Abstract
Background and Aims
Carnivorous plants of the genus Nepenthes possess modified leaves that form pitfall traps in order to capture prey, mainly arthropods, to make additional nutrients available for the plant. These pitchers contain a digestive fluid due to the presence of hydrolytic enzymes. In this study, the composition of the digestive fluid was further analysed with regard to mineral nutrients and low molecular-weight compounds. A potential contribution of microbes to the composition of pitcher fluid was investigated.
Methods
Fluids from closed pitchers were harvested and analysed for mineral nutrients using analytical techniques based on ion-chromatography and inductively coupled plasma–optical emission spectroscopy. Secondary metabolites were identified by a combination of LC-MS and NMR. The presence of bacteria in the pitcher fluid was investigated by PCR of 16S-rRNA genes. Growth analyses of bacteria and yeast were performed in vitro with harvested pitcher fluid and in vivo within pitchers with injected microbes.
Key Results
The pitcher fluid from closed pitchers was found to be primarily an approx. 25-mm KCl solution, which is free of bacteria and unsuitable for microbial growth probably due to the lack of essential mineral nutrients such as phosphate and inorganic nitrogen. The fluid also contained antimicrobial naphthoquinones, plumbagin and 7-methyl-juglone, and defensive proteins such as the thaumatin-like protein. Challenging with bacteria or yeast caused bactericide as well as fungistatic properties in the fluid. Our results reveal that Nepenthes pitcher fluids represent a dynamic system that is able to react to the presence of microbes.
Conclusions
The secreted liquid of closed and freshly opened Nepenthes pitchers is exclusively plant-derived. It is unsuitable to serve as an environment for microbial growth. Thus, Nepenthes plants can avoid and control, at least to some extent, the microbial colonization of their pitfall traps and, thereby, reduce the need to vie with microbes for the prey-derived nutrients.
Keywords: Antimicrobial activity, carnivorous plants, defensive proteins, digestive pitcher fluid, naphthoquinones, Nepenthes spp., mineral nutrients, pitfall traps
INTRODUCTION
Carnivorous or insectivorous plants fascinate scientists, in particular botanists, since the times of Charles Darwin. He was the first to write a book on this topic, which still represents the standard work about insectivorous plants (Darwin, 1875). Although in that work Darwin described plants that are equipped with pitfall traps, so-called pitchers, to catch their prey such as Sarracenia spp. and Darlingtonia californica, he never saw species of the genus Nepenthes occurring basically in south-east Asia. Like other carnivorous plants, Nepenthes spp. grow on poor soil. Therefore, they need to complement their mineral nutrients – primarily with nitrogen and phosphorus – from caught and digested prey. When visiting the pitfall traps, the attracted prey, mainly arthropods, falls into the trap (Gaume et al., 2002; Bohn and Federle, 2004), drowns and is digested by the enzyme cocktail of the pitcher fluid (Heslop-Harrison, 1975; Juniper et al., 1989). Because the digestive liquid can be easily harvested from the pitcher, Nepenthes plants are ideal objects to study enzymes and other compounds involved in this plant carnivory. As a consequence, compared with other carnivorous taxa, the protein composition of the digestive fluid of Nepenthes is fairly well analysed and documented (Mithöfer, 2011).
In Nepenthes, the trap-bearing leaf consists of a photosynthetic part, originally the enlarged leaf base, and a tendril that at its end might develop the pitfall trap, which is formed from a leaf by epiascidiation, i.e. by in-rolling of the adaxial leaf surface followed by marginal fusion (Juniper et al., 1989; Owen and Lennon, 1999). The digestive fluid can already be collected from young, still closed and prey-free pitchers (Fig. 1). Due to the fact that closed pitchers have no direct contact to the environment it has been widely claimed that (a) their pitcher fluid is sterile and (b) all proteins and compounds identified in this pitcher fluid are solely plant-derived. Interestingly, only two experiments have been conducted to demonstrate the sterility of pitcher liquid: fluid taken from a closed pitcher was plated either on plain nutrient agar (Hepburn, 1918) or on meat agar plates (Lüttge, 1964) and incubated for several days. In no case was any bacterial colony detected growing and the authors concluded that the pitcher fluid is sterile. However, this conclusion was challenged, at least for N. alata, when a few bacteria were found in closed pitchers by Sota et al. (1998). Moreover, for Sarracenia spp. the presence of endophytic fungi has been described (Glenn and Brodi, (2012). In any case, the presence of microbes cannot be excluded completely by such simple experiments because most micro-organisms cannot be grown in culture (Riesenfeld et al., 2004).
Fig. 1.

Closed pitcher of Nepenthes alata.
In closed as well as in open pitchers any contamination by unwanted microbes will stress the plant and, probably more important, represent a population of organisms that compete with the plant for nutrients from the pitcher fluid. Thus, it seems necessary to generate an environment that is at least unsuitable or even hostile to microbes. The presence of pathogen-related proteins in the pitcher fluid that have no obvious hydrolytic function in the digestion of caught prey suggests that these proteins might be directed against micro-organisms (Hatano and Hamada, 2008; Mithöfer, 2011). Moreover, inducible low molecular-weight compounds with antimicrobial properties could be identified in the fluid of N. khasiana, droserone and 5-O-methyl-droserone (Eilenberg et al., 2010). These findings strongly suggest that Nepenthes plants try to keep their pitchers free from bacteria as long as possible to avoid the growth of microbial competitors and cheaters to ensure that all nutrients available from the pitchers only benefit the plant. However, such questions have not been addressed experimentally.
Here, we analysed the composition of Nepenthes digestive fluid from closed pitchers to examine whether or not pitchers are really sterile inside and how these plants manage to keep microbial growth under control. Therefore, beyond proteins, inorganic ion compositions as well as secondary metabolites were studied. In addition, the effect of pitcher fluid on microbial growth was investigated. Our results reveal that the fluid of closed Nepenthes pitchers is so composed as to gain anti-microbial growth conditions.
MATERIALS AND METHODS
Plants
Nepenthes plants (N. alata, N. fusca, N.gracilis, N. mirabilis, N. superba, N. thorelii and N. ventricosa) were grown either in the greenhouses of the Botanical Gardens in Jena and Munich, Germany, or at the MPI for Chemical Ecology. Plants grew at 24–30 °C, at a humidity of 60–90 %, with a minimum of 12 h and a maximum of 16 h light. Due to the limited number of well-developed but still closed pitchers and the limited volumes of pitcher fluid (often <0·5 mL) available from them, various Nepenthes species had to be included in this study. However, as will be seen from the results, in all experiments where different species were investigated in parallel, the results obtained were strikingly similar suggesting that all results are very likely representative for the genus Nepenthes.
Analysis of Nepenthes pitcher fluid for the presence of bacterial 16S-rDNA
The digestive fluids of closed Nepenthes pitchers of N. alata, N. fusca, N. mirabilis, N. superba, N. thorelii and N. ventricosa were sampled with sterile syringes and stored at –80 °C till further analysis. For lyses of bacterial cells, the pitcher fluid was mixed 1 : 1 with the Lyse and Go reagent (Thermo Scientific, Rockford, IL, USA) and treated according to the manufacturer's instructions. Two microlitres of the treated pitcher fluid was added to a final volume of 25 µL PCR mixture [1 mm MgCl2, 0·2 mm dNTPs, 10 mm Tris–HCl, 50 mm KCl, 0·8 % (v/v) Nonidet P40, 0·025 U Taq polymerase and 4 µm forward (E334F: 5'-AGA CTC CTA CGG GAG GCA GC-3'; Baker and Smith; 2003, modified from Rudi et al., 1997) and reverse primer (U529R: 5'-ACC GCG GCK GCT GGC-3'; DasSarma and Fleischmann, 1995; Baker and Smith, 2003)]. The bacterial 16S-rRNA gene was amplified as follows: unitial denaturation at 94 °C for 3 min, followed by 25 cycles of denaturation at 94 °C for 30 s, annealing at 60·5 °C for 1 min, amplification at 72 °C for 1 min and a final amplification step at 72 °C for 7 min. No-templates were included as negative controls in every PCR. Positive controls contained 2 µL of pitcher fluid and Escherichia coli cells. Reactions were checked for positive amplification by gel electrophoresis on 1 % agarose gels stained with ethidium bromide.
Microbial growth analyses
Pitcher fluids were tested in vitro for antimicrobial effects on the growth of E. coli and Pseudomonas syringae pv. syringae, as well as on yeast Saccharomyces cerevisiae. Therefore, 500 µL of overnight cultures from these strains were inoculated in 10 mL fresh LB-medium (Luria Bertani medium: 5 g L−1 yeast extract, 10 g L−1 trypton, 10 g L−1 NaCl) for E. coli and P. syringae, and ME-medium (malt extract broth: 17 g L−1 malt extract, 3 g L−1 pepton) for S. cerevisiae, respectively, and incubated at 37 °C (E. coli) or 30 °C (P. syringae, S. cerevisiae), shaking at 220 rpm up to an OD600 of 0·5. For microbial-growth analyses, 20 µL of these cultures plus 80 µL LB- or ME-medium and 100 µL of pitcher fluid from closed or just opened (i.e. maximum 16 h after opening) Nepenthes pitchers were incubated in cavities of a 96-well microtiter plate at the respective temperatures. As a positive control we used 100 µL H2O (pH 4) instead of pitcher fluid; as a negative control 100 µL of pitcher fluid plus 100 µL LB/ME-medium was used. The respective cultures' growth was determined for up to 20 h at OD600 using a SPECTRAmax 250-Photometer (Molecular Devices, Sunnyvale, CA, USA) against a reference (100 µL H2O plus 100 µL LB-/ME-medium) and documented every hour. Six technical replicates were carried out and the experiments were repeated independently three times.
For in vivo growth analyses, 100 µL of S. cerevisiae and P. syringae cultures (OD600 approx. 0·5), respectively, were injected into the fluid of closed pitchers and gently slewed. Immediately after injection, 100 µL of this suspension was harvested (0 h, t0) and after 72 h (t72) a second sample was taken to determine the number of living bacteria and yeast cells. For a control, cultures were also injected into sterile pitcher fluid of closed pitchers after removal from the living plant and left with the Nepenthes plants in the greenhouse to have identical growth conditions. The collected samples were diluted and plated on LB-/ME-medium on Petri dishes. After incubation for 24 h, the colony-forming units (cfu) were counted and the ratio of cfu t72/t0 was calculated. Data are from experiments carried out in triplicate.
Anions and nitrogen analyses
Fluoride, chloride, bromide, nitrate, phosphate and sulfate measurements were performed on the ion chromatography system DX-500 (Dionex, Idstein, Germany), equipped with an electrical conductivity detector (CD40) with auto-regenerating anion suppressor (ASRS® 300) and UV/VIS-detector (AD20). The stationary phase consisted of a guard column (IonPac® AG14, 4 × 50 mm; Dionex) and an analytical column (IonPac® AS14, 4 × 250 mm; Dionex). As the mobile phase, a 3·5-mm sodium carbonate/1·0-mm sodium bicarbonate solution with a flow rate of 1·2 mL min−1 was used. An integrated sample pre-treatment and pre-concentration unit working with an OnGuard® cartridge (OnGuard® II RP; Dionex) and ultra-low-pressure trace-anion-concentrator column (TAC-ULP1®; Dionex) allowed detection of small amounts of the analytes in samples, which were potentially contaminated with aromatic dyes, lipids, aromatic carboxylic acids, hydrocarbons, surfactants or other contaminants.
Sample measurements were performed in duplicate, using a sample volume of 50 µL for each injection. Because of different ionic concentrations from traces up to several hundred mg L−1, pitcher fluids were analysed in different dilution steps. Statistical parameters of the measurements such as the limit of detection and the limit of quantification were calculated using the signal-to-noise ratio and the corresponding multi-point calibrations, respectively. Acceptable instrument performance, long-term precision and accuracy of measurements were verified by analysing quality-control samples, e.g. anion standard solutions (Fluka, grade: TraceCERT®, for ion chromatography; Sigma-Aldrich Corporation, St Louis, MO, USA), and certified reference material, e.g. river water MISSIPPI-03 (Environment Canada, Burlington, Canada).
Measurements of total bound nitrogen were performed using the sum parameter analyser TN-100 (Mitsubishi; a1-envirosciences GmbH, Düsseldorf, Germany) which was equipped with a high-temperature reaction unit where both inorganic and organic nitrogen compounds were oxidized to NOx and converted subsequently into NO (temperature, 800 °C; catalyst, platinum; carrier/reaction gas, oxygen 5·0). After drying, NO reacts in the chemiluminescence cell with ozone to NO2 and hν. This emitted light was detected using a photomultiplier tube. Pitcher fluids were measured in triplicate with a selectable sample volume of 30 µL for each injection. Using a multi-point calibration, statistical parameters (limits of detection and quantification) were calculated. Acceptable instrument performance, long-term precision and accuracy of measurements were verified by analysing quality-control samples, e.g. NH4+ standard solution (CertiPUR®; Merck KGaA, Darmstadt, Germany) and certified reference material, e.g. Nutrients QCI-042-2 (RT Corp, Laramie, WY, USA).
Inductively coupled plasma–optical emission spectroscopy
To analyse the elements in the pitcher fluid, an OptimaTM 3300 DV inductively coupled plasma–optical emission spectroscope (PerkinElmer Inc., Shelton, CT, USA) equipped with a 40-MHz, free-running RF-generator and an array detector allowing for the simultaneous determination of the elements was used. According to Boumans (1987) and DIN EN ISO 11885 (1998), the wavelengths listed in Supplementary Data Table S1 were selected for element determination.
A two-point calibration (at 0·25 mg L−1 and 2·5 mg L−1 for Al, B, Cu, Fe, Mn, Ni, Si, Sr and Zn; at 5·0 and 50 mg L−1 for Na, P and S; at 10 and 100 mg L−1 for Mg; and at 25 and 250 mg L−1 for Ca and K) was carried out using a multi-element standard solution (Spex CertiPrep, Metuchen, NJ, USA). Except for K and Na, which were analysed using the radial plasma viewing mode, all other elements were analysed using the axial viewing plasma mode. Other instrumental operating parameters are summarized in Supplementary Data Table S2. The fluid from five closed N. alata pitchers was collected and combined for the analyses.
Secondary-metabolite analysis of Nepenthes pitcher fluid
Pitcher fluid collected from N. ventricosa was transferred into a 150-mL Erlenmeyer flask and 50 mL of dichloromethane was added. The flask was subsequently sealed with a piece of aluminium foil and kept on a shaker at room temperature overnight. Then, the organic phase was transferred into a 100-mL round bottom flask and removed under vacuum to dryness. The residue was reconstituted with 1 mL of methanol and subjected to HPLC-MS and NMR analyses.
HPLC-MS analysis
Metabolites extracted from Nepenthes pitcher juices were separated on a Nucleodur Isis RP18ec column (250 × 4·6 mm, 5-μm particle size; Macherey Nagel, Düren, Germany) using an Agilent 1100 series HPLC (Agilent Technologies GmbH, Böblingen, Germany). The flow rate for chromatographic separation was 0·8 mL min−1. Column temperature was maintained at 25 °C. The metabolites were separated using 0·1 % (v/v) formic acid and methanol as mobile phases A and B, respectively, with the following elution profile: 0–1 min, 100 % A; 1–41 min, 0–100 % B in A; 41–50 min 100 % B; 50–55 min, 100–0 % B in A and 55–60 min 100 % A. Compound detection and quantification were accomplished with an Esquire 3000 ESI ion-trap mass spectrometer (MS) (Bruker Daltonics, Bremen, Germany). Flow coming from the column was diverted in a ratio of 3 : 1 before entering the mass spectrometer electrospray chamber. The MS was operated in positive mode scanning m/z between 150 and 1500 with an optimal target mass of 200 m/z. The mass spectrometer was operated using the following specifications: skimmer voltage, 60 V; capillary voltage, 4000 V; nebulizer pressure, 35 psi; drying gas, 12 L min−1; gas temperature, 330 °C. Capillary exit potential was kept at 121 V. Compounds in chromatograms were identified based on retention time and their m/z ratio.
NMR analysis
1H NMR, 13C NMR, 1H-1H COSY, HMBC and HSQC spectra were measured on a Bruker Avance 500 NMR spectrometer (Bruker Biospin, Karlsruhe, Germany), operating at 500·13 MHz for 1H and 125·75 MHz for 13C. A TCI cryoprobe (5 mm) was used to measure spectra at a probe temperature of 300 K. Spectra are referenced to the residual solvent signals of acetone-d6 at δ 2·04/206·0 ppm and compared with spectra of standard compounds. Standard tubes (5 mm i.d.) and capillary tubes (2 mm i.d.) were used for all NMR measurements.
Structural modelling
For homology modelling of protein structure, 3-dimensional models of thaumatin-like protein (TLP) were calculated using SWISS-MODEL, an automated comparative modelling program (http://swissmodel.expasy.org/workspace) (Guex and Peitsch, 1997; Schwede et al., 2003; Arnold et al., 2006). As homologous protein template for Nepenthes gracilis TLP (EMBL: ABC73397·1), the crystal structure of TLP from banana (Musa acuminata) (Ban-TLP, 1Z3Q_A, (Leone et al., 2006) was used as a template.
RESULTS
Sterility and microbial growth properties of closed pitcher fluids from Nepenthes
Due to the fact that often less than half of bacterial phylotypes present in a sample grow on plates, a molecular approach was employed to identify bacteria in the fluid of closed pitchers. Using a PCR technique with domain-specific primers of highly conserved bacterial 16S-rDNA sequences that cover about half a million bacteria species (Baker and Smith, 2003), it was only in the positive E. coli control that the bacterial DNA could be proved to be the expected 200-bp PCR product detected (Fig. 2). None of the six Nepenthes species tested showed amplification of DNA fragments. Based on dilution series of E. coli, the detection limit for this method was determined to be 150 cells.
Fig. 2.

Proof of the absence of bacteria in Nepenthes pitcher fluids. PCR-based amplification of highly conserved 16S-rDNA sequence (200 bp) using selected domain-specific primers. Lanes: 1, N. alata; 2, N. fusca; 3, N. mirabilis; 4, N. superba; 5, N. thorelii, 6, N. ventricosa; M: 100-bp DNA ladder; NC, negative control without cells; PC, positive control using E. coli cells.
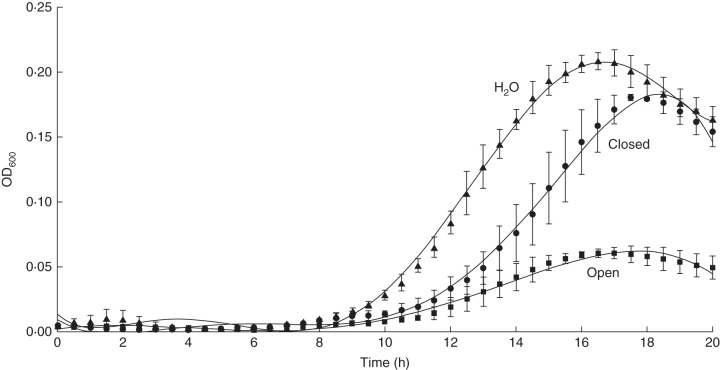
Further, to analyse the impact of closed pitcher fluid on microbial growth, in vitro growth curves with two bacteria species, Pseudomonas syringae pv. syringae (Fig. 3A) and E. coli (Fig. 3B), were recorded. Therefore, pitcher fluid from N. mirabilis, growth medium, and bacteria suspension cultures were incubated in a 10 : 8 : 2 ratio. Both bacterial species showed typical growth behaviour. No obvious difference in growth was detected when the pitcher fluid was replaced by water, pH 4, indicating that the liquid did not affect the growth conditions (Fig. 3). To exclude bacterial contamination of the liquid, growth medium and pitcher fluid was mixed 1 : 1 and analysed. No growth was detected (Fig. 3). To test fungal growth in addition, Saccharomyces cerevisiae (baker's yeast), was used in a similar approach (Fig. 4). Here, the presence of fluid from closed pitchers clearly affected, by delaying, yeast growth. Because open pitchers are expected to contain higher biological activities, the experiment was repeated with fluid from just opened pitfalls. As shown in Fig. 4, in this case, inhibition of S. cerevisiae growth was even stronger. This result forced us to carry out similar experiments in vivo, i.e. within closed N. mirabilis pitchers. Pseudomonas syringae as well as S. cerevisiae cultures were injected into closed pitchers. An aliquot was harvested directly (t0, 0 h) and 72 h (t72) after injection to determine the number of colony forming units (cfu). The ratio of cfu (t72/t0) was calculated to deduce the growth of the particular microbes. For P. syringae, in the pitchers the ratio (t72/t0) was determined to be 0·1 ± 0·06; for S. cerevisiae, this ratio was 1·1 ± 0·2. These results indicate that bacteria incubated within the pitfall trap died because the ratio (t72/t0) was lower than 1·0; whereas the yeast cells with a ratio (t72/t0) of about 1·0, did not die but they also did not grow. In the controls where the cultures were injected in removed pitcher fluid, both P. syringae and S. cerevisiae grew with ratios (t72/t0) of 18·1 and 2·7, respectively. Thus, we concluded that the pitcher fluid has bactericide as well as fungistatic properties if challenged with the injection of microbes.
Fig. 3.

Bacterial growth analyses (measured as OD600) in the presence and absence of Nepenthes pitcher fluid: (A) Pseudomonas syringae pv. syringae; (B) E. coli. Growth was monitored for 9 h in a total volume of 200 µL, consisting of 100 µL growth medium + 20 µL bacterial suspension culture + 80 µL pitcher fluid or water, pH 4, as indicated in the key. For a control of contamination, 100 µL growth medium + 100 µL pitcher fluid were incubated.
Fig. 4.
Saccharomyces cerevisiae growth analysis (measured as OD600) in the presence and absence of Nepenthes pitcher fluid. Growth was monitored for 20 h in a total volume of 200 µL, consisting of 100 µL growth medium + 20 µL S. cerevisiae suspension culture + 80 µL closed pitcher fluid, or open pitcher fluid, or water, as indicated on the graph. The 100 µL growth medium + 100 µL pitcher fluids did not show any growth.
Anion and element composition in Nepenthes pitcher fluid
To understand better why the microbes hardly grow or even die in the pitcher fluid, a detailed investigation of the inorganic ion composition in closed pitchers was performed for three different Nepenthes species with a focus on nitrogen- and phosphorus-containing anions. Because the differences among the three species tested were quite small their results are combined and summarized in Table 1 showing the average anion composition in Nepenthes. Both phosphate and nitrate were hardly detectable, although the total nitrogen content (inorganic + organic) could be determined at a low concentration of 2·5 ± 2·0 mg L−1. Using inductively coupled plasma–optical emission spectroscopy, the element composition in fluid collected from pitchers of N. alata was also analysed (Table 2). Actually, the liquid represents an approx. 25-mm KCl solution with 2·5 mm calcium, 1·0 mm magnesium, sodium, sulfate and traces of a few other elements.
Table 1.
Anion composition of pitcher fluids from the genus Nepenthes
Mean values ± s.d. are given, calculated from the values of three species, N. alata, N. gracilis and N. mirabilis.
Trace: below the detection limit of 0·1 mg L−1.
Table 2.
Mineral nutrients composition of fluid from closed Nepenthes alata pitchers
| Nutrient | mg L−1 |
|---|---|
| Elements | |
| Al | nd |
| B | 1·36 |
| Ca | 99·3 |
| Cu | 0·08 |
| Fe | 0·48 |
| K | 1019 |
| Mg | 26·7 |
| Mn | 0·35 |
| Na | 39·8 |
| Ni | 0·02 |
| P | nd |
| S | 2·41 |
| Si | 0·35 |
| Sr | 0·10 |
| Zn | 0·04 |
| Anions | |
| F− | Trace |
| Cl− | 995 |
| Br− | 0·61 |
| NO3− | Trace |
| PO43– | nd |
| SO42– | 5·44 |
nd, not detectable. Trace: below the detection limit of 0·1 mg L−1.
Antimicrobial compounds in Nepenthes pitcher fluid
The limitation of mineral nutrients (N, P) in the pitcher liquid can explain why the yeast cells did not grow. However, the question why the bacteria died had to be solved. The presence of defence-related proteins including pathogenesis-related proteins has already been described (Mithöfer, 2011). Among them, cDNAs and/or encoding genes of TLP were isolated from pitchers for nine different Nepenthes species, including all Nepenthes species used in the present study (Hatano and Hamada, 2008; Rottloff et al., 2009). A comparison of all the respective amino acid sequences showed a similarity of at least 92 %. Because antimicrobial activity has yet to be demonstrated for any of these TLPs, an in silico approach was chosen to test whether or not the Nepenthes TLPs are structurally similar to other known TLPs with biological activities. Structural modelling using the N. gracilis TLP revealed that they all belong to the group of L-type TLPs showing the 16 conserved cysteine residues of the Thaumatin_2-motif (Prosite no. PS51367) that can form eight putative disulfide bonds which stabilize the protein structure (Liu et al., 2010). In addition, it consists of three domains (I, II and III). The spatial structure of this N. gracilis TLP was modelled by comparative modelling based on the known X-ray crystallography-solved structure of TLPs from banana (Ban TLP, 1Z3Q_A; Leone et al., 2006) (Fig. 5). In both TLPs, a highly similar surface-exposed electronegative cleft is formed between domains I and II, which could be responsible for antifungal activities (Leone et al., 2006; Wang et al., 2011). This suggests an antimicrobial activity for Nepenthes TLPs as well.
Fig. 5.

Calculated 3-D structure of the thaumatin-like protein (TLP) from Nepenthes gracilis. The structure was suggested by the SWISSMODEL web server upon comparative modelling using a TLP from banana (Musa acuminate; Ban-TLP, 1Z3Q_A) as the homologous template structure. The model shows three domains (I, II and III): domain I which is a β-sandwich consisting of 11 β-sheets; domain II which contains one α-helix and one β-sheet; domain III which forms a hair-pin, built by two β-sheets.
In addition to such defensive proteins, low molecular-weight compounds that belong to the naphthoquinones were identified in the pitcher fluid of N. ventricosa. Both compounds, plumbagin and 7-methyl-juglone (Fig. 6), could be detected, especially in the liquid of open pitchers containing debris of formerly trapped insects, and only in very low concentrations in the fluid from closed pitchers. Since both compounds show a (m/z) of 189 in positive-mode ESI-MS, further structure determination by NMR was necessary. Both naphthoquinones exhibit distinct signals in the 1H NMR spectrum (Fig. 6). For plumbagin, four characteristic multiple signals appear at δ 7·73, δ 7·58, δ 7·27 (all dd, J = 7·5/1·0 Hz), together with another signal at δ 6·92 (dd, J = 3·0/1·5 Hz). The latter couples to a methyl doublet (J = 1·5 Hz) at δ 2·15. Characteristic signals for 7-methyl-juglone appear at δ 7·37, δ 7·13 (both broad singlets), and at δ 7·02 and δ 6·98 (both d, J = 10·0 Hz). The methyl singlet appears at δ 2·47. Both structures were further determined by 2-D heteronuclear experiments. As described for other naphthoquinones, such compounds exhibit antimicrobial activities (Didry et al., 1994; Eilenberg et al., 2010). However, droserone and 5-O-methyldroserone, described for N. khasiana (Eilenberg et al., 2010), could not be detected in our study.
Fig. 6.
Structures and characteristic 1H-NMR signals of plumbagin and 7-methyl-juglone isolated from N. alata pitcher tissue. Characteristic multiple signals for plumbagin: four signals at δ 7·73, δ 7·58, δ 7·27 (dd, J = 7·5/1·0 Hz) and at δ 6·92 (dd, J = 3·0/1·5 Hz). The latter corresponds to a methyl signal (d, J = 1·5 Hz) at δ 2·15. Characteristic signals for 7-methyl-juglone: at δ 7·37 and δ 7·13 (broad singlets), together with two signals (d, J = 10·0 Hz) at δ 7·04 and δ 7·00. A methyl singlet appears at δ 2·44. The positions in the molecule and their respective 1H-NMR signals are indicated by different colours (red, plumbagin; blue. 7-methyl-juglone).
DISCUSSION
Sterility of pitcher fluid
It has been previously claimed that the fluid from closed Nepenthes pitchers is sterile (Hepburn, 1918; Lüttge, 1964). Unfortunately, the growth assays used for those studies were not suitable to prove the absence of micro-organisms since it is not possible to cultivate most of them (Riesenfeld et al., 2004). Recently, there has been some debate about this issue because, using microscopic techniques, bacteria have been found in unopened N. alata pitchers (Sota et al., 1998). However, these authors themselves critically discussed this finding as they could not rule out contamination. As a consequence, the application of an assay based on molecular traits such as the PCR-amplification of highly conserved 16S-rDNA seemed to be the better approach. As shown in Fig. 2, neither in the fluid from N. alata nor from any other Nepenthes species was the amplification of bacterial DNA detected. The number of bacteria detected in the work of Sota et al. (1998), 1·6 × 107 cells mL−1, would have been easily detected by our approach as the detection limit was about 150 cells, suggesting that it was indeed contamination. Thus, the bacteria-free nature of fluid from closed Nepenthes pitchers suggests that the closed pitchers are sterile inside.
Nepenthes pitcher fluid and microbial growth
The ability of Nepenthes pitcher fluid to affect microbial growth was further investigated both in vitro with collected fluid and in vivo within the pitchers. As shown in Fig. 3, in vitro, bacterial growth was not affected by the fluid as E. coli as well as P. syringae grew as the controls did. However, S. cerevisiae growth was only slightly inhibited in the presence of fluid from closed pitchers but was more so in the presence of fluid from open pitchers (Fig. 4). These results suggested that the composition of the pitcher fluid was changing, probably due to the particular developmental stage or challenge. Further experiments did support this idea, when either bacteria or yeast cells were injected into closed pitchers and, after 3 d of incubation, the number of microbial cell was determined and compared with the particular controls. Clearly, bacterial cells died; yeast cells did not grow inside the pitcher but survived. These results reveal two features of Nepenthes pitcher fluid: (1) it is a dynamic system that is able to react to the challenge of microbe injections; (2) the fluid can exhibit bactericide as well as fungistatic properties.
There are two conceivable reasons why the yeast did not grow within the pitcher: (1) the presence of compounds that actively inhibit the growth; and (2) there are not sufficient nutrients in the fluid. To follow up this latter hypothesis, the mineral content of the pitcher fluid was analysed. Up to now only two studies addressed such an analysis: Morrissey (1955) quantified the chloride concentration in newly opened pitchers as between 600 and 900 mg L−1 (17–26 mm) and Nemček et al. (1966) found a chloride concentration of 19·5 mm in closed pitchers; both represented the same range detected by us (Table 1). In addition to chloride, we also determined other anions and found that there were actually neither phosphate nor nitrate detectable (Table 1). The total amount of bound nitrogen present in inorganic and organic forms – 2·5 mg L−1 – was very low. Actually, when compared with the data from Table 2, the pitcher fluid is a 25-mm KCl solution with few additional ions. Moreover, the NMR analysis showed that there were almost no detectable sugar signals (C. Paetz, MPI Chemical Ecology, Jena, Germany, pers. comm.) Taken together, these results indicate that the fluid from closed or newly opened pitchers per se is an extremely poor substrate, lacking phosphate and nitrogen sources and thus unsuitable for microbial growth.
However, this finding cannot completely explain why bacteria died during inoculation within the pitchers. Here, additional components are necessary. The presence of antimicrobial compounds in the fluid such as the naphthoquinones, droserone and 5-O-methyldroserone (Eilenberg et al., 2010), can provide this explanation. Both compounds are described as inducible (Eilenberg et al., 2010; Raj et al., 2011), a fact that strengthens our finding that the bacteria only die when inoculated within the pitcher, not when grown outside. It might be interesting to mention that in N. ventricosa pitcher fluid we did not find the naphthoquinones described for N. khasiana but two other derivatives, plumbagin and 7-methyl-juglone (Fig. 6), indicating the metabolic plasticity of the genus Nepenthes.
It is generally accepted that pitcher fluid is responsible for the digestion of trapped prey to make nutrients available for the plant. This is due to the activities of various hydrolytic enzymes. However, these proteins also play a role in affecting microbial growth within the pitcher fluid (Eilenberg et al., 2006; Hatano and Hamada, 2008, 2012; Mithöfer, 2011). Similar to the naphthoquinones, some of those enzymes are inducible by prey or chitin such as the type I basic chitinases Nkchit1b in N. khasiana, the class III acid endochitinase NrChit1 in N. mirabilis, and class III peroxidase from N. alata (Eilenberg et al., 2006; Rottloff et al., 2011; Hatano and Hamada, 2012). Such a point of view is supported by the presence of additional proteins in the pitcher fluid, which have no known hydrolytic activities but are antimicrobial, e.g. the pathogenesis-related TLPs (Hatano and Hamada, 2008; Rottloff et al., 2009; Mithöfer, 2011). It is tempting to speculate that the TLPs also contribute to the antimicrobial activities in the pitcher fluid. On-going experiments with fruit flies as prey have already shown that a 2-fold induction of TLP–mRNA could be observed in N. mirabilis. All these results, as well as the results with the naphthoquinones, demonstrate how dynamically Nepenthes plants can react in order to adapt the fluid to the particular needs.
Open pitchers, microbes and food webs
Once the pitchers are open, unwanted micro-organisms can enter the traps just by chance. They are introduced by rain or directly from the air into the traps. Probably, most of the microbes are carried by caught prey where they live on the surface or inside the body. Besides the various micro-organisms such as bacteria, yeast, algae and protozoa, many arthropod species are often present (Shivas and Brown, 1989; Adlassnig et al., 2011). Eventually, when opened Nepenthes pitchers grow older, they are often colonized and even food webs might be generated (Beaver, 1985; Kitching, 2001; Sota et al., 1998). Interestingly, this is possible although the pitcher fluid appears toxic or unsuitable for most organisms. But, obviously, some species are adapted to this environment, live inside the pitcher and subsist on the captured prey. Such colonizers might contribute to the digestion of prey but they are also consumers of nutrients and, therefore, competitors for nutrients. In particular, microbes are competing with the plants, whereas many arthropods feed on the micro-organisms (Ratsirarson and Silander, 1996; Sota et al., 1998). Once the arthropods die inside the pitcher, they are digested and available for the plant again. Therefore, the presence of micro-communities in pitchers is not necessarily negative for the plant. However, it seems that, at least in the beginning when the pitfall traps start to open and to catch prey, the plant tries to control the microbial colonization. Actually the selective influence of the pitcher fluid provides a possibility of affecting and, maybe, selecting certain organisms. For instance, after prey capture the pH of the pitcher fluid decreases in Nepenthes. This occurs when the number of bacteria has reached its maximum so as to inhibit further bacterial growth (Higashi et al., 1993), which is in agreement with our results of the dynamic defence reactions in Nepenthes. Only later can the number of microbes be too high and not manageable any more. In such a situation, more investment in defensive compounds could be too costly for the plant and might also affect other members of the food web. An established food web might suppress high microbial densities more efficiently by predation (Sota et al., 1998). This aspect will be investigated in further studies.
Conclusions
In the present study, we addressed some old but still-open questions concerning the sterility and putative antimicrobial properties of the digestive fluid from closed and just-opened pitchers of the genus Nepenthes. Using different independent approaches, we analysed the chemical composition of the fluid, on one hand, and, on the other hand, we investigated its abilities to affect microbes. Our results reveal that the secreted fluid of Nepenthes pitchers is a bacteria-free, very likely sterile liquid and is unsuitable of serving as an environment for microbial growth. Thus, the plants can avoid, at least to some extent, the growth of microbes that compete with the plant for the prey-derived nutrients that are available in the pitcher.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank Birgit Arnold and the whole greenhouse team at the MPI for Chemical Ecology for growing Nepenthes plants, the Botanical Gardens in Jena and Munich for providing plants and samples, Florian Turini for collecting pitcher fluid, and Wilhelm Boland and the Max Planck Society for support. We also thank the two anonymous reviewers for their stimulating comments on our manuscript.
LITERATURE CITED
- Adlassnig W, Peroutka M, Lendl T. Traps of carnivorous pitcher plants as a habitat: composition of the fluid, biodiversity and mutualistic activities. Annals of Botany. 2011;107:181–194. doi: 10.1093/aob/mcq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS MODEL Workspace: a web based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Baker GC, Smith JJ. Review and re-analysis of domain-specific 16S primers. Journal of Microbiological Methods. 2003;55:541–555. doi: 10.1016/j.mimet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Beaver RA. Geographical variation in food web structure in Nepenthes pitcher plants. Ecological Entomology. 1985;10:241–248. [Google Scholar]
- Bohn HF, Federle W. Insect aquaplaning: Nepenthes pitcher plants capture prey with the peristome, a fully wettable water-lubricated anisotropic surface. Proceedings of the National Acadamy of Science of the USA. 2004;101:14138–14143. doi: 10.1073/pnas.0405885101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boumans PWJM. Inductively coupled plasma emission spectrometry. Part I. New York, NY: John Wiley & Sons; 1987. [Google Scholar]
- Darwin C. Insectivorous plants. London: John Murray; 1875. [Google Scholar]
- DasSarma S, Fleischmann EF. Archaea: a laboratory manual – halophiles. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1995. [Google Scholar]
- Didry N, Dubrevil L, Pinkas M. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Pharmazie. 1994;49:481–483. [PubMed] [Google Scholar]
- DIN EN ISO 11885. Determination of 33 elements by inductively coupled plasma atomic emission spectroscopy. Brussels: European Committee for Standardization; 1998. [Google Scholar]
- Eilenberg H, Pnini-Cohen S, Schuster S, Movtchan A, Zilberstein A. Isolation and characterization of chitinase genes from pitchers of the carnivorous plant Nepenthes khasiana. Journal of Experimental Botany. 2006;57:2775–2784. doi: 10.1093/jxb/erl048. [DOI] [PubMed] [Google Scholar]
- Eilenberg H, Pnini-Cohen S, Rahamim Y, et al. Induced production of antifungal naphthoquinones in the pitchers of the carnivorous plant Nepenthes khasiana. Journal of Experimental Botany. 2010;61:911–922. doi: 10.1093/jxb/erp359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaume L, Gorb S, Rowe N. Function of epidermal surfaces in the trapping efficiency of Nepenthes alata pitchers. New Phytologist. 2002;156:479–489. doi: 10.1046/j.1469-8137.2002.00530.x. [DOI] [PubMed] [Google Scholar]
- Glenn A, Brodi MS. Fungal endophyte diversity in Sarracenia. PLoS One. 2012;7 doi: 10.1371/journal.pone.0032980. e32980. http://dx.doi.org/10.1371/journal.pone.0032980 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guex N, Peitsch MC. SWISS MODEL and the Swiss PdbViewer: an environment for comparative protein modelling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- Hatano N, Hamada T. Proteome analysis of pitcher fluid of the carnivorous plant Nepenthes alata. Journal of Proteome Research. 2008;7:809–816. doi: 10.1021/pr700566d. [DOI] [PubMed] [Google Scholar]
- Hatano N, Hamada T. Proteomic analysis of secreted protein induced by a component of prey in pitcher fluid of the carnivorous plant Nepenthes alata. Journal of Proteomics. 2012;75:4844–4852. doi: 10.1016/j.jprot.2012.05.048. [DOI] [PubMed] [Google Scholar]
- Hepburn JS. Biochemical studies of the pitcher liquor of Nepenthes. Proceedings of the American Philosophical Society. 1918;57:112–129. [Google Scholar]
- Heslop-Harrison Y. Enzyme release in carnivorous plants. In: Dingle JT, Dean RT, editors. Lysozymes in biology and pathology. Amsterdam: North Holland Publishing Company; 1975. pp. 525–578. [PubMed] [Google Scholar]
- Higashi S, Nakashima A, Ozaki H, Abe M, Uchiumi T. Analysis of feeding mechanism in a pitcher of Nepenthes hybrida. Journal of Plant Research. 1993;106:47–54. [Google Scholar]
- Juniper BE, Robins RJ, Joel DM. The carnivorous plants. London: Academic Press; 1989. [Google Scholar]
- Kitching RL. Food webs in phytotelmata. Annual Reviews of Entomology. 2001;46:729–760. doi: 10.1146/annurev.ento.46.1.729. [DOI] [PubMed] [Google Scholar]
- Leone P, Menu-Bouaouiche L, Peumans WJ, et al. Resolution of the structure of the allergenic and antifungal banana fruit thaumatin-like protein at 1·7-Å. Biochimie. 2006;88:45–52. doi: 10.1016/j.biochi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Liu J, Sturrock R, Ekramoddoullah AKM. The superfamily of Thaumatin-like proteins: its origin, evolution, and expression towards biological function. Plant Cell Reports. 2010;29:419–436. doi: 10.1007/s00299-010-0826-8. [DOI] [PubMed] [Google Scholar]
- Lüttge U. Untersuchungen zur Physiologie der Carnivoren-Drüsen. Planta. 1964;63:103–117. [Google Scholar]
- Mithöfer A. Carnivorous pitcher plants: insights in an old topic. Phytochemistry. 2011;72:1678–1682. doi: 10.1016/j.phytochem.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Morrissey S. Chloride ions in the secretion of the pitcher plant. Nature. 1955;176:1220–1221. [Google Scholar]
- Nemček O, Sigler K, Kleinzeller A. Ion transport in the pitcher of Nepenthes henryana. Biochimica et Biophysica Acta. 1966;126:73–80. [Google Scholar]
- Owen TP, Jr, Lennon KA. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae) American Journal of Botany. 1999;86:1382–1390. [PubMed] [Google Scholar]
- Raj G, Kurup R, Hussain AA, Baby S. Distribution of naphthoquinones, plumbagin, droserone and 5-O-methyl droserone in chitin-induced and uninduced Nepenthes khasiana: molecular events in prey capture. Journal of Experimental Botany. 2011;62:5429–5436. doi: 10.1093/jxb/err219. [DOI] [PubMed] [Google Scholar]
- Ratsirarson J, Silander JA., Jr Structure and dynamics in Nepenthes madagascariensis pitcher plant micro-communities. Biotropica. 1996;28:218–227. [Google Scholar]
- Riesenfeld CS, Schloss PD, Handelsman J. Metagenomics: genomic analysis of microbial communities. Annual Reviews of Genetics. 2004;38:525–552. doi: 10.1146/annurev.genet.38.072902.091216. [DOI] [PubMed] [Google Scholar]
- Rottloff S, Müller U, Kilper R, Mithöfer A. Micropreparation of single secretory glands from the carnivorous plant Nepenthes. Analytical Biochemistry. 2009;394:135–137. doi: 10.1016/j.ab.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Rottloff S, Stieber R, Maischak H, Turini FG, Heubl G, Mithöfer A. Functional characterization of a class III acid endochitinase from the traps of the carnivorous pitcher plant genus. Nepenthes. Journal of Experimental Botany. 2011;62:4639–4647. doi: 10.1093/jxb/err173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudi K, Skulberg OM, Larsen F, Jacobsen KS. Strain classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from variable regions V6, V7 and V8. Applied and Environmental Microbiology. 1997;63:2593–2599. doi: 10.1128/aem.63.7.2593-2599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwede T, Kopp J, Guex N, Peitsch MC. SWISS MODEL: an automated protein homology modeling server. Nucleic Acids Research. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivas RG, Brown JF. Yeasts associated with fluid in pitchers of Nepenthes. Mycological Research. 1989;93:96–100. [Google Scholar]
- Sota T, Mogi M, Kato K. Local and regional-scale food web structure in Nepenthes alata pitchers. Biotropica. 1998;30:82–91. [Google Scholar]
- Wang Q, Li F, Zhang X, et al. Purification and characterization of a CkTlp protein from Cynanchum komarovii seeds that confers antifungal activity. PLoS One. 2011;6 doi: 10.1371/journal.pone.0016930. e16930. http://dx.doi.org/10.1371/journal.pone.0016930 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.