Abstract
Objective: To evaluate the efficacy of combination cryotherapy and imiquimod 3.75% cream versus cryotherapy alone in the treatment of hypertrophic actinic keratosis on the dorsal hand and forearm. Methods: Twenty subjects with at least three hypertrophic actinic keratoses on each dorsal hand or forearm underwent cryotherapy treatment to hypertrophic actinic keratoses. Following cryotherapy, subjects were randomized to have either their right or left dorsal hand or forearm treated with imiquimod 3.75% cream to begin on the same day as cryotherapy treatment. Subjects then utilized the two weeks on, two weeks off, two weeks on regimen of imiquimod 3.75% cream application. Local skin reactions were also assessed. Results: For the cryotherapy/imiquimod 3.75% arm, the median total hypertrophic actinic keratosis reduction was −5.12 and for the cryotherapy alone arm, the median total hypertrophic actinic keratosis reduction was −2.24 (P<0.0094). Limitations: Local skin reactions unbind the investigator. Conclusion: Cryotherapy plus imiquimod 3.75% cream resulted in a statistically significant improvement in the reduction of hypertrophic actinic keratoses than cryotherapy alone at 14 weeks.
Actinic keratoses (AKs) are common cutaneous lesions associated with chronic ultraviolet radiation exposure.1 Ultraviolet radiation produces local and systemic immunosuppression, mutations in the p53 tumor suppressor gene, and deoxyribonucleic acid pyrimidine covalent dimmers, each of which is believed to contribute to the dysplasia seen in AK.2–4 While most authorities consider AK as a premalignant lesion, gene expression studies and other evidence is accumulating that AKs are part of a spectrum of lesions ranging from sun-damaged skin to squamous cell carcinoma in situ (SCC).2,5–7 The risk for progression to SCC for an individual AK is reportedly low, but highly variable. In one large study, the risk of progression of AK to primary SCC (invasive or in situ) was 0.60 percent at one year and 2.57 percent at four years. Additionally, approximately 65 percent of all primary SCCs and 36 percent of all primary BCCs diagnosed in the study cohort arose in lesions that previously were diagnosed clinically as AKs.7 However, as patients often have multiple AKs, the overall risk for progression over a lifetime can be significant, and thus treatment of AKs is warranted.5,7,8 In addition, the skin around clinically obvious AK lesions has been subject to the same chronic ultraviolet exposure, resulting in genetic damage and mutations, resulting in field cancerization. Subclinical AKs may progress to clinical AKs, or even de novo invasive SCCs.
The current therapies for the treatment of AK include excisional surgery, cryotherapy, electrodessication and curettage, topical chemotherapy, and photodynamic therapy. Though there are no standard guidelines for cryotherapy with liquid nitrogen, liquid nitrogen has been effectively used for decades and several studies have assessed efficacy after complete freezing of actinic keratoses.9 Efficacy can be improved by increasing the freeze time, but this is often associated with greater discomfort, more severe skin necrosis, and increased risk of post-treatment hypopigmentation. In addition, as with other lesion-directed therapies, cryotherapy does not treat subclinical lesions in the surrounding skin. In a study comparing cryotherapy, imiquimod 5%, and 5-fluorouracil (5-FU), sustained clearance at 12 months was 28 percent (7/25) for cryotherapy, 54 percent (16/24) for 5-FU, and 73 percent (19/26) for imiquimod 5%. Sustained clearance of the total treatment field was 33 percent (8/24) for 5-FU and 73 percent (19/26) for imiquimod 5%.10 A significant limitation of this study is a small number of patients treated.
Imiquimod is a topical immune response modifier that activates the innate immune system via Toll-like receptor 7, as well as enhances the acquired immune system. Initially approved in the 5% form, imiquimod demonstrated 84-percent median lesion reductions of AKs after one 12-week treatment course.11 More recently, a newer 3.75% pump formulation was approved for the treatment of AKs on the face or balding scalp. Subjects in the Swanson et al12 trial applied cream daily to the entire face or balding scalp for two, two-week treatment cycles, separated by a two-week “rest period” (“2 weeks on, 2 weeks off, 2 weeks on”). Subjects achieved a median lesion reduction of 82 percent, and more than one-third demonstrated complete clearance.12 Topical imiquimod treatment may also reduce subclinical lesions in the treatment area, resulting in fewer new AK lesions developing over the same period of time when compared to local treatment, such as cryotherapy. In fact, more than 80 percent of subjects in the Swanson et al12 trial demonstrated subclinical lesions. Tan et al13 reported that while application of imiquimod or vehicle following cryotherapy resulted in comparable target, AK clearance rates at 12 weeks of 79 percent versus 76 percent, respectively, the imiquimod group had fewer total AKs and fewer subclinical AKs. One recent randomized, doubleblind, placebo-controlled, multicenter study evaluating cryotherapy followed by 3.75% imiquimod cream also found that a short, cyclical treatment course of field-directed daily 3.75% imiquimod cream following lesion-directed cryotherapy was well tolerated and provided additional therapeutic benefits to cryotherapy alone.14
Treatment of AKs with cryotherapy followed by imiquimod appears to be logical, as cryotherapy has cytodestructive effects with immediate short-term efficacy on treated AKs, while imiquimod has a slower onset. Imiquimod is also able to treat the entire field via an immunological mechanism with good long-term efficacy outcomes and may treat subclinical lesions that might evolve to clinically visible lesions over time. Thus, using imiquimod post cryotherapy should combine the immediate effects of cryotherapy and the long-term benefits of the imiquimod. Use of imiquimod 3.75% daily allows for a shorter imiquimod treatment duration (6 weeks) than the currently approved 16-week (United States) and eight-week (Europe) regimen for imiquimod 5%. Additionally, imiquimod 3.75% has demonstrated safety and efficacy for the treatment of a 200cm2 area (full face or balding scalp) as compared to a patch treatment of 25cm2 for the currently approved 5% imiquimod.
MATERIALS AND METHODS
Men or women older than 18 years of age with a clinical diagnosis of at least three HAKs on each dorsal hand or forearm were enrolled in this single-blind study held in the dermatology department at Mount Sinai School of Medicine from October 2010 to August 2011. All patients provided written consent to participate after receiving detailed information on the purpose and design of the study. The latter was approved by the Institutional Review Board at the Mount Sinai School of Medicine. Eligibility requirements for enrollment included the following: willingness to forego any other treatments on the dorsum of the hands and/or forearms (including tanning bed use and excessive sun exposure). Additionally, women of childbearing potential must have a negative urine pregnancy test result prior to study treatment initiation and must agree to use an approved method of birth control while enrolled in the study. Those with a history of melanoma anywhere on the body, a nonmelanoma skin cancer on the dorsum of the hands or forearms, or any dermatological disease in the treatment area that may be exacerbated by the treatment proposed or that might impair the evaluation of AKs, were excluded. Subjects who had previously been treated with imiquimod on the dorsum of the hands or forearms in the past six months or outside of the study area within the past 30 days were also excluded. Women who were pregnant, lactating, or planning to become pregnant and those who have known allergies to any excipient in the study cream were prevented from study participation. Those who had utilized topical prescription medications in the study area within 30 days prior to study treatment initiation or treatment with interferon or interferon inducers, cytotoxic drugs, immunomodulators or immunosuppressive therapies (inhaled/intranasal steroids were permitted), oral or parenteral corticosteroids, topical corticosteroids if greater than 2g/day, or any dermatological procedures or surgeries on the study area (including any AK treatments) within 90 days prior to study treatment initiation were also excluded.
STUDY DESIGN AND PROTOCOL
The trial included a six-week treatment period with imiquimod 3.75% and an eight-week follow-up period. Each qualifying subject had at least three hypertrophic AKs, defined as more than 3mm in thickness, on each dorsal hand and/or forearm treated with cryotherapy. Cryotherapy was standardized in all patients and for all treated lesions, using two sprays, five seconds each, with a five-second rest interval. Following cryotherapy, subjects were randomized to treat either their right or left dorsal hand or forearm with imiquimod 3.75% cream. Subjects treated AKs on only one forearm/dorsal hand with imiquimod 3.75% cream starting on the same day as the cryotherapy (Day 0). Subjects utilized the two weeks on, two weeks off, two weeks on regimen as directed for imiquimod 3.75% cream use. Subjects were followed every two weeks during treatment (Weeks 2, 4, and 6) and then four and eight weeks post last-imiquimod application (Weeks 10 and 14). At each visit, each subject had a thorough clinical examination, photographs of bilateral treatment areas were taken, and a manual count of each hypertrophic and nonhypertrophic AK was tabulated. Assessments for local skin reactions were conducted (Tables 1 and 2).
TABLE 1.
Local skin reaction scale
| LOCAL SKIN REACTION | SEVERITY DEFINITIONS | |||
|---|---|---|---|---|
| 0 | 1 (MILD) | 2 (MODERATE) | 3 (SEVERE) | |
| Erythema | None | Faint to mild redness | Moderate redness | Intense redness |
| Edema | None | Mild visible or barely palpable swelling/induration | Easily palpable swelling/induration | Gross swelling/induration |
| Weeping/exudate | None | Minimal exudate | Moderate exudate | Heavy exudate |
| Flaking/scaling/dryness | None | Mild dryness/flaking | Moderate dryness/flaking | Severe dryness/flaking |
| Scabbing/crusting | None | Crusting | Serous scab | Eschar |
TABLE 2.
Local skin reaction scale (erosion/ulceration)
| LOCAL SKIN REACTION | SEVERITY DEFINITIONS | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Erosion/ulceration | None | Erosion | Ulceration |
EFFICACY AND SAFETY EVALUATION
Primary efficacy endpoints included percent change from baseline in total HAK and non-HAK lesion count at end of study. All AK lesions on the forearm/dorsal hand were included in the analysis, including treated and untreated AK lesions at baseline (defined as AK lesion count just prior to cryotherapy) and new lesions that appear after baseline.
STATISTICAL ANALYSIS
Differences in AK counts between different time points were evaluated by the analysis of variance (ANOVA) test. The significance level was P<0.05. Data are expressed as mean (standard deviation [SD]).
RESULTS
Twenty subjects were enrolled to participate in this study. One subject was terminated early, and two subjects were lost to follow up. Of the remaining 17 subjects, the forearm was the target location for 14 subjects and the dorsal hand was targeted in the remaining three. The average age of the subjects enrolled was 73.4. All 20 subjects were Caucasian. There were 17 men and three women. The average number of HAKs on the right arm was 6.0 and 5.8 on the left arm. The average number of non-HAKs on the right arm was 7.1 and 8.1 on the left arm.
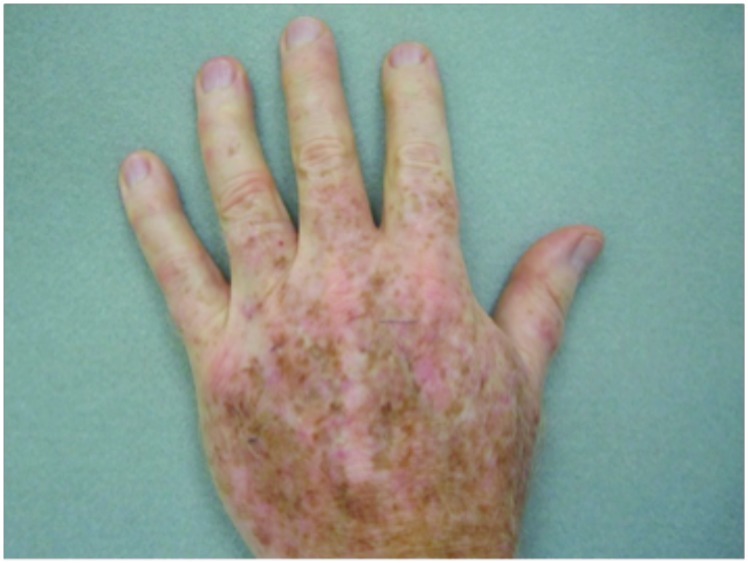
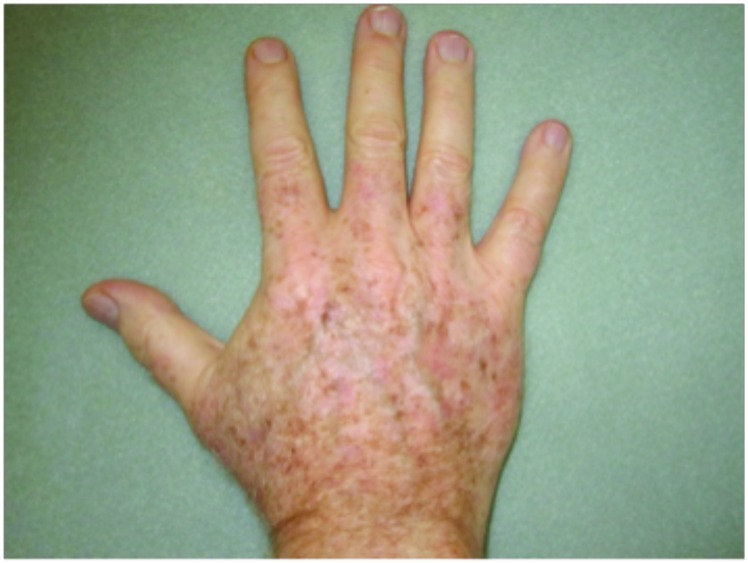
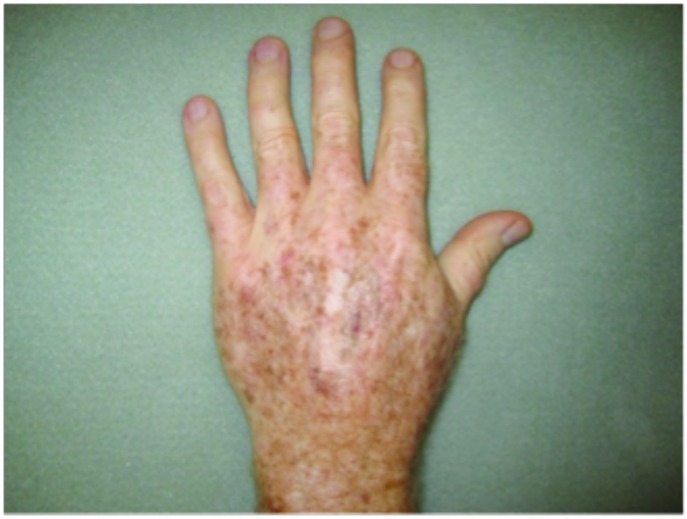
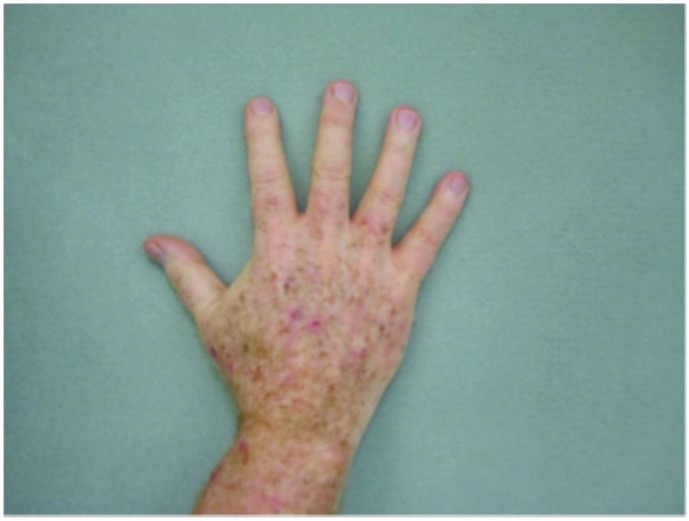
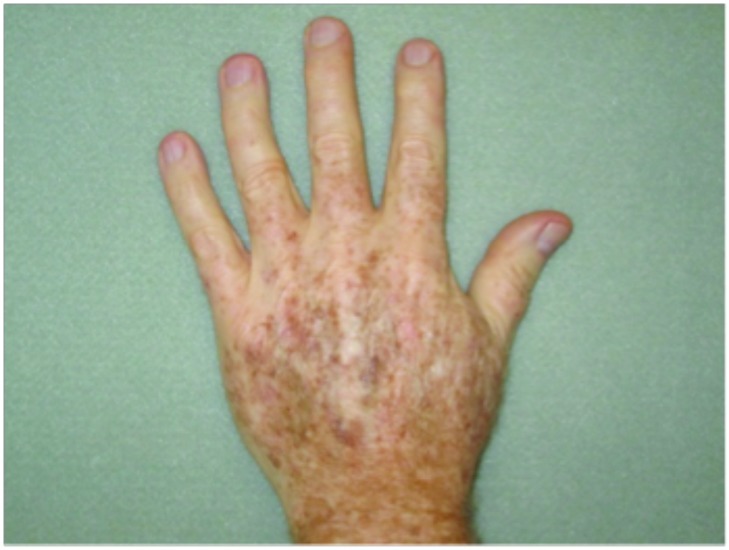
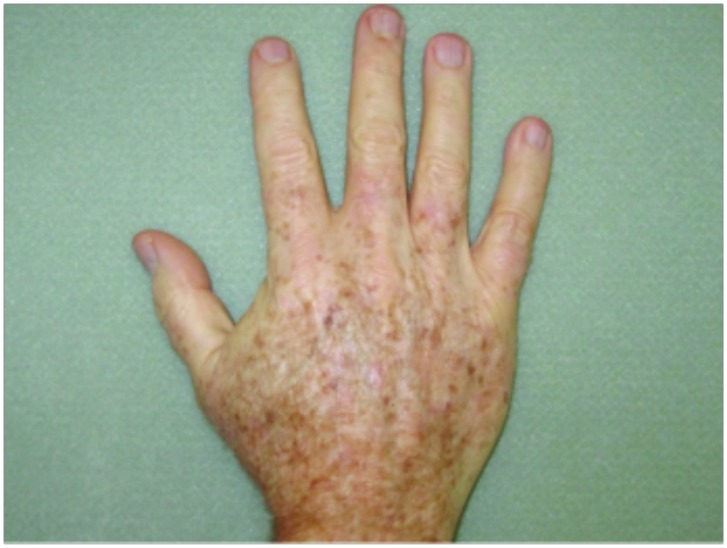
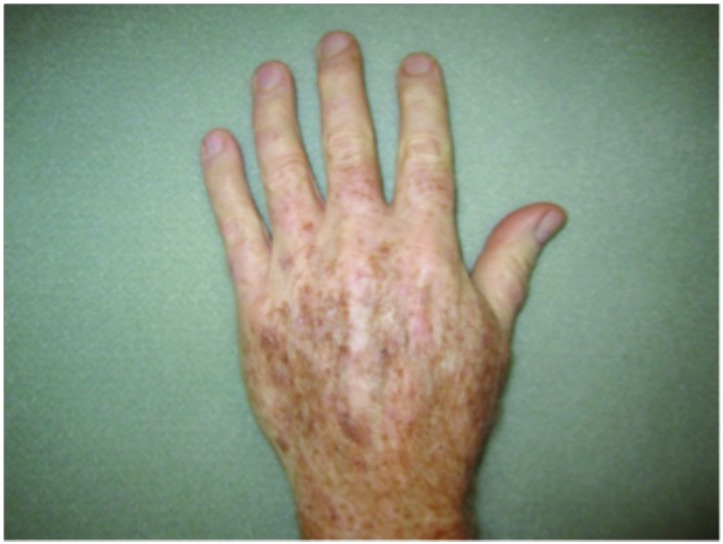
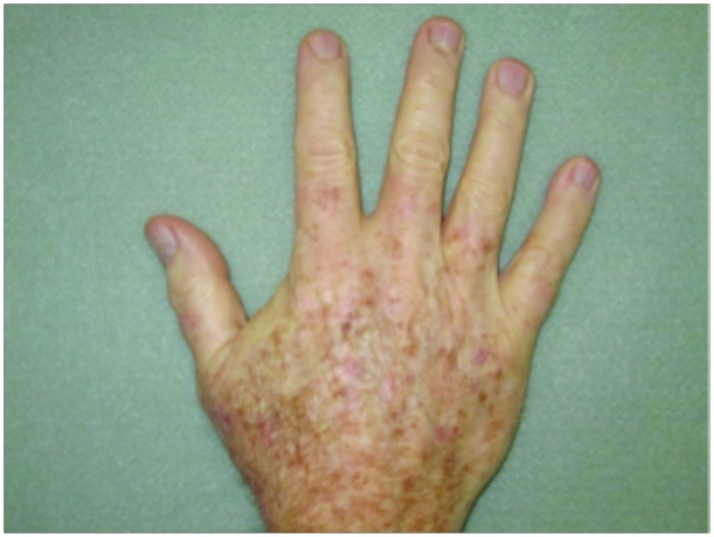
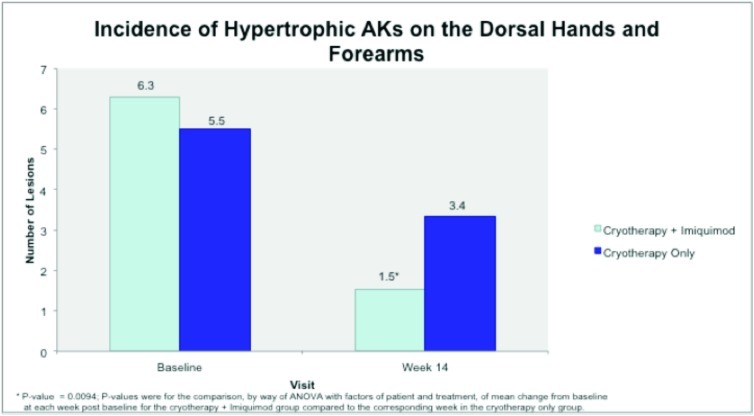
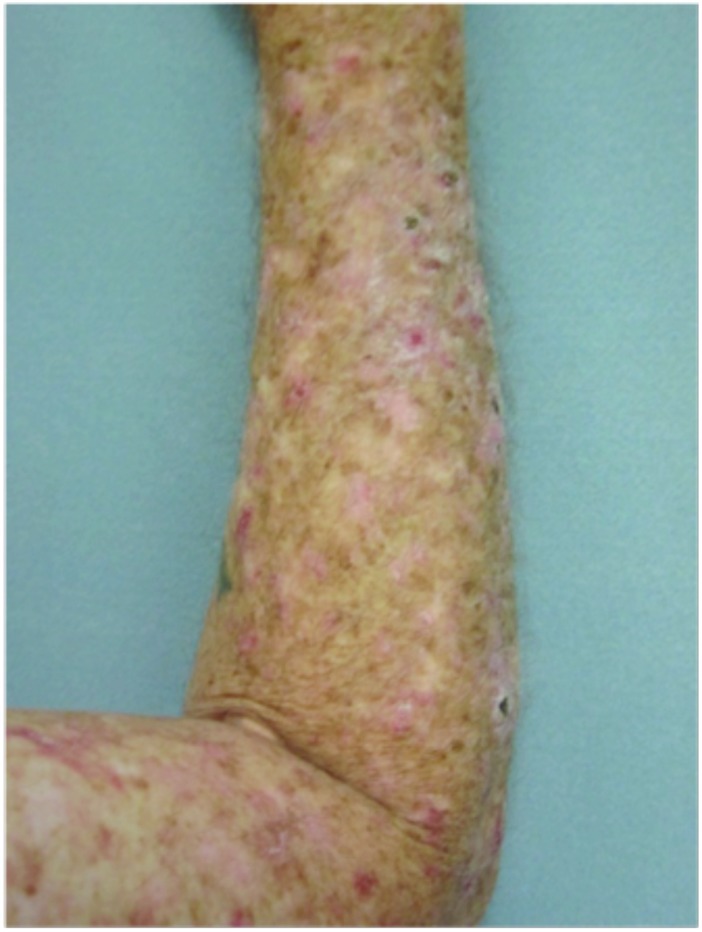
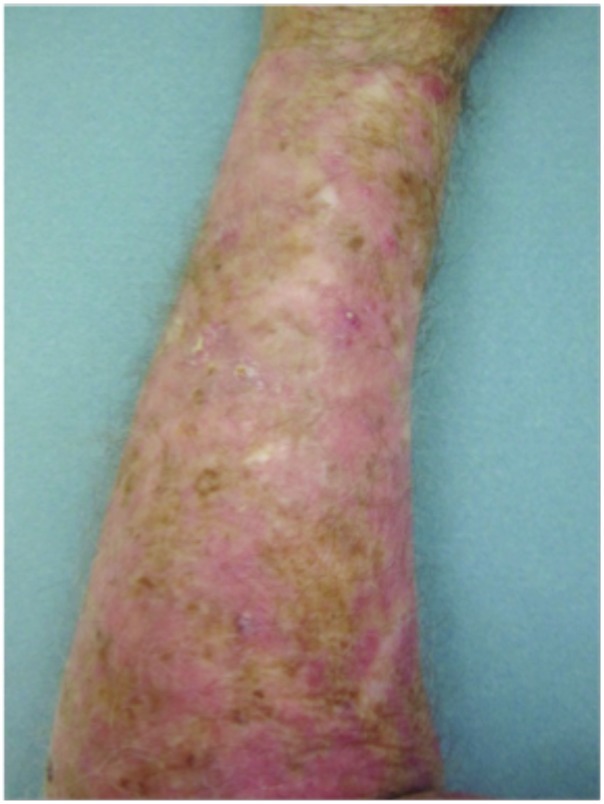
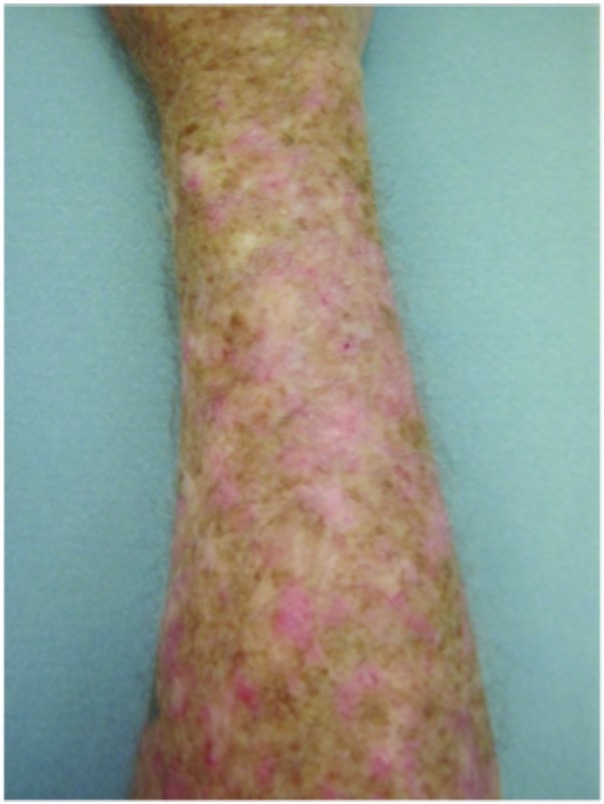
Hypertrophic actinic keratoses. The incidence of HAKs on the dorsal hands and forearms in those receiving combination therapy of cryotherapy plus imiquimod as compared to those receiving cryotherapy alone is presented in Table 3. The number of lesions among subjects with HAKs in both treatment groups appeared to decrease over time with an apparently more pronounced treatment effect observed at Weeks 10 and 14 in the cryotherapy/imiquimod group. Statistical comparison of the rates of decrease in lesion incidence at each week versus baseline via ANOVA using factors of patient and treatment to account for inter-subject variability in bilateral lesion count confirmed this treatment effect starting at Week 10 (p=0.0169) and continuing through the end of study visit at Week 14 (p=0.0094). For the cryotherapy/imiquimod 3.75% group, the median total HAK reduction was −5.12 and for the cryotherapy alone group, the median total HAK reduction was −2.24 (P<0.0094) (Figures 1–3).
Figure 2.
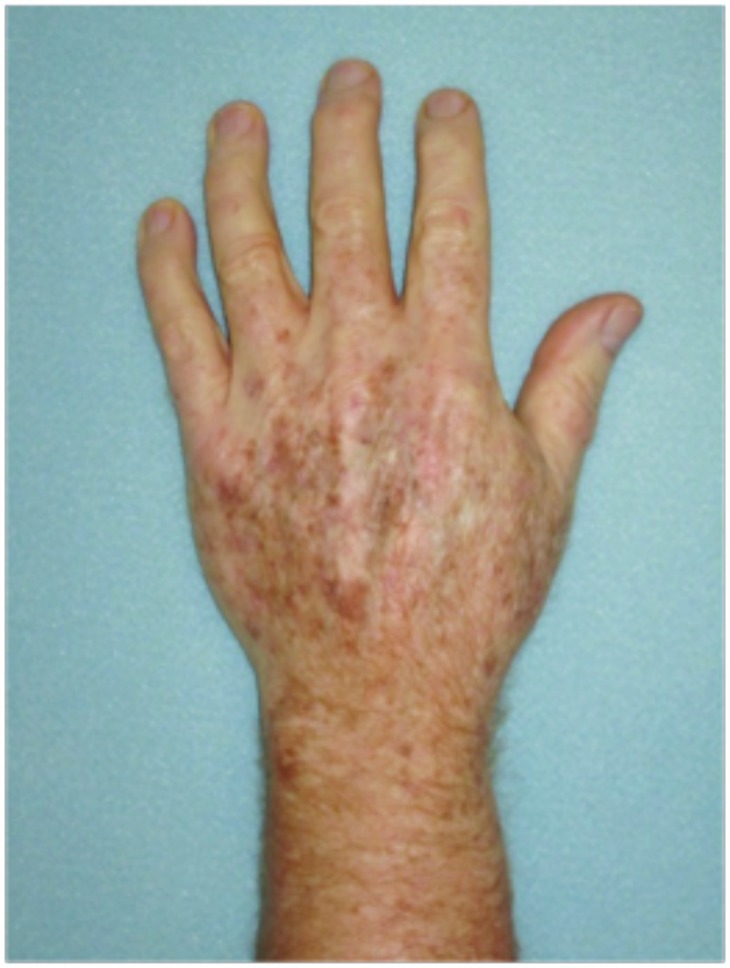
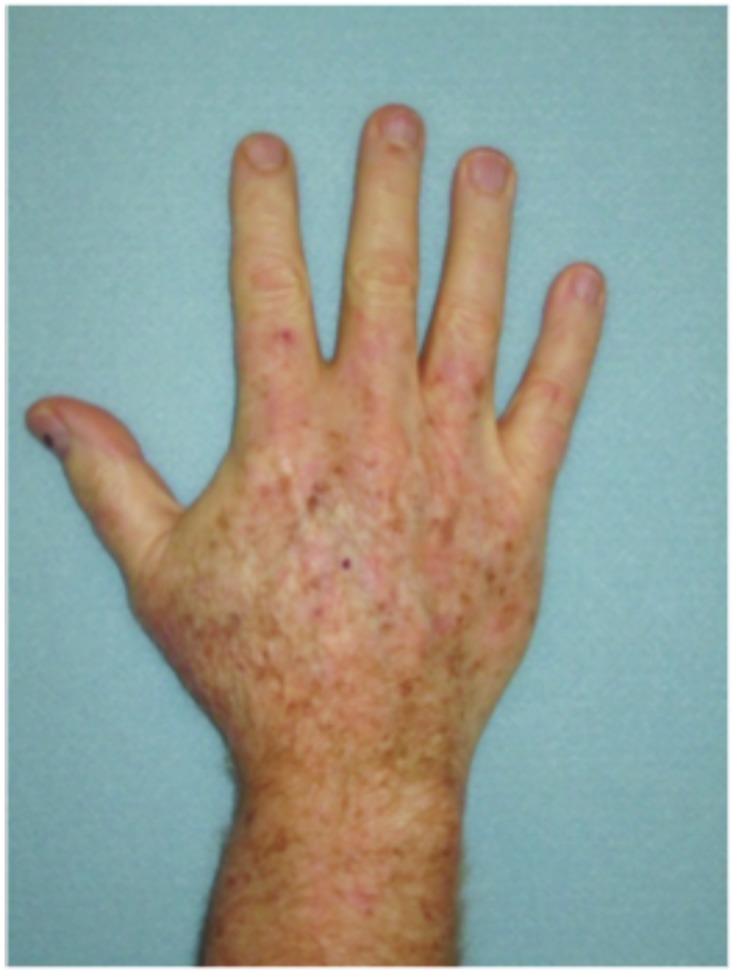
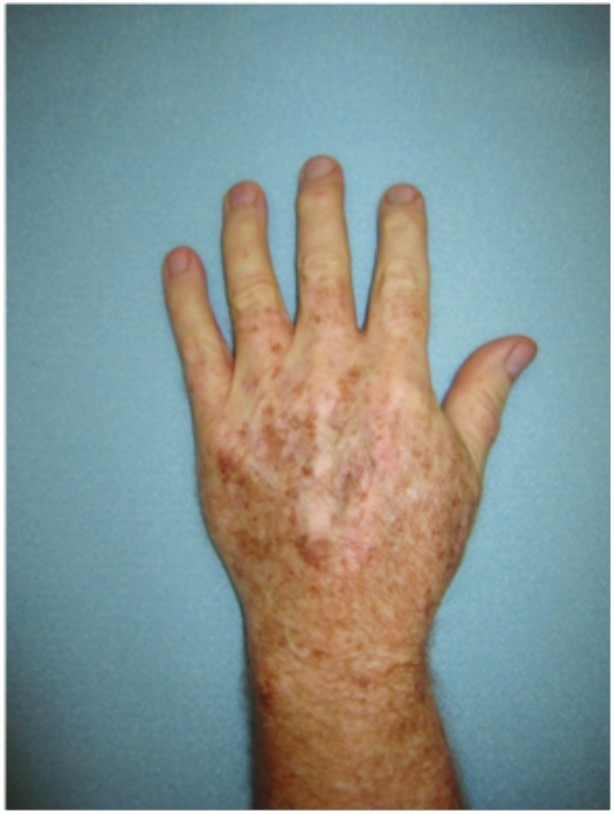
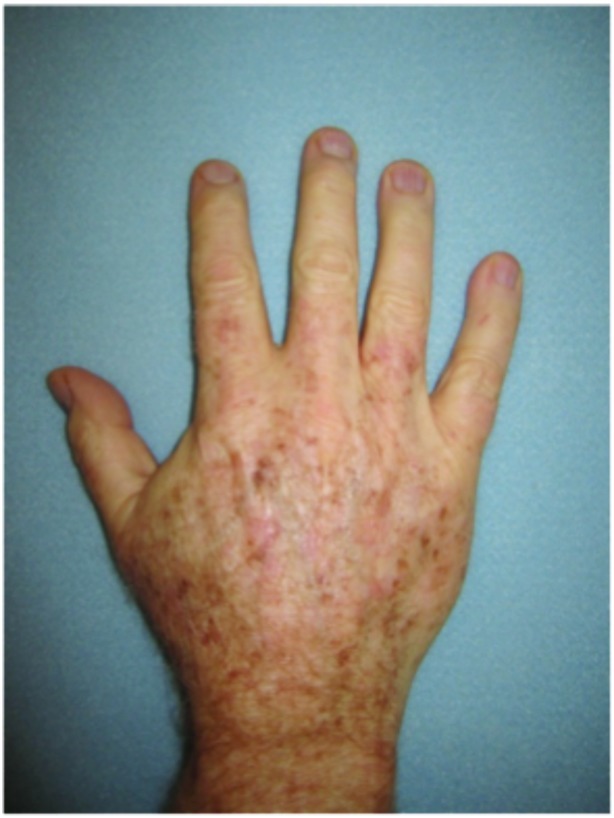
Dorsal hands. Both hands were treated with cryotherapy. The right hand was additionally treated with imiquimod 3.75% for two weeks on, two weeks off, and two weeks on.
TABLE 3.
Incidence of hypertrophic actinic keratoses on the dorsal hands and forearms of cryotherapy plus imiquimod recipients relative to recipients of cryotherapy only
| NUMBER OF LESIONS | CHANGE FROM BASELINE IN NUMBER OF LESIONS VERSUS CONTROL | |||||||
|---|---|---|---|---|---|---|---|---|
| TREATMENT | NUMBER OF OBSERVATIONS | TIMEPOINT | NUMBER OF SUBJECTS | MEAN | SD | MEAN | SD | P-VALUEa |
| Cryotherapy plus imiquimod | 20 | Baseline | 20 | 6.3000000 | 3.5850567 | — | — | — |
| Week 2 | 18 | 6.1666667 | 3.8994721 | 0.28 | 2.70 | 0.0913 | ||
| Week 4 | 18 | 5.3888889 | 3.8369548 | −1.06 | 3.10 | 0.9248 | ||
| Week 6 | 17 | 3.6470588 | 2.9987743 | −2.94 | 4.87 | 0.3816 | ||
| Week 10 | 15 | 1.8666667 | 1.3020131 | −5.00 | 3.57 | 0.0169 | ||
| Week 14 | 17 | 1.5294118 | 1.4627734 | −5.12 | 3.84 | 0.0094 | ||
| Cryotherapy only | 20 | Baseline | 20 | 5.5000000 | 2.5649459 | — | — | — |
| Week 2 | 19 | 4.3684211 | 2.4085617 | −1.11 | 1.29 | — | ||
| Week 4 | 18 | 4.5000000 | 2.4554861 | −1.00 | 2.33 | — | ||
| Week 6 | 17 | 3.5294118 | 2.2112679 | −1.94 | 2.22 | — | ||
| Week 10 | 16 | 3.3750000 | 2.7049338 | −2.13 | 2.78 | — | ||
| Week 14 | 17 | 3.3529412 | 2.8049326 | −2.24 | 3.19 | — | ||
P-values were for the comparison, by way of ANOVA with factors of patient and treatment, of mean change from baseline at each week post-baseline for the cryotherapy plus imiquimod group compared to the corresponding week in the cryotherapy only group.
Figure 1.
Incidence of hypertrophic actinic keratoses on dorsal hands and forearms at baseline and after treatment with cryotherapy + imiquimod or cryotherapy alone
Figure 3.
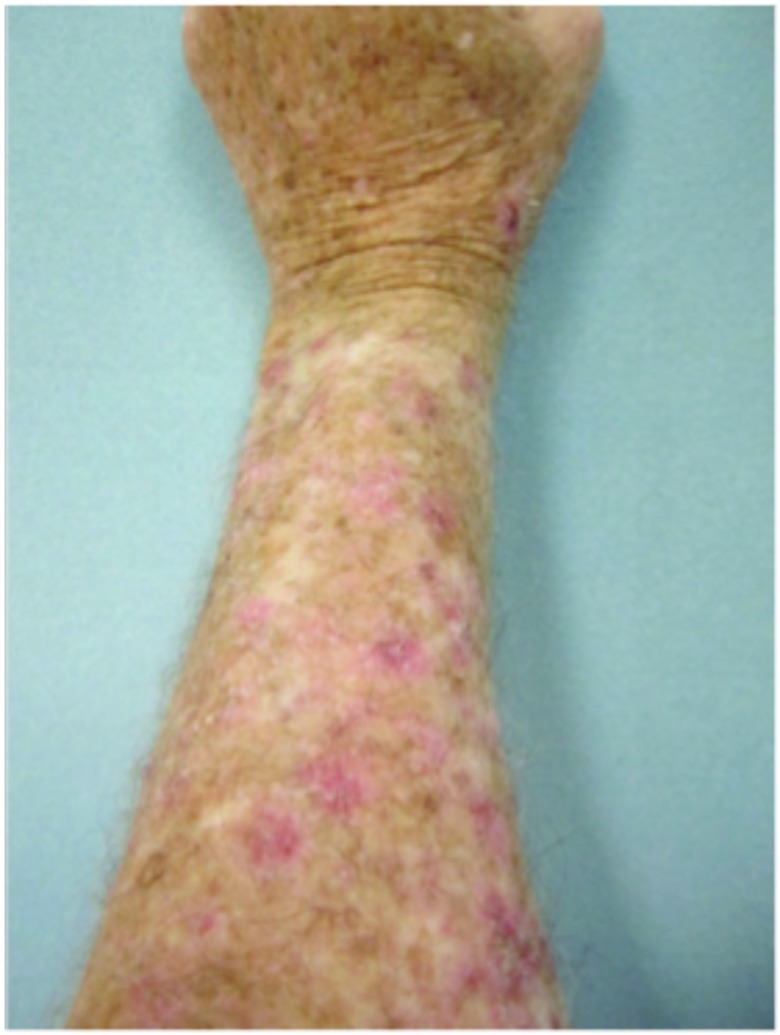
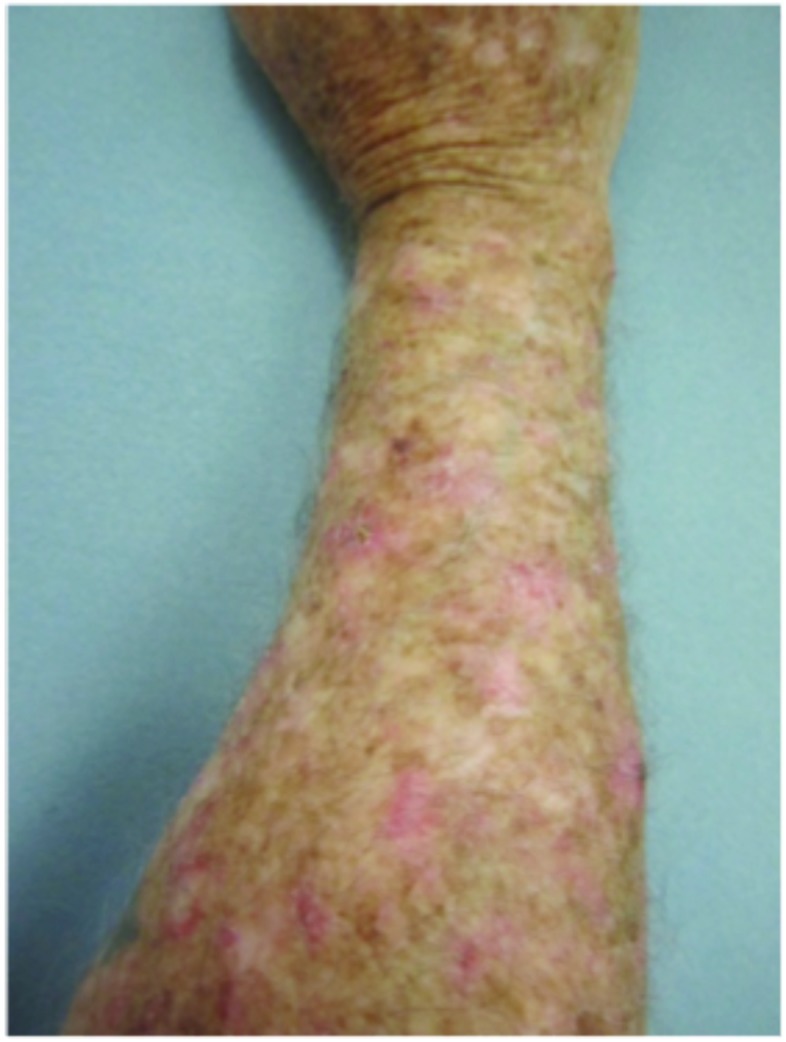
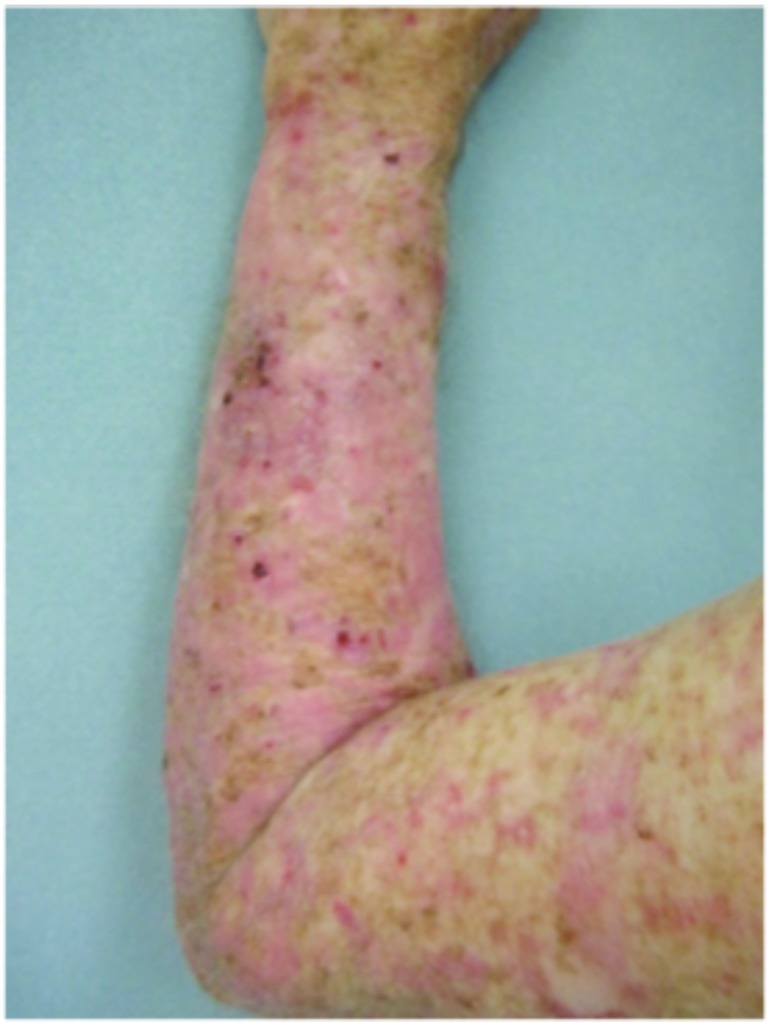
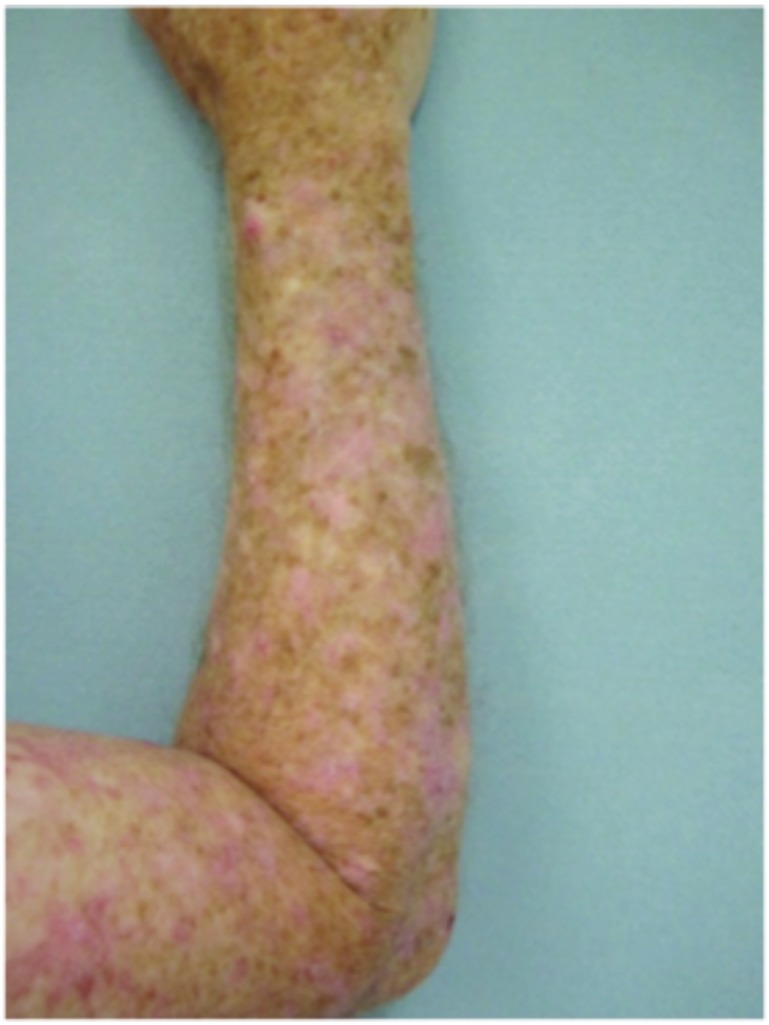
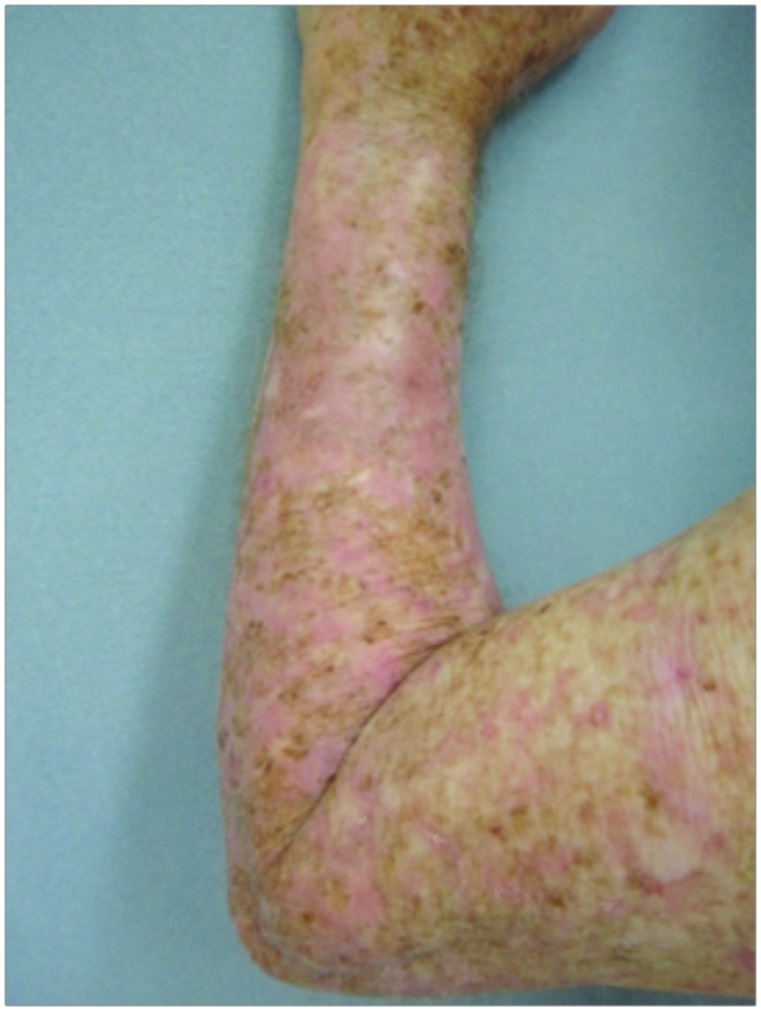
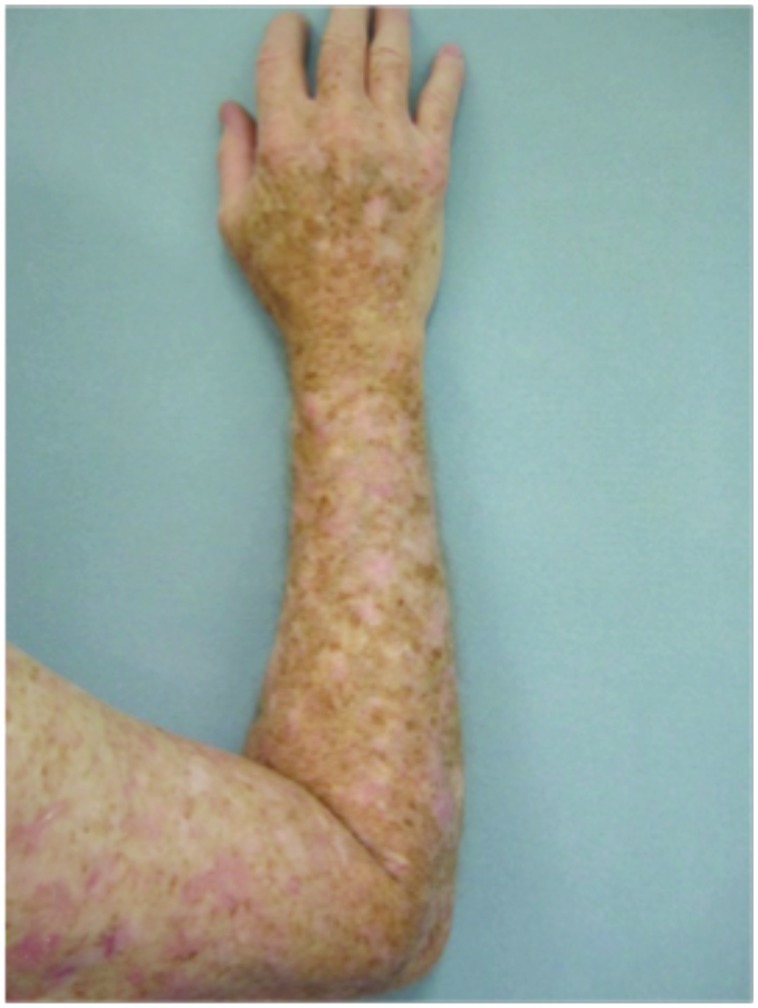
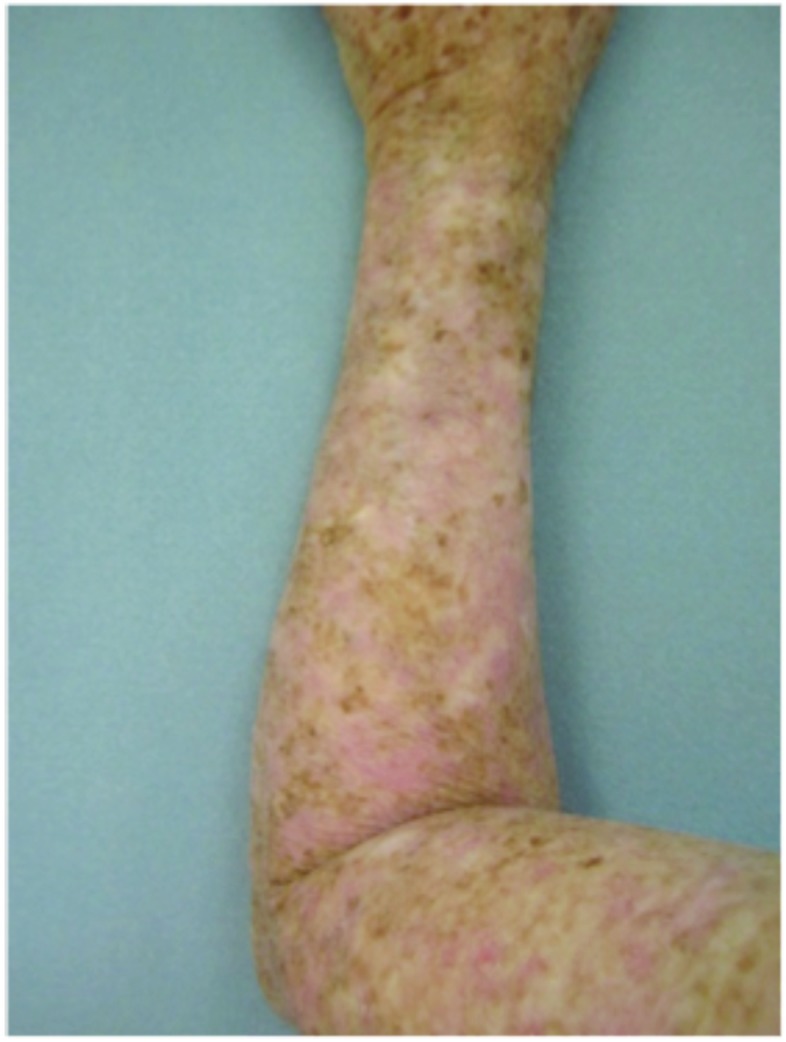
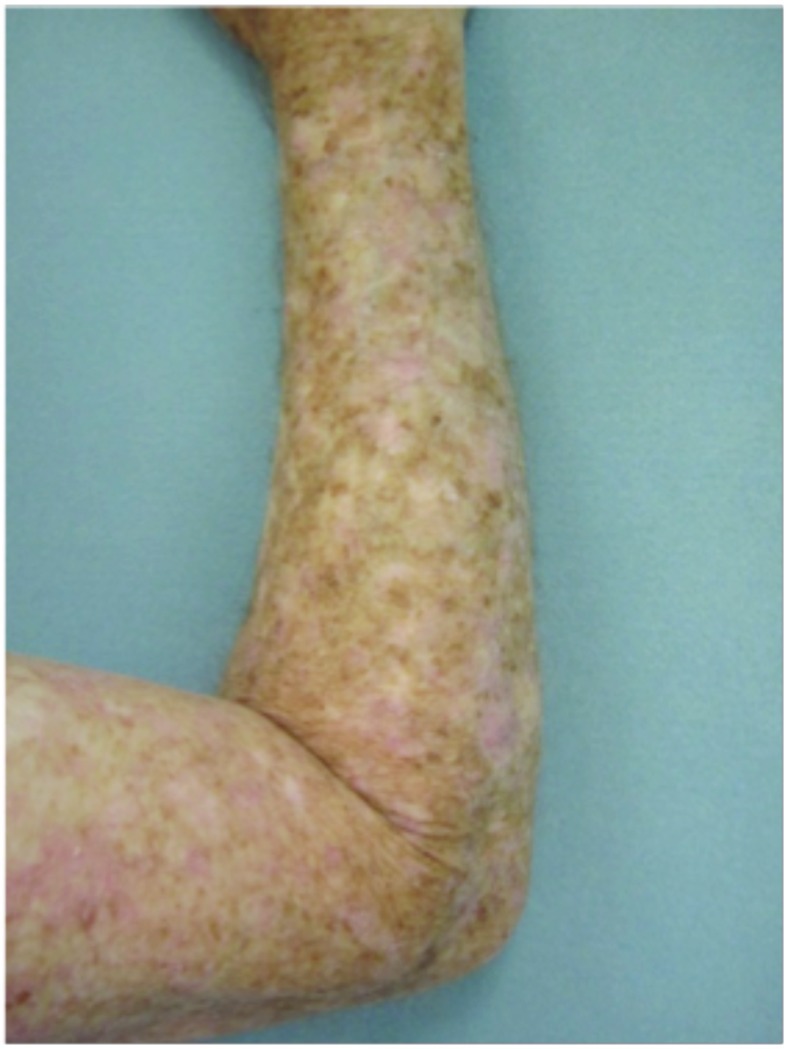
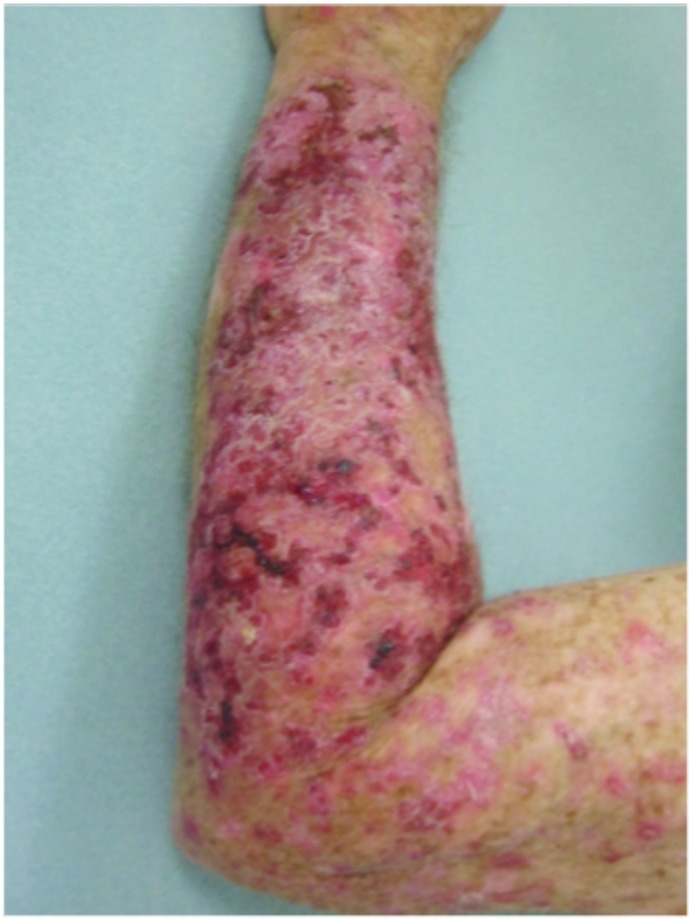
Forearms. Both forearms were treated with cryotherapy. The left forearm was additionally treated with Imiquimod 3.75% for two weeks on, two weeks off, and two weeks on.
Nonhypertrophic actinic keratoses (nHAKs). The incidence of nHAKs on the dorsal hands and forearms in those receiving combination therapy of cryotherapy plus imiquimod as compared to those receiving cryotherapy alone is presented in Table 4. The number of lesions in the combination therapy group increased at Week 6 and then decreased over time. Lesion rates decreased in the cryotherapy alone group. No statistically significant difference was observed between treatment groups at any week relative to baseline and both groups had a similar number of AKs at the end of the study period.
TABLE 4.
Incidence of non-hypertrophic actinic keratoses on the dorsal hands and forearms of cryotherapy plus imiquimod recipients relative to recipients of cryotherapy only
| NUMBER OF LESIONS | CHANGE FROM BASELINE IN NUMBER OF LESIONS VERSUS CONTROL | |||||||
|---|---|---|---|---|---|---|---|---|
| TREATMENT | NUMBER OF OBSERVATIONS | TIMEPOINT | NUMBER OF SUBJECTS | MEAN | SD | MEAN | SD | P-VALUEa |
| Cryotherapy plus imiquimod | 20 | Baseline | 20 | 7.6000000 | 5.3054094 | — | — | — |
| Week 2 | 18 | 5.5555556 | 4.5012707 | −1.06 | 3.81 | 0.2810 | ||
| Week 4 | 17 | 5.9411765 | 4.9177051 | ≈−0.59 | 3.86 | 0.0457 | ||
| Week 6 | 17 | 8.0588235 | 5.3673852 | 1.29 | 6.02 | 0.0178 | ||
| Week 10 | 15 | 4.9333333 | 4.5113613 | −2.00 | 4.69 | 0.8570 | ||
| Week 14 | 17 | 2.9411765 | 4.9177051 | −4.29 | 4.98 | — | ||
| Cryotherapy only | 20 | Baseline | 20 | 7.6000000 | 4.5468323 | — | — | — |
| Week 2 | 19 | 5.0526316 | 3.3743095 | −2.16 | 3.50 | — | ||
| Week 4 | 17 | 4.4444444 | 3.5183924 | −2.76 | 3.07 | — | ||
| Week 6 | 17 | 4.5294118 | 3.0437979 | −2.41 | 4.06 | — | ||
| Week 10 | 16 | 3.4375000 | 2.3935678 | −4.13 | 4.01 | — | ||
| Week 14 | 17 | 3.2352941 | 3.8653818 | −4.12 | 3.31 | — | ||
P-values were for the comparison, by way of ANOVA with factors of patient and treatment, of mean change from baseline at each week post-baseline for the cryotherapy plus imiquimod group compared to the corresponding week in the cryotherapy only group
DISCUSSION
In this study, the authors show a benefit of using imiquimod 3.75% immediately after cryotherapy for the treatment of HAKs. The number of HAKs treated with cryotherapy decreased more in the group that used imiquimod when compared to the group that underwent cryotherapy alone; median reduction was −5.12 HAKs in the cryotherapy/imiquimod group and −2.24 in the cryotherapy alone group (P<0.0094).
The authors did not find a statistically significant difference in the clearance of nHAKs. The group treated with imiquimod showed an increase in number of nHAKs at Week 6 and did show a decrease in number of nHAKs from baseline to end of the study (baseline−7.6 nHAKs; Week 6–8.05; Week 14–2.94 nHAKs). Interestingly, there was a decrease of nHAKs in the group that did not receive any treatment of nHAKs (baseline−7.6nHAKs; Week 14–3.23nHAKs). One potential explanation for this is the systemic effect of imiquimod. When imiquimod cream is applied to the skin, keratinocytes are directly affected, as are a multitude of other inflammatory mediators, because of the inflammatory cytokines and chemokines that are produced locally at the site of application.15 This can further amplify the effects of this topical immune response modifier and possibly lead to the systemic effects the authors saw in their study. The authors propose that treatment with imiquimod on one hand/forearm therefore benefited the subjects’ other hand/forearm because of this amplification of the systemic immune response.
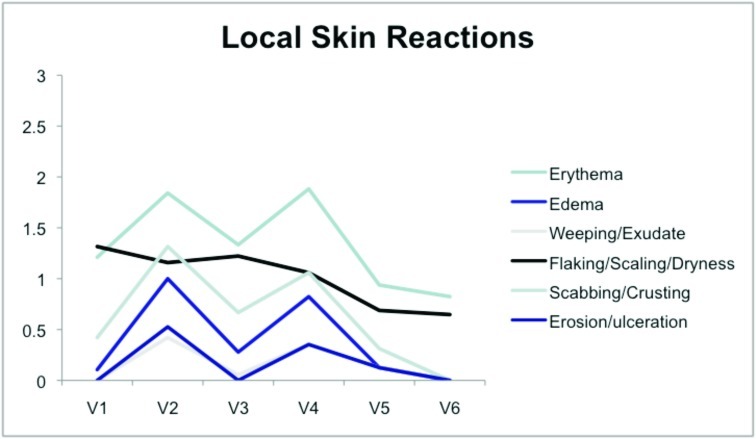
Tolerability was good with no serious adverse events reported (Figure 4). The most common adverse events noted were local skin reactions, such as erythema, edema, scabbing, and crusting. In Jorizzo et al,14 imiquimod was applied approximately two weeks after cryotherapy. In the study herein, imiquimod was applied on the same day as cryotherapy was performed. No serious adverse events were reported. The investigators also believe there is therapeutic benefit to using imiquimod immediately after cryotherapy— imiquimod is a large molecule, and epidermal spongiosis caused by cryotherapy potentiates absorption. This is especially true in hypertrophic lesions. It is common for patients to experience LSRs, such as redness, burning, and crusting, with treatment. Indeed, some practitioners believe that the larger the LSR, the higher the clearance rates of skin lesions.16,17 Systemic adverse events are rare, as systemic absorption is reportedly seen in a minority of cases (<1%).18,19 The majority of these reported AEs include influenza-like or gastrointestinal symptoms or induction of nondermatological disorders.20,21 This rare systemic response is thought to occur because imiquimod induces cytokines in the area of application and can potentially cause systemic augmentation of the immune response.19,21 This systemic reaction potentially could explain clearance of nHAKs in the cryotherapy alone group.
Figure 4.
Local skin reactions
CONCLUSION
For patients with HAKs, combination treatment of cryotherapy plus imiquimod is superior to cryotherapy alone in reducing lesion count.
Footnotes
DISCLOSURE: Dr. Goldenberg received honoraria and study support from Medicis and Graceway. Dr. Frankel, Dr. Linkner, and Ms. Singer report no relevant conflicts of interest. This study was supported by a grant from Graceway Pharmaceuticals and Medicis Pharmaceuticals.
REFERENCES
- 1.Goldberg L. Review of actinic keratosis. Part 1: etiology, epidemiology and clinical presentation. J Drug Dermatol. 2010;9:1125–1132. [PubMed] [Google Scholar]
- 2.Stockfleth E, Ferrandiz C, Grob JJ, et al. Development of a treatment algorithm for actinic keratoses: a European consensus. Eur J Dermatol. 2008;18(6):651–659. doi: 10.1684/ejd.2008.0514. [DOI] [PubMed] [Google Scholar]
- 3.Padilla RS, Sebastian S, Jiang Z, et al. Gene expression patterns of normal human skin, actinic keratosis, and squamous cell carcinoma: a spectrum of disease progression. Arch Dermatol. 2010;146(3):288–293. doi: 10.1001/archdermatol.2009.378. [DOI] [PubMed] [Google Scholar]
- 4.Campione E, Diluvio L, Paternö EJ, Chimenti S. Topical treatment of actinic keratoses with piroxicam 1% gel: a preliminary open-label study utilizing a new clinical score. Am J Clin Dermatol. 2010;11(1):45–50. doi: 10.2165/11311170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.Anderson L, Schmieder GJ, Werschler WP, et al. Randomized, double-blind, double-dummy, vehicle-controlled study of ingenol 14. mebutate gel 0.025% and 0.05% for actinic keratosis. J Am Acad Dermatol. 2009;60(6):934–943. doi: 10.1016/j.jaad.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Siller G, Gebauer K, Welburn P, et al. PEP005 (ingenol mebutate) gel, a novel agent for the treatment of actinic keratosis: results of a randomized, double-blind, vehicle-controlled, multicentre, phase IIa study. Australas J Dermatol. 2009;50(1):16–22. doi: 10.1111/j.1440-0960.2008.00497.x. [DOI] [PubMed] [Google Scholar]
- 7.Criscione V, Weinstock M, Naylor M, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Treinoin Chemoprevention Trial. Cancer. 2009;115(11):2523–2530. doi: 10.1002/cncr.24284. [DOI] [PubMed] [Google Scholar]
- 8.Neidecker MV, Davis-Ajami ML, Balkrishnan R, et al. Pharmacoeconomic considerations in treating actinic keratosis. Pharmacoeconomics. 2009;27(6):451–164. doi: 10.2165/00019053-200927060-00002. [DOI] [PubMed] [Google Scholar]
- 9.Silapunt S, Goldberg LH, Alam M. Topical and light-based treatments for actinic keratoses. Semin Cutan Med Surg. 2003;22(3):162–170. doi: 10.1016/S1085-5629(03)00040-3. [DOI] [PubMed] [Google Scholar]
- 10.Krawtchenko N, Roewert-Huber J, Ulrich M, et al. A randomized study of topical 5% imiquimod vs. topical 5-fluorouracil vs. cryosurgery in immunocompetent patients with actinic keratoses: a comparison of clinical and histological outcomes including 1 year follow-up. Br J Dermatol. 2007;157(Suppl 2):34–40. doi: 10.1111/j.1365-2133.2007.08271.x. [DOI] [PubMed] [Google Scholar]
- 11.Stockfleth E, Meyer T, Benninghoff B, et al. A randomized, double-blind, vehicle-controlled study to assess 5% imiquimod cream for the treatment of multiple actinic keratoses. Arch Dermatol. 2002;138(11):1498–1502. doi: 10.1001/archderm.138.11.1498. [DOI] [PubMed] [Google Scholar]
- 12.Swanson N, Abramovits W, Berman B, et al. Imiquimod 2.5% and 3.75% for the treatment of actinic keratoses: results of two placebo-controlled studies of daily application to the face and balding scalp for two 2-week cycles. J Am Acad Dermatol. 2010;62(4):582–590. doi: 10.1016/j.jaad.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Tan JK, Thomas DR, Poulin Y, et al. Efficacy of imiquimod as an adjunct to cryotherapy for actinic keratoses. J Cutan Med Surg. 2007;1196:195–201. doi: 10.2310/7750.2007.00033. [DOI] [PubMed] [Google Scholar]
- 14.Jorizzo JL, Markowitz O, Lebwohl MG, et al. A randomized, double-blinded, placebo-controlled, multicenter efficacy and safety study of 3.75% imiquimod cream following cryosurgery for the treatment of actinic keratoses. J Drugs Dermatol. 2010;9:1101–1108. [PubMed] [Google Scholar]
- 15.Gaspari AA. Potential roles of an immune response modifier. Cutis. 2007;79(Suppl 4):36–45. [PubMed] [Google Scholar]
- 16.Geisse J, Caro I, Lindholm J, et al. Imiquimod 5% cream for the treatment of superficial basal cell carcinomas: results from two phase III randomized, vehicle-controlled studies. J Am Acad Dermatol. 2004;50:722–733. doi: 10.1016/j.jaad.2003.11.066. [DOI] [PubMed] [Google Scholar]
- 17.Stockfleth E, Sterry W, Carey-Yard M, Bichel J. Multicentre, open-label study using imiquimod 5% cream in one or two 4-week courses of treatment for multiple actinic keratoses on the head. Br J Dermatol. 2007;157(Suppl 2):41–46. doi: 10.1111/j.1365-2133.2007.08272.x. [DOI] [PubMed] [Google Scholar]
- 18.Goldstein D, Hertzog P, Tomkinson E, et al. Administration of imiquimod, an interferon inducer in asymptomatic human immunodeficiency virus-infected persons to determine safety and biologic response modification. J Infect Diseases. 1998;178:858–861. doi: 10.1086/515343. [DOI] [PubMed] [Google Scholar]
- 19.Heikkinen AK, Susittaival P. Severe systemic reaction to topical imiquimod. Acta Derm Venereol. 2011;91:594–595. doi: 10.2340/00015555-1121. [DOI] [PubMed] [Google Scholar]
- 20.Yi Ba, Nirenberg MJ, Goldstein SM, et al. Chronic neuropathic pain associated with imiquimod: report of 2 cases. J Am Acad Dermatol. 2005;52:57–58. doi: 10.1016/j.jaad.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Bhushan K, Tarun N. Local and systemic adverse effects to topical imiquimod due to systemic immune stimulation. Sex TransmInfect. 2011;87:432. doi: 10.1136/sextrans-2011-050025. [DOI] [PubMed] [Google Scholar]