Abstract
Our objective was to survey neonatologists regarding international practice of red cell transfusion thresholds for premature infants with <1000-g birth weight and/or <28-week gestation. An invitation to fill out an 11-question web-based survey was distributed to neonatologists through their professional societies in 22 countries. Physicians were asked about which specific factors, in addition to hemoglobin levels, influenced their decisions about transfusing premature infants. These factors included gestational age, postnatal age, oxygen need, respiratory support, reticulocyte count, and inotropic support. Physicians were presented with 5 scenarios and asked to identify hemoglobin cutoff values for transfusing infants with <1000-g birth weight and/or <28-week gestation. One thousand eighteen neonatologists responded: the majority were from the United States (67.5%), followed by Germany (10.7%), Japan (8.0%), the United Kingdom (4.9%), Spain (3.9%), Italy (2.6%), Colombia (0.6%), Argentina (0.4%), Canada (0.4%), Belgium (0.1%), and the Netherlands (0.1%). Half of the respondents (51.1%) reported having a written policy with specific red cell transfusion guidelines in their unit. Factors considered “very important” regarding the need to administer blood transfusions included degree of oxygen requirement (44.7%) and need for respiratory support (44.1%). Erythropoietin was routinely used to treat anemia by 26.0% of respondents. Delayed cord clamping or cord milking was practiced by 29.1% of respondents. The main finding was of a wide variation in the hemoglobin values used to transfuse infants, regardless of postnatal age. Step-wise increments in the median hemoglobin cutoffs directly paralleled an increase in the need for levels of respiratory support. In the first week of life, there was a wider range in the distribution of hemoglobin transfusion thresholds for infants requiring no respiratory support and full mechanical ventilation compared with the thresholds used in the second, third, and fourth weeks of life. An international survey using hypothetical scenarios shows that red blood cell transfusion practices vary widely among practicing neonatologists in participating countries.
Keywords: infant, extremely premature infant, extremely low birth weight, red blood cell transfusion, erythrocyte transfusion, international practices
Extremely premature infants (<1000-g birth weight) are the most highly transfused birth weight group within the neonatal intensive care unit (NICU). This is primarily the result of anemia from an immature hematopoietic system and frequent iatrogenic laboratory loss.1
Controversy in the NICU remains regarding when to transfuse these infants. Maintaining higher transfusion thresholds may increase oxygen-carrying capacity particularly to the brain,2 decrease apnea and periodic breathing,3–6 and increase metabolic potential for better weight gain.7,8 However, it is possible that the risks associated with red cell transfusion outweigh any potential benefit. The potential risks of blood transfusions include higher death rates9; an increased iron or mercury load10–12; continued risk for retinopathy of prematurity13; and the association between necrotizing enterocolitis and transfusions.14,15
There are only few randomized data on which to base decisions for administering red cell transfusions. Four recent randomized trials comparing restrictive and liberal transfusion criteria in premature infants have been reported and summarized in a Cochrane review.16 Although the design and results of the 2 largest trials differ, they each provide valuable data. The Premature Infants in Need of Transfusion (PINT) trial reported that different thresholds for transfusion did not affect rates of early clinically relevant end points at discharge from the NICU, composed of death and/or bronchopulmonary dysplasia, retinopathy of prematurity (ROP), and brain injury.17 Conversely, Bell et al, in secondary post hoc analyses, found higher rates of adverse neurologic events in infants in the restrictive transfusion group.4 These concerns are compounded by results at 18- to 21-month follow-up of the PINT cohort that, although not statistically significant in the primary a priori outcome, suggest better outcomes in the higher threshold group in a post hoc analysis.18
The publication of these clinical trials left serious uncertainty as to the long-term outcomes attributable to the use of restrictive versus liberal transfusion thresholds, and a further larger trial, adequately powered for reporting long-term neurodevelopmental outcomes, was proposed. Integral to the design of this was the determination of current conventional thresholds used by neonatologists after the publication of the earlier trials. We sought to determine current practice patterns with respect to transfusion thresholds in different countries.
Methods
Neonatologists in 22 countries were invited to fill out an 11-question web-based survey via Survey Monkey (SurveyMonkey.com, LLC, Palo Alto, CA; www.surveymonkey.com). For each country, nominated collaborators were asked to forward a letter of invitation to take part in a neonatal red cell transfusion survey to all eligible neonatal physicians. Physicians were asked to identify their country of practice, whether their unit had standard guidelines for transfusion, whether they routinely used erythropoietin, and whether they practiced delayed clamping of the umbilical cord. Physicians were also asked to identify which factors influence their decision to transfuse extremely premature infants (<1000-g birth weight and/or <28-week gestation) during the period from birth to first hospital discharge. The options given were gestational age, postnatal age, degree of oxygen requirement, need for respiratory support, reticulocyte count, and inotropic support. Finally, physicians were asked to consider the minimal hemoglobin at which they would transfuse an infant during each of the first 4 weeks of life given the following increasingly severe 5 levels of respiratory support:
Infant is not on added oxygen and does not need any respiratory support
Infant is only on 21%–30% oxygen by low nasal cannula (<2 liters per minute [LPM])
Infant is on <30% oxygen by low (<2LPM) or high cannula flow (<2LPM)
Infant is on a noninvasive mechanical support such as continuous positive airway pressure, synchronized inspiratory positive airway pressure, biphasic intermittent positive airway pressure, or nasal intermittent mandatory ventilation.
Infant is on full mechanical ventilation with an endotracheal tube.
Median and interquartile range (IQR) for hemoglobin cutoff values was calculated for each of the first 4 weeks of life in each scenario. Hemoglobin values are expressed in grams per deciliter (g/dL).
Results
One thousand eighteen neonatologists from 11 countries responded with the majority from the United States (67.5% of neonatologists), followed by Germany (10.7%), Japan (8.0%), the United Kingdom (4.9%), Spain (3.9%), Italy (2.6%), Colombia (0.6%), Argentina (0.4%), Canada (0.4%), Belgium (0.1%), and the Netherlands (0.1%). Half of the respondents (51.1%) reported having a written policy with specific red cell transfusion guidelines in their unit. Erythropoietin was routinely used by 26.0% of respondents. The obstetrical colleagues of 29.1% of respondents routinely practiced delayed cord clamping or cord milking. Factors considered “very important” regarding the need to administer blood transfusions included degree of oxygen requirement (44.7%) and need for respiratory support (44.1%). Postnatal age (36.5%), reticulocyte count (32.7%), and whether the infant was receiving inotropic support (30.9%) were considered “important” modifiers.
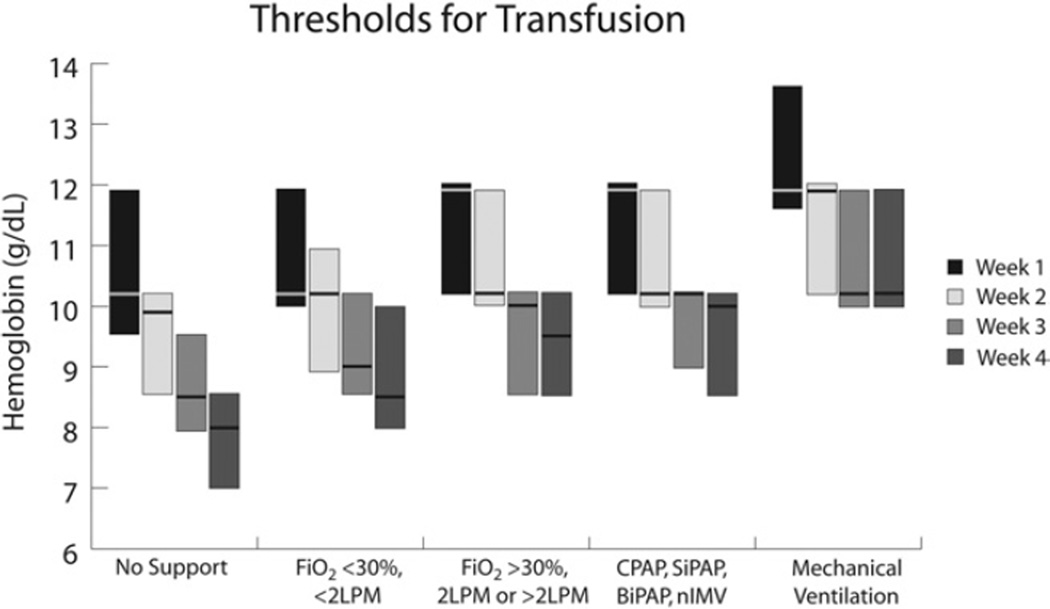
There was a wide variation in the hemoglobin value used to decide when to transfuse infants in all weeks of life (Fig. 1). Step-wise increments in the median hemoglobin cutoffs paralleled the increasing levels of respiratory support. For an infant without any mechanical support in the first week of life, the median hemoglobin threshold for transfusion was 10.2 g/dL (IQR: 9.5–11.9 g/dL). For an infant on full mechanical ventilation with an endotracheal tube, the median hemoglobin threshold for transfusion in the first week of life increased to 11.9 g/dL (IQR: 11.6–13.6 g/dL). Similar step-wise increments with respiratory support were seen in hemoglobin thresholds for the second, third, and fourth weeks of life. There was a step-wise reduction in the median hemoglobin cutoff used for transfusion with increasing postnatal age. Higher thresholds were seen in the first week of life compared with the fourth week of life, irrespective of degree of respiratory support. For an infant in the first week of life on full mechanical ventilation with an endotracheal tube, the median hemoglobin threshold for transfusion was 11.9 g/dL (IQR: 11.6–13.6 g/dL). For an infant in the fourth week of life on full mechanical ventilation with an endotracheal tube, the median hemoglobin threshold for transfusion dropped to 10.2 g/dL (IQR: 10–11.9 g/dL). Similar decrements in hemoglobin thresholds with increasing postnatal age were seen for each level of respiratory support. The IQR was widest for transfusion thresholds used in the first week of life for infants requiring no respiratory support (IQR: 9.5–11.9 g/dL) and for infants on full mechanical ventilation (IQR: 11.6–13.6 g/dL) compared with the thresholds used in the second, third, and fourth weeks of life and the thresholds for intermediate levels of respiratory support.
Figure 1.
Thresholds for red cell transfusion for infants weighing <1000 g at birth and/or <28-week GA for each of the first 4 weeks of life given 5 different levels of respiratory support. Each box represents the interquartile range (25th–75th percentile). The median value intersects each box. BiPAP, biphasic intermittent positive airway pressure; CPAP, continuous positive airway pressure; FiO2, fraction of inspired oxygen; LPM, liters per minute; nIMV, nasal intermittent mandatory ventilation; SiPAP, synchronized inspiratory positive airway pressure.
Discussion
Red blood cell transfusion practices vary widely among practicing neonatologists worldwide. The current available data to guide clinical management of red blood cell transfusions in extremely premature infants are limited. Two moderate-sized, randomized trials have compared restrictive versus liberal transfusion criteria in extremely low-birth-weight infant population: the Iowa and PINT trials.17,18 Both trials used transfusion thresholds that varied with patient status, respiratory support, and, in the PINT trial, postnatal age. The Iowa trial was a single-center study designed to test whether using more restrictive transfusion criteria would reduce the number of transfusions received by preterm infants with birth weight 500–1300 g. The transfusion thresholds ranged from 7.3 to 11.3 g/dL in the “restrictive” group for infants without respiratory support or supplemental oxygen and from 10 to 15.3 g/dL in the “liberal” group for infants receiving mechanical ventilation. Infants in the low-threshold group received fewer transfusions. On secondary post hoc analysis, major head ultrasound anomalies were found to be more frequent in the restrictive-threshold group. Infants in this group also had more frequent apnea. The PINT trial was a 3-country multicenter study with 10 participating centers designed to examine the impact of transfusions on the incidence of the composite outcome of death, ROP, bronchopulmonary dysplasia, or brain injury on cranial ultrasonography in infants weighing <1000 g at birth. The hemoglobin transfusion thresholds ranged from 6.8 to 11.5 g/dL in the “restrictive” threshold group, and from 7.7 to 13.5 g/dL in the “liberal” threshold group. The PINT trial reported there were no differences in the primary outcome between the 2 groups. Infants in the higher threshold group received more transfusions without evidence of long-term benefit. Although follow-up at 18–21 months’ corrected age of infants enrolled in the PINT trial did not show a significant difference in the combined outcome of death or severe adverse neurodevelopmental impairment, the difference in cognitive delay (<2 standard deviation [SD] below the mean) approached statistical significance. A post hoc analysis with redefined cognitive delay, indicated by an Mental Developmental Index (MDI) score of <1 SD below the mean, statistically favored the liberal threshold group. A third, small single-center randomized trial examined the effect of restrictive versus liberal transfusion criteria in 36 very low-birth-weight infants (<1500 g).6 The transfusion thresholds ranged from a hematocrit of 22% to 35% in the “restrictive” threshold group, and from 30% to 45% in the “liberal” threshold group. Infants in the restrictive threshold group received fewer transfusions. There were no significant differences between the 2 groups in the proportion of patients with respiratory distress syndrome, patent ductus arteriosus, severe intraventricular hemorrhage, ROP, or chronic lung disease.
At the time of this survey, the results of the clinical trials (although not the Cochrane review) had been published. Nevertheless, it was unclear what the hemoglobin threshold for transfusion should be for extremely low-birth-weight infants. Clinicians in the present survey were highly influenced by an infant’s degree of oxygen requirement and need for respiratory support, as well as postnatal age. Even when making decisions based on these clinical factors, there was a large variation among clinicians as to what hemoglobin threshold to use for red cell transfusion. In this survey, the largest variation occurred in the first week of life for infants at the low and high ends of respiratory support. Larger studies with sufficient power to address short- and long-term outcomes are needed to better define the optimal threshold for transfusion. Such studies must also carefully assess the risk:benefit ratio more fully to allow clinicians and parents to make more informed decisions. Although the present study does not provide this vital information, these results will provide relevant clinical practice data needed to inform future red blood cell transfusion trials.
References
- 1.Maier RF, Sonntag J, Walka MM, et al. Changing practices of red blood transfusions in infants with birth weights less than 1000g. J Pediatr. 2000;136:220–224. doi: 10.1016/s0022-3476(00)70105-3. [DOI] [PubMed] [Google Scholar]
- 2.van Hoften JC, Verhagen EA, Keating P, et al. Cerebral tissue oxygen saturation and extraction in preterm infants before and after blood transfusion. Arch Dis Child Fetal Neonatal Ed. 2010;95:F352–F358. doi: 10.1136/adc.2009.163592. [DOI] [PubMed] [Google Scholar]
- 3.Joshi A, Gerhardt T, Shandloff P, et al. Blood transfusion effect on the respiratory pattern of preterm infants. Pediatrics. 1987;80:79–84. [PubMed] [Google Scholar]
- 4.Bell EF, Strauss RG, Widness JA, et al. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–1691. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valieva OA, Strandjord TP, Mayock DE, et al. Effects of transfusions in extremely low birth weight infants: A retrospective study. J Pediatr. 2009;155:331–337. doi: 10.1016/j.jpeds.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HL, Tseng HI, Lu CC, et al. Effect of blood transfusions on the outcome of very low body weight preterm infants under two different transfusion criteria. Pediatr Neonatol. 2009;50:110–116. doi: 10.1016/S1875-9572(09)60045-0. [DOI] [PubMed] [Google Scholar]
- 7.Ross MP, Christensen RD, Rothstein G, et al. A randomized trial to develop criteria for administering erythrocyte transfusions to anemic preterm infants 1 to 3 months of age. J Perinatol. 1989;9:246–253. [PubMed] [Google Scholar]
- 8.Meyer J, Sive A, Jacobs P. Empiric red cell transfusion in asymptomatic preterm infants. Acta Paediatr. 1993;82:30–34. doi: 10.1111/j.1651-2227.1993.tb12510.x. [DOI] [PubMed] [Google Scholar]
- 9.dos Santos AM, Guinsburg R, de Almeida MF, et al. Red blood cell transfusions are independently associated with intra-hospital mortality in very low birth weight preterm infants. J Pediatr. 2011;159:371–376. doi: 10.1016/j.jpeds.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 10.Hirano K, Morinobu T, Kim H, et al. Blood transfusion increases radical promoting non-transferrin bound iron in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2001;84:F188–F193. doi: 10.1136/fn.84.3.F188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng PC, Lam CWK, Lee CH, et al. Hepatic iron storage in very low birthweight infants after multiple blood transfusions. Arch Dis Child Fetal Neonatal Ed. 2001;84:F101–F105. doi: 10.1136/fn.84.2.F101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elabiad MT, Hook RE. Mercury content of blood transfusions for infants with extremely low birth weight. Pediatrics. 2011;128:331–334. doi: 10.1542/peds.2010-3712. [DOI] [PubMed] [Google Scholar]
- 13.Dani C, Martelli E, Bertini G, et al. Effect of blood transfusions on oxidative stress in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F408–F411. doi: 10.1136/adc.2003.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mally P, Golombek SG, Mishra R, et al. Association of necrotizing enterocolitis with elective packed red blood cell transfusions in stable, growing, premature neonates. Am J Perinatol. 2006;23:451–458. doi: 10.1055/s-2006-951300. [DOI] [PubMed] [Google Scholar]
- 15.Paul DA, Mackley A, Novitsky A, et al. Increased odds of necrotizing enterocolitis after transfusion of red blood cells in premature infants. Pediatrics. 2011;127:635–641. doi: 10.1542/peds.2010-3178. [DOI] [PubMed] [Google Scholar]
- 16.Whyte RK, Kirpalani H. Low versus high haemoglobin concentration threshold for blood transfusion for preventing morbidity and mortality in very low birth weight infants. Cochrane Database Syst Rev. 2011;11 doi: 10.1002/14651858.CD000512.pub2. CD000512. [DOI] [PubMed] [Google Scholar]
- 17.Kirpalani H, Whyte RK, Andersen C, et al. The premature infants in need of transfusion (PINT) study: A randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 18.Whyte RK, Kirpalani H, Asztalos EV, et al. Neurodevelopmental outcome of extremely low birthweight infants randomly assigned to restrictive or liberal hemoglobin thresholds for blood transfusions. Pediatrics. 2009;123:207–213. doi: 10.1542/peds.2008-0338. [DOI] [PubMed] [Google Scholar]