Abstract
Behavior genetic studies of brain activity associated with complex cognitive operations may further elucidate the genetic and physiological underpinnings of basic and complex neural processing. In the present project, monozygotic (N=51 pairs) and dizygotic (N=48 pairs) twins performed a visual oddball task with dense-array EEG. Using spatial PCA, two principal components each were retained for targets and standards; wavelets were used to obtain time-frequency maps of eigenvalue-weighted event-related oscillations for each individual. Distribution of inter-trial phase coherence (ITC) and single trial power (STP) over time indicated that the early principal component was primarily associated with ITC while the later component was associated with a mixture of ITC and STP. Spatial PCA on point-by-point broad sense heritability matrices revealed data-derived frequency bands similar to those well established in EEG literature. Biometric models of eigenvalue-weighted time-frequency data suggest a link between physiology of oscillatory brain activity and patterns of genetic influence.
Keywords: visual oddball, spatial PCA, time-frequency EEG, twin study
Introduction
Comprehensive evaluation of genetic influences on neural activity in normal cognitive neuroscience studies is important for many reasons. First, brain activity measures are closer to the primary gene products than are molar-level behaviors (Iacono & Clementz, 1993), so they may have simpler genetic profiles. Second, activity in particular brain regions and of particular types may provide more specific information than molar-level behaviors about genetic variance in specific neural circuitries supporting complex cognitive operations. Third, studying genetic variation at the level of neural activity may provide useful information about the genetics of cognitive control that is independent of particular task demands. Most studies of genetic influence on human behavior focus on personality and/or characteristics associated with psychopathologies (Frederick & Iacono, 2006; Lykken, 2006). Neuroimaging studies (fMRI and EEG/MEG) investigating genetic variance of neural activations supporting cognition in normal twins are uncommon, with many imaging genetic studies focusing on twins discordant for certain pathologies (Gottesman & Gould, 2003).
The present study used a classical twin design to evaluate genetic influences on spectral components of brain responses that are associated with both simple and complex cognitive operations elicited by a visual oddball task (Katsanis et al., 1997; Steinhauer et al., 1987; Simson et al., 1977). Multiple brain regions are activated during such tasks, including visual, parietal, inferior temporal, anterior cingulate, and prefrontal cortices (Linden, 2005). A prominent brain response, called the P300, has been the focus of most twin studies using similar target detection tasks (e.g., van Baal et al., 1998; van Beijsterveldt et al., 1998; 2001; Begleiter et al., 1998; Almasy et al., 1999; Anohkin et al., 2001; Carlson & Iacono, 2006;). The P300 is generally associated with context updating in working memory and target evaluation (Linden, 2005). Meta-analytic studies show that about 60% of the variance in P300 amplitude and 50% of the variance in latency is attributable to genes (van Beijsterveldt & van Baal, 2002). These analyses, however, were mostly limited to evaluations of voltage at single sensors (Pz) so they may have incompletely described the genetic and environmental variance constituents of this neurophysiologically complex trait.
Considerable effort has been devoted to studying genetic influences on P300, including its endophenotype characteristics (e.g., Bestelmeyer et al., 2009; Hall et al., 2009; Schulze et al., 2008; Yoon et al., 2006; Bramon et al., 2005; Carlson, Iacono, & McGue, 2004), but other brain responses elicited during the same tasks have received considerably less attention. Indeed, the degree of genetic influence on other brain responses in visual oddball paradigms is, at best, uncertain (e.g., Katsanis et al., 1997; Almasy et al., 1999; Smit et al., 2007). The standard N100 appears to be moderately heritable (Almasy et al., 1999; Smit et al., 2007), while, surprisingly, the target N100 seems to possess limited genetic variance (Katsanis et al., 1997). Katsanis and colleagues (1997) also reported significant genetic variance for P200 and N200 neural responses. Like most P300 investigations, these studies also used few sensors, and measured neural activity at a small number of time points, limiting their ability to completely describe genetic contributions to these complex neural responses.
Brain processes can be measured in many ways. Investigations of genetic influences on amplitude and latency of individual ERP peaks (at a limited set of individual time points) have been useful. Such ERP peak measurements in the time domain, even for brain responses to simple stimuli, however, incompletely describe the complexity of neural responding that is evident in time-frequency representations (Klimesch et al., 2007). Indeed, quantifying oscillatory phenomena captures an important aspect of neural processing that is a predominant characteristic of brain function (e.g. Privman et al., 2011; Samar et al., 1995; Gray & Singer, 1989).
There are few investigations of event related oscillations (EROs) in twins (Gilmore et al., 2010a; 2010b). Most studies reporting heritability statistics on the spectral characteristics of EEG and ERP activity come from resting state paradigms with no external stimulus. In the resting state, heritability has been established for delta, theta, alpha, and beta bands (Lykken et al., 1982; Smit et al., 2005; 2010; Posthuma et al., 2001; van Baal et al., 1996; van Beijsterveldt et al., 1998; Zietsch et al., 2007). Genetic influences on oscillations occurring in response to an external stimulus or task have been useful in studies of twin pairs discordant for specific pathologies (Hall et al., 2011; Smit et al., 2009), but are infrequent in the normal cognitive neuroscience literature. Reports on oddball task EROs often focus entirely on the P300, and many use ERP-related methods for quantification, such as grand-averaging and/or restricted time ranges (Basar-Eroglu at al., 2001; Devrim et al., 1999; Ergen et al., 2008; Gilmore et al., 2010a; Ucles, Mendez, & Garay, 2009; but see Demiralp et al., 1999), despite evidence that alternative approaches may be useful (Andrew & Fein, 2010). For instance, single trial analyses, a method increasingly used in ERP studies can parse neural contributions to multiple ERP components (Mouraux & Iannetti, 2008; Hu et al., 2010). Because neural oscillatory activity changes over the course of stimulus processing, quantifying heritability changes in frequency space during stimulus processing could be useful. This paper quantifies genetic influences on neural oscillatory activity during cognitive processing over time using point-by-point broad spectrum heritability of whole head neural activity.
The purpose of the present investigation was to investigate the utility of a novel approach for studying genetic variance on brain activations during complex cognitive processing. Two specific modifications to the typical approach used in twin studies were implemented here. First, spatial principal components analysis (PCA) was used to reduce whole head EEG data to components that efficiently captured neural activation variance in response to task stimuli. These components were then subjected to frequency decomposition over time using Morlet wavelets. Significant time-frequency regions of heritable neural activity were submitted to Cholesky decomposition to evaluate sources of genetic and environmental variance for brain oscillatory behavior during cognitive processing. This approach (i) used minimal data processing adjustments to impose the fewest restrictions possible on data analyses, (ii) integrated information across a large number (61) of EEG sensors, allowing for maximal use of available data, (iii) rather than arbitrarily selecting sensors for analyses, used spatial PCA to empirically derive a multi-sensor neural response that could be quantified with enhanced signal-to-noise ratio and improved reliability of measurement (Braboszcz & Delorme, 2011), (iv) used broad sense heritability information to derive frequency bands with similar heritability patterns, and (v) evaluated neural responses over the entire time range of stimulus processing to provide a closer approximation to the actual functional characteristics of brain activations supporting cognitive operations during visual target detection.
Materials and Methods
Participants
The Minnesota Twin Family Study, begun in 1990, is an epidemiological study of same sex twin pairs born in the state of Minnesota during selected birth years. For the present study, 51 MZ (24 female) and 48 DZ (22 female) twin pairs from this project were used, all 29 yrs of age (see Iacono et al., 1999 for a description). The total cohort included 457 twin pairs (259 female). Twin pairs were randomly selected as every 5th pair in chronological order from a subgroup of subjects with relatively artifact-free EEG data and no significant neurological history. No exclusions were made for psychopathology in order to preserve the representativeness of the sample. Twenty-two subjects (12.6%), 13 of them females, met DSM IV criteria for major depressive disorder for the 5-year interval since their last assessment, 18 subjects (10.3%, 6 of them female) were nicotine dependent for this interval, while 8 (5.0%, 1 of them female) met DSM-IV criteria for alcohol dependence in this interval. Five subjects met DSM-IV criteria for a diagnosis of cannabis (all males) or psychostimulant (4 males) dependence in the 5–6 years since their last assessment.
Procedure
In the EEG environment, participants performed the rotated heads task developed by Begleiter and colleagues (1984; see also Carlson & Iacono, 2006). Subjects viewed a sequence of 240 stimuli. The stimuli were presented for 100 ms with an inter-stimulus interval of 3–4 sec (rectangular distribution). One third of the stimuli (targets) showed a top-down view of a schematic head, with nose and one ear depicted by a triangle and a small oval, respectively. The remaining 160 trials (standards) showed a simple oval with no corresponding head features. Participants were asked to respond to targets and indicate whether they saw a left or right ear by pressing a corresponding left or right response button. For half of the targets, the nose pointed upward and the task was relatively straightforward. For the other half of the targets, the head was rotated 180 degrees to make the nose point downward, making the task of indicating left or right more difficult. There was no required response for standard trials. Target stimuli to which subjects failed to respond were immediately re-presented, preceded by two standards to maintain the ratio of target to standard responses (Gilmore et al., 2009).
ERP Recording
EEG was continuously recorded and digitized at 1024 Hz, with a 5th-order Bessel anti-aliasing filter at 205 Hz, using a 61-channel BioSemi system with sensors placed according to the International 10/10 system (Chatrian et al., 1985). Recording included two earlobe sensors, two sensors on the outer canthus of each eye, and one sensor each above and below the right eye to record eye movements. All sensors were referenced to a monopolar reference feedback loop (connecting a driven right leg passive sensor and common mode sense active sensor, both located on posterior scalp).
EEG Data Analysis
Raw data were visually inspected offline for bad sensor recordings. Bad sensors were interpolated using a spherical spline interpolation method as implemented in BESA 5.1 (MEGIS Software, Gräfelfing, Germany). Trials with sensor amplitudes exceeding 100 μV were not used for data analysis. Data were transformed to an 81 sensor average reference montage in order to provide measurement for interpolated area in the lower occipital region between Oz and the lower CP1 and CP2 sensors on the original cap. This area is important to measure as it captures much of the activity associated with early visual processes in occipital cortex, and thus necessitates an equal representation in sensor space, particularly when submitted to the spatial PCA. Averages were digitally filtered from .5–100 Hz (6 and 12 db/octave rolloff, respectively; zero-phase) and baseline corrected using the 200 ms pre-stimulus interval. Grand averages were then calculated for each individual. Twin pairs in which one or both twins' grand average data exceeded 3 standard deviations from the mean were removed from further analysis, leaving 44 DZ pairs (20 female) and 48 MZ pairs (23 female). Furthermore, one MZ twin pair was removed due to extreme latency of response to targets in one individual, exceeding 3 standard deviations from the mean, leaving a final total of 47 MZ pairs (23 female).
Grand averages over all remaining individuals were computed separately for targets and standards. To avoid response-related motor potentials (average response latency was 808.3 ms, see behavioral results), epochs were limited to −200 to 600 ms. Previous work with this paradigm has shown no significant differences in heritability estimates or P300 characteristics for the easy vs. hard target tasks (Katsanis et al., 1997), so data were collapsed over these two conditions to create one “target” condition. Errors are uncommon in this task, so all trials were included in the analysis.
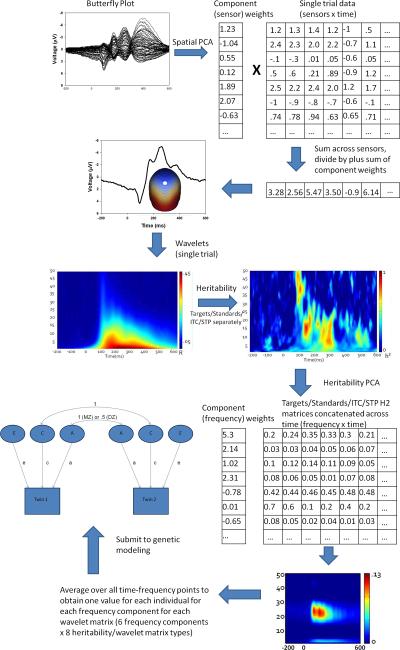
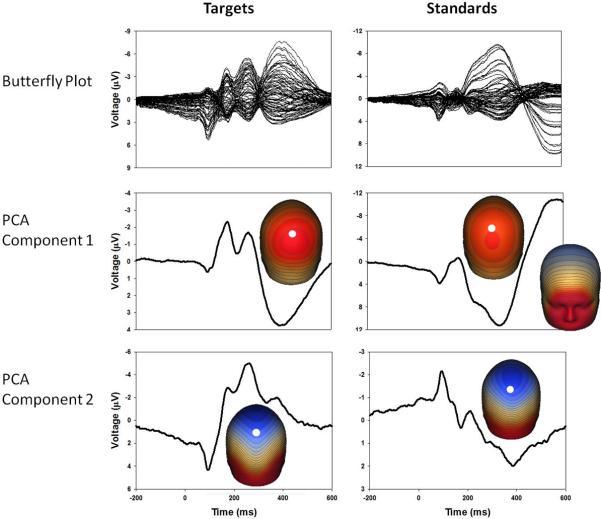
To integrate data recorded from every sensor, spatial principal components analysis (PCA) was implemented. For each group average and condition, an 81×81 covariance matrix was calculated (using time points as observations) and PCA was calculated with promax vector rotation and Kaiser normalization (Dien, 2006). For both target and standard PCA results, component weights were multiplied by each subject's single trial data VEP, summed across sensors, and divided by the sum of the component weights. This reduced waveforms from one for each sensor to one waveform per component for each subject for targets and standards (see Figure 1 for a diagram of all PCA-related methods and Figure 2 for component waveforms). For both targets and standards, the first component captured later activity (the traditional P3b for targets and the late slow wave for standards), and the second component mostly captured earlier more sensory-registration-related activity.
Figure 1.
Pictorial representation of data reduction methods utilized in this project, including matrix manipulation for spatial and heritability PCAs. Step 1 begins at the upper left corner (Butterfly plot) and proceeds in a serpentine, arrow-guided manner to the final step (Submit to genetic modeling).
Figure 2.
Grand average butterfly plots, PCA component waveforms and component topographies for targets and standards. PCA component 1 for both conditions shows the largest deflection and has component topographies representative of late processing. PCA component 2 for both conditions has component topographies more representative of early stimulus registration. For orientation purposes, white dots on topographies indicate the position of sensor Pz.
Wavelet Analysis
PCA weighted waveforms for each subject were then analyzed trial by trial using Morlet wavelets with a frequency resolution of 1 Hz. To balance time resolution in the lower frequencies with stability in the higher frequencies (Busch & VanRullen, 2010), wavelets were calculated using a linearly increasing cycle length from 1 cycle at the lowest frequency (2 Hz) to 5 cycles at the highest (50 Hz). Wavelets provide a measure of single trial power (STP) and inter-trial coherence (ITC) which can be used to evaluate the relative amplitude of response at a particular frequency and how stable the response is in time across trials, respectively (Delorme & Makeig, 2004). STP and ITC values were averaged over trials for each individual and transformed into time-frequency plots (Hamm et al., 2012; Figure 3).
Figure 3.
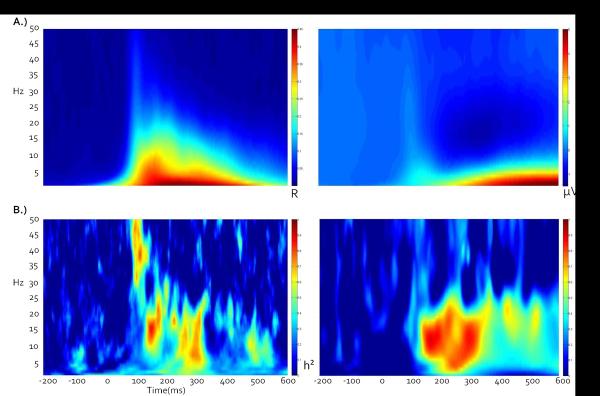
A) Grand average ITC and STP time-frequency plots across all twins, conditions, and components indicate that early stimulus registration is primarily accompanied by an increase in ITC, while late processing primarily consists of a large low-frequency STP synchronization and corresponding beta desynchronization relative to baseline. B) Corresponding broad sense heritability plots, calculated point-by-point as twice the difference between MZ and DZ intraclass correlations. Note that peak heritability values do not necessarily correspond to peak ITC or STP amplitudes.
Biometrical Analysis
For time-frequency data, intraclass correlations (ICCs) were calculated at each time point for each frequency for MZ and DZ twins. Generally, genetic effects may be suspected if MZ ICCs are higher than those of DZ twins, although other components such as shared environmental variance may contribute to these differences. Broad sense heritability (Falconer's h2) was then calculated at each point as the doubled difference between MZ and DZ ICCs, with h2 bound between 0 and 1 (Figure 3).
To examine patterns of heritability in time-frequency space, grand average h2 plots across all twins for each measurement type (ITC and STP), condition (targets and standards) and PCA component (late versus early activations) were concatenated and submitted to a heritability-based PCA following the same guidelines as for the voltage-based PCA, except with frequencies rather than sensors as variables. In general, STP heritability-related variance was later in stimulus processing and ITC heritability-related variance was earlier in stimulus processing.
PCA weights from the heritability-based decomposition were then applied to each individual's ITC and STP time-frequency space data and averaged across all time-frequency points to obtain a component-specific (and thus frequency-specific) quantification for each individual as a function of condition (standard, target) and voltage-based PCA decomposition (late versus early activations) generating one value per individual per frequency band per heritability-based component. In order to better assess potential for additive vs. dominance variance, intraclass correlations were calculated on each frequency band component (Table 1). To control for potential order effects within twin pairs causing unequal within-pair variances, component values were double entered into covariance calculations, with a corresponding reduction in degrees of freedom. MZ and DZ covariance matrices for these heritability-based component values were then analyzed for genetic and environmental effects using the standard univariate ACE and ADE twin models and their reduced models (AE, CE, and E, as appropriate) using Mx (Neale et al., 2003). These models used Cholesky decomposition to partition genetic variance into additive (A, small single-allele effects across multiple loci), non-additive or dominance (D, interactions between allele effects on a single locus) genetic effects, as well as shared (C) and unique (E) environmental variance. The E component also encompasses measurement error. The reduced DE model is never tested, because additive genetic variance necessarily exists when dominance variance is present (Eaves, 1986). The present study does not have the power to test for D, so the ADE models, despite showing evidence in some cases of strong dominance variance and non-significant additive variance (Tables 2 and 3), never fit better than a reduced nested AE model.
Table 1.
Intraclass correlations for MZ and DZ twins for each component and trial type
| Component | Targets | Standards | ||
|---|---|---|---|---|
|
|
||||
| MZ | DZ | MZ | DZ | |
|
|
||||
| ITC Sensory (Early) | ||||
| Delta/Theta | .45** | .29* | .53*** | .47** |
| Alpha | .67*** | .17 | .51*** | .23 |
| Low Beta | .45** | −.01 | .34** | .03 |
| High Beta | .19 | −.05 | .30* | .02 |
| Low Gamma | .13 | −.28 | .29* | .14 |
| High Gamma | .14 | −.08 | .39** | .03 |
|
|
||||
| ITC P300/Slow Wave (Late) | ||||
| Delta/Theta | .45** | .26* | .64*** | .33* |
| Alpha | .67*** | .34* | .69*** | .39** |
| Low Beta | .40** | .09 | .47** | .16 |
| High Beta | −.02 | 17 | .26* | .33* |
| Low Gamma | −.12 | .16 | .16 | −.09 |
| High Gamma | −.01 | .10 | .29* | .22 |
|
|
||||
| STP Sensory (Early) | ||||
| Delta/Theta | .34** | .26* | .39** | .13 |
| Alpha | .62*** | .33* | .66*** | .32* |
| Low Beta | .33* | .12 | .21 | .05 |
| High Beta | −.11 | .23 | .03 | −.13 |
| Low Gamma | .07 | −.08 | −.06 | −.30 |
| High Gamma | −.09 | .13 | .03 | −.23 |
|
|
||||
| STP P300/Slow Wave (Late) | ||||
| Delta/Theta | .26* | .05 | .32* | .19 |
| Alpha | .61*** | .22 | .57*** | .29* |
| Low Beta | .65*** | .35** | .58*** | .37** |
| High Beta | .26* | .41** | .15 | .23 |
| Low Gamma | .13 | .13 | .26* | .01 |
| High Gamma | .10 | .29* | .24* | .08 |
p<.05
p<.01
p<.001
Table 2.
Best fitting model results for target stimuli weighted by heritability PCA component weights.
| Genetic | Shared | Non-shared | % variance accounted for (base model- ACE or ADE) | % variance accounted for (best fitting model) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| Best-fitting Model | Goodness of Fit χ2 (df)* | V | SE | V | SE | V | SE | a2 (95% CI) | d2 (95% CI) | c2 (95% CI) | e2 (95% CI) | a2 (95% CI) | c2 (95% CI) | e2 (95% CI) | |
|
|
|||||||||||||||
| Target Accuracy | - | - | - | - | - | - | - | - | - | - | - | - | |||
| Target Latency | AE | 0.49(2) | 108.4 | 136 | 106.6 | 99 | 29 (0–66) | - | 20 (0–56) | 51 (34–75) | 51 (29–67) | - | 49 (33–71) | ||
|
|
|||||||||||||||
| COMPONENT | |||||||||||||||
| Target ITC Sensory (Early) | |||||||||||||||
| Delta/Theta | AE | 1.47(2) | .19 | .03 | 0 | .19 | .02 | 49 (0–67) | 1 (0–47) | 50 (33–80) | 50 (26–67) | 50 (33–74) | |||
| Alpha | AE | 1.33(2) | .24 | .03 | 0 | .18 | .02 | 0 (0–75) | 66 (0–78) | - | 34 (22–53) | 64 (44–77) | - | 36 (23–56) | |
| Low Beta | AE | 2.57(2) | .07 | .01 | 0 | .09 | .009 | 0 (0–54) | 41 (0–60) | - | 59 (40–84) | 37 (12–57) | - | 63 (43–88) | |
| High Beta | E | 1.93(3) | 0 | 0 | .08 | .004 | 0 (0–37) | 17 (0–42) | - | 83 (58–100) | - | - | 100 | ||
| Low Gamma | E | 4.18(3) | 0 | 0 | .05 | .003 | 0 (0–25) | 5 (0–32) | - | 95 (68–100) | - | - | 100 | ||
| High Gamma | E | 1.07(3) | 0 | 0 | .07 | .004 | 0 (0–31) | 10 (0–36) | - | 90 (64–100) | - | - | 100 | ||
|
|
|||||||||||||||
| Target ITC P300 (Late) | |||||||||||||||
| Delta/Theta | AE | 0.03(2) | .17 | .02 | 0 | .18 | .02 | 40 (0–63) | - | 5 (0–49) | 55 (37–80) | 45 (22–63) | - | 55 (37–78) | |
| Alpha | AE | 0.21(2) | .26 | .03 | 0 | .18 | .02 | 54 (0–79) | 14 (0–78) | - | 32 (21–50) | 68 (50–79) | - | 32 (21–50) | |
| Low Beta | AE | 0.61(2) | .07 | .01 | 0 | .09 | .008 | 0 (0–55) | 39 (0–58) | - | 61 (42–87) | 36 (11–56) | - | 64 (44–89) | |
| High Beta | E | 1.74(3) | 0 | 0 | .07 | .004 | 0 (0–30) | - | 8 (0–28) | 92 (70–100) | - | - | 100 | ||
| Low Gamma | E | 2.19(3) | 0 | 0 | .04 | .002 | 0 (0–20) | - | 0 (0–20) | 100 (80–100) | - | - | 100 | ||
| High Gamma | E | 0.88(3) | 0 | 0 | .06 | .003 | 0 (0–29) | - | 5 (0–25) | 95 (71–100) | - | - | 100 | ||
|
|
|||||||||||||||
| Target STP Sensory (Early) | |||||||||||||||
| Delta/Theta | CE | 1.01(2) | 0 | .71 | .13 | 1.09 | .08 | 3 (0–51) | - | 27 (0–47) | 70 (49–91) | - | 29 (9–47) | 71 (53–91) | |
| Alpha | AE | 2.59(2) | 2.10 | .22 | - | 1.80 | .17 | 38 (0–71) | - | 19 (0–61) | 43 (29–63) | 58 (39–71) | - | 42 (29–61) | |
| Low Beta | AE | 0.62(2) | .66 | .15 | 0 | 1.03 | .09 | 18 (0–50) | 12 (0–51) | - | 70 (49–95) | 29 (5–50) | - | 71 (50–95) | |
| High Beta | - | - | - | - | - | - | - | - | - | - | - | - | |||
| Low Gamma | - | - | - | - | - | - | - | - | - | - | - | - | |||
| High Gamma | - | - | - | - | - | - | - | - | - | - | - | - | |||
|
|
|||||||||||||||
| Target STP P300 (Late) | |||||||||||||||
| Delta/Theta | AE | 1.26(2) | .96 | .27 | 0 | 1.86 | .16 | 0 (0–43) | 23 (0–45) | - | 77 (55–100) | 21 (0–43) | - | 79 (57–100) | |
| Alpha | AE | 3.61(2) | 2.63 | .29 | 0 | 2.35 | .22 | 53 (0–69) | 2 (0–69) | - | 45 (31–65) | 55 (35–69) | - | 45 (31–65) | |
| Low Beta | AE | 1.46(2) | 1.01 | .10 | 0 | .80 | .08 | 46 (0–74) | - | 15 (0–60) | 39 (26–59) | 61 (43–74) | - | 39 (26–57) | |
| High Beta | CE | 1.69(2) | - | .56 | .09 | .80 | .06 | 0 (0–44) | - | 32 (0–50) | 68 (50–87) | - | 32 (13–50) | 68 (50–87) | |
| Low Gamma | - | - | - | - | - | - | - | - | - | - | - | - | |||
| High Gamma | - | - | - | - | - | - | - | - | - | - | - | - | |||
V indicates the raw variance component associated with each latent factor.
Chi square goodness of fit indices are all non-significant at the α<.05 level, indicating a lack of divergence of observed values from model parameters. Missing data indicates a poor model fit for all tested models (significant Chi square fit index). Standard errors are not calculable on variance parameters fixed at zero. Model parameter estimates are reported for the best fitting model, while heritability estimates (% variance accounted for) are reported for the base model and best fitting models. Nested DE models are not fit to the data (see Methods for explanation), so base models indicating genetic variance of either type (A or D) most often reduced to the AE model.
Table 3.
Best fitting model results for standard stimuli weighted by heritability PCA component weights.
| Genetic | Shared | Non-shared | % variance accounted for (base model-ACE or ADE) | % variance accounted for (best fitting model) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||||
| COMPONENT | Best-fitting Model | Goodness of Fit χ2 (df)* | V | SE | V | SE | V | SE | a2 (95% CI) | d2 (95% CI) | c2 (95% CI) | e2 (95% CI) | a2 (95% CI) | c2 (95% CI) | e2 (95% CI) |
|
|
|||||||||||||||
| Standard ITC Sensory (Early) | |||||||||||||||
| Delta/Theta | CE | 0.17(2) | 0 | .18 | .02 | .18 | .01 | 12 (0–65) | - | 40 (0–63) | 48 (31–68) | - | 49 (32–63) | 51 (37–68) | |
| Alpha | AE | 0.05(2) | .21 | .03 | 0 | .22 | .02 | 49 (0–66) | - | 0 (0–47) | 51 (34–74) | 49 (27–66) | - | 51 (34–73) | |
| Low Beta | AE | 0.98(2) | .06 | .01 | 0 | .09 | .008 | 0 (0–49) | 33 (0–55) | - | 67 (45–95) | 29 (2–51) | - | 71 (49–98) | |
| High Beta | AE | 2.01(2) | .04 | .01 | 0 | .06 | .006 | 0 (0–48) | .31 (0–55) | - | 69 (45–99) | 25 (0–50) | - | 75 (50–100) | |
| Low Gamma | AE | 0.44(2) | .02 | .005 | 0 | .03 | .003 | 31 (0–53) | - | 0 (0–37) | 69 (47–96) | 31 (4–53) | - | 69 (47–96) | |
| High Gamma | AE | 2.52(2) | .03 | .007 | 0 | .05 | .005 | 0 (0–54) | 40 (0–61) | - | 60 (39–89) | 34 (6–57) | - | 66 (43–94) | |
|
|
|||||||||||||||
| Standard ITC Slow Wave( Late) | |||||||||||||||
| Delta/Theta | AE | 0.01(2) | .74 | .08 | 0 | .56 | .06 | 61 (0–76) | 3 (0–75) | - | 36 (23–55) | 64 (45–76) | - | 36 (24–55) | |
| Alpha | AE | 0.35(2) | .33 | .03 | 0 | .23 | .02 | 56 (5–79) | - | 11 (0–54) | 33 (21–50) | 68 (51–79) | - | 32 (21–49) | |
| Low Beta | AE | 1.41(2) | .23 | .03 | 0 | .27 | .02 | 28 (0–60) | 14 (0–60) | - | 58 (40–81) | 41 (19–60) | - | 59 (40–81) | |
| High Beta | CE | 0.19(2) | 0 | .03 | .007 | .05 | .004 | 0 (0–47) | - | 28 (0–46) | 72 (51–92) | - | 28 (8–46) | 72 (54–92) | |
| Low Gamma | E | 1.93(3) | 0 | 0 | .03 | .002 | 0 (0–34) | 13 (0–41) | - | 87 (59–100) | - | - | 100 | ||
| High Gamma | AE | 0.28(2) | .03 | .006 | 0 | .04 | .004 | 16 (0–51) | - | 13 (0–43) | 71 (49–95) | 31 (6–51) | - | 69 (49–94) | |
|
|
|||||||||||||||
| Standard STP Sensory (Early) | |||||||||||||||
| Delta/Theta | AE | 0.27(2) | .63 | .12 | 0 | .85 | .08 | 13 (0–56) | 25 (0–57) | - | 62 (43–88) | 36 (11–56) | - | 64 (44–89) | |
| Alpha | AE | 0.14(2) | 1.7 | .17 | 0 | 1.27 | .12 | 61 (0–76) | 2 (0–75) | - | 36 (24–54) | 64 (46–76) | - | 36 (24–54) | |
| Low Beta | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| High Beta | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| Low Gamma | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| High Gamma | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
|
|
|||||||||||||||
| Standard STP Slow Wave (Late) | |||||||||||||||
| Delta/Theta | AE | 0.09(2) | 1.24 | .25 | 0 | 1.76 | .16 | 22 (0–52) | - | 8 (0–43) | 70 (48–94) | 31 (6–52) | - | 69 (48–94) | |
| Alpha | AE | 1.59(2) | 2.53 | .29 | 0 | 2.25 | .21 | 40 (0–68) | - | 13 (0–57) | 47 (32–69) | 53 (33–68) | - | 47 (32–67) | |
| Low Beta | AE | 0.58(2) | .76 | .08 | 0 | .65 | .06 | 37 (0–71) | - | 19 (0–59) | 44 (29–65) | 58 (38–72) | - | 42 (28–62) | |
| High Beta | CE | 5.06(2) | 0 | .31 | .09 | .65 | .05 | 1 (0–49) | - | 18 (0–38) | 81 (51–100) | - | 18 (0–38) | 82 (62–100) | |
| Low Gamma | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| High Gamma | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Chi square goodness of fit indices are all non-significant at the α>.05 level, indicating a lack of divergence of observed values from model parameters. Missing data indicates a poor model fit for all tested models (significant Chi square fit index). Standard errors are not calculable on variance parameters fixed at zero. Model parameter estimates are reported for the best fitting model, while heritability estimates (% variance accounted for) are reported for the base model and the best fitting model. Nested DE models are not fit to the data (see Methods for explanation), so base models indicating genetic variance most often reduced to the AE model.
Best fitting models were identified using a chi-square difference test and the Akaike information criterion (AIC). All best fitting models had a non-significant (p > .05) chi-square goodness of fit index, indicating that the model did not significantly differ from the observed variance structure. The base model (ACE or ADE) was chosen as the model that minimized the AIC. Then, appropriate nested models with non-significant chi-square indices were compared to the chosen base model using chi-square difference tests. If the difference was significant, it was assumed the base model provided a better fit to the data so the base model was retained. If the difference was non-significant, the more parsimonious nested model was retained. Best fitting models were also those that minimized the AIC; small AIC indicates good model fit together with parsimony, in that this criterion includes a penalty for model complexity.
Results
Behavioral
For response latency, MZ (M=815.3 ms, SD=152.6 ms) and DZ (M=801.3 ms, SD=152.1 ms) twins did not differ, t(180)=.62, p=.54. Likewise, for response accuracy, MZ (M=98.5%, SD=2.1%) and DZ (M=97.7%, SD=3.1%) twins did not differ, t(180)=1.88, p=.06.
Spatial PCA
Scree plots (Cattell, 1966) for each condition always identified 2 components, with the spatial distributions of these components and amount of variance accounted for (always over 90%) being nearly identical between zygosities and within condition. For the target condition, the first (late) and second (early) components accounted for 67.7% and 25.8%. For the standard condition, the first (late) and second (early) components accounted for 88.7% and 7.2% of the variance, respectively.
Wavelets
In the 100–300 ms following stimulus onset, neural activity in response to both targets and standards was characterized by high ITCs and low STPs (Figure 3). This time range encompasses the traditional early (and more sensory) ERP components such as the P1, N1, and P2 (Figure 2). The 300–600ms time range showed some ITC enhancement, but was dominated by an alpha/beta power decrease (de-synchronization) and accompanying lower frequency power increase. This time range captures the traditional P300 ERP component in the target condition and the late slow wave in the standard condition.
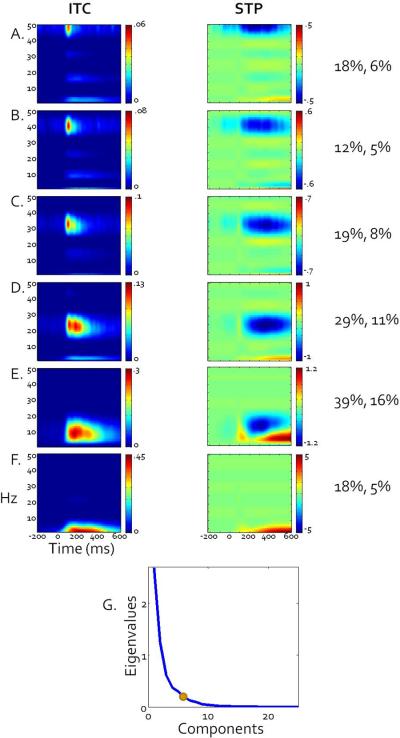
Heritability based PCA analyses revealed 6 components that captured relatively traditional frequency band subdivisions (Figure 4; for relative separation of bands see covariance matrix in Figure 5). In order of percent variance accounted for, these subdivisions were: alpha (peaking at 13Hz, 39% of variance, Fig 3E), low beta (peaking at 26 Hz, 29% of variance, Fig 3D), high beta (peaking at 34 Hz, 19% of variance, Fig 3C), delta/theta (peaking at 4 Hz, 18% of variance, Fig 3F) low gamma (peaking at 42 Hz, 18% of variance, Fig 3B), high gamma (peaking at 50 Hz, 12% of variance, Fig 3A). Subsequent analyses used these subdivisions for estimating genetic and environmental variance contributing to neural activations during visual target detection. These analyses were conducted separately for the voltage-based late (P300, slow wave) and early (sensory registration) processing components as a function of stimulus type (targets versus standards) using phase stability (ITC) and single trial power (STP) metrics.
Figure 4.
Grand average ITC (left column) and STP (right column) plots weighted by broad sense heritability PCA components, revealing a data-driven distribution of frequency bands that closely resemble traditional bands defined in the EEG literature. Scale is provided for each plot but denotes weighted values so is not directly comparable with raw ITC or STP values. Percent variance accounted for by each rotated component, ignoring other components, followed by percent variance uniquely accounted for by each component, is presented to the right of each set of plots. A). High gamma. B). Low gamma. C). High beta. D). Low beta. E). Alpha. F). Theta/Delta G). Scree plot of unrotated eigenvalues. The dot indicates where the final component retained is located.
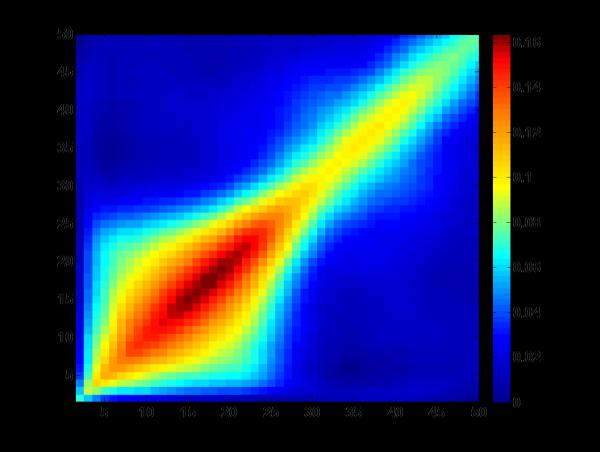
Figure 5.
Covariance matrix for heritability of full concatenated data set as input into the frequency PCA. Although there is a degree of overlap in the lower frequencies, frequency bands in the delta/theta, alpha, high and low beta, and high and low gamma can be seen based on relative covariance between and within bands. Similarities in power across time between evoked delta/theta and alpha frequency bands might be expected in this task, as they are primary contributors to the large ERPs such as P3 and slow wave (Basar et al., 1999). The similarities between the heritability patterns and those commonly seen in raw power values bolster the connection between genetic effects and the underlying biology of the distinctions between frequency bands.
Twin Correlations
Intraclass correlation values for frequency band components (Table 1) indicate significant correlations within MZ and DZ twin pairs for low frequencies and some evidence of significant MZ correlations for higher frequencies. For targets, instances where MZ correlations were more than twice those of DZ twins occurred primarily in the lower frequency bands. For standards, the high frequency bands tended to show MZ correlations much more than twice those of DZ twins.
Genetic Models
No frequency band component or behavioral variable was best fit by the full ACE or ADE base model; models estimating only one genetic variance source appeared to more parsimoniously account for these data (See Tables 2 and 3 for variance parameter estimates). Because of the large confidence intervals in the full models due to relatively small sample size, evidence for genetic variance was considered to be significant if the resulting best fitting nested model (see “% variance accounted for – best fitting model” in Tables 2 and 3) incorporated a genetic (A) variance parameter with a confidence interval that did not encompass zero.
Behavioral
Response accuracy did not fit with any of the standard genetic models, most likely due to a ceiling effect, as most participants scored close to or at 100% accuracy for this relatively easy task. Response latency to targets best fit with the AE model, suggesting additive genetic influence for reaction time.
Targets Sensory (Early) Voltage Component
For ITC, significant genetic influence was found for the delta/theta though high beta frequency bands (AE model), with the higher frequency bands being best fit by the unique environmental variance model (E). For STP, only the alpha and low beta frequency bands showed a significant genetic influence (AE model). The delta/theta band was best fit by the common environmental influences model (CE). The remaining frequency bands were not fit by any of the standard models.
Targets P300 (Late) Voltage Component
For ITC, significant genetic influence was found for the delta/theta through low beta frequency bands (AE model), with higher frequency bands being best fit by the unique environmental influences model (E). For STP, significant genetic influence also was found for the alpha through low beta frequency bands (AE models) while the high beta band was best fit by the common environmental influences model (CE) after the removal of one MZ pair outlier in this single band. The higher frequencies were not fit by any of the standard models.
Standards Sensory (Early) Voltage Component
For ITC, all frequency bands showed a significant genetic influence (AE model), except for the high beta band, which best fit the AE model but the additive variance was not significant, and for the delta/theta band, which was best fit by the common environmental influences model (CE). For STP, significant genetic influences were found for the delta/theta and alpha bands (AE model), with the higher frequencies not fitting any of the standard models.
Standards Slow Wave (Late) Voltage Component
For ITC, significant genetic influence was found for the delta/theta through low beta frequency bands (AE model), with the high beta and low gamma frequency bands being best fit by the common (CE) and unique (E) environmental influences models, respectively. The high gamma band showed significant genetic influence with a best fit to the AE model. For STP, the delta/theta through low beta frequency bands were best fit by the additive genetic model (AE), while the high beta band best fit to the CE model and the gamma bands did not fit to any of the tested models.
Discussion
This study yielded two notable findings. First, heritable brain responses follow patterns closely associated with the prototypical frequency bands. This finding suggests that genetic influences on oscillatory brain activity can be explained based on biological mechanisms for specific types of neural oscillations or frequency bands. Second, peaks in heritability do not necessarily correspond to peaks in EEG amplitude, indicating that a comprehensive analysis of the entire time-frequency spectrum is important to develop a more complete understanding of the connections between gene expression and EEG phenotypes.
When decomposed into frequency variables, broad sense heritability patterns break down into groups which closely follow established frequency band subdivisions. These patterns are encouraging for molecular genetics because they suggest a connection between biological mechanisms of oscillatory brain activity and specific patterns of inheritance. Activity in these data-defined frequency bands largely fits with classic twin models, (with some exceptions primarily in higher frequency bands for STP where there is little variation from zero), indicating that composite broad sense heritability and frequency information may serve as a useful organizational guide for more specific genetic modeling.
Since heritability patterns and brain oscillations were linked, differences in narrow sense heritability patterns may also shed some light on differences in neural recruitment during stimulus processing. For example, gamma ITC is only heritable during processing of standard stimuli, with late ITC perhaps indicating a change in gene expression in glutamatergic NMDA receptors or their modulatory systems (Traub et al., 2004). This high frequency-related change in glutamatergic modulation may account for some portion of late stimulus evaluation separate from the low frequency ERP patterns observable in the standard late slow wave. In addition, modulatory dopaminergic effects on lower frequency bands have been reported for the oddball task. Late theta power increases to novel stimuli show a dependency on COMT Val allele loading, an effect which also interacts with DRD4 genotype (Marco-Pallares et al., 2010). Evoked delta and theta power to visual oddball targets also show linkage to two separate SNPs on the CHRM2 gene, which codes for the cholinergic muscarinic receptor 2 (Jones et al., 2004). Both delta and theta bands contribute significantly to P300 generation and acetylcholine has been shown to affect P300 amplitude (Callaway, 1983).
One of the most consistently heritable components across all measurements was the low beta frequency band. Beta activity has been associated with processing of salience information (Kisley and Cornwell, 2006), suggesting that the early evaluation of salience of targets versus standards and its subsequent later cognitive processing is largely guided by genetic factors. Resting beta activity has shown linkage to areas containing the GABRB1, GABRA2, and GABRA4 receptors, which are involved in regulation of inhibitory interneurons (Porjesz et al., 2002; Ghosh et al., 2002). This connection also has been demonstrated using association mapping (Roy-Gagnon, Mathias, and Wilson, 2005). While heritability of resting EEG has been well established (van Beijesterveldt et al., 1996; Zietsch et al., 2007), genetic influences on evoked power spectra are less well understood. Evoked alpha and beta frequency bands have been relatively neglected in the genetic literature; the discrepancy between studies on resting and evoked power in the alpha band is particularly large. The general robustness of heritability in the alpha and low beta bands in the current study suggests that these frequencies may be of interest for understanding genetic influences on complex cognitive operations.
While heritability of early and late components of target processing has been fairly well established (van Beijesterveldt and vab Baal, 2002), ERP findings in the literature vary on heritability of responses to nontarget (standard) stimuli. Additive genetic effects have been reported for standard N1 amplitude (Almasy et al., 1999; Smit et al., 2007). O'Connor and colleagues (1994) found a mixture of additive and dominant genetic effects for standard N1 latency, although this study utilized auditory oddball stimuli, and there is some suggestion that heritability may not translate across modalities (Katsanis et al., 1997). However, bands of heritability within the N1 time window but with differing frequency and genetic profiles may shine some light on the mixed model findings of O'Connor and colleagues (1994) on ERP amplitude, which is a composite of all frequency bands. Relatively little is known about late processing of standards, although there are some reports of significant heritability of P3 amplitude to standard stimuli in the rotated heads oddball task, which elicits this neural response to both standard and target stimuli (van Baal et al., 1998; van Beijsterveldt et al., 1998). Both of these groups utilized a 35 Hz low pass filter to examine late activity. Heritable gamma activity to standards in the current study suggests that ERP heritability findings may be influenced by low-pass filtering. Filtering out high beta and gamma frequencies may remove valuable sources of genetic variation that may differentiate late processing in different conditions. The late component lower frequency bands in the target condition also show mixed ACE and ADE model fits, showing support for genetic influences on the P300 component in both single trial power and ITC (but more consistently in ITC).
Target gamma and high beta frequency bands were most frequently best fit by E models. This may be due to a number of factors. First, single trial power for gamma was very low, perhaps causing a floor effect for variability. Second, in any measure of gamma that includes the whole head, the possibility of myogenic artifact must be acknowledged. Although these data were relatively free of visible muscle artifact, high frequency noise from minute muscle movement in the scalp could contribute to random variability in the genetic models. Third, preparation to make a response may synchronize beta and gamma activity in motor preparation areas in a manner that is locked to responses (Salenius et al., 1996). Late response-locked activity in the target condition may be relatively desynchronized relative to stimulus onset at the individual level and may present as randomness in low gamma ITC at the group level, reducing heritability.
Generally, the later occurring neural activations were more associated with variations in single trial power than were the earlier stimulus registration-related activations (Figure 3). Figures 3 and 4 are consistent with the theory that early voltage-related activity in ERP recordings is accounted for by increased phase locking within a neural mass (Moratti et al., 2007; Sayers et al., 1974; Jansen et al., 2003; Klimesch et al., 2004; Gruber et al., 2005; Makeig et al., 2002). As previously shown, early ERPs such as the P1 and N1 are primarily accounted for by phase locking in lower frequencies (Moratti et al., 2007; Sayers et al., 1974; Jansen et al., 2003; Klimesch et al., 2004; Gruber et al., 2005; Makeig et al., 2002). Later components such as the P300 and late slow wave show a mixture of activity in the STP and ITC plots, with the late slow wave almost entirely associated with variation in single trial power. Interestingly, the peaks in the heritability plots do not cleanly follow the peaks in the time-frequency plots, particularly for STP (Figure 3). This discrepancy suggests that although the primary mechanism behind early ERP peaks may be ITC, MZ twins show more similarity than DZ twins in evoked power during these early time ranges. It stands to reason that evoked power during the early oscillatory response should not be ignored as an important and heritable component of healthy cognitive function.
The lack of complete overlap between peaks in activation and peaks in heritability suggests that genetic analyses in the frequency domain should not be limited to restricted time ranges around ERP peaks or even restricted time-frequency ranges. Characteristic differences in P300 response have been linked to several disorders with genetic components, including schizophrenia (Bestelmeyer et al., 2009; Hall et al., 2009; Bramon et al., 2005) and substance abuse (Begleiter et al., 1984; Iacono et al., 1999; 2000; 2003). The early and late components of the visual oddball task have been relatively neglected, but may provide complementary information about genetic influences on a variety of cognitive processes. By decomposing the ERP into its constituent frequency properties, more direct physiological mechanisms of ERP generation and their potential genetic influences can be studied.
In this report, additive and dominance effects differed as a function of stimulus type and frequency band. There is evidence from other neuroimaging studies for nonadditive genetic effects on brain function (Tan et al., 2009). Indeed, some of the components that fit the ADE showed evidence for the genetic influence coming primarily from the dominance component, with a minimal loading on the additive component. It has been suggested that more similar environments for MZ over DZ twins could mimic dominance, emergenic, or epistatic effects (Christian et al., 1975). Similar ICCs for MZs reared together and those reared apart (van Beijsterveldt & Boomsma, 1994) indicate that postnatal environments do not unduly influence variations in brain activity for MZ twins. Other data do implicate prenatal environment in determining variations in brain activity. For instance, MZ twins with monochorionic placentas are more alike for measures of type A behavior (Reed et al., 1991), personality scores (Sokol et al., 1995), IQ scores (Melnic et al., 1978), and may have higher concordance for schizophrenia (Davis et al., 1995). DZ twins are dichorionic, so it would seem that additional covariance between monochorionic MZs over dichorionic DZs may inflate heritability estimates and mimic dominance effects. Since approximately 66% of MZ twins are monochorionic, the total phenotypic variance of MZs across placental types may be greater than that of DZs, which may actually downwardly bias heritability estimates (Christian et al., 1996). If so, nonadditive genetic effects could be even higher than those estimated here. This will be an important issue to consider in future studies with large samples.
Future studies could also benefit from some methodological refinements. First, higher sensor densities with more recording locations below the cantho-meatal line would help fully capture visual cortex-related neural activities. Second, not with standing the heavier computational demands imposed by dense array ERP analysis, larger sample sizes would provide more power to evaluate the fit of different models as well as more precise heritability estimates. Larger sample sizes would also determine if the lack of biometric model fit and low ICCs that occurred in some of the higher frequency bands, particularly in STP, were a result of low amplitude responses where most of scores were near zero, thus restricting the range of variance in these particular variables. ITC is not dependent on amplitude of response, and good model fit for ITC for the higher frequencies inspires confidence in those results. Given the growing interest in high frequency oscillatory responses in brain research, more study with larger samples will be necessary in order to better resolve whether environmental influences or quantification factors contribute significantly to biometric modeling of these variables.
Nevertheless, the present methodology and results illustrate the possible advantages of the current general approach over those typically encountered in studies addressing the genetics of brain activations. The present study illustrates the importance of considering multiple measures of brain activity, rather than voltage measurements from a single sensor alone, in the search for heritable and refined phenotypes for complex cognitive operations. Single peaks of brain activity do not occur in isolation. Complementing single peak amplitude measures with single sensors to more complex analysis of brain activity may better represent the complex organization of the neural response to a given stimulus and task. In turn, refining phenotypes using data reduction/integration techniques may more accurately characterize gene effects on certain complex cognitive operations.
Highlights
Heritable brain responses follow patterns associated with common frequency bands.
Connection between biological mechanisms of brain oscillations and inheritance.
Peaks in heritability do not necessarily correspond to peaks in EEG amplitude.
Composite broad sense heritability as a guide for specific genetic modeling.
Data reduction/integration methods may more accurately characterize gene effects.
Acknowledgments
Funding This work was supported by the National Institutes of Health (DA 05147, AA 09367, and DA 024417 to W.G.I and K01 AA015621 to S.M.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almasy L, Porjesz B, Blangero J, Chorlian DB, O'Connor SJ, Kuperman S, et al. Heritability of event-related brain potentials in families with a history of alcoholism. Am J Med Genet. 1999;88(4):383–390. [PubMed] [Google Scholar]
- Andrew C, Fein G. Event-related oscillations versus event-related potentials in a P300 task as biomarkers for alcoholism. Alcohol Clin Exp Res. 2010;34(4):669–680. doi: 10.1111/j.1530-0277.2009.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin AP, van Baal GC, van Beijsterveldt CE, de Geus EJ, Grant J, Boomsma DI. Genetic correlation between the P300 event-related brain potential and the EEG power spectrum. Behav Genet. 2001;31(6):545–554. doi: 10.1023/a:1013341310865. [DOI] [PubMed] [Google Scholar]
- Basar E, Demiralp T, Schurmann M, Basar-Eroglu C, Ademoglu A. Oscillatory brain dynamics, wavelet analysis, and cognition. Brain and Language. 1999;66:146–183. doi: 10.1006/brln.1998.2029. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Demiralp T, Schurmann M, Basar E. Topological distribution of oddball `P300' responses. Int J Psychophysiol. 2001;39(2–3):213–220. doi: 10.1016/s0167-8760(00)00142-2. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225(4669):1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, et al. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol. 1998;108(3):244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Bestelmeyer PE, Phillips LH, Crombie C, Benson P, St Clair D. The P300 as a possible endophenotype for schizophrenia and bipolar disorder: Evidence from twin and patient studies. Psychiatry Res. 2009;169(3):212–219. doi: 10.1016/j.psychres.2008.06.035. [DOI] [PubMed] [Google Scholar]
- Bramon E, McDonald C, Croft RJ, Landau S, Filbey F, Gruzelier JH, et al. Is the P300 wave an endophenotype for schizophrenia? A meta-analysis and a family study. Neuroimage. 2005;27(4):960–968. doi: 10.1016/j.neuroimage.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG. Heritability of P300 amplitude development from adolescence to adulthood. Psychophysiology. 2006;43(5):470–480. doi: 10.1111/j.1469-8986.2006.00450.x. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Iacono WG, McGue M. P300 amplitude in nonalcoholic adolescent twin pairs who become discordant for alcoholism as adults. Psychophysiology. 2004;41(6):841–844. doi: 10.1111/j.1469-8986.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- Chatrian GE, Lettich E, Nelson PL. Ten percent electrode system for topographic studies of spontaneous and evoked EEG activity. Am J EEG Technol. 1985;25:83–92. [Google Scholar]
- Christian JC, Feinleib M, Norton JA., Jr. Letters to the editor: Statistical analysis of genetic variance in twins. Am J Hum Genet. 1975;27(6):807. [PMC free article] [PubMed] [Google Scholar]
- Christian JC, Morzorati S, Norton JA, Jr., Williams CJ, O'Connor S, Li TK. Genetic analysis of the resting electroencephalographic power spectrum in human twins. Psychophysiology. 1996;33(5):584–591. doi: 10.1111/j.1469-8986.1996.tb02435.x. [DOI] [PubMed] [Google Scholar]
- Davis JO, Phelps JA, Bracha HS. Prenatal development of monozygotic twins and concordance for schizophrenia. Schizophr Bull. 1995;21(3):357–366. doi: 10.1093/schbul/21.3.357. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Demiralp T, Yordanova J, Kolev V, Ademoglu A, Devrim M, Samar VJ. Time-frequency analysis of single-sweep event-related potentials by means of fast wavelet transform. Brain Lang. 1999;66(1):129–145. doi: 10.1006/brln.1998.2028. [DOI] [PubMed] [Google Scholar]
- Devrim M, Demiralp T, Ademoglu A, Kurt A. A model for P300 generation based on responses to near-threshold visual stimuli. Brain Res Cogn Brain Res. 1999;8(1):37–43. doi: 10.1016/s0926-6410(99)00007-5. [DOI] [PubMed] [Google Scholar]
- Dien J. Progressing towards a consensus on PCA of ERPs. Clin Neurophysiol. 2006;117(3):699–702. doi: 10.1016/j.clinph.2005.09.029. author reply 703-697. [DOI] [PubMed] [Google Scholar]
- Eaves LJ. Dominance alone is not enough. Behav Gen. 1986;18(1):27–33. doi: 10.1007/BF01067073. [DOI] [PubMed] [Google Scholar]
- Ergen M, Marbach S, Brand A, Basar-Eroglu C, Demiralp T. P3 and delta band responses in visual oddball paradigm in schizophrenia. Neurosci Lett. 2008;440(3):304–308. doi: 10.1016/j.neulet.2008.05.054. [DOI] [PubMed] [Google Scholar]
- Fell J, Dietl T, Grunwald T, Kurthen M, Klaver P, Trautner P, et al. Neural bases of cognitive ERPs: more than phase reset. J Cogn Neurosci. 2004;16(9):1595–1604. doi: 10.1162/0898929042568514. [DOI] [PubMed] [Google Scholar]
- Frederick JA, Iacono WG. Beyond the DSM: defining endophenotypes for genetic studies of substance abuse. Curr Psychiatry Rep. 2006;8(2):144–150. doi: 10.1007/s11920-006-0014-2. [DOI] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2009;47(1):123–132. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Bernat EM, Iacono WG. Relationship between the P3 event-related potential, its associated time-frequency components, and externalizing psychopathology. Psychophysiology. 2010a;47(1):123–132. doi: 10.1111/j.1469-8986.2009.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Malone SM, Iacono WG. Brain electrophysiological endophenotypes for externalizing psychopathology: a multivariate approach. Behav Genet. 2010b;40(2):186–200. doi: 10.1007/s10519-010-9343-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gruber WR, Klimesch W, Sauseng P, Doppelmayr M. Alpha phase synchronization predicts P1 and N1 latency and amplitude size. Cereb Cortex. 2005;15(4):371–377. doi: 10.1093/cercor/bhh139. [DOI] [PubMed] [Google Scholar]
- Hall MH, Schulze K, Rijsdijk F, Kalidindi S, McDonald C, Bramon E, et al. Are auditory P300 and duration MMN heritable and putative endophenotypes of psychotic bipolar disorder? A Maudsley Bipolar Twin and Family Study. Psychol Med. 2009;39(8):1277–1287. doi: 10.1017/S0033291709005261. [DOI] [PubMed] [Google Scholar]
- Hall MH, Spencer KM, Schulze K, McDonald C, Kalidindi S, Kravariti E, et al. The genetic and environmental influences of event-related gamma oscillations on bipolar disorder. Bipolar Disord. 2011;13(3):260–271. doi: 10.1111/j.1399-5618.2011.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Ethridge LE, Shapiro JR, Stevens MC, Boutros NN, Summerfelt AT, et al. Spatio-temporal and frequency domain analysis of auditory paired stimuli processing in schizophrenia and psychotic bipolar disorder. Psychophys. 2012 doi: 10.1111/j.1469-8986.2011.01327.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselbrock V, Begleiter H, Porjesz B, O'Connor S, Bauer L. P300 event-related potential amplitude as an endophenotype of alcoholism--evidence from the collaborative study on the genetics of alcoholism. J Biomed Sci. 2001;8(1):77–82. doi: 10.1007/BF02255974. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM. Identifying a multivariate endophenotype for substance use disorders using psychophysiological measures. Int J Psychophysiol. 2000;38(1):81–96. doi: 10.1016/s0167-8760(00)00132-x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59(8):750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11(4):869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Clementz BA. A strategy for elucidating genetic influences on complex psychopathological syndromes (with special reference to ocular motor functioning and schizophrenia) Prog Exp Pers Psychopathol Res. 1993;16:11–65. [PubMed] [Google Scholar]
- Jansen BH, Agarwal G, Hegde A, Boutros NN. Phase synchronization of the ongoing EEG and auditory EP generation. Clin Neurophysiol. 2003;114(1):79–85. doi: 10.1016/s1388-2457(02)00327-9. [DOI] [PubMed] [Google Scholar]
- Katsanis J, Iacono WG, McGue MK, Carlson SR. P300 event-related potential heritability in monozygotic and dizygotic twins. Psychophysiology. 1997;34(1):47–58. doi: 10.1111/j.1469-8986.1997.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Klimesch W, Schack B, Schabus M, Doppelmayr M, Gruber W, Sauseng P. Phase-locked alpha and theta oscillations generate the P1–N1 complex and are related to memory performance. Brain Res Cogn Brain Res. 2004;19(3):302–316. doi: 10.1016/j.cogbrainres.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Kilmesch W, Sauseng P, Hanslmayr S, Gruber W, Freunberger R. Event-related phase reorganization may explain evoked neural dynamics. Neurosci Biobehav Rev. 2007;31(7):1003–1016. doi: 10.1016/j.neubiorev.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Linden DE. The p300: where in the brain is it produced and what does it tell us? Neuroscientist. 2005;11(6):563–576. doi: 10.1177/1073858405280524. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. Event-related potentials: Methodology and quantification. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic principles, clinical applications, and related fields. 3rd ed. Williams & Wilkins; Baltimore: 1993. pp. 877–886. [Google Scholar]
- Lykken DT. The mechanism of emergenesis. Genes Brain Behav. 2006;5(4):306–310. doi: 10.1111/j.1601-183X.2006.00233.x. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Tellegen A, Iacono WG. EEG spectra in twins: Evidence for a neglected mechanism of genetic determination. Physiological Psychology. 1982;10:60–65. [Google Scholar]
- MacQueen JB. Some methods for classification and analysis of multivariate observations. Proceedings of 5th Berkeley Symposium on Mathematical Statistics and Probability; University of California Press; 1967. pp. 281–297. [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295(5555):690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Melnick M, Myrianthopoulos NC, Christian JC. The effects of chorion type on variation in IQ in the NCPP twin population. Am J Hum Genet. 1978;30(4):425–433. [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7(10):818–827. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Moratti S, Clementz BA, Gao Y, Ortiz T, Keil A. Neural mechanisms of evoked oscillations: stability and interaction with transient events. Hum Brain Mapp. 2007;28(12):1318–1333. doi: 10.1002/hbm.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 2003 [Google Scholar]
- Norusis M. IBM SPSS Statistics 19 Statistical Procedures Companion. Pearson; New York: 2011. [Google Scholar]
- O'Connor S, Morzorati S, Christian JC, Li TK. Heritable features of the auditory oddball event-related potential: peaks, latencies, morphology and topography. Electroencephalogr Clin Neurophysiol. 1994;92(2):115–125. doi: 10.1016/0168-5597(94)90052-3. [DOI] [PubMed] [Google Scholar]
- Polich J, Burns T. P300 from identical twins. Neuropsychologia. 1987;25(1B):299–304. doi: 10.1016/0028-3932(87)90143-6. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Neale MC, Boomsma DI, de Geus EJ. Are smarter brains running faster? Heritability of alpha peak frequency, IQ, and their interrelation. Behav Genet. 2001;31(6):567–579. doi: 10.1023/a:1013345411774. [DOI] [PubMed] [Google Scholar]
- Reed T, Carmelli D, Rosenman RH. Effects of placentation on selected Type A behaviors in adult males in the National Heart, Lung, and Blood Institute (NHLBI) twin study. Behav Genet. 1991;21(1):9–19. doi: 10.1007/BF01067663. [DOI] [PubMed] [Google Scholar]
- Salenius S, Salmelin R, Neuper C, Pfurtscheller G, Hari R. Human cortical 40 Hz rhythm is closely related to EMG rhythmicity. Neurosci Lett. 1996;213:75–78. doi: 10.1016/0304-3940(96)12796-8. [DOI] [PubMed] [Google Scholar]
- Salvador S, Chan P. Determining the number of clusters/segments in hierarchical clustering/segmentation algorithms. Proceedings of the 16th IEEE International Conference on Tools with Artificial Intelligence (ICTAI 2004).2004. pp. 576–584. [Google Scholar]
- Sayers BM, Beagley HA, Henshall WR. The mechansim of auditory evoked EEG responses. Nature. 1974;247(441):481–483. doi: 10.1038/247481a0. [DOI] [PubMed] [Google Scholar]
- Schulze KK, Hall MH, McDonald C, Marshall N, Walshe M, Murray RM, et al. Auditory P300 in patients with bipolar disorder and their unaffected relatives. Bipolar Disord. 2008;10(3):377–386. doi: 10.1111/j.1399-5618.2007.00527.x. [DOI] [PubMed] [Google Scholar]
- Simson R, Vaughn HG, Jr., Ritter W. The scalp topography of potentials in auditory and visual discrimination tasks. Electroencephalogr Clin Neurophysiol. 1977;42(4):528–535. doi: 10.1016/0013-4694(77)90216-4. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Boersma M, van Beijsterveldt CE, Posthuma D, Boomsma DI, Stam CJ, et al. Endophenotypes in a dynamically connected brain. Behav Genet. 2010;40(2):167–177. doi: 10.1007/s10519-009-9330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, De Geus EJ. Heritability of anterior and posterior visual N1. Int J Psychophysiol. 2007;66(3):196–204. doi: 10.1016/j.ijpsycho.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, De Geus EJ. Phenotypic and genetic correlations between evoked EEG/ERP measures during the response anticipation period of a delayed response task. Psychophysiology. 2009;46(2):344–356. doi: 10.1111/j.1469-8986.2008.00777.x. [DOI] [PubMed] [Google Scholar]
- Smit DJ, Posthuma D, Boomsma DI, Geus EJ. Heritability of background EEG across the power spectrum. Psychophysiology. 2005;42(6):691–697. doi: 10.1111/j.1469-8986.2005.00352.x. [DOI] [PubMed] [Google Scholar]
- Sokol DK, Moore CA, Rose RJ, Williams CJ, Reed T, Christian JC. Intrapair differences in personality and cognitive ability among young monozygotic twins distinguished by chorion type. Behav Genet. 1995;25(5):457–466. doi: 10.1007/BF02253374. [DOI] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Crit Rev Neurobiol. 2000;14(3–4):199–224. [PubMed] [Google Scholar]
- Steinhauer SR, Hill SY, Zubin J. Event-related potentials in alcoholics and their first-degree relatives. Alcohol. 1987;4(4):307–314. doi: 10.1016/0741-8329(87)90028-0. [DOI] [PubMed] [Google Scholar]
- Surwillo WW. Cortical evoked potentials in monozygotic twins and unrelated subjects: comparisons of exogenous and endogenous components. Behav Genet. 1980);10(2):201–209. doi: 10.1007/BF01066270. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Prefrontal cognitive systems in schizophrenia: towards human genetic brain mechanisms. Cogn Neuropsychiatry. 2009;14(4–5):277–298. doi: 10.1080/13546800903091665. [DOI] [PubMed] [Google Scholar]
- Ucles P, Mendez M, Garay J. Low-level defective processing of non-verbal sounds in dyslexic children. Dyslexia. 2009;15(2):72–85. doi: 10.1002/dys.360. [DOI] [PubMed] [Google Scholar]
- van Baal GC, De Geus EJ, Boomsma DI. Genetic architecture of EEG power spectra in early life. Electroencephalogr Clin Neurophysiol. 1996;98(6):502–514. doi: 10.1016/0013-4694(96)95601-1. [DOI] [PubMed] [Google Scholar]
- van Baal GC, de Geus EJ, Boomsma DI. Longitudinal study of genetic influences on ERP-P3 during childhood. Devel Neuropsych. 1998;14(1):19–45. [Google Scholar]
- van Beijsterveldt CE, Boomsma DI. Genetics of the human electroencephalogram (EEG) and event-related brain potentials (ERPs): a review. Hum Genet. 1994;94(4):319–330. doi: 10.1007/BF00201587. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Individual differences in P300 amplitude: a genetic study in adolescent twins. Biol Psychol. 1998;47(2):97–120. doi: 10.1016/s0301-0511(97)00025-2. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61(1–2):111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC, Molenaar PC, Boomsma DI, de Geus EJ. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behav Genet. 2001;31(6):533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- Yoon HH, Iacono WG, Malone SM, McGue M. Using the brain P300 response to identify novel phenotypes reflecting genetic vulnerability for adolescent substance misuse. Addict Behav. 2006;31(6):1067–1087. doi: 10.1016/j.addbeh.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Zietsch BP, Hansen JL, Hansell NK, Geffen GM, Martin NG, Wright MJ. Common and specific genetic influences on EEG power bands delta, theta, alpha, and beta. Biol Psychol. 2007;75(2):154–164. doi: 10.1016/j.biopsycho.2007.01.004. [DOI] [PubMed] [Google Scholar]