Abstract
Therapeutic mild hypothermia has been widely used in brain injury. It has been evaluated in numerous clinical trials, and there is strong evidence for the use of hypothermia in treating patients with several types of ischemic/reperfusional (I/R) injuries, the examples being cardiac arrest and neonatal hypoxic-ischemic encephalopathy. In spite of many basic research projects demonstrating effectiveness, therapeutic hypothermia has not been proved effective for the heterogeneous group of patients with traumatic brain injury (TBI) in multicenter clinical trials. In the latest clinical trial, however, researchers were able to demonstrate the significant beneficial effects of hypothermia in one specific group; patients with mass evacuated lesions. This suggested that mild therapeutic hypothermia might be effective for I/R related TBI. In this article, we have reviewed much of the previous literature concerning the mechanisms of I/R injury to the protective effects of mild therapeutic hypothermia.
Introduction
Historically, hypothermia was induced before surgery to assist procedures that caused prolonged ischemia, including open heart surgery (Lewis, 1955; Swan, 1956; Sealy et al., 1958) and various organ transplants (Moossa et al., 1976). Within its first decade, hypothermia was applied to multiple emergency situations that were characterized by ischemia such as stroke (De Georgia et al., 2004; Hemmen et al., 2010), myocardial infarction (Miki et al., 1998; Dae et al., 2002), and cardiac arrest (Abella et al., 2004; Janata and Holzer, 2009).
The specific protective mechanisms of hypothermia in ischemic injury remain unclear. Most assume its effects are demonstrated in the reperfusion stage of the injury. The best clinical evidence to date comes from studying hypothermia in cardiac arrest patients (Bernard et al., 2002; HACA Study Group, 2002). In light of these trials, more attention was focused on the specific ability of hypothermia therapy to blunt the effects of reperfusion injury after ischemia and act as a neuroprotectant.
Acute subdural hematomas (ASDHs) represent a prevalent traumatic brain injury (TBI) with ischemic/reperfusional (I/R) pathophysiology. In the experimental model, ischemia occurs during ASDH expansion and is followed by reperfusion after removal (Kuroda and Bullock, 1992). In the National Acute Brain Injury Study: Hypothermia II (NABIS: H II), the latest clinical trial to confirm the efficacy of very early hypothermia in patients with severe brain injury, preoperative- and prereperfusion-induced hypothermia proved efficacious for a subset of patients with evacuated mass lesions, a large number of which were ASDH (Clifton et al., 2011). Though this pointed to a potentially beneficial role for hypothermia in TBI, further elucidation was needed.
To clarify the appropriate design of further clinical trials using therapeutic mild hypothermia in traumatic I/R brain injury, we reviewed and summarized several articles discussing the mechanisms of I/R injury and the efficacy of therapeutic hypothermia, especially in traumatic I/R brain injury.
The Mechanisms of I/R Brain Injury and Hypothermia Treatment
Despite much research, the exact mechanisms of the I/R injury itself remain unclear. Reperfusion after ischemia can cause neurovascular injury leading to detrimental changes in blood brain barrier permeability (Abulrob et al., 2011), cerebral edema (Elali et al., 2011), brain hemorrhage (Natarajan et al., 2010), and neuronal death by apoptosis/necrosis (Dietrich, 1994). These complications clearly limit the benefits of reperfusional therapies. For example, in the clinical situation, reperfusion after brain ischemia often leads to hemorrhage with relatively poor outcomes (De Marchis et al., 2011).
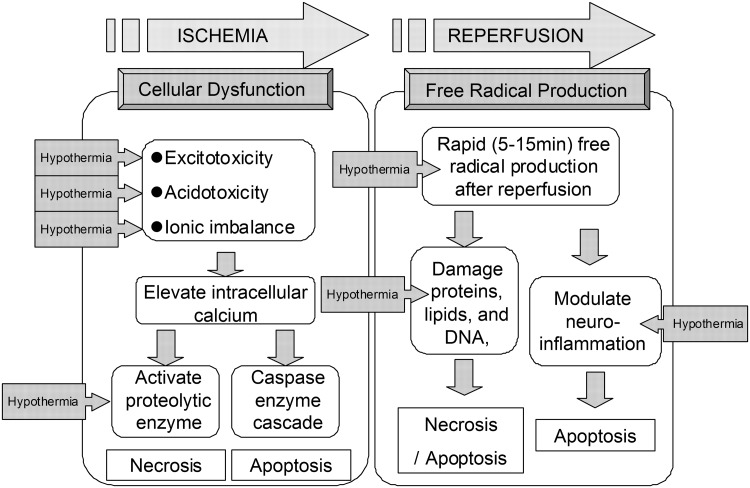
The processes leading to cellular damage after I/R injury are complex and multi-factorial. At this point, the pathology of I/R injury has been separated into two distinct mechanisms. One is the cell death after cellular dysfunction, that is, excitotoxcity, acidotoxicity, ionic imbalance, and abnormal cell signaling. This first process is seen primarily in the ischemic phase. The other type of injury comes from free radical production, and this becomes particularly bad during the reperfusion phase (Lampe and Becker, 2011). Together, these mechanisms create a complicated picture of injury (Fig. 1).
FIG. 1.
Schematic illustrating the mechanisms of ischemic/reperfusional (I/R) injury and the effects of therapeutic hypothermia. We illustrate the schema of mechanisms of I/R brain injury and the effective point of hypothermia treatment. The pathology of I/R injury is approximately separated as two mechanisms, that is, the cell death after cellular dysfunction in ischemic phase, and the free radical production in reperfusion phase. The boxed arrow with entered “Hypothermia” means the estimated effective points in I/R cascade.
In the ischemic phase, brain ischemia initiates a cascade of destructive and often irreversible processes that destroy brain cells and tissue. One example of this is the intracellular conversion to anaerobic metabolism (Polderman, 2009). Depletion of adenosine triphosphate (ATP) in the absence of oxidative metabolism leads to failure of the Na+/K+ ATPase pump. This causes depolarization of the cell membrane leading to activation of voltage-gated calcium channels and an influx of intracellular calcium (Badruddin et al., 2011). Moreover, with the anerobic metabolism induced, intracellular and extracellular acidosis contributes to the calcium influx. This rapid increase in intracellular calcium causes release of large amounts of the excitatory neurotransmitter glutamate, which further stimulates calcium influx in postsynaptic cells (Simon, 2006). In addition to what has been mentioned, calcium triggers activation of phospholipase, nitric oxide synthase, proteases, endonucleases, and oxidase enzymes (Wahlgren and Ahmed, 2004). These activated molecules can easily damage other cell proteins and lipid membranes causing necrosis (Leker and Shohami, 2002). Furthermore, recent studies have demonstrated the production of superoxide radicals by N-methyl-D-aspartate receptor-mediated nicotinamide adenine dinucleotide phosphate oxidase activation (Brennan et al., 2009). Such events amplify reactive oxygen species production, mitochondrial dysfunction, and proapoptotic protein activation. Intracellular calcium accumulation itself also triggers initiation of mitochondrial dysfunction and fragmentation leading to activation of proapoptotic proteins such as the caspases (Eldadah and Faden, 2000).
Reperfusion to this ischemic tissue results in a short period of excessive free radical production (Tuttolomondo et al., 2009). Experimental measurements of the reperfusion phase demonstrate that oxygen- and carbon-centered free radicals peak within 5 minutes of reperfusion (Bolli et al., 1989) and that hydroxyl generation peaks within 15 minutes (Khalid and Ashraf, 1993). This oxidative stress can damage proteins, lipids, and DNA, possibly leading to necrosis and apoptosis (Halliwell, 1994; Sugawara and Chan, 2003). Oxidants also modulate neuro-inflammation (Wong and Crack, 2008) leading to increased levels of neuronal apoptosis in adjacent cells (Huang et al., 2007; Jung et al., 2010; Lv et al., 2011).
Despite much basic and clinical research using hypothermia in I/R brain injury, the mechanisms of its neuronal protection remain unclear. Therapeutic hypothermia is a promising neuroprotective intervention shown to improve outcome from brain ischemia in humans, and the neuroprotective effects of hypothermia has been well established in experimental animals (Yanamoto et al., 1999; Kawai et al., 2000; Miyazawa et al., 2003). Although the key mechanisms have not been clarified, hypothermic neuroprotection may provide insight into I/R pathophysiology and suggest a novel therapeutic target (Shintani et al., 2010). Most believe it to act through a multitude of different pathways. Mitochondrial-free radical production might be an important target, and it provides a possible window of opportunity for hypothermia treatment (Kil et al., 1996). Supporting this point, hypothermia has been shown to decrease abnormal production of free radicals (Shao et al., 2010). Another potential mechanism of hypothermia involves reduction of the inflammatory cascade including neutrophil infiltrations (Wang et al., 2002) and cell death pathways of apoptosis and necrosis (Yang et al., 2009; Yenari and Hemmen, 2010).
Hypothermia also reduces cellular metabolism and oxygen demand while maintaining acceptable ATP levels (Erecinska et al., 2003). Likewise, it improves cellular ion handling and cellular pH balance (Polderman, 2009). In the issue of cell signaling, for example, nuclear factor κ B, hypothermia also gives preventable effects for neural cell death after ischemia (Han et al., 2003).
In Figure 1, we illustrate the schema of mechanisms of I/R injury and the estimated points where hypothermia treatment can effect.
I/R Pathophysiology in TBI
The ischemic mechanism has been considered one of the important parts of TBI pathophysiology. Actually, some primary and secondary brain damages are surely related to ischemic insults. For example, in severe fluid percussion injury rat model, Dietrich et al. (1998) revealed the severe reduction of local cerebral blood flow (CBF) in post-traumatic phase with an autoradiographic and histopathological study. In the post-traumatic phase, systemic hypotension and hypothermia certainly deteriorate cerebral ischemia after severe brain injury (Chesnut et al., 1993). On the other hand, to mitigate this secondary ischemic insult, post-traumatic hypothermia therapy has been said to be effective (Matsushita et al., 2001).
I/R pathophysiology in TBI patients was first described by Muizelaar (1993). They used stable Xenon-computed tomography and measured CBF in 26 patients with traumatic head injury (Muizelaar, 1993). They advocated that I/R pathophysiology was a significant part of certain traumatic brain injuries.
In 1990, ischemic brain damage was first described in the ASDH rat model by Miller et al. They injected autologous blood into the subdural space and induced ASDH. They confirmed the ischemic change with histological estimations (Miller et al., 1990). Kuroda and Bullock also used a similar rat ASDH model to autoradiographically map regional CBF before and after removal of hematoma. They concluded that a major cause of hemisphere swelling, seen after hematoma removal, was due to enlargement of the zone experiencing focal tissue ischemia, just beneath the hematoma, and that neuronal damage continued even after decompressive craniotomy (Kuroda and Bullock, 1992). Epidural hematomas are related to this I/R pathophysiology as well. In one experimental study, a rat epidural balloon compression model was used, and researchers examined CBF measured with laser doppler flowmetry, brain tissue oxygenation (PtiO2), magnetic resonance imaging, and histological changes in brain tissue. In the time period during balloon inflation, the value of CBF and PtiO2 decreased, recovering with balloon deflation. This study also demonstrated that intraischemic hypothermia might reduce the ischemia associated tissue damage and hippocampal neuronal cell injury (Burger et al., 2004).
The evidence from these previous reports allows us to say that traumatic focal brain injury concurrent with evacuated mass lesions, that is, subdural or epidural hematomas, represents an I/R injury.
The Appropriate Timing of Hypothermia Induction
The appropriate and effective timing of hypothermia induction in brain injury is also in controversy. The previous studies have shown that hypothermia should be achieved within 2 to 6 hours of severe hypoxic-ischemic injury in sheep, gerbils, and rats to afford protection. For example, cooling sheep to 34°C for 72 hours gave good protection if started 90 minutes after the injury, was partly effective if started at 5.5 hours, and ineffective if started at 8.5 hours (Gunn, 2000). On the other hand, some experimental reports with delayed induction of therapeutic hypothermia in ischemic rat model hypothermia also exist. In the study of Colbourne et al. (1999), a 48-hour period of mild hypothermia was induced starting 6 hours after 10 minutes of severe four-vessel occlusion ischemia in rats. Untreated normothermic ischemia resulted in total CA1 cell loss (99%), whereas delayed hypothermia treatment reduced neuronal loss to 14% at a 28-day survival. These results also indicates the potential of late, but prolonged hypothermia in reperfusional phase might be effective in I/R rat model (Colbourne and Corbett, 1994; Colbourne et al., 1999).
Most clinical trials have suggested that the earlier that mild hypothermia is initiated, the more likely beneficial effects may be obtained. Clinical data further show that mild hypothermia within 6 hours after injury may be effective (Shann, 2003). Hypothermia is currently being induced by surface cooling with use of cooling blankets, which usually requires 4 to 8 hours to get the target hypothermia temperature (33°C to 35°C) (Schwab et al., 1998; Jiang et al., 2000; Clifton et al., 2001; Bernard and Buist, 2003).
Bernard et al. (2002) reported that cooling can be achieved more rapidly (2°C over 30 minutes) by intravenous administration of iced (4°C) crystalloid solution. This use of cold intravenous fluids may represent a logical strategy for future clinical trials for hypothermia in severe TBI.
Therapeutic Window for Hypothermia Treatment and the Rewarming Phase
Since the I/R type of injury seen in ASDH and epidural hematomas differs from the other injuries commonly encountered in TBI, there are no clear conclusions on the optimal initiation and length of hypothermia treatment in this I/R subgroup of TBI. The I/R injury resembles the cellular injury seen in acute stroke patients and, therefore, might share some elements for this type of hypothermia treatment. In a recent review of thirteen clinical studies on the use of hypothermia in acute stroke, the treatment was initiated between 6 and 22 hours after onset of symptoms and maintained for a mean of 40.9 hours (range 24–67h) (Groysman et al., 2011). Previous animal studies have showed that hypothermia should be achieved within 2 to 6 hours of severe hypoxic-ischemic injury to afford protection. Cooling of sheep to 34°C for 72 hours gave good protection if initiated 90 minutes after the injury, was partly effective if started at 5.5 hours, and ineffective if started at 8.5 hours (Gunn, 2000). In rat global forebrain ischemia model, intraischemic brain hypothermia therapy (30°C) provided chronic protection to the hippocampus after transient brain ischemia (Dietrich et al., 1993). Moreover, this showed that delayed cooling was not permanently protective in this rat global ischemia model. On the contrary, as just described, delayed but prolonged cooling method could have benefits for the neuroprotection in gerbil experimental model (Colbourne and Corbett, 1994, 1995). This topic is still controversial. The optimum duration of hypothermia for hypoxic-ischemic injury may depend on the severity of the injury and the delay before therapeutic hypothermia is achieved. Within limits, longer cooling could compensate for the severity of injury or delay in initiating treatment.
In the I/R injury, the evacuated mass lesion relieves the ICP, and the main intention of hypothermia is to blunt the reperfusion injury and the cascades set off by the ischemia. In a combined injury mechanism, there is still risk for delayed edema with an increased risk of progression for several days after injury. In fact, beneficial effects on secondary injury mechanisms may have occurred in patients treated with mild or moderate hypothermia for longer than 48 hours, despite the established risks of complications from prolonged moderate hypothermia, because cerebral swelling and brain edema are often greatest at 48 hours after injury in this patient group. Hypoxic-ischemic damage occurs in 90% patients who die from TBI, which suggests that such patients benefit from early-induced hypothermia of sufficient depth and duration in order to minimize hypoxic-ischemic damage (Shann, 2003). In a randomized prospective trial by Clifton et al., 2001 hypothermia was initiated in 199 patients within 6h of injury. The duration of the mild hypothermia was 48 hours, which may have been too short to control brain edema and intracranial hypertension failing to demonstrate the beneficial effects on outcome in unstratified TBI patients (Clifton et al., 2001). Jiang et al. (2009) performed a randomized study to compare the effect of long-term (5 days) mild hypothermia versus short-term (2 days) mild hypothermia, suggesting that mild hypothermia may improve the outcome in a series of 215 severe adult TBI patients, when cooling is maintained for longer than 48 hours. Clinical data further show that mild hypothermia within 6 hours after injury may be effective in TBI patients (Shann, 2003). Based on these results, patients with severe TBI may benefit from being cooled for longer than patients with pure I/R injury, where an early-induced hypothermia seem to be beneficial. Considering both experimental and clinical literature on the rate of rewarming is an important variable for influencing the protective effects of the hypothermia therapy. In the experimental setting, post-traumatic hypothermia followed by slow rewarming appears to provide maximal protection in terms of traumatically induced axonal damage, microvascular damage and dysfunction, and contusional expansion (Matsushita et al., 2001; Suehiro et al., 2003). In contrast, hypothermia followed by rapid rewarming not only reverses the protective effects associated with hypothermic intervention, but also, in many cases, exacerbates the traumatically induced pathology and its functional consequences (Suehiro et al., 2003; Hayashi 2009; Povlishock and Wei, 2009).
The Results of Multicenter Clinical Trials of Hypothermia and Their Interpretation
Previous clinical trials have been unable to establish the efficacy of hypothermia. In a multicenter trial using hypothermia as an intervention (Clifton et al., 2001), 392 patients with acute brain injury were randomized to normothermia or surface-induced hypothermia groups. Unfortunately, no improvement in outcome was noted between temperature groups (NABIS: H). However, there was some weak evidence of improved outcomes in patients who were hypothermic on admission and treated with continued hypothermia (Clifton et al., 2001). This same group then tried to determine the efficacy of very early hypothermia in patients with severe brain injury; NABIS: H II (Clifton et al., 2011). In NABIS: H II, the early-induced hypothermia similarly did not demonstrate efficacy when researchers looked at mortality and morbidity data. On the other hand, in a sub-population analysis that divided the diffuse brain injury patients and those with surgical hematoma evacuation, early-induced hypothermia proved efficacious for the later group (poor outcome ratio; 33% in hypothermia group vs. 69% in normothermia group, (p=0.02). Authors concluded that one explanation was the different pathophysiology between diffuse brain injury and hematoma. As just mentioned, ischemia occurs during ASDH expansion followed by reperfusional injury after removal (Kuroda and Bullock, 1992; Muizelaar, 1993). This injury has similar pathophysiology to that seen in patients with cardiac arrest—a group that has been successfully treated with hypothermia (Janata and Holzer, 2009). As previously mentioned, encouraging experimental data demonstrated that intraischemic hypothermia before hematoma removal was associated with improved outcome (Burger et al., 2004). Diffuse brain injury is not characterized by ischemia in in vitro studies and would not be a good candidate for hypothermia treatment (Farkas and Povlishock, 2007). The authors of NABIS: H II concluded that their finding of improved outcome in patients with evacuated hematomas warranted further study.
Future Implications of Early-Induced Hypothermia Study for Traumatic I/R Brain Injury
As just described, the effectiveness of early- or preoperative-induced hypothermia especially in patients with evacuated hematomas should be verified with a larger multicenter clinical study in near future. Clifton et al. (2011) Therefore, we also should know feasibility and safety in the treatment of preoperative hypothermia. However, unfortunately, there have never been randomized clinical trials for preoperative-induced hypothermia in I/R TBI such as ASDH. In reviewing several reports that used intraoperative hypothermia in neurosurgical procedures involving craniotomy, much can be learned about temperature reduction between pre-intraoperative periods. (Table 1) (Clifton and Christensen, 1992; Baker et al., 1994; Hindman et al., 1999, 2010; Steinberge et al., 2004; Todd et al., 2005; Choi et al., 2009). The basic issues of feasibility and safety of mild hypothermia in a neurosurgical setting were first described by Baker et al. (1994). They concluded that intraoperative mild hypothermia can be easily and safely achieved.
Table 1.
Clinical Studies Using Intraoperative Hypothermia for Neurosurgical Procedure
| Authors and year | No. of cases | Operative procedure (number) | Cooling method | Complication | Mean target temperature (°C) | Mean duration of hypothermia (minutes) | Mean rewarming rate (°C/hour) | Mean rewarming temperature (°C) | Effectiveness of hypothermia |
|---|---|---|---|---|---|---|---|---|---|
| Baker et al., 1994 | 30 (Normo 17, Hypo 13) | Elective craniotomy for supratentorial tumor resection (14), aneurysm repair (14) other (2) | WB | Shivering (Normo 0 case vs. Hypo 7 cases, p=0.002) no severe complications | 34.3±0.4 | NR | 0.7±0.6 | 35.8±1.0 | NR |
| Clifton and Christensen, 1992 | 21 Hypo | Aneurysm surgery with elective craniotomy (21) | WB | No complications | 32.0 | NR | NR | NR | NR |
| Hindman et al., 1999 | 114 (Normo 57, Hypo 57) | SAH clipping (52), unruptured aneurysm clipping (62) | AC | No significant difference between Normo and Hypo. No severe complications | 33.7 (33.2–34.2) | NR | NR | 35.7 (34.9–36.4) | NS |
| Sato et al., 2000 | 60 (Normo 28, Hypo 32) | SAH clipping | AC and WB | NR | 34.0 | NR | Time: 115 minutes (45–250 minutes) | 36.2 | NR |
| Steinberg et al., 2004 | 153 Hypo | Elective open craniotomy for unruptured cerebral aneurysm | WB (61) versus endo(92) | Postoperative infection 4.3% endo versus 4.9% WB, NS. No severe complications in all. | 33.0 | 274 | 1.88 (WB) versus 0.69 (endo) | (35–36) | NS between WB and endo |
| Todd et al., 2005 | 1000 (Normo 501, Hypo 499) | SAH clipping | AC | Postoperative bacteremia (5% Hypo vs. 3% Normo, p=0.05), no severe complications in all | 33.0 (32.5–33.5) | 324±120 | NR | 36.4±1.0 | NS |
| Hindman et al., 2010 | 441 (Normo 233, Hypo 208) | SAH patients undergoing temporary clipping | AC | NR | 33.3°C–0.8°C | NR | Time: 120 minutes | 36.7°–0.5°C | NS |
This table is partially quoted from the literature of Choi et al., 2009. Ranges are listed inside the parentheses.
Normo, normothermia; Hypo, hypothermia; SAH, subarachnoidal hemorrhage; WB, water blanket cooling; AC, air cooling; endo, endovascular cooling; comp, complication; NA, not applicable; NR, not reported; NS, not significant.
Surgical operations on cerebral aneurysms create a substantial risk of cerebral ischemia. During such procedures, temporary clips are often used to control proximal blood flow. This creates a situation that mirrors the I/R model. The Intraoperative Hypothermia for Aneurysm Surgery Trial was designed to specifically compare the effects of intraoperative temperatures of 32.5°C–33.5°C versus 36°C–37°C on long-term neurologic outcome in patients with documented aneurysmal subarachnoid hemorrhage (Hindman et al., 2010). In total, 1001 patients with ruptured aneurysms were randomized to the temperature groups. Researchers found no clear benefit from the hypothermia, but at the same time, they noted no significant adverse effects. This research group mentioned that the short duration of cooling (only in the operating theater) and quick rewarming after finishing anesthesia might be a reason that their model had no effect on outcome (Todd et al., 2005).
These studies can teach us important lessons in planning future clinical trials using hypothermia in traumatic I/R brain injured patients. Specifically, we have learned that (1) perioperative induced hypothermia is feasible and safe, and (2) careful consideration should be used in determining the cooling and rewarming durations. All previous hypothermia studies describe no severe complications from the perioperative induced hypothermia. One should note that their cooling and rewarming durations were all relatively short (Table 1).
With the results from NABIS: H II, we have been interested in developing a randomized clinical trial for early-induced, preoperative hypothermia in traumatic I/R brain injury. Using the considerations obtained from the previous reports on perioperative hypothermia, we believe that preoperative hypothermia could be induced feasibly and safely. We also believe that continuous hypothermia maintenance after surgery would be needed for a more robust, protective effect.
As just described, based on the results from the NABIS: H II, a large multicenter clinical trial is warranted to further investigate the effect of early hypothermia in I/R TBI. Important consideration should be given, as several researchers have pointed out, to cooling rate, period of hypothermia, rewarming rate, and volumes of intravenous fluid for such a clinical trial to commence (Honeybul et al., 2011; Nichol et al., 2011; Polderman and Andrews, 2011; Takeuchi et al., 2011).
Conclusion
We reviewed previous literature relating to the mechanisms of I/R brain injury and mild therapeutic hypothermia with a focus on the treatment of I/R related TBI. We referred to our experience with mild therapeutic hypothermia in an experimental rat model. Based on the results from our animal studies and the clinical data from the NABIS: H II, a multicenter clinical trial is needed to establish early, preoperative hypothermia as a neuroprotective intervention in patients with evacuated hematomas.
Disclosure Statement
No competing financial interests exist.
References
- Abella BS. Zhao D. Alvarado J. Hamann K. Vanden Hoek TL. Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109:2786–2791. doi: 10.1161/01.CIR.0000131940.19833.85. [DOI] [PubMed] [Google Scholar]
- Abulrob A. Brunette E. Slinn J. Baumann E. Stanimirovic D. In vivo optical imaging of ischemic blood-brain barrier disruption. Methods Mol Biol. 2011;763:423–439. doi: 10.1007/978-1-61779-191-8_29. [DOI] [PubMed] [Google Scholar]
- Badruddin A. Taqi MA. Abraham MG. Dani D. Zaidat OO. Neurocritical care of a reperfused brain. Curr Neurol Neurosci Rep. 2011;11:104–110. doi: 10.1007/s11910-010-0156-9. [DOI] [PubMed] [Google Scholar]
- Baker KZ. Young WL. Stone JG. Kader A. Baker CJ. Solomon RA. Deliberate mild intraoperative hypothermia for craniotomy. Anesthesiology. 1994;81:361–367. doi: 10.1097/00000542-199408000-00014. [DOI] [PubMed] [Google Scholar]
- Bernard SA. Buist M. Induced hypothermia in critical care medicine: a review. Crit Care Med. 2003;31:2041–2051. doi: 10.1097/01.CCM.0000069731.18472.61. [DOI] [PubMed] [Google Scholar]
- Bernard SA. Gray TW. Buist MD. Jones BM. Silvester W. Gutteridge G. Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- Bolli R. Jeroudi MO. Patel BS. Aruoma OI. Halliwell B. Lai EK. McCay PB. Marked reduction of free radical generation and contractile dysfunction by antioxidant therapy begun at the time of reperfusion. Evidence that myocardial “stunning” is a manifestation of reperfusion injury. Circ Res. 1989;65:607–622. doi: 10.1161/01.res.65.3.607. [DOI] [PubMed] [Google Scholar]
- Brennan AM. Suh SW. Won SJ. Narasimhan P. Kauppinen TM. Lee H. Edling Y. Chan PH. Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger R. Bendszus M. Vince GH. Solymosi L. Roosen K. Neurophysiological monitoring, magnetic resonance imaging, and histological assays confirm the beneficial effects of moderate hypothermia after epidural focal mass lesion development in rodents. Neurosurgery. 2004;54:701–711. doi: 10.1227/01.neu.0000108784.80585.ee. discussion 711–702. [DOI] [PubMed] [Google Scholar]
- Chesnut RM. Marshall SB. Piek J. Blunt BA. Klauber MR. Marshall LF. Early and late systemic hypotension as a frequent and fundamental source of cerebral ischemia following severe brain injury in the Traumatic Coma Data Bank. Acta Neurochir Suppl (Wien) 1993;59:121–125. doi: 10.1007/978-3-7091-9302-0_21. [DOI] [PubMed] [Google Scholar]
- Choi R. Andres RH. Steinberg GK. Guzman R. Intraoperative hypothermia during vascular neurosurgical procedures. Neurosurg Focus. 2009;26:E24. doi: 10.3171/2009.3.FOCUS0927. [DOI] [PubMed] [Google Scholar]
- Clifton GL. Christensen ML. Use of moderate hypothermia during elective craniotomy. Tex Med. 1992;88:66–69. [PubMed] [Google Scholar]
- Clifton GL. Miller ER. Choi SC. Levin HS. McCauley S. Smith KR., Jr Muizelaar JP. Wagner FC., Jr Marion DW. Luerssen TG. Chesnut RM. Schwartz M. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556–563. doi: 10.1056/NEJM200102223440803. [DOI] [PubMed] [Google Scholar]
- Clifton GL. Valadka A. Zygun D. Coffey CS. Drever P. Fourwinds S. Janis LS. Wilde E. Taylor P. Harshman K. Conley A. Puccio A. Levin HS. McCauley SR. Bucholz RD. Smith KR. Schmidt JH. Scott JN. Yonas H. Okonkwo DO. Very early hypothermia induction in patients with severe brain injury (the National Acute Brain Injury Study: Hypothermia II): a randomised trial. Lancet Neurol. 2011;10:131–139. doi: 10.1016/S1474-4422(10)70300-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F. Corbett D. Delayed and prolonged post-ischemic hypothermia is neuroprotective in the gerbil. Brain Res. 1994;654:265–272. doi: 10.1016/0006-8993(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Colbourne F. Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci. 1995;15:7250–7260. doi: 10.1523/JNEUROSCI.15-11-07250.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbourne F. Li H. Buchan AM. Indefatigable CA1 sector neuroprotection with mild hypothermia induced 6 hours after severe forebrain ischemia in rats. J Cereb Blood Flow Metab. 1999;19:742–749. doi: 10.1097/00004647-199907000-00003. [DOI] [PubMed] [Google Scholar]
- Dae MW. Gao DW. Sessler DI. Chair K. Stillson CA. Effect of endovascular cooling on myocardial temperature, infarct size, and cardiac output in human-sized pigs. Am J Physiol Heart Circ Physiol. 2002;282:H1584–H1591. doi: 10.1152/ajpheart.00980.2001. [DOI] [PubMed] [Google Scholar]
- De Georgia MA. Krieger DW. Abou-Chebl A. Devlin TG. Jauss M. Davis SM. Koroshetz WJ. Rordorf G. Warach S. Cooling for Acute Ischemic Brain Damage (COOL AID): a feasibility trial of endovascular cooling. Neurology. 2004;63:312–317. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- De Marchis GM. Jung S. Colucci G. Meier N. Fischer U. Weck A. Mono ML. Galimanis A. Mattle HP. Schroth G. Gralla J. Arnold M. Brekenfeld C. Intracranial hemorrhage, outcome, and mortality after intra-arterial therapy for acute ischemic stroke in patients under oral anticoagulants. Stroke. 2011;42:3061–3066. doi: 10.1161/STROKEAHA.111.615476. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. Morphological manifestations of reperfusion injury in brain. Ann N Y Acad Sci. 1994;723:15–24. [PubMed] [Google Scholar]
- Dietrich WD. Alonso O. Busto R. Prado R. Zhao W. Dewanjee MK. Ginsberg MD. Posttraumatic cerebral ischemia after fluid percussion brain injury: an autoradiographic and histopathological study in rats. Neurosurgery. 1998;43:585–593. doi: 10.1097/00006123-199809000-00105. discussion 593–584. [DOI] [PubMed] [Google Scholar]
- Dietrich WD. Busto R. Alonso O. Globus MY. Ginsberg MD. Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab. 1993;13:541–549. doi: 10.1038/jcbfm.1993.71. [DOI] [PubMed] [Google Scholar]
- Elali A. Doeppner TR. Zechariah A. Hermann DM. Increased blood-brain barrier permeability and brain edema after focal cerebral ischemia induced by hyperlipidemia: role of lipid peroxidation and calpain-1/2, matrix metalloproteinase-2/9, and RhoA overactivation. Stroke. 2011;42:3238–3244. doi: 10.1161/STROKEAHA.111.615559. [DOI] [PubMed] [Google Scholar]
- Eldadah BA. Faden AI. Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma. 2000;17:811–829. doi: 10.1089/neu.2000.17.811. [DOI] [PubMed] [Google Scholar]
- Erecinska M. Thoresen M. Silver IA. Effects of hypothermia on energy metabolism in Mammalian central nervous system. J Cereb Blood Flow Metab. 2003;23:513–530. doi: 10.1097/01.WCB.0000066287.21705.21. [DOI] [PubMed] [Google Scholar]
- Farkas O. Povlishock JT. Cellular and subcellular change evoked by diffuse traumatic brain injury: a complex web of change extending far beyond focal damage. Prog Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- Groysman LI. Emanuel BA. Kim-Tenser MA. Sung GY. Mack WJ. Therapeutic hypothermia in acute ischemic stroke. Neurosurg Focus. 2011;30:E17. doi: 10.3171/2011.4.FOCUS1154. [DOI] [PubMed] [Google Scholar]
- Gunn AJ. Cerebral hypothermia for prevention of brain injury following perinatal asphyxia. Curr Opin Pediatr. 2000;12:111–115. doi: 10.1097/00008480-200004000-00004. [DOI] [PubMed] [Google Scholar]
- HACA Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- Han HS. Karabiyikoglu M. Kelly S. Sobel RA. Yenari MA. Mild hypothermia inhibits nuclear factor-kappaB translocation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:589–598. doi: 10.1097/01.WCB.0000059566.39780.8D. [DOI] [PubMed] [Google Scholar]
- Hayashi N. Management of pitfalls for the successful clinical use of hypothermia treatment. J Neurotrauma. 2009;26:445–453. doi: 10.1089/neu.2008.0648. [DOI] [PubMed] [Google Scholar]
- Hemmen TM. Raman R. Guluma KZ. Meyer BC. Gomes JA. Cruz-Flores S. Wijman CA. Rapp KS. Grotta JC. Lyden PD. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41:2265–2270. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindman BJ. Bayman EO. Pfisterer WK. Torner JC. Todd MM. No association between intraoperative hypothermia or supplemental protective drug and neurologic outcomes in patients undergoing temporary clipping during cerebral aneurysm surgery: findings from the intraoperative hypothermia for aneurysm surgery trial. Anesthesiology. 2010;112:86–101. doi: 10.1097/ALN.0b013e3181c5e28f. [DOI] [PubMed] [Google Scholar]
- Hindman BJ. Todd MM. Gelb AW. Loftus CM. Craen RA. Schubert A. Mahla ME. Torner JC. Mild hypothermia as a protective therapy during intracranial aneurysm surgery: a randomized prospective pilot trial. Neurosurgery. 1999;44:23–32. doi: 10.1097/00006123-199901000-00009. discussion 32–23. [DOI] [PubMed] [Google Scholar]
- Honeybul S. Ho K. Lind C. Gillett G. Hypothermia in patients with brain injury: the way forward? Lancet Neurol. 2011;10:405–406. doi: 10.1016/S1474-4422(11)70086-2. author reply 406–407. [DOI] [PubMed] [Google Scholar]
- Huang Y. Rabb H. Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janata A. Holzer M. Hypothermia after cardiac arrest. Prog Cardiovasc Dis. 2009;52:168–179. doi: 10.1016/j.pcad.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Jiang J. Yu M. Zhu C. Effect of long-term mild hypothermia therapy in patients with severe traumatic brain injury: 1-year follow-up review of 87 cases. J Neurosurg. 2000;93:546–549. doi: 10.3171/jns.2000.93.4.0546. [DOI] [PubMed] [Google Scholar]
- Jiang J. Clinical study of mild hypothermia treatment for severe traumatic brain injury. J Neurotrauma. 2009;26:399–406. doi: 10.1089/neu.2008.0525. [DOI] [PubMed] [Google Scholar]
- Jung JE. Kim GS. Chen H. Maier CM. Narasimhan P. Song YS. Niizuma K. Katsu M. Okami N. Yoshioka H. Sakata H. Goeders CE. Chan PH. Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol. 2010;41:172–179. doi: 10.1007/s12035-010-8102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai N. Okauchi M. Morisaki K. Nagao S. Effects of delayed intraischemic and postischemic hypothermia on a focal model of transient cerebral ischemia in rats. Stroke. 2000;31:1982–1989. doi: 10.1161/01.str.31.8.1982. discussion 1989. [DOI] [PubMed] [Google Scholar]
- Khalid MA. Ashraf M. Direct detection of endogenous hydroxyl radical production in cultured adult cardiomyocytes during anoxia and reoxygenation. Is the hydroxyl radical really the most damaging radical species? Circ Res. 1993;72:725–736. doi: 10.1161/01.res.72.4.725. [DOI] [PubMed] [Google Scholar]
- Kil HY. Zhang J. Piantadosi CA. Brain temperature alters hydroxyl radical production during cerebral ischemia/reperfusion in rats. J Cereb Blood Flow Metab. 1996;16:100–106. doi: 10.1097/00004647-199601000-00012. [DOI] [PubMed] [Google Scholar]
- Kuroda Y. Bullock R. Local cerebral blood flow mapping before and after removal of acute subdural hematoma in the rat. Neurosurgery. 1992;30:687–691. [PubMed] [Google Scholar]
- Lampe JW. Becker LB. State of the art in therapeutic hypothermia. Annu Rev Med. 2011;62:79–93. doi: 10.1146/annurev-med-052009-150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leker RR. Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: neuroprotective opportunities. Brain Res Brain Res Rev. 2002;39:55–73. doi: 10.1016/s0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Lewis FJ. Hypothermia in cardiac and general surgery. Minn Med. 1955;38:77–81. [PubMed] [Google Scholar]
- Lv M. Liu Y. Zhang J. Sun L. Liu Z. Zhang S. Wang B. Su D. Su Z. Roles of inflammation response in microglia cell through Toll-like receptors 2/interleukin-23/interleukin-17 pathway in cerebral ischemia/reperfusion injury. Neuroscience. 2011;176:162–172. doi: 10.1016/j.neuroscience.2010.11.066. [DOI] [PubMed] [Google Scholar]
- Matsushita Y. Bramlett HM. Alonso O. Dietrich WD. Posttraumatic hypothermia is neuroprotective in a model of traumatic brain injury complicated by a secondary hypoxic insult. Crit Care Med. 2001;29:2060–2066. doi: 10.1097/00003246-200111000-00004. [DOI] [PubMed] [Google Scholar]
- Miki T. Liu GS. Cohen MV. Downey JM. Mild hypothermia reduces infarct size in the beating rabbit heart: a practical intervention for acute myocardial infarction? Basic Res Cardiol. 1998;93:372–383. doi: 10.1007/s003950050105. [DOI] [PubMed] [Google Scholar]
- Miller JD. Bullock R. Graham DI. Chen MH. Teasdale GM. Ischemic brain damage in a model of acute subdural hematoma. Neurosurgery. 1990;27:433–439. doi: 10.1097/00006123-199009000-00016. [DOI] [PubMed] [Google Scholar]
- Miyazawa T. Tamura A. Fukui S. Hossmann KA. Effect of mild hypothermia on focal cerebral ischemia. Review of experimental studies. Neurol Res. 2003;25:457–464. doi: 10.1179/016164103101201850. [DOI] [PubMed] [Google Scholar]
- Moossa AR. Zarins CK. Skinner DB. In situ kidney preservation for transplantation with use of profound hypothermia (5 to 20 degrees C.) with an intact circulation. Surgery. 1976;79:60–64. [PubMed] [Google Scholar]
- Muizelaar JP. Cerebral ischemia-reperfusion injury after severe head injury and its possible treatment with polyethyleneglycol-superoxide dismutase. Ann Emerg Med. 1993;22:1014–1021. doi: 10.1016/s0196-0644(05)82744-1. [DOI] [PubMed] [Google Scholar]
- Natarajan SK. Karmon Y. Snyder KV. Ohta H. Hauck EF. Hopkins LN. Siddiqui AH. Levy EI. Prospective acute ischemic stroke outcomes after endovascular therapy: a real-world experience. World Neurosurg. 2010;74:455–464. doi: 10.1016/j.wneu.2010.06.035. [DOI] [PubMed] [Google Scholar]
- Nichol AD. Trapani T. Murray L. Vallance S. Cooper DJ. Hypothermia in patients with brain injury: the way forward? Lancet Neurol. 2011;10:405. doi: 10.1016/S1474-4422(11)70085-0. author reply 406–407. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–S202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- Polderman KH. Andrews PJ. Hypothermia in patients with brain injury: the way forward? Lancet Neurol. 2011;10:404–405. doi: 10.1016/S1474-4422(11)70084-9. [DOI] [PubMed] [Google Scholar]
- Povlishock JT. Wei EP. Posthypothermic rewarming considerations following traumatic brain injury. J Neurotrauma. 2009;26:333–340. doi: 10.1089/neu.2008.0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K. Yoshimoto T. Systemic and cerebral haemodynamics during craniotomy under mild hypothermia in patients with acute subarachroid haemorrhage. Acta neurochirurgica. 2000;142:1013–1020. doi: 10.1007/s007010070056. [DOI] [PubMed] [Google Scholar]
- Schwab S. Schwarz S. Spranger M. Keller E. Bertram M. Hacke W. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998;29:2461–246. doi: 10.1161/01.str.29.12.2461. [DOI] [PubMed] [Google Scholar]
- Sealy WC. Brown IW., Jr Young WG., Jr A report on the use of both extracorporeal circulation and hypothermia for open heart surgery. Ann Surg. 1958;147:603–613. doi: 10.1097/00000658-195805000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shann F. Hypothermia for traumatic brain injury: how soon, how cold, and how long? Lancet. 2003;362:1950–1951. doi: 10.1016/S0140-6736(03)15083-0. [DOI] [PubMed] [Google Scholar]
- Shao ZH. Sharp WW. Wojcik KR. Li CQ. Han M. Chang WT. Ramachandran S. Li J. Hamann KJ. Vanden Hoek TL. Therapeutic hypothermia cardioprotection via Akt- and nitric oxide-mediated attenuation of mitochondrial oxidants. Am J Physiol Heart Circ Physiol. 2010;298:H2164–H2173. doi: 10.1152/ajpheart.00994.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y. Terao Y. Ohta H. Molecular mechanisms underlying hypothermia-induced neuroprotection. Stroke Res Treat. 2010:2011. doi: 10.4061/2011/809874. Article ID 809874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RP. Acidotoxicity trumps excitotoxicity in ischemic brain. Arch Neurol. 2006;63:1368–1371. doi: 10.1001/archneur.63.10.1368. [DOI] [PubMed] [Google Scholar]
- Steinberg GK. Ogilvy CS. Shuer LM. Connolly ES., Jr Solomon RA. Lam A. Kassell NF. Baker CJ. Giannotta SL. Cockroft KM. Bell-Stephens TE. Allgren RL. Comparison of endovascular and surface cooling during unruptured cerebral aneurysm repair. Neurosurgery. 2004;55:307–314. doi: 10.1227/01.neu.0000129683.99430.8c. discussion 314–305. [DOI] [PubMed] [Google Scholar]
- Suehiro E. Ueda Y. Wei EP. Kontos HA. Povlishock JT. Posttraumatic hypothermia followed by slow rewarming protects the cerebral microcirculation. J Neurotrauma. 2003;20:381–390. doi: 10.1089/089771503765172336. [DOI] [PubMed] [Google Scholar]
- Sugawara T. Chan PH. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- Swan H. Hypothermia for general and cardiac surgery; with techniques of some open intracardiac procedures under hypothermia. Surg Clin North Am. 1956:1009–1024. [PubMed] [Google Scholar]
- Takeuchi S. Nawashiro H. Otani N. Hypothermia in patients with brain injury: the way forward? Lancet Neurol. 2011;10:404. doi: 10.1016/S1474-4422(11)70083-7. [DOI] [PubMed] [Google Scholar]
- Todd MM. Hindman BJ. Clarke WR. Torner JC. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135–145. doi: 10.1056/NEJMoa040975. [DOI] [PubMed] [Google Scholar]
- Tuttolomondo A. Di Sciacca R. Di Raimondo D. Arnao V. Renda C. Pinto A. Licata G. Neuron protection as a therapeutic target in acute ischemic stroke. Curr Top Med Chem. 2009;9:1317–1334. doi: 10.2174/156802609789869646. [DOI] [PubMed] [Google Scholar]
- Wahlgren NG. Ahmed N. Neuroprotection in cerebral ischaemia: facts and fancies—the need for new approaches. Cerebrovasc Dis. 2004;171(Suppl):153–166. doi: 10.1159/000074808. [DOI] [PubMed] [Google Scholar]
- Wang GJ. Deng HY. Maier CM. Sun GH. Yenari MA. Mild hypothermia reduces ICAM-1 expression, neutrophil infiltration and microglia/monocyte accumulation following experimental stroke. Neuroscience. 2002;114:1081–1090. doi: 10.1016/s0306-4522(02)00350-0. [DOI] [PubMed] [Google Scholar]
- Wong CH. Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- Yanamoto H. Nagata I. Nakahara I. Tohnai N. Zhang Z. Kikuchi H. Combination of intraischemic and postischemic hypothermia provides potent and persistent neuroprotection against temporary focal ischemia in rats. Stroke. 1999;30:2720–2726. doi: 10.1161/01.str.30.12.2720. discussion 2726. [DOI] [PubMed] [Google Scholar]
- Yang D. Guo S. Zhang T. Li H. Hypothermia attenuates ischemia/reperfusion-induced endothelial cell apoptosis via alterations in apoptotic pathways and JNK signaling. FEBS Lett. 2009;583:2500–2506. doi: 10.1016/j.febslet.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Yenari MA. Hemmen TM. Therapeutic hypothermia for brain ischemia: where have we come and where do we go? Stroke. 2010;41:S72–S74. doi: 10.1161/STROKEAHA.110.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]