Abstract
Background and Aims
There is a conspicuous increase of poikilohydric organisms (mosses, liverworts and macrolichens) with altitude in the tropics. This study addresses the hypothesis that the lack of bryophytes in the lowlands is due to high-temperature effects on the carbon balance. In particular, it is tested experimentally whether temperature responses of CO2-exchange rates would lead to higher respiratory carbon losses at night, relative to potential daily gains, in lowland compared with lower montane forests.
Methods
Gas-exchange measurements were used to determine water-, light-, CO2- and temperature-response curves of net photosynthesis and dark respiration of 18 tropical bryophyte species from three altitudes (sea level, 500 m and 1200 m) in Panama.
Key Results
Optimum temperatures of net photosynthesis were closely related to mean temperatures in the habitats in which the species grew at the different altitudes. The ratio of dark respiration to net photosynthesis at mean ambient night and day temperatures did not, as expected, decrease with altitude. Water-, light- and CO2-responses varied between species but not systematically with altitude.
Conclusions
Drivers other than temperature-dependent metabolic rates must be more important in explaining the altitudinal gradient in bryophyte abundance. This does not discard near-zero carbon balances as a major problem for lowland species, but the main effect of temperature probably lies in increasing evaporation rates, thus restricting the time available for photosynthetic carbon gain, rather than in increasing nightly respiration rates. Since optimum temperatures for photosynthesis were so fine tuned to habitat temperatures we analysed published temperature responses of bryophyte species worldwide and found the same pattern on the large scale as we found along the tropical mountain slope we studied.
Keywords: Altitudinal gradient, bryophytes, carbon balance, dark respiration, gas-exchange measurements, hepatics, liverworts, mosses, net photosynthesis, photosynthesis-response curves, temperature, tropical rain forest
INTRODUCTION
In the wet tropics, bryophytes (mosses and liverworts) decrease strongly in abundance (Frahm, 1990; Wolf, 1993) and diversity (Seifriz, 1924; Pócs, 1980; Gradstein and Pocs, 1989) with decreasing altitude. The poor development of these plants in the lowlands contradicts the intuitive expectation of a prospering bryophyte flora all over the wet tropics, i.e. areas where precipitation rarely drops below 100 mm month−1 (e.g. Richards, 1996). The reasons for this conspicuous altitudinal pattern are unknown, though several untested hypotheses have been put forward over the years (summarized in Wagner et al., 2012).
A plausible, though untested, physiological explanation for the lack of bryophytes in the warm lowlands was put forward almost 30 years ago by Richards (1984 and see also Frahm, 1987; Zotz, 1999; Wagner et al., 2012), and was later extended to macrolichens (Zotz and Winter, 1994). Both bryophytes and lichens are poikilohydric organisms, their moisture content rapidly equilibrating with environmental conditions and physiological activity varying with moisture content (Green and Lange, 1994). Substantial carbon losses due to respiration can occur when poikilohydric organisms are wet in the dark (e.g. Proctor, 1982; Lange, 2003b). In the lowland tropics, afternoon rains are common and nights are warm, so that respiration rates are high and a large proportion of assimilated carbon may be lost at night. Conversely, during the day CO2 uptake of poikilohydric organisms is restricted by low light intensities, especially in the understorey and after rainstorms, or by fast drying, especially at exposed sites (Zotz and Winter, 1994; Zotz et al., 1997, 1998). Due to these high losses and low gains, poikilohydric organisms have difficulties obtaining a positive carbon balance. One of the implicit assumptions in this hypothesis is that temperature responses of dark respiration in lowland bryophyte species are comparable to those from montane regions, so that the ratio of dark respiration to potential net photosynthesis (RD/NP) at ambient temperatures is highest in the lowlands and decreases with altitude. We intend to test this assumption in the present study.
An additional assumption, put forward by Zotz (1999), has been addressed experimentally in a separate study (S. Wagner, G. Zotz and M. Y. Bader, unpubl. res.): lower montane species cannot acclimatize their metabolic rates, dark respiration in particular, to warmer (lowland) conditions. This assumption is also essential because, according to a hypothesis centred on the dark respiration rate, inherent acclimation of these rates in montane species could allow them to fill open niches in the lowlands and would thus allow a higher diversity in the lowlands than is actually observed.
If the altitudinal patterns described are indeed caused by temperature, climatic warming could be directly detrimental for tropical bryophytes, further excluding them from the lowlands and diminishing biomass and diversity in montane areas. However, there is very little information on the physiology of tropical bryophytes (Wagner et al., 2012), which would allow us to test Richard's notion rigorously, and predictions are thus hardly possible. This motivated the present study. Here, we present temperature-response curves of net photosynthesis (NP) and dark respiration (RD) of 18 species of mosses and hepatics from tropical lowland to lower-montane rainforest in Panama, including the first such curves published for lowland bryophytes. We also report light- and water-response curves for these species and CO2-response curves for a subset of three species. In the discussion we will put our findings in the context of global patterns of the temperature responses of bryophytes, presenting a meta-analysis of published temperature optima for photosynthesis.
MATERIALS AND METHODS
Study areas
Sampling took place along the Caribbean slope of the continental divide in Western Panama. The lower study area, Bocas del Toro (BT), was in the lowland rainforest of Isla Colon in the Bocas del Toro archipelago (08 °47'N, 082·13'W, sea level). The middle area, Palo Seco (PS), was also situated in lowland rainforest, but at a higher altitude (approx. 500 m a.s.l.) in the Bosque Protector Palo Seco, Bocas Del Toro Province (08°48′N, 082°11′W). The highest study area was in the Reserva Forestal Fortuna (F), Chiriquí Province (08°43′N, 82°14′W, approx.1200 m a.s.l.), which is covered by lower montane rainforest. Mean annual rainfall ranged from 3300 mm year−1 (2006–2009) in Bocas del Toro (STRI data for 2012) to 5400 mm year−1 (1997–2009) in Fortuna (C. Espinosa, Panama, Smithsonian Tropical Research Institute, pers. comm.). Long-term rainfall data are not available for PS, hence precipitation was monitored with a rain gauge (HOBO Data logging rain gauge RG3) from 12 November 2009 to 17 May 2010 (2900 mm) and from 11 November 2010 to 5 May 2011 (2700 mm), yielding an estimated total of approx. 5700 mm year−1 (not taking into account possible seasonal differences). Temperature was also monitored at the study sites with HOBO Pendant G Temperature/Lux data loggers and HOBO U23 Pro v2 Temperature/Relative Humidity data logger (Onset Computer Corporation). Measuring intervals lasted from November 2009 to May 2011 (BT and PS) and from June 2011 to December 2011 (F). Data loggers were installed inside the forest at about breast height. Mean daytime temperature decreased from 26·0 °C (n = 4) in BT over 22·6 °C (n = 6) in PS to 20·2 °C (n = 3) in Fortuna. Night-time temperature averaged 24·0 °C in BT, 21·4 °C in PS and 17·8 °C in Fortuna.
Study species
Six locally common species, including both mosses and hepatics, were chosen from each altitude. Different life-forms (sensu Mägdefrau, 1982) (Table 1) were chosen to cover a substantial portion of the variety of bryophytes in the study areas. In BT and PS all species were from the forest understorey, while in Fortuna five out of six species were from forest edges. The latter habitats were 1·2 °C warmer during the day, but had similar temperature during the night compared with Fortuna understorey plots (S. Wagner, G. Zotz and M. Y. Bader, unpubl. res.). This might result in upward shifts in temperature responses compared with understorey species, independent of altitudinal origin. Therefore, the expected lower temperature optima in the Fortuna species might be masked to some extent by the exposure difference, and optima in undergrowth species are expected to be somewhat lower than those found in the exposed species.
Table 1.
Bryophyte species from tropical Panama for which gas-exchange response curves were determined
| Location | Species | Family | Exposure | Life-form |
|---|---|---|---|---|
| BT | Octoblepharum pulvinatum (Dozy & Molk.) Mitt. | Leucobryaceae | Understorey | Cushion |
| BT | Orthostichopsis tetragona (Sw. ex Hedw.) Broth. | Pterobryaceae | Understorey | Pendant |
| BT | Plagiochila sp. 1 | Plagiochilaceae | Understorey | Cover/fan |
| BT | Stictolejeunea squamata (Willd. ex F. Weber) Schiffn. | Lejeuneaceae | Understorey | Cover/fan |
| BT | Symbiezidium spp. | Lejeuneaceae | Understorey | Cover |
| BT | Zelometeorium patulum (Hedw.) Man. | Meteoriaceae | Understorey | Pendant |
| PS | Bryopteris filicina (Sw.) Nees | Lejeuneaceae | Understorey | Pendant/fan |
| PS | Lepidopilum polytrichoides (Hedw.) Brid. | Pilotrichaceae | Understorey | Cover/felt |
| PS | Pilotrichum bipinnatum (Schwä.) Brid. | Pilotrichaceae | Understorey | Pendant/fan |
| PS | Plagiochila sp. 2 | Plagiochilaceae | Understorey | Cover/fan |
| PS | Symbiezidium spp. | Lejeuneaceae | Understorey | Cover |
| PS | Syrrhopodon incompletus Schwä. | Calymperaceae | Understorey | Cushion |
| F | Durmortiera hirsuta subsp. hirsuta (Sw.) Nees | Marchantiaceae | Understorey | thalloid |
| F | Frullania mirabilis Jack & Steph. | Frullaniaceae | Forest edge | Pendant |
| F | Herbertus divergens (Steph.) Herz. | Herbertaceae | Forest edge | Loose turf |
| F | Leucobryum antillarum Schimp. ex Besch. | Leucobryaceae | Forest edge | Cushion |
| F | Phyllogonium fulgens (Hedw.) Brid. | Phyllogoniaceae | Forest edge | Pendant |
| F | Phyllogonium viscosum (P. Beauv.) Mitt. | Phyllogoniaceae | Forest edge | Pendant |
Locations: BT, Bocas del Toro, 0 m; PS, Palo Seco, 500 m; F, Fortuna, 1200 m.
Life-forms after Mägdefrau (1982). Note there are two very similar species of Symbiezidium in the study region which are very difficult to distinguish.
Except for Durmortierra hirsuta, which is a terrestrial species, all bryophytes are epiphytes and were collected from branches, buttress roots (Octoblepharum pulvinatum) or tree trunks (Syrrhopodon incompletus) at heights up to approx. 3 m. Only specimens with no other species intermixed were sampled, although the S. incompletus samples were later found to contain a small proportion of Cyclolejeunea luteola. Voucher specimens of all the mosses and hepatics studied were deposited at the herbarium of the University of Panama (PMA), Republic of Panama.
Gas-exchange measurements
After collection, the bryophyte samples were brought to the field stations and stored outside in the shade for up to 1 week. One day prior to measurement they were wetted to ensure recovery of the vital metabolic processes. Dirt and dead biomass were removed, taking care to maintain the original shape and density of the samples as much as possible.
A portable open flow infrared gas analyser (GFS 3000, Walz, Effeltrich, Germany) with a special moss cuvette was used to determine light, water and temperature-response curves of net photosynthesis (NP) and dark respiration (RD). At least five replicates per species and response type were used. The following conditions were used: CO2 concentration at 380 µmol mol−1, relative humidity at 85 %, photosynthetic photon flux density (PPFD) near saturation for photosynthesis (Table 2; except for light-response curves), and temperature close to local ambient values (Table 2; except for temperature-response curves). Additionally, CO2-response curves were measured with a subset of three species in the laboratory in Oldenburg, Germany. These samples grew in glass jars at 20 °C for approx. 9 months (Herbertus divergens and Leucobryum antillarum, two montane Fortuna species) or in the green house at approx. 26/20 °C (day/night) for >3 years (Orthostichopsis tetragona, a lowland species). These samples were then treated like those from the field and measured with the same gas-exchange equipment using the same settings (Table 2). After the measurements, all samples were dried for 3 d at 90 °C to determine dry weight. All CO2-exchange rates are expressed relative to dry weight.
Table 2.
Settings of temperature and light conditions during gas-exchange measurements at the three altitudes
| Location | Altitude (m a.s.l.) | Measuring temperature (°C) | Measuring light (μmol m2 s−1) | NP (% max) |
|---|---|---|---|---|
| Bocas del Toro | 0 | 29 | 500 | 76 ± 5 |
| Palo Seco | 500 | 25 | 400 | 75 ± 3 |
| Fortuna | 1200 | 22 | 300 | 65 ± 9 |
Temperatures during measurements approximated ambient temperatures at the three locations.
Light intensity was near saturation for photosynthesis, based on the modelled maximum rate of photosynthesis of each species (means ± s.d.).
First, the response of CO2 exchange to changing plant water content (WC) was determined. After submerging samples in distilled water for up to 2 min, surface water was removed by vigorous shaking. Samples were then enclosed in the cuvette and CO2-exchange rates were recorded when steady state was reached. A typical measurement lasted from 2 to 5 min at most. Samples were then weighed to determine WC and left to dry until the next measurement approx. 30 min later. This procedure was repeated until the weight no longer changed.
Light-response and CO2-response curves were determined in samples with water contents slightly above the optimum because of inevitable water loss during the experiment. After enclosure in the cuvette, samples were light-induced at 300–500 µmol m2 s−1 and 380 µmol mol−1 CO2 for 5 min. Light was then changed in 15 steps from 0 to 1500 µmol m2 s−1, or CO2 from 0–1500 µmol mol−1 in 11 steps, each step lasting approx. 3 min to reach steady state. Before and after the measurement, WC was determined to make sure that it was still near the optimum.
For the temperature-response curves the entire gas-exchange system had to be heated or cooled to prevent condensation in the tubing or cuvette. This was achieved by placing the system in a custom-built, temperature-controlled box. The moss samples were sprayed to reach near-optimal WC before being enclosed in the cuvette, where their CO2 exchange was recorded at the adjusted temperature after approx. 3 min. The temperature was set to the next step and the sample was measured again after checking/adjusting its water content. Temperature ranges differed between altitudes: BT, 20–37·5 °C; PS, 17·5–33 °C and F, 10–35 °C. The temperatures of the moss samples were probably somewhat below those of the cuvette due to evaporative cooling.
No cold trap was used in the gas exchange measurement, so that the raw data was corrected for the effect of water vapour. A correction is already included in the equipment software, but we tested if additional corrections were necessary when measuring wet mosses by measuring ‘CO2 exchange’ of tissue paper soaked in distilled water. This test revealed that net photosynthesis was underestimated (and dark respiration overestimated) by 0·13 ± 0·03 (mean ± s.d., n = 7) μmol CO2 (before taking dry weight into account). Thus, all data from light-, temperature- and water-response curves were corrected with this mean value. For water-response curves we assumed that the correction factor decreases linearly from the optimum towards lower water contents. In spite of this correction, the absolute rates of CO2 exchange are still somewhat uncertain. The interpretation therefore stresses relative differences. The CO2-response curves were not corrected because the equipment had been serviced before these measurements and the wet tissue test no longer showed ‘false’ respiration.
Tissue chemistry
Nitrogen contents of moss samples, only including green parts as used in the gas-exchange measurements, were determined at the University of Oldenburg with a CHNS Analyzer (FLASHEA, 1112 Series; CE Elantech, Inc., Lakewood, USA). Chlorophyll a and b contents were determined at the Bocas del Toro field station, using methanol as extractant. Fresh plant material (20–100 mg) was added to a vial and heated in a water bath for 10 min at 60 °C. The extract was decanted and the procedure was repeated. The gathered extracts were mixed well and measured in a photospectrometer (650 and 665 nm). The samples were then dried at 90 °C and the chlorophyll content was related to dry weight.
Data analysis
Using R version 2·14·0 (R Development Core Team, 2011) models were fitted to the response data and the main cardinal points (optimum, maximum, compensation point) were calculated from the modelled curves. Water-response curves were fitted to all measuring points, for several samples per species (n ≥ 5). Respiration rates reaching values >30 % of the general maximum at high water contents were considered artefacts and were excluded from the analysis (0–3 points per sample). Light-, CO2- and temperature-response curves were fitted to means of several samples per species (n ≥ 5). Best-fit model types were chosen for the water and temperature-response curves and are only meaningful in the range of measurement.
| (1) |
| (2) |
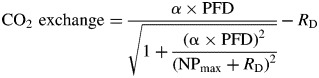 |
(3) |
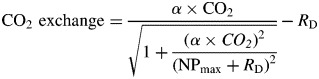 |
(4) |
| (5) |
| (6) |
***Equations 1 (hyperbolic function) and 2 (Michaelis Menten) are models for the water-response curves in the presence of light and in dark, respectively. WC is the water content of the bryophyte samples. In the cases of the light- and CO2-response curves the modified ‘Smith-function’ (eqns 3 and 4) (Tenhunen et al., 1976) was fitted, where α represents the initial slope of the curve, NPmax the maximum rate of net photosynthesis and RD the dark respiration rate. PPFD is the incident light in μmol m−2 s−1 and CO2 the CO2 concentration in μmol mol−1. Equations 5 and 6 were fitted to the temperature-response data, again in the light and in the dark. Temp is the temperature of the cuvette in °C. As the lower bounds of the optimal temperature ranges were frequently lower than the lowest experimental temperature, and since the model used was not suitable for downward extrapolation, the lowest temperature measured in these cases is reported (Table 3). Additionally, based on the temperature-response curves, we calculated the ratio of RD to NP at mean local night and day temperatures, respectively, as well as under three warming scenarios, assuming no acclimation (Table 4). Cardinal points of photosynthesis (Tables 3 and 4) were compared among altitudes using type I one-way ANOVA, followed, when appropriate, by Tukey HSD tests. Means are reported ± s.d.
Table 3.
Cardinal points of the photosynthesis response curves of 18 tropical bryophyte species from three locations in Panama
| Location | Species | WCopt (90 %) (% of d. wt) | WCmax (% of d. wt) | Depression (% of max NP) | NPmax (nmol g1 s−1) | LCP (μol m2 s−1) | RD/NPmax (%) | Topt (°C) | Topt (90 %) (°C) | TNP=RD (°C) | Tcomp (°C) | Q10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BT | Octoblepharum pulvinatum | 10·8 | 22 | 23 | 27 | 21–32 | 33 | >37·5 | ||||
| BT | Orthostichopsis tetragona | 210–808 | 1294 | 66 | 22·0 | 35 | 37 | 25 | <20–30 | 36 | >37·5 | 3·6 |
| BT | Plagiochila sp. 1 | 256–597 | 768 | 68 | 14·5 | 42 | 53 | 24 | <20–29 | 33 | 37 | 1·6 |
| BT | Stictolejeunea squamata | 152–479 | 967 | 36 | 12·5 | 69 | 63 | 25 | 21–29 | 30 | 36 | 2·1 |
| BT | Symbiezidium spp. | 262–523 | 814 | 12 | 16·0 | 47 | 48 | 27 | 24–31 | 32 | 37 | 2·1 |
| BT | Zelometeorium patulum | 244–1007 | 1558 | 70 | 18·6 | 48 | 53 | 25 | 19–30 | 35 | >37·5 | 2·2 |
| PS | Bryopteris filicina | 184–418 | 663 | 40 | 16·0 | 43 | 50 | 25 | 19–29 | 30 | >33 | 2·3 |
| PS | Lepidopilum polytrichoides | 281–669 | 1092 | 38 | 19·8 | 34 | 48 | 24 | 20–28 | 28 | >33 | 2·8 |
| PS | Pilotrichum bipinnatum | 226–745 | 1065 | 70 | 16·1 | 27 | 38 | 23 | <18–28 | 30 | >33 | 2·5 |
| PS | Plagiochila sp. 2 | 232–530 | 756 | 55 | 16·1 | 27 | 38 | 26 | 21–31 | 31 | >33 | 2·4 |
| PS | Symbiezidium spp. | 217–519 | 869 | 34 | 19·1 | 38 | 42 | 25 | 20–30 | 31 | >33 | 3·6 |
| PS | Syrrhopodon incompletus | 187–404 | 617 | 41 | 16·1 | 20 | 29 | 27 | 22–31 | 30 | >33 | 3·2 |
| F | Durmortiera hirsuta | 701–998 | 1082 | 67 | 28·5 | 29 | 38 | 20 | <10–26 | 28 | >35 | 1·9 |
| F | Frullania mirabilis | 127–289 | 464 | 36 | 12·5 | 159 | 63 | 16 | <10–21 | 19 | 29 | 3·1 |
| F | Herbertus divergens | 177–334 | 426 | 57 | 4·8 | 77 | 77 | 21 | 14–26 | 26 | 35 | 3·8 |
| F | Leucobryum antillarum | 667–1286 | 1711 | 52 | 12·9 | 38 | 71 | 23 | 15–28 | 31 | >35 | 3·5 |
| F | Phyllogonium fulgens | 201–466 | 645 | 63 | 9·3 | 79 | 77 | 17 | <10–23 | 23 | 33 | 2·3 |
| F | Phyllogonium viscosum | 324–849 | 1110 | 72 | 12·8 | 30 | 37 | 23 | 16–28 | 29 | >35 | 4·1 |
| ANOVA for location | N/A | n.s. | n.s. | n.s. | n.s. | n.s. | *** | N/A | ** | N/A | n.s. | |
Locations: BT, Bocas del Toro (0 m); PS, Palo Seco (500 m); F, Fortuna (1200 m).
Parameters from left to right: WCopt (90 %) = range of water contents (WC) where net photosynthesis (NP) >90 % of the maximum NP rate; WCmax = maximum WC, i.e. WC at full saturation; Depression = % depression of maximum NP at the WCmax; NPmax = maximum rate of net photosynthesis (based on light-response curve); LCP = light compensation point; RD/NP = ratio of RD to NPmax (last two based on light-response curve, rates at standard measuring temperature; see Table 2); Topt = temperature optimum of NP; Topt (90 %) = optimum temperature range where NP >90 % NPmax; TNP = RD = temperature where NP = RD; Tcomp = high-temperature compensation point of NP; Q10 = Q10 of RD, calculated at ±3 °C of local mean night temperature (BT = 24 °C, PS = 21 °C, F = 18 °C).
Values are means of at least five samples per response curve and species.
Where appropriate and possible, a Type I one-way ANOVA was conducted (significance levels: ***, P < 0·001; **, P < 0·01; *, P < 0·05; n.s., not significant; N/A, ANOVA not done).
Table 4.
Ratio of dark respiration (RD) to net photosynthesis (NP) of tropical bryophytes calculated for the mean local night/day temperature based on temperature-response curves
| Location | RD/NP ( %) at mean T | RD/NP (%) + 1 °C | RD/NP (%) + 3 °C | RD/NP (%) + 6 °C |
|---|---|---|---|---|
| Bocas del Toro | 31 ± 16 | 35 ± 17 | 44 ± 19 | 72 ± 34 |
| Palo Seco | 40 ± 5 | 44 ± 5 | 52 ± 6 | 75 ± 11 |
| Fortuna | 46 ± 26 | 52 ± 31 | 68 ± 45 | 118 ± 110 |
Data are means ± s.d. of six species.
RESULTS
Water response
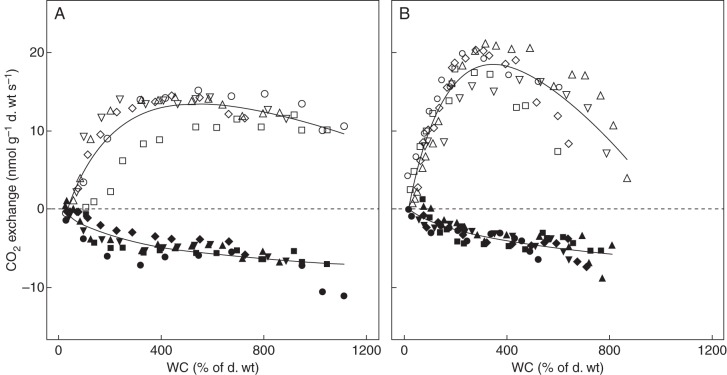
The responses of CO2 exchange to water content (WC) in the 18 studied species were quite variable quantitatively, but similar qualitatively, with strong depressions of both net photosynthesis (NP) and dark respiration (RD) at low water contents, and a variable depression of NP (12–72 % of the max, mean = 52 ± 17 %, n = 18) and no depression of RD at high water contents (Fig. 1 and Table 3). The fitted models for NP had r2 values ranging from 0·49 to 0·81 and for RD from 0·22 to 0·78. The optimum WC for NP (where NP exceeded 90 % of NPmax) differed considerably amongst species, ranging from approx. 150 to 750 %. Species with high maximum water contents generally had greater optimum ranges as well (r = 0·96 for WCmax – WCopt lower and r = 0·93 WCmax – WCopt upper). Optimum water contents for photosynthesis did not differ significantly between altitudes (P = 0·39).
Fig. 1.
Changes in net photosynthesis (NP) and dark respiration (RD) as a function of thallus water content in (A) Phyllogonium viscosum (Fortuna) and (B) Symbiezidium spp. (Palo Seco). The open symbols denote NP, closed symbols are RD. The different symbol shapes refer to different samples. Dashed lines indicate zero net gas exchange.
Light and CO2 response
The light responses of all 18 species were described well by the modified Smith function (r2 ≥ 0·99). Maximum rates of NP varied 6-fold from 4·8 nmol g−1 d. wt s−1 in the turf H. divergens to 28·5 nmol g−1 d. wt s−1 in the thallose terrestrial liverwort D. hirsuta (Table 3). Light compensation points were rather high relative to light conditions in the natural habitat in all species. The lowest light compensation point was found in the understorey species S. incompletus (20 µmol m2 s−1; Fig. 2A) and the highest in the exposed-growing species Frullania mirabilis (159 µmol m2 s−1). Altitude had no significant effect on any parameter of the light-response curve (P = 0·13–0·49). The ratio of NP to RD was rather variable and ranged from 1·3 to 4·0.
Fig. 2.
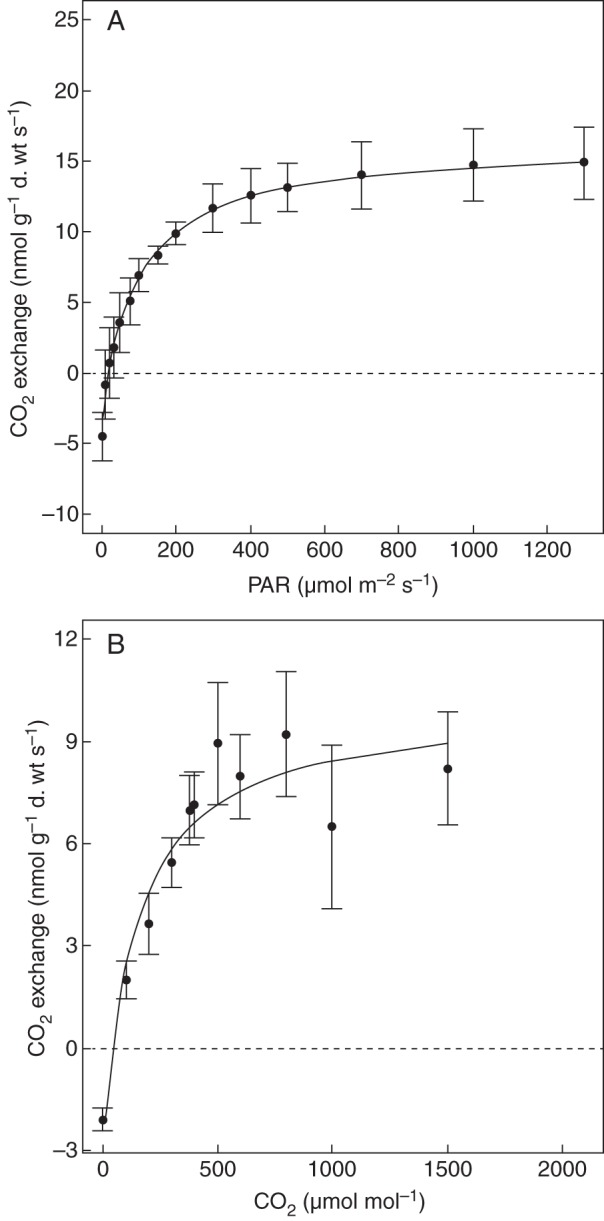
(A) Net photosynthesis (NP) as a function of light in the moss Syrrhopodon incompletus (Palo Seco, Panama, 500 m). (B) NP as a function of ambient CO2 concentration in Leucobryum antillarum (Fortuna, Panama, 1200 m). Data are means ± s.d., n = 5. Dashed lines indicate zero net gas exchange.
A second modified Smith function appropriately described the response of NP to varying CO2 concentrations (r2 = 0·62–0·97; Fig. 2B). Carbon dioxide concentrations can be up to approx. 480 µmol mol−1 in the moss phylloplane (Wagner et al., 2012), but the effects of this small increase in CO2 on NP are moderate when compared with atmospheric concentrations of approx. 380 µmol mol−1: exchange rates rise from 3·1 to 3·5 nmol g−1 s−1 in H. divergens, from 6·5 to 7·1 nmol g−1 s−1 in L. antillarum and from 3·8 to 4·6 nmol g−1 s−1 in O. tetragona (all n = 5). Actual rates of net photosynthesis in the natural habitat of the moss may thus be 9–20 % higher than rates determined at CO2 concentrations in the air of 380 µmol mol−1.
Temperature response
Temperature optima differed predictably between altitudes (Figs 3 and 4 and Table 3). The fitted models for NP had r2 values from 0·72 to 0·99, for RD r2 = 0·79–0·99. The optimum ranges for net photosynthesis (with NP > 90 % NPmax) usually spanned approx. 10 °C and included the mean daytime averages of temperature experienced at the respective altitudes.
Fig. 3.
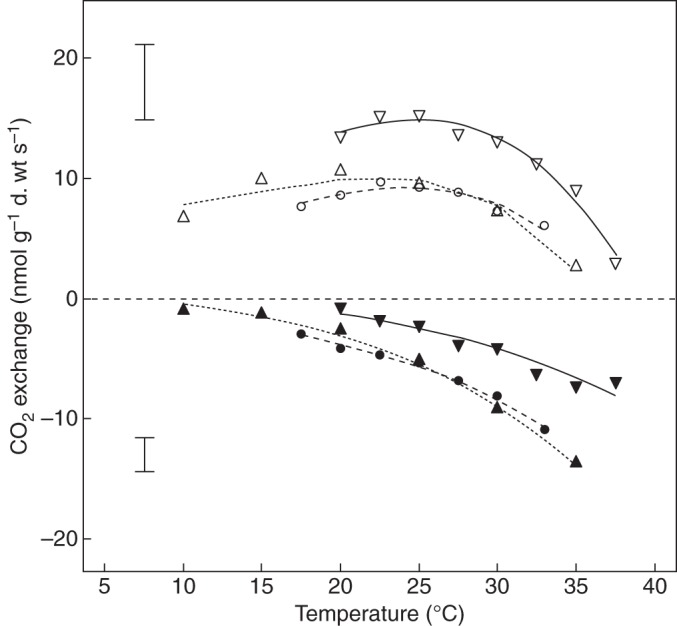
Net photosynthesis (NP; open symbols) and dark respiration (RD; closed symbols) as a function of temperature of three pendant bryophyte species from three altitudes in Panama: Orthostichopsis tetragona, continuous lines, downward-pointing triangles (0 m, Bocas del Toro); Bryopteris filicina, dashed lines, downward-pointing triangles (500 m, Palo Seco); Phyllogonium viscosum, dotted lines, upward-pointing triangles (1200 m, Fortuna). n = 5 for each species, error bars indicate mean standard deviations of NP and RD for the three species.
Fig. 4.
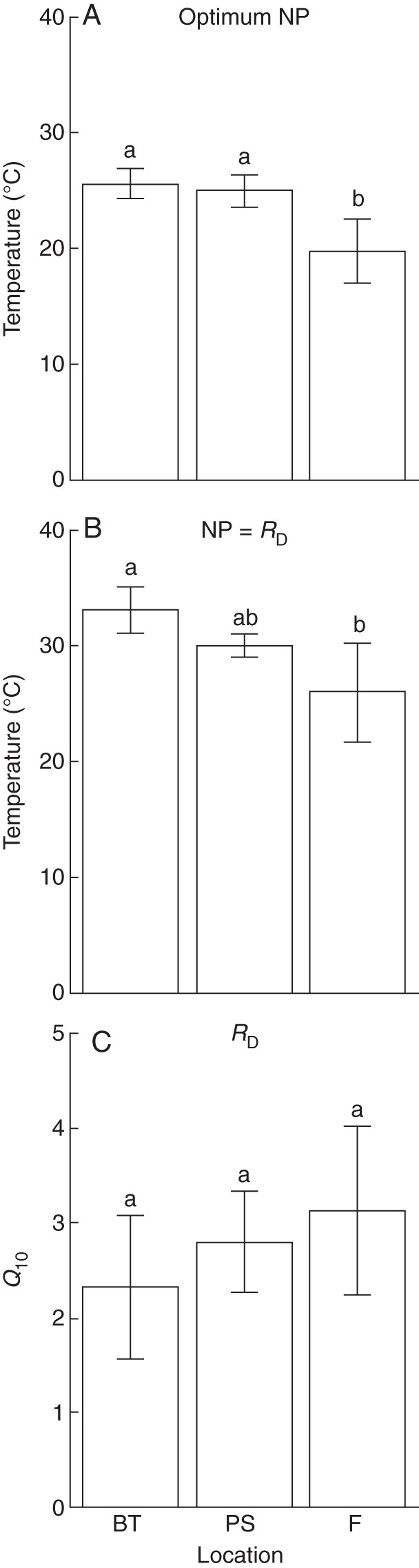
Metabolic temperature-response parameters for bryophyte species from sea level (BT), 500 m (PS) and 1200 m (F) in Panama: (A) temperature optima of net photosynthesis (NP; n = 6 species; (B) temperature where NP equals dark respiration rates (RD; n = 6 species); (C) Q10 of RD (n = 6 species, except BT, where n = 5 species, calculated at ± 3 °C of mean night temperature, BT = 24 °C, PS = 21 °C, F = 18 °C). Error bars indicate s.d. Letters indicate significant differences between altitudes (P < 0·001 for optimum temperature and P < 0·01 for NP = RD, one-way ANOVA followed by Tukey HSD-test).
Differences in optimum temperatures for NP (means of six species) were significant between the lower montane rainforest site (F; 20 °C) and the two lowland sites (BT and PS; 26 and 25 °C) (Fig. 4). The only species that was measured at two altitudes (Symbiezidium sp.) showed a trend to a somewhat higher optimum temperature in BT compared with PS (27 °C vs. 25 °C, P = 0·26), possibly suggesting either acclimation or divergence between populations (genetic variation).
The temperature at which NP equalled RD was also significantly higher in BT compared with F (P < 0·01), while the differences between PS and F and between PS and BT were not significant (P = 0·06 and 0·14, respectively). The upper temperature compensation points were beyond the range of measurements for most species. Q10 values for dark respiration, based on a temperature interval of ±3 °C around the local mean night temperature, tended to increase with increasing altitude, but this trend was not significant (Fig. 4; P = 0·23). Also Q10 values were calculated using the same temperature range for all species (20–30 °C), which yielded smaller and non-significant differences (BT = 2·3 ± 0·7, n = 5; PS = 2·6 ± 0·7, n = 6 and F = 2·5 ± 0·3, n = 6).
The ratio of RD to NP at mean local night and day temperatures (Table 4) did not differ between altitudes (P = 0·40). NP was determined at lower PPFD for the Fortuna samples (Table 2). Correcting for this, the mean value of RD/NP for Fortuna samples decreased from 46 % to 37 %, i.e. ratios are probably even more similar along the altitudinal gradient. Effects of warming on RD/NP were smallest at sea level and tended to increase with altitude although these differences were also not significant. A warming of 6 °C, which is approximately equivalent to a transfer from Fortuna to Bocas del Toro, would more-or-less double respiration for all species, with little reduction in photosynthesis.
Tissue chemistry
The average nitrogen content for all species was 12·1 ± 3·5 mg g−1 d. wt, with up to 3-fold differences between species (Table 5). Chlorophyll contents (chlorophyll a + b) varied more than 20-fold (Table 5). None of the parameters (N, total Chl or Chl a/b) differed significantly between altitudes (one-way ANOVA, P = 0·10, 0·16 and 0·09, respectively). Linear regressions of NPmax as a function of N and total chlorophyll content showed only a very weak positive relationship (r2 = 0·18 for both N and Chl).
Table 5.
Nitrogen and chlorophyll content of green biomass of tropical bryophytes from three different altitudes (0 m, BT; 500 m PS; 1200 m, Fortuna) in Panama
| Location | Species | N (mg g−1d. wt) | Total Chl (mg chloro g−1 d. wt) | Chl a/b |
|---|---|---|---|---|
| BT | Octoblepharum pulvinatum | 15·7 ± 1·0 | 7·7 ± 1·3 | 1·4 ± 0·1 |
| BT | Orthostichopsis tetragona | 12·4 ± 1·3 | 4·0 ± 0·5 | 1·7 ± 0·1 |
| BT | Plagiochila sp. 1 | 9·9 ± 1·9 | 1·2 ± 0·5 | 0·9 ± 0·3 |
| BT | Stictolejeunea squamata | 12·1 ± 1·0 | 2·1 ± 0·4 | 1·4 ± 0·1 |
| BT | Symbiezidium sp. 1 | 9·5 ± 0·4 | 1·6 ± 0·7 | 1·1 ± 0·3 |
| BT | Zelometeorium patulum | 11·0 ± 0·9 | 4·3 ± 0·9 | 1·5 ± .0·1 |
| PS | Bryopteris filicina | 12·9 ± 3·7 | 1·1 ± 1·0 | 1·1 ± 0·6 |
| PS | Lepidopilum polytrichoides | 21·0 ± 2·2 | 9·9 ± 2·0 | 1·1 ± 0·1 |
| PS | Pilotrichum bipinnatum | 16·2 ± 2·1 | 8·4 ± 2·6 | 1·1 ± 0·1 |
| PS | Plagiochila sp. 2 | 14·3 ± 0·9 | 3·9 ± 0·4 | 1·1 ± 0·4 |
| PS | Symbiezidium sp. 2 | 11·9 ± 1·0 | 3·7 ± 2·0 | 1·3 ± 0·5 |
| PS | Syrrhopodon incompletus | 9·8 ± 0·8 | N/A | N/A |
| F | Durmortiera hirsuta | 12·8 ± 0·9 | 5·2 ± 1·9 | 1·3 ± 0·3 |
| F | Frullania mirabilis | 14·3 ± 0·5 | 0·5 ± 0·4 | 1·9 ± 0·5 |
| F | Herbertus divergens | 5·8 ± 0·8 | 0·4 ± 0·1 | 1·5 ± 0·1 |
| F | Leucobryum antillarum | 10·9 ± 0·7 | 2·6 ± 1·8 | 1·2 ± 0·2 |
| F | Phyllogonium fulgens | 8·4 ± 0·8 | 1·6 ± 0·3 | 1·9 ± 1·3 |
| F | Phyllogonium viscosum | 8·4 ± 0·2 | 2·4 ± 0·2 | 1·3 ± 0·1 |
Means means ± s.d., n = 3, except for chlorophyll content of Herbertus divergens, where n = 2.
DISCUSSION
The response of net CO2 uptake to varying temperatures in the tropical mosses and liverworts studied shows a clear adaptation, or acclimatization, to mean habitat temperatures, while light responses and water responses show no such relationship with altitude and are more species specific. The change in optimum temperatures for photosynthesis (26, 25, 20 °C) closely matches ambient temperatures between sea level and 1200 m a.s.l. (26, 23, 20 °C), and also approximates the moist adiabatic lapse rate of the atmosphere (approx. 0·6 °C/100 m; Richards, 1996). In spite of this appealing coincidence, we cannot completely exclude that the lower optimum temperature in the Fortuna species is due to their more exposed growing sites. However, the higher temperature and brighter light here would usually result in higher rather than lower temperature optima (Niinemets et al, 1999) so that including understorey species or samples for Fortuna might, arguably, have yielded an even stronger gradient
The Q10 of dark respiration declines with increasing temperatures in a range of plant species from ecosystems worldwide, probably due to a shift from enzyme-limitation at lower temperatures to substrate or energy-demand limitation at higher temperatures (Atkin and Tjoelker, 2003). This pattern seems to hold true for tropical bryophytes, where dark respiration tended to respond more strongly to temperature (higher Q10) in the lower montane species, i.e. at lower measuring temperatures, than in the lowland species, though differences in our study were not significant. This altitudinal trend disappeared when the Q10 of all species was calculated for the same temperature range, suggesting no special adaptation or acclimation of Q10 to altitude (i.e. ambient temperatures).
In contrast to our expectation, the ratio between dark respiration and net photosynthesis rates at ambient temperatures did not differ between altitudes (Table 4). High respiration rates due to warm nights thus do not appear to be a distinguishing feature of the tropical lowland species compared with species from more bryophyte-rich lower montane areas. In fact, lowland species appear to be, if anything, better acclimatized or adapted to their ambient temperatures in terms of potential metabolic rates than montane species. However, these instantaneous rates are affected also by factors other than temperature, so that integrated daily carbon gains cannot be predicted from temperature responses alone.
Respiratory carbon losses are not restricted to the night, but net losses can also occur during the day in times of suboptimal water contents and low light or during resaturation bursts following rewetting, all of which could lower the integrated carbon gain (Lange et al., 2000, 2004). The major limitation of bryophyte net carbon gain is the availability of moisture. Drought tolerance per se does not appear to limit altitudinal distributions, because tolerances of both lowland and montane species in our study area far exceed the maximum duration of dry periods in most lowland forests (Bader et al., 2012; Pardow and Lakatos, 2012) and is also high in tropical bryophytes from other regions (Proctor, 2002; León-Vargas et al., 2006). However, the timing and duration of moisture availability determines physiological activity and, consequently, carbon gains and losses (Norris, 1990). Actual carbon gains and losses will be much lower than suggested by instantaneous rates derived from response curves (see Table 4) due to sub-optimal moisture conditions during most of the day. Based on integrated daily courses of in situ gas exchange, Zotz et al. (1997) estimated that montane bryophytes in Fortuna lost, on average, 60 % of daily gains to nightly respiration. This is comparable to values for the European lichen Lecanora muralis (lost 57 % on average) measured over the course of 1 year (Lange, 2003a). For the lowland lichen Parmotrema endosulphureum, however, nightly losses averaged 90 % with very frequent negative diel carbon balances (Zotz et al., 2003). For this lichen the temperature optimum for photosynthesis was also low, at 22 °C, and similar to that of the montane lichens Dictyonema glabratum (Lange et al., 1994) and Pseudocyphellaria aurata (Lange et al., 2004). The bryophytes we studied appear to be somewhat better-adapted physiologically to the high lowland temperatures than P. endosulphureum. They do, however, face the same challenges regarding the timing and duration of their physiological activity, which would be intensified by warmer temperatures (Bates, 1998; Kürschner et al., 1999).
At higher temperatures, evaporation rates increase through higher vapour pressure deficits, causing faster drying and shorter activity times in poikilohydric organisms, such as many epiphytic bryophytes which do not have an effective boundary layer (Jonsson et al., 2008). In contrast to mosses and liverworts in many montane areas, bryophytes growing in the lowlands generally cannot benefit from the frequent passage of clouds and fog (Cavelier et al., 1996). Such lateral precipitation is especially beneficial when it occurs during the morning, as it often does, allowing activity at times of high light. As a case in point, a prospering bryophyte flora occurs in those rare lowland forests where morning fog is common (Gradstein et al., 2010). Especially for loose life forms like wefts and pendants, where activity is already limited to 2 or 3 h after wetting (Zotz et al., 1997; Proctor, 2002; Romero et al., 2006; Wagner et al., 2012), a reduced period of activity would severely limit carbon gain. Faster drying could also lead to more frequent rewetting causing carbon losses through carbohydrate leaching (Coxson et al., 1992). Whether bursts of respiration at rewetting are really irrelevant ecologically, as suggested by Zotz et al. (1998) for the tropical lichen Sticta tomentosa, needs to be tested for other mosses and lichens.
Our study showed a clear relationship between mean temperatures experienced and temperature responses of net photosynthesis. The positive relationship between ambient temperature and temperature response can also be extended to bryophytes worldwide: the temperature optima of net photosynthesis of 61 bryophyte species scale significantly with the mean temperatures during the growing season (Fig. 5). One species, Rhacomitrium lanuginosum, did not follow this trend and has a somewhat strange behaviour. A study by Kallio and Heinonen (1975) showed that the temperature optimum of net photosynthesis in this boreal species was always 5 °C, irrespective of latitude (latitudinal range: 47–79 °N and 53 °S). This is a very low temperature compared with the optima of other species, even from the arctic, and the homogeneity suggests virtually no acclimatization or adaptation potential. On the other hand, the optimum temperature of the same species measured in south-west England (approx. 50 °N) was nearly threefold higher (see Supplementary Data Table S1 and Tallis, 1959). In spite of uncertainties in the temperature estimations (exact locations and harvesting times were not reported in all sources, even though the latter seems especially important in seasonal climates; Hicklenton and Oechel, 1977; Larigauderie and Körner, 1995), and methodological differences between data sources (although we only included measurements under ambient CO2 concentrations), the relationship is clear. Temperature responses of net photosynthesis, and of respiration rates, commonly closely match climatic conditions.
Fig. 5.
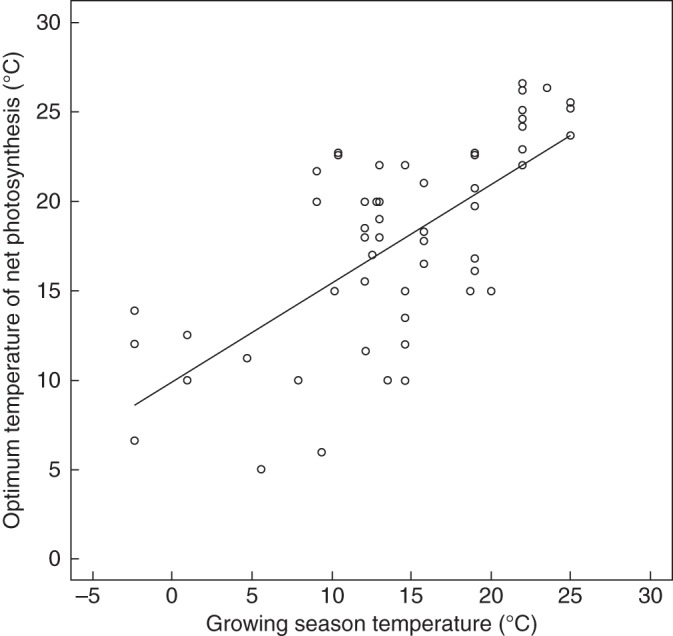
Temperature optima of net photosynthesis of 61 bryophyte species in relation to mean temperature of the habitat during the growing season, based on 19 studies from a wide range of ecosystems: polar, alpine, temperate, desert and tropical regions. Each data point represents one species. Growing-season temperatures are based on data from the nearest climate station (monthly averages from 1970–2009 or less). Mean temperatures of the study sites were adjusted according to altitude (moist adiabatic lapse rate: 0·6 °C 100 m−1). Where ranges were reported the arithmetic mean of the range was used for the analysis. Optimum temperatures were averaged where species showed seasonal acclimation or came from different altitudes. The line represents a linear regression model (r2 = 0·46, P < 0·001). All studies included net photosynthesis measured at normal atmospheric CO2 concentrations and at close to saturating irradiances. For details and references see Supplementary Data Table S1 online.
Such an adaption could also restrict species to certain climatic ranges (e.g. montane tropics), though this restriction would be lifted if rates can acclimatize to ambient temperatures. A recent study found no metabolic acclimation to higher temperatures in a range of tropical bryophytes from 500 and 1200 m a.s.l., although some samples survived nearly2 years at higher temperatures and recovered growth rates after initial dieback during this time (S. Wagner, G. Zotz and M. Y. Bader, unpubl. res.). The lack of warmer areas to which samples could be transplanted to precluded the inclusion of lowland species in that study. The question of the acclimation potential of tropical bryophytes is thus still not resolved. If lowland species were already at their physiological limit, predicted temperature increases could severely threaten bryophytes in the tropical lowlands.
In conclusion, this study showed that tropical bryophytes, in contrast to lichens, are well adapted physiologically to the prevalent temperatures of their habitat. This refutes a common explanation for the observed altitudinal gradient in the tropics. An alternative explanation for altitudinal sorting, differences in desiccation tolerance, has been dismissed recently because the tolerance of montane species to prolonged desiccation is fully sufficient for them to survive even under lowland conditions (Bader et al., 2012). We suggest that the timing and duration of hydration, more than metabolic rates, are most likely the main drivers for the precarious carbon balance and, consequently, the low biomass of lowland bryophytes. Restricted photosynthetic activity may also be the main limitation for montane bryophytes to establish at lower altitudes. Thus, future research on the causes of bryophyte abundance patterns in the tropics should focus on activity times during day and night.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Lina Lüdemann for measuring CO2-response curves, Steve González and Carlos Espinosa for field work. Permission to work in Panama was granted by local authorities (ANAM; SC/P-7-11, SEX/P-62-11, SEX/P-7-10). This work was supported by the German Research Foundation–DFG (grant number BA 3843/3-1).
LITERATURE CITED
- Atkin OK, Tjoelker MG. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends in Plant Science. 2003;8:343–351. doi: 10.1016/S1360-1385(03)00136-5. [DOI] [PubMed] [Google Scholar]
- Bader MY, Reich T, Wagner S, González González AS, Zotz G. Differences in desiccation tolerance do not explain altitudinal distribution patterns of tropical bryophytes. Journal of Bryology. 2012 in press. http://dx.doi.org/10.1179/1743282012Y.0000000033. [Google Scholar]
- Bates JW. Is ‘life-form’ a useful concept in bryophyte ecology? Oikos. 1998;82:223–237. [Google Scholar]
- Cavelier J, Solis D, Jaramillo MA. Fog interception in montane forests across the Central Cordillera of Panamá. Journal of Tropical Ecology. 1996;12:357–369. [Google Scholar]
- Coxson DS, McIntyre DD, Vogel HJ. Pulse release of sugars and polyols from canopy bryophytes in tropical montane rain forest (Guadeloupe, French West Indies) Biotropica. 1992;24:121–133. [Google Scholar]
- Frahm J-P. Ökologische Studien über die epiphytische Moosvegetation in Regenwäldern NO-Perus. Beihefte zur Nova Hedwigia. 1987;88:143–158. [Google Scholar]
- Frahm J-P. Bryophyte phytomass in tropical ecosystems. Botanical Journal of the Linnean Society. 1990;104:23–33. [Google Scholar]
- Gradstein SR, Pocs T. Bryophytes. In: Lieth H, Werger MJA, editors. Tropical rainforest ecosystems. Amsterdam: Elsevier; 1989. pp. 311–325. [Google Scholar]
- Gradstein SR, Obregon A, Gehrig C, Bendix J. Tropical lowland cloud forest: a neglected forest type. In: Bruijnzeel LA, Scatena FN, Hamilton LS, editors. Tropical montane cloud forests. New York, NY: Cambridge University Press; 2010. pp. 130–133. [Google Scholar]
- Green TGA, Lange OL. Photosynthesis in poikilohydric plants: a comparison of lichens and bryophytes. In: Schulze ED, Caldwell MM, editors. Ecophysiology of photosynthesis. Berlin: Springer; 1994. pp. 319–341. [Google Scholar]
- Hicklenton PR, Oechel WC. Influence of light-intensity and temperature on field carbon-dioxide exchange of Dicranum fuscens in subarctic. Arctic and Alpine Research. 1977;9:407–419. [Google Scholar]
- Jonsson AV, Moen J, Palmqvist K. Predicting lichen hydration using biophysical models. Oecologia. 2008;156:259–273. doi: 10.1007/s00442-008-0990-5. [DOI] [PubMed] [Google Scholar]
- Kallio P, Heinonen S. CO2 exchange and growth of Rhacomitrium lanuginosum and Dicranum elongatum. In: Wielgolaski FE, editor. Fennoscandian tundra ecosystems. Part 1. Plants and microorganisms. New York, NY: Springer; 1975. [Google Scholar]
- Kürschner H, Frey W, Parolly G. Patterns and adaptive trends of life forms, life strategies and ecomorphological structures in tropical epiphytic bryophytes–a pantropical synopsis. Nova Hedwigia. 1999;69:73–99. [Google Scholar]
- Lange OL. Photosynthetic productivity of the epilithic lichen Lecanora muralis: long-term field monitoring of CO2 exchange and its physiological interpretation. II. Diel and seasonal patterns of net photosynthesis and respiration. Flora. 2003a;198:55–70. [Google Scholar]
- Lange OL. Photosynthetic productivity of the epilithic lichen Lecanora muralis: long-term field monitoring of CO2 exchange and its physiological interpretation. II. Diel and seasonal patterns of net photosynthesis and respiration. Flora. 2003b;198:55–70. [Google Scholar]
- Lange OL, Büdel B, Zellner H, Zotz G, Meyer A. Field measurements of water relations and CO2 exchange of the tropical, cyanobacterial basidiolichen Dictyonema glabratum in a Panamanian rainforest. Botanica Acta. 1994;107:279–290. [Google Scholar]
- Lange OL, Büdel B, Meyer A, Zellner H, Zotz G. Lichen carbon gain under tropical conditions: water relations and CO2 exchange of three Leptogium species of a lower montane rain forest in Panama. Flora. 2000;195:172–190. [Google Scholar]
- Lange OL, Büdel B, Meyer A, Zellner H, Zotz G. Lichen carbon gain under tropical conditions: water relations and CO2 exchange of Lobariaceae species of a lower montane rain forest in Panama. Lichenologist. 2004;36:329–342. [Google Scholar]
- Larigauderie A, Körner C. Acclimation of leaf dark respiration to temperature in alpine and lowland plant species. Annals of Botany. 1995;76:245–252. [Google Scholar]
- León-Vargas Y, Engwald S, Proctor MCF. Microclimate, light adaptation and desiccation tolerance of epiphytic bryophytes in two Venezuelan cloud forests. Journal of Biogeography. 2006;33:901–913. [Google Scholar]
- Mägdefrau K. Life-forms of bryophytes. In: Smith AJE, editor. Bryophyte ecology. London: Chapman and Hall; 1982. pp. 45–58. [Google Scholar]
- Niinemets Ü, Oja V, Kull O. Shape of leaf photosynthetic electron transport versus temperature response curve is not constant along canopy light gradients in temperate deciduous trees. Plant, Cell & Environment. 1999;22:1497–1513. [Google Scholar]
- Norris DH. Bryophytes in perennial moist forests of Papua New Guinea: ecological orientation and predictions of disturbance effects. Botanical Journal of the Linnean Society. 1990;104:281–291. [Google Scholar]
- Pardow A, Lakatos M. Desiccation tolerance and global change: implications for tropical bryophytes in lowland forests. Biotropica. 2012 in press. http://dx.doi.org/10.1111/j.1744-7429.2012.00884.x. [Google Scholar]
- Pócs T. The epiphytic biomass and its effect on the water balance of two rain forest types in the Uluguru Mountains (Tanzania, East Africa) Acta Botanica Academiae Scientiarium Hungaricae. 1980;26:143–167. [Google Scholar]
- Proctor MCF. Ecophysiological measurements on two pendulous forest mosses from Uganda, Pilotrichella ampullacea and Floribundaria floribunda. Journal of Bryology. 2002;24:223–232. [Google Scholar]
- Proctor MCF. Physiological ecology: water relations, light and temperature responses, carbon balance. In: Smith AJE, editor. Bryophyte ecology. London: Chapman and Hall; 1982. pp. 333–381. [Google Scholar]
- R Development Core Team. R: a language and environment for statistical computing. 2011 v.2.14.0. Vienna, Austria. http://r-project.org . [Google Scholar]
- Richards PW. The ecology of tropical forest bryophytes. In: Schuster RM, editor. New manual of bryology. Nichinan: The Hattori Botanical Laboratory; 1984. pp. 1233–1277. [Google Scholar]
- Richards PW. The tropical rain forest: an ecological study. Cambridge: Cambridge University Press; 1996. [Google Scholar]
- Romero C, Putz FE, Kitajima K. Ecophysiology in relation to exposure of pendant epiphytic bryophytes in the canopy of a tropical montane oak forest. Biotropica. 2006;38:35–41. [Google Scholar]
- Seifriz W. The altitudinal distribution of lichens and mosses on Mt. Gedeh, Java. Journal of Ecology. 1924;12:307–313. [Google Scholar]
- Tallis JH. Studies in the biology and ecology of Rhacomitrium lanuginosum Brid. 2. Growth, reproduction and physiology. Journal of Ecology. 1959;47:325–350. [Google Scholar]
- Tenhunen JD, Weber JA, Yocum CS, Gates DM. Development of a photosynthesis model with an emphasis on ecological application. II. Analysis of a data set describing the PM surface. Oecologia. 1976;26:101–119. doi: 10.1007/BF00582889. [DOI] [PubMed] [Google Scholar]
- Wagner S, Zotz G, Bader MY. Physiological ecology of tropical bryophytes. In: Hanson D, Rice S, editors. Photosynthesis of bryophytes and early land plants. New York, NY: Springer; 2012. (in press) [Google Scholar]
- Wolf JHD. Diversity patterns and biomass of epiphytic bryophytes and lichens along an altitudinal gradient in the northern Andes. Annals of the Missouri Botanical Garden. 1993;80:928–960. [Google Scholar]
- Zotz G. Altitudinal changes in diversity and abundance of non-vascular epiphytes in the tropics: an ecophysiological explanation. Selbyana. 1999;20:256–260. [Google Scholar]
- Zotz G, Winter K. Photosynthesis and carbon gain of the lichen, Leptogium azureum, a lowland tropical forest. Flora. 1994;189:179–186. [Google Scholar]
- Zotz G, Budel B, Meyer A, Zellner H, Lange OL. Water relations and CO2 exchange of tropical bryophytes in a lower montane rain forest in Panama. Botanica Acta. 1997;110:9–17. [Google Scholar]
- Zotz G, Büdel B, Meyer A, Zellner H, Lange OL. In situ studies of water relations and CO2 exchange of the tropical macrolichen, Sticta tomentosa. New Phytologist. 1998;139:525–535. [Google Scholar]
- Zotz G, Schultz S, Rottenberger S. Are tropical lowlands a marginal habitat for macrolichens? Evidence from a field study with Parmotrema endosulphureum in Panama. Flora. 2003;198:71–77. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.