Abstract
Background
A moss sporophyte inherits a haploid set of genes from the maternal gametophyte to which it is attached and another haploid set of genes from a paternal gametophyte. Evolutionary conflict is expected between genes of maternal and paternal origin that will be expressed as adaptations of sporophytes to extract additional resources from maternal gametophytes and adaptations of maternal gametophytes to restrain sporophytic demands.
Interpretation
The seta and stomata of peristomate mosses are interpreted as sporophytic devices for increasing nutrient transfer. The seta connects the foot, where nutrients are absorbed, to the developing capsule, where nutrients are needed for sporogenesis. Its elongation lifts stomata of the apophysis above the boundary layer, into the zone of turbulent air, thereby increasing the transpirational pull that draws nutrients across the haustorial foot. The calyptra is interpreted as a gametophytic device to reduce sporophytic demands. The calyptra fits tightly over the intercalary meristem of the sporophytic apex and prevents lateral expansion of the meristem. While intact, the calyptra delays the onset of transpiration.
Predictions
Nutrient transfer across the foot, stomatal number and stomatal aperture are predicted to be particular arenas of conflict between sporophytes and maternal gametophytes, and between maternal and paternal genomes of sporophytes.
Keywords: Moss, calyptra, seta, stomata, transpiration, parent–offspring conflict
INTRODUCTION
Sporophytes of mosses, liverworts and hornworts are often described as parasites on the gametophytes to which they are attached and from which they are nourished (Haberlandt, 1886; Vaizey, 1888; Goebel, 1905; Jennings, 1928; Raven, 2002b). Roth (1969) rejected this label because gametophyte and sporophyte were two stages of a single life history that underwent coordinated growth whereas parasitism was properly an antagonistic interaction between individuals of different species. He preferred to describe relations between haploid and diploid generations as gonotrophy (nutrition from a progenitor). Matrotrophy (nutrition from a mother) is a term of similar meaning (Graham and Wilcox, 2000). Whatever label is used, relations between diploid progeny (sporophytes) and haploid progenitors (gametophytes) are expected to be neither perfectly harmonious nor purely antagonistic whenever sporophytes possess paternal alleles that are absent in maternal gametophytes (Haig and Wilczek, 2006). Sporophytic fitness will often be maximized by transfer of more resources than maximizes maternal gametophytic fitness.
Church (1919) proposed that the original function of transpiration was parasitic absorption of food from gametophytes. His hypothesis is worth quoting at length:
“Why should the diploid sporophyte initiate a transpiration current, and its associated stomatal mechanism, when the homologous gametophyte, which started on equal terms failed to do so? A little consideration, however, suggests that such a current was never initiated directly for the purpose of absorbing water; that any elementary organism can recognize the nature of its physiological problems and solve them directly is too much to expect. The successful solution of any problem, as in the case of all biological mechanism, is always of the nature of an adaptation of something pre-existing. The transpiration-current, in other words, traces its origin to the haustorial absorption of food rather than water … food-supply direct from the gametophyte is the first need of a parasitic zygote; and in so inducing a haustorial drain, an upward current may be initiated which may continue to take water. … [The] transpiration-current which marks the essentially new physiological mechanism allowing existence in less and less saturated air was never ‘invented’ de novo for its special purpose; it began as a mechanism of parasitic nutrition … ” (pp. 71–72).
Transpiration of modern tracheophytes continues long after a sporophyte is nutritionally self-sufficient and no longer receives nutrients from maternal sources. Adult transpiration clearly does not constitute parasitism on maternal gametophytes. Ancestral tracheophytes, however, may have possessed permanently dependent sporophytes. Aerial axes of early tracheophytes possess stomata concentrated at the base of sporangia (Edwards et al., 1996), an arrangement compatible with apoplastic delivery of nutrients to the sporangial base followed by symplastic transport to spore-forming tissues (Edwards et al., 1998). Boyce (2008) calculated that many Cooksonia stems are too narrow to have been photosynthetically self-sufficient once allowance is made for cuticular, transport and support functions. Therefore, he concluded that such fossils are remnants of dependent sporophytes of unpreserved gametophytes and that stomata functioned in transpiration-driven transport of solutes to sporangia. Church's hypothesis thus receives some support from early tracheophytes. This paper proposes a role of transpiration in the ‘parasitic’ nutrition of the sporophytes of peristomate mosses.
GENETIC CONFLICTS IN MOSS DEVELOPMENT
There are two ways to conceptualize genetic individuals (genets) when a sporophyte grows attached to a gametophyte. The more familiar is to recognize the two generations as distinct individuals. The less familiar is to recognize the maternal haploid genet as extending across the gametophyte–sporophyte boundary into the sporophyte. In the orthodox account, the boundary between genets separates diploid from haploid tissues. In the heterodox account, two haploid genets (mum and dad) are physically fused in the sporophyte but nevertheless maintain distinct genetic interests. All mum's genes are present in both generations and benefit from the same outcomes whether a particular gene is expressed in the gametophyte or sporophyte. By contrast, paternal genes are absent from mum and, for this reason, are subject to different selective forces from those experienced by maternal genes. Here, and in the remainder of this paper, I adopt the convention that haploid parents are mums and dads (monosyllabic) to distinguish them from diploid mothers and fathers (bisyllabic) of tracheophytes.
A sporophyte's maternal genome is transmitted in its entirety to all other sexual and asexual offspring produced by its mum, but the sporophyte's paternal genome may be absent from the mum's other offspring, either because these are produced asexually or because they are sired by a different dad. Therefore, genes of maternal origin will favour allocations of limited resources among multiple offspring that maximize mum's fitness, whereas genes of paternal origin will favour greater investment in their particular sporophyte at the expense of other sporophytes or asexual propagules produced by mum. Interactions between the generations are expected to exhibit a high degree of coordination, because a sporophyte and its mum have a mutual interest in each other's well-being, on account of the genes they share, but have divergent interests because sporophytes also inherit genes from dads (Haig and Wilczek, 2006).
Three genetic factions with distinct interests can be identified in sporophyte genomes (Haig, 2006). The first contains genes that are selected to maximize maternal fitness. These include genes that are expressed in the maternal gametophyte and maternally inherited genes expressed in the sporophyte, including genes on X chromosomes or in the genomes of maternally inherited organelles (McDaniel et al., 2007; Natcheva and Cronberg, 2007). Maternally expressed imprinted genes of the sporophyte also belong to this faction. The second contains genes that are selected to maximize paternal fitness. These include Y-linked genes and paternally expressed imprinted genes. The third contains unimprinted autosomal genes of the sporophyte. These genes evolve to favour a compromise between maternal and paternal interests.
From a genetic perspective, conflict between genes of maternal and paternal origin will play out partly between haploid and diploid tissues at the gametophyte–sporophyte interface and partly within diploid tissues of the sporophyte. From a phenotypic perspective, sporophytes are predicted to exhibit adaptations for overcoming maternal constraints on nutrient transfer and maternal gametophytes are predicted to possess adaptations for regulating resource transfer to sporophytes. These predictions extend the theory of parent–offspring conflict to the case of a diploid offspring nourished by a haploid parent (Trivers, 1974; Westoby and Rice, 1982; Queller, 1983; Trivers and Burt, 1999; Haig and Wilczek, 2006).
GAMETOPHYTE–SPOROPHYTE RELATIONS
The most recent common ancestor of extant mosses is much older than the most recent common ancestor of angiosperms. By this criterion, mosses encompass much greater phylogenetic diversity than flowering plants, including substantial variation in relations between gametophytes and sporophytes. This paper cannot adequately review that diversity. Therefore, I will often make general statements that are true of many taxa but not of all. Comparative studies, especially studies that seek to understand exceptions to general rules, will be an important means of testing ideas about intergenerational conflict presented in this paper.
Diploid development begins when an egg is fertilized and the resulting zygote divides to produce an embryo enclosed within the haploid epigonium. Sporophytes of most peristomate mosses possess an intercalary meristem that generates a seta between the foot and precursors of the future capsule (Fig. 1A). Mitotic divisions of this meristem promote elongation of the embryonic axis and thereby facilitate penetration of the foot into gametophytic tissues (Vaizey, 1888; Uzawa and Higuchi, 2010). Downward penetration requires that the sporophyte elongates faster than the epigonium and that gametophytic tissues provide less resistance to expansion in the downward than upward direction. Setal elongation eventually causes the epigonium to rupture into two parts, an upper calyptra and lower vaginula. Subsequent elongation results in upward growth of the sporophyte carrying the calyptra aloft (Fig. 1B). The calyptra often remains tightly appressed to the apex of the sporophyte. Capsule maturation and sporogenesis occur only after the calyptra is split or shed.
Fig. 1.
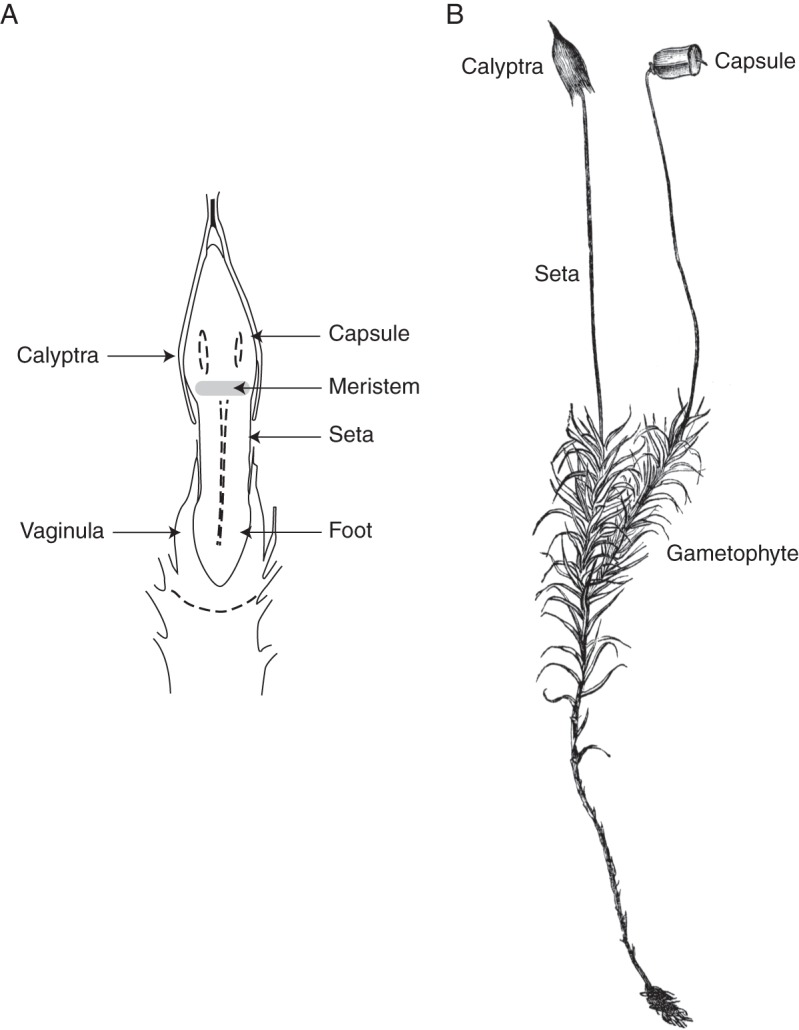
(A) Longitudinal section of developing sporophyte and associated gametophytic structures (Phascum cuspidatum, modified from Roth, 1969). Gametophytic structures are labelled on the left and sporophytic structures on the right. (B) Habit of Polytrichum commune (modified from Goebel, 1905). The leafy gametophyte bears two sporophytes. The sporophyte on the left retains its gametophytic calyptra whereas the sporophyte on the right has shed its calyptra.
Terminal differentiation of the intercalary meristem produces a region at the base of the developing capsule known as the neck or apophysis (Kreulen, 1975; French and Paolillo, 1975a). Stomata are restricted to the apophysis in many peristomate mosses (Valentine, 1839; Haberlandt, 1914; Paton and Pearce, 1957). These stomata are initially occluded by the calyptra and a transpiration stream is not established until the calyptra is displaced from the apophysis.
Distinctive features of the development of peristomate mosses can be identified by comparisons with corresponding processes in liverworts and non-peristomate mosses. Sporophytes of liverworts complete spore maturation while enclosed within the epigonium, close to the source of maternal nutrients. Mature capsules of most liverworts are then elevated on a short-lived sporophytic seta produced by elongation of existing cells without cell division. The epigonium is ruptured by setal elongation as the capsule is elevated for spore dispersal. Neither gametophytes nor sporophytes of liverworts possess stomata.
Sporophytes of Sphagnum, Andreaea and Andreaeobryum also develop enclosed within the epigonium until late in spore maturation. Mature capsules of Sphagnum and Andreaea are then elevated on a gametophytic pseudopodium whereas capsules of Andreaeobryum are elevated on a short sporophytic seta that resembles the setae of liverworts before cell elongation more than the setae of peristomate mosses (Steere and Murray, 1976; Murray, 1988). Andreaea and Andreaeobryum lack stomata at all stages of their life cycle. Spore capsules of Sphagnum bear numerous pseudostomata but these lack open pores and do not function in transpiration (Boudier, 1988; Cox et al., 2004; Duckett et al., 2009a). The capsules of these mosses (and liverworts) develop enclosed within the epigonium until immediately prior to spore dispersal. Therefore, the absence of stomata-associated transpiration makes functional sense.
Capsules of Takakia rupture the epigonium and are elevated on a sporophytic seta before meiosis (Renzaglia et al., 1997). In this sense, Takakia resembles peristomate mosses. However, its sporophyte lacks stomata. Sporophytes of Oedipodium possess numerous stomata on an elongated neck, or pseudoseta, and capsules are exposed prior to maturity (Crum, 2007; Shimamura and Deguchi, 2008).
The calyptra of peristomate mosses is typically a robust, tightly fitting structure that covers the capsule-forming part of the sporophyte. A structure that corresponds to a calyptra (i.e. an upper detached part of the epigonium) has a variable form in other mosses. Calyptras of Sphagnum and Andreaea are relatively flimsy whereas calyptras of Andreaeobryum and Takakia are more substantial structures; the former covers the entire capsule, the latter the upper part of the capsule only (Braithwaite, 1893; Schofield, 1985; Smith and Davison, 1993; Renzaglia et al., 1997). Calyptras of Andreaeobryum and Takakia can be removed without adverse effects on sporophyte development (Murray, 1988; Renzaglia et al., 1997). The calyptra of Oedipodium is small and readily detached (Crum, 2007).
Figure 2 presents a phylogenetic hypothesis of relations among major embryophyte taxa (based on Chang and Graham, 2011) onto which the presence of stomata are mapped. Liverworts form a clade sister to all other embryophytes with mosses sister to a hornwort–tracheophyte clade, and hornworts sister to tracheophytes (Qiu et al., 2007). Molecular data suggest Oedipodium is the sister group of peristomate mosses (Cox et al., 2004) although this has been disputed on morphological grounds (Ligrone and Duckett, 2011).
Fig. 2.
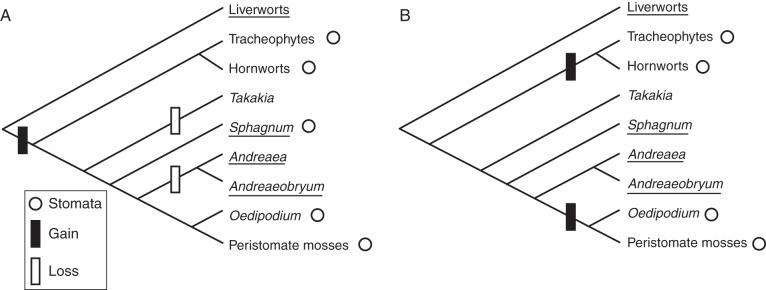
Phylogeny of major groups of mosses with the presence of stomata indicated by open circles. Taxa in which the sporophyte is enclosed within the epigonium until after meiosis are underlined. (A) Hypothesis in which there is a single origin of stomata from which pseudostomata of Sphagnum were derived. (B) Hypothesis in which stomata evolved twice and in which pseudostomata are not homologous to stomata.
There are at least three competing scenarios of the evolutionary origin of stomata. (1) Stomata have a single origin in a common ancestor of mosses and the hornwort–tracheophyte clade (Ligrone et al., 2012). Recent discoveries of shared mechanisms of stomatal control in mosses and tracheophytes have been interpreted as supporting this hypothesis (Bowman, 2011; Chater et al., 2011; Fig. 2A). This scenario implies secondary loss of stomata in Takakia, Andreaea and Andreaeobryum, and loss of a stomatal role in transpiration in Sphagnum. (2) Stomata evolved twice: once in an ancestor of peristomate mosses and once in an ancestor of hornworts and tracheophytes (Cox et al., 2004; Duckett et al., 2009a; Fig. 2B). In this scenario, pseudostomata of Sphagnum and stomata of other mosses are not homologous. (3) Stomata of hornworts and tracheophytes are not homologous (Pressel et al., 2011). This implies at least three origins of stomata. The resolution of this question – of one or more origins of stomata – or revisions of the phylogenetic hypothesis of Fig. 2 should not substantially affect the functional arguments of subsequent sections.
STOMATA AND SETAE
Setae position capsules above the boundary layer of still air and thereby facilitate long-distance dispersal of spores (Niklas, 2000; Raven, 2002a). The capsules of liverworts, Sphagnum, Andreaea and Andreaeobryum are elevated on setae or pseudopodia only after spores are mature. Why should peristomate mosses elongate their seta before spore maturation, distancing the developing spores from their source of nutrition, and placing them in a more desiccating environment? The paradox deepens when it is noted that the ephemeral seta of liverworts is produced at less cost than the more robust seta of mosses.
The paradox is resolved if setal elongation enhances spore nutrition. The moss seta can be compared with a waxed drinking straw that functions as a low-resistance conduit for water transport from the foot, where nutrients are absorbed, to the developing capsule, where nutrients are unloaded for sporogenesis. Setal elongation lifts the stomata of the apophysis above the boundary layer, thereby increasing evaporative water loss and strengthening the transpirational pull that draws maternal nutrients across the haustorial foot. Sporophytes suck.
Stomatal function is commonly viewed as mediating a trade-off between influx of CO2 (benefit) and efflux of water vapour (cost). However, evaporative water loss may benefit sporophytes if transpiration brings nutrients to the developing capsule (Ligrone and Gambardella, 1988). Transpiration could serve both functions, delivery of nutrients and maintenance of high rates of carbon fixation, but one can ask what is the relative importance of these processes in any particular case. If the principal function of transpiration is to replace water lost as a side-effect of photosynthesis, then natural selection will promote efficient use of water relative to amount of carbon fixed. If, on the other hand, its principal function is to draw nutrients from the maternal gametophyte, then water use will be profligate relative to photosynthetic carbon gain.
Moss sporophytes are covered by a waxy cuticle on all surfaces except the foot (Vaizey, 1887). Stomata regulate the exchange of gases between the atmosphere and internal spaces of the apophysis by opening and closing pores in this otherwise impermeable epidermis. Even if the support of photosynthesis is a major function of transpiration, the theory of parent–offspring conflict predicts that moss sporophytes should maintain open stomata beyond the point that is optimal for maternal fitness. An analogy with hemiparasitic angiosperms and their hosts is useful. Stomata of hemiparasites are able to remain open when host stomata close because the costs of parasite transpiration are borne by the host. In this way, a hemiparasite can continue to photosynthesize when its host's stomata close to cope with parasite-exacerbated water stress. Parasites gain additional advantages of maintaining open stomata if nutrients are obtained via the transpiration stream (Press et al., 1988; Shen et al., 2006). Moss stomata, like the stomata of hemiparasites (Press et al., 1988), are often open at night (Paton and Pearce, 1957; Garner and Paolillo, 1973; Renzaglia et al., 2007) and close only when the capsule itself starts to dehydrate (Paton and Pearce, 1957). Moss sporophytes remain turgid as gametophytes wilt (Vaizey, 1887).
Desiccation of the capsule in preparation for spore dispersal has been proposed to be the original function of stomata with regulation of gas exchange acquired as a secondary function early in the history of land plants (Duckett et al., 2009a). The proportion of permanently open stomata increases as capsules of Funaria hygrometrica mature, contributing to desiccation of the capsule prior to release of spores (Garner and Paolillo, 1973). Pseudostomata of Sphagnum are the primary site of water loss before discharge of the desiccated capsule (Duckett et al., 2009a). Hornwort stomata open once and then remain open as the capsule desiccates (Lucas and Renzaglia, 2002; Duckett et al., 2009b; Pressel et al., 2011).
Capsule desiccation may be the primary function of stomata of hornworts and pseudostomata of Sphagnum because vapour loss in these taxa is not replaced by transpiration. Stomata may also play an important role in desiccation during the final stages of capsule maturation of peristomate mosses, a process that would be facilitated by interruption of transpiration. However, the maintenance of transpiration at earlier stages of development suggests stomata serve some other function at these stages.
BORDER ZONES
There are two disjunct interfaces between maternal and offspring tissues after rupture of the epigonium. At one end of the sporophyte, the foot is embedded in the vaginula (basal interface) whereas, at the other end, the future capsule and intercalary meristem are enclosed by the calyptra (distal interface). Interactions at both interfaces are expected to show traces of a mixed history of conflict and collaboration, analogous to the interplay of conflict and cooperation in mammalian placentas (Haig, 1993, 2010).
Basal interface
Moss placentas have attracted much recent interest with particular attention paid to the distribution of ‘transfer cells’ (Ligrone and Gambardella, 1988; Ligrone et al., 1993; Frey et al., 2001). Transfer cells are defined by the presence of wall ingrowths that increase a cell's surface area to volume ratio (i.e. the ratio of plasma membrane to cytoplasm) and are believed to facilitate rapid secretion into, or rapid absorption from, the placental space (Gunning and Pate, 1969; Browning and Gunning, 1979a). Most mosses have transfer cells on both sides of the placenta. Placentas of Andreaea, Andreaeobryum and Polytrichales possess sporophytic transfer cells, but not gametophytic transfer cells, and Sphagnum has transfer cells on neither side of the placenta. Ligrone et al. (1993) commented that this variation ‘has no obvious (or even obscure) functional meaning’.
Without knowing what transfer cells secrete, and what they absorb, their function is difficult to interpret in terms of either intergenerational cooperation or conflict. The theory of parent–offspring conflict suggests sporophytic transfer cells might secrete substances that increase sporophytic access to maternal resources whereas gametophytic transfer cells might, in some circumstances, secrete substances that inhibit actions of sporophytic factors.
Limited data exist on the function of sporophytic transfer cells. The gradient of cellular degeneration in advance of the foot of Funaria hygrometrica suggests the secretion of hydrolytic enzymes. Excised sporophytes of this species absorb sucrose and glucose from the medium, and rapidly convert glucose to sucrose (Browning and Gunning, 1979a, b, c). Excised haustoria of Polytrichum formosum acidify the medium, generating a proton gradient, which is used to drive uptake of amino acids (Caussin et al., 1983; Renault et al., 1989).
If the principal function of sporophytic transfer cells is to extract resources from maternal gametophytes then one might predict conflict between maternal and paternal genomes within sporophytes over their development. Transfer cells, however, are reported from apogamous sporophytes of Physcomitrium coorgense (Lal and Narang, 1985). This seems to argue against a major role of imprinted genes in the development of sporophytic transfer cells because apogamous sporophytes develop without a paternal genome.
Even less is known about the function of gametophytic transfer cells. The vaginula of Funaria fills with water when the sporophyte is removed (Bopp and Weniger, 1971), possibly an osmotic response to solutes secreted by gametophytic transfer cells. Auxotrophic mutants of Physcomitrella patens growing on supplemented media provide evidence of a barrier to free transport of metabolites from maternal gametophytes to early embryos (Courtice et al., 1978). In these experiments, mutants growing on supplemented media were vigorous and cross-fertile but self-sterile. Therefore, metabolites moved freely from the medium into gametophytes but not from gametophytes to young embryos. Cross-fertility showed that viable gametes were formed and that sporophytes could develop if the mutant maternal allele of sporophytes was complemented by a wild-type allele from dad.
The placental space is littered with cellular debris as cell walls disintegrate in advance of the sporophytic foot (Browning and Gunning, 1979a; Frey et al., 2001; Uzawa and Higuchi, 2010). This gross morphology suggests the aftermath of a struggle between the generations. One might argue that maternal gametophytes facilitate ‘creative destruction’ to nourish their offspring, but similar cell death is not observed when gametophytes supply nutrients to asexual propagules such as gemmae (Ligrone et al., 1996) nor in the placenta of ferns where cells of the two generations are closely interdigitated (Ligrone et al., 1993; Duckett and Ligrone, 2003). Haig and Wilczek (2006) have suggested this difference may be explained by reduced intergenerational conflict in ferns (relative to mosses) because a fern mum is committed to a single sporophyte and lacks other options for reproductive investment, whereas a moss mum may invest in multiple sporophytes as well as asexual progeny.
Considerable variation exists in the depth to which the sporophytic foot penetrates maternal tissues (Roth, 1969; Ligrone et al., 1993; Uzawa and Higuchi, 2010). The presence or absence of barriers to unfettered flow between maternal and offspring tissues is also variable. Apoplastic continuity between gametophytic and sporophytic hydroids is present in Bryum capillare (Ligrone and Gambardella, 1988) and Funaria hygrometrica (Browning and Gunning, 1979c) whereas the two sets of hydroids are separated by a layer of sporophytic transfer cells in Timmiella barbuloides (Ligrone et al., 1982). The foot does not penetrate as far as the maternal vascular strand in other mosses (Uzawa and Higuchi, 2010).
The foot of Funaria hygrometrica is regionally differentiated with a basal part, consisting of an epidermis with weakly developed wall ingrowths plus a central core of hydroids, embedded in the central vascular strand of the gametophyte. This region appears specialized for water uptake and apoplastic transport. Above this region, epidermal cells have strongly developed wall ingrowths and lie adjacent to vaginular cells with similar ingrowths. At this level, plasmodesmata connect epidermal cells of the foot to each other and to the parenchymatous cortex present between epidermis and hydroids. This region appears specialized for nutrient uptake from the vaginula and symplastic transport. As the foot grades into the basal seta, epidermal cells lose wall ingrowths and surround a core of stereids, leptoids and hydroids (Wiencke and Schulz, 1975, 1978; Schulz and Wiencke, 1976).
Distal interface
The upper part of the epigonium of Sphagnum is torn irregularly as the capsule expands (Valentine, 1837; Boudier, 1988). The calyptra of most peristomate mosses, by contrast, is a robust structure that separates from the vaginula along a regular line of abscission. In Funaria hygrometrica, the calyptra remains alive for months after its separation from the rest of the maternal gametophyte (True, 1906). In this section I will discuss protective and morphogenetic roles that have been ascribed to the calyptra, before considering how these purported functions interact with the conflicting interests of maternal and paternal genomes.
Sporophyte development takes place partially or completely enclosed within the epigonium and its descendant parts, the calyptra and vaginula. Earlier separation of the calyptra would result in shallower penetration of the foot into maternal tissues, and setal elongation accelerates once the calyptra separates from the vaginula, just as capsule expansion accelerates once the constraining bonds of the calyptra are broken. Thus, a sporophyte's external form can be moulded by variation in the resistance of gametophytic tissues to sporophytic expansion.
Funaria hygrometrica is by far the best-studied species with respect to calyptral effects on development. Its calyptra is 2–5 mm long with a distal rostrum, an enlarged sac-like middle, and a short basal collar that clasps the seta tightly. The intercalary setal meristem is initially located within the close-fitting rostrum. Apical expansion begins when the sporophytic apex is withdrawn from the rostrum into the calyptral sac. The apophysis expands first, with differentiation of stomata, followed by expansion of the capsule and rupture of the calyptra (True, 1906; Garner and Paolillo, 1973; Paolillo, 1968; French and Paolillo, 1975a, b, c, 1976; Budke et al., 2011).
The seta of Funaria bends to gain leverage for withdrawal of the sporophytic apex from the calyptral rostrum (True, 1906; Paolillo, 1968; similar bending of the seta accompanies rupture of the calyptra of Pohlia nutans, see fig. 4 of Kreulen, 1975). Interactions between the calyptra and sporophyte may not be purely physical, however. Oehlkers and Bopp (1957) isolated mutants causing premature withdrawal of the sporophyte from the rostrum. This effect was determined by the calyptra's genotype, not the sporophyte's, and was absent when calyptras were killed and then replaced.
Experimental manipulations provide direct evidence of a morphogenetic role of the calyptra in shaping young sporophytes. Bopp (1957) reported extensive experiments on the removal and replacement of the calyptra of Funaria hygrometrica. The intercalary meristem of the sporophyte was closely appressed to the inner surface of the calyptra. When the calyptra was replaced by a slightly larger calyptra from the same or a different species, the meristem broadened until it tightly fitted the calyptra. Setal thickening was similarly inhibited by calyptras that had been boiled in alcohol or distilled water before being replaced on the sporophyte. When the tip of a calyptra was cut off, the setal meristem broadened as soon as it had been pushed through the open end of the calyptra. Growth-inhibitory substances were also detected in calyptras, but Bopp's experiments indicated that the mechanical constraint provided by the calyptra was sufficient to prevent setal thickening. Removal of the calyptra and broadening of the seta increased transpiration through the seta (Bopp and Stehle, 1957).
Bopp's experiments were replicated by French and Paolillo (1975b, c, 1976), with similar results and conclusions. Capsule expansion was accelerated when calyptras were removed from older sporophytes whereas removal from younger sporophytes resulted in prolonged intercalary growth without differentiation of a capsule. The meristem expanded laterally when the calyptra was removed with an increase in the number of meristematic cells in transverse sections. Stomata are usually oriented with their long axis parallel to the sporophytic axis but stomatal orientation was random when calyptras were removed before division of guard cell mother cells. These effects of the calyptra on sporophyte development were determined largely by mechanical restraints because substitution of killed, chemically extracted, calyptras allowed normal growth and capsule expansion.
Experimental results in Funaria cannot be generalized to all peristomate mosses. Removal of the calyptra has variable effects among species (Bopp, 1956). Physcomitrella patens and Pyramidula tetragona both belong to the Funariaceae and both possess short setae. The calyptra of Physcomitrella is loosely connected to the sporophyte and easily removed without morphogenetic effects (Hohe et al., 2002) whereas the inflated calyptra of Pyramidula tetragona never separates from the vaginula and the sporophyte matures within an intact epigonium (Kara et al., 2008). Excised sporophytes of Mnium cuspidatum never form capsules if the calyptra is retained but often form capsules if the calyptra is removed (Lowry, 1954). The calyptra of Polytrichum juniperinum splits along one side and thus determines the plane of bilateral symmetry of the capsule. If the calyptra is prematurely removed, there is minimal thickening of the seta but capsules develop with radial symmetry (Paolillo, 1968). Capsules of Atrichum rhystophyllum are malformed when the calyptra splits at atypical locations (Suzuki, 1982).
Calyptras have been proposed to protect the sporophytic apex from desiccation (Goebel, 1895; True, 1906; Zielinski, 1909; Irmscher, 1912; Janzen, 1917; Bopp and Stehle, 1957). Thus, the thick cuticle of the calyptra of Funaria hygrometrica has been interpreted as a form of maternal care to prevent drying of the sporophyte's tender tip (Budke et al., 2011). The sporophytic tip wilts if the calyptra is prematurely removed because the sporophyte only develops an effective cuticle on surfaces as they are exposed during the course of normal development (Budke et al., 2012). Parental care enabling offspring helplessness is a recurring theme in evolutionary biology.
Normal sporophyte development depends on the presence of the calyptra and its premature removal has adverse consequences for sporophyte fitness. But some effects of the calyptra seem ‘designed’ to restrict nutrient transfer to the sporophyte. More copious transpiration, and greater flow of nutrients, could be maintained through a thicker seta but the calyptra tightly constrains lateral expansion of the intercalary meristem and limits setal thickness. Moreover, transpiration is strongly inhibited while the calyptra covers the apophysis. Such a mixture of maternal solicitude and restraint is precisely what is predicted by modern evolutionary theory (Haig, 2010).
Biphasic nutrition
The properties of the calyptra suggest that postembryonic nutrition of the sporophyte is biphasic. Most nutrients are probably transported by a symplastic route while the calyptra occludes stomata of the apophysis. Once stomata are exposed, apoplastic transport via hydroids is predicted to become a major route by which nutrients are translocated to the developing capsule.
Such a biphasic pattern has been reported in Funaria hygrometrica: setal leptoids degenerate before meiosis but transpiration in hydroids is maintained for another month (Schulz and Wiencke, 1976; Wiencke and Schulz, 1978). Leptoids probably degenerate about the time stomata become functional after calyptral rupture (cf. Garner and Paolillo, 1973). Wiencke and Schulz (1978) emphasized nutrient transport via leptoids in the first phase and water transport via hydroids in the second, but hydroids probably play a role in nutrient transport during the second phase (cf. Ligrone and Gambardella, 1988).
TESTING THE PREDICTIONS
The major prediction of this paper is that moss sporophytes have evolved to take more nutrients from maternal gametophytes than maternal gametophytes have evolved to supply, resulting in ongoing evolutionary conflict. If transpiration has a major role in sporophytic nutrition, then sporophytes should possess adaptations to increase transpiration and maternal gametophytes adaptations to reduce transpiration.
Setal elongation before spore maturation is predicted to place stomata above the boundary layer and thus increase transpiration and the delivery of nutrients to the developing capsule. Because nutrient translocation is predicted to be an important function of transpiration, moss sporophytes are predicted to exhibit low water-use efficiencies with stomata open at night. Before stomata are exposed to the atmosphere, however, nutrients must be transferred to the growing tip of the sporophyte by other means. During this phase, sporophytes are predicted to maintain lower osmotic potentials than gametophytes to facilitate movement of water and solutes into the sporophyte from the gametophyte.
Maternal adaptations are predicted at the placental interface to control rates of nutrient transfer and these adaptations are predicted to be opposed by sporophytic or paternal adaptations to increase transfer. Moss developmental genetics is in its infancy but distinct roles are predicted for genes of maternal and paternal origin in placental and stomatal development and physiology.
Comparative studies hold particular promise for testing the ideas presented in this paper. Information about sporophyte nutrition is missing for key taxa. Takakia has capsules borne on setae that elongate before spore maturity (like peristomate mosses) but lack stomata. Oedipodium possesses numerous stomata on an elongated ‘pseudoseta’ (Crum, 2007; Shimamura and Deguchi, 2008). Hornwort sporophytes possess stomata but exhibit rapid external conduction of water (Isaac, 1941). Studies of sporophytic water relations and nutrition in these taxa will be of particular interest.
Considerable diversity exists among peristomate mosses in relations between calyptras and sporophytes, in length of setae, and in number and distribution of stomata. Sporophytes in the Polytrichaceae, for example, typically possess many, large stomata associated with well-developed assimilative tissue in the apophysis, but some members of the family have capsules without stomata (Paton and Pierce, 1957). An understanding of how sporophyte nutrition differs between taxa with and without stomata will be of particular interest. Archidium species, to take another example, produce the largest spores of any moss in setaless capsules, without stomata, covered by a flimsy ‘calyptra’ that tears irregularly as the capsule expands (Brown and Lemmon, 1985). Why this combination of unusual features? Archidium is monoicous. The answer may, in part, be related to diminished conflict associated with frequent self-fertilization of Archidium gametophytes.
Dioicous mosses produce unisexual gametophytes, either male or female, whereas monoicous mosses produce bisexual gametophytes. When a bisexual gametophyte fertilizes itself, a sporophyte's dad is also its mum. The sporophyte and its single haploid parent are genetically identical at all loci, except that each locus is present in double dose in the sporophyte. Therefore, the genetic interests of maternal and paternal genes will converge as the frequency of gametophytic selfing increases and the degree of conflict will correspondingly diminish. Other things being equal, sporophytes of monoicous mosses are predicted to have shorter setae, smaller capsules, fewer and smaller stomata, and to be less profligate in their use of water than the sporophytes of dioicous mosses.
ACKNOWLEDGMENTS
Special thanks to Kirsten Bomblies and Richard Bondi for help with translations and to Jessica Budke, Jonathan Shaw and Robert Trivers for comments on the manuscript. The paper, in its multiple incarnations, has benefited from the comments of several anonymous reviewers.
LITERATURE CITED
- Bopp M. Die Bedeutung der Kalyptra für die Entwicklung der Laubmoossporogone. Berichte der deutschen Botanischen Gesellschaft. 1956;69:455–468. [Google Scholar]
- Bopp M. Entwicklungsphysiologische Untersuchungen an Moosmutanten. I. Zur Wirkung der Laubmooskalyptra. Zeitschrift für induktive Abstammungs- und Vererbungslehre. 1957;88:600–607. [Google Scholar]
- Bopp M, Stehle E. Zur Frage der Wasserleitung im Gametophyten und Sporophyten der Laubmoose. Zeitschrift für Botanik. 1957;45:161–174. [Google Scholar]
- Bopp M, Weniger HP. Wassertransport vom Gametophyten zum Sporophyten bei Laubmoosen. Zeitschrift für Pflanzenphysiologie. 1971;64:190–198. [Google Scholar]
- Boudier P. Différenciation structurale de l'épiderme du sporogone chez Sphagnum fimbriatum Wilson. Annales des Sciences Naturelles, Botanique (thirteenth series) 1988;8:143–156. [Google Scholar]
- Bowman JL. Stomata: active portals for flourishing on land. Current Biology. 2011;21:R540–R541. doi: 10.1016/j.cub.2011.06.021. [DOI] [PubMed] [Google Scholar]
- Boyce CK. How green was Cooksonia? The importance of size in understanding the early evolution of physiology in the vascular plant lineage. Paleobiology. 2008;34:179–194. [Google Scholar]
- Braithwaite R. On the anatomy of mosses. Journal of the Royal Microscopical Society. 1893;13:137–144. [Google Scholar]
- Brown RC, Lemmon BE. Phylogenetic aspects of sporogenesis in Archidium. Monographs in Systematic Botany from the Missouri Botanical Garden. 1985;11:25–39. [Google Scholar]
- Browning AJ, Gunning BES. Structure and function of transfer cells in the sporophyte haustorium of Funaria hygrometrica Hedw. I. The development and ultrastructure of the haustorium. Journal of Experimental Botany. 1979a;30:1233–1246. [Google Scholar]
- Browning AJ, Gunning BES. Structure and function of transfer cells in the sporophyte haustorium of Funaria hygrometrica Hedw. II. Kinetics of uptake of labelled sugars and localization of absorbed products by freeze-substitution and autoradiography. Journal of Experimental Botany. 1979b;30:1247–1264. [Google Scholar]
- Browning AJ, Gunning BES. Structure and function of transfer cells in the sporophyte haustorium of Funaria hygrometrica Hedw. III. Translocation of assimilate into the attached sporophyte and along the seta of attached and excised sporophytes. Journal of Experimental Botany. 1979c;30:1265–1273. [Google Scholar]
- Budke JM, Goffinet B, Jones CS. A hundred-year-old question: is the moss calyptra covered by a cuticle? A case study of Funaria hygrometrica. Annals of Botany. 2011;107:1279–1286. doi: 10.1093/aob/mcr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke JM, Goffinet B, Jones CS. The cuticle on the gametophyte calyptra matures before the sporophyte cuticle in the moss Funaria hygrometrica (Funariaceae) American Journal of Botany. 2012;99:14–22. doi: 10.3732/ajb.1100311. [DOI] [PubMed] [Google Scholar]
- Caussin C, Fleurat-Lessard P, Bonnemain JL. Absorption of some amino acids by sporophytes isolated from Polytrichum formosum and ultrastructural characteristics of the haustorium transfer cells. Annals of Botany. 1983;51:167–173. [Google Scholar]
- Chang Y, Graham SW. Inferring the higher-order phylogeny of mosses (Bryophyta) and relatives using a large, multigene plastid data set. American Journal of Botany. 2011;98:839–849. doi: 10.3732/ajb.0900384. [DOI] [PubMed] [Google Scholar]
- Chater C, Kamisugi Y, Movahedi M, et al. Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Current Biology. 2011;21:1025–1029. doi: 10.1016/j.cub.2011.04.032. [DOI] [PubMed] [Google Scholar]
- Church AH. Thalassiophyta and the subaerial transmigration. London: Oxford University Press; 1919. [Google Scholar]
- Courtice GRM, Ashton NW, Cove DJ. Evidence for the restricted passage of metabolites into the sporophyte of the moss Physcomitrella patens (Hedw.) Br. Eur. Journal of Bryology. 1978;10:191–198. [Google Scholar]
- Cox CJ, Goffinet B, Shaw AJ, Boles SB. Phylogenetic relationships among the mosses based on heterogenous Bayesian analysis of multiple genes from multiple genomic compartments. Systematic Botany. 2004;29:234–250. [Google Scholar]
- Crum HA. Flora of North America, Volume 27. Bryophytes: Mosses, part 1. New York: Oxford University Press; 2007. Oedipodiaceae Steere; pp. 116–117. [Google Scholar]
- Duckett JG, Ligrone R. The structure and development of haustorial placentas in leptosporangiate ferns provides a clear-cut distinction between euphyllophytes and lycophytes. Annals of Botany. 2003;92:513–521. doi: 10.1093/aob/mcg160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckett JG, Pressel S, P'ng KMY, Renzaglia KS. Exploding a myth: the capsule dehiscence mechanism and the function of pseudostomata in Sphagnum. New Phytologist. 2009a;183:1053–1063. doi: 10.1111/j.1469-8137.2009.02905.x. [DOI] [PubMed] [Google Scholar]
- Duckett JG, P'ng KMY, Renzaglia KS, Pressel S. The function and evolution of stomata in bryophytes. Field Bryology. 2009b;101:38–40. [Google Scholar]
- Edwards D, Abbott GD, Raven JA. Cuticles of early land plants: a palaeoecophysiological evaluation. In: Kerstiens G, editor. Plant cuticles. Oxford: BIOS Scientific; 1996. pp. 1–31. [Google Scholar]
- Edwards D, Kerp H, Hass H. Stomata in early land plants: an anatomical and ecophysiological approach. Journal of Experimental Botany. 1998;49:255–278. (special issue) [Google Scholar]
- French JC, Paolillo DJ. Intercalary meristematic activity in the sporophyte of Funaria (Musci) American Journal of Botany. 1975a;62:86–96. doi: 10.1002/j.1537-2197.1975.tb12342.x. [DOI] [PubMed] [Google Scholar]
- French JC, Paolillo DJ. On the role of the calyptra in permitting expansion of capsules in the moss Funaria. Bryologist. 1975b;78:438–446. [Google Scholar]
- French JC, Paolillo DJ. The effect of the calyptra on the plane of guard cell mother cell division in Funaria and Physcomitrium capsules. Annals of Botany. 1975c;39:233–236. [Google Scholar]
- French JC, Paolillo DJ. Effect of the calyptra on intercalary meristematic activity in the sporophyte of Funaria (Musci) American Journal of Botany. 1976;63:492–498. doi: 10.1002/j.1537-2197.1975.tb12342.x. [DOI] [PubMed] [Google Scholar]
- Frey W, Hofmann M, Hilger HH. The gametophyte–sporophyte junction: unequivocal hints for two evolutionary lines of archegoniate land plants. Flora. 2001;196:431–445. [Google Scholar]
- Garner D, Paolillo DJ. On the functioning of stomates in Funaria. Bryologist. 1973;76:423–427. [Google Scholar]
- Goebel K. Archegoniatenstudien. 7. Über die Sporenausstreuung bei den Laubmoosen. Flora. 1895;80:459–486. [Google Scholar]
- Goebel K. Oxford: Clarendon Press; 1905. Organography of plants, especially of the Archegoniatae and Spermophyta. Part II. Special organography. [Google Scholar]
- Graham LKE, Wilcox LW. The origin of alternation of generations in land plants: a focus on matrotrophy and hexose transport. Philosophical Transactions of the Royal Society of London B. 2000;355:757–766. doi: 10.1098/rstb.2000.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning BES, Pate JS. “Transfer cells.” Plant cells with wall ingrowths, specialized in relation to short distance transport of solutes—their occurrence, structure, and development. Protoplasma. 1969;68:107–133. [Google Scholar]
- Haberlandt G. Die Assimilationssystem der Laubmoos-Sporogonien. Flora. 1886;69:45–47. [Google Scholar]
- Haberlandt G. Physiological plant anatomy. London: Macmillan; 1914. [Google Scholar]
- Haig D. Genetic conflicts in human pregnancy. Quarterly Review of Biology. 1993;68:495–532. doi: 10.1086/418300. [DOI] [PubMed] [Google Scholar]
- Haig D. Intragenomic politics. Cytogenetic and Genome Research. 2006;113:68–74. doi: 10.1159/000090816. [DOI] [PubMed] [Google Scholar]
- Haig D. Fertile soil or no man's land: cooperation and conflict in the placental bed. In: Pijnenborg R, Brosens I, Romero R, editors. Placental bed disorders. Cambridge: Cambridge University Press; 2010. pp. 165–173. [Google Scholar]
- Haig D, Wilczek A. Sexual conflict and the alternation of haploid and diploid generations. Philosophical Transactions of the Royal Society of London B. 2006;361:335–343. doi: 10.1098/rstb.2005.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohe A, Rensing SA, Mildner M, Lang D, Reski R. Day length and temperature strongly influence sexual reproduction and expression of a novel MADS-box gene in the moss Physcomitrella patens. Plant Biology. 2002;4:595–602. [Google Scholar]
- Irmscher E. Über die Resistenz der Laubmoose gegen Austrocknung und Kälte. Jahrbücher für wissenschaftliche Botanik. 1912;50:387–449. [Google Scholar]
- Isaac I. The structure of Anthoceros laevis in relation to its water supply. Annals of Botany. 1941;5:339–351. [Google Scholar]
- Janzen P. Die Haube der Laubmoose. Hedwigia. 1917;58:156–280. [Google Scholar]
- Jennings OE. The ancestry of the mosses. Bryologist. 1928;31:10–15. [Google Scholar]
- Kara R, Ezer T, Düzenli A. Pyramidula tetragona (Funariaceae) new to Turkey. Bryologist. 2008;111:494–495. [Google Scholar]
- Kreulen DJW. Notes on the early development of the sporophyte in the Bryales. Lindbergia. 1975;3:1–13. [Google Scholar]
- Lal M, Narang A. Ultrastructural and histochemical studies of transfer cells in the callus and apogamous sporophytes of Physcomitrium coorgense Broth. New Phytologist. 1985;100:225–231. [Google Scholar]
- Ligrone R, Duckett JG. Morphology versus molecules in moss phylogeny: new insights (or controversies) from placental and vascular anatomy in Oedipodium griffithianum. Plant Systematics and Evolution. 2011;296:275–282. [Google Scholar]
- Ligrone R, Gambardella R. The sporophyte–gametophyte junction in bryophytes. Advances in Bryology. 1988;3:225–274. [Google Scholar]
- Ligrone R, Gambardella R, de Lucia Sposito ML. Ultrastructure and development of the sporophyte foot–gametophyte vaginula complex in Timmiella barbuloides (Brid.) Moenk. Planta. 1982;154:414–425. doi: 10.1007/BF01267808. [DOI] [PubMed] [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. The gametophyte–sporophyte junction in land plants. Advances in Botanical Research. 1993;19:231–317. [Google Scholar]
- Ligrone R, Duckett JG, Gambardella R. Development and liberation of cauline gemmae in the moss Aulacomnium androgynum (Hedw.) Schwaegr. (Bryales): an ultrastructural study. Annals of Botany. 1996;78:559–568. [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. Major transitions in the evolution of early land plants: a bryological perspective. Annals of Botany. 2012;109:851–871. doi: 10.1093/aob/mcs017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry RJ. Observations upon the regeneration of Mnium cuspidatum setae. Bryologist. 1954;57:147–149. [Google Scholar]
- Lucas JR, Renzaglia KS. Structure and function of hornwort stomata. Microscopy and Microanalysis. 2002;8:1090–1091. (abstract) [Google Scholar]
- McDaniel SF, Willis JH, Shaw AJ. A linkage map reveals a complex basis of segregation distortion in an interpopulation cross in the moss Ceratodon purpureus. Genetics. 2007;176:2489–2500. doi: 10.1534/genetics.107.075424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray BM. Systematics of the Andreaeopsida (Bryophyta): two orders with links to Takakia. Beihefte zum Nova Hedwigia. 1988;90:289–336. [Google Scholar]
- Natcheva R, Cronberg N. Maternal transmission of cytoplasmic DNA in interspecific hybrids of peat mosses, Sphagnum (Bryophyta) Journal of Evolutionary Biology. 2007;20:1613–1616. doi: 10.1111/j.1420-9101.2007.01341.x. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. The evolution of plant body plans—a biomechanical perspective. Annals of Botany. 2000;85:411–438. [Google Scholar]
- Oehlkers F, Bopp M. Entwicklungsphysiologische Untersuchungen an Moosmutanten. II. Die Korrelation zwischen Sporogon und Kalyptra bei Mutanten von Funaria und Physcomitrium. Zeitschrift für induktive Abstammungs- und Vererbungslehre. 1957;88:608–618. [Google Scholar]
- Paolillo DJ. The effect of the calyptra on capsule symmetry in Polytrichum juniperinum Hedw. Bryologist. 1968;71:327–334. [Google Scholar]
- Paton JA, Pearce JV. The occurrence, structure and functions of the stomata in British bryophytes. Transactions of the British Bryological Society. 1957;3:228–259. [Google Scholar]
- Press MC, Graves JD, Stewart GR. Transpiration and carbon acquisition in root hemiparasitic angiosperms. Journal of Experimental Botany. 1988;39:1009–1014. [Google Scholar]
- Pressel S, Renzaglia K, J Duckett J. Hornworts: a new look at stomatal evolution. XVIII International Botanical Congress. 2011 abstract book, pp.237. http://www.ibc2011.com/downloads/IBC2011_Abstract_Book.pdf . [Google Scholar]
- Queller DC. Kin selection and conflict in seed maturation. Journal of Theoretical Biology. 1983;100:153–172. [Google Scholar]
- Qiu YL, Li L, Wang B, et al. A nonflowering plant land phylogeny inferred from nucleotide sequences of seven chloroplast, mitochondrial, and nuclear genes. International Journal of Plant Science. 2007;168:691–708. [Google Scholar]
- Raven JA. Selection pressures on stomatal evolution. New Phytologist. 2002a;153:371–386. doi: 10.1046/j.0028-646X.2001.00334.x. [DOI] [PubMed] [Google Scholar]
- Raven JA. Putting the fight in bryophytes. New Phytologist. 2002b;156:321–323. doi: 10.1046/j.1469-8137.2002.00545.x. [DOI] [PubMed] [Google Scholar]
- Renault S, Despeghel-Caussin C, Bonnemain JL, Delrot S. The proton electrochemical transmembrane gradients generated by the transfer cells of the haustorium of Polytrichum formosum and their use in the uptake of amino acids. Plant Physiology. 1989;90:913–920. doi: 10.1104/pp.90.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renzaglia KS, McFarland KD, Smith DK. Anatomy and ultrastructure of the sporophyte of Takakia ceratophylla (Bryophyta) American Journal of Botany. 1997;84:1337–1350. [PubMed] [Google Scholar]
- Renzaglia KS, Schuette S, Duff EJ, et al. Bryophyte phylogeny: advancing the molecular and morphological frontiers. Bryologist. 2007;110:179–213. [Google Scholar]
- Roth D. Embryo und Embryotheca bei den Laubmoosen. Eine histogenetische und morphologische Untersuchung. Bibliotheca Botanica. 1969;129:1–49. [Google Scholar]
- Schofield WB. Caldwell, NJ: Blackburn Press; 1985. Introduction to bryology. [Google Scholar]
- Schulz D, Wiencke C. Sporophytenentwicklung von Funaria hygrometrica Sibth. II. Differenzierung des Wasser- und Stoffleitungssystems in der Seta. Flora. 1976;165:47–60. [Google Scholar]
- Shen H, Ye W, Hong L, et al. Progress in parasitic plant biology: host selection and nutrient transfer. Plant Biology. 2006;8:175–185. doi: 10.1055/s-2006-923796. [DOI] [PubMed] [Google Scholar]
- Shimamura M, Deguchi H. Sporophyte anatomy of Oedipodium griffithianum (Oedipodiaceae) In: Mohamed H, Baki BB, Nasrulhaq-Boyce A, Lee PKY, editors. Bryology in the new millenium. Kuala Lumpur: University of Malaya; 2008. pp. 319–325. [Google Scholar]
- Smith DK, Davison PG. Antheridia and sporophytes in Takakia ceratophylla (Mitt.) Grolle: evidence for reclassification among the mosses. Journal of the Hattori Botanical Laboratory. 1993;73:263–271. [Google Scholar]
- Steere WC, Murray BM. Andreaeobryum macrosporum, a new genus and species of Musci from northern Alaska and Canada. Phytologia. 1976;33:407–410. [Google Scholar]
- Suzuki Y. The calyptra rupture and capsule form in Atrichum rhystophyllum. Proceedings of the Bryological Society of Japan. 1982;3:72–73. [Google Scholar]
- Trivers RL. Parent–offspring conflict. American Zoologist. 1974;14:249–264. [Google Scholar]
- Trivers R, Burt A. Kinship and genomic imprinting. In: Ohlsson R, editor. Genomic imprinting. An interdisciplinary approach. Berlin: Springer; 1999. pp. 1–21. [Google Scholar]
- True RH. Notes on the physiology of the sporophyte of Funaria and of Mnium. Beihefte zum Botanischen Centralblatt (Erste Abteilung) 1906;19:34–44. [Google Scholar]
- Uzawa M, Higuchi M. Comparative development of the sporophyte–gametophyte junction in six moss species. Journal of Plant Research. 2010;123:777–787. doi: 10.1007/s10265-010-0339-0. [DOI] [PubMed] [Google Scholar]
- Vaizey JR. On the absorption of water and its relation to the constitution of the cell-wall in mosses. Annals of Botany. 1887;1:147–152. [Google Scholar]
- Vaizey JR. On the anatomy and development of the sporogonium of mosses. Journal of the Linnean Society (Botany) 1888;24:262–285. [Google Scholar]
- Valentine W. Observations on the development of the theca, and on the sexes of mosses. Transactions of the Linnean Society. 1837;17:465–484. [Google Scholar]
- Valentine W. On the existence of stomata in mosses. Transactions of the Linnean Society. 1839;18:239–245. [Google Scholar]
- Westoby M, Rice B. Evolution of the seed plants and inclusive fitness of plant tissues. Evolution. 1982;36:713–724. doi: 10.1111/j.1558-5646.1982.tb05437.x. [DOI] [PubMed] [Google Scholar]
- Wiencke C, Schulz D. Sporophytenentwicklung von Funaria hygrometrica Sibth. I. Strukturelle Grundlagen der Wasser- und Nährstoffaufnahme im Haustorium. Protoplasma. 1975;86:107–117. [Google Scholar]
- Wiencke C, Schulz D. The development of transfer cells in the haustorium of the Funaria hygrometrica sporophyte. In: Suire C, editor. Congrès International de Bryologie. Bryophytorum Bibliotheca, Volume 13. Vaduz: J. Cramer. 1978. pp. 147–167. [Google Scholar]
- Zielinski F. Beiträge zur Biologie des Archegoniums und der Haube der Laubmoose. Flora. 1909;100:1–36. [Google Scholar]


