Abstract
Aim
Tacrolimus is an immunosuppressant used in transplantation. This article reports the validation of the authors’ recently developed genetics-based tacrolimus equation that predicts troughs.
Methods
Validation was performed in an independent cohort of 795 kidney transplant recipients receiving tacrolimus. The performance of the equation to predict initial troughs was assessed by calculating the bias and precision of the equation. For all troughs in the first 6 months post-transplant, a comparison was made between the troughs predicted using the equation versus those predicted using a basic apparent clearance model with no covariates.
Results
For initial troughs, the equation had a low bias (0.2 ng/ml) and high precision (1.8 ng/ml). For all troughs, the equation predicted troughs significantly better than the basic apparent clearance model.
Conclusion
The tacrolimus equation had good bias and precision in predicting initial troughs and performed better than a basic apparent clearance model for all the troughs.
Keywords: clearance equation, CYP3A5, kidney transplant, pharmacogenetics, pharmacokinetics, polymorphism, tacrolimus, trough, validation
Interindividual variability in drug exposure and response is a major limitation to drug therapy [1]. Although it is generally unclear as to why patients do not respond to therapy, a substantial portion of these individuals may have genetic variability that influences drug efficacy, pharmacology and/or systemic exposure [1,2]. Genetic differences in metabolism enzymes and mechanistic pathways are well known to impact pharmacokinetics, efficacy and toxicity of warfarin. To reduce interpatient variability and improve efficacy, several dosing equations for warfarin that incorporate genetic polymorphisms in CYP2C9 and VKORC1 along with clinical factors (e.g., age, body surface area, smoking status, race, age and concomitant medications) have been successfully developed [3–7]. Dosing of war farin by genotype status has been given a level A rating by the pharmacogenetics implement ation consortium, which highlights the potential importance of genotype in dose determination [8]. Several studies have shown that genotype-guided warfarin dosing resulted in reduced time to stable anticoagulation and faster achievement of therapeutic international normalized ratio, and fewer and smaller dose changes [5,9].
Tacrolimus is a widely used maintenance immunosuppressant in kidney transplantation [10]. It is rapidly absorbed from the GI tract and has a poor oral bioavailability. It is highly bound to plasma proteins and mainly eliminated through metabolism by CYP3A5 [11]. Several pharmacokinetic studies have estimated the tacrolimus apparent clearance (CL/F) to range from 21 to 35 l/h [12–14]. Its use is complicated by its narrow therapeutic window and large interindividual variability in pharmacokinetics [15,16]. Elevated trough concentrations are associated with increased risk of toxicity while low troughs are associated with increased risk of rejection [17]. In the clinical setting, initial tacrolimus doses are based on bodyweight and subsequent doses are adjusted by therapeutic drug monitoring (TDM) of trough concentrations [15]. Despite these efforts, a large number of patients achieve troughs that are above or below the targeted therapeutic range, particularly in the early days post-transplant [18–20]. To address this problem, the authors’ group developed an equation using 681 kidney transplant recipients, enrolled through a multicenter consortium, using clinical factors and genetic variants that individualize tacrolimus dosing [21]. This equation estimates an apparent CL/F for an individual based on days post-transplant, CYP3A5*1 genotype status, transplantation at a steroid-sparing transplantation center, age at the time of transplant and the use of calcium channel blocker (Box 1; Equation 1). Following this, the total daily dose requirement for any desired tacrolimus trough target, can be determined using the CL/F estimate (Box 2; Equation 2).
A prerequisite for the clinical use of this clearance equation is to demonstrate its validity in predicting troughs. Therefore, the objective of this study was to evaluate the predictive performance of the clearance equation to predict troughs relative to actual observed troughs using an independent cohort of kidney transplant recipients. Validation of this equation will provide the additional confidence that the equation can be safely used, may reduce the number of out-of-range trough concentrations and will ultimately reduce the number of trough concentrations necessary to achieve optimal immune suppression. Randomized clinical trials would be a final step to demonstrate the clinical utility of the equation.
Methods
Study design
Patients in which the equation was developed and validated were recruited from the multicenter observational trial DeKAF Genomics study [21]. Details of the DeKAF Genomics study have been published elsewhere [22–24] and are registered at ClinicalTrials.gov (NCT00270712) [101]. The equation was developed in 681 subjects who were selected from the first 1000 subjects enrolled in DeKAF Genomics. The 795 subjects reported here were selected from the next 1000 subjects and were used to validate the equation. Thus, the validation cohort was comprised of a completely separate set of transplant recipients from the development cohort. Approval was obtained from the Institutional Review Board Human Subjects Committees at each of the parti cipating centers. All subjects provided written informed consent. Subject inclusion and exclusion criteria, data collection and analysis for the validation cohort were identical to that of the cohort of patients used for development of the equation [21]. Briefly, patients with end-stage renal dysfunction undergoing kidney or kidney–pancreas transplant who were ≥18 years of age and received tacrolimus (Prograf) at any time in the first 6-months post-transplant were selected for this validation analysis. Clinical data were prospectively collected from the medical records. Tacrolimus trough concentrations were obtained as part of clinical care at the treating center after either once- or twice-daily oral dosing of tacrolimus. Data were collected for the first 6-months post-transplant. Initial tacrolimus doses were based on bodyweight (mg/kg) as per standard clinical practice at each site. Subsequent doses were adjusted by TDM to achieve institution-specific targets (in general, troughs of 8–12 ng/ml in the first 3 months post-transplant and 6–10 ng/ml in months 3–6 post-transplant). Two trough concentrations were extracted from the medical record in each of weeks 1–8 post-transplant and twice in each of months 3, 4, 5 and 6 post-transplant. Identical to the dosing equation development, only troughs after day 2 post-transplant were included in this validation to ensure that tacrolimus was at or near steady state. Genotyping for CYP3A5*1 was conducted in all individuals and was previously described [21].
Statistical analysis
The data were analyzed using the R 12.2.0 statistical package. For each observed tacrolimus trough (Cobs) in the validation cohort, a corresponding CL/F was estimated using the previously developed tacrolimus equation (Equation1). The authors’ previously developed equation estimated CL/F, since tacrolimus was given by the oral route. From each CL/F estimate, a predicted trough (Cpred) was then determined for each Cobs in the validation cohort (Equation 3).
| (3) |
where dose administered is the total daily dose of tacrolimus administered in mg divided by 24 h and CL/F is in l/h.
Predictive performance of the equation was evaluated in two ways: absolute performance for the initial trough concentrations prior to dose adjustments based on TDM information; and relative performance for all trough concentrations over the entire 6-months post-transplant compared with a basic apparent clearance model. The initial trough was defined as the first trough measured at steady state in the first week post-transplant. To ensure that tacrolimus was at or near steady state, only troughs after day 2 post-transplant were used in this validation. The mean half-life of tacrolimus is approximately 12 h, and therefore, steady state would be achieved in approximately 60 h (2.5 days) [25]. The concomitant use of CYP450 inhibitors or inducers (e.g., steroids) to make a determination of steady state was not considered. However, the steroid effect in the development of the equation using the transplant center as a covariate, that is, having a different CL/F for a recipient receiving transplant at a steroid-sparing center versus not, was indirectly accounted for. The use of steroids (or not) was a center-specific practice. The predictive performance of initial and all troughs was assessed by calculation of the bias and precision of the equation using prediction errors.
First, for the initial troughs (n = 412 troughs), the absolute predictive performance was evaluated with prediction errors and given as bias and precision of the troughs predicted by the equation relative to the Cobs. Prediction error (PEi) was defined to be the difference between Cpred obtained from the equation and the Cobs (which were derived from weight-based dosing [mg/kg]) (Equation 4). Bias and precision was calculated as the median prediction error (MPE) (Equation 5) and the median absolute prediction error (MAPE) (Equation 6), respectively. A 95% confidence interval for these medians was calculated based on binomial probabilities [26].
| (4) |
where Cpred,i is the equation-predicted ith trough, and Cobs,i is the observed ith trough.
| (5) |
| (6) |
Second, the relative predictive performance was evaluated over the entire 6 months (n = 13,698 troughs). Relative performance of this model was assessed by comparing it with a basic apparent clearance model (derived from the original model before incorporation of clinical and genetic variables into the model, (Equation 7) [21].
Basic tacrolimus apparent clearance,
| (7) |
The assessment was carried out using the Wilcoxon signed-rank test applied to the paired differences (Di) of the median errors (Equation 8) for relative bias, and to the paired differences of the median absolute error (Equation 9) for relative precision [27].
| (8) |
| (9) |
where PE1i was the prediction error from the equation and PE2i was the prediction error from the basic apparent clearance model in the ith trough concentration (Equation 7).
For assessing relative bias, the null hypothesis (H0) was that the median of the prediction errors of the two methods (equation and basic apparent clearance model) are the same. For the assessment of precision, the H0 was that the median of the absolute prediction errors of the two methods (equation and basic apparent clearance model) are the same. A p < 0.001 was evidence in favor of rejection of H0 and was indicative of a difference in bias and precision of the two methods.
Results
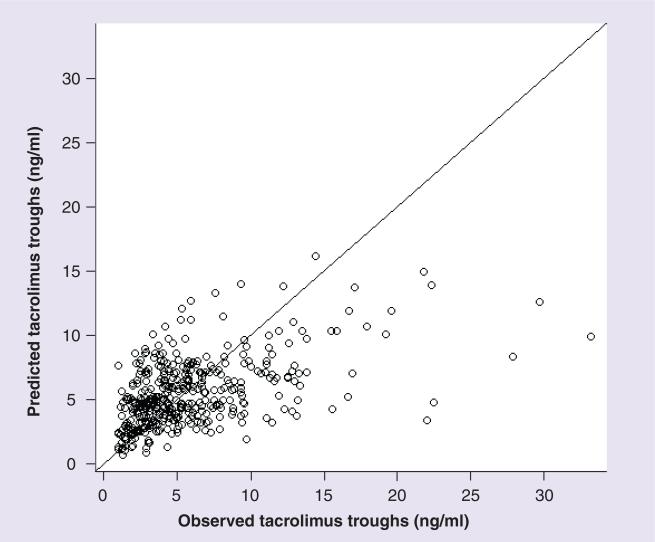
The demographics of subjects in the development and validation cohorts are shown in Table 1. The observed versus predicted troughs for the initial troughs are shown in Figure 1. The absolute predictive performance of the equation for the initial troughs is given in Box 3. For the initial troughs, the equation had a MPE (bias) of 0.2 ng/ml and a MAPE (precision) of 1.8 ng/ml. Thus, the initial troughs were slightly overpredicted by the equation on average by 0.2 ng/ml compared with the actual Cobs. The precision was within ± 18% for a trough of 10 ng/ml and within ± 15% for a trough of 12 ng/ml. The relative predictive performance of the equation for all the troughs in the first 6 months post-transplant is given in Table 2. The relative performance of the equation was assessed relative to a basic apparent clearance model (Equation 7). The predictive performance of the equation to predict troughs had a MPE (bias) of 0.3 ng/ml and a MAPE (precision) of 2.9 ng/ml. Both MPE (0.5 ng/ml) and MAPE (3.6 ng/ml) were higher for the basic apparent clearance model in predicting trough as compared with the equation. From the Wilcoxon signed-rank test for relative bias and precision, the p-value was <0.001 for bias as well as precision. This supported the rejection of the H0 and indicated that the median differences from the two methods were different. Since the bias and precision were better for the equation as compared with a basic apparent clearance model, the equation was superior to the basic apparent clearance model in predicting the trough concentration for all the observed trough concentrations in the first 6-months post-transplant.
Table 1.
Characteristics of subjects in the equation development and validation cohorts.
| Characteristic | Development cohort | Validation cohort |
|---|---|---|
| Subjects (n) | 681 | 795 |
| Age (years) of recipient† | 50.2 ± 12.2 | 50.5 ± 13.2 |
| Baseline weight (kg) of recipient† | 81.3 ± 18.7 | 83.0 ± 19.3 |
| Gender of recipient, male/female (%) | 429 (63)/252 (37) | 499 (63)/96 (37) |
| Race (non-African–American/African–American) | 538 (79)/143 (21) | 652 (82)/143 (18) |
| Transplanted at a steroid-sparing center, n (%) | 205 (30) | 100 (12.6) |
| Living donor, n (%) | 398 (59) | 465 (58.5) |
| Troughs (n) | 11,823 | 13,698 |
| Mean tacrolimus daily dose (mg/kg)‡ | 0.08 ± 0.05 | 0.09 ± 0.05 |
| Mean tacrolimus trough (ng/ml)‡ | 8.31 ± 3.48 | 8.53 ± 3.41 |
| Patients with troughs <8 ng/ml, n (%) | ||
| – Week 1 | 413 (75) | 476 (60) |
| – Week 2 | 331 (55) | 375 (47) |
| Calcium channel blocker use§, n (%) | 5082 (43) | 5843 (43) |
| CYP3A5 genotype (rs776746), n (%) | ||
| – *1/*1 | 72 (11) | 75 (9.4) |
| – *1/*3 | 129 (19) | 147 (18.5) |
| – *3/*3 | 476 (70) | 573 (72.1) |
Reported age and baseline weight are those measured at the time of transplant and are given as the mean ± standard deviation.
Doses and troughs are over the 6-month study period and are given as the mean ± standard deviation.
Number of troughs collected when calcium channel blocker was used.
Figure 1.
Initial observed troughs versus the initial troughs predicted by the clearance equation (n = 412).
Table 2.
Absolute predictive performance in predicting observed tacrolimus troughs using the tacrolimus equation relative to a basic apparent clearance model with no covariates in the first 6 months post-transplant.
| Absolute predictive performance | Equation | Basic clearance model |
|---|---|---|
| MPE† (95% CI) | 0.3 (0.2–0.4) | 0.5 (0.4–0.6) |
| MAPE‡ (95% CI) | 2.9 (2.8–2.10) | 3.6 (3.5–3.7) |
13,698 troughs in 795 subjects.
Mean prediction error indicates the bias of the equation in predicting all troughs in ng/ml.
Median absolute prediction errors indicates the precision of the equation in predicting all troughs in ng/ml.
MAPE: Median absolute prediction error; MPE: Mean predictor error.
Discussion
Low tacrolimus troughs in the early post-transplant period have been associated with a higher rate of acute rejection [20]. Mean trough concentrations in the first week post-transplant have been shown to be significantly different between rejectors and nonrejectors [28,29]. Assuring that all patients are within the therapeutic target in the early post-transplant period would improve therapy [29]. Therefore, how closely the equation could predict the actual observed initial tacrolimus trough concentrations was tested. The bias of the equation for the initial troughs was low (0.2 ng/ml). Moreover, the precision for the equation (1.8 ng/ml) was good (i.e., less than ± 20% for a trough of 10 ng/ml).
The performance of the equation was also evaluated using all troughs from the first 6-months post-transplant relative to troughs predicted using a basic apparent clearance model that was derived from the authors’ previous work in kidney transplant recipients (Equation 7). This is a naive predictor of CL/F since it does not take into account clinical or genetic factors that may affect apparent clearance. The bias and precision of the equation in predicting troughs was significantly better compared with the basic apparent clearance model. This is likely because the equation accounts for important clinical factors such as age, comedications (such as calcium channel blocker) and genetic factors such as the CYP3A5*1 status [30]. A naive CL/F is similar to current clinical practice where all subjects receive approximately the same tacrolimus dose (0.08–0.09 mg/kg/day) based on bodyweight. In the authors’ previous analyses, the authors were unable to show that weight was an important determinant of trough. Therefore, the use of weight-based dosing for tacrolimus is probably of limited benefit in tailoring therapy [18,19,21]. For all troughs in the first 6-months post-transplant, the equation had a bias of 0.3 ng/ml and a precision of 2.9 ng/ml (i.e., less than ± 30% for a trough of 10 ng/ml). It is believed that the relative performance may be more relevant than the absolute performance for all troughs since the bias and imprecision is expected to be inflated. This inflation is likely because the reference is a trough measurement that is derived from TDM, which incorporates all available clinical information. It is not un expected that it would have better performance than the equation, which is based on a few covariates. The lower predictive performance of the equation using all troughs might be attributable to factors such as unknown drug interactions, noncompliance and development of comorbid conditions that are more likely to occur later post-transplant. In addition, dosing is less closely supervised in the later months post-transplant, which may affect the exactness of the trough measurements thereby reducing performance.
Predictive performance analyses suffer from the limitation that reasons for a low predictive performance is never clear. In this case, the lower predictive performance of the equation for all troughs may be attributed to the equation itself or inaccuracies in the reference (which in this case is the Cobs). For example, the Cobs may not have been obtained at the ideal trough time point, the patient might be noncompliant, unknown drug interactions might be occurring and there might be substantial variability in the assay. When there is inaccuracy in Cobs, the bias and imprecision of the equation is overestimated [27].
Other possible reasons for poor predictive performance is that the equation is misspecified or lacks one or more relevant clinical or genetic factors. The equation does not contain adjustments for every possible medication that may interact with tacrolimus. However, many of the common potential interacting medications were tested during the development phase of the equation (e.g., ACE inhibitors and antiviral agents) and did not influence CL/F [18,21]. There is a known drug interaction between tacrolimus and mycophenolic acid (MPA) that results in alterations in the MPA concentrations (not tacrolimus) and therefore, concomitant MPA use is not relevant towards the equation [31]. In addition, disease states that might affect absorption and/or gastrointestinal transit time (e.g., gastroparesis, malabsorption and diarrhea) was not recorded. The authors previously tested for race in the cohorts and it was not a significant covariate. However, African–American race is highly correlated with CYP3A5*1 genotype. Therefore, it was difficult to distinguish between effects of race and genotype. Consequently, it was not possible to quantify these types of effects with the available data.
In general, the validity of equations can be tested by using either an internal or independent external population. The authors chose to validate the equation using an external cohort of subjects, which is considered more rigorous than an internal validation. However, it has the caveat that if the predictive performances are not reasonable, it is unfeasible to ascertain whether the unreasonable performance is due to a difference between the populations (development and validation cohort) or due to a model misspecification [32].
Conclusion
In conclusion, we conducted a validation of our equation in an independent cohort of kidney transplant recipients. The bias and precision was good for the initial troughs. The low bias (overprediction of 0.2 ng/ml) and high precision (error of less than ± 20% for troughs of 10 ng/ml) demonstrated the ability of the equation to predict initial troughs. Consequently, we believe that the equation can be safely used for predicting initial troughs. Acute rejection is an early event with two-thirds of events occurring within the first 3-months post-transplant and fewer between months 4 and 6 post-transplant. It has been shown that low exposure to tacrolimus as early as the first week post-transplant is a predictor of early acute rejection [29]. Therefore, any tools to predict troughs with an equation such as the one we developed may get patients to reach the therapeutic dose faster and reduce the number of out-of-range troughs and dose changes required. The ability to predict troughs based on an equation instead of the traditional trial and error dosing approach may lower overall costs of monitoring. The cost associated with genotyping may be recovered by the reduction in the number of trough levels obtained and staff utilization. A study to prospectively apply the equation to dose selection is needed in the future. Comparison of the cost–benefit ratio of TDM with and without this equation was outside the scope of this study. The future of pharmacogenomics is dependent upon developing simple clinical tools that can translate genetic association findings into the clinical environment. Studies such as this demonstrate the potential value of genetics-based dosing equations.
Box 1. Equation 1.
CCB: Calcium channel blocker; CL/F: Apparent clearance.
Box 2. Equation 2.
CL/F: Apparent clearance; TDD: Total daily dose.
Box 3. Absolute predictive performance of tacrolimus equation in predicting observed initial troughs.
■ MPE†: 0.2 (95% CI: 0–0.5)
■ MAPE‡: 1.8 (95% CI: 1.6–2.0)
n = 412 troughs in 412 subjects.
†MPE indicates the bias of the equation in predicting the initial troughs in ng/ml.
‡MAPE indicates the precision of the equation in predicting the initial troughs in ng/ml.
MAPE: Median absolute prediction error; MPE: Mean predictor error.
Executive summary.
Tacrolimus pharmacogenomics
■ Tacrolimus has a narrow therapeutic range and low troughs are associated with rejection and increased troughs are associated with toxicity.
■ CYP3A5 primarily metabolizes tacrolimus and the genetic variation in the CYP3A5 gene is associated with significant changes in clearance of tacrolimus.
■ We recently developed a dosing equation for tacrolimus that estimates an apparent tacrolimus clearance for an individual using the following clinical and genetic factors: days post-transplant, CYP3A5*1 genotype status, transplantation at a steroid-sparing transplantation center, age at the time of transplant and the use of calcium channel blocker. From the calculated tacrolimus clearance, the tacrolimus dose can be calculated.
Validation of dosing equation
■ Validation was done by predictive performance of the equation using bias and precision.
■ For initial troughs, the equation had a bias of 0.2 ng/ml and a precision of 1.8 ng/ml. The equation did less well for all troughs in the first 6-months post-transplant and had a bias of 0.3 ng/ml and a precision of 2.9 ng/ml.
■ The clearance equation performed better than a basic tacrolimus clearance model, which assumes the same apparent clearance for all transplant recipients.
Conclusion
■ The equation can be safely used for predicting initial clearance from which dose can be determined. For predicting all troughs, the equation performs better than a basic apparent clearance model.
Acknowledgements
The authors would like to acknowledge the contributions of the DeKAF investigators in this study.
This work was supported by 5U19-A1070119 from the National Institute of Allergy and Infectious Disease (MD, USA).
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References/Website
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Wolf CR, Smith G, Smith RL. Science, medicine, and the future: pharmacogenetics. BMJ. 2000;320(7240):987–990. doi: 10.1136/bmj.320.7240.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadée W, Dai Z. Pharmacogenetics/genomics and personalized medicine. Hum. Mol. Genet. 2005;14(Suppl. 2):R207–R214. doi: 10.1093/hmg/ddi261. [DOI] [PubMed] [Google Scholar]
- 3.Gage BF, Eby C, Johnson JA, et al. Use of Pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin. Pharmacol. Ther. 2008;84(3):326–331. doi: 10.1038/clpt.2008.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sconce EA, Khan TI, Wynne HA, et al. The impact of CYP2C9 and VKORC1 genetic polymorphism and patient characteristics upon warfarin dose requirements: proposal for a new dosing regimen. Blood. 2005;106(7):2329–2333. doi: 10.1182/blood-2005-03-1108. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JL, Horne BD, Stevens SM, et al. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation. 2007;116(22):2563–2570. doi: 10.1161/CIRCULATIONAHA.107.737312. [DOI] [PubMed] [Google Scholar]
- 6■.Marin-Leblanc M, Perreault S, Bahroun I, et al. Validation of warfarin pharmacogenetic algorithms in clinical practice. Pharmacogenomics. 2012;13(1):21–29. doi: 10.2217/pgs.11.120. [Explains how warfarin algorithms have been validated and serves as an example for this study.] [DOI] [PubMed] [Google Scholar]
- 7■.Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N. Engl. J. Med. 2009;360(8):753–764. doi: 10.1056/NEJMoa0809329. [An example of predicting warfarin doses using clinical and genetic factors. The authors of this article are trying to do the same for tacrolimus.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JA, Gong L, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin. Pharmacol. Ther. 2011;90(4):625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caraco Y, Blotnick S, Muszkat M. CYP2C9 genotype-guided warfarin prescribing enhances the efficacy and safety of anticoagulation: a prospective randomized controlled study. Clin. Pharmacol. Ther. 2007;83(3):460–470. doi: 10.1038/sj.clpt.6100316. [DOI] [PubMed] [Google Scholar]
- 10.Bowman LJ, Brennan DC. The role of tacrolimus in renal transplantation. Expert Opin. Pharmacother. 2008;9(4):635–643. doi: 10.1517/14656566.9.4.635. [DOI] [PubMed] [Google Scholar]
- 11.Vicari-Christensen M, Repper S, Basile S, Young D. Tacrolimus: review of pharmacokinetics, pharmacodynamics, and pharmacogenetics to facilitate practitioners’ understanding and offer strategies for educating patients and promoting adherence. Prog. Transplant. 2009;19(3):277–284. doi: 10.1177/152692480901900315. [DOI] [PubMed] [Google Scholar]
- 12.Staatz CE, Willis C, Taylor PJ, Tett SE. Population pharmacokinetics of tacrolimus in adult kidney transplant recipients. Clin. Pharmacol. Ther. 2002;72(6):660–669. doi: 10.1067/mcp.2002.129304. [DOI] [PubMed] [Google Scholar]
- 13.Staatz CE, Willis C, Taylor PJ, Lynch SV, Tett SE. Toward better outcomes with tacrolimus therapy: population pharmacokinetics and individualized dosage prediction in adult liver transplantation. Liver Transpl. 2003;9(2):130–137. doi: 10.1053/jlts.2003.50023. [DOI] [PubMed] [Google Scholar]
- 14.Tada H, Tsuchiya N, Satoh S, et al. Impact of CYP3A5 and MDR1(ABCB1) C3435T polymorphisms on the pharmacokinetics of tacrolimus in renal transplant recipients. Transplant. Proc. 2005;37(4):1730–1732. doi: 10.1016/j.transproceed.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 15.Wallemacq P, Armstrong VW, Brunet M, et al. Opportunities to optimize tacrolimus therapy in solid organ transplantation: report of the European consensus conference. Ther. Drug Monit. 2009;31(2):139–152. doi: 10.1097/FTD.0b013e318198d092. [DOI] [PubMed] [Google Scholar]
- 16.Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin. Pharmacokinet. 2004;43(10):623–653. doi: 10.2165/00003088-200443100-00001. [DOI] [PubMed] [Google Scholar]
- 17.Laskow DA. An open-label, concentration-ranging trial of FK506 in primary kidney transplantation: a report of the United States Multicenter FK506 Kidney Transplant Group. Transplantation. 1996;62(7):900–905. doi: 10.1097/00007890-199610150-00005. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson PA, Oetting WS, Brearley AM, et al. Novel polymorphisms associated with tacrolimus trough concentrations: results from a Multicenter Kidney Transplant Consortium. Transplantation. 2011;91(3):300–308. doi: 10.1097/TP.0b013e318200e991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ekberg H, Mamelok RD, Pearson TC, Vincenti F, Tedesco-Silva H, Daloze P. The challenge of achieving target drug concentrations in clinical trials: experience from the Symphony study. Transplantation. 2009;87(9):1360–1366. doi: 10.1097/TP.0b013e3181a23cb2. [DOI] [PubMed] [Google Scholar]
- 20■.Staatz C, Taylor P, Tett S. Low tacrolimus concentrations and increased risk of early acute rejection in adult renal transplantation. Nephrol. Dial. Transpl. 2001;16(9):1905–1909. doi: 10.1093/ndt/16.9.1905. [Illustrates the importance of achieving therapeutic troughs in the early post-transplant period, and is the basis for the authors’ interest in accurately predicting the initial troughs in this article.] [DOI] [PubMed] [Google Scholar]
- 21.Passey C, Birnbaum AK, Brundage RC, Oetting WS, Israni AK, Jacobson PA. Dosing equation for tacrolimus using genetic variants and clinical factors. Br. J. Clin. Pharmacol. 2011;72(6):948–957. doi: 10.1111/j.1365-2125.2011.04039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation. 2010;90(1):68–74. doi: 10.1097/TP.0b013e3181e065de. [DOI] [PubMed] [Google Scholar]
- 23.Gaston RS, Kasiske BL, Fieberg AM, et al. Use of cardioprotective medications in kidney transplant recipients. Am. J. Transpl. 2009;9(8):1811–1815. doi: 10.1111/j.1600-6143.2009.02696.x. [DOI] [PubMed] [Google Scholar]
- 24.Matas AJ, Leduc R, Rush D, et al. Histopathologic clusters differentiate subgroups within the nonspecific diagnoses of CAN or CR: preliminary data from the DeKAF study. Am. J. Transpl. 2010;10(2):315–323. doi: 10.1111/j.1600-6143.2009.02943.x. [DOI] [PubMed] [Google Scholar]
- 25.Jusko WJ, Piekoszewski W, Klintmalm GB, et al. Pharmacokinetics of tacrolimus in liver transplant patients. Clin. Pharmacol. Ther. 1995;57(3):281–290. doi: 10.1016/0009-9236(95)90153-1. [DOI] [PubMed] [Google Scholar]
- 26.Conover WJ. Practical Nonparametric Statistics. 2nd Edition Wiley; NY, USA: 1980. [Google Scholar]
- 27■■.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J. Pharmacokinet. Biopharma. 1981;9(4):503–512. doi: 10.1007/BF01060893. [Serves as the basis of this ana lysis. It describes how predictive performances should be assessed and the common mistakes that usually occur.] [DOI] [PubMed] [Google Scholar]
- 28.O'Seaghdha CM, Mcquillan R, Moran AM, et al. Higher tacrolimus trough levels on days 2–5 post-renal transplant are associated with reduced rates of acute rejection. Clin. Transpl. 2009;23(4):462–468. doi: 10.1111/j.1399-0012.2009.01021.x. [DOI] [PubMed] [Google Scholar]
- 29.Borobia AM, Romero I, Jimenez C, et al. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther. Drug Monit. 2009;31(4):436–442. doi: 10.1097/FTD.0b013e3181a8f02a. [DOI] [PubMed] [Google Scholar]
- 30■.Wang P, Mao Y, Razo J, et al. Using genetic and clinical factors to predict tacrolimus dose in renal transplant recipients. Pharmacogenomics. 2010;11(10):1389–1402. doi: 10.2217/pgs.10.105. [Emphasizes the use of pharmacogenomics and clinical factors in predicting the doses for tacrolimus in renal transplantation.] [DOI] [PubMed] [Google Scholar]
- 31.Zucker K, Rosen A, Tsaroucha A, et al. Unexpected augmentation of mycophenolic acid pharmacokinetics in renal transplant patients receiving tacrolimus and mycophenolate mofetil in combination therapy, and analogous in vitro findings. Transplant Immunol. 1997;5(3):225–232. doi: 10.1016/s0966-3274(97)80042-1. [DOI] [PubMed] [Google Scholar]
- 32.Ralph LD, Sandstrom M, Twelves C, Dobbs NA, Thomson AH. Assessment of the validity of a population pharmacokinetic model for epirubicin. Br. J. Clin. Pharmacol. 2006;62(1):47–55. doi: 10.1111/j.1365-2125.2006.02584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.A study of factors that affect long-term kidney transplant function (DeKAF) http://clinicaltrials.gov/ct2/show/NCT00270712.