Abstract
Seborrheic dermatitis is a multifactorial skin disease characterized by a chronic course with periods of exacerbation and remission. Although topical corticosteroids have been the mainstay of treatment, alternative therapies are often needed to avoid protracted use of topical corticosteroid therapy in order to avert side effects and to sustain control of the disorder. Topical pimecrolimus, a calcinuerin inhibitor, is a safe alternative for seborrheic dermatitis and is more ideal for long-term use. More specifically, topical pimecrolimus not only has an attractive safety profile with no risk of many of the potential side effects seen with topical corticosteroids, but also has favorable efficacy data, including more data on long-term use. This is a review of literature evaluating the efficacy and safety profile of topical pimecrolimus 1% cream for the treatment of seborrheic dermatitis.
Seborrheic dermatitis (SD) is a common, mild, chronic dermatitis confined to areas with high sebum production affecting 1 to 3 percent of the adult population.1–3 It is characterized by mild (pink) erythema with well-developed, fine, white-silver scales affecting common sites of predilection, such as the scalp, periauricular area, glabellar region, eyebrows, and the paranasal region. Other sites that are sometimes affected include the mid chest, genitalia, and groin folds. SD is reported to be more prevalent in males than in females, although both genders are commonly affected, and its incidence peaks in infants, adolescents, and adults over the age of 50 years.3 There is also a higher rate of occurrence in immunocompromised patients ranging from 30 to 83 percent in human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) patients and 18 to 50 percent in individuals with Parkinson’s disease.1,3 Historically, treatment of SD has relied heavily on keratolytics, antifungals, and corticosteroids.3 Topical antifungals (i.e., imidazoles, ciclopirox) have been used effectively to treat SD by decreasing the colonization of Malassezia species as the proliferation of this commensal organism has been shown in some reports to correlate with exacerbation of SD.3 The Malassezia spp that have been most commonly associated with SD are M. globosa and M. restricta, both of which are commensal yeasts that require an exogenous source of lipids.1–3 From a clinical perspective, however, the antifungal agents exhibit a relatively slow onset of anti-inflammatory effect when treating a flare of SD as compared to a topical corticosteroid (TC).4,5 TCs, including mid-potency and low-potency agents, rapidly clear SD in most cases due to their broad range anti-inflammatory effects; however, side effects with prolonged application and the common finding of early relapse after their discontinuation limit the usage of TCs for SD primarily to short courses for treatment of flares.5 Although approved by the United States Food and Drug Administration (FDA) for the treatment of atopic dermatitis in affected patients two years of age or older, topical calcineurin inhibitors (i.e., pimecrolimus, tacrolimus) have become more popular as an alternative therapy for chronic inflammatory skin disorders, such as SD, due to their efficacy and favorable safety profiles. Pimiecrolimus has been evaluated more extensively as the cream formulation is more acceptable for facial application than the ointment vehicle that is used with topical tacrolimus.
IMMUNOLOGICAL FACTORS IN SEBORRHEIC DERMATITIS
Although the pathogenesis of SD is complex and is not fully understood, immunological evaluations of SD have shown an inadequate or abnormal host immune response to yeast, toxins produced by yeast, or other irritant byproducts of yeast. Studies have shown a marked depression of interleukin (IL)-2 and interferon-gamma and an increase in IL-10 production with lymphocytes in SD patients in response to Malassezia furfur extracts.6 This may reflect a disturbed cellular immune response that may be inherent in some predisposed individuals.6 Other studies using in situ immunohistochemical staining have indicated that SD may be the result of a combination of inflammatory and immunogenic responses of the host to Malassezia furfur.7 Malassezia yeasts induce an inflammatory reaction by production of toxic lipid-soluble mediators that affect neutrophil chemotaxis and induce an inflammatory response in the host referred to as lipid-like leukocytes (LILA).8 LILA induces an inflammatory response and produces irritant fatty acids and arachidonic acid, which appear to contribute to the inflammatory response seen in SD.9
M. globosa and M. restricta have been reported to degrade lipids in sebum with production of free fatty acids and triglycerides, followed by consumption of certain saturated fatty acids.2,3 The remaining modified unsaturated short-chain fatty acids are believed to penetrate into skin more easily and subsequently can induce an inflammatory response.3 It has also been suggested than an aberrant host immune process or response occurs in those with SD. The increase in natural killer cells (NK1+) and CD16+ cells, increase in inflammatory interleukins, and activation of complement in lesional SD skin as compared to nonlesional skin in patients with SD and healthy control human skin suggests an augmented inflammatory response in individuals with SD.2,3
BACKGROUND ON PIMECROLIMUS
Pimecrolimus is a topical immunomodulatory agent FDA-approved for the treatment of atopic dermatitis, but has also been used to treat other inflammatory skin diseases (i.e., SD, inverse psoriasis, genital lichen planus). It is a semisynthetic derivative of ascomycin, a macrolactam that was isolated from Streptomyces hygroscopivus var ascomyceticus. It belongs to a family of calcineurin inhibitors that affect T-cell activation, which is a critical step in the cascades inflammation involved in many skin disorders. There is also evidence to suggest that pimecrolimus has direct effects on the release of exogenous IL-2.10 The second major effect of pimecrolimus is the inhibition of pro-inflammatory mediators released from mast cells, such as histamine, serotonin, and B-hexosaminidase.11,12 Because of these anti-inflammatory properties, topical pimecrolimus has been used with varying degrees of success for many inflammatory dermatosis and was developed specifically to treat inflammatory skin conditions, such as atopic dermatitis.10 Both of the available topical calcineurin inhibitors are important to dermatologists because they provide nonsteroidal alternatives that are reasonably effective for the treatment of atopic dermatitis, SD, inverse psoriasis, and mucosal lichen planus.
EFFICACY OF TOPICAL PIMECROLIMUS IN SEBORRHEIC DERMATITIS
The immunomodulatory and anti-inflammatory effects of topical pimecrolimus, its availability in a cream vehicle, its favorable skin tolerability, and its overall safety profiles make this an ideal agent for the treatment of SD. There have been many studies that have evaluated the efficacy of topical pimecrolimus 1% for the treatment of SD. A randomized, double-blind, vehicle-controlled study was completed in patients with moderate-to-severe facial SD (N=96) treated with topical pimecrolimus 1% cream twice daily for four weeks.13 Significant mean changes from baseline in total area score at Week 2 (P<0.01) and at Week 4 (P<0.05) were reported.13 There were no adverse events that warranted the need to discontinue therapy with topical pimecrolimus. In another randomized, investigator-blinded trial of patients with facial SD (N=40), 83 percent of patients achieved complete clearance after two weeks of twice-daily application of pimecrolimus 1% cream.14
Use of topical pimecrolimus has also been reported for treatment of SD in HIV-affected patients. Although topical pimecrolimus is an immunomodulatory drug, it does not appear to alter the immune status of HIV/AIDS patients, likely due to limited systemic absorption after topical application, especially with limited application to SD on the facial area. A single-center, open-label study assessed outcomes with the use of pimecrolimus 1% cream in HIVpositive patients with mild-to-severe facial SD.1 Investigators noted marked improvement in erythematous scaling by Day 7 of treatment with more than 90 percent of participants clearing within two weeks.1 All patients responded to therapy with no apparent systemic effects observed.
COMPARISON OF TOPICAL PIMECROLIMUS WITH OTHER TOPICAL AGENTS USED FOR TREATMENT OF SEBORRHEIC DERMATITIS
Pimecrolimus has been shown in studies to be just as efficacious as TCs and also effective in refractory cases of SD, although the onset of clinical efficacy is slower than with TCs. Published data, although limited, suggest that final outcomes with the use of topical pimecrolimus are comparable to those achieved with TCs.15–17 One open-label study compared the efficacy of pimecrolimus 1% cream twice daily (n=11) with betamethasone valerate 0.1% cream twice daily (n=9) in patients with SD (N=20).15 All patients in the trial were instructed to discontinue further application of their study medication as soon as they noted the absence of signs and symptoms of SD at the sites that were being treated in the study. In the pimecrolimus study group, improvements in erythema, scaling, and pruritus were noted within nine days.15 Although those patients treated with betamethasone valerate 0.1% cream showed improvement in erythema and scaling quicker than patients treated with pimecrolimus 1% cream, there was no statistical difference between the groups found at Day 9 (p>0.05). However, the number of patients in the study was small, thus making the statistical analysis potentially less relevant to the clinical setting without further study in more patients.15 An interesting observation noted by the investigators was that erythema, scaling, and pruritus recurred more commonly at Day 15 in the betamethasone valerate study group compared with what was observed in the pimecrolimus study group, the latter experiencing signs of relapse (erythema, scaling), but without reemergence of pruritus at Day 21.15 The absence of pruritus in pimecrolimus-treated patients may suggest a possible advantage of a longer duration of symptom suppression with this agent; however, more data are needed before this can be definitively concluded.15
Pimecrolimus 1% cream has also been used successfully in case reports of patients with SD who were refractory to both topical corticosteroids and ketoconazole. In a case report collection inclusive of two patients with SD who were refractory to TC therapy (hydrocortisone acetate and desonide), pimecrolimus 1% cream achieved clearance after six weeks and 14 weeks, respectively.16 Another case report demonstrated complete clearance of SD within 28 days with use of topical pimecrolimus in an 18-year-old patient who failed prior therapy with topical ketoconazole and hydrocortisone.17
SIDE EFFECT PROFILE OF TOPICAL PIMECROLIMUS
Unlike TCs, which exert a broad range of anti-inflammatory and inhibitory effects on a wide variety of cell types, pimecrolimus exhibits T-lymphocyte (T-cell) selectivity through calcineurin inhibition. This results in inhibition of T-cell activation, which causes downregulation of the release of proinflammatory cytokines without alteration of fibroblast activity or vasculature proliferation.11–13 Therefore, application of topical pimecrolimus does not cause dermal atrophy or telangiectasia formation. With regard to more prolonged durations of therapy, topical application of calcineurin inhibitors, such as pimecrolimus, exhibit a more favorable safety profile compared to TC application.18 Clinical studies in adults and children with atopic dermatitis have demonstrated a very good safety profile.19,20 Skin atrophy was not observed in patients treated with pimecrolimus 1% cream for up to 26 weeks.20 In another study designed to quantify cutaneous atrophy before and after application of different topical agents, healthy subjects (N=16) applied pimecrolimus 1% cream, betamethsone valerate 0.1% cream, triamcinolone 0.1% cream, and vehicle cream (control) to designated sites on the volar forearm twice daily six days a week for four weeks.18 Topical pimecrolimus did not induce atrophy after four weeks as compared to the TC agents. In this study, skin thickness was determined by ultrasound examination, clinical signs of atrophy were assessed by stereomicroscopy, and epidermal thickness was evaluated by histological examination. Both TCs caused marked reductions in skin thickness as compared with pimecrolimus 1% cream and vehicle, with the latter two being equivalent. The differences in skin thickness as measured by ultrasound examination between the TC-treated sites and sites treated with topical pimecrolimus were significant from Day 8 onward. Histological analysis performed at Day 29 showed marked epidermal thinning with both TCs compared with pimecrolimus 1% cream and with the vehicle.18
Overall, the most common adverse effect reported with use of pimecrolimus 1% cream has been transient application site irritation in some patients, although most note no problems with application to disease-affected skin.7 In clinical trials of patients with SD treated with pimecrolimus 1% cream, 10 to 37 percent of patients complained of mild burning on initiation of treatment, which almost always subsided within the first 24 to 72 hours of therapy and was not often problematic for the affected patients.15,21,22 Application-site reactions are not a common cause for discontinuation of use of pimecrolimus 1% cream; however, the importance of educating patients with inflamed “dermatitic” skin on proper gentle skin care is vital as use of harsh skin care products can impair the epidermal permeability barrier and increase the likelihood of irritation with use of other topical products. Nevertheless, patients with inflamed and/or eczematous skin are overall more prone to application-site reactions to topical products in general so proper skin care education and product selection cannot be overemphasized. One study found a statistically significant higher rate of burning in the pimecrolimus group compared with the hydrocortiosone group (p<0.05).14 Another side effect that occurs less commonly with the use of topical calcineurin inhibitors, such as pimecrolimus, is facial flushing after alcohol ingestion, which has been treated with prostaglandin inhibitors.23 There have also been sporadic reports of rosaceiform dermatitis shortly after initiation with both topical pimecrolimus and tacrolimus that usually resolves with cessation of the drug and treatment with an oral tetracycline agent, with minocycline used in one report.23 Although the cause of the rosaceiform facial eruption is unknown, possible vasoactive properties of calcinuerin inhibitors or overgrowth of follicular Demodex mites have been suggested.24
DOES USE OF PIMECROLIMUS 1% CREAM EXERT CLINICALLY RELEVANT SYSTEMIC IMMUNOSUPPRESSIVE EFFECTS?
Systemic administration of the calcineurin inhibitors cyclosporin and tacrolimus are known to exhibit immunosuppressive effects, which is why these agents are used to suppress rejection of a transplanted organ (e.g., kidney) in transplant recipients. One concern has been the extent of systemic absorption with application of a topical calcineurin inhibitor. With pimecrolimus 1% cream, low blood concentrations (<0.08ng/mL), below the level of detection in 98 percent of blood level evaluations (900/918), were found in adults with moderate-to-severe atopic dermatitis (N=40) who were applying topical pimecrolimus over up to 20 percent of body surface area (BSA).25 Patients applied pimecrolimus 1% cream twice daily as needed to active eczematous areas over a one-year period.25 Even in patients with extensive atopic dermatitis (BSA<69%) and in children treated for atopic dermatitis, there has been a conspicuous absence of untoward reports with elevated serum concentrations of pimecrolimus.25–28 To add, in vitro analysis using static Franz-type diffusion cells found that while both tacrolimus and pimecrolimus penetrate the epidermis well, they appear to sequester more in the dermis rather than permeating in large quantities into the systemic circulation.29 Another long-term study with use of topical calcineurin inhibitors in patients with atopic dermatitis showed no increase in infection rates and serious adverse events compared to control subjects.30 Additionally, studies with HIV-positive patients using topical calcineurin inhibitors demonstrated no increase in viral load or adverse effects on CD4+ and CD8+ T-cell counts after two weeks of treatment.1
IS THERE A CORRELATION BETWEEN USE OF TOPICAL PIMECROLIMUS AND DEVELOPMENT OF MALIGNANCY?
Data from animal studies documenting cases of malignancy with exposure to calcinuerin inhibitors in very high concentrations has been the basis of concern for potential carcinogenicity in humans associated with topical application of tacrolimus and/or pimecrolimus. In 2005, the FDA placed a cautionary black box warning on the basis of toxicology studies in animals, including cynomolgus monkeys given oral pimecrolimus for 39 consecutive weeks.31 In this study, oral pimecrolimus resulted in immunosuppression-related lymphoproliferative disorders associated with lymphocryptovirus.31 However, this would be analogous to topical treatment in humans exceeding 30 times the maximal recommended dose over an extensive BSA over 9 to 10 months.32,33 The black box warning was based on speculative inference, and to date over the ensuing seven years there has been no correlation between use of topical calcineurin therapy and development of malignancy in children or adults.
Toxicity data have demonstrated a high safety margin for topical use of both topical calcineurin inhibitors.3,33,34 To add, the American Academy of Dermatology Association Task Force has stated, “there is no causal proof that topical calcineurin inhibitors cause lymphoma or non-melanoma skin cancer,” and systemic immunosuppression after short-term or intermittent long-term topical application is an unlikely operative mechanism.34
INTEGRATION OF TOPICAL PIMECROLIMUS INTO THE MANAGEMENT OF SEBORRHEIC DERMATITIS OF INVOLVING GLABROUS SKIN (FACE, OTHER SITES)
Although SD commonly affects hair-bearing skin, glabrous skin involvement is common on many regions of the face, including the paranasal region and nasal alar folds (Figure 1), inner cheeks, the auricular region (Figure 2), the eyebrows and glabella (central lower forehead) region (Figure 3), anterior hairline (upper forehead), and the marionette lines extending inferiorly from the oral commissures, with the creases of the latter more pronounced in some older individuals.3 TCs formulated in solutions, suspensions, aqueous-based gels or foams (that easily liquefy on application), and sprays may be used for treatment of SD involving hair-bearing scalp.3 Shampoos can also be utilized on the scalp as well as the trunk. Available shampoos include those containing an antifungal agent (e.g., ketoconazole, ciclopirox, zinc pyrithione), which decreases Malassezia spp and may have mild antiinflammatory activity; a desmolytic agent (e.g., salicylic acid) to remove scaling and promote physiological desquamation; or the nonsteroidal topical device shampoo formulation (piroctone olamine biocide, multiple antioxidants, emollients/conditioners), which has been shown to exhibit anti-inflammatory and antifungal properties against Malassezia spp with a cream formulation of the same topical device shown to be effective for facial SD.3,35
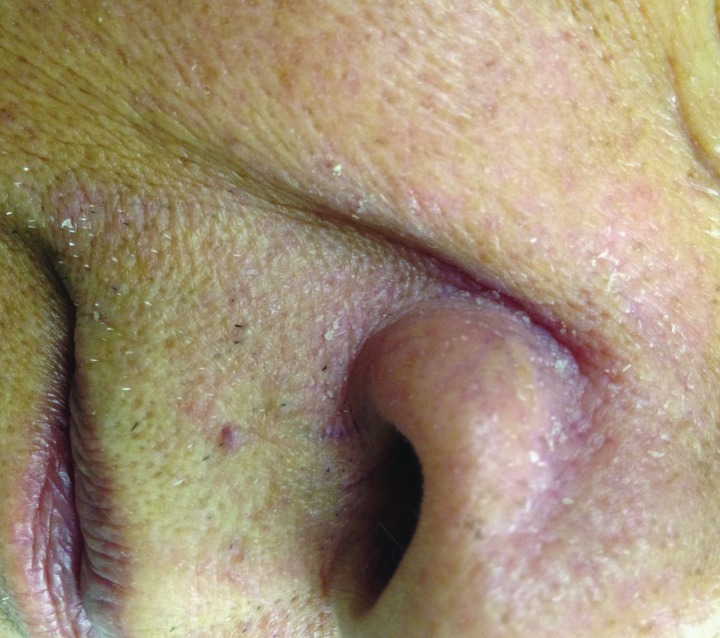
Figure 1.
Seborrheic dermatitis involving the nasal alar region, paranasal area, and inner cheeks
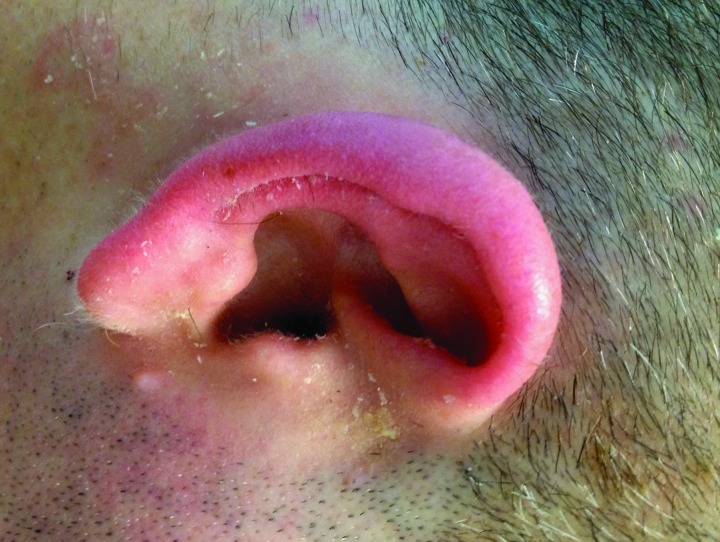
Figure 2.
Auricular and periauricular involvement with seborrheic dermatitis
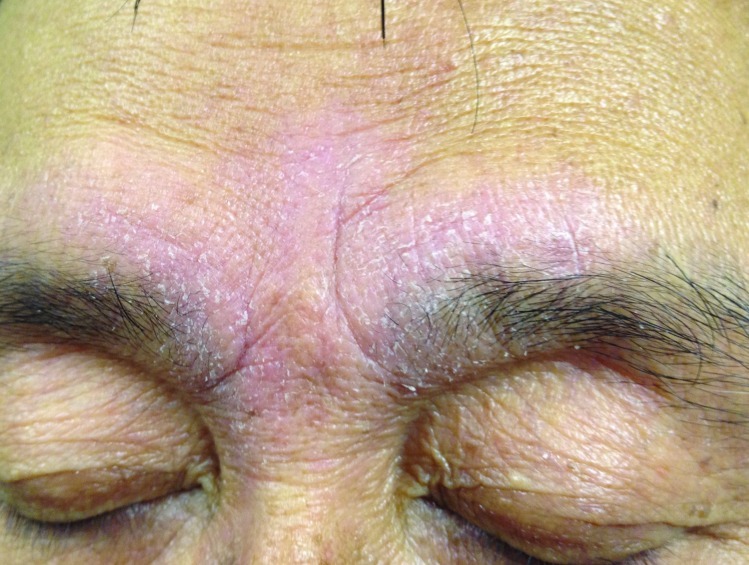
Figure 3.
Marked confluent involvement of the eyebrows and central lower forehead (glabella) region with seborrheic dermatitis
For SD of the head (including bald scalp), neck, chest, axilla, and genital regions, a TC may be used to rapidly control a flare of SD; however, it should be used for a short course (i.e., 1–2 weeks) to avoid adverse reactions. Relapse of SD is to be expected and may be more common after use of even a low-potency TC for SD.3,15,35 In order to sustain control of SD without exacerbation, especially in patients prone to frequent flares and/or persistence of SD (often low-grade but consistently visible with erythema and scaling), selection of a nonsteroidal therapy is rational as treatment can be continued beyond short term with negligible risk of any significant adverse effects. Nonsteroidal leave-on options include a topical antifungal agent (e.g., ketoconazole, ciclopirox), a topical calcineurin inhibitor (pimecrolimus 1% cream, tacrolimus 0.1% ointment), or the nonsteroidal topical device cream. Among these choices, although all may be effective in many cases, topical pimecrolimus is well-suited to control flares of lower intensity and for long-term use to maintain control of SD based on the available efficacy and tolerability data with the cream formulation adaptable for use on the facial and intertriginous areas. Figures 4 and 5 depict how pimecrolimus 1% cream may be integrated into the management of SD involving facial skin and other affected anatomic sites where little to know terminal hair is present. Studies are not available to address all choices and frequencies of application with different agents used for SD, especially with intermittent use, which is based on the proactive therapy concept used with topical fluticasone or topical tacrolimus, applied twice weekly for long-term management of atopic dermatitis after the flare is controlled.36
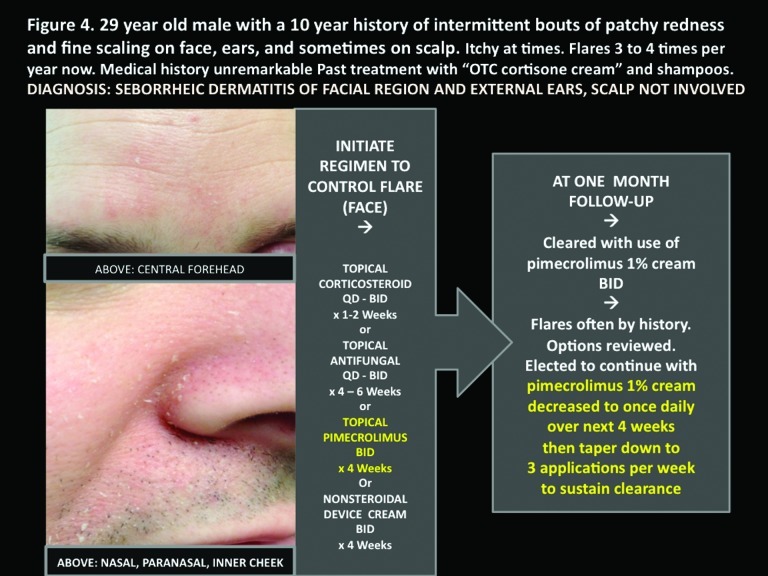
Figure 4.
Pictured here is a 29-year-old man with a 10-year history of intermittent bouts of patchy redness and fine scaling on face, ears, and sometimes scalp that is itchy at times and flares 3 to 4 times per year now. The patient’s medical history was unremarkable, and past treatment was with over-the-counter cortisone cream and shampoos. The patient’s diagnosis: seborrheic dermatitis of facial region and external ears; scalp not involved.
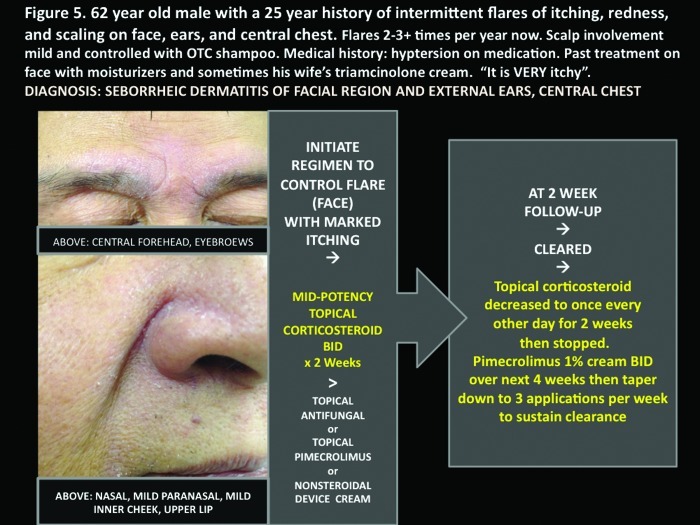
Figure 5.
A 62-year-old man with a 25-year history of intermittent flares of itching, redness, and scaling on face, ears, and central chest. Flares occur 2 to 3 times per year now. Scalp involvement is mild and controlled with over-the-counter shampoo. The patient’s medical history included hyptersion for which the patient was taking medication. The patient’s past treatment on face included moisturizers and sometimes his wife’s triamcinolone cream, which he said was “very itchy.” The patient’s diagnosis: seborrheic dermatitis of facial region, external ears, and central chest.
CONCLUSION
Topical corticosteroids and antifungal medications have been the mainstay of SD treatment. However, topical calcineurin inhibitors are becoming a more favorable option as a safe and effective alternative, especially when a nonsteroidal agent is deemed necessary by the clinician. In cases with SD refractory to other therapies, such as TCs and antifungal agents, pimecrolimus 1% cream may often be a rational choice to initiate. Studies have revealed that topical calcineurin inhibitors are comparable in efficacy to a TC provided an adequate duration of therapy is completed (i.e., 2–4 weeks as a guide), without the risk of atrophy, telangiectasia formation, and TC-associated perioral dermatitis. Although studies have shown that both topical pimecrolimus and tacrolimus can incur an overall higher drug cost, they may be associated with fewer secondary drug costs compared to TCs.37 Available studies including long-term data support the use of a topical calcineurin inhibitor for disease control and for use in SD recalcitrant to other topical therapies. Pimecrolimus 1% cream is favored over topical tacrolimus in most cases due to the cream formulation being more adaptable for facial use and twice-daily application and more data available in the literature. As SD is a very common chronic, recurrent inflammatory skin disorder that often starts early in life and afflicts individuals over a course of many years, clinicians need an effective and safe long-term therapeutic option, such as topical pimecrolimus, for their patients. Importantly, many possible nonsteroidal options are available, and the clinician is encouraged to gain experience with many if not all of them in order to acquire their own “working feel” for how these nonsteroidal agents may be optimally utilized for treatment of SD in their patients. In addition, it is important not to forget the incorporation of proper adjunctive skin care including a gentle cleanser and well-formulated moisturizer/barrier repair cream as an integral component of the therapeutic regimen.
Footnotes
DISCLOSURE: Dr. Kim reports no relevant conflicts of interest. Dr. Del Rosso has served as a consultant, speaker, and/or researcher for Allergan, Bayer HealthCare Pharmaceuticals, Dermira, Galderma, Medicis, Onset Dermatologies, Obagi Medical Products, Pharmaderm, Primus, Promius, Ranbaxy, Taro Pharma, TriaBeauty, Unilever, Valeant, and Warner-Chilcott. Specifically regarding topical pimecrolimus, Dr. Del Rosso has occasionally served as a consultant, advisory board member, and/or speaker related to pimecrolimus 1% cream and currently serves as a consultant with Valeant (the current owner of Elidel, brand of pimecrolimus 1 % cream) and many other companies that market products for the treatment of seborrheic dermatitis and also skin care (all listed above). Neither of the authors received any form of compensation for developing, writing, or submitting this article.
REFERENCES
- 1.de Moraeas AP, Arruda E, Vitoriano MAV, et al. An open-label efficacy pilot study with pimecrolimus cream 1% in adults with facial seborrhoeic dermatitis infected with HIV. J Eur Acad Dermatol Venereol. 2007;21(5):596–601. doi: 10.1111/j.1468-3083.2006.01923.x. [DOI] [PubMed] [Google Scholar]
- 2.Faergemann J, Bergbrant IM, Dohse M. Seborrhoeic dermatitis and Pityrosporum (Malassezia) folliculitis: characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br J Dermatol. 2001;144:549–556. doi: 10.1046/j.1365-2133.2001.04082.x. [DOI] [PubMed] [Google Scholar]
- 3.Del Rosso JQ. Adult seborrheic dermatitis: a status report on practical topical management. J Clin Aesthet Dermatol. 2011;4(5):32–38. [PMC free article] [PubMed] [Google Scholar]
- 4.Pierard-Franchimont C, Pierard GE. A double-blind placebo-controlled study of ketoconazole + desonide gel combination in the treatment of facial seborrheic dermatitis. Dermatology. 2002;204:344–347. doi: 10.1159/000063382. [DOI] [PubMed] [Google Scholar]
- 5.Faergemann J. Management of seborrheic dermatitis and pityriasis versicolor. Am J Clin Dermatol. 2000;1:75–80. doi: 10.2165/00128071-200001020-00001. [DOI] [PubMed] [Google Scholar]
- 6.Neuber K, Kroger S, Gruseck E, et al. Effects of Pityrosproum ovale on proliferation, immunoglobulin (IgA, G, M) synthesis and cytokine (IL-2,IL-10, IFN gamma) production of peripheral blood mononuclear cells from patients with seborrheic dermatitis. Arch Dermatol Res. 1996;288:532–536. [PubMed] [Google Scholar]
- 7.Cook BA, Warshaw EM. Role of topical calcineurin inhibitors in the treatment of seborrheic dermatitis. Am J Clin Dermatol. 2009;10(2):103–118. doi: 10.2165/00128071-200910020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Kahlke B, Brasch J, Christophers E, et al. Dermatophytes contain a novel lipid-like leukocytes activator. J Invest Dermatol. 1996;107(1):108–112. doi: 10.1111/1523-1747.ep12298332. [DOI] [PubMed] [Google Scholar]
- 9.Mayser P, Haze P, Papvassilis C, et al. Differentiation of Malassezia species selectivity of Cremophor EL castor oil and ricinoleic acid for. M. furfur. Brit Dermatol. 1997;137(2):208–213. doi: 10.1046/j.1365-2133.1997.18071890.x. [DOI] [PubMed] [Google Scholar]
- 10.Grassberger M, Baumrauker T, Enz A, et al. A novel antiinflammatory drug, SDZ ASM 981, for the treatment of skin diseases; in vitro pharmacology. Br J Dermatol. 1999;141(2):264–273. doi: 10.1046/j.1365-2133.1999.02974.x. [DOI] [PubMed] [Google Scholar]
- 11.Graham-Brown RA, Grassberger M. Pimecrolimus: a review of pre-clinical and clinical data. Int J Clin Pract. 2003;57(4):319–327. [PubMed] [Google Scholar]
- 12.Grassberger M, Steinhoff M, Schneider D, et al. Pimecrolimus: an anti-inflammatory drug targeting the skin. Exp Dermatol. 2004;13(12):721–730. doi: 10.1111/j.0906-6705.2004.00269.x. [DOI] [PubMed] [Google Scholar]
- 13.Warshaw EM, Wohlhuter RJ, Liu A, et al. Results of a randomized, double blind, vehicle-controlled efficacy trial of pimecrolimus cream 1% for the treatment of moderate to severe facial seborrheic dermatitis. J Am Acad Dermatol. 2007;57(2):257–264. doi: 10.1016/j.jaad.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Firooz A, Solpour A, Gorouhi F, et al. Pimecrolimus cream 1% vs. hydrocortisone acetate cream 1%, in the treatment of facial seborrheic dermatitis: a randomized open-label clinical trial. Br J Dermatol. 2004;151:1071–1075. [Google Scholar]
- 15.Rigopoulos D, Ioannides D, Kalogeromitros D, et al. Pimecrolimus cream 1% vs. betamethasone 17-valerate 0.1% cream in the treatment of seborrhoeic dermatitis: a randomized open-label clinical trial. Br J Dermatol. 2004;151:1071–1075. doi: 10.1111/j.1365-2133.2004.06208.x. [DOI] [PubMed] [Google Scholar]
- 16.Cunha PR. Pimecrolimus cream 1% is effective in seobrrhoeic dermatitis refractory to treatment with topical coricosteroids. Acta Derm Venereol. 2006;86(1):69–70. doi: 10.1080/00015550510040040. [DOI] [PubMed] [Google Scholar]
- 17.Brownell I, Quan LT, Hsu S. Topical pimecrolimus in the treatment of seborrheic dermatitis. Dermatol Online J. 2003;9(3):13. [PubMed] [Google Scholar]
- 18.Queille-Roussel C, Paul C, Duteil L, et al. The new topical ascomycin derivative SDZ ASM 981 does not induce skin atrophy when applied to normal skin for 4 weeks: a randomized, doubleblind controlled study. Br J Dermatol. 2001;144:507–513. doi: 10.1046/j.1365-2133.2001.04076.x. [DOI] [PubMed] [Google Scholar]
- 19.Meurer M, Folster-Holst R, Wozel G, et al. CASM-DE-01 study group. Pimecrolimus cream in the long-term management of atopic dermatitis in adults: a six-month study. Dermatology. 2002;205:217. doi: 10.1159/000065863. [DOI] [PubMed] [Google Scholar]
- 20.Langley RG, Eichenfield LF, Lucky AW, et al. Sustained efficacy and safety of pimecrolimus cream 1% when used long-term (up to 26 weeks) to treat children with atopic dermatitis. Pediatr Dermatol. 2008;25(3):301–307. doi: 10.1111/j.1525-1470.2008.00671.x. [DOI] [PubMed] [Google Scholar]
- 21.Rallis E, Naiopoulou A, Kouskoukis C, et al. Pimecrolimus cream 1% can be an effective treatment for seborrheic dermatitis of the face and trunk. Drugs Exp Clin Res. 2004;30:191–195. [PubMed] [Google Scholar]
- 22.Meshkinpour A, Sun J, Weinstein G. An open pilot study using tacrolimus ointment in the treatment of seborrheic dermatitis J 31. Am Acad Dermatol. 2003;49:145–147. doi: 10.1067/mjd.2003.450. [DOI] [PubMed] [Google Scholar]
- 23.Ehst BD, Warshaw EM. Alcohol-induced application site erythema after topical immunomodulator use and its inhibition by aspirin. Arch Dermatol. 2004;140(8):1014–1015. doi: 10.1001/archderm.140.8.1014. [DOI] [PubMed] [Google Scholar]
- 24.Antille C, Saurat JH, Lubbe J. Induction of raosaceiform dermatitis during treatment of facial inflammatory dermatoses with tacrolimus ointment. Arch Dermatol. 2004;140(4):457–460. doi: 10.1001/archderm.140.4.457. [DOI] [PubMed] [Google Scholar]
- 25.Van Leent Ej, Ebelin ME, Burtin P, et al. Low systemic exposure after repeated topical application of pimecrolimus (Elidel, SDZ ASM 981) in patients with atopic dermatitis. Dermatology. 2002;204(1):63–68. doi: 10.1159/000051813. [DOI] [PubMed] [Google Scholar]
- 26.Staab D, Pariser D, Gottliev AB, et al. Low systemic absorption and good tolerability of pimecrolimus, administered as 1% cream (Elidel) in infants with atopic dermatitis: a mulitcenter, 3 week, open-label study. Pediatr Dermatol. 2005;22(5):465–471. doi: 10.1111/j.1525-1470.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 27.Allen BR, Lakhanpaul M, Morris A, et al. Systemic exposure, tolerability and efficacy of pimecrolimus cream 1% in atopic dermatitis patients. ArchDis Child. 2003;88(11):969–973. doi: 10.1136/adc.88.11.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harper J, Green A, Scoot G, et al. First experience of topical SDZ ASM 981 in children with atopic dermatitis. Br J Dermatol. 2001;144(4):781–787. doi: 10.1046/j.1365-2133.2001.04133.x. [DOI] [PubMed] [Google Scholar]
- 29.Billich A, Aschauer H, Aszodi A, et al. Percutaneous absorption of drugs used in atopic eczema: pimecrolimus permeates less through skin than corticosteroids and tacrolimus. Int J Pharm. 2004;269(1):29–35. doi: 10.1016/j.ijpharm.2003.07.013. [DOI] [PubMed] [Google Scholar]
- 30.Wahn U, Bos JD, Goodfield M, et al. Efficacy and safety of pimecrolimus cream in the long term management of atopic dermatitis in children. Pediatrics. 2002 doi: 10.1542/peds.110.1.e2. 110(1Pt1):e2. [DOI] [PubMed] [Google Scholar]
- 31. [November 24, 2008]. http://www.fda.gov/fdac/departs/2006/206_upd.html#eczema FDA. Pharmacokinetics/toxicokinetics: brief summary. 2005:1-18 [online].
- 32.Patel TS, Greer SC, Skinner RB. Cancer concerns with topical immunomodulators in atopic dermatitis: overview of data and recommendations to clinicians. Am J Clin Dermatol. 2007;8(4):189–194. doi: 10.2165/00128071-200708040-00001. [DOI] [PubMed] [Google Scholar]
- 33.Hultsch T, Kapp A, Spergel J. Immunomodulation and safety of topical calcineurin inhibitors for the treatment of atopic dermatitis. Dermatology. 2005;211(2):174–187. doi: 10.1159/000086739. [DOI] [PubMed] [Google Scholar]
- 34.Berger TG, Duvic M, Van Voorhees AS, et al. The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol. 2006;54:818–823. doi: 10.1016/j.jaad.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 35.Elewski B. An investigator-blinded, randomized, 4-week, parallel-group, multicenter pilot study to compare the safety and efficacy of a nonsteroidal cream (Promiseb Topical Cream) and desonide cream 0.05% in the twice-daily treatment of mild to moderate seborrheic dermatitis of the face. Clinics Dermatol. 2009;27:S10–S15. doi: 10.1016/j.clindermatol.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Wollenberg A, Ehmann LM. Long term treatment concepts and proactive therapy for atopic eczema. Ann Dermatol. 2012;24(3):253–260. doi: 10.5021/ad.2012.24.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ellis C, Drake LA, Predergast MM, et al. Cost-effectiveness analysis of tacrolimus ointment versus high-potency topical corticosteroids in adults with moderate to severe atopic dermatitis. J Am Acad Dermatol. 2003;48(4):553–563. doi: 10.1067/mjd.2003.240. [DOI] [PubMed] [Google Scholar]