Abstract
Background and Aims
Daytime root-zone temperature may be a significant factor regulating water flux through plants. Water flux can also occur during the night but nocturnal stomatal response to environmental drivers such as root-zone temperature remains largely unknown.
Methods
Here nocturnal and daytime leaf gas exchange was quantified in ‘Shiraz’ grapevines (Vitis vinifera) exposed to three root-zone temperatures from budburst to fruit-set, for a total of 8 weeks in spring.
Key Results
Despite lower stomatal density, night-time stomatal conductance and transpiration rates were greater for plants grown in warm root-zones. Elevated root-zone temperature resulted in higher daytime stomatal conductance, transpiration and net assimilation rates across a range of leaf-to-air vapour pressure deficits, air temperatures and light levels. Intrinsic water-use efficiency was, however, lowest in those plants with warm root-zones. CO2 response curves of foliar gas exchange indicated that the maximum rate of electron transport and the maximum rate of Rubisco activity did not differ between the root-zone treatments, and therefore it was likely that the lower photosynthesis in cool root-zones was predominantly the result of a stomatal limitation. One week after discontinuation of the temperature treatments, gas exchange was similar between the plants, indicating a reversible physiological response to soil temperature.
Conclusions
In this anisohydric grapevine variety both night-time and daytime stomatal conductance were responsive to root-zone temperature. Because nocturnal transpiration has implications for overall plant water status, predictive climate change models using stomatal conductance will need to factor in this root-zone variable.
Keywords: Water-use efficiency, leaf gas exchange, CO2 assimilation, grapevine, Vitis vinifera, stomatal conductance, root-zone temperature, ‘Shiraz’, grapevine, soil temperature
INTRODUCTION
Rising global temperatures can be accompanied by smaller, but significant, changes in soil surface temperatures (van Gestel et al., 2011) and this has the potential to affect root physiology and root activity. In particular, a gradual rise in night-time air temperature over the past few decades (Vose et al., 2005) has probably resulted in an increase in soil temperature during the night and early morning, depending on soil depth. Root growth (Larson, 1970; Kasper and Bland, 1992), water uptake (Wan et al., 1999), nutrient uptake and availability (MacDonald et al., 1995; BassiriRad, 2000; Melillo et al., 2002; Pregitzer and King, 2005) and signal transduction (Zhang et al., 2008; Field et al., 2009) are all influenced by the temperature of the soil. These processes affect above-ground growth (Lopushinsky and Kaufmann, 1984; Dawes et al., 2011; Rogiers et al., 2011b) and productivity (He et al., 2001). As a result, analogous to air temperature, soil temperature can limit the geographical distribution of plants and crops (Fosaa et al., 2004).
Leaf gas exchange is particularly responsive to the root-zone environment. While there are many studies characterizing the consequences of soil moisture on leaf gas exchange, soil temperature has also been found to affect stomatal conductance and photosynthesis in perennials (Cai and Dang, 2002; Wan et al., 2004; Wu et al., 2012) and annuals (He and Lee, 2001; He et al., 2001; Zhang et al., 2008). Root-zone temperature can restrict photosynthesis through alterations in photosynthetic reactions (Cai and Dang, 2002; Zhou et al., 2004; Erice et al., 2006) or changes in stomatal conductance (Wan et al., 2004). For instance, boreal and alpine plants are often exposed to low soil temperature stress and in conifer species the maximum rate of carboxylation (Vc,max) and the maximum rate of electron transport (Jmax) increased with soil temperature up to an optimum and then declined (Cai and Dang, 2002). Conversely, it was found that when Populus tremuloides was exposed to low temperatures soil net assimilation was limited by stomatal conductance (Wan et al., 1999). As a consequence, it was suggested that similar root-to-shoot signalling pathways may operate in plants subjected to drought and low root temperature (Wan et al., 2004). As such, it was found that roots of Cucurbitaceae species can regulate stomatal behaviour in response to root-zone temperature by modifying delivery of abscisic acid (ABA) to shoots and leaves (Zhang et al., 2008).
Those environmental parameters regulating stomatal conductance (g) and transpiration (E) during the day may also exert some control during the night. Stomata can remain partially open during the night and, although the function of night-time transpiration is yet to be resolved, this can result in substantial night-time transpiration if the vapour pressure difference between the leaf and air (VPD) is high. In some species and varieties, nocturnal transpiration can even cause disequilibrium in plant water status (Caird et al., 2007; Dawson et al., 2007; Fisher et al., 2007; Kavanagh et al., 2007). For instance, Vitis vinifera ‘Grenache’, an isohydric grapevine variety, has low g both during the day and night. This is in contrast to ‘Semillon’, an anisohydric variety, with comparatively high g throughout the diurnal cycle and as a result incomplete plant rehydration prior to dawn especially during warm, windy nights (Rogiers et al., 2009). Aside from inherent genetic factors, nocturnal g appears to be influenced by abiotic factors such as VPD, wind, atmospheric CO2 concentration and soil moisture (Ludwig et al., 2006; Howard and Donovan, 2007; Zeppel et al., 2010, 2012). Another key environmental factor which may regulate nocturnal g is root-zone temperature. To date, the consequences of root-zone temperature on nocturnal stomatal conductance, to our knowledge, are unknown. It is therefore difficult to incorporate soil temperature into crop production models because there is little information available on how soil temperature affects daytime and especially night-time foliar gas exchange.
We assessed the foliar gas exchange response of ‘Shiraz’, an anisohydric grapevine variety (Schultz, 2003) originating from temperate Bordeaux, France, and grown widely in warm climates across southern Australia and Argentina. ‘Shiraz’ is grown across a broad range of latitudes, elevations and topographic conditions that expose this variety to a wide range in root-zone temperatures. Slope orientation and the extent of ground cover in the inter-row area by cover crops or weeds will also affect the temperature of the soil. Other factors such as rain patterns, irrigation and soil type influence rooting depth, and therefore root-zone temperature, and this will also affect plant response. The objective of this work was to clarify the role of root-zone temperature on both nocturnal and daytime g in ‘Shiraz’. Using well-watered grapevines grown in three different root-zone temperatures for 2 months we assessed the response of g to this below-ground parameter during both the day and the night. The three root-zone temperatures consisted of ambient, 5 °C below or 5 °C above ambient levels to encompass the natural variation that can occur across a vineyard site in spring.
MATERIALS AND METHODS
Plant material
Six-year-old ‘Shiraz’ grapevines (n = 60) on their own roots (Vitis vinifera L., clone 1654) were re-potted during dormancy into 40-litre pots containing a well-draining potting mix. The plants were placed outdoors in a bird-proof enclosure in a randomized block design surrounded on all sides by buffer plants. The root-zone temperature treatments were administered by circulating water at controlled temperatures through a 1·1-m long coil of 13-mm polyethylene irrigation tubing inserted within the pot and embedded through the root-zone to a depth of 30 cm. This tubing was attached to 50-mm DWV pipe connected to a recirculating water system (UPS 32-80 N 180; Grundfos, Bjerringbro, Denmark) and one of three temperature-controlled 1200-litre water tanks. The sides and bottom of the pots were insulated with blue 10-mm dense foam to minimize heat exchange between the soil and air or ground. At the time of re-potting, a soil temperature probe (DS18B20; Maxim Integrated Products, Sunnyvale, CA, USA) was inserted into the centre of the root mass of every vine. A TDR soil moisture probe (Trace; Soil Moisture Equipment Corp., Santa Barbara, CA, USA) was also inserted in the same location in nine pots per treatment. Both these probes were logged at 30-min intervals (Mini Trase data logger; Soil Moisture Equipment Corp.). A weather station, equipped with an air temperature probe (Intercap HMP50; Vaisala, Hawthorn, VIC, Australia) and a quantum sensor (Apogee, SQ-110; Logan UT, USA) logging at half hourly intervals, was placed at canopy height within the trial. Ten weeks prior to bud-burst, the plants were pruned to two spurs, each carrying two buds. After bud-burst the plants were irrigated daily and excess water was allowed to drain from the bottom of the pot. Beginning 25 d after bud-burst plants were fertilized with 200 mL tap water containing 1 mL nutrient concentrate (Megamix Plus, equivalent to N/P/K 130 : 100 : 150 mg per plant) every 5 d until veraison, then every 10 d thereafter.
Root-zone temperature treatments
Three root-zone temperature treatments were initiated at the onset of budburst (E-L Stage 4) and terminated at fruit-set (E-L stages 27–29). The root-zone temperatures were allowed to fluctuate diurnally, with the ambient treatment mimicking the nearby vineyard under-vine soil at a depth of 15 cm. Maximum root-zone temperatures over the 63-d treatment period averaged 16·9 ± 0·3 °C for the cool, 22·6 ± 0·5 °C for the ambient and 24·8 ± 0·4 °C for the warm treatment, while minimum root-zone temperatures averaged 13·3 ± 0·3, 17·8 ± 0·5 °C and 22·6 ± 0·2 °C, respectively. Air temperature at the canopy level (approx. 1 m above the soil surface) was not altered by the treatments and ranged between an average minimum of 10·3 °C and maximum of 28·5 °C. VPD ranged between 1·97 and 0·17 kPa while daily maximum photosynthetically active radiation (PAR) averaged 1200 µmol m−2 s−1. Soil moisture over the 63-d treatment period averaged 21·9 ± 1·3 % for the cool, 21·5 ± 1·4 % for the ambient and 21·1 ± 1·4 % for the warm treatment.
Plant measurements
Instantaneous net assimilation, stomatal conductance, transpiration and leaf intercellular CO2 concentrations were measured using a portable photosynthesis system (LI-6400; LI-COR Biosciences Inc., Lincoln NE, USA). Midday measurements were carried out weekly from bud-burst to fruit-set. An artificial red–blue light source attached to the chamber head was used to illuminate the leaf at 1500 µmol m−2 s−1 PAR. The CO2 concentration of the incoming air was maintained at 400 µmol mol−1 and the block temperature was set at 25 °C. On each shoot (18 plants per treatment with four shoots per plant), the leaf opposite the basal inflorescence (most often located at the fourth or fifth node from the base) was measured. Nocturnal measurements (nine plants per treatment) were carried out every 2 weeks on the same leaves prior to dawn with the chamber light turned off, incoming CO2 concentration set at 400 µmol mol−1 and the block temperature set to 18 °C. Time-integrated values of transpiration were calculated based on a normal distribution of leaf stomatal transpiration (Rogiers et al., 2009), over a 15-h light period and constant E during the 9-h dark period. Light, temperature and VPD response curves were carried out at midday, 43–50 d after the onset of the root-zone temperature treatments, on four to six plants per treatment. VPD was not controlled in the temperature or light response curves.
Leaf chlorophyll was assessed non-destructively with a chlorophyll meter (SPAD-502; Minolta Co., Ltd, Tokyo, Japan) twice weekly from 30 d after bud-burst to fruit-set. The meter readings were converted to chlorophyll content using a calibration curve derived from paired meter and spectrophotometer readings. The spectrophotometer readings were converted to an estimate of chlorophyll content following the method of Steele et al. (2008). Measurements were carried out on the leaf opposite the basal inflorescence and all leaves opposite the basal tendril in the three-node repeating metamer pattern that occurs along V. vinifera shoots. Stomatal density was assessed on these same leaves on six plants per treatment according to the method of Rogiers et al. (2011a).
A/Ci responses to root-zone temperatures
Measurements of photosynthetic responses to cool and warm root-zone temperatures were carried out 50 d after onset of treatments on four plants per treatment, targeting fully expanded leaves opposite the basal inflorescence. The leaf respiration/internal CO2 concentration (A/Ci) response was measured according to Ainsworth et al. (2002). Photosynthesis was initially measured at 400 µmol mol−1 CO2 under saturating light (1500 µmol mol−1 s−1) and a block temperature of 25 °C, until A was steady-state. The CO2 concentration surrounding the leaf (Ca) was then reduced to 50 µmol mol−1 over nine steps with A and Ci recorded as soon as Ca was stable. Ca was then returned to 400 µmol mol−1 and increased over eight steps to 2000 µmol mol−1. Each A/Ci curve was processed to obtain Jmax, the maximum rate of electron transport, Vc,max, the in vivo maximum rates of Rubisco activity, and Rd, the day respiration, according to the method of Greer and Weedon (2012) using SAS 9·1 (SAS Institute Inc., Cary, NC, USA).
Statistical analysis
SigmaPlot graphing and statistics software (version 12·3; SPSS Inc., Chicago, IL, USA) was used to fit linear or non-linear regressions of gas exchange parameters with soil temperature, air temperature, VPD and PAR. The three-dimensional mesh plots of nocturnal leaf stomatal conductance (gn) and nocturnal leaf stomatal conductance (En) with soil moisture and root-zone temperature were created with smoothed data calculated from running averages using SigmaPlot. An analysis of variance (GenStat release 15·0; VSN, Hertfordshire, UK) was used for comparison of treatment effects during the root-zone temperature treatments and least significant differences were calculated at 5 % significance. Results of the statistical analyses are presented in the tables and figures. Values presented in the text are means ± s.e.
RESULTS
Nocturnal gas exchange
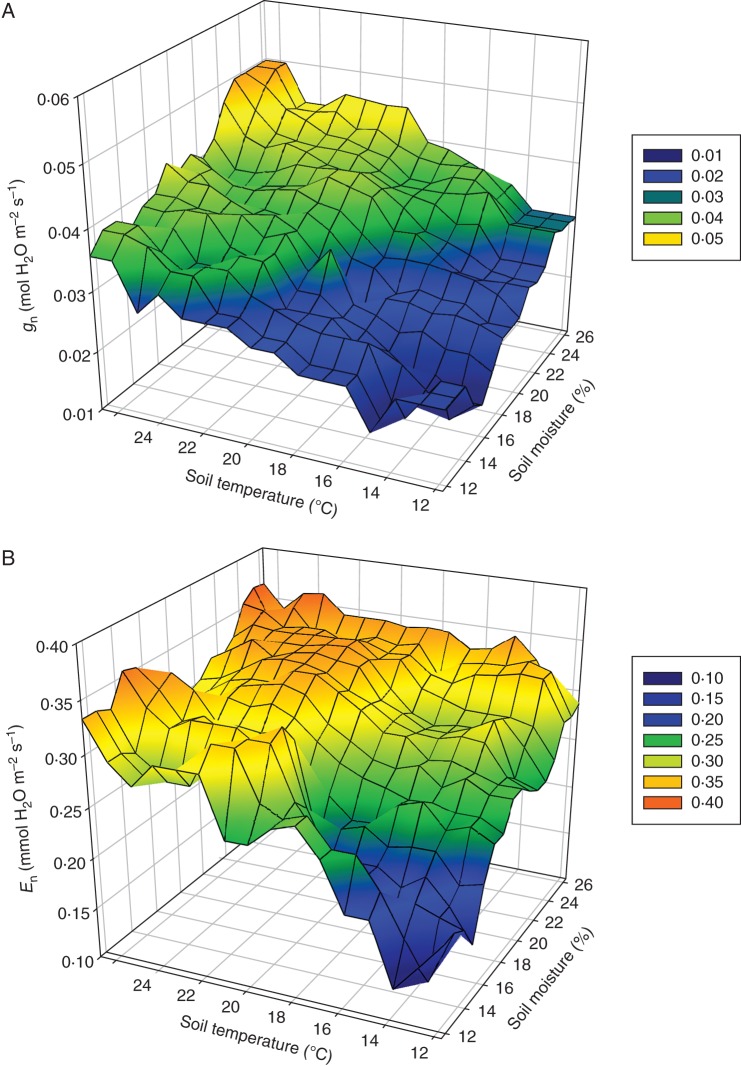
Nocturnal leaf stomatal conductance, gn, (P < 0·001) and transpiration, En, (P < 0·001) responded to root-zone temperature (Table 1). In the cool root-zone treatment, gn was 14·5 % lower than in the ambient treatment and 20·7 % less than in the warm treatment. Similarly, compared with the ambient and warm root-zone treatments, En was 13·8 and 19·8 % lower, respectively, in the cool treatment. This decline was apparent without any treatment differences in soil moisture (P = 0·105) which averaged at 20·9 % at the time of measurement. A linear regression of gn with soil moisture at the time of the gas exchange measurements (P < 0·01) explained only 4 % of the variance in the data while a regression of this parameter with root-zone temperature (P < 0·002) accounted for 3 % of the variance. Further detailed examination of these two environmental parameters, however, showed that vines exposed to high root-zone temperatures combined with high soil moistures had highest gn and En values while those exposed to low temperatures and low moisture responded with low gn and En (Fig. 1). The combined factors of leaf age, leaf temperature, soil moisture and soil temperature accounted for 31 % of the variance in gn (P < 0·001) and 58 % of the variance in En (P < 0·002).
Table 1.
Nocturnal leaf A, gn, En and Ci of grapevines under cool, ambient or warm root-zone conditions
| Treatment | Root-zone temperature (°C) | Soil moisture (%) | A (μmol CO2 m−2 s−1) | gn (mol H2O m−2 s−1) | En (mmol H2O m−2 s−1) | Ci (μmol mol−1) |
|---|---|---|---|---|---|---|
| Cool | 14·8a | 21·2a | –0·53a | 0·0314a | 0·275a | 455·2a |
| Ambient | 20·8b | 20·9a | –0·45a | 0·0367b | 0·319b | 437·8b |
| Warm | 24·2c | 19·7a | –0·48a | 0·0396b | 0·343b | 435·5b |
| LSD | 0·2 | 1·8 | 0·11 | 0·0034 | 0·025 | 11 |
| F-test, P < | 0·001 | 0·179 | 0·199 | 0·001 | 0·001 | 0·001 |
Values are means of three sampling dates over a 60-d treatment period (n = 9 on each sampling date). Root-zone temperatures and soil moistures represent the means at the time the gas exchange measurements were made. Means followed by different letters are significantly different at P < 0·05.
Fig. 1.
Nocturnal leaf (A) gn and (B) En as a function of root-zone temperature and soil moisture. ‘Shiraz’ plants were treated with cool, ambient or warm root-zone conditions over a 60-d period from bud-burst to fruit-set. Smoothed data represent three sampling dates (n = 9 on each sampling date).
Leaf respiration (–A) was not affected (P = 0·20) by root-zone temperatures and averaged 0·49 µmol CO2 m−2 s−1 (Table 1). Internal CO2 concentrations (Ci), were higher by about 20 µmol mol−1 in cool root-zone temperatures compared with the other treatments (P < 0·001), although this was apparently not linked to gn because a linear regression of Ci with gn was not significant (P = 0·15).
Midday gas exchange
As leaves matured over a 20-d period of rapid shoot growth, midday stomatal conductance (gd) increased (P < 0·001) and had doubled by the time full lamina expansion had taken place. This occurred in all three treatments within 1 month after bud-burst (Fig. 2). On each measuring date, gd was substantially less in the cool root-zone as compared with the two other treatments. As the season progressed, air temperatures rose, the soil temperatures of the ambient and the warm treatments converged, and so did leaf gd of plants grown in those treatments.
Fig. 2.
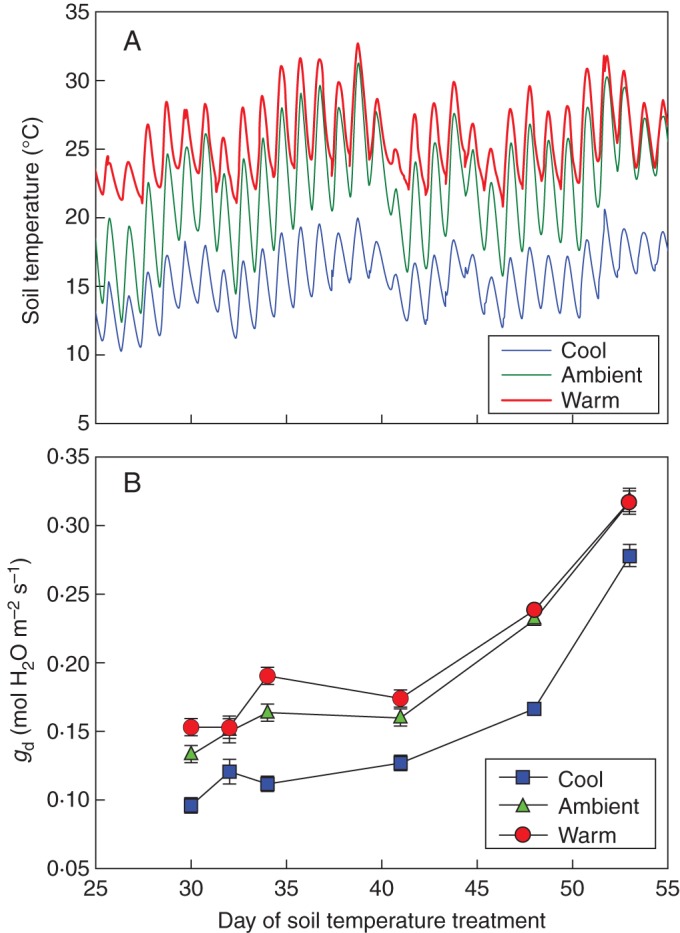
(A) Average root-zone temperature of the cool, ambient and warm treatments (n = 18), and (B) midday leaf stomatal conductance (gd) in response to cool, ambient and warm root-zone temperatures applied over a 60-d period from bud-burst to fruit-set in ‘Shiraz’ vines. The first measurements were made when leaves opposite the basal inflorescence had obtained an approximate area of 10 cm2.
Rates of CO2 assimilation (A) were highest in the warm and lowest in the cool root-zone temperatures (P < 0·001), each diverging by about 5 % from the ambient treatment (Table 2). This was not the result of earlier bud-burst in the warm or later bud-burst in the cool treatment because bud-burst occurred at roughly the same time for all the treatments (P = 0·37). Leaf development after bud-burst may have proceeded at a greater rate in the warm root-zone treatment, but even though leaves of plants grown in this treatment were larger (Table 4), lamina length and A were not correlated (P = 0·822). Higher A was also not due to higher chlorophyll levels in warm plants because chlorophyll levels in the leaves from nodes 2 to 6 over this period were not different from each other in the cool and warm treatments (Table 3). Soil moisture can also not explain these differences because, at the time gas exchange was measured, this parameter varied only slightly between the treatments with lower levels in the warm treatment (22·8 ± 0·5 % for cool, 22·6 ± 0·5 % for ambient and 20·6 ± 0·5 % for warm) which would result in stomatal closure and decline in A rather than an increase.
Table 2.
Midday leaf A, gd, Ed, A/g, A/E and Ci of grapevines under cool, ambient or warm root-zone conditions
| Treatment | Root-zone temperature (°C) | A (μmol CO2 m−2 s−1) | gd (mol H2O m−2 s−1) | Ed (mmol H2O m−2 s−1) | A/g (μmol CO2 mol−1 H2O) | A/E (μmol CO2 mmol−1 H2O) | Ci (μmol CO2 mol−1 air) |
|---|---|---|---|---|---|---|---|
| Cool | 16·7a | 8·83a | 0·155a | 2·70a | 62·0a | 3·74a | 262·3a |
| Ambient | 23·1b | 9·17b | 0·198b | 3·26b | 50·5b | 3·24b | 280·0b |
| Warm | 26·5c | 9·60c | 0·201b | 3·25b | 53·8c | 3·35b | 275·4b |
| LSD | 0·3 | 0·30 | 0·006 | 0·09 | 2·7 | 0·13 | 5·0 |
| F-test, P < | 0·001 | 0·001 | 0·001 | 0·001 | 0·001 | 0·001 | 0·001 |
Values are means of seven sampling dates over a 60-d treatment period (n = 18 on each sampling date). Root-zone temperatures represent the means at the time the gas exchange measurements were made. Means followed by different letters are significantly different at P < 0·05.
Table 4.
Stomatal density of leaves that emerged during the root-zone temperature treatments and of those leaves that emerged after the cessation of the treatments
| Stomatal density (stomata mm−2) |
Lamina length (mm) |
|||
|---|---|---|---|---|
| Treatment | During treatments | Following treatments | During treatments | Following treatments |
| Cool | 127·93a | 127·04a | 83·6a | 77·2a |
| Ambient | 123·15ab | 120·08a | 87·0b | 79·5b |
| Warm | 116·67b | 129·70a | 90·6c | 81·8c |
| LSD | 8·26 | 9·76 | 3·0 | 1·4 |
| F-test, P < | 0·018 | 0·127 | 0·001 | 0·001 |
Data during the treatments are the means of leaves up to and including node 6, where gas exchange measurements were made (n = 5 plants, four shoots per plant). Data of leaves that emerged after the treatments were discontinued up to node 27. Means followed by different letters are significantly different at P < 0·05.
Table 3.
Leaf chlorophyll concentrations and leaf photosynthetic characteristics derived from intercellular CO2 response curves of mature leaves that emerged during the cool or warm rootzone treatments (n = 4)
| Treatment | Chlorophyll (mg m−2) | Jmax (μmol m−2 s−1) | Vc,max (μmol m−2 s−1) | Rd (μmol m−2 s−1) |
|---|---|---|---|---|
| Cool | 424·4a | 91·0a | 34·8a | 0·29a |
| Warm | 424·9a | 97·1a | 33·0a | 0·58a |
| LSD | 2·56 | 17·4 | 9·15 | 0·45 |
| F-test, P < | 0·756 | 0·442 | 0·655 | 0·18 |
Jmax is the maximum rate of electron transport, Vc,max is the in vivo maximum rates of Rubisco activity and Rd is the daytime respiration rate. Chlorophyll concentrations represent averages of nodes 2–6 of weekly measurements during the temperature treatments (n = 18 plants, four shoots per plant). Means followed by different letters are significantly different at P < 0·05.
Despite no differences in soil moisture between the cool and the ambient treatments, midday gd (P < 0·001) and Ed (P < 0·001) were lower by 22 and 17 %, respectively, in the cool treatment (Table 2). While there were no differences in gd or Ed between the ambient and warm treatments, regressions of gd and Ed with root-zone temperature were highly (P < 0·001) significant (Fig. 3). During a 9-h period of darkness, total En amounted to 160 mL H2O m−2 leaf area in the cool, 186 mL H2O m−2 in the ambient and 200 mL H2O m−2 in the warm treatment. During the 15-h light period, total Ed amounted to 1312 mL H2O m−2 in the cool, 1584 mL H2O m−2 in the ambient and 1580 mL H2O m−2 in the warm treatment. Daily En as a proportion of Ed varied by only 1 % between the treatments (12·2 % for the cool treatment, 11·7 % for the ambient treatment and 12·7 % for the warm treatment).
Fig. 3.
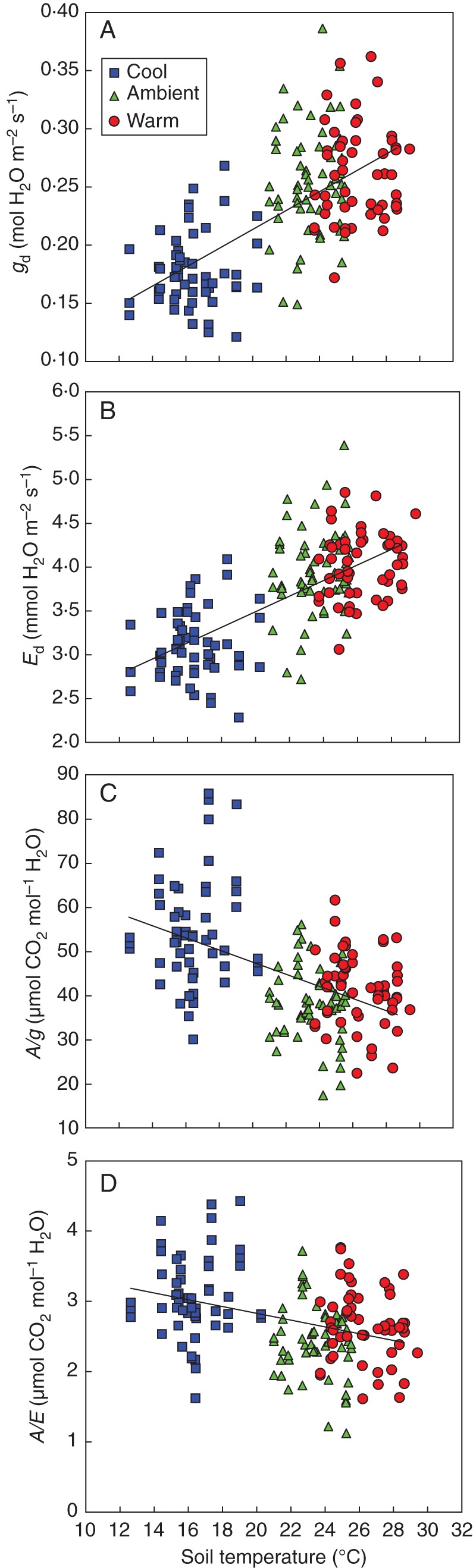
Midday leaf gd, Ed, A/g and A/E as a function of root-zone temperature 48 d after the onset of treatments. Soil moisture was not different between the treatments (F-test, P = 0·72) and averaged 22·6 ± 1·4 % at the time of measurement. F-test of linear regression for g = 0·001, E = 0·001, A/g = 0·001 and A/E = 0·001; variance accounted for in g = 0·47, E = 0·49, A/g = 0·34 and A/E = 0·19.
Plants grown with cool root-zones had 15 % greater transpiration efficiency (A/E) (P < 0·001) and 23 % greater intrinsic transpiration efficiency (A/g) (P < 0·001) than plants grown in ambient soil (Table 2). Those grown in the warm soil also had somewhat higher A/g (6 %) but this may have been an artefact of the slightly reduced soil moistures in this treatment. While there were no differences in A/E between the ambient and warm treatments (Table 2), the regression of A/E with soil temperature was significant due to the strong cool treatment effect (Fig. 3). Similar to gd, midday leaf Ci was somewhat less (7 %) in the cool treatment, indicating depleted CO2 concentrations and a stomatal limitation to photosynthesis.
Light, temperature and VPD response curves
Air temperature, light and VPD response curves (Figs 4–6) provide more extensive data of how leaf gas exchange was affected by root-zone temperatures. In both cool and warm root-zones, stomata were progressively more closed across an air temperature range of 18–38 °C (Fig. 4). VPD was not controlled over this temperature range and Ed increased linearly (P < 0·001) with air temperature in both root-zone temperature treatments. However, A did not change with this environmental parameter in either the cool (P = 0·38) or the warm (P = 0·73) root-zones. Ci followed similar trends to that of gd with lower values in the cool root-zones.
Fig. 4.
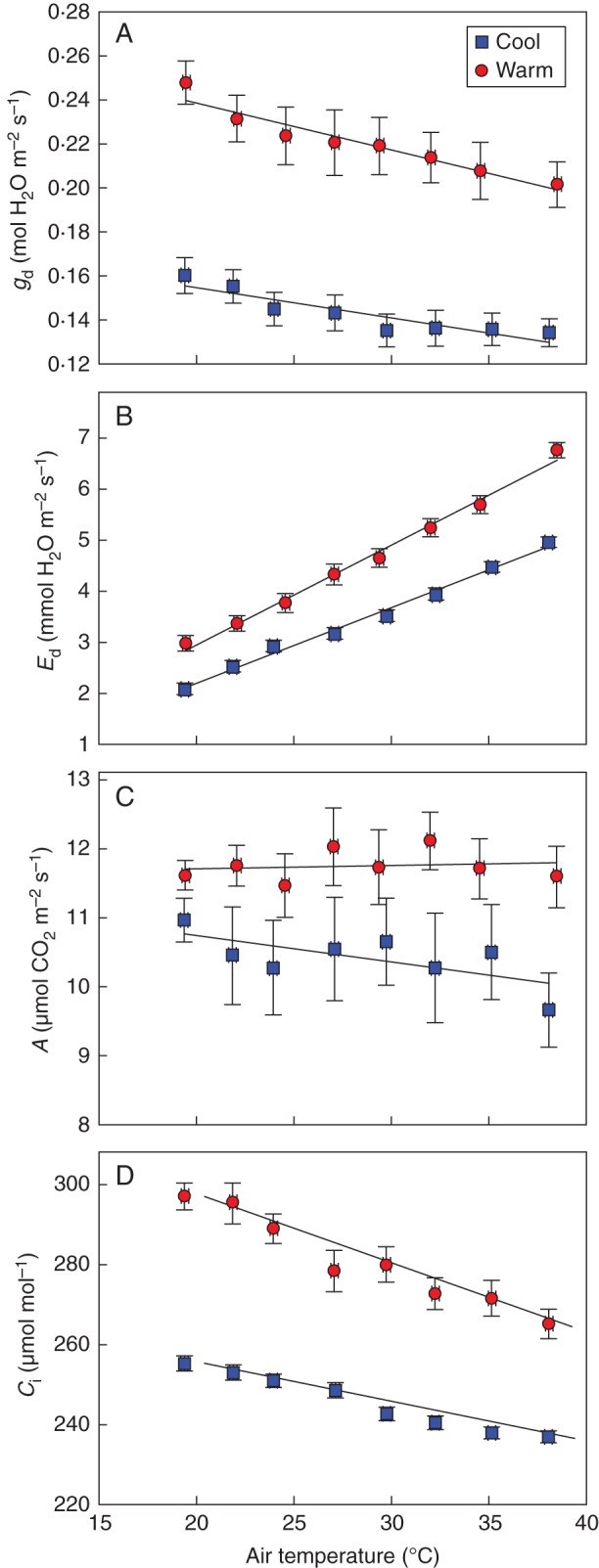
Midday leaf gd, Ed, A and Ci in response to air temperature (n = 4) of vines grown in either cool or warm soil. At the time of measurement, root-zone temperature of the warm treatment was 26·9 ± 1·3 °C and of the cool treatment was 15·0 ± 1·0 °C (P < 0·001). Soil moisture was not different between the treatments (F-test, P = 0·72) and averaged 17·6 ± 0·6 %. The intercepts and slopes of the regressions for the cool and warm cohorts are different from each other for gd (P < 0·001), Ed (P < 0·001), A (P < 0·001) and Ci (P < 0·001).
Fig. 5.
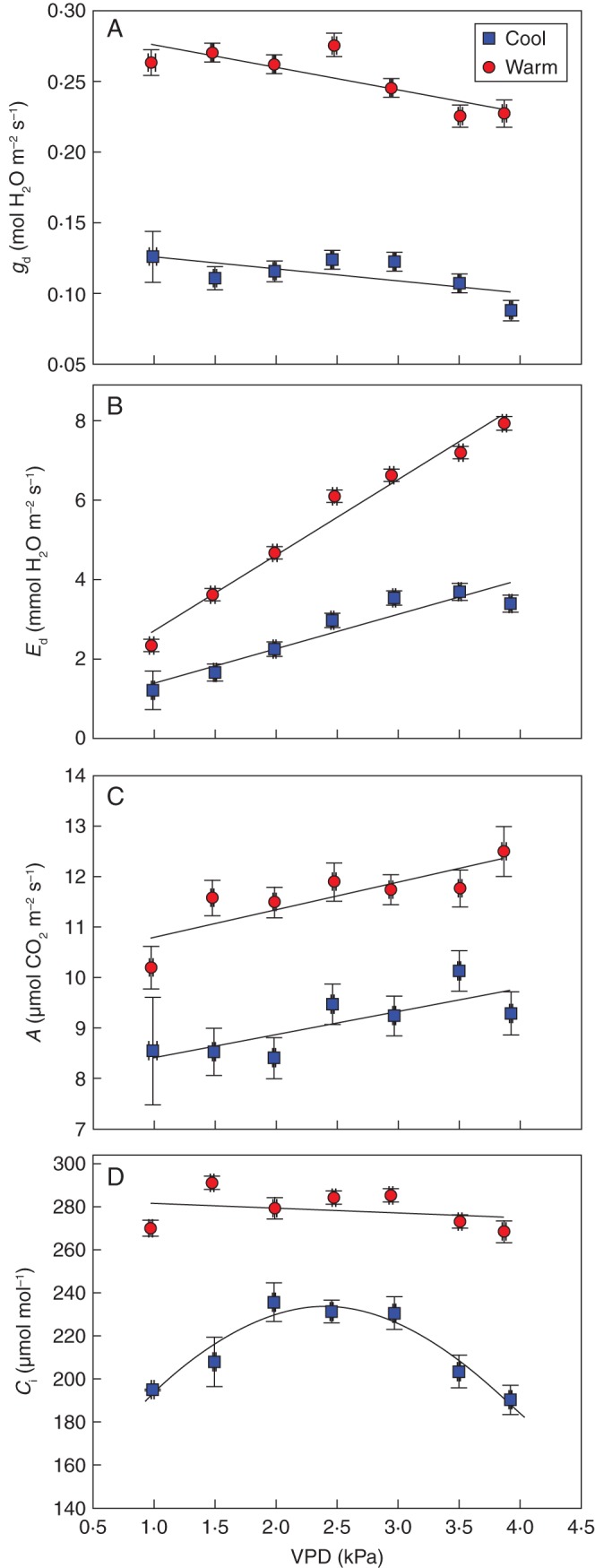
Midday leaf gd, Ed, A and Ci in response to VPD (n = 4) of vines grown in either cool or warm soil. At the time of measurement, root-zone temperature of the warm treatment was 20·3 ± 0·9 °C and of the cool treatment was 13·3 ± 0·7 °C (P < 0·001). Soil moisture was not different between the treatments (F-test, P = 0·50) and averaged 16·8 ± 0·5 %. The intercepts and slopes of the regressions for the cool and warm cohorts are different from each other for Ed (P < 0·001), but only the intercept for gd (P < 0·001) and A (P < 0·001). A non-linear curve was fitted to the Ci data of the cool root-zone temperature treatment (P < 0·001).
Fig. 6.
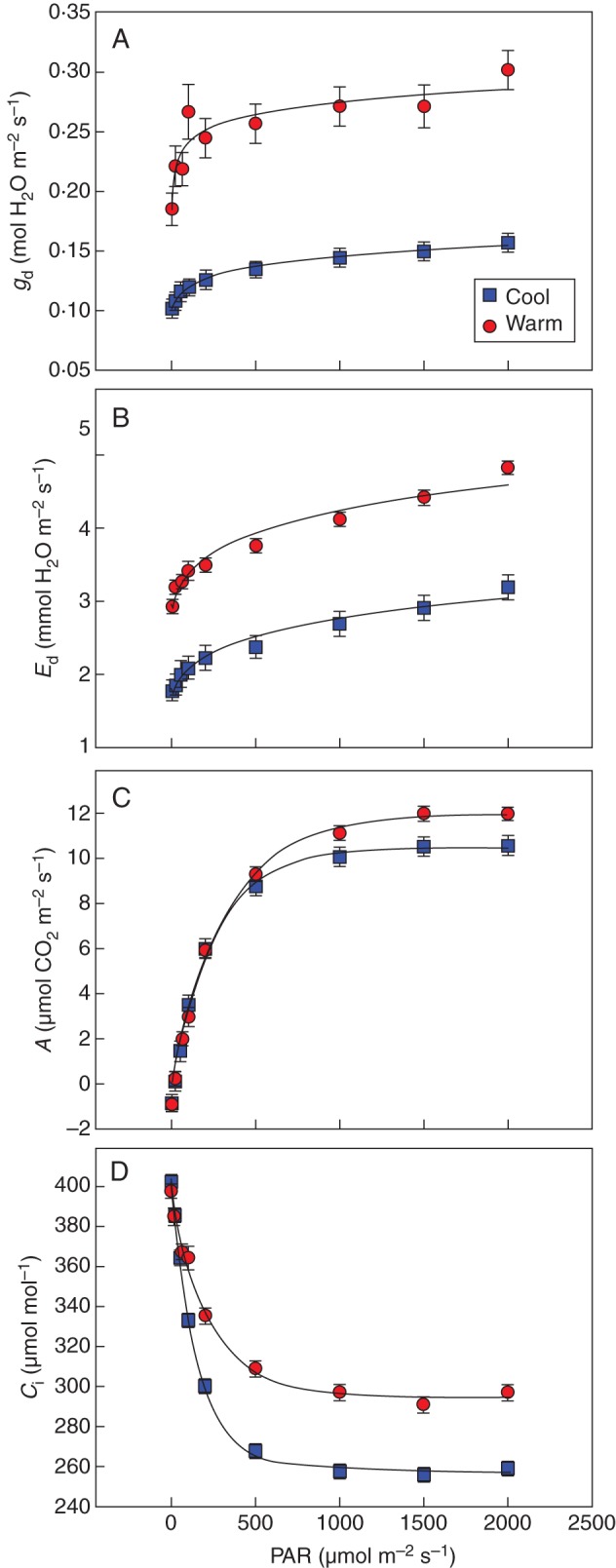
Midday leaf gd, Ed, A and Ci in response to PAR (n = 6) of vines grown in either cool or warm soil. At the time of measurement, root-zone temperature of the warm treatment was 24·2 ± 1·1 °C and of the cool treatment was 13·3 ± 0·7 °C (P < 0·001). Soil moisture was not different between the treatments (F-test, P = 0·63) and averaged 17·6 ± 0·9 %.
There were similar trends in gd and Ed with increasing VPD (Fig. 5). While gd declined, Ed increased linearly across the VPD range of 1–4 kPa. Increases in Ed in the warm root-zones were double (slope 1·9 mmol H2O m−2 s−1 kPa−1) that in the cool root-zones (slope 0·86 mmol H2O m−2 s−1 kPa−1) (P < 0·001). Despite stomatal closure, A increased slightly with VPD in both root-zone treatments. Ci was lower in the cool root-zones and responded in a curvilinear manner to VPD, unlike in the warm root-zone treatment.
As with the air temperature and VPD curves, light response curves showed good separation between the root-zone treatments with higher values in the warm treatment as compared with the cool treatment (Fig. 6). Both gd and Ed increases were curvilinear and did not plateau until the highest PAR value tested (2000 µmol m−2 s−1). A increased steeply in response to rising PAR and leaves from the warm root-zone reached a 15 % higher plateau at 1000 µmol m−2 s−1 than in the cool root-zone. Conversely, Ci values declined with increasing PAR and above 1500 µmol m−2 s−1 Ci settled at 300 µmol mol−1 in the warm and 260 µmol mol−1 in the cool root-zone treatment.
Leaf and photosynthetic characteristics
A/Ci curves in response to root-zone temperatures are presented in Fig. 7. Further analyses of these curves indicate there were no differences in Jmax (P = 0·44) or Vc,max (P = 0·65) between the treatments and they averaged 94·1 ± 7·5 and 33·0 ± 4·0 µmol m−2 s−1, respectively (Table 3). Daytime dark respiration was also not different between the treatments (P = 0·18) and averaged 0·4 ± 0·2 µmol m−2 s−1. Furthermore, leaf chlorophyll concentrations did not differ (P = 0·75) and averaged 424·6 ± 1·3 mg m−2.
Fig. 7.
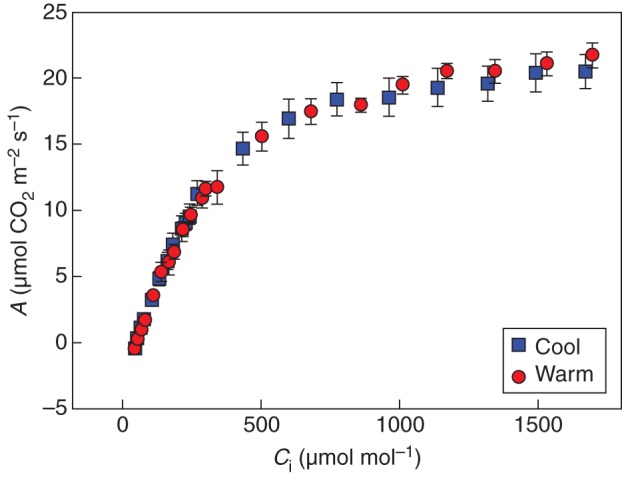
A/Ci curves of grapevines grown in warm or cool soil (n = 4). At the time of measurement, root-zone temperature of the warm treatment was 25·9 ± 0·8 °C and of the cool treatment was 15·1 ± 1·3 °C (P < 0·001). Soil moisture was not different between the treatments (F-test, P = 0·12) and averaged 18·0 ± 0·5 %.
One leaf characteristic that did differ between the treatments was stomatal density. Of those leaves that emerged during the treatments, stomatal density was 10 % (P < 0·05) higher in the cool treatment as compared with the warm treatment (Table 4). Despite 8 % larger leaves in the warm treatment as compared with the cool treatment (Table 4), there was no significant relationship between stomatal density and leaf area (F-test, P = 0·10 for a data set with combined treatments), and therefore other factors aside from leaf area appear to be involved. There were no differences in stomatal density of those leaves that emerged after the discontinuation of the treatments and they averaged 125·6 ± 5 stomata mm−2. Note, however, that stomatal density of newly emerging leaves of plants grown in the warm treatment increased by 11 % after that treatment had been removed. As averaged over 60 d following the termination of the treatments, leaf length remained 6 % larger in the warm treatment than the cool treatment (P < 0·001).
Gas exchange after termination of the treatments
After discontinuation of the treatments the midday root-zone temperatures averaged at 20·6 ± 0·3 °C and varied by less than 4 % (Table 5). Despite equal amounts of water applied, soil moisture was slightly (3 %) higher in those plants that received the cool treatment as compared with the warm treatment. This is probably because the cool plants had less total leaf area (Table 4) and therefore less whole plant transpiration and lower rates of soil water utilization. Leaf A, gd and Ed were no longer different between plants that had previously received the three treatments.
Table 5.
Midday leaf A, gd, Ed and Ci of grapevines after root-zone temperature treatments were discontinued
| Treatment | Root-zone temperature (°C) | Soil moisture (%) | A (μmol CO2 m−2 s−1) | gd (mol H2O m−2 s−1) | Ed (mmol H2O m−2 s−1) | Ci (μmol mol−1) |
|---|---|---|---|---|---|---|
| Cool | 21·0a | 23·6a | 10·3a | 0·223a | 3·70a | 286·2a |
| Ambient | 20·2b | 20·1b | 10·2a | 0·225a | 3·74a | 288·9ab |
| Warm | 20·7a | 20·0c | 10·5a | 0·218a | 3·66a | 283·0b |
| LSD | 0·3 | 2·1 | 0·4 | 0·008 | 0·14 | 4·5 |
| F-test, P < | 0·007 | 0·001 | 0·314 | 0·703 | 0·573 | 0·038 |
Values are means of three sampling dates over a 54-d period after the discontinuation of the treatments (n = 12 on each sampling date). Root-zone temperatures and soil moistures represent the means at the time the gas exchange measurements were made. Means followed by different letters are significantly different at P < 0·05.
DISCUSSION
Nocturnal stomatal conductance
Our study confirms that daytime stomatal conductance of grapevines, like many other species, responds to root-zone temperature. Our present findings extend this observation to nocturnal stomatal conductance. There is very little experimental work on the interaction of root-zone temperatures with nocturnal gas exchange and we believe this is one of the first reports to characterize this. Cool root-zones resulted in a sustained suppression in g and E during both the day and the night and leaves transpired nearly 20 % less than those grown in warm root-zones. Aside from the stomatal limitation evident in our data, others have shown that low E can be the result of reductions in both soil and plant hydraulic conductance (Kramer and Boyer, 1995; Fennel and Markhart, 1998; Berndt et al., 1999) possibly through an increase in water viscosity (Muhsin and Zwiazek, 2002). After discontinuation of the treatments, differences in leaf gd and Ed were no longer apparent. This signifies that the root-zone temperature effect on gas exchange was not due to a permanent anatomical change but rather a transient physiological effect, perhaps relayed through signals targeted at the stomata. Another study on aspen has demonstrated that low root-zone temperatures can reduce g through pH, ion and ABA signals transported in the xylem sap (Wan et al., 2004). It would therefore be expected that these signals persist throughout the diurnal cycle, including the night. The role of such signals was not addressed in this study, however, and further work is required to verify their contribution to gd and gn in grapevines.
The resultant reduction in gn of plants growing in cool root-zones may thus be an after-effect of daytime changes, or it may offer a physiological advantage such as hastened plant rehydration through concomitant decreases in En. Nocturnal transpiration, assessed in this study under mild spring-time conditions, was generally about 12 % of Ed. This is similar to other species grown in wetter environments (Caird et al., 2007; Dawson et al., 2007) and less than the 40–75 % found in desert species (Ogle et al., 2012). Substantial night-time transpiration as a consequence of elevated gn in warm night-time root-zones may prevent complete plant rehydration prior to dawn (Kavanagh et al., 2007), resulting in greater water stress during the day as evaporative demand increases. Because our plants were well watered in all treatments with soil moistures not dropping below 20 %, soil moisture was not a stressor. In grapevines, wilting tendrils at the shoot tips are the first signs of water stress (Winkler et al., 1974) and we did not observe this in any of our plants. In a field situation, however, under the absence of irrigation or under reduced water allocations, plants may experience daytime water stress due to elevated night-time soil temperatures, especially in warm, dry climates. It follows then that daily minimum soil temperature may be just as critical as daily maximum or average soil temperature in determining plant water relations.
Absence of acclimation
One of most significant findings of this study was that stomatal conductance remained low in the cool root-zones throughout the 2-month treatment period, with apparently no temperature acclimation. Sustained reduction in g may be required over weeks if plants are to prevent dehydration, especially with rising midday and nocturnal VPD driving E as the season progresses.
Other factors controlling nocturnal stomatal conductance
Because nocturnal gas exchange has not yet received the same depth of attention as daytime gas exchange, far less is known about the plasticity of gn to environmental factors. We found that aside from root-zone temperature, soil moisture was another variable that impinged on nocturnal stomatal conductance. Nocturnal stomatal conductance was found to decline with drying soil (Cavender-Bares et al., 2007; Howard and Donovan, 2007) and increasing VPD (Barbour and Buckley, 2007) in other studies. VPD did not explain any of the variance in gn in our ‘Shiraz’ plants but this may be because the leaf chamber temperature was held constant and the resultant VPD range was fairly narrow.
In the first few weeks after bud-burst gn increased with leaf age, mimicking trends in gd. Leaf maturity thus appears to be another variable controlling gn. When leaves first emerge there is a progressive increase in gd as stomata mature and the stomatal apertures expand (England and Attiwill, 2011) and we reason that a similar developmental effect persists on gn during the night. We did not measure gn during the latter half of the season but ageing and senescing leaves often have lower gd and thus probably gn as well, but this is yet to be verified. Other factors that might affect gn and En include developmental stage of the plant and plant nutrient status (Ludwig et al., 2006).
Root-zone temperature regulates photosynthesis
Rates of CO2 assimilation were highest in the warm and lowest in the cool root-zones, and this was apparent across a wide range in PAR, VPD and air temperatures. The decline in Ci values under cool root-zone temperatures points to a stomatal limitation and therefore a curtailed CO2 supply to photosynthesis under these conditions. There were no apparent limitations at the chloroplast level as chlorophyll content and both Jmax and Vc,max did not differ between the treatments. This indicates that the low photosynthesis of the cool root-zones was not due to Rubisco or RuBP limitations. Cai and Dang (2002) found that root-zone temperature had an effect on both Jmax and Vc,max in conifer species, although a much wider temperature range (5–35 °C) was employed. These parameters had a maximum at around 25 °C, declining at supra-optimal temperatures. Similar to our grapevines, dark respiration was not affected by root-zone temperature in any of the four species tested.
The key point is that soil temperature did influence A, and furthermore A was more sensitive to soil temperature than to an air temperature range of 20–35 °C. Similarly, photosynthesis of red spruce saplings grown in a cold region was limited more by minimum soil temperature than minimum air temperature, especially in spring (Schwarz et al., 1997). If this is the case for grapevines grown in cool regions, it would be advantageous for the plant to direct root growth to a depth where diurnal temperature fluctuations are dampened. When field grapevines are drip irrigated, however, most of the roots can be found in the top layers (Stevens and Douglas, 1994) and therefore they are exposed to significant fluctuations in temperature. Natural daily cycling in temperature is often overlooked in root-zone studies (Pregitzer et al., 2000), but to mimic field conditions we allowed soil temperature to fluctuate diurnally by approximately 5 °C. Diurnal soil temperature fluctuations would also be relevant to newly established seedlings with small root systems as well as shallow-rooted species growing on impenetrable soils.
Transpiration efficiency responds to root-zone temperature
Despite greater A in warm root-zones, both A/g and A/E were inversely proportional to root-zone temperature. In other words, leaves of plants grown in the warmer root-zones lost greater amounts of water per unit of carbon gained. This is similar to the drop in transpiration efficiency of grapevine varieties grown in high VPD (Düring, 1987; Pou et al., 2008) or ample soil moisture (Cuevas et al., 2006). There is wide varietal diversity in transpiration efficiency of grapevines (Schultz, 1996; Bota et al., 2001; Gibberd et al., 2001; Rogiers et al., 2009). While the adoption of irrigation technology that confers water savings has improved water-use efficiency at the vineyard level, further gains can be made by planting inherently efficient varieties (Condon et al., 2004). Varietal selection therefore must not only take into consideration VPD and soil moisture responses but, as shown here, soil temperature as well.
Stomatal density responds to root-zone temperature
Stomatal density of concurrently formed leaves was lowest in the warm root-zone treatment and highest in the cool treatment. It would seem intuitive that the high stomatal densities of the cold treatment might lead to higher gd and gn, but as is apparent here, there was no such relationship. The low g characteristic of the cool root-zone treatment is therefore not due to low stomatal density and must be related to stomatal aperture only.
In a previous study on ‘Chardonnay’ grapevines it was found that root-zone temperature and atmospheric carbon dioxide both impinged on stomatal density (Rogiers et al., 2011a). Because this leaf parameter was closely and inversely correlated with starch concentration in roots and trunks we suggested that the carbohydrate reserve status of the plant may be an important endogenous determinant of stomatal density. Depleted starch reserves, elicited by several weeks of high metabolism in the warm root-zones, would require replenishment and indeed we find that, in the current study, stomatal density of newly emerging leaves of grapevines grown in warm soil increased after the treatment had been removed.
Summary
We report that En of anisohydric grapevines can be a small (12 %) but significant part of daily transpiration. Similar to gd, gn was responsive to root-zone temperature and thus those regulatory signals that are operative during the day also persist during the night. Removal of the cool or warm treatments brought g back to ambient levels, indicating the presence of a system that is able to react to environmental conditions. The limitation in photosynthesis induced by cool root-zones was the result of a stomatal limitation rather than limitations at the chloroplast level, and accompanying increases in stomatal density brought on by the cool root-zones were not able to offset this stomatal limitation. Intrinsic transpiration efficiency declined with increasing root-zone temperature. Therefore, it is likely that carbon assimilation will occur at the expense of a high water loss when anisohydric grapevines are grown in warm climates with warm soils.
ACKNOWLEDGEMENTS
We are grateful to Beverly Orchard for generating the experimental design and Mark Weedon for the development and implementation of the soil temperature sensors. We thank Kirstina Lamont and Robert Lamont for their contributions to trial maintenance and data collection. Our appreciation also goes to Dr Dennis Greer for assistance with A/Ci curve analyses. This work was supported by the grapegrowers and winemakers of Australia through their investment body, the Grape and Wine Research Development Corporation, with matching funds from the federal government.
LITERATURE CITED
- Ainsworth EA, Davey PA, Hymus GJ, Drake BG, Long SP. Long-term response of photosynthesis to elevated carbon dioxide in a Florida scrub-oak ecosystem. Ecological Applications. 2002;12:1267–1275. [Google Scholar]
- Barbour MM, Buckley TN. The stomatal response to evaporative demand persists at night in Ricinus communis plants with high nocturnal conductance. Plant, Cell and Environment. 2007;30:711–721. doi: 10.1111/j.1365-3040.2007.01658.x. [DOI] [PubMed] [Google Scholar]
- BassiriRad H. Kinetics of nutrient uptake by roots: responses to global change. New Phytologist. 2000;147:155–169. [Google Scholar]
- Berndt ML, McCully ME, Canny MJ. Is xylem embolism and refilling involved in the rapid wilting and recovery of plants following root cooling and rewarming? A cryo-microscope investigation. Plant Biology. 1999;1:506–515. [Google Scholar]
- Bota J, Flexas J, Medrano H. Genetic variability of photosynthesis and water use in Balearic grapevine cultivars. Annals of Applied Biology. 2001;138:353–365. [Google Scholar]
- Cai T, Dang Q-L. Effects of soil temperature on parameters of a coupled photosynthesis–stomatal conductance model. Tree Physiology. 2002;22:819–827. doi: 10.1093/treephys/22.12.819. [DOI] [PubMed] [Google Scholar]
- Caird MA, Richards JH, Donovan LA. Night-time stomatal conductance and transpiration in C3 and C4 plants. Plant Physiology. 2007;143:4–10. doi: 10.1104/pp.106.092940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavender-Bares J, Sack L, Savage J. Atmospheric and soil drought reduce nocturnal conductance in live oaks. Tree Physiology. 2007;27:611–620. doi: 10.1093/treephys/27.4.611. [DOI] [PubMed] [Google Scholar]
- Condon AG, Richards RA, Rebetzke GJ, Farquhar GD. Breeding for high water-use efficiency. Journal of Experimental Botany. 2004;55:2447–2460. doi: 10.1093/jxb/erh277. [DOI] [PubMed] [Google Scholar]
- Cuevas E, Baeza P, Lissarrague JR. Variation in stomatal behaviour and gas exchange between mid-morning and mid-afternoon of north-south oriented grapevines (Vitis vinifera L. cv. Tempranillo) at different levels of soil water availability. Scientia Horticulturae. 2006;108:173–180. [Google Scholar]
- Dawes MA, Hagedorn F, Zumbrunn T, et al. Growth and community responses of alpine dwarf shrubs to in situ CO2 enrichment and soil warming. New Phytologist. 2011;191:806–818. doi: 10.1111/j.1469-8137.2011.03722.x. [DOI] [PubMed] [Google Scholar]
- Dawson TE, Burgess SS, Tu KP, et al. Night-time transpiration in woody plants from contrasting ecosystems. Tree Physiology. 2007;27:561–575. doi: 10.1093/treephys/27.4.561. [DOI] [PubMed] [Google Scholar]
- Düring H. Stomatal responses to alterations of soil and air humidity in grapevines. Vitis. 1987;26:9–18. [Google Scholar]
- England JR, Attiwill PM. Changes in stomatal frequency, stomatal conductance and cuticle thickness during leaf expansion in the broad-leaved evergreen species, Eucalyptus regnans. Trees. 2011;25:987–996. [Google Scholar]
- Erice G, Irigoyen JJ, Pérez P, Martínez-Carrasco R, Sánchez-Díaz M. Effect of elevated CO2, temperature and drought on photosynthesis of nodulated alfalfa during a cutting regrowth cycle. Physiologia Plantarum. 2006;126:458–468. [Google Scholar]
- Fennel A, Markhart AH. Rapid acclimation of root hydraulic conductivity to low temperature. Journal of Experimental Botany. 1998;49:879–884. [Google Scholar]
- Field ST, Smith JP, Holzapfel BP, Emery RJN, Hardie WJ. Grapevine response to soil temperature: xylem cytokinins and carbohydrate reserve mobilization from budbreak to anthesis. American Journal of Enology and Viticulture. 2009;60:164–172. [Google Scholar]
- Fisher JB, Baldocchi DD, Misson L, Dawson TE, Goldstein AH. What the towers don't see at night: nocturnal sap flow in trees and shrubs at two Ameriflux sites in California. Tree Physiology. 2007;27:597–610. doi: 10.1093/treephys/27.4.597. [DOI] [PubMed] [Google Scholar]
- Fosaa AM, Sykes MT, Lawesson JE, Gaard M. Potential effects of climate change on plant species in the Faroe Islands. Global Ecology and Biogeography. 2004;13:427–437. [Google Scholar]
- Gibberd M, Walker R, Blackmore D, Condon A. Transpiration efficiency and carbon-isotope discrimination of grapevines grown under well-watered conditions in either glasshouse or vineyard. Australian Journal of Grape and Wine Research. 2001;7:110–117. [Google Scholar]
- Greer DH, Weedon MM. Modelling photosynthetic responses to temperature of grapevine (Vitis vinifera cv. Semillon) leaves on vines grown in a hot climate. Plant, Cell and Environment. 2012;35:1050–1064. doi: 10.1111/j.1365-3040.2011.02471.x. [DOI] [PubMed] [Google Scholar]
- Howard AR, Donovan LA. Helianthus nighttime conductance and transpiration respond to soil water but not nutrient availability. Plant Physiology. 2007;143:145–155. doi: 10.1104/pp.106.089383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Lee SK. Relationship among photosynthesis, ribulose-1,5-bisphosphate carboxylase (Rubisco) and water relations of the subtropical vegetable Chinese broccoli grown in the tropics by manipulation of root-zone temperature. Environmental and Experimental Botany. 2001;46:119–128. [Google Scholar]
- He J, Lee SK, Dood IC. Limitations to photosynthesis of lettuce grown under tropical conditions: alleviation by root-zone cooling. Journal of Experimental Botany. 2001;52:1323–1330. [PubMed] [Google Scholar]
- Kasper TC, Bland WL. Soil temperature and root growth. Soil Science. 1992;154:290–299. [Google Scholar]
- Kavanagh KL, Pangle R, Schotzko AD. Nocturnal transpiration causing disequilibrium between soil and stem predawn water potential in mixed conifer forests of Idaho. Tree Physiology. 2007;27:621–629. doi: 10.1093/treephys/27.4.621. [DOI] [PubMed] [Google Scholar]
- Kramer PJ, Boyer JS. Water relations of plants and soil. San Diego: Academic Press; 1995. [Google Scholar]
- Larson MM. Root regeneration and early growth of red oak seedlings: influence of soil temperature. Forest Science. 1970;16:442–446. [Google Scholar]
- Lopushinsky W, Kaufmann MR. Effects of cold soil on water relations and spring growth of Douglas-fir seedlings. Forest Science. 1984;30:628–634. [Google Scholar]
- Ludwig F, Jewitt RA, Donovan LA. Nutrient and water addition effects on day- and night-time conductance and transpiration in a C-3 desert annual. Oecologia. 2006;148:219–225. doi: 10.1007/s00442-006-0367-6. [DOI] [PubMed] [Google Scholar]
- MacDonald NW, Zak DR, Pregitzer KS. Temperature effects on kinetics of microbial respiration and net nitrogen and sulfur mineralization. Soil Science Society of America Journal. 1995;59:233–240. [Google Scholar]
- Melillo JM, Steudler PA, Aber JD, et al. Soil warming and carbon-cycle feedbacks to the climate system. Science. 2002;298:2173–2176. doi: 10.1126/science.1074153. [DOI] [PubMed] [Google Scholar]
- Muhsin T, Zwiazek JJ. Ectomycorrhizas increase apoplastic water transport and root hydraulic conductivity in Ulmus americana seedlings. New Phytologist. 2002;153:153–158. [Google Scholar]
- Ogle K, Lucas RW, Bentley LP, et al. Differential daytime and night-time stomatal behavior in plants from North American deserts. New Phytologist. 2012;194:464–476. doi: 10.1111/j.1469-8137.2012.04068.x. [DOI] [PubMed] [Google Scholar]
- Pou A, Flexas J, del Mar AM, et al. Adjustments of water use efficiency by stomatal regulation during drought and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris) Physiologia Plantarum. 2008;134:313–323. doi: 10.1111/j.1399-3054.2008.01138.x. [DOI] [PubMed] [Google Scholar]
- Pregitzer KS, King JS. Effects of soil temperature on nutrient uptake. In: Bassirirad H, editor. Nutrient Acquisition by plants: an ecological perspective. Heidelberg: Springer; 2005. pp. 277–310. [Google Scholar]
- Pregitzer KS, King JS, Burton AJ, Brown SE. Responses of tree fine roots to temperature. New Phytologist. 2000;147:105–115. [Google Scholar]
- Rogiers SY, Greer DH, Hutton RJ, Landsberg JJ. Does night-time transpiration contribute to anisohydric behaviour in a Vitis vinifera cultivar? Journal of Experimental Botany. 2009;60:3751–3763. doi: 10.1093/jxb/erp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogiers SY, Hardie WJ, Smith JP. Stomatal density of grapevine leaves (Vitis vinifera L.) responds to soil temperature and atmospheric carbon dioxide. Australian Journal of Grape and Wine Research. 2011a;17:147–152. [Google Scholar]
- Rogiers SY, Smith JP, Holzapfel BP, Hardie WJ. Soil temperature moderates grapevine carbohydrate reserves after bud-break and conditions fruit-set responses to photoassimilatory stress. Functional Plant Biology. 2011b;38:899–909. doi: 10.1071/FP10240. [DOI] [PubMed] [Google Scholar]
- Schultz HR. Water relations and photosynthetic responses of two grapevine cultivars of different geographical origin during water stress. Acta Horticulturae. 1996;427:251–266. [Google Scholar]
- Schultz HR. Differences in hydraulic architecture account for near-isohydric and anisohydric behaviour of two field-grown Vitis vinifera L. cultivars during drought. Plant Cell and Environment. 2003;26:1393–1405. [Google Scholar]
- Schwarz PA, Fahey TJ, Dawson TE. Seasonal air and soil temperature effects on photosynthesis in red spruce (Picea rubens) saplings. Tree Physiology. 1997;17:187–194. doi: 10.1093/treephys/17.3.187. [DOI] [PubMed] [Google Scholar]
- Steele M, Gitelson AA, Rundquist D. Nondestructive estimation of leaf chlorophyll content in grapes. American Journal of Enology and Viticulture. 2008;59:299–305. [Google Scholar]
- Stevens RM, Douglas T. Distribution of grapevine roots and salt under drip and full-ground cover microjet irrigation systems. Irrigation Science. 1994;15:147–152. [Google Scholar]
- van Gestel NC, Schwilk DW, Tissue DT, Zak JC. Reductions in daily soil temperature variability increase soil microbial biomass C and decrease soil N availability in the Chihuahuan Desert: potential implications for ecosystem C and N fluxes. Global Change Biology. 2011;17:3564–3576. [Google Scholar]
- Vose RS, Easterling DR, Gleason B. Maximum and minimum temperature trends for the globe: an update through 2004. Geophysical Research Letters. 2005;32:L23822. [Google Scholar]
- Wan X, Landhäusser SM, Zwiazek JJ, Lieffers VJ. Root water flow and growth of aspen (Populus tremuloides) at low root temperatures. Tree Physiology. 1999;19:879–884. doi: 10.1093/treephys/19.13.879. [DOI] [PubMed] [Google Scholar]
- Wan X, Landhäusser SM, Zwiazek JJ, Lieffers VJ. Stomatal conductance and xylem sap properties of aspen (Populus tremuloides) in response to low soil temperature. Physiologia Plantarum. 2004;122:79–85. [Google Scholar]
- Winkler AJ, Cook JA, Kliewer WM, Lider LA. Berkeley: University of California Press; 1974. General viticulture. [Google Scholar]
- Wu SH, Jansson P-E, Kolari P. The role of air and soil temperature in the seasonality of photosynthesis and transpiration in a boreal Scots pine ecosystem. Agricultural and Forest Meteorology. 2012;156:85–103. [Google Scholar]
- Zeppel M, Tissue D, Taylor D, Macinnis-Ng C, Eamus D. Rates of nocturnal transpiration in two evergreen temperate woodland species with differing water-use strategies. Tree Physiology. 2010;30:988–1000. doi: 10.1093/treephys/tpq053. [DOI] [PubMed] [Google Scholar]
- Zeppel MJ, Lewis JD, Chaszar B, et al. Nocturnal stomatal conductance responses to rising [CO2], temperature and drought. New Phytologist. 2012;193:929–938. doi: 10.1111/j.1469-8137.2011.03993.x. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Qiao YX, Zhang YL, Zhou YH, Yu JQ. Effects of root temperature on leaf gas exchange and xylem sap abscisic acid concentrations in six Cucurbitaceae species. Photosynthetica. 2008;46:356–362. [Google Scholar]
- Zhou YH, Yu JQ, Huang LF, Nogués S. The relationship between CO2 assimilation, photosynthetic electron transport and water-water cycle in chill-exposed cucumber leaves under low light and subsequent recovery. Plant Cell and Environment. 2004;27:1503–1514. [Google Scholar]