | 10 (53) | 9 (47) | ||
| 2 | 29 (85) | 5 (15) | ||
| 3 | 18 (95) | 1 (5) | 0.003* | |
| Ki67 negative | Ki67 positive | |||
| 1 | 6 (32) | 13 (68) | ||
| 2 | 20 (59) | 14 (41) | ||
| 3 | 15 (79) | 4 (21) | 0.012* | |
| CK5 negative | CK5 positive | |||
| 1 | 8 (42) | 11 (58) | ||
| 2 | 26 (77) | 8 (24) | ||
| 3 | 16 (84) | 3 (16) | 0.009* |
Notes: The patients are divided into Groups 1, 2 and 3 according to the time of relapse after primary diagnosis. Categorical Pearson’s Chi-squared test was used. Group 1 includes patients with a recurrence or metastasis detected within 2 years of diagnosis. Groups 2 and 3 include patients with a recurrence or metastasis detected at 5–10 years, and after 10 years of follow-up, respectively. Group 1 (n = 19), Group 2 (n = 34), Group 3 (n = 19).
Statistically significant.
Table 6.
Dependence of the time of tumor recurrence on different factors.
| Variable | Wald sig. | OR | 95% confidence interval |
|---|---|---|---|
| Model 1 | |||
| Size (2–90 mm) | 0.011 | 1.07 | 1.02–1.13 |
| HER2– | 0.027 | 0.19 | 0.04–0.83 |
| ER– | 0.228 | 2.03 | 0.64–6.41 |
| CK5– | 0.146 | 0.35 | 0.09–0.69 |
| Ki67– | 0.344 | 0.53 | 0.15–1.96 |
| Node negative | 0.431 | 1.64 | 0.48–5.60 |
| Grade 1 | 0.647 | 0.62 | 0.08–4.77 |
| Gade 2 | 0.799 | 0.83 | 0.19–3.58 |
| Model 2 | |||
| Size (2–90 mm) | 0.013 | 1.07 | 1.01–1.12 |
| Non-luminal A | 0.049 | 3.26 | 1.01–10.59 |
| Non-HER2 overexpressing | 0.211 | 0.34 | 0.06–1.85 |
| Node negative | 0.593 | 1.39 | 0.41–4.67 |
| Grade 1 | 0.153 | 0.26 | 0.04–1.65 |
| Grade 2 | 0.277 | 0.47 | 0.12–1.84 |
Note: Model 1 includes clinicopathological parameters and HER2, ER, CK5, Ki-67. Model 2 includes the clinicopathological parameters and the breast cancer subtypes defined by IHC: Luminal A and HER2-overexpressing. Regression ordinal test was used. IHC subtypes: luminal A (ER or PR+HER2–), HER2 overexpressing (ER–PR–HER+).
CK5
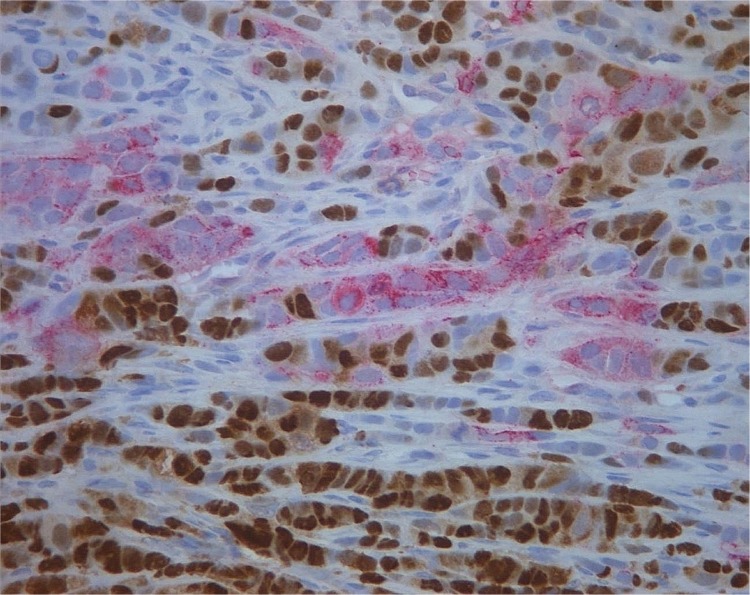
In the whole tumor set of 72 breast cancers, there were significantly more CK5 positive (Fig. 1) primary tumors than recurrences/metastases. There were 22 (31%) CK5 positive tumors in the primary tumors and 8 (11%) in their metastases (P = 0.0001, Table 3). There was also a significant loss of CK5 positivity in the recurrent/metastases of Group 1 (P = 0.010, Table 3). CK5 positivity associated with early tumor recurrence (P = 0.009, Table 4). There were 11 (58%) primary tumors and 5 (26%) recurrent positive tumors in the early relapsing tumor group (Group 1). There were only 3 CK5 positive primary tumors in the latest relapsing tumor group (Group 3), and in this group the metastases were all CK5 negative (Table 3).
Figure 1.
CK5 (red)/PR(brown) dual staining in an early relapsing breast cancer of Group 1, magnification × 400.
Table 3.
Expression of CK5, ER, PR, HER2 and Ki-67 as compared in primary and metastatic tumors of 72 breast cancer patients.
| Primary positive n (%) | Recurrent/metastatic positive n (%) | P | |
|---|---|---|---|
| ER | |||
| All cases | 49 (68) | 47 (65) | 0.596 |
| Group | |||
| 1 | 9 (47) | 6 (32) | 0.083 |
| 2 | 24 (71) | 25 (47) | 0.711 |
| 3 | 16 (84) | 16 (84) | 1.000 |
| PR | |||
| All cases | 38 (53) | 24 (33) | 0.005* |
| Group | |||
| 1 | 6 (32) | 4 (21) | 0.331 |
| 2 | 19 (56) | 13 (38) | 0.110 |
| 3 | 13 (68) | 7 (37) | 0.030* |
| HER2 | |||
| All cases | 15 (21) | 15 (21) | 1.000 |
| Group | |||
| 1 | 9 (47) | 8 (42) | 0.331 |
| 2 | 5 (15) | 5 (15) | 1.000 |
| 3 | 1 (5) | 2 (11) | 0.331 |
| Ki67 | |||
| All cases | 31 (43) | 39 (54) | 0.059 |
| Group | |||
| 1 | 13 (68) | 14 (74) | 0.667 |
| 2 | 14 (42) | 21 (62) | 0.017* |
| 3 | 4 (21) | 4 (21) | 1.000 |
| CK5 | |||
| All cases | 22 (31) | 8 (11) | 0.0001* |
| Group | |||
| 1 | 11 (58) | 5 (26) | 0.010* |
| 2 | 8 (24) | 3 (9) | 0.058 |
| 3 | 3 (16) | 0 | 0.083 |
Notes: The patients are divided into Groups 1, 2 and 3, according to the time of relapse after primary diagnosis. Paired samples t-test was used. Group 1 (n = 19) includes patients with a recurrence/metastasis detected within 2 years of diagnosis. Groups 2 (n = 34) and 3 (n = 19) include patients with a recurrence/metastasis detected at 5–10 years, or after 10 years of follow-up, respectively.
Statistically significant
CK5 positivity in the primary tumors correlated significantly with metastasis in axillary lymph nodes (P = 0.025), with a high tumor grade (P = 0.0001), with ductal histological type of tumor (P = 0.003), with ER negativity (P = 0.029), and with Ki-67 positivity (P = 0.0001) (data not shown). In the metastases, CK5 positivity correlated with ER negativity (P = 0.0001), and with PR negativity (P = 0.034) (data not shown).
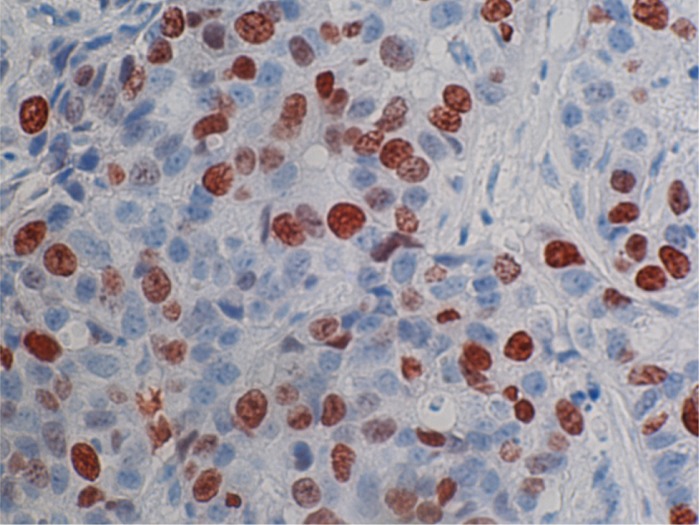
ER
There were 49 ER positive (68%) primary tumors in the whole tumor set (72) and 47 (65%) in the metastases. ER positivity (Fig. 2) changed mostly in the early relapsing tumor Group 1, where three ER positive tumors were ER negative in the metastases (15%, Table 3). ER positivity was associated with late tumor recurrence (P = 0.047, Table 4) in univariate analysis. In multivariate analysis, ER was not a significant risk factor for late relapse. ER positivity in the primary tumors was associated with axillary node negativity (P = 0.014), with low grade (P = 0.024) and with HER2 negativity (P = 0.049), data not shown. In metastases, ER positivity correlated with PR positivity (P = 0.005) and HER2 negativity (P = 0.0001) (data not shown).
Figure 2.
ER staining in a metastatic breast cancer of the late relapsing tumors of Group 3, magnification × 400.
PR
There were significantly more PR positive (Fig. 1) primary tumors in the whole tumor set (72) than in the metastases. There were 38 (53%) PR positive primary tumors compared to 24 (33%) positive metastases, (P = 0.005, Table 3). In the late metastasizing tumors of Group 3, there were significantly more PR positive primary tumors (13, 68%) than metastases (7, 37%; P = 0.030, Table 3).
There were no significant differences in PR expression between the groups. PR positivity was associated with node negativity (P = 0.017) in primary tumors and with HER2 negativity in metastases (P = 0.014), data not shown.
HER2
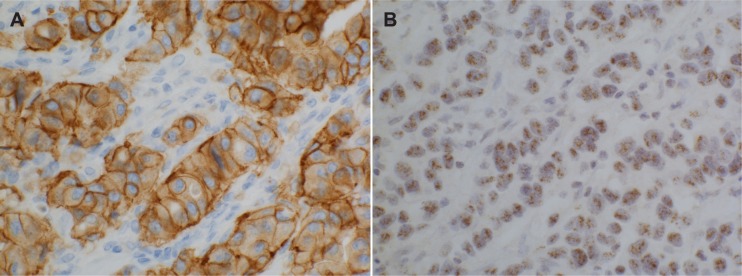
There was a high concordance (97%) of HER2 overexpression by IHC and CISH (Fig. 3) between the primary tumors and the corresponding metastasis in all three groups (Table 3). There were 15 (21%) HER2 positive primary and 15 (21%) positive recurrent cancers in the whole tumor set. In the whole tumor material of paired primary and metastatic tumors, there were only three pairs with discordant HER2 status. One primary case, belonging to the early relapsing Group 1 was HER2 positive by IHC and CISH, but CISH negative in the recurrent lesion. Another case of discordance was also from Group 1, in which the primary tumor was HER2 positive by both IHC and CISH, but was negative (1+) in the metastasis by IHC and CISH positive. In the third case of discordance, a primary tumor from Group 3 was negative for HER2 by both IHC and CISH, but the metastasis was positive by both IHC and CISH. One tumor of all CISH positive cases showed low amplification. By testing with the chromosome 17 centromere probe, the case showed an average HER2/Chr17 ratio over two, and turned out to be HER2 gene amplified.
Figure 3.
HER2 overexpression in an early relapsing breast cancer of Group (A) IHC, (B) CISH, magnification × 400.
HER2 overexpression was associated with early tumor recurrence (P = 0.003, Table 4). In multivariate analysis, HER2 negativity significantly lowered the risk of early tumor relapse (OR 0.19 95% CI 0.04–0.83, Table 6) or inversely, HER2 positivity increased the risk of early tumor relapse by 1/0.19 = 5.3. HER2 negativity was associated with node negativity (P = 0.018), low tumor grade (P = 0.021) and with Ki-67 negativity (P = 0.008) in primary the tumors (data not shown). HER2 positivity in metastases correlated with ER negativity (P = 0.0001) and PR negativity (P = 0.014) (data not shown).
Ki-67
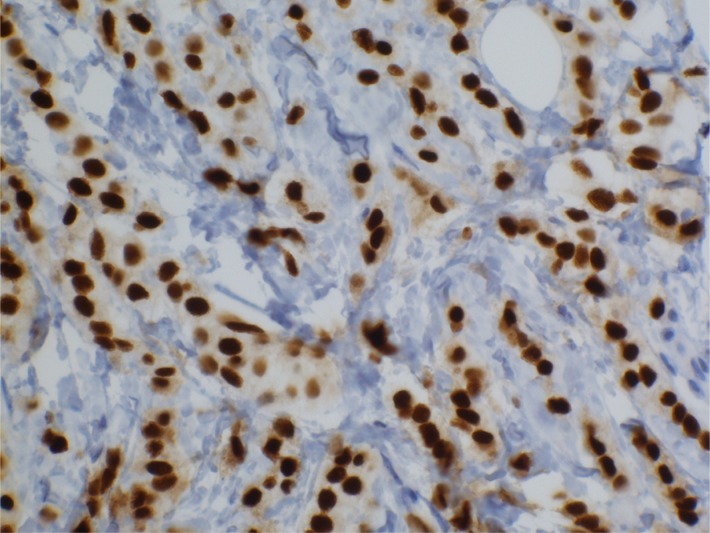
There were 31 (43%) Ki-67 positive (Fig. 4) primary tumors and 39 (54%) Ki-67 positive recurrent/metastatic lesions in the whole tumor set. There were significantly more Ki-67 positive metastases in Group 2 compared to the primary cancers (P = 0.017, Table 3). The Ki-67 positivity associated with early tumor recurrence (P = 0.012, Table 4). There was a gradual decline in the expression level of Ki-67 from the early relapsing tumor group (Group 1) toward the late relapsing tumor groups (Group 2 and 3). In the multivariate test, Ki-67 was not a significant risk factor of early tumor relapse. Ki-67 positivity in primary tumors was associated with late relapse in luminal A type of tumors, but not in luminal B type of cancers (P = 0.0001 and P = 0.364, respectively), (data not shown). Ki-67 positivity associated with axillary node positivity (P = 0.027), with high grade (P = 0.0001), with ductal histological type (P = 0.026) and HER2 positivity (P = 0.008) (data not shown).
Figure 4.
Ki-67 positive staining in an early relapsing breast cancer of Group 1, magnification × 400.
Relationship of the subgroups, luminal A (ER or PR+HER2–), luminal B (ER or PR+HER2+), HER2 overexpressing (ER–PR–HER2+), triple-negative (ER–PR–HER2–), basal-like (ER–PR–HER2–CK5+), unclassified (ER–PR–HER2–CK–) and lumino-basal (ER or PR+CK+) to early and late recurrence of tumors
The luminal A subtype of tumors was associated with late recurrence in the univariate (P = 0.0001, Table 4) analysis. The other subtypes were rare and they mostly represented early relapsing tumors, but the differences were not significant. In multivariate analysis, the non-luminal A phenotype of tumors significantly increased the risk of early tumor relapse (OR 3.26 95% CI 1.01–10.59) (Table 6) ie, the subtype luminal A with this changing coefficient of 0.3067 (1/3.26 = 0.3067) lowers the risk 100*(1 – 0.3067) = 69.33%.
CK5 positive triple negative subtype, which in this study is the same as basal-like (ER–PR–HER2–CK5+) type of tumors tended to associate with early tumor relapse, compared to the CK5 negative triple negative type of tumors, which is the same as unclassified (ER–PR–HER2–CK5–) type (P = 0.055, Table 5). There were altogether 11 (14%) triple negative primary tumors in the whole tumor set, 4 (21%) in the early relapsing tumor group (Group 1), 5 (15%) in Group 2 (relapse at 5–10 years) and 2 (11%) in the late relapsing tumor Group 3 (recurrence/metastasis detected after 10 years of follow-up). There were 4 (21%) CK5+ triple negative primary tumors in the early relapsing tumors (Group 1) and no CK5+ triple negative type tumors among the late relapsing tumors (Group 3).
Table 5.
Distribution of seven subtypes of tumors defined by IHC, in primary tumors of 72 breast cancer patients.
| ‘Luminal A’ | All others | P | |
|---|---|---|---|
| Group 1 (n = 19) | 2 (11) | 17 (90) | |
| Group 2 (n = 34) | 20 (59) | 14 (41) | |
| Group 3 (n = 19) | 13 (68) | 6 (32) | 0.0001 |
| ‘Luminal B’ | All others | ||
| Group 1 (n = 19) | 2 (11) | 17 (90) | |
| Group 2 (n = 34) | 2 (6) | 32 (94) | |
| Group 3 (n = 19) | 0 | 19 (100) | 0.364 |
| ‘Her2 enriched’ | All others | ||
| Group 1 (n = 19) | 5 (26) | 14 (74) | |
| Group 2 (n = 34) | 2 (6) | 32 (94) | |
| Group 3 (n = 19) | 1 (5) | 18 (95) | 0.049 |
| ‘Luminobasal’ | All others | ||
| Group 1 (n = 19) | 6 (32) | 13 (68) | |
| Group 2 (n = 34) | 5 (15) | 29 (85) | |
| Group 3 (n = 19) | 3 (16) | 16 (84) | 0.296 |
| ‘Basal-like’ | All others | ||
| Group 1 (n = 19) | 4 (21) | 15 (79) | |
| Group 2 (n = 34) | 3 (9) | 31 (91) | |
| Group 3 (n = 19) | 19 (100) | 0 | 0.088 |
| ‘Non classified’ | All others | ||
| Group 1 (n = 19) | 0 | 19 (100) | |
| Group 2 (n = 34) | 2 (6) | 32 (94) | |
| Group 3 (n = 19) | 2 (11) | 17 (90) | 0.364 |
| Triple negative CK5+ | Triple negative CK5− | ||
| Group 1 (n = 19) | 4 (100) | 0 | |
| Group 2 (n = 34) | 3 (60) 2 (40) | 2 (40) | |
| Group 3 (n = 19) | 0 | 2 (100) | 0.055 |
Notes: The patients are divided into 3 groups, according to the time of relapse after primary diagnosis. Categorical Pearson’s Chi-squared test was used. IHC subtypes: Luminal A (ER or PR+HER2−), Luminal B (ER or PR+HER2+), HER2-over-expressing (ER–PR–HER2+), Triple-negative (ER–PR–HER2–), Basal-like (ER–PR–HER2–CK5+), Non-classified (ER–PR–HER2–CK5–), Luminobasal (ER or PR+CK5+). Group 1 (n = 19) includes patients with a recurrence or metastasis detected within 2 years of diagnosis. Groups 2 (n = 34) and 3 (n = 19) include patients with a recurrence or metastasis detected at 5–10 years, or after 10 years of follow-up, respectively.
Statistically significant.
Discussion
In this study we describe the status of the established prognostic biomarkers, ER, PR, HER2 and Ki-67, used in breast cancer diagnosis, together with basal type cytokeratin CK5 in 72 primary breast cancers and their corresponding recurrent/metastatic tumors in early and late relapsing tumors. Of these cancers, 19 were early relapsing and 54 late relapsing (34 after 5 years, and 19 after 10 years, respectively). In addition, the tumors were divided into seven IHC surrogates to the genetically defined subtypes, luminal A, luminal B, HER2 overexpressing, triple-negative, basal-like, unclassified, and luminobasal.27 The expression levels and the distribution of the subgroups were compared between primary tumors and metastases, between the different groups, and with the clinicopathological parameters, in order to analyze their role in breast cancer progression.
CK5
The basal type cytokeratin CK5 expression correlated with poor prognostic features, such as early recurrence, axillary lymph node positivity, high tumor grade, Ki-67 positivity, and ER negativity. Our results are in concordance with those of Banerjee et al28 and Choccalingam et al29 who also demonstrated that basal-like breast cancer expression, defined by basal cytokeratin expression, correlated with negative hormonal status and shorter disease-free intervals. They also analyzed triple negative breast cancers and obtained results similar to ours, by showing that patients with CK5+ triple negative breast cancers tended to have shorter disease-free intervals than the subgroup of CK5- triple negative cancers.29
Contrary to our results, Tot (2000) demonstrated a high concordance of CK5 expression between primary and paired tumors of 31 breast cancer patients.30 However, the breast cancers in their study were all medullary type, while there were no medullary type of tumors in our study. Alterations of cytokeratin expression and partial loss of the normal regulation of cytokeratin expression during carcinogenesis and tumor progression has been demonstrated.31
ER
ER positivity in this study was associated with very late tumor relapse, which is in accordance with previous reports.32,33 The heterogeneity of ER expression within an individual tumor, between different tumors and also between the primary tumor and their metastases, is a well known phenomenon. Breast cancers are known to express epithelial cell-associated antigens in a heterogeneous manner.34 The discordance of the receptor status between primary and metastatic breast cancers has been described for over 30 years.35 Such discordance in estrogen and progesterone receptors can occur in as many as 40% of breast cancer cases.36 In our tumor material, there was a 15% loss of positivity in the early relapsing tumor Group 1, from 9 (47%) ER positive primary tumors to 6 (32%) ER positive metastases. In a study of 75 patients, Sari et al37 demonstrated that 8% of ER positive tumors turned out not to express ER in their metastases, and in contrast 5% of ER negative primary tumors were positive in the metastases. In our study, only one (3%) primarily ER negative tumor (in Group 2) had an ER positive metastasis. Hoefnagel et al38 showed inversion of primary ER positive to negative metastases in 10.7% of their cases, and from negative to positive in 3.4%. The corresponding numbers in the study of Thomson et al were 8% from positive to negative, and vice versa in 2.2% of the cases.39
PR
Positive estrogen receptor status in breast cancer is associated with a good response to hormonal therapy and to a good prognosis, a long disease-free and overall survival.40 The additional prognostic and predictive value of the progesterone receptor has remained controversial. The value of PR for prognosis and the response to tamoxifen was examined in a population-based series of 4,046 invasive early stage breast cancer patients.41 Survival analyses for both the whole cohort and ER positive cases that were given tamoxifen therapy showed that patients with PR positive tumors had better breast cancer specific survival. IGF-I (insulin-like growth factor-1) has been shown to inhibit progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/akt/mammalian target of the rapamycin pathway, and low PR expression may serve as an indicator of activated growth factor signaling in breast cancer cells.42 Therefore it has been suggested that low PR expression may serve as an indicator of activated growth factor signaling in breast cancer cells, and represent an aggressive tumor phenotype resistant against hormonal therapy.42 PR expression may define a subpopulation of breast cancer patients who have a stronger dependence on hormone receptor-associated growth, and therefore a superior response to hormone therapy.43
In our study, PR positivity between metastases and primary tumors changed most in Group 3, where 6 out of 13 PR positive tumors turned out to be negative in the metastases. The role of progesterone in early or late tumor recurrence in the present study remains unclear.
HER2
HER2 protein and gene overexpression in our study was associated with aggressive tumor features, such as early tumor relapse, axillary node positivity, high tumor grade and large tumor size. This finding supports earlier reports.11 HER2 protein and gene overexpression in our study showed a high concordance between the primary tumors and their metastases (97%). Previous studies have shown that the HER2 status in primary tumors remains highly conserved, when compared to their corresponding metastases.44–46 In previous studies, the discordance in the expression of HER2 in primary cancers and relapsing/metastasizing tumors was found to be between 0% and 34%.37 The high concordance of HER2 status in our tumor material was also conserved in the late relapsing cancers. The only case in which the primarily HER2 negative tumor changed to HER2 positive, was a tumor of the late relapsing Group 3 (relapse after 10 years). Of 31 breast carcinomas with corresponding lymph node and distant metastases, HER2 amplification and overexpression de novo was demonstrated in 9.7% in distant metastases at a late disease state.47
Ki-67
Ki-67 positivity in our study was associated with early tumor recurrence, and the expression of Ki-67 gradually decreased from early relapsing tumors to late recurring ones. Numerous studies have shown that breast cancers overexpressing Ki-67 in more than 20%–50% of the cells are at high risk of developing recurrent disease, showing a statistically significant correlation with clinical outcome, such as disease-free survival or overall survival.48–50
In our study, the low Ki-67 expression distinguished the luminal A type of tumors from the luminal B type. This is in agreement with the study of Cheang and colleagues, who described an immunopanel of ER, PR, HER2, and Ki-67 that can segregate the luminal A and B subtypes.6 Luminal breast cancers with Ki-67 levels of at least 14% had a worse prognosis for both breast cancer recurrence and death, compared with tumors with Ki-67 levels of less than 14%.
Conclusions
Our study showed that large tumor size and HER2 positivity are significant risk factors for rapid tumor recurrence, and conversely, that the luminal A subtype of tumor significantly lowers the risk of breast cancer recurrence. The basal-like subtype of tumors defined as triple-negative CK5 positive cancers were able to distinguish the early relapsing tumors from the slower recurring triple-negative CK5 negative, unclassified tumors. Ki-67 positivity with a cut-off point of 14% associated with early tumor recurrence and correlated linearly with tumor progression, since Ki-67 positivity declined gradually from early-relapsing toward late-recurring tumors. In our study, luminal A-type tumors were associated with low Ki-67 expression, and Ki-67 positivity was associated with late tumor recurrence in luminal A type tumors.
Acknowledgments
We thank Eija Heiliö for excellent technical assistance and Antti Nevanlinna, MSc, for assistance with the statistics. We thank Terttu Kaustia for reviewing the English of this manuscript. This work was supported by the Helsinki University Central Hospital Research Foundation, the Finnish Cancer Foundation, The Sigrid Juselius Foundation and the Finnish Breast Cancer Group.
Footnotes
Author Contributions
Conceived and designed the experiments: KJ, PH. Analysed the data: KBH, KJ, PH. Wrote the first draft of the manuscript: KJ. Contributed to the writing of the manuscript: KJ, LCA, PH. Agree with manuscript results and conclusions: MK. Jointly developed the structure and arguments for the paper: ML. Made critical revisions and approved final version: KJ, ML, MK, LCA, KBH, PH. All authors reviewed and approved of the final manuscript.
This article is available from http://www.la-press.com.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Brackstone JL, Townson AF, Chambers AF. Tumor dormancy in breast cancer: an update. Breast Cancer Res. 2007;9(3):208. doi: 10.1186/bcr1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 5.van de Rijn M, Perou CM, Tibshirani R, et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am J Pathol. 2002;161(6):1991–6. doi: 10.1016/S0002-9440(10)64476-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheang MC, Chia SK, Voduc D, et al. Ki-67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–50. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.King CR, Kraus MH, Aaronson SA. Amplification of a novel v-erB-related gene in a human mammary carcinoma. Science. 1985;229:974–6. doi: 10.1126/science.2992089. [DOI] [PubMed] [Google Scholar]
- 8.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 9.van de Vijver MJ, Peterse JL, Mooi WJ, et al. Neu-protein overexpression in breast cancer. Association with comedo-type ductal carcinoma in situ and limited prognostic value in stage II breast cancer. N Engl J Med. 1988;319(19):1239–45. doi: 10.1056/NEJM198811103191902. [DOI] [PubMed] [Google Scholar]
- 10.Gusterson BA, Gelber RD, Goldhirsch A, et al. Prognostic importance of c-erbB-2 expression in breast cancer. International (Ludwig) Breast Cancer Study Group. J Clin Oncol. 1992;10(7):1049–56. doi: 10.1200/JCO.1992.10.7.1049. [DOI] [PubMed] [Google Scholar]
- 11.Ross JS, Fletcher JA. HER-2/neu (c-erb-B2) gene and protein in breast cancer. Am J Clin Pathol. 1999;112(1 Suppl 1):53–67. [PubMed] [Google Scholar]
- 12.Joensuu H, Bono P, Kataja V, et al. Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol. 2009;27(34):5685–92. doi: 10.1200/JCO.2008.21.4577. [DOI] [PubMed] [Google Scholar]
- 13.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 14.Gerdes J, Li L, Schlueter C, et al. Immunobiochemical and molecular biologic characterization of the cell proliferation-associated nuclear antigen that is defined by monoclonal antibody Ki-67. Am J Pathol. 1991;138(4):867–73. [PMC free article] [PubMed] [Google Scholar]
- 15.Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984;133(4):1710–5. [PubMed] [Google Scholar]
- 16.Sahin AA, Ro J, Ro JY, et al. Ki-67 immunostaining in node-negative stage I/II breast carcinoma. Significant correlation with prognosis. Cancer. 1991;68(3):549–7. doi: 10.1002/1097-0142(19910801)68:3<549::aid-cncr2820680318>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 17.Keshgegian AA, Cnaan A. Proliferation markers in breast carcinoma. Mitotic figure count, S-phase fraction, proliferating cell nuclear antigen, Ki-67 and MIB-1. Am J Clin Pathol. 1995;104(1):42–9. doi: 10.1093/ajcp/104.1.42. [DOI] [PubMed] [Google Scholar]
- 18.Taylor-Papadimitriou J, Stampfer M, Bartek J, et al. Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J Cell Sci. 1989;94(Pt 3):403–13. doi: 10.1242/jcs.94.3.403. [DOI] [PubMed] [Google Scholar]
- 19.AbdEl-Rehim DM, Pinder SE, Paish CE, et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J Pathol. 2004;203(2):661–71. doi: 10.1002/path.1559. [DOI] [PubMed] [Google Scholar]
- 20.Cheang MC, Voduc D, Bajdik C, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenoptype. Clin Cancer Res. 2008;14(5):1368–76. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 21.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295(21):2492–502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 22.Joensuu K, Heikkilä P, Andersson LC. Tumor dormancy: elevated expression of stanniocalcins in late relapsing breast cancer. Cancer Lett. 2008;265(1):76–83. doi: 10.1016/j.canlet.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 24.Haughian JM, Pinto MP, Harrell JC, et al. Maintenance of hormone responsiveness in luminal breast cancers by suppression of Notch. Proc Natl Acad Sci U S A. 2012;109(8):2742–7. doi: 10.1073/pnas.1106509108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammond MEH, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch pathol Lab Med. 2010;134(7):907–22. doi: 10.5858/134.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–45. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 27.Laakso M, Tanner M, Nilsson J, et al. Basoluminal carcinoma: a new biologically and prognostically distinct entity between basal and luminal breast cancer. Clin Cancer Res. 2006;12(14 Pt 1):4185–91. doi: 10.1158/1078-0432.CCR-06-0353. [DOI] [PubMed] [Google Scholar]
- 28.Banerjee S, Reis-Filho JS, Ashley S, et al. Basal-like breast carcinomas: clinical outcome and response to chemotherapy. J Clin Pathol. 2006;59(7):729–35. doi: 10.1136/jcp.2005.033043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choccalingam C, Rao L, Rao S. Clinico-Pathological Characteristics of Triple Negative and Non Triple Negative High Grade Breast Carcinomas with and Without Basal Marker (CK5/6 and EGFR) Expression at a Rural Tertiary Hospital in India. Breast Cancer. 2012;6:21–9. doi: 10.4137/BCBCR.S8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tot T. The cytokeratin profile of medullary carcinoma of the breast. Histopathology. 2000;37(2):175–81. doi: 10.1046/j.1365-2559.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- 31.Su L, Morgan PR, Lane EB. Expression of cytokeratin messenger RNA versus protein in the normal mammary gland and in breast cancer. Hum Pathol. 1996;27(8):800–6. doi: 10.1016/s0046-8177(96)90452-9. [DOI] [PubMed] [Google Scholar]
- 32.Hess KR, Pusztai L, Buzdar AU, Hortobagyi GN. Estrogen receptors and distinct patterns of breast cancer relapse. Breast Cancer Res Treat. 2003;78(1):105–8. doi: 10.1023/a:1022166517963. [DOI] [PubMed] [Google Scholar]
- 33.Brewster AM, Hortobagyi GN, Broglio KR, et al. Residual risk of breast cancer recurrence 5 years after adjuvant therapy. J Natl Cancer Inst. 2008;100(16):1179–83. doi: 10.1093/jnci/djn233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charpin C, Martin PM, De Victor B, et al. Multiparametric study (SAMBA 200) of estrogen receptor immunocytochemical assay in 400 human breast carcinomas: analysis of estrogen receptor distribution heterogeneity in tissues and correlations with dextran coated charcoal assays and morphological data. Cancer Res. 1988;48(6):1578–86. [PubMed] [Google Scholar]
- 35.Brennan MJ, Donegan WL, Appleby DE. The variability of estrogen receptors in metastatic breast cancer. Am J Surg. 1979;137(2):260–2. doi: 10.1016/0002-9610(79)90159-4. [DOI] [PubMed] [Google Scholar]
- 36.Li BD, Byskosh A, Molteni A, Duda RB. Estrogen and progesterone receptor concordance between primary and recurrent breast cancer. J Surg Oncol. 1994;57(2):71–7. doi: 10.1002/jso.2930570202. [DOI] [PubMed] [Google Scholar]
- 37.Sari E, Guler G, Hayran M, Gullu I, Altundag K, Ozisik Y. Comparative study of the immunohistochemical detection of hormone receptor status and HER-2 expression in primary and paired recurrent/metastatic lesions of patients with breast cancer. Med Oncol. 2011;28(1):57–63. doi: 10.1007/s12032-010-9418-2. [DOI] [PubMed] [Google Scholar]
- 38.Hoefnagel LD, van de Vijver MJ, van Slooten HJ, et al. Receptor conversion in distant breast cancer metastases. Breast Cancer Res. 2010;12(5):R75. doi: 10.1186/bcr2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson AM, Jordan LB, Quinlan P, et al. Prospective comparison of switches in biomarker status between primary and recurrent breast cancer: the Breast Recurrence In Tissues Study (BRITS) Breast Cancer Res. 2010;12(6):R92. doi: 10.1186/bcr2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vollenweider-Zerargui L, Barrelet L, Wong Y, Lemarchand-Béraud T, Gómez F. The predictive value of estrogen and progesterone receptors’ concentrations on the clinical behaviour of breast cancer in women. Clinical correlation on 547 patients. Cancer. 1986;57(6):1171–80. doi: 10.1002/1097-0142(19860315)57:6<1171::aid-cncr2820570618>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 41.Liu S, Chia SK, Mehl E, et al. Progesterone receptor is a significant factor associated with clinical outcomes and effect of adjuvant tamoxifen therapy in breast cancer patients. Breast Cancer Res Treat. 2010;119(1):53–61. doi: 10.1007/s10549-009-0318-0. [DOI] [PubMed] [Google Scholar]
- 42.Cui X, Zhang P, Deng W, et al. Insulin-like growth factor-I inhibits progesterone receptor expression in breast cancer cells via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin pathway: progesterone receptor as a potential indicator of growth factor activity in breast cancer. Mol Endocrinol. 2003;17(4):575–88. doi: 10.1210/me.2002-0318. [DOI] [PubMed] [Google Scholar]
- 43.Cui X, Schiff R, Arpino G, Osborne CK, Lee AV. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J Clin Oncol. 2005;23(30):7721–35. doi: 10.1200/JCO.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 44.Tanner M, Järvinen P, Isola J. Amplification of HER-2/neu and topoisomerase IIalpha in primary and metastatic breast cancer. Cancer Res. 2001;61(14):5345–8. [PubMed] [Google Scholar]
- 45.Tapia C, Savic S, Wagner U, et al. HER2 gene status in primary breast cancers and matched distant metastases. Breast Cancer Res. 2007;9(3):R31. doi: 10.1186/bcr1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strien L, Leidenius M, von Smitten K, Heikkilä P. Concordance between HER-2 and steroid hormone receptor expression between primary breast cancer, sentinel node metastases, and isolated tumor cells. Pathol Res Pract. 2010;206(4):253–8. doi: 10.1016/j.prp.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 47.Regitnig P, Schippinger W, Lindbauer M, Samonigg H, Lax SF. Change of HER-2/neu status in a subset of distant metastases from breast carcinomas. J Pathol. 2004;203(4):918–26. doi: 10.1002/path.1592. [DOI] [PubMed] [Google Scholar]
- 48.Assersohn L, Salter J, Powles TJ, et al. Studies of the potential utility of Ki-67 as a predictive molecular marker of clinical response in primary breast cancer. Breast Cancer Res Treat. 2003;82(2):113–23. doi: 10.1023/B:BREA.0000003968.45511.3f. [DOI] [PubMed] [Google Scholar]
- 49.Locker AP, Birrell K, Bell JA, et al. Ki-67 immunoreactivity in breast carcinoma: relationships to prognostic variables and short term survival. Eur J Surg Oncol. 1992;18(3):224–9. [PubMed] [Google Scholar]
- 50.Veronese SM, Gambacorta M, Gottardi O, Scanzi F, Ferrari M, Lampertico P. Proliferation index as a prognostic marker in breast cancer. Cancer. 1993;71(12):3926–31. doi: 10.1002/1097-0142(19930615)71:12<3926::aid-cncr2820711221>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]