Abstract
Background and Aims
Previous research has suggested a trade-off between the capacity of plants to downregulate their phosphorus (P) uptake capacity and their efficiency of P resorption from senescent leaves in species from P-impoverished environments.
Methods
To investigate this further, four Australian native species (Banksia attenuata, B. menziesii, Acacia truncata and A. xanthina) were grown in a greenhouse in nutrient solutions at a range of P concentrations [P]. Acacia plants received between 0 and 500 µm P; Banksia plants received between 0 and 10 µm P, to avoid major P-toxicity symptoms in these highly P-sensitive species.
Key Results
For both Acacia species, the net P-uptake rates measured at 10 µm P decreased steadily with increasing P supply during growth. In contrast, in B. attenuata, the net rate of P uptake from a solution with 10 µm P increased linearly with increasing P supply during growth. The P-uptake rate of B. menziesii showed no significant response to P supply in the growing medium. Leaf [P] of the four species supported this finding, with A. truncata and A. xanthina showing an increase up to a saturation value of 19 and 21 mg P g−1 leaf dry mass, respectively (at 500 µm P), whereas B. attenuata and B. menziesii both exhibited a linear increase in leaf [P], reaching 10 and 13 mg P g−1 leaf dry mass, respectively, without approaching a saturation point. The Banksia plants grown at 10 µm P showed mild symptoms of P toxicity, i.e. yellow spots on some leaves and drying and curling of the tips of the leaves. Leaf P-resorption efficiency was 69 % (B. attenuata), 73 % (B. menziesii), 34 % (A. truncata) and 36 % (A. xanthina). The P-resorption proficiency values were 0·08 mg P g−1 leaf dry mass (B. attenuata and B. menziesii), 0·32 mg P g−1 leaf dry mass (A. truncata) and 0·36 mg P g−1 leaf dry mass (A. xanthina). Combining the present results with additional information on P-remobilization efficiency and the capacity to downregulate P-uptake capacity for two other Australian woody species, we found a strong negative correlation between these traits.
Conclusions
It is concluded that species that are adapted to extremely P-impoverished soils, such as many south-western Australian Proteaceae species, have developed extremely high P-resorption efficiencies, but lost their capacity to downregulate their P-uptake mechanisms. The results support the hypothesis that the ability to resorb P from senescing leaves is inversely related to the capacity to downregulate net P uptake, possibly because constitutive synthesis of P transporters is a prerequisite for proficient P remobilization from senescing tissues.
Keywords: Downregulation, nutrient-poor soils, phosphorus toxicity, phosphorus-uptake capacity, Proteaceae, remobilization, resorption, Banksia attenuata, B. menziesii, Acacia truncata, A. xanthina
INTRODUCTION
Soils in Australia generally have low concentrations of phosphate (Beadle, 1966), especially in south-western Australia (McArthur, 1991; Lambers et al., 2010), where bicarbonate-extractable phosphorus (P) values (‘plant-available’ P) range from 0·9 to 47 mg kg−1 (Singh and Gilkes, 1991). The readily available P concentration [P] of these soils is extremely low, because they have developed from low-P parental material (such as sandstones and beach sand) and because these landscapes have been climatically buffered since the Jurassic, without glaciations for millions of years (Hopper, 2009; Lambers et al., 2010). The species occurring on soils low in P exhibit several traits that allow them to survive in these environments. These include maximizing P acquisition, P-use efficiency and conservation of P within the plant. Specific adaptations include associations with mycorrhizal fungi, development of cluster roots, exudation of chemicals that ‘unlock’ P from soil particles, high photosynthesis per unit of P, sclerophylly, high leaf longevity and highly efficient P resorption from senescing leaves (Lamont, 1982; Lambers et al., 2010).
Plants usually respond to P addition with a positive response in biomass and leaf [P] if this nutrient is limiting (Elser et al., 2007; Ostertag, 2010). However, when P is supplied in excess of what is required for growth, the plant's P-uptake capacity tends to be downregulated, as demonstrated by the effect of the addition of inorganic phosphorus (Pi) during growth (Dong et al., 1999; Shane et al., 2004b). Species with a low capacity to downregulate their P uptake may develop P-toxicity symptoms when supplied with P above the normal levels in soil (Shane et al., 2004a, b, 2008; Shane and Lambers, 2006). Phosphorus-toxicity symptoms are commonly found in Australian and South African species that naturally occur on P-impoverished soil when plants are exposed to slightly elevated soil P levels (Handreck, 1991; Lambers et al., 2002; Hawkins et al., 2008).
Plants may remobilize nutrients from leaves during senescence, and these nutrients are transported to sinks, including growing leaves (Veneklaas et al., 2012). Resorption of both nitrogen (N) and P tends to increase with decreasing leaf nutrient status (Vergutz et al., 2012) and is a nutrient-conservation mechanism, albeit of less significance than leaf longevity (Escudero et al., 1992; Reich et al., 1995; Aerts, 1996). In south-western Australian species, both high resorption efficiency (Wright and Westoby, 2003; Denton et al., 2007) and high leaf longevity (Wright et al., 2004; Lambers et al., 2012a) are common, as expected given the P-impoverished status of the environment (Hopper, 2009).
When studying nutrient resorption from senescing tissues, there are two complementary parameters that can be calculated: nutrient-resorption efficiency (the percentage of nutrients that a plant can remove from its senescing leaves compared with how much is in its adult leaves), and nutrient-resorption proficiency (how little nutrient is left, in absolute terms, in the senesced leaves). Killingbeck (1996) concluded that efficiency values are best suited for resolving issues related to the conservation of nutrients and, therefore, reduction of subsequent nutrient uptake. Proficiency values, on the other hand, appear to be a more objective measure of the degree to which selection has acted to minimize nutrient loss. Resorption of nutrients from senescing leaves, with the exception of calcium, decreases with increasing leaf nutrient status (Vergutz et al., 2012).
The Proteaceae are a conspicuous family in south-western Australia (Pate et al., 2001), and they are typically non-mycorrhizal and dominant on soils lowest in P (Lambers et al., 2006, 2010). The vast majority of Proteaceae species make cluster roots, which release carboxylates to make sorbed P available for uptake (Shane and Lambers, 2005). The Fabaceae are also a common and diverse family in south-western Australia, and some species are also able to form cluster roots (Lamont, 1972; Adams et al., 2002). Most Fabaceae species in south-western Australia are mycorrhizal (Brundrett, 2009) and a large proportion form root nodules (Hansen and Pate, 1987; Adams et al., 2002). Fabaceae tend to be more tolerant of higher levels of soil P than Proteaceae (Handreck, 1997), presumably due to their ability to downregulate their P-uptake capacity. In the present study, we hypothesized that there is an inverse relationship between a species' capacity to downregulate its P-uptake capacity and its P-resorption efficiency and proficiency. Our hypothesis is that Banksia attenuata and B. menziesii (Proteaceae) will not be able to downregulate their P-uptake capacity significantly, and hence accumulate P in their leaves and show symptoms of P toxicity. On the other hand, they are expected to have a very high P-resorption efficiency and withdraw P from senescing leaves to an extremely low level (high proficiency). In contrast, we expect Acacia truncata and A. xanthina (Fabaceae) to be able to downregulate their P-uptake capacity strongly, but to be less efficient and proficient at P resorption. To test our hypothesis, we measured P uptake at a standard [P] for plants of all four species grown at a range of P supplies in a glasshouse. We also measured the [P] in fully mature and recently senesced leaves on plants of the same species growing in their natural habitat.
MATERIALS AND METHODS
Glasshouse plant cultivation
Acacia truncata (Burm.f.) Hoffmanns, A. xanthina Benth. (Fabaceae: Mimosoideae), and Banksia attenuata R.Br. and B. menziesii R.Br. (Proteaceae) were chosen for this experiment because they are endemic to south-western Australia and because they belong to families that are abundant and species rich in Australia.
Seeds were purchased from a local nursery (Nindethana Australian Seeds) and germinated in Petri dishes with moist filter paper at 15 °C. When the radicles emerged, the seedlings were transferred to trays containing sterilized, washed sand placed in a glasshouse. When they had reached the size of 3 cm (11 weeks), they were removed from the sand, gently washed and transplanted to individual pots in an aerated hydroponic system in a glasshouse on 29 June (beginning of winter).
The glasshouse was equipped with root-cooling tanks that kept the nutrient solutions at 16 °C. The relative humidity of the glasshouse varied between 50 and 70 % at night and decreased to 30–50 % during the day. The temperature ranged from 5 to 15 °C at night and from 18 to 26 °C during the day for the duration of the experiment. The area in which the plants were located received around 70 % of external sunlight, with a daily peak radiation of 800–1650 µmol m−2 s−1. Plants were grown for 10 weeks at five P supplies with six replicates per treatment.
The nutrient solution contained (in μm): 200 CaNO3, 100 K2SO4, 54 MgSO4, 20 KCl, 2 Fe-EDTA, 2·4 H3BO3, 0·3 Na2MoO4, 0·24 MnSo4, 0·1 ZnSO4 and 0·02 CuSO4, made up with deionized water and with a pH of 5·8 (Shane and Lambers, 2006), but the addition of KH2PO4 for the P treatments differed with a range of [P] supplied to the plants for 10 weeks. Based on preliminary experiments, we used a range from zero to 10 µm P for the Banksia species; for the Acacia species, expected to be more tolerant of P, we used a range from zero to 500 µm P. The P-sensitive B. attenuata and B. menziesii were grown at: 0, 0·1, 1, 5 and 10 µm P; the more P-tolerant A. xanthina and A. truncata were grown at: 0, 0·5, 5, 50 and 500 µm P. All plants were grown as one plant per 2 L pot. Nutrient solutions were replaced daily.
Net P-uptake determination
After 10 weeks, all plants were transferred to a no-P basal nutrient solution for 20 h. On the day of harvest, they were all supplied with a [P] of 10 µm (in a total volume of 200 mL). The optimal concentration and volume of solution to measure P uptake were based on preliminary experiments and published experiments (Shane et al., 2004a).
To measure P uptake of each individual plant, samples (1 mL) of each solution were taken every 30 min from 1030 until 1230 h, and then hourly until 1630 h. These samples were stored at 5 °C until analysed using the malachite green method (Motomizu et al., 1983) in a microplate spectrophotometer (MultiSkan, Thermo Scientific, MA, USA). Only the values in the linear phase of P uptake collected between 1030 and 1230 h were used to calculate net P-uptake rates. Rates of P uptake of each individual plants were divided by root fresh weight to obtain specific P-uptake rates.
All plants were harvested and had their stems and roots separated. Shoots and roots were weighed for fresh weight (f. wt), and then dried at 60 °C for 3 d and weighed to determine their dry weight (d. wt).
Leaf P concentration of experimental plants
To calculate the leaf [P], all individuals had their leaves dried, ground using a mortar and pestle, and homogenized. They were then subjected to a nitric–perchloric acid digestion, diluted and analysed using the malachite green method (Motomizu et al., 1983).
Resorption
To calculate the P-resorption proficiency of B. attenuata, B. menziesii, A. truncata, A. xanthina, Grevillea crithmifolia and Hakea prostrata, both fully expanded leaves (green, mature and not visibly damaged) and recently senesced leaves (either completely dry but still on the plant or on the top layer of litter) were collected in Bold Park (31·95 °S, 115·77 °E), a native bushland in the Perth metropolitan area. Samples from field specimens had to be collected for these analyses because the leaves of these slow-growing species live for ≥2 years, and hence experimental plants had no senescing leaves at the end of the study. In addition to those four species, samples of Hakea prostrata (Bold Park, Western Australia), Acacia suaveolens and Banksia serrata (Blue Mountains National Park, New South Wales, Australia) were collected and analysed. Three replicates of both mature and senesced leaves were taken for each species (each replicate was from a separate shrub or tree no closer than 5 m to one another). Each replicate consisted of leaves taken from various points of the same plant. Leaves were gently brushed to remove sand and dust and then dried in an oven over 3 d at 60 °C. The dried material was ground using a mortar and pestle, digested with nitric and perchloric acid, and then analysed by inductively coupled plasma mass spectrometry (ICP-OES, model Optima 7300 DV, Perkin Elmer, MA, USA).
The P-resorption efficiency was calculated as the difference between mature and senesced leaf [P] divided by mature leaf [P]. Phosphorus-resorption proficiency, or the amount of P present in the senesced leaves, was also determined. All [P] values in this study are expressed on a leaf dry mass basis.
Statistics
Single-factor analyses of variance (ANOVAs) considering the P treatments as factors were conducted for all of the data, species by species, and then grouped by genus, finally with all species together. All weight measurements were log-transformed prior to the ANOVAs, since they did not have equal variances, and the residuals ‘fanned out’. These analyses were performed with Genstat 12th Edition, 2009 (VSN International Ltd). Means are presented with standard errors.
RESULTS
Effect of P supply on growth
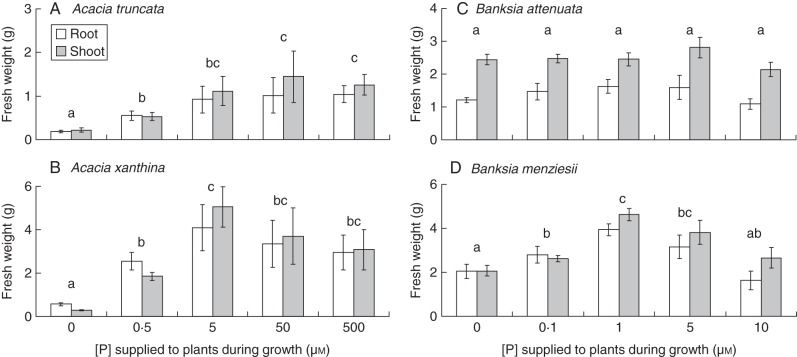
Acacia truncata, A. xanthina and B. menziesii exhibited a positive growth response to increasing P supply in the root environment up to an intermediate treatment level, and no further growth response with higher levels of P (Fig. 1). Acacia xanthina accumulated the most biomass of all species (5·04 g shoot f. wt and 4·08 g root f. wt), and A. truncata the least (1·45 g shoot f. wt and 1·04 g root f. wt). Banksia menziesii reached values (4·63 g shoot f. wt and 3·93 g root f. wt) approximately twice those of B. attenuata (2·80 g shoot f. wt and 1·61 g root f. wt). With the exception of B. attenuata, all species had higher biomass at an intermediate P supply (P < 0·05). For A. truncata, 50 µm P resulted in the most root and shoot weight. The P concentration that was optimal for growth for A. xanthina was 5 µm, and for B. menziesii it was 1 µm P. When expressed on a dry weight basis, the same trends were found (data not shown).
Fig. 1.
Effects of P concentration on fresh weight of (A) Acacia truncata, (B) A. xanthina, (C) Banksia attenuata and (D) B. menziesii. Plants were grown at five P concentrations (0, 0·5, 5, 50 and 500 µm P for Acacia, and 0, 0·1, 1, 5 and 10 µm P for Banksia) during 10 weeks in nutrient solution. Root and shoot fresh weights are as indicated in the key in (A). Bars indicate standard errors, n = 6. Letters above the bars indicate the pairwise comparison of means (P = 0·05) and show that roots and shoots reached a maximum fresh weight at an intermediate P concentration for three species. Banksia attenuata was the exception, showing no growth response to P supply. Note that the scales on the y-axes differ between panels.
The root weight ratio (RWR; g root f. wt g−1 plant f. wt) also differed between treatments for all species except B. attenuata (Fig. 2), but only showed a significant decrease trend for B. menziesii. The RWR varied between 0·41 and 0·51 for A. truncata; 0·44 and 0·66 for A. xanthina; 0·33 and 0·39 for B. attenuata; and 0·36 and 0·51 for B. menziesii.
Fig. 2.
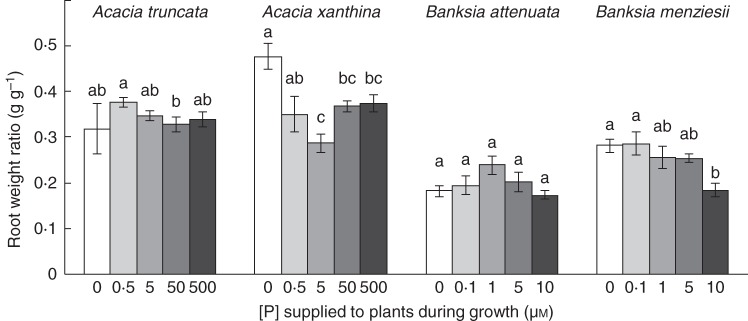
Effects of P concentration on root weight ratio (total root dry weight as a fraction of total plant dry weight) for Acacia truncata, A. xanthina, Banksia attenuata and B. menziesii grown at five P concentrations in nutrient solution (0, 0·5, 5, 50 and 500 µm P for Acacia, and 0, 0·1, 1, 5 and 10 µm P for Banksia). The root weight ratio of B. menziesii was the only one showing a significant decrease along P treatments (P < 0·001, n = 6). Letters above the bars indicate significant difference in a HSD Tukey test.
Leaf P concentration
In the Acacia species, leaf [P] increased linearly with increasing P supply for the three lowest P concentrations (0, 0·5 and 5 µm) (Fig. 3A), but reached a saturation point of approx. 16 mg P g−1 leaf d. wt. At approx. 10 µm P, further accumulation of P in the leaves was only slight, regardless of the nutrient solution concentration (Fig. 3A); from that point on, A. truncata increased its leaf [P] from 11 to 15 mg P g−1 leaf d. wt, and A. xanthina from 12 to 17 mg P g−1 leaf d. wt. Conversely, in both Banksia species, leaf [P] increased linearly with increasing P supply during growth (Fig. 3B). Banksia menziesii accumulated 1·3 times more P than B. attenuata in response to 10 µm P in the growth medium (Fig. 3B).
Fig. 3.
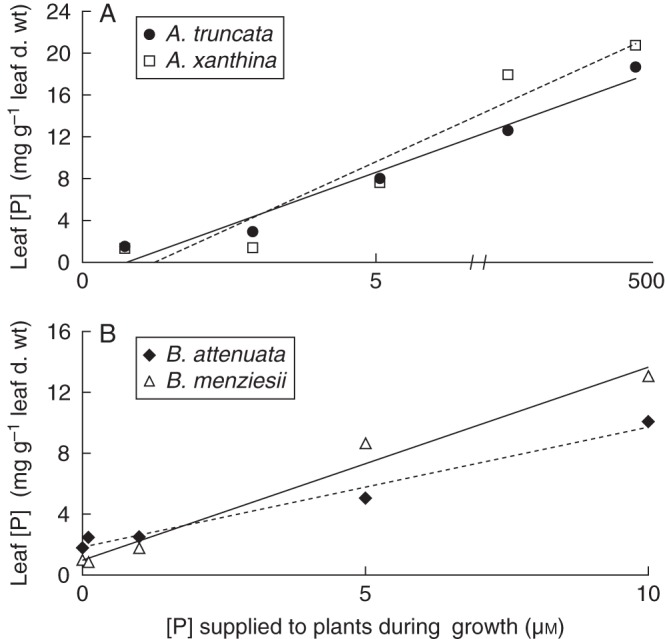
Leaf phosphorus concentration ([P]) plotted against the [P] at which the plants were grown (n = 6). (A) Leaf [P] in Acacia truncata and A. xanthina, as indicated. (B) Banksia attenuata and B. menziesii, as indicated. The equations for the fitted trend lines, R2 and significance levels are: Acacia truncata [y = 1·04Ln(x) + 8·38; R2 = 0·76; P < 0·001]; Acacia xanthina [y = 1·28Ln(x) + 9·34; R2 = 0·69; P < 0·001]; Banksia attenuata (y = 0·79x + 1·82; R2 = 0·98; P < 0·001); Banksia menziesii (y = 1·27x + 0·96; R2 = 0·98; P < 0·001).
Phosphorus uptake
In both Acacia species, P-uptake rates from a 10 µm P solution decreased significantly when plants were grown at higher [P] (Fig. 4A). In A. truncata, the P-uptake rate decreased from 0·26 to 0·03 nmol P g−1 root f. wt s−1; in A. xanthina it declined from 0·08 to –0·03 nmol P g−1 root f. wt s−1. Interestingly, six individuals of Acacia (three of each species) grown at the highest [P] (500 µm) did not take up any P during the measurement period; in fact, they showed P efflux from the root system into the solution during the measurement period, resulting in a negative average of P-uptake rate for A. xanthina. In contrast to the response of the Acacia species, B. attenuata and B. menziesii did not show a reduction in their P-uptake rate from the standard 10 µm P solution at an increased P supply during growth (Fig. 4B). Banksia attenuata grown without external P showed a P-uptake rate measured at 10 µm P of 0·023 nmol P g−1 root f. wt s−1; when grown at the highest [P] in the medium (10 µm P), a P-uptake rate at 10 µm P of 0·036 nmol P g−1 root f. wt s−1 was found. Similarly, B. menziesii showed P-uptake rates at 10 µm P of 0·026 (no P) and 0·027 nmol P g−1 root f. wt s−1 (10 µm P).
Fig. 4.
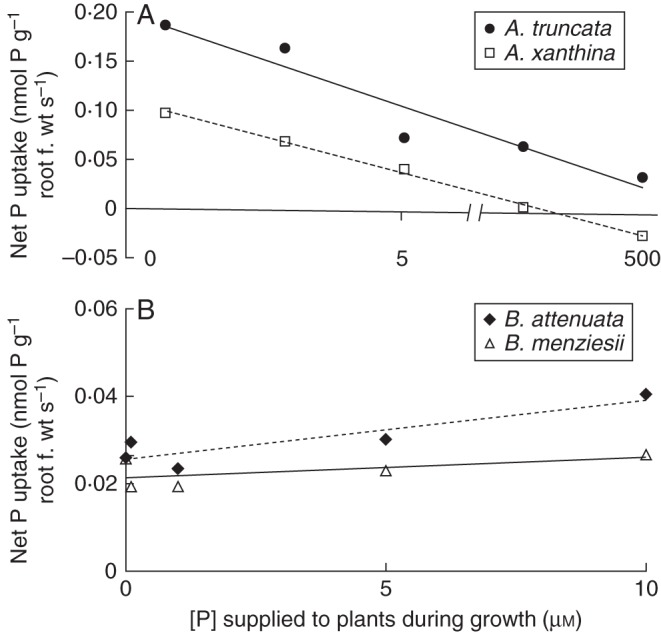
Net P-uptake rates calculated from P depletion from a standard solution containing 10 µm P (n = 6). The x-axis shows the P concentration ([P]) at which plants were grown for 10 weeks. (A) Net P-uptake rates of Acacia truncata and A. xanthina, as indicated. (B) P-uptake rate of Banksia attenuata and B. menziesii, as indicated. The difference between the two genera is statistically significant (significance value of 0·05), but that between the two species of the same genus is not. The equations for the fitted trendlines, R2 and significance levels are as follows: Acacia truncata [y = –0·02Ln(x) + 0·13; R2 = 0·96; P = 0·02]; Acacia xanthina [y = –0·01Ln(x) + 0·04; R2 = 0·79; P < 0·01]; Banksia attenuata (y = 0·001x + 0·03; R2 = 0·75; P = 0·05); Banksia menziesii (y = 0·0003x + 0·02; R2 = 0·13; P = 0·55). Note the difference in y-axis scale.
Resorption of leaf P
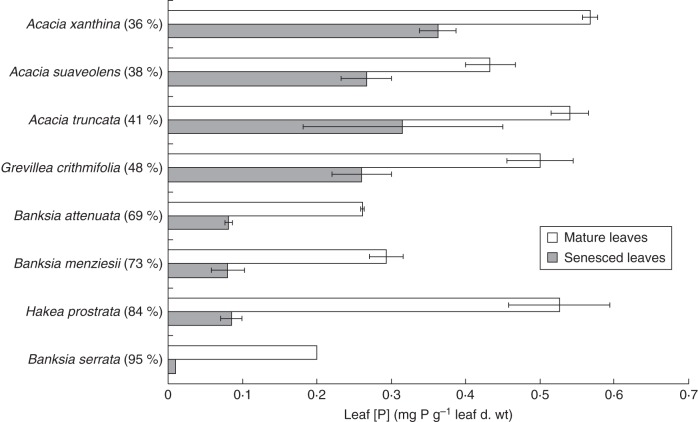
Figure 5 shows the [P] both in green, fully expanded, mature leaves and in recently senesced leaves collected from plants growing in their natural habitat. The average mature and senesced leaf [P] values for A. truncata were 0·54 and 0·32 mg P g−1 leaf d. wt, respectively. For A. xanthina, the values were 0·57 and 0·36 mg P g−1 leaf d. wt for mature and senesced leaves, respectively. The mature leaf [P] values were not significantly different (significance level of 5 %) between both Acacia species, and neither were the senesced leaf [P] values. Similarly, no significant difference was found for leaves of a similar age between the two Banksia species, with mature leaf [P] being 0·26 (B. attenuata) and 0·29 mg P g−1 leaf d. wt (B. menziesii) and senesced leaf [P] being 0·08 mg P g−1 leaf d. wt for both species.
Fig. 5.
Leaf P concentrations for mature and senesced leaves for each species listed along the y-axis. Columns represent averages (n = 3) of mature and senesced leaves, as indicated; bars represent standard errors. Samples were collected from Bold Park, Perth (S31·95, E115·77), with the exception of Acacia suaveolens and Banksia serrata, which were collected in Blue Mountains National Park, New South Wales, Australia). The percentage values shown in parentheses represent the P-remobilization efficiency.
Phosphorus-resorption efficiency was not significantly different between A. truncata and A. xanthina or between B. menziesii and B. attenuata, but was remarkably different between Acacia (34 and 41 %) and Banksia (69 and 73 %). The senesced leaf [P] of Banksia was much lower than that of Acacia; the Banksia species resorbed most P from their leaves during leaf senescence before leaf shedding. The P-resorption proficiency of Banksia was also high, with an average of only 0·08 mg P g−1 dry leaf d. wt left in the senesced leaves for both species.
On average, B. attenuata and B. menziesii drew their leaf [P] down to 0·08 mg P g−1 leaf d. wt. The senesced leaves of A. xanthina, however, retained 0·36 mg P g−1 leaf d. wt, and A. truncata 0·32 mg P g−1 leaf d. wt, showing a 4-fold variation in P-resorption proficiency between Acacia on the one hand and Banksia and Hakea on the other. Resorption efficiencies were also determined for two other species in south-western Australia: G. crithmifolia and H. prostrata (Proteaceae). Grevillea crithmifolia showed a resorption efficiency of only 48 %, somewhere between the values for Acacia and Banksia species. Hakea prostrata showed a much higher P-resorption efficiency: 84 %, the highest efficiency for plants collected in Bold Park. Banksia serrata and A. suaveolens, species native to the Blue Mountains in New South Wales, Australia, were also analysed for mature and senesced leaf [P], with calculated resorption efficiencies of 95 and 38 %, respectively.
Phosphorus-toxicity symptoms
After 10 weeks of growth at a range of P supplies, Banksia leaves in the 10 µm P treatment had dried and curled leaf tips, and some showed dark spots on the leaves (Fig. 6). These symptoms of P toxicity were more pronounced on the oldest leaves, but not exclusive to them.
Fig. 6.

Symptoms of toxicity due to excess phosphorus. (A) Banksia attenuata with the leaf tips dried and curled; (B) B. menziesii with dark spots on the leaves; (C) B. menziesii with chlorophyll loss and dried leaf margins. Photographs of healthy Banksia leaves without P-toxicity symptoms are included in some of our previous papers (Lambers et al., 2002, 2012a).
Relationship between P uptake and P resorption
Figure 7 shows data on downregulation of P uptake and P-remobilization efficiency for the four species assessed in the present study combined with data on P uptake of two additional species available from the literature (Shane et al., 2004b; Shane and Lambers, 2006). Phosphorus-mobilization efficiency, as determined on plants growing in their natural habitat, was strongly correlated with the capacity to downregulate P-uptake capacity, as assessed in laboratory experiments.
Fig. 7.
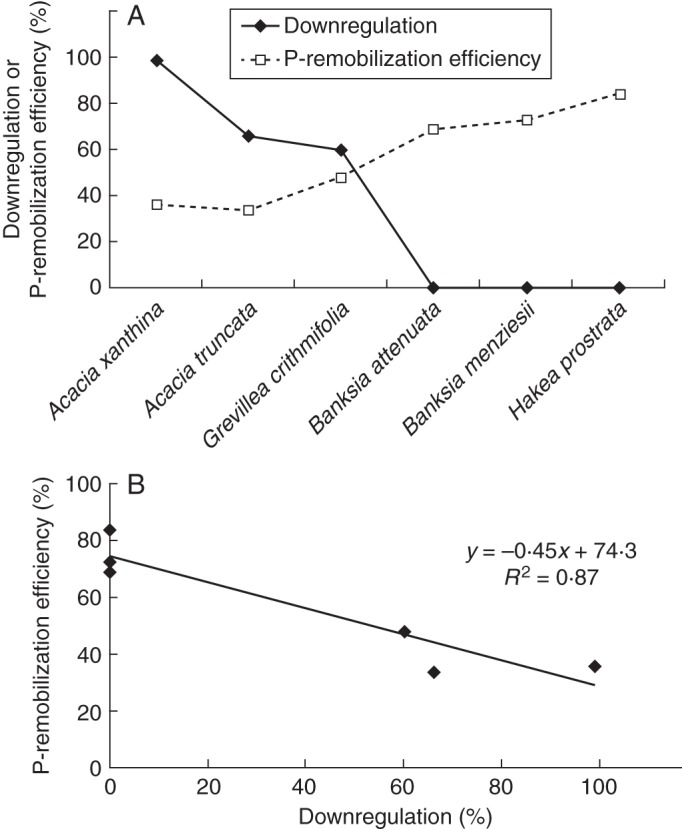
(A) Downregulation of P-uptake capacity and P-remobilization efficiency and (B) the relationship between them. Downregulation of P-uptake capacity was calculated as the difference of the rate of P uptake from a standard 10 µm P solution for plants grown at the lower and that for plants grown at the higher P concentrations during growth. Phosphorus-remobilization efficiency was assessed for plants growing in their natural habitat. Data on P-uptake rates of Grevillea crithmifolia and Hakea prostrata were taken from the literature (Shane et al., 2004b; Shane and Lambers, 2006).
DISCUSSION
An increase in P supply to B. attenuata, B. menziesii, A. truncata and A. xanthina resulted in an increase in biomass only when the P supply was relatively low, but at higher P supply a decrease in biomass was observed and symptoms of P toxicity became apparent for some species, as previously observed for a range of Australian species from severely nutrient-impoverished environments (Specht, 1963; Grundon, 1972; Groves and Keraitis, 1976; Handreck, 1997; Ozanne and Specht, 1981). The level of external [P] at which this happens varies among species (Groves and Keraitis, 1976) as they have different P demands and different capacities to downregulate their P-uptake (Shane et al., 2004b, 2008; Shane and Lambers, 2006).
Phosphorus-resorption efficiencies and proficiencies in situ
Banksia attenuata and B. menziesii both have very low mature leaf [P], about half the average leaf [P] for Australian plants (0·49 mg g−1) and a quarter of the world average (1·02 mg g−1) (Lambers et al., 2010). The explanation for this low leaf [P] when these plants show relatively high rates of photosynthesis is still under investigation, but one reason is that mature leaves of these Banksia species replace phospholipids by galactolipids and sulfolipids (Lambers et al., 2012b). Phospholipids represent a major fraction of total leaf [P] when plants are grown at a low P supply (Veneklaas et al., 2012). Despite starting with low [P], the Banksia leaves resorbed a very large fraction of their leaf P during leaf senescence, with P-resorption efficiencies (relative to their initial P content) of 69 and 73 %, respectively. Hakea prostrata, another P-sensitive species (Shane et al., 2004a), exhibited a similar pattern to B. attenuata and B. menziesii: its P-resorption efficiency was 84 %. Acacia xanthina and A. truncata exhibited a mature leaf [P] of 0·55 mg g−1 d. wt, twice as much as the Banksia species, but their P-resorption efficiencies were much lower, 36 and 41 %, respectively. In a study of 73 Australian evergreen taxa from nutrient-poor, water-limited sites, the mean P-resorption efficiency was 63 % (Wright and Westoby, 2003), which is higher than the present results for A. truncata and A. xanthina, but lower than the efficiency of B. attenuata, B. menziesii and H. prostrata. Data on P-resorption efficiency and proficiency in the literature and as presented herein should be considered as actual values; that is, they do not necessarily show values a species may express under conditions that maximize resorption (Reed et al., 2012; Vergutz et al., 2012). In the present study, for instance, the P-resorption efficiency for B. attenuata was 69 %, whereas in a different study with collections in 2005 at different sites, the value was only 27 % (Denton et al., 2007). The differences between resorption efficiencies can be due to water availability, timing of abscission, leaf nutrient status or shade (Killingbeck, 1996; Vergutz et al., 2012). Interestingly, Denton et al.'s study included B. menziesii with a P-resorption efficiency of 72 %, similar to the value found in the present study.
The two genera studied here differed in their P-resorption proficiency (absolute measure of how little is left in the senesced leaves), B. attenuata and B. menziesii being more proficient at resorbing P from senescing leaves than the two Acacia species studied. The differences in proficiency between species of the same genus were not statistically significant, but they were significantly different between genera. The proficiency values found for B. attenuata and B. menziesii in this study (80 mg P g−1 d. wt) fall within the published range for Banksia species: 29–128 mg P g−1 d. wt (Denton et al., 2007). They are very similar to the values found (85 mg P g−1 d. wt) for H. prostrata, another slow-growing Proteaceae species, which is P sensitive, like B. attenuata and B. menziesii. Acacia xanthina and A. truncata showed P-resorption proficiencies of only 360 and 320 mg P g−1 d. wt, respectively, four times less proficient at resorbing P than the two studied Banksia species. They were still proficient when considered in a global context (450 mg P g−1 d. wt) (Killingbeck, 1996), but less proficient than the average of 180 mg P g−1 d. wt for Australian evergreen species (Wright and Westoby, 2003).
Unlike the P-sensitive B. attenuata and B. menziesii, A. truncata and A. xanthina had greater tolerance of higher levels of P which would allow them to occupy niches in the landscape with slightly higher nutrient concentrations, such as drainage areas and disturbed edges of the vegetation formation (Hopper and Maslin, 1978; http://florabase.dec.wa.gov.au). Given their faster growth and higher shoot [P], the Acacia species also had a greater demand for P which allows them to be successful at early stages following fires (Bell and Koch, 1980). On the other hand, A. xanthina and A. truncata were not as proficient in P resorption which might explain why they disappear at a later stage after fire, when soil P has declined and most P would be locked up in the biomass again.
Previous studies with large numbers of species and considering environmental fertility variation indicate that mature leaf nutrient concentration is an important determinant of senesced leaf [P], and P-resorption efficiency declines with increasing green leaf [P] (Kobe et al., 2005; Vergutz et al., 2012). In agreement with this, both Acacia species in our study showed higher leaf [P] than the Banksia species, and also showed a lower P-resorption proficiency and efficiency.
Effects of P supply on plant growth
Acacia truncata, A. xanthina and B. menziesii plants produced most biomass at an intermediate P supply. The highest P supply did not result in additional biomass production or inhibited growth for these species and led to P-toxicity symptoms in Banksia leaves. Banksia attenuata did not produce any additional biomass with increased P supply. The seedlings of B. attenuata have a very high P content in their cotyledons (Denton et al., 2007) and grow very slowly, which explains why no increase in biomass was observed. However, a clear increase in leaf [P] was observed with increasing P supply.
Effect of P supply on leaf [P]
After 10 weeks of growth at high P supply, Banksia leaves showed the typical symptoms of P toxicity (Groves and Keraitis, 1976; Parks et al., 2000; Lambers et al., 2002). This effect was most pronounced on the oldest leaves. The leaves of Acacia, unlike those of Banksia, were not visually damaged in any way, even at the highest P supply, which was considerably higher than that used for Banksia. The results on leaf [P] provide evidence that A. truncata and A. xanthina were able to downregulate their P-uptake capacity, in contrast to B. attenuata and B. menziesii.
Net P-uptake rates
Both Acacia species studied were able to downregulate their P-uptake rates. Acacia xanthina also showed downregulation of its P uptake from a standard solution. However, plants grown in the no-P solution showed a net P-uptake rate of 0·08 nmol P g−1 root f. wt s−1, much lower than that of A. truncata grown under similar conditions. Its P-uptake rate decreased with increasing P supply to 0·07, 0·04, 0·03 and –0·03 (plants grown at 0·5, 5, 50 and 500 µm P, respectively); the negative value shows that the plants grown at 500 µm released P into the standard solution, suggesting the net P-uptake rate comprised a major efflux component. Therefore, downregulation of net P uptake in Acacia may be based on a decreased activity of plasma membrane P transporters as well as an increased efflux. Efflux of P represents a major component of net P uptake in Pinus species endemic to P-deficient soils (Topa and Sisak, 1997). Banksia attenuata increased its P-uptake rate with increasing P supply. The B. attenuata individuals grown at the highest P supply for 10 weeks had a P-uptake rate approx. 60 % greater (0·036 nmol P g−1 root f. wt s−1) than the plants grown with no P (0·023 nmol P g−1 root f. wt s−1).
Banksia menziesii, like B. attenuata, did not downregulate its P uptake, and the difference between P treatments was not statistically significant. The P-uptake rates of B. menziesii ranged between 0·02 and 0·027 nmol P g−1 root f. wt s−1. Shane et al. (2004a) studied the P-uptake rates and P toxicity in H. prostrata (also native to south-western Australia) and determined the uptake rates at 5 µm P. Those plants were grown at eight P concentrations, ranging from 0 to 100 µm P. Although H. prostrata almost doubled its P-uptake rate when comparing plants grown at 0 and 0·2 µm P, its uptake rate then stabilized at 0·05 nmol P g−1 f. wt s−1 in the 0·2–1 µm P range. Interestingly, the uptake rate then decreased by half at the highest [P] (10, 50 and 100 µm P), coinciding with a suppression of cluster-root formation. Such a decrease in uptake was not observed in the present experiment with B. attenuata and B. menziesii.
Linking P uptake and P resorption
As hypothesized, we observed a strong inverse correlation between P-resorption efficiency and the downregulation of P-uptake capacity. This is the first attempt to search for such a correlation and, even though only six species were included in the analysis, they represent four genera (Acacia, Banksia, Grevillea and Hakea) of two major families in Australian heathlands.
Downregulation of P-uptake systems is important to avoid P toxicity, whereas upregulation is unlikely to enhance the root's P uptake significantly, because in soil P acquisition is limited by processes determining P mobility, rather than P-uptake kinetics (Silberbush and Barber, 1983; Lambers et al., 2006). Therefore, the genes that control downregulation of net P-uptake capacity are likely to be basal, and their loss would be secondary. High P-resorption efficiency and proficiency, on the other hand, appear to be traits acquired more recently (Killingbeck, 1996). We surmise that there is a link between the genetic attributes that allow for the plant to possess a very high P-remobilization efficiency and those responsible for lack of downregulation of P uptake. Both traits would involve P transporters, and if the genes encoding these transporters are expressed constitutively, this presumably allows both greater resorption and less controllable uptake. This would account for the correlation between P sensitivity and P-resorption efficiency. Pei et al. (2012) overexpressed a gene encoding a vacuolar H+-pyrophosphatase from Thellungiella halophila in Zea mays. This enzyme maintains vacuolar pH and provides energy for tonoplast transport. The transgenic maize plants exhibited more vigorous root growth under P-sufficient as well as P-deficient conditions, and they were more tolerant of low-P stress than the wild type. Their work shows that modifying membrane transport properties may indirectly enhance a plant's performance under P-limiting conditions. Further work focusing on the expression of specific transporters might shed further light on the putative link between P-resorption efficiency and a low capacity to downregulate P-uptake mechanisms.
Concluding remarks
As hypothesized, we observed an inverse correlation between the P-resorption efficiency and the ability to downregulate P-uptake capacity in the species studied here. The species included in this study fall into two groups: one with highly specialized physiology aiding survival in P-impoverished soil conditions (B. attenuata, B. menziesii and H. prostrata); and the second with greater tolerance to P supply but with a less efficient P-conservation mechanism (A. truncata, A. xanthina and G. crithmifolia).
The species we have studied are representative of two contrasting survival strategies within nutrient-impoverished landscapes, but we envisage a continuum of species showing different degrees of capacity to downregulate their P-uptake systems. At one end of this continuum, plants possess a low capacity for P resorption from senescing leaves but control their uptake (avoiding P toxicity); at the other end, plants are extremely efficient at P resorption, but, perhaps as a consequence, cannot downregulate their P uptake. The species used in this study co-occur in some areas in south-western Australia, possibly reflecting the complex mosaic of soils or time since disturbance (fire), providing different niches. By studying traits relating to P transport into and inside the plant, in combination with other ecophysiological traits, we may better understand intricate details of the functioning of species-rich regions in Australia's biodiversity hotspot of global significance.
ACKNOWLEDGEMENTS
Thanks are due to the University of Western Australia for a doctorate scholarship (IPRS/SIRF) to M.C.R.dC. and the School of Plant Biology for funding and infrastructure. We thank David Elsworth for collecting and sending leaf material of species that occur in Blue Mountains National Park near Sydney, Australia. This research was supported by the Australian Research Council (ARC) (to H.L.) and additional grants were provided by ANZ Holsworth Wildlife Foundation and The Mary Janet Lindsay of Yanchep Memorial Fund (to M.C.R.dC.). We also to thank our colleagues who helped with the experiment and made valuable suggestions.
LITERATURE CITED
- Adams MA, Bell TL, Pate JS. Phosphorus sources and availability modify growth and distribution of root clusters and nodules of native Australian legumes. Plant, Cell and Environment. 2002;25:837–850. [Google Scholar]
- Aerts R. Nutrient resorption from senescing leaves of perennials: are there general patterns? Journal of Ecology. 1996;84:597–608. [Google Scholar]
- Beadle NCW. Soil phosphate and its role in molding segments of the Australian flora and vegetation, with special reference to xeromorphy and sclerophylly. Ecology. 1966;47:992–1007. [Google Scholar]
- Bell DT, Koch JM. Post-fire succession in the northern jarrah forest of Western Australia. Australian Journal of Ecology. 1980;5:9–14. [Google Scholar]
- Brundrett MC. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil. 2009;320:37–77. [Google Scholar]
- Denton MD, Veneklaas EJ, Freimoser FM, Lambers H. Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilization of phosphorus. Plant, Cell and Environment. 2007;30:1557–1565. doi: 10.1111/j.1365-3040.2007.01733.x. [DOI] [PubMed] [Google Scholar]
- Dong B, Ryan PR, Rengel Z, Delhaize E. Phosphate uptake in Arabidopsis thaliana: dependence of uptake on the expression of transporter genes and internal phosphate concentrations. Plant, Cell and Environment. 1999;22:1455–1461. [Google Scholar]
- Elser JJ, Bracken MES, Cleland EE, et al. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters. 2007;10:1135–1142. doi: 10.1111/j.1461-0248.2007.01113.x. [DOI] [PubMed] [Google Scholar]
- Escudero A, del Arco JM, Sanz IC, Ayala J. Effects of leaf longevity and retranslocation efficiency on the retention time of nutrients in the leaf biomass of different woody species. Oecologia. 1992;90:80–87. doi: 10.1007/BF00317812. [DOI] [PubMed] [Google Scholar]
- Groves RH, Keraitis K. Survival and growth of seedlings of three sclerophyll species at high levels of phosphorus and nitrogen. Australian Journal of Botany. 1976;24:681–690. [Google Scholar]
- Grundon NJ. Mineral nutrition of some Queensland heath plants. Journal of Ecology. 1972;60:171–181. [Google Scholar]
- Handreck K. Interactions between iron and phosphorus in the nutrition of Banksia ericifolia L.f var ericifolia (Proteaceae) in soil-less potting media. Australian Journal of Botany. 1991;39:373–384. [Google Scholar]
- Handreck KA. Phosphorus requirements of Australian native plants. Australian Journal of Soil Research. 1997;35:241–290. [Google Scholar]
- Hansen AP, Pate JS. Comparative growth and symbiotic performance of seedlings of Acacia spp. in defined pot culture or as natural understorey components of a eucalypt forest ecosystem in S.W. Australia. Journal of Experimental Botany. 1987;38:13–25. [Google Scholar]
- Hawkins H-J, Hettasch H, Mesjasz-Przybylowicz J, Przybylowicz W, Cramer MD. Phosphorus toxicity in the Proteaceae: a problem in post-agricultural lands. Scientia Horticulturae. 2008;117:357–365. [Google Scholar]
- Hopper SD. OCBIL theory: towards an integrated understanding of the evolution, ecology and conservation of biodiversity on old, climatically buffered, infertile landscapes. Plant and Soil. 2009;322:49–86. [Google Scholar]
- Hopper SD, Maslin BR. Phytogeography of Acacia in Western Australia. Australian Journal of Botany. 1978;26:63–78. [Google Scholar]
- Killingbeck KT. Nutrients in senesced leaves: keys to the search for potential resorption and resorption proficiency. Ecology. 1996;77:1716–1727. [Google Scholar]
- Kobe RK, Lepczyk CA, Iyer M. Resorption efficiency decreases with increasing green leaf nutrients in a global data set. Ecology. 2005;86:2780–2792. [Google Scholar]
- Lambers H, Juniper D, Cawthray GR, Veneklaas EJ, Martínez-Ferri E. The pattern of carboxylate exudation in Banksia grandis (Proteaceae) is affected by the form of phosphate added to the soil. Plant and Soil. 2002;238:111–122. [Google Scholar]
- Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ. Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Annals of Botany. 2006;98:693–713. doi: 10.1093/aob/mcl114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Brundrett MC, Raven JA, Hopper SD. Plant mineral nutrition in ancient landscapes: high plant species diversity on infertile soils is linked to functional diversity for nutritional strategies. Plant and Soil. 2010;334:11–31. [Google Scholar]
- Lambers H, Bishop JG, Hopper SD, Laliberté E, Zúñiga-Feest A. Phosphorus-mobilisation ecosystem engineering: the roles of cluster roots and carboxylate exudation in young P-limited ecosystems. Annals of Botany. 2012a;110:329–348. doi: 10.1093/aob/mcs130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambers H, Cawthray GR, Giavalisco P, et al. Proteaceae from severely phosphorus-impoverished soils extensively replace phospholipids with galactolipids and sulfolipids during leaf development to achieve a high photosynthetic phosphorus-use efficiency. New Phytologist. 2012b;196:1098–1108. doi: 10.1111/j.1469-8137.2012.04285.x. [DOI] [PubMed] [Google Scholar]
- Lamont BB. ‘Proteoid’ roots in the legume Viminaria juncea. Search. 1972;3:90–91. [Google Scholar]
- Lamont BB. Mechanisms for enhancing nutrient uptake in plants, with particular reference to mediterranean South Africa and Western Australia. Botanical Review. 1982;48:597–689. [Google Scholar]
- McArthur WM. Reference soils of south-western Australia. Department of Agriculture Western Australia: South Perth; 1991. [Google Scholar]
- Motomizu S, Wakimoto T, Toei K. Spectrophotometric determination of phosphate in river waters with molybdate and malachite green. Analyst. 1983;108:361–367. doi: 10.1016/0039-9140(84)80269-6. [DOI] [PubMed] [Google Scholar]
- Ostertag R. Foliar nitrogen and phosphorus accumulation responses after fertilization: an example from nutrient-limited Hawaiian forests. Plant and Soil. 2010;334:85–98. [Google Scholar]
- Ozanne PG, Specht RL. Mineral nutrition of heathlands: phosphorus toxicity. In: Specht RL, editor. Ecosystems of the world. Amsterdam: Elsevier Scientific; 1981. pp. 209–213. [Google Scholar]
- Parks SE, Haigh AM, Cresswell GC. Stem tissue phosphorus as an index of the phosphorus status of Banksia ericifolia L. f. Plant and Soil. 2000;227:59–65. [Google Scholar]
- Pate JS, Verboom WH, Galloway PD. Cooccurrence of Proteaceae, laterite and related oligotrophic soils: coincidental associations or causative inter-relationships? Australian Journal of Botany. 2001;49:529–560. [Google Scholar]
- Pei L, Wang J, Li K, et al. Overexpression of Thellungiella halophila H+-pyrophosphatase gene improves low phosphate tolerance in maize. PLoS ONE, 2012;7:pe43501. doi: 10.1371/journal.pone.0043501. http://dx.doi.org/10.1371/journal.pone.0043501 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SC, Townsend AR, Davidson EA, Cleveland CC. Stoichiometric patterns in foliar nutrient resorption across multiple scales. New Phytologist, 2012;196:173–180. doi: 10.1111/j.1469-8137.2012.04249.x. [DOI] [PubMed] [Google Scholar]
- Reich PB, Ellsworth DS, Uhl C. Leaf carbon and nutrient assimilation and conservation in species of differing successional status in an oligotrophic Amazonian forest. Functional Ecology, 1995;9:65–76. [Google Scholar]
- Shane MW, Lambers H. Cluster roots: a curiosity in context. Plant and Soil. 2005;274:101–125. [Google Scholar]
- Shane MW, Lambers H. Systemic suppression of cluster-root formation and net P-uptake rates in Grevillea crithmifolia at elevated P supply: a proteacean with resistance for developing symptoms of ‘P toxicity. Journal of Experimental Botany. 2006;57:413–423. doi: 10.1093/jxb/erj004. [DOI] [PubMed] [Google Scholar]
- Shane MW, McCully ME, Lambers H. Tissue and cellular phosphorus storage during development of phosphorus toxicity in Hakea prostrata (Proteaceae) Journal of Experimental Botany. 2004a;55:1033–1044. doi: 10.1093/jxb/erh111. [DOI] [PubMed] [Google Scholar]
- Shane MW, Szota C, Lambers H. A root trait accounting for the extreme phosphorus sensitivity of Hakea prostrata (Proteaceae) Plant, Cell and Environment. 2004b;27:991–1004. [Google Scholar]
- Shane MW, Cramer MD, Lambers H. Root of edaphically controlled Proteaceae turnover on the Agulhas Plain, South Africa: phosphate uptake regulation and growth. Plant, Cell and Environment. 2008;31:1825–1833. doi: 10.1111/j.1365-3040.2008.01889.x. [DOI] [PubMed] [Google Scholar]
- Silberbush M, Barber SA. Sensitivity of simulated phosphorus uptake to parameters used by a mechanistic–mathematical model. Plant and Soil. 1983;74:93–100. [Google Scholar]
- Singh B, Gilkes RJ. Phosphorus sorption in relation to soil properties for the major soil types of south-western Australia. Australian Journal of Soil Research. 1991;29:603–618. [Google Scholar]
- Specht R. Dark Island heath (Ninety-mile plain, South Australia). VII. The effect of fertilizers on composition and growth, 1950–60. Australian Journal of Botany. 1963;11:67–94. [Google Scholar]
- Topa MA, Sisak CL. Characterization of phosphorus uptake in slow- and fast-growing southern pine seedlings grown in solution culture. Plant and Soil. 1997;190:317–329. [Google Scholar]
- Veneklaas EJ, Lambers H, Bragg J, et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytologist. 2012;195:306–320. doi: 10.1111/j.1469-8137.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- Vergutz L, Manzoni S, Porporato A, Novais RF, Jackson RB. Global resorption efficiencies and concentrations of carbon and nutrients in leaves of terrestrial plants. Ecological Monographs. 2012;82:205–220. [Google Scholar]
- Wright IJ, Reich PB, Westoby M, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- Wright IJ, Westoby M. Nutrient concentration, resorption and lifespan: leaf traits of Australian sclerophyll species. Functional Ecology. 2003;17:10–19. [Google Scholar]