Abstract
Aberrant epidermal growth factor receptor (EGFR) expression promotes the pathogenesis of malignant peripheral nerve sheath tumors (MPNSTs), the most common malignancy associated with neurofibromatosis type 1, but the mechanisms by which EGFR expression promotes MPNST pathogenesis are poorly understood. We hypothesized that inappropriately expressed EGFRs promote MPNST invasion and found that these kinases are concentrated in MPNST invadopodia in vitro. EGFR knockdown inhibited the migration of unstimulated MPNST cells in vitro and exogenous EGF further enhanced MPNST migration in a substrate-specific manner, promoting migration on laminin and, to a lesser extent, collagen. Thus, in this setting, EGF acts as a chemotactic factor. We also found that the 7 known EGFR ligands (EGF, betacellulin, epiregulin, heparin-binding EGF, transforming growth factor α [TGFα], amphiregulin, and epigen) variably enhanced MPNST migration in a concentration-dependent manner, with TGFα being particularly potent. With the exception of epigen, these factors similarly promoted the migration of non-neoplastic Schwann cells. Although transcripts encoding all 7 EGFR ligands were detected in human MPNST cells and tumor tissues, only TGFα was consistently overexpressed and was found to colocalize with EGFR in situ. These data indicate that constitutive EGFR activation, potentially driven by autocrine or paracrine TGFα signaling, promotes the aggressive invasive behavior characteristic of MPNSTs.
Keywords: ErbB membrane tyrosine kinases, Nerve sheath tumor, Neurofibromatosis type 1, Schwann cell, Tumor cell invasion
INTRODUCTION
Neoplastic Schwann cells within benign plexiform neurofibromas commonly undergo malignant transformation and give rise to highly aggressive spindle cell malignancies referred to as malignant peripheral nerve sheath tumors (MPNSTs). In the general population, MPNSTs are rare neoplasms but they are relatively common in patients with the autosomal dominant tumor predisposition syndrome neurofibromatosis type 1 (NF1) – an NF1 patient’s lifetime risk of developing an MPNST has been estimated at 8% to 13% (1) and 5.9% to 10.3% (2). Further, when NF1 patients develop MPNSTs, the clinical management of their tumors is problematic. Radiotherapy does not prolong the survival of these patients (3). Chemotherapy is typically ineffective in adults with MPNSTs (4), although chemotherapy may have some efficacy in children with unresectable MPNSTs (5). Given the limitations of radiotherapy and chemotherapy, it is not surprising that surgery is the primary means of treating MPNSTs. However, a surgical cure is often not possible because MPNSTs aggressively invade adjacent non-neural tissues and frequently metastasize to a variety of organ sites (6). The molecular pathways mediating MPNST invasion and metastasis are largely unknown. Identifying the critical signaling molecules in these pathways is essential because it would allow the development of novel therapies designed to restrict these neoplasms to their site of origin thereby rendering them amenable to surgical resection.
Several lines of evidence indicate that inappropriate expression of the epidermal growth factor (EGF) receptor ([EGFR], also known as HER1 and erbB1) promotes the pathogenesis of neurofibromas and MPNSTs. Although this membrane tyrosine kinase is not expressed by neonatal Schwann cells (7), EGFR is present in neoplastic Schwann cells isolated from human neurofibromas and MPNSTs (8), surgically resected MPNSTs (8), and cells derived from tumors arising in a genetically engineered mouse model of MPNST pathogenesis (Nf1+/−;p53+/− cis mice) (9). In 2 large series, amplification of the EGFR gene was identified in 26% (10) and 28% (11) of the MPNSTs that were studied. This has clinical relevance because patients with an MPNST that overexpresses EGFRs have a higher prevalence of advanced stage tumors, a lower probability of 5-year, disease-free survival and lower 5-year survival rates (12). Further, transgenic mice inappropriately expressing EGFRs in Schwann cells develop several abnormalities reminiscent of the early stages of neurofibroma pathogenesis, including Schwann cell hyperplasia, increased collagen deposition, mast cell accumulation and disruption of axon-Schwann cell interactions (13, 14). A role for EGFR signaling in MPNST pathogenesis is also supported by the observation that introducing an EGFR hypomorphic mutation into Nf1+/−; p53+/− cis mice improves the survival of these animals (14).
Although the observations noted above clearly support the hypothesis that inappropriate expression of the EGFR contributes to neurofibroma and MPNST pathogenesis, the precise mechanisms by which this overexpression facilitates tumorigenesis remain poorly understood. In particular, it is not known whether EGFR signaling in peripheral nerve sheath tumors promotes the aggressive invasive behavior characteristic of MPNST cells. Consequently, here we examine the role that the EGFR and each of its 7 known ligands play in MPNST invasion.
MATERIALS AND METHODS
Human Cases
Experiments using human tissues were reviewed and approved by the University of Alabama at Birmingham Institutional Review Board for Human Use. Surgically resected MPNST tumor tissues were obtained from the Southern Division of the Cooperative Human Tissue Network and the UAB Comprehensive Cancer Center Tissue Procurement Shared Facility (Director: William E. Grizzle, MD, PhD).
Reagents and Antibodies
EGF (#E4127), betacellulin (#B3670), transforming growth factor-α ([TGFα]; #T7924), heparin binding-epidermal growth factor (HB-EGF; #E4643), amphiregulin (#A7080) and epiregulin (#E8780) were purchased from Sigma-Aldrich (St. Louis, MO). Epigen (#1127-EP) was obtained from R&D Systems (Minneapolis, MN). The rabbit polyclonal anti-EGFR antiserum and the rabbit monoclonal anti-EGFR antibody used for immunostaining and immunoblotting were from Millipore (#06-847; Billerica, MA) and Cell Signaling Technologies (#4267; Danvers, MA), respectively. A mouse monoclonal anti-TGFα antibody (clone 213-4.4) was purchased from Abcam (Cambridge, MA). An anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) monoclonal antibody (clone 6C5) was obtained from Fitzgerald Antibodies and Antigens (Concord, MA). Fluorescein isothiocyanate- and horseradish peroxidase-conjugated donkey anti-mouse and anti-rabbit secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA).
Culture of Adult Schwann Cells and Tumor Cell Lines
Non-neoplastic human Schwann cells were used for real-time polymerase chain reaction (PCR) analyses of EGFR ligand mRNA expression. Because only a limited quantity of human Schwann cells was available to us, migration assays comparing the effects of different EGFR ligands were performed using rat Schwann cells. Our previously described methodology was used to prepare cultures of wild-type Schwann cells from the sciatic nerves of adult Sprague-Dawley rats (15). Rat Schwann cell cultures were propagated in Dulbecco’s modification of Eagle’s medium (DMEM) containing 10% heat-inactivated fetal calf serum, 10 IU/mL penicillin and 10 µg/mL streptomycin (DMEM10); this media was additionally supplemented with 50 nM neuregulin-1β (NRG1β) and 2 µM forskolin. Cultures of human Schwann cells prepared from sciatic nerve collected from organ donors were kindly provided by Dr. Mary Bunge (Miami Project to Cure Paralysis, Miami, FL). These cells were also maintained in DMEM10 supplemented with 50 nM NRG1β and 2 µM forskolin. We have previously described the sources of the ST88-14, STS-26T, YST-1, NMS2, NMS2-PC, 90-8, S462 and T265-2c human MPNST cell lines used in this study (16–18). All MPNST cell lines were maintained in DMEM10.
Immunoblotting Assays
Cultures of human MPNST cells were homogenized in situ with 1 mL of ice-cold HES (20 mM HEPES [pH 7.4], 1 mM EDTA, 250 mM sucrose) buffer supplemented with protease (Sigma #P8340), serine/threonine phosphatase (Sigma #P2850) and tyrosine phosphatase (Sigma #P5726) inhibitors diluted 1:100. Protein concentrations were determined with a modified Lowry assay (DC Protein Assay; Bio-Rad, Hercules, CA). Equal quantities of protein lysates were resolved on sodium dodecyl sulfate polyacrylamide gels, immunoblotted per our previously described protocol (22), and probed with an antibody recognizing the EGFR (1:20,000 dilution; Cell Signaling). Immunoreactive species were detected by enhanced chemiluminescence (Pierce). Membranes were subsequently reprobed with an anti-GAPDH antibody (1:20,000 dilution) to verify equal protein loading.
Knockdown of EGFR Expression with shRNA Expressing Lentiviral Vectors and Plasmids
To express doxycycline-inducible miR-like shRNAs in MPNST cells, we used single lentivector for inducible knockdown (pSLIK) lentiviral vectors (19), which express these shRNAs coordinately with green fluorescent protein (GFP). Oligonucleotides encoding EGFR shRNA sequences were designed using the RNAi Codex algorithm (http://katahdin.cshl.org:9931/portal/scripts/main2.pl). Oligonucleotides were annealed and ligated to BfuAI digested pen_TTGmiRc2. The resulting plasmids were then recombined with pSLIK-hygro vector using LR Clonase per the manufacturer’s recommendations (Invitrogen, Carlsbad, CA). Two lentiviral vectors targeting distinct EGFR sequences were constructed in this way: pSLC667 and pSLC668, which target nucleotides 1245–1265 and 3504–3524 of NM_005228.3, respectively. To identify possible non-specific activation of RISC, a pSLIK vector was also constructed that contained an shRNA sequence not present in the human, mouse or rat genomes (pSLC727).
To prepare lentivirus, pSLIK vectors together with helper plasmids (pPLP1, pPLP2, pVSVg) were transfected into 293FT cells using Polyfect reagent (Qiagen, Valencia, CA) per the manufacturer’s recommendations. At 72 hours post-transfection, virus-containing media was collected and stored at −80°C until use. To stably transduce lentiviral vectors into MPNST cells, virus-containing supernatant and 6 µg/mL Polybrene (Sigma-Aldrich) was added to cells plated in 6 well plates containing DMEM10 medium, and 24 hours later, the virus-containing medium was replaced with tetracycline-free DMEM10. At 48 to 72 hours post-transduction, wells were split into three 100-mm dishes and hygromycin selection begun. Colonies arose approximately 2 weeks later, at which time they were picked and individually expanded. Lines with appropriate regulation of the shRNA/GFP cassette were identified by establishing that they had no GFP fluorescence in the absence of doxycycline and strong GFP signals following the addition of doxycycline.
Plasmids expressing shRNAs targeting EGFR mRNA or a nonsense sequence were purchased from SABiosciences (#KH00138G, Valencia, CA). To transiently transfect these plasmids, log phase (~70% confluent) T265-2c cells maintained in DMEM10 were removed from their substrate with Cell Stripper (Invitrogen), and then resuspended in DMEM with 1% fetal calf serum at a concentration of 2 × 106 cells/mL; 400 µl of this suspension together with 12 µg of a shRNA-expressing plasmid was placed in a 4-mm-gap width cuvette. Using a BTX ECM 830 Square Wave electroporator (Harvard Apparatus, Inc., Holliston, MA), cells were pulsed 3 X at 675 V, with each pulse lasting for 300 µs and with a 100-ms pause between pulses. Electroporated cells were allowed to recover in the cuvette for 2 minutes at room temperature (RT) prior to being transferred into the DMEM10 containing wells of a 6-well plate. After allowing the cells to attach overnight, the media were changed. At 72 hours post-electroporation, a portion of the cells was used for migration assays. The remainder of the electroporated cells was used to prepare lysates that were immunoblotted and probed for EGFR protein to verify knockdown.
Migration Assays
MPNST cells were allowed to migrate for 6 hours on substrate-coated Transwell migration filters using our previously described methodology (17). Filters were stained overnight at 4°C with crystal violet dissolved in ethanol. Filters were then washed 4 X with phosphate-buffered saline (PBS) and cells on the top surface of the filter removed using a PBS-soaked cotton-tipped applicator. Cells on the lower surface of the filter were photographed by bright-field microscopy, with 5 distinct 20× fields per filter captured for quantification. Experiments were repeated at least 6 times. Numbers of cells per photographed field were established using Image Pro Plus (version 4.1) software (Media Cybernetics; Silver Springs, MD). Resulting data were analyzed with a one-way ANOVA followed by a Tukey post-hoc test, with p values < 0.05 considered significant.
Real-Time PCR Assays
An ABI 7500 Real Time PCR System (Applied Biosystems, Inc., Foster City, CA) was used to perform real-time quantitative PCR per our previously described protocol (20). Levels of EGF, TGFα, betacellulin, HB-EGF, amphiregulin, epiregulin and epigen transcript sequences were assayed using TaqMan MGB probes labeled with FAM dye (ABI assays Hs01099999_m1, Hs00608187_m1, Hs01101204_m1, Hs00961131_m1, Hs00950669_m1, Hs00914313_m1 and Hs02385424_m1, respectively). 18S ribosomal cDNA levels were assayed using TaqMan MGB probes labeled with VIC dye (Applied Biosystems # 4319413E). Assays were performed in triplicate, with target transcript levels normalized to levels of 18S cDNA in the same reverse transcription reaction. Controls lacking added template were performed in parallel to verify an absence of contamination.
Immunocytochemical and Immunohistochemical Localization of EGFRs and TGFα
To examine potential EGFR localization in invadopodia, MPNST cells were plated on poly-L-lysine/laminin-coated FluoroBlok filters with 8-µm pores (#351152; BD Biosciences, Bedford, MA) and allowed to migrate for 1 hour. Cells were then fixed for 20 minutes at RT in 4% paraformaldehyde in PBS. Fixed cells were washed 3 X with PBS and then incubated in TBST (Tris-buffered saline containing 2% bovine serum albumin and 0.1% Triton X-100) for 20 minutes at RT. Following these washes, cells were incubated with anti-EGFR antibody diluted in TBST (1:100 dilution) overnight at 4°C. The next morning, filters were washed 3 X with PBS (15 minutes/wash) and then incubated for 1 hour at RT with fluorescein isothiocyanate-conjugated anti-rabbit secondary antibody (1:100 dilution in TBST). Cells were washed 3 more times with PBS (15 minutes/wash) and then counterstained with 2 µg/mL bisbenzamide (10 minutes at RT). Filter membranes were removed, mounted under coverslips with 1:1 PBS:glycerol and examined by indirect immunofluorescence microscopy.
Double label immunohistochemistry for TGFα and EGFRs was performed on tumor sections using a modification of our previously described methodology (16, 17), with the primary difference being that tyramide signal amplification was used to detect antigen signals.
RESULTS
EGFRs Are Present in MPNST Cell Invadopodia and Promote Migration of Unstimulated MPNST Cells
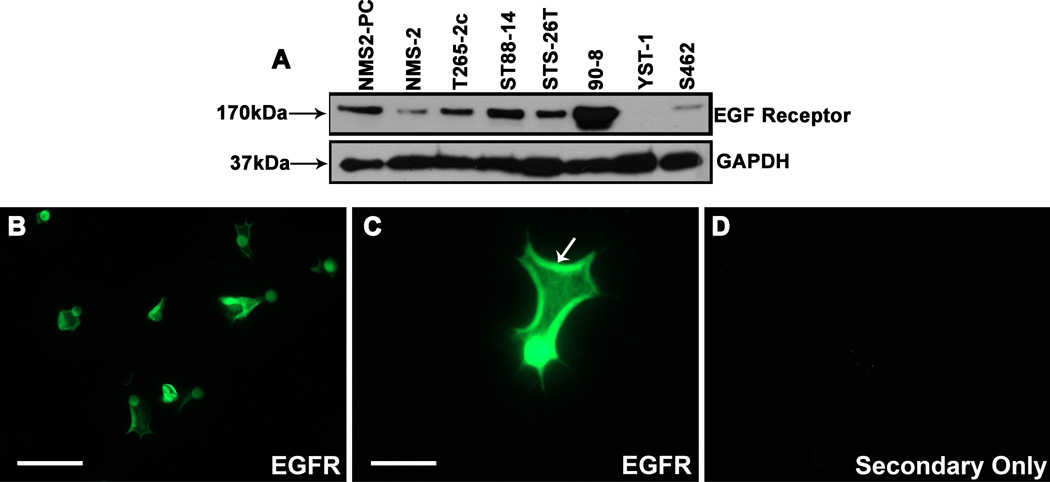
EGFR amplification is variably present in human MPNSTs in vivo. To ensure that the MPNST cell lines we used to examine the role of the EGFR in migration expressed this membrane tyrosine kinase, we screened lysates prepared from 6 cell lines derived from MPNSTs arising in NF1 patients (NMS2-PC, NMS2, T265-2c, ST88-14, 90-8 and S462) and 2 lines derived from sporadic MPNSTs (STS-26T, YST-1) for the expression of EGFRs. Upon immunoblotting these lysates and probing them with a rabbit monoclonal anti-EGFR antibody, we detected a band of the expected 170-kDa mass in all of these lines except YST-1 cells (Fig. 1A).
Figure 1.
Epidermal growth factor receptors (EGFRs) are present in the invadopodia of malignant peripheral nerve sheath tumor (MPNST) cells. (A) Expression of the 170 kDa EGFR in a panel of human MPNST cell lines. Lines are indicated above the panels. The lower panel is the same lysates probed for glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (37 kDa) to demonstrate equivalent loading. (B–D) Human ST88-14 MPNST cells plated on FluoroBlok filters were allowed to migrate for 1 hour, fixed and then immunostained for EGFR. Examination of the undersurface of these filters via conventional immunofluorescence microscopy shows numerous EGFR-positive processes protruding through the pores of the filter (B). Examination of the EGFR immunoreactive invadopodia at higher power shows that EGFR immunoreactivity is concentrated at the edges of the processes extended through the filter pores (C, arrow). In contrast, in negative controls, the undersurface of FluoroBlok filter shows no immunoreactive MPNST processes (D). Scale bars: B–D, 20 µm.
Invadopodia are specialized processes that contain cell adhesion molecules, cytoplasmic signaling molecules and proteases, which allow tumor cells to adhere to and degrade the extracellular matrix during invasion (21). We have previously shown that the NRG1 receptors erbB3 and erbB4, which are members of the EGFR subfamily of membrane tyrosine kinases, are concentrated in MPNST cell invadopodia (17). To determine whether EGFRs are similarly present in MPNST cell invadopodia, ST88-14 cells were plated on the upper surface of FluoroBlok filters coated with poly-L-lysine/laminin, a substrate that supports MPNST migration (17). These cells were allowed to migrate for 1 hour, a period we empirically determined is sufficient for the initial protrusion of invadopodia through filter pores (17). The filters were then immunostained for EGFR and their undersurfaces examined by immunofluorescence microscopy. Because FluoroBlok filters are opaque at the wavelengths used for immunofluorescence microscopy, the portion of the cells still present in the upper chamber is masked from view by the filter membrane and only invadopodia that have extended through membrane pores are visualized with this approach. EGFR immunoreactivity was present within the invadopodia of ST88-14 cells (Fig. 1B) and was particularly evident at the edges of protruding processes (Fig. 1C). This pattern of immunoreactivity was absent in negative controls (Fig. 1D). We conclude that EGFRs are present in MPNST invadopodia and are thus appropriately localized to promote MPNST migration and invasion.
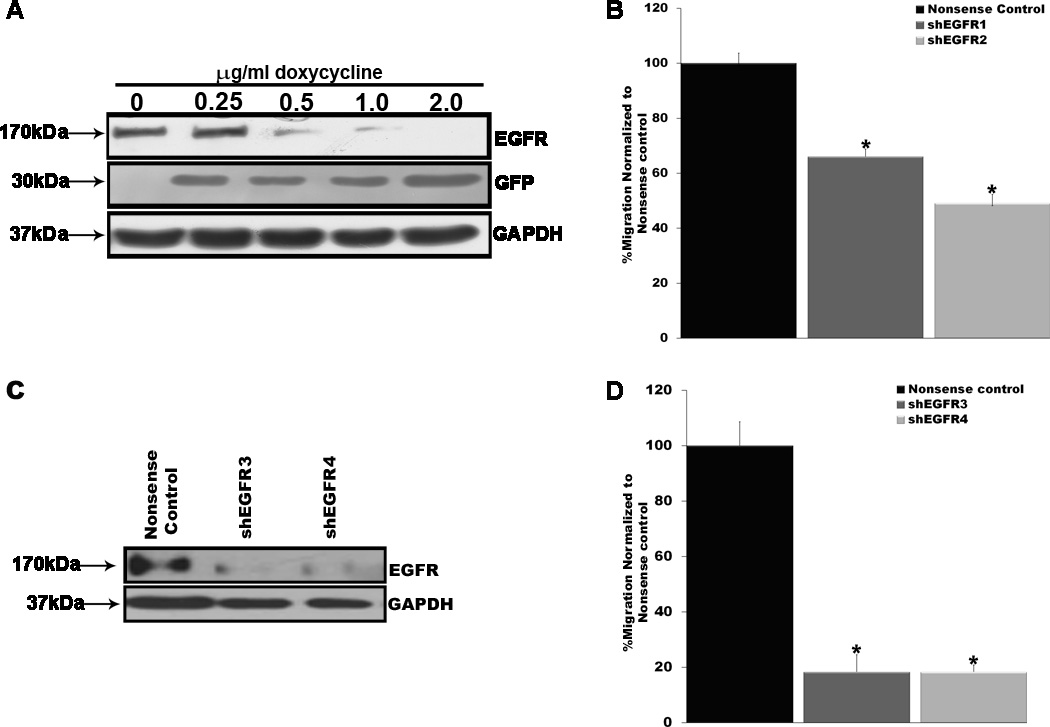
We next asked whether ablating EGFR expression in MPNST cells would inhibit their migration. To test this, we cloned shRNA sequences targeting human EGFR mRNA or a nonsense sequence into the doxycycline-inducible lentiviral vector pSLIKhygro, which expresses shRNAs coordinately with GFP (19). These lentiviral vectors were then stably transduced into a representative MPNST line (ST88-14 cells). To verify that shRNA and GFP expression in the selected cells was appropriately regulated and to establish the optimal conditions for knockdown of EGFR expression, transformants were challenged with 0, 0.25, 0.5, 1.0 or 2.0 µg/mL doxycycline for 72 hours. Lysates of cells stimulated with varying doxycycline concentrations were then immunoblotted and probed with antibodies recognizing GFP or EGFR (Fig. 2A). GFP expression was effectively induced with the lowest concentration of doxycycline used. In contrast, knockdown of EGFR expression increased in a concentration-dependent manner, with the greatest level of knockdown achieved with 2 µg/mL doxycycline. Comparing the extent of EGFR knockdown 24, 48, 72 and 96 hours after induction with 2 µg/mL doxycycline, we found that shRNA effects were maximal by 72 hours (data not shown). Consequently, in subsequent experiments induction was performed using cells treated for 72 hours with 2 µg/mL doxycycline.
Figure 2.
Epidermal growth factor receptors (EGFRs) are required for the migration of unstimulated malignant peripheral nerve sheath tumor (MPNST) cells. (A) ST88-14 MPNST cells stably transduced with a pSLIK lentivirus coordinately expressing an EGFR shRNA and green fluorescent protein (GFP) were treated with a range of doxycycline concentrations for 72 hours. These lysates were then probed for EGFR (upper panel) and GFP (30 kDa; middle panel) to assess EGFR knockdown and cassette activation, respectively. The lower panel is a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) loading control. (B) Migration of ST88-14 MPNST cells 72 hours after inducing the expression of 2 distinct EGFR shRNAs (shEGFR1, shEGFR2) or a nonsense control shRNA. Migration in the EGFR shRNA-expressing cells was normalized to migration in cells expressing the nonsense control shRNA. SEM are indicated for each condition; *p < 0.05 vs. the nonsense control. (C) T265-2c MPNST cell lysates were prepared 72 hours after transfection with plasmids expressing 2 distinct EGFR shRNAs (shEGFR3, shEGFR4) or a nonsense control shRNA and probed for EGFR expression to demonstrate knockdown (upper panel). The lower panel is a GAPDH loading control. (D) Migration assays with T265-2c MPNST cells transiently transfected with the same shRNA-expressing plasmids expressing shRNAs presented in (C). Numbers of shEGFR transfected cells migrating to filter undersurfaces are normalized to numbers of nonsense shRNA transfected cells migrating to filter undersurfaces under the same conditions. SEM are indicated, with asterisk (*) indicating conditions for which p < 0.05 for comparisons with the nonsense control.
To determine whether knockdown of EGFR expression inhibited the migration of ST88-14 MPNST cells, shRNA expression was induced in cells carrying lentiviral vectors expressing 2 distinct EGFR shRNAs or a nonsense control. Transwell migration assays were then performed on filters coated with poly-L-lysine/laminin. After allowing the cells to migrate for 6 hours, numbers of EGFR shRNA-expressing cells that migrated to the undersurface of the filters were determined and normalized to the number of nonsense control shRNA-expressing cells that migrated to the filter undersurface (Fig. 2B). We found that knocking down EGFR expression significantly inhibited the migration of these MPNST cells, producing approximately 40% and 50% decreases in migration in ST88-14 cells expressing the 2 different EGFR shRNAs. In control experiments, induction of the nonsense control shRNA had no effect on the migration of these cells. Treatment of the parent line with 2 µg/mL doxycycline likewise had no effect on migration, indicating that the decreased migration observed following induction of EGFR shRNA expression is not due to the presence of doxycycline.
To assess the role that the EGFR plays in MPNST cell migration further, we transiently transfected plasmids expressing shRNAs targeting 2 distinct EGFR sequences or a nonsense control sequence into a different MPNST line (T265-2c cells). Relative to the nonsense control shRNA, both of these EGFR shRNAs effectively knocked down EGFR expression (Fig. 2C). Transwell migration assays performed as described above 72 hours post-transfection showed that migration was decreased more than 80% in T265-2c cells transiently transfected with EGFR shRNA plasmids relative to cells transfected with the nonsense control (Fig. 2D). Taken together, these experiments demonstrate that EGFR expression promotes the migration of unstimulated MPNST cells.
EGF Promotes the MPNST Cell Migration in a Substrate-Dependent Manner
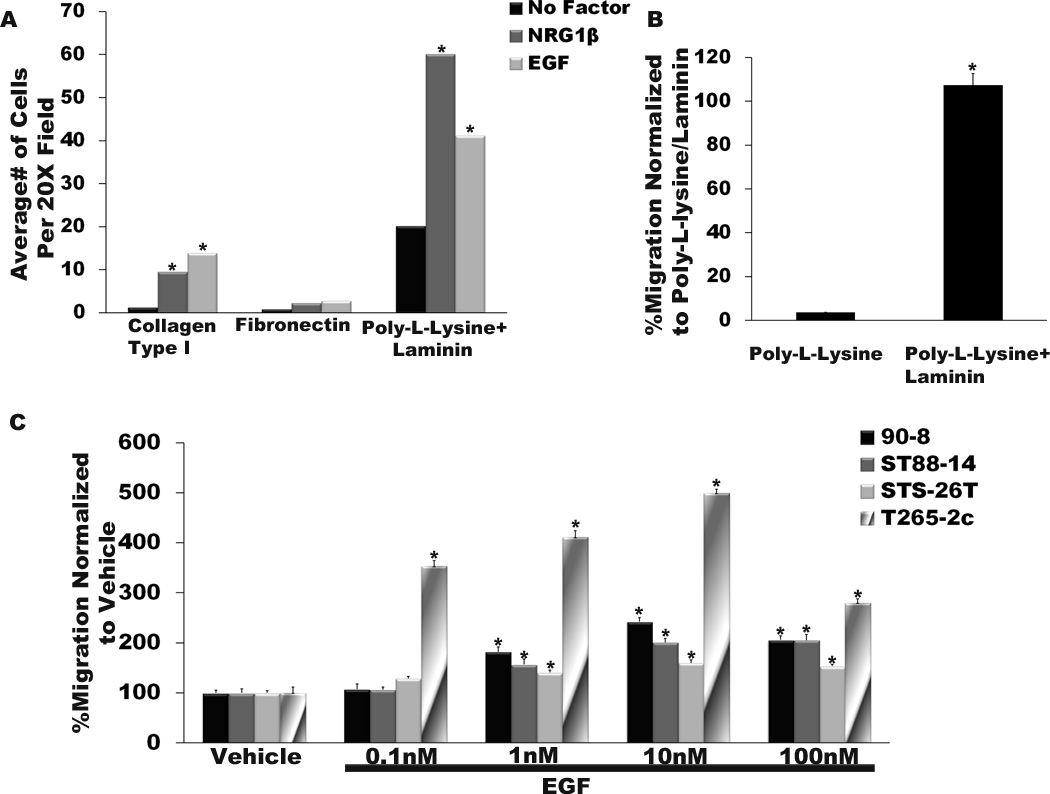
We have previously shown that NRG1β, a growth factor that acts through membrane tyrosine kinases (erbB2, erbB3 and/or erbB4) closely related to the EGFR, preferentially promotes MPNST migration on a laminin substrate (17). Therefore, we first determined whether the migration of ST88-14 MPNST cells in response to exogenous EGF is similarly dependent upon the presence of specific substrates including collagen type I, fibronectin and poly-L-lysine/laminin substrates. These substrates were chosen because they support the migration of wild-type Schwann cells (22, 23) and neoplastic Schwann cells derived from ethylnitrosourea-induced MPNSTs (24). They are also present within Schwann cell basement membranes and extracellular matrix in normal nerve (25), neurofibromas and MPNSTs (26), as well as being widely expressed at common sites of MPNST metastasis. In these experiments, serum-starved ST88-14 cells were plated on the upper surface of Transwell filter inserts the undersurfaces of which had been coated with the test substrates. The MPNST cells were challenged with vehicle, a positive control (NRG1β) or EGF and allowed to migrate for 6 hours. Cells that had migrated to the filter undersurface were then counted. We found that collagen type I, fibronectin, and poly-L-lysine/laminin all supported the migration of unstimulated ST88-14 cells, although this migration was maximal on poly-L-lysine/laminin (Fig. 3A). However, when 10 nM EGF was added to the lower chamber of test wells, a clear substrate preference became evident. Ten nM EGF induced a significant increase in the migration of ST88-14 cells on both poly-L-lysine/laminin and collagen type I (Fig. 3A), but the overall number of migrating cells was much higher on the poly-L-lysine/laminin substrate. In contrast, EGF did not significantly increase the number of migrating MPNST cells when they were plated on fibronectin. ST88-14 cells challenged with 0.1 nM NRG1β (a concentration that optimally induces the migration of these cells) showed a similar preference for the poly-L-lysine/laminin substrate (Fig. 3A), in keeping with our previous observations (17).
Figure 3.
Epidermal growth factor (EGF), like neuregulin-1β (NRG1β), optimally stimulates malignant peripheral nerve sheath tumor (MPNST) cell migration assays on a laminin substrate. (A) Human ST88-14 MPNST cells were plated on the upper surface of Transwell filter inserts coated with collagen type I, fibronectin or poly-L-lysine/laminin and allowed to migrate for 6 hours. Vehicle, 0.1 nM NRG1β (a concentration we have previously determined optimally promotes the migration of this line), or 10 nM EGF was added to the bottom chamber. The bar graph indicates the average number of ST88-14 MPNST cells migrating to the undersurface of filters coated with the indicated substrates. *p < 0.05 vs. cells receiving vehicle and migrating on the same substrate. (B) ST88-14 cells were plated on the upper surface of Transwell filter inserts coated with poly-L-lysine alone or poly-L-lysine/laminin and allowed to migrate for 6 hours. Migration on poly-L-lysine/laminin is considered 100%, with migration on poly-L-lysine normalized to this value. *p < 0.05 for the comparison of these 2 conditions. (C) Bar graphs indicating the relative migration of 4 MPNST cell lines challenged with vehicle or 0.1 to 100 nM concentrations of EGF. SEM are indicated for each bar. *p < 0.05 vs. the migration of the same cell lines receiving vehicle rather than EGF.
To establish which of the components in the poly-L-lysine/laminin substrate was required for optimal EGF-induced migration, we next compared the migration of ST88-14 cells challenged with 10 nM EGF on poly-L-lysine alone or on poly-L-lysine/laminin (Fig. 3B). While the poly-L-lysine/laminin substrate again supported migration, there was only a very low level of migration on poly-L-lysine alone, indicating that laminin is the substrate component that is primarily responsible for promoting EGF-induced migration of MPNST cells.
Having identified a substrate that optimally supports EGF-induced migration, we next asked if increased migration in response to EGF stimulation was widely characteristic of MPNST cells and, if so, what concentration of EGF optimally promoted migration. For this purpose, Transwell migration assays were performed on a poly-L-lysine/laminin substrate using 3 lines derived from NF1-associated MPNSTs (ST88-14, 90-8 and T265-2c cells), and a sporadic MPNST cell line (STS-26T cells). The cells were challenged with EGF concentrations ranging from 0.1 to 100 nM. Although all of these cell lines were EGF-responsive (Fig. 3C), the maximal level of migration varied from line to line, with T265-2c showing the greatest extent of migration (approximately 500% above vehicle), and the other 3 lines demonstrated somewhat lower maximal levels of migration (150 to 250% of vehicle). For all 4 lines, the EGF concentration required to induce the maximal level of migration (10 nM) was the same. These observations indicate that enhanced migration in response to stimulation with exogenous EGF is characteristic of MPNST cells.
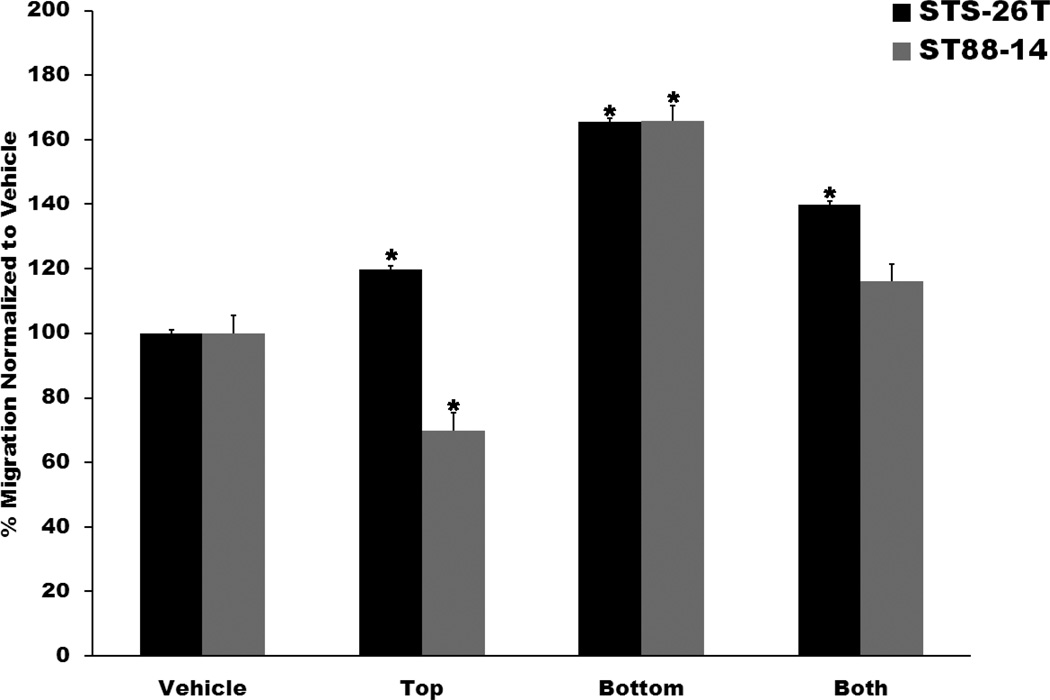
EGF Promotes MPNST Cell Migration in a Chemotactic Fashion
Growth factors that promote tumor cell migration can do so by enhancing motility in a non-directed manner (a chemokinetic effect), or by directing cellular movement toward an area of higher growth factor concentration (a chemotactic effect). To establish whether EGF acts in a chemokinetic or a chemotactic manner, Transwell assays were performed in which ST88-14 and STS-26T cells were challenged with 10 nM EGF in the bottom chamber only, the top chamber only, or in both the top and bottom chambers. After 6 hours exposure to EGF, the number of cells migrating to filter undersurfaces were quantified, with the number of cells detected normalized to the number of cells seen in wells containing vehicle alone. For both ST88-14 and STS-26T cells, the highest level of migration was observed when EGF was added only to the bottom chamber (Fig. 4). In contrast, the number of cells migrating to filter undersurfaces was lowest when EGF was placed in the top chamber, with an intermediate level of migration seen when EGF was added to both chambers. Together, these findings indicate that EGF acts upon MPNST cell migration in a predominantly chemotactic manner.
Figure 4.
Epidermal growth factor (EGF) promotes malignant peripheral nerve sheath tumor (MPNST) migration in a predominantly chemotactic manner. Human STS-26T (black bars) and ST88-14 (gray bars) MPNST cells were plated in the upper chamber of Transwell filter inserts. Ten nM EGF was then added to the top chamber only (Top), the bottom chamber only (Bottom) or both chambers (Both). Bars indicate average migration ± SEM vs. cells receiving vehicle alone. Asterisks above bars indicate a significant change in migration (p < 0.05) relative to cells challenged with vehicle.
EGFR Ligands Differ in their Ability to Promote MPNST Cell Migration
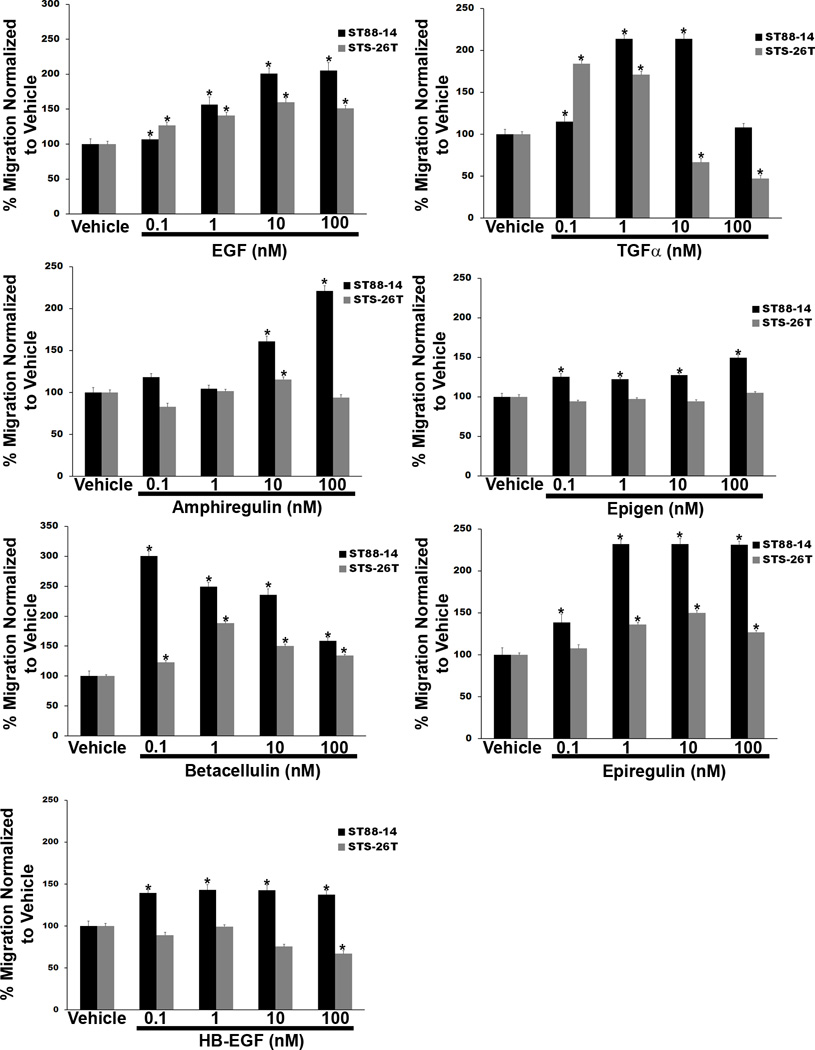
In addition to EGF, multiple ligands are capable of activating the EGFR. These growth factors include 4 ligands that bind solely to the EGFR (EGF, TGFα, amphiregulin and epigen) and 3 ligands (HB-EGF, betacellulin, and epiregulin), which are capable of activating both the EGFR and the related membrane tyrosine kinase erbB4. Therefore, we next compared the relative ability of each of these ligands to promote MPNST cell migration by performing Transwell migration assays in which representative NF1-associated (ST88-14) and sporadic (STS-26T) MPNST lines were challenged with 0.1 to 100 nM concentrations of each ligand. Cells migrating to the undersurfaces of filters 6 hours after adding growth factor to the lower chamber of the well were then counted and the counts normalized to the level of migration observed with cells challenged with vehicle. All 7 of the EGFR ligands induced migration in at least one of the MPNST lines (Fig. 5), but the degree of migration and the concentration of each growth factor that maximally induced migration differed considerably among the different ligands. EGF induced significantly increased levels of migration at every concentration tested in both ST88-14 and STS-26T cells, with maximal migration in both lines again achieved with a 10-nM concentration of this growth factor. In contrast, 0.1 and 1 nM TGFα maximally stimulated the migration of STS-26T and ST88-14 cells, respectively, with higher concentrations of this ligand producing significant decreases in the migration of STS-26T cells. Amphiregulin was less potent than both EGF and TGFα; significant increases in the migration of ST88-14 cells were first observed with a 10 nM concentration of amphiregulin, with a 100-nM concentration of this factor inducing a still higher level of migration. In STS-26T cells, only the 10 nM concentration of amphiregulin produced a significant increase in migration, and that effect was quite modest. Epigen was similarly ineffective, producing modest increases in the migration of ST88-14 cells at all concentrations tested and having no significant effect on the migration of STS-26T cells.
Figure 5.
The migration of human malignant peripheral nerve sheath tumor (MPNST) cells is enhanced in a concentration-dependent manner by multiple epidermal growth factor receptor (EGFR) ligands. Transwell migration assays in which human STS-26T (gray bars) and ST88-14 (black bars) MPNST cells were stimulated with vehicle or 0.1 to 100 nM concentrations of the EGFR ligands EGF, transforming growth factor-α (TGFα), amphiregulin, heparin-binding EGF (HB-EGF), betacellulin, epiregulin or epigen are shown. Numbers of cells migrating in wells receiving EGFR ligands are normalized to numbers of cells migrating in wells receiving vehicle. Bars represent the normalized average migration ± SEM. An asterisk above a bar indicates a different level of migration (p < 0.05) vs. cells challenged with vehicle.
The group of ligands capable of activating both the EGFR and erbB4 had similarly variable effects on the migration of ST88-14 and STS-26T cells (Fig. 5). Betacellulin and epiregulin had particularly potent effects on ST88-14 cells, with 0.1 nM and 1 nM concentrations of betacellulin and epiregulin, respectively, being sufficient to stimulate maximally the migration of this line; this is in contrast to the 10 nM concentration of EGF that is required to promote maximally the migration of this line. In STS-26T cells, the concentrations of betacellulin and epiregulin that maximally induced migration were an order of magnitude higher than that required with ST88-14 cells and the level of migration achieved was lower, although still significant. In contrast, HB-EGF enhanced the migration of ST88-14, but not STS-26T cells, and the maximal increase induced was less than 50% higher than in cells challenged with vehicle. Overall, these observations indicate that although multiple EGFR ligands are capable of enhancing MPNST migration, these growth factors differ in their relative ability to induce migration and different MPNST lines demonstrate distinct responses to these factors.
EGFR Ligands Differently Promote the Migration of Non-Neoplastic Schwann Cells
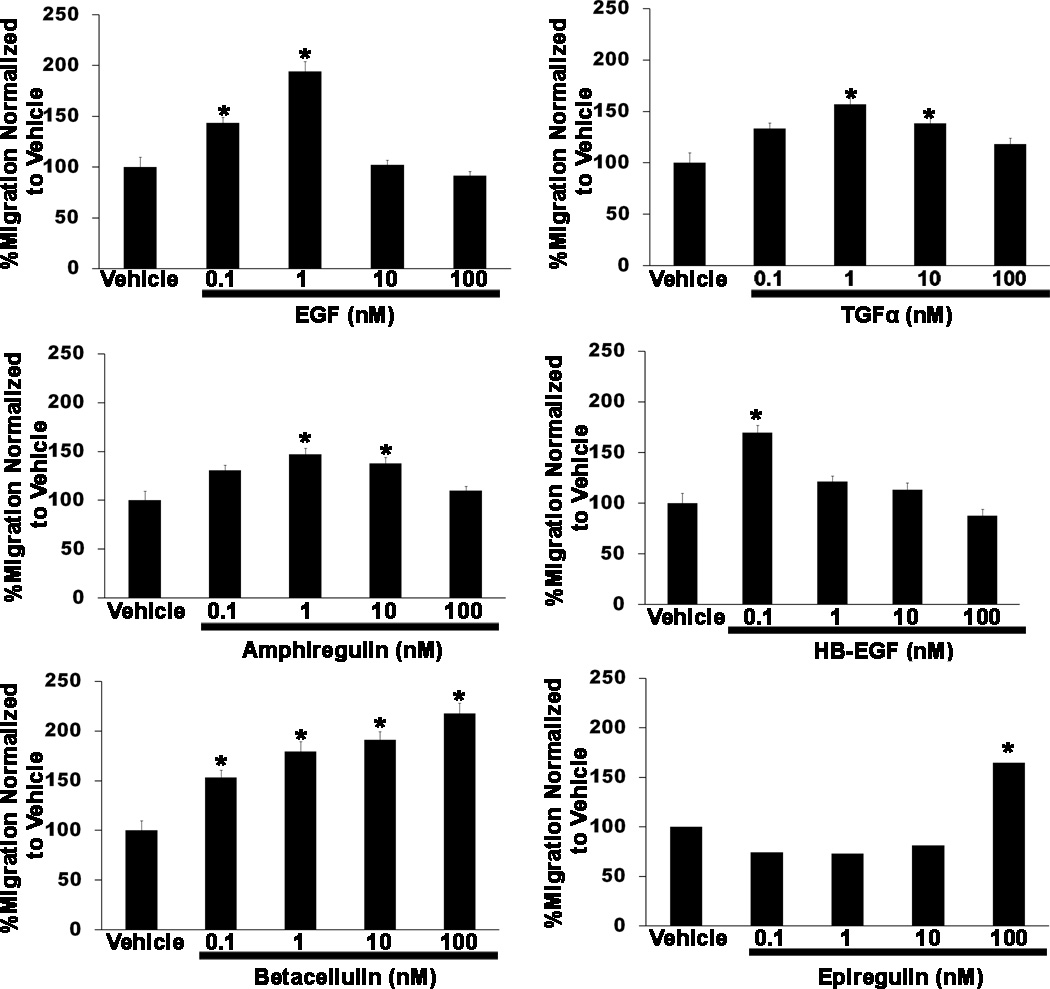
We previously demonstrated that adult (but not neonatal) Schwann cells express the EGFR (7), and thus are potentially responsive to ligands activating this membrane tyrosine kinase. Therefore, we next asked whether the same EGFR ligands that promote MPNST migration also enhance the migration of non-neoplastic adult Schwann cells. Using the same conditions employed with MPNST cells, we performed Transwell migration assays in which these glia were stimulated with vehicle or 0.1 to 100 nM concentrations of each of the 7 EGFR ligands. We found that 3 of the 4 EGFR-specific ligands increased the migration of adult Schwann cells. However, the Schwann cell responses to these factors differed in some aspects from those of MPNST cells (Fig. 6). For example, EGF enhanced Schwann migration only at 0.1 and 1 nM concentrations, whereas ST88-14 and STS-26T MPNST cells, which demonstrated statistically significant increases at these same concentrations, showed increasing levels of migration at higher EGF concentrations. TGFα effects on the migration of non-neoplastic Schwann cells (which were maximal at a 1 nM concentration) were similar to those observed in MPNST cells stimulated with this factor. In contrast, non-neoplastic Schwann cells were more sensitive to amphiregulin than the MPNST lines; amphiregulin maximally promoted Schwann cell migration at 1 nM, whereas concentrations an order of magnitude higher were required to achieve the same effect in MPNST cells. The fourth EGFR-specific ligand, epigen, had no effect on Schwann cell migration.
Figure 6.
Exogenous epidermal growth factor receptor (EGFR) ligands variably enhance the migration of non-neoplastic Schwann cells in a concentration-dependent manner. Transwell migration assays in which rat Schwann cells were stimulated with vehicle or 0.1 to 100 nM concentrations of the EGFR ligands EGF, transforming growth factor-α (TGFα), amphiregulin, heparin-binding EGF (HB-EGF), betacellulin or epiregulin are shown. Epigen had no effect on the migration of non-neoplastic Schwann cells (not shown). Numbers of cells migrating in wells receiving EGFR ligands were normalized to the number of cells migrating in wells receiving vehicle. Bars represent the normalized average migration ± SEM. Asterisks above bars indicate a significant change in migration (p < 0.05) vs. cells challenged with vehicle.
We also found that all 3 of the ligands capable of activating both the EGFR and erbB4 promoted Schwann cell migration (Fig. 6). However, again there were some clear differences between the effects these growth factors had on non-neoplastic Schwann cells and on MPNST cells. HB-EGF, which enhanced the migration of ST88-14 cells (but not STS-26T cells) at all concentrations tested, produced a significant increase in the migration of Schwann cells only at a 0.1-nM concentration. Concentrations of 0.1 to 100 nM betacellulin produced concentration-dependent increases in Schwann migration; in contrast, betacellulin effects on ST88-14 and STS-26T MPNST cells peaked at 0.1 and 1 nM, respectively. Epiregulin induced a significant increase in Schwann cell migration only at the highest concentration tested (100 nM). This is strikingly different from ST88-14 and STS-26T cells, which showed increased migration at concentrations 2 orders of magnitude lower. Thus, non-neoplastic Schwann cells are responsive to EGFR ligands and that most of these growth factors promote Schwann cell migration. However, non-neoplastic Schwann cells and the MPNST lines tested do differ in their relative sensitivity to these ligands.
EGFR Ligands Are Variably Expressed in MPNST Cell Lines and Resected MPNSTs
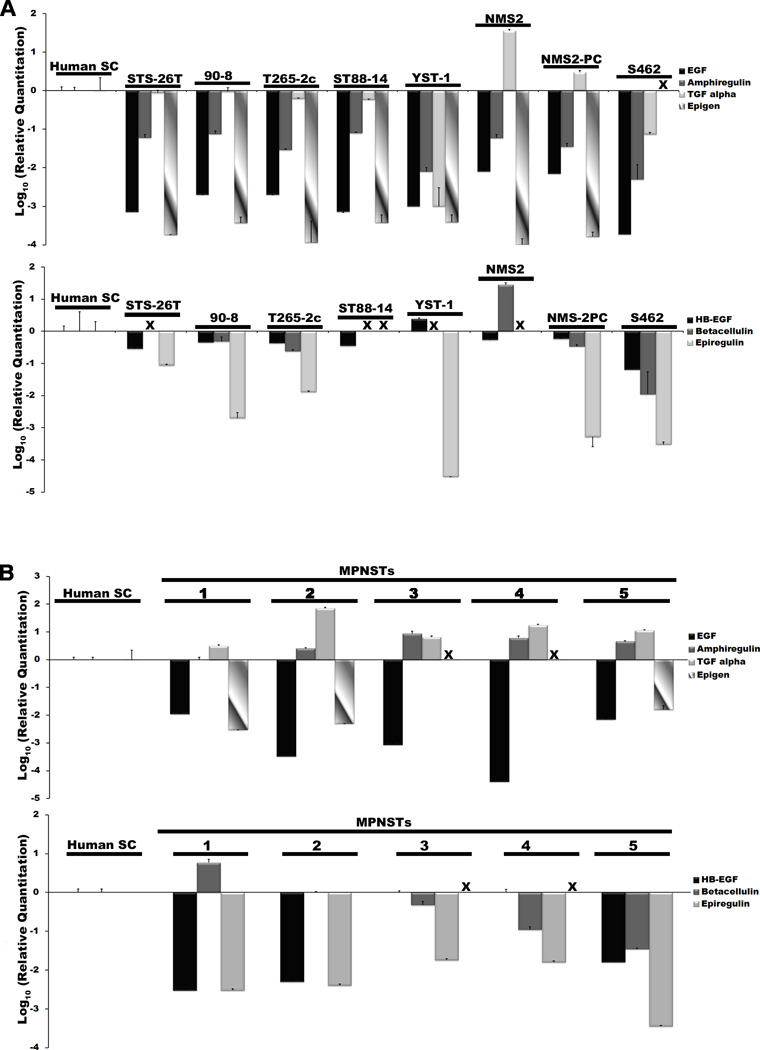
Our finding that the EGFR promotes the migration of unstimulated MPNST cells indicated that this membrane tyrosine kinase is constitutively activated in MPNST cells. This in turn raised the question of whether ligands activating this membrane tyrosine kinase are expressed in MPNST cells and thus promote MPNST migration via autocrine or paracrine signaling. To determine whether EGFR ligands are expressed in MPNST cells and, if so, which ones, we used real-time PCR to quantify the expression of transcripts encoding these growth factors in the same 8 human MPNST cell lines that we tested for EGFR expression. The level of EGFR ligand mRNA expression in these lines was compared to that in non-neoplastic human Schwann cells. We found that Schwann cells expressed readily detectable levels of transcripts encoding all 7 of these growth factors (Fig. 7A). In contrast, expression of EGFR ligand mRNAs was more variable in MPNST cells, i.e. in 6 of the 8 lines, mRNA for the EGFR-specific ligand TGFα was expressed at levels similar to or, in the case of NMS2, NMS2-PC, higher than that seen in non-neoplastic Schwann cells (Fig. 7A, top graph). Transcripts encoding the other 3 EGFR-specific growth factors (EGF, amphiregulin, epigen) were present at levels 10- to more than 1000-fold lower than that detected in Schwann cells. In the group of growth factors that target both the EGFR and erbB4 (Fig. 7A, lower graph), HB-EGF transcripts were most commonly detected, being present in all 8 MPNST lines at levels typically 1.7- to 15-fold lower than was detected in Schwann cells. Betacellulin mRNA was detected in 5 MPNST lines; with the exception of NMS2 cells (28-fold higher than Schwann cells), this expression was 2- to 90-fold lower than that observed in Schwann cells. Transcripts encoding epiregulin, which we detected in 6 MPNST lines, were 10- to 10,000-fold lower in these lines than in Schwann cells.
Figure 7.
Epidermal growth factor receptor (EGFR) ligands are variably expressed by malignant peripheral nerve sheath tumor (MPNST) cells and in surgically resected MPNSTs. (A) Real-time quantitative PCR assays quantifying the expression of mRNAs encoding EGFR-specific ligands (EGF, amphiregulin, transforming growth factor-α [TGFα] and epigen [upper graph]) and ligands binding both EGFR and erbB4 [heparin binding-epidermal growth factor (HB-EGF)], betacellulin and epiregulin (lower graph)] in a panel of MPNST cell lines (STS-26T, 90-8, T265-2c, ST88-14, YST-1, NMS2, NMS2-PC and S462 cells). These values are normalized to expression in non-neoplastic human sciatic nerve-derived Schwann cells. (B) Real-time quantitative PCR comparing the expression of transcripts encoding these same EGFR ligands in a panel of surgically resected human MPNSTs to non-neoplastic human Schwann cells. Bars indicate relative levels of expression, with expression in non-neoplastic human Schwann cells designated as 1 in (A) and (B). Values are expressed on a log10 scale, with 95% confidence intervals indicated. X = not detectable.
To determine whether EGFR ligand mRNAs had a similar pattern of expression in situ, we performed real-time quantitative PCR with cDNAs derived from 5 surgically resected human MPNSTs, again comparing the signals obtained in these tumors to those evident in non-neoplastic human Schwann cells (Fig. 7B). Among the EGFR-specific factors, TGFα transcripts were consistently expressed at increased levels (3- to 71-fold higher) relative to Schwann cells. However, in contrast to our observations in MPNST lines, amphiregulin mRNA accumulation was also equal to or higher (2.5- to 8.6-fold) in the tumors than was observed in Schwann cells. The expression of all of the other EGFR ligand transcripts (EGF, epigen, HB-EGF, betacellulin and epiregulin) was variable in these tumors, typically being present at levels lower than were observed in Schwann cells. Considered jointly, these observations suggest that TGFα expressed by MPNST cells in vitro and in situ is potentially capable of activating EGFRs expressed by these tumor cells in an autocrine or paracrine fashion. Amphiregulin derived from an as yet unknown cellular source may similarly act upon MPNST cells in vivo.
TGF-α and EGFR Colocalize in MPNSTs In Situ
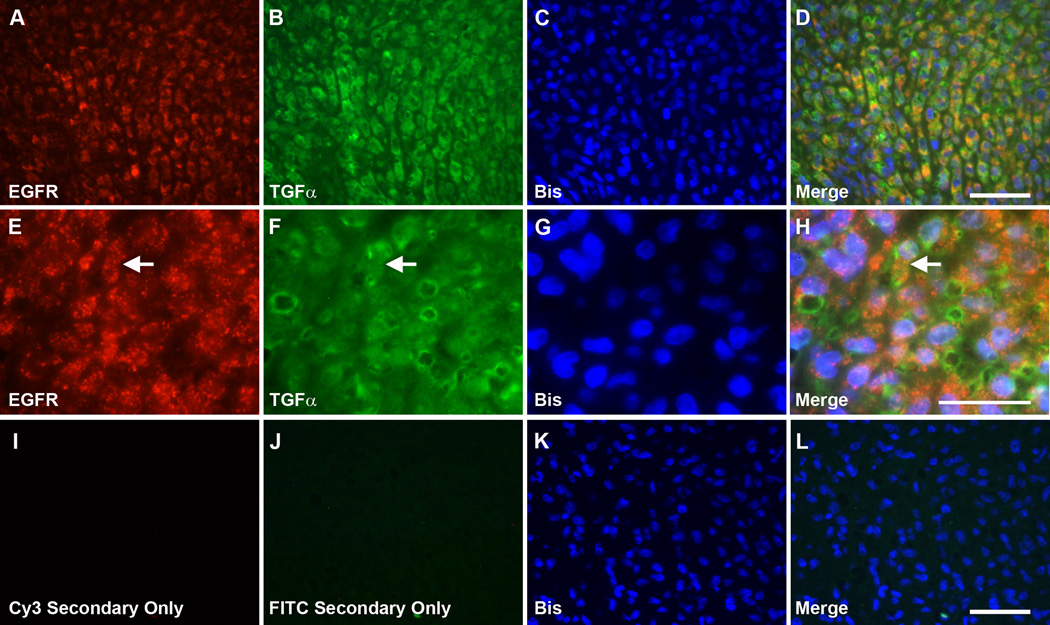
The real-time PCR assays described above indicated that TGFα was the most highly expressed EGFR ligand in MPNSTs, and was thus the factor most likely to be responsible for inducing constitutive EGFR activation and baseline migration in MPNST cells. If autocrine or paracrine TGFα signaling does indeed promote MPNST invasion in vivo, we would expect that TGFα and EGFRs would be closely localized in MPNST tumor tissue. To test this, we performed double label immunohistochemistry for TGFα and EGFRs in sections from 3 surgically resected MPNSTs using a mouse monoclonal anti-TGFα antibody and a rabbit polyclonal antibody recognizing EGFRs. Highly similar patterns of staining were evident in all 3 neoplasms. Immunoreactivity for EGFRs was associated with the plasma membranes of the tumor cells (Fig. 8A, E). Prominent punctate staining was also evident in the cytoplasm of the tumor cells, a distribution that has been previously observed and has been suggested to represent EGFRs that have internalized following activation by a ligand (27). Prominent TGFα immunoreactivity was also present in tumor cells, demonstrating a distribution that was strikingly similar to that of the EGFRs (Fig. 8B, F). Indeed, upon comparing the distribution of EGFR and TGFα immunoreactivity, we found that these molecules showed extensive colocalization (Fig. 8D, H). Staining for both EGFRs and TGFα was absent in negative control preparations (Fig. 8I–L). These findings indicate that EGFRs and the EGFR ligand TGFα are located in close proximity to one another in MPNSTs in vivo and thus are appropriately positioned to promote constitutive EGFR activation and tumor cell invasion.
Figure 8.
Epidermal growth factor receptors (EGFRs) and transforming growth factor-α (TGFα) colocalize in malignant peripheral nerve sheath tumor (MPNST) cells in situ. (A–D) Double label immunohistochemistry for EGFRs (A) and TGFα (B) in a representative surgically resected MPNST. Preparations were counterstained with the nuclear dye bisbenzamide (C). A merged image of these stains shows that EGFRs and TGFα extensively colocalize in this tumor (D). Scale bar in D applies to A–D and is 50 µm. (E–H) Higher power view of the immunohistochemical preparations shown in (A–D). Numerous cells expressing both EGFRs and TGFα are present in this field; one of these cells is indicated by an arrow in (E), (F) and (H). Scale bar in H applies to (E–H) and is 10 µm. (I–L) Staining for EGFRs and TGFα is absent in negative control preparations. Scale bar in (L) applies to (I–L) and is 50 µm.
DISCUSSION
Previous studies have presented evidence that inappropriate EGFR expression promotes MPNST pathogenesis by stimulating the proliferation of neoplastic Schwann cells (8, 9) but it has not been known whether inappropriate EGFR expression exerts other protumorigenic effects in MPNSTs. Here, we have shown that activated EGFRs also promote the invasion of MPNST cells, indicating that this membrane tyrosine kinase plays an additional, previously unappreciated role in the biology of these spindle cell malignancies. Considered collectively, our findings demonstrate that EGFRs enhance MPNST cell chemotaxis, acting as part of a highly complex signaling system the action of which is modulated by key molecules in the extracellular matrix and by multiple growth factors, some of which are produced by the tumor cells themselves and others that are likely derived from target tissues. These findings raise intriguing questions regarding the mechanism(s) by which EGFRs are activated in MPNST cells, the significance of the distinct responses induced by different EGFR ligands in MPNST cells and the sources of these factors in vivo. These observations also have important implications for the design of therapies targeting MPNSTs.
We found that ablating EGFR expression potently impaired the migration of both ST88-14 and T265-2c MPNST cells. Because the cells used in our initial experiments had not been challenged with exogenous EGF, this observation implies that the EGFRs expressed by MPNST cells are constitutively activated. There are multiple non-mutually exclusive mechanisms that conceivably could drive the constitutive activation of EGFRs in MPNST cells. First, we have found that mRNAs encoding several EGFR ligands are expressed in MPNST cells and in surgically resected MPNSTs. This suggests that the EGFRs expressed by MPNST cells could be activated when these factors act upon the tumor cells in an autocrine or paracrine fashion. Alternatively, because overexpression of erbB kinases has been shown to result in increased basal levels of receptor activation in other tumor types (28), it is possible that the overexpression of EGFRs in MPNST cells contributes to constitutive EGFR activation. Finally, we cannot yet exclude the possibility that as yet undetected activating mutations are present in the gene encoding the EGFR in some MPNSTs. In future studies, it will be of great interest to examine the relative contribution each of these mechanisms makes to EGFR activation in MPNSTs.
When we compared the ability of the 7 EGFR ligands to promote migration, we found that all of these factors enhanced the migration of at least one of the MPNST lines tested. In principle, any of these growth factors could therefore promote MPNST invasion and/or metastasis in vivo. Nonetheless, there are 2 reasons to think that that these factors do not play an equally important role in MPNST invasion. The first is that several of the EGFR ligands are not highly expressed in MPNSTs. As potential sources of these molecules include the tumor cells themselves and other cell types in the tumor microenvironment, we examined the expression of transcripts encoding EGFR ligands in both cultured tumor cells and in surgically resected MPNSTs. Strikingly, we found that transcripts encoding TGFα were typically expressed at increased levels relative to non-neoplastic Schwann cells, whereas mRNAs for the other EGFR ligands were, with a few exceptions, much lower. Likewise, in MPNST tumor tissue, TGFα and amphiregulin transcripts were expressed at levels higher than we observed in non-neoplastic Schwann cells. Because TGFα mRNA was consistently overexpressed in vitro and in situ, these observations suggest that basal EGFR activation may reflect, in part, autocrine or paracrine TGFα action in these cells. In keeping with this, we also found that TGFα and EGFRs colocalized in tumor tissue. Nonetheless, these observations do not exclude a role for other EGFR ligands in MPNST invasion. For example, it is conceivable that the invasion of a subset of MPNSTs may be driven by HB-EGF or betacellulin because we found that mRNAs encoding these factors were expressed at levels similar to those seen in Schwann cells in some of the MPNST lines examined. In addition, we found that all of the EGFR ligands tested enhanced MPNST migration. Consequently, target tissues rich in either TGFα or other EGFR ligands may facilitate MPNST invasion or metastasis.
An additional reason to predict that different EGFR ligands do not play an equally important role in MPNST invasion is that we found that these factors differ in their relative ability to induce MPNST migration. Among the EGFR-specific ligands, the MPNST cell lines we tested showed the greatest sensitivity and responsiveness to EGF and TGFα; amphiregulin required concentrations 1 to 2 orders of magnitude higher than EGF and TGFα to achieve maximal levels of migration whereas epigen had only a modest effect that was apparent in only 1 line. The factors capable of activating both EGFR and erbB4 similarly differed in their ability to promote MPNST cell migration. Betacellulin was particularly potent in our migration assays, producing a maximal effect at 0.1 nM, with epiregulin being only slightly less potent (maximal effect achieved with a 1 nM concentration). HB-EGF also induced MPNST cell migration but its effect was significantly less, inducing a maximal 40% increase in 1 MPNST line and having no effect in the other line tested. Given that betacellulin and epiregulin are both capable of activating both erbB4 and the EGFR and that we have previously shown that activation of NRG1 receptors (erbB3 and/or erbB4) also induces MPNST migration, it is at present unclear whether the migratory responses observed with betacellulin and epiregulin reflect solely activation of the EGFR or whether erbB4 activation also contributes to the responses induced by these factors.
In comparing the ability of the different EGFR ligands to induce the migration of non-neoplastic Schwann cells, we found that (with the exception of epigen) the same EGFR ligands that promoted MPNST migration also promoted Schwann cell migration. It has been previously suggested that the expression of EGFRs is an early event in Schwann cell transformation, in part because this receptor is not present in neonatal Schwann cells (8). However, we have shown that adult Schwann cells isolated from axotomized sciatic nerve do express EGFRs (7). We have now expanded upon our initial observations by showing that the EGFRs expressed by adult Schwann cells are functional as demonstrated by the fact that these glia respond to multiple EGFR ligands with increased migration. Thus, it is clear that the expression of functional EGFRs does occur in non-neoplastic Schwann cells and is not necessarily indicative of a neoplastic phenotype. Having said that, however, we recognize that there is strong evidence indicating that EGFR expression makes an important contribution to the pathogenesis of peripheral nerve sheath tumors. Consequently, we would suggest that it will be of great interest to establish whether the acquisition of EGFR expression occurs as an early step in the progenitor population that gives rise to neurofibromas. Answering this question will require that we first establish the identity of this progenitor population, which remains a topic of controversy (29–31).
We found that EGF optimally promotes MPNST cell migration on a laminin substrate, raising the question of whether the EGFR interacts with laminin receptors during this physiologic response. Of note, we previously showed that the EGFR-related molecules erbB3 and erbB4, which serve as direct NRG1 receptors, associate with the laminin receptor subunit β1 integrin during MPNST invasion and have a similar substrate requirement for optimal induction of migration by NRG1β (17). These observations argue that the activated EGFR signaling complex likely does involve interactions with β1 integrin. In future studies, it will be of interest to examine these signaling complexes in detail to identify additional proteins that are involved in their formation.
In summary, we found that the EGFR plays a previously unsuspected role in promoting the aggressive invasive behavior characteristic of MPNSTs. This response likely reflects the combined effect of a complex mixture of factors including the increased expression of EGFRs seen in these tumor cells, interaction with critical substrate molecules and the variable activation of EGFRs by multiple EGFR ligands expressed by the tumor cells themselves, other cells in the tumor microenvironment or target tissues that are invaded by the tumor cells. From a clinical perspective, these observations suggest that the EGFR and/or its ligands and dimerization partners may be important therapeutic target in MPNSTs. Successfully impeding MPNST invasion or metastasis would thus potentially facilitate the ability to achieve a surgical cure in patients with these clinically problematic neoplasms.
ACKNOWLEDGMENTS
We thank UAB Neuroscience Core Facilities for technical assistance (P30 NS57098 and P30 NS474466). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
Sources of Support: This work was supported by National Institute of Neurological Diseases and Stroke Grants R01 NS048353 (to S.L.C.) and F30 NS063626 (to N.M.B.); National Cancer Institute Grants R01 CA122804 (to S.L.C.) and R01 CA134773 (to K.A.R. and S.L.C.); and Department of Defense Grants X81XWH-09-1-0086 and X81XWH-12-1-0164 (to S.L.C.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Evans DG, Baser ME, McGaughran J, et al. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39:311–314. doi: 10.1136/jmg.39.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCaughan JA, Holloway SM, Davidson R, et al. Further evidence of the increased risk for malignant peripheral nerve sheath tumour from a Scottish cohort of patients with neurofibromatosis type 1. J Med Genet. 2007;44:463–466. doi: 10.1136/jmg.2006.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferner RE, O'Doherty MJ. Neurofibroma and schwannoma. Curr Opin Neurol. 2002;15:679–684. doi: 10.1097/01.wco.0000044763.39452.aa. [DOI] [PubMed] [Google Scholar]
- 4.Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62:1573–1577. [PubMed] [Google Scholar]
- 5.Carli M, Ferrari A, Mattke A, et al. Pediatric malignant peripheral nerve sheath tumor: the Italian and German soft tissue sarcoma cooperative group. J Clin Oncol. 2005;23:8422–8430. doi: 10.1200/JCO.2005.01.4886. [DOI] [PubMed] [Google Scholar]
- 6.Ducatman BS, Scheithauer BW, Piepgras DG, et al. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57:2006–2021. doi: 10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Frohnert PW, Stonecypher MS, Carroll SL. Constitutive activation of the neuregulin-1/ErbB receptor signaling pathway is essential for the proliferation of a neoplastic Schwann cell line. Glia. 2003;43:104–118. doi: 10.1002/glia.10232. [DOI] [PubMed] [Google Scholar]
- 8.DeClue JE, Heffelfinger S, Benvenuto G, et al. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest. 2000;105:1233–1241. doi: 10.1172/JCI7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li H, Velasco-Miguel S, Vass WC, et al. Epidermal growth factor receptor signaling pathways are associated with tumorigenesis in the Nf1:p53 mouse tumor model. Cancer Res. 2002;62:4507–4513. [PubMed] [Google Scholar]
- 10.Perry A, Kunz SN, Fuller CE, et al. Differential NF1, p16, and EGFR patterns by interphase cytogenetics (FISH) in malignant peripheral nerve sheath tumor (MPNST) and morphologically similar spindle cell neoplasms. J Neuropathol Exp Neurol. 2002;61:702–709. doi: 10.1093/jnen/61.8.702. [DOI] [PubMed] [Google Scholar]
- 11.Holtkamp N, Malzer E, Zietsch J, et al. EGFR and erbB2 in malignant peripheral nerve sheath tumors and implications for targeted therapy. Neuro-oncology. 2008;10:946–957. doi: 10.1215/15228517-2008-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keizman D, Issakov J, Meller I, et al. Expression and significance of EGFR in malignant peripheral nerve sheath tumor. J Neurooncol. 2009;94:383–388. doi: 10.1007/s11060-009-9862-z. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Crimmins JT, Monk KR, et al. Perinatal epidermal growth factor receptor blockade prevents peripheral nerve disruption in a mouse model reminiscent of benign world health organization grade I neurofibroma. Am J Pathol. 2006;168:1686–1696. doi: 10.2353/ajpath.2006.050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ling BC, Wu J, Miller SJ, et al. Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell. 2005;7:65–75. doi: 10.1016/j.ccr.2004.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frohnert PW, Stonecypher MS, Carroll SL. Lysophosphatidic acid promotes the proliferation of adult Schwann cells isolated from axotomized sciatic nerve. J Neuropathol Exp Neurol. 2003;62:520–529. doi: 10.1093/jnen/62.5.520. [DOI] [PubMed] [Google Scholar]
- 16.Stonecypher MS, Byer SJ, Grizzle WE, et al. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene. 2005;24:5589–5605. doi: 10.1038/sj.onc.1208730. [DOI] [PubMed] [Google Scholar]
- 17.Eckert JM, Byer SJ, Clodfelder-Miller BJ, et al. Neuregulin-1 beta and neuregulin-1 alpha differentially affect the migration and invasion of malignant peripheral nerve sheath tumor cells. Glia. 2009;57:1501–1520. doi: 10.1002/glia.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byer SJ, Eckert JM, Brossier NM, et al. Tamoxifen inhibits malignant peripheral nerve sheath tumor growth in an estrogen receptor-independent manner. Neuro-oncology. 2011;13:28–41. doi: 10.1093/neuonc/noq146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin KJ, Wall EA, Zavzavadjian JR, et al. A single lentiviral vector platform for microRNA-based conditional RNA interference and coordinated transgene expression. Proc Natl Acad Sci U S A. 2006;103:13759–13764. doi: 10.1073/pnas.0606179103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stonecypher MS, Chaudhury AR, Byer SJ, et al. Neuregulin growth factors and their ErbB receptors form a potential signaling network for schwannoma tumorigenesis. J Neuropathol Exp Neurol. 2006;65:162–175. doi: 10.1097/01.jnen.0000199575.93794.2f. [DOI] [PubMed] [Google Scholar]
- 21.Buccione R, Orth JD, McNiven MA. Foot and mouth: podosomes, invadopodia and circular dorsal ruffles. Nat Rev Mol Cell Biol. 2004;5:647–657. doi: 10.1038/nrm1436. [DOI] [PubMed] [Google Scholar]
- 22.Muir D. Differences in proliferation and invasion by normal, transformed and NF1 Schwann cell cultures are influenced by matrix metalloproteinase expression. Clin Exp Metastasis. 1995;13:303–314. doi: 10.1007/BF00133486. [DOI] [PubMed] [Google Scholar]
- 23.Ahmed Z, Brown RA. Adhesion, alignment, and migration of cultured Schwann cells on ultrathin fibronectin fibres. Cell Motil Cytoskeleton. 1999;42:331–343. doi: 10.1002/(SICI)1097-0169(1999)42:4<331::AID-CM6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.McCarthy JB, Palm SL, Furcht LT. Migration by haptotaxis of a Schwann cell tumor line to the basement membrane glycoprotein laminin. J Cell Biol. 1983;97:772–777. doi: 10.1083/jcb.97.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 26.Haraida S, Nerlich AG, Bise K, et al. Comparison of various basement membrane components in benign and malignant peripheral nerve tumours. Virchows Archiv A, Pathological anatomy and histopathology. 1992;421:331–338. doi: 10.1007/BF01660980. [DOI] [PubMed] [Google Scholar]
- 27.Lantini MS, Cossu M, Isola M, et al. Subcellular localization of epidermal growth factor receptor in human submandibular gland. J Anat. 2006;208:595–599. doi: 10.1111/j.1469-7580.2006.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–6487. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Williams JP, Rizvi TA, et al. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer Cell. 2008;13:105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zheng H, Chang L, Patel N, et al. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer Cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Le LQ, Liu C, Shipman T, et al. Susceptible Stages in Schwann Cells for NF1-Associated Plexiform Neurofibroma Development. Cancer Res. 2011;71:4686–4695. doi: 10.1158/0008-5472.CAN-10-4577. [DOI] [PMC free article] [PubMed] [Google Scholar]