Abstract
Many signaling, cytoskeletal, and transport proteins have to be localized to the plasma membrane (PM) in order to carry out their function. We surveyed PM-targeting mechanisms by imaging the subcellular localization of 125 fluorescent protein–conjugated Ras, Rab, Arf, and Rho proteins. Out of 48 proteins that were PM-localized, 37 contained clusters of positively charged amino acids. To test whether these polybasic clusters bind negatively charged phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] lipids, we developed a chemical phosphatase activation method to deplete PM PI(4,5)P2. Unexpectedly, proteins with polybasic clusters dissociated from the PM only when both PI(4,5)P2 and phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] were depleted, arguing that both lipid second messengers jointly regulate PM targeting.
Small guanosine triphosphatases (GTPases) from the Ras, Rho, Arf, and Rab subfamilies often exert their role at the PM where they control diverse signaling, cytoskeletal, and transport processes (1–3). KRas, CDC42, and other family members require a cluster of positively charged amino acids for PM localization and activity (2, 4). In vitro studies indicate that the physiological PM binding partner of such polybasic clusters could be phosphatidylserine, which has one negative charge, or the less abundant lipid second messenger PI(4,5)P2, which has four negative charges (5–7). We took a genomic survey approach and investigated PM-targeting mechanisms by confocal imaging of 125 cyan fluorescent protein (CFP)–tagged constitutively active small GTPases (8). Expression in NIH3T3 and HeLa cells showed that 48 small GTPases were fully or partially localized to the PM (Fig. 1A and fig. S1).
Fig. 1.
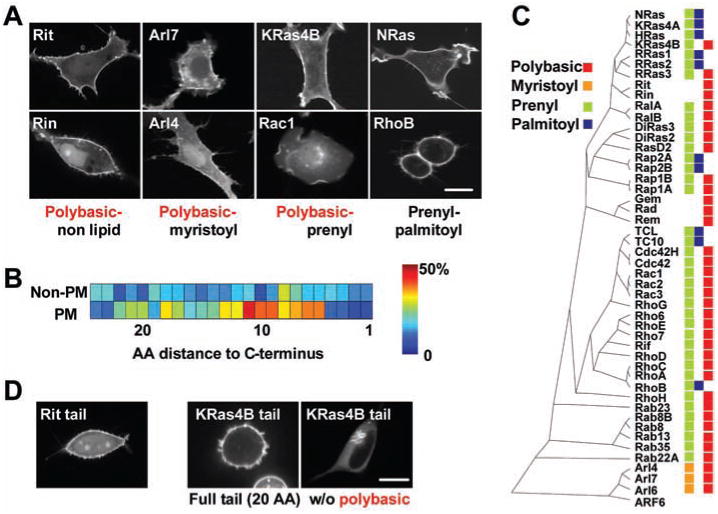
A survey of the subcellular localization of 125 small GTPases shows that most PM-localized small GTPases have targeting motifs with clusters of polybasic amino acids. (A) Confocal images of the subcellular localization of CFP-conjugated small GTPases in NIH3T3 cells (full set of images in NIH3T3 and HeLa cells in fig. S1). The four main PM-targeting motifs are represented in the image panels. (B) Correlation between PM localization and the presence of lysine residues in a region 5 to 20 amino acids from the C terminus. (C) Phylogenetic tree of 48 small GTPases that were identified to be partially or fully localized to the PM. Individual membrane targeting elements are color coded: red for polybasic clusters and blue, green, and orange for palmitoyl, prenyl, and myristoyl consensus sequences, respectively. (D) Twenty-amino-acid-long C-terminal tail fragments of Rit and KRas are PM-localized. Lack of PM targeting of a KRas tail fragment without the polybasic region (right). Scale bars, 10 μm.
Thirty-seven of these PM-localized small GTPases had C-terminal polybasic clusters consisting of four or more Lys or Arg residues at positions 5 to 20 from the C terminus (Fig. 1B and fig. S1). Polybasic clusters were found in three forms: They were present together with N-terminal myristoylation consensus sequences (as in Arl4) (9) or with C-terminal prenylation consensus sequences (as in KRas) (5, 6, 10), or they lacked lipid modifications (as in Rit) (11). We called these three combinations polybasic-myristoyl, polybasic-prenyl, and polybasic-nonlipid PM-targeting motifs, respectively. A number of remaining PM-targeted small GTPases had a combined prenylation and palmitoylation consensus sequence that mediated PM targeting without requiring polybasic amino acids (as does that of HRas) (Fig. 1, A and C) (5, 12). Arf6 lacked a specific targeting motif and was only localized to the PM in its guanosine triphosphate (GTP)–bound form (fig. S2) (13). The sequence homology comparison of PM-localized small GTPases in Fig. 1C shows that closely homologous small GTPases can have different targeting motifs. We also confirmed, for the examples of Rit and KRas, that polybasic targeting motifs alone can be sufficient for PM targeting (Fig. 1D).
To test whether polybasic clusters are anchored to the PM by binding to PI(4,5)P2(14), we hydrolyzed PM PI(4,5)P2 by rapid targeting of Inp54p, a 5′ specific PI(4,5)P2 phosphatase (15), to the PM. This method is based on a PM-localized FK506-binding protein (FKBP12)– rapamycin–binding (FRB) construct and a cytosolic Inp54p enzyme conjugated with FKBP12 (CF-Inp) that can be translocated to the PM by chemical heterodimerization by using a rapamycin analog, iRap (16).
In experiments where we monitored PI(4,5)P2 using a yellow fluorescent protein (YFP)– conjugated pleckstrin homology (PH) domain from phospholipase Cδ (PLCδ) (17), PM trans-location of CF-Inp triggered a rapid and near complete dissociation of the YFP-PLCδ-PH domain from the PM (Fig. 2A). Despite this marked reduction in PI(4,5)P2 concentration, only a small fraction of the polybasic-nonlipid tail fragment of Rit dissociated from the PM (Fig. 2B), which suggests that PI(4,5)P2 is not alone responsible for PM targeting. Parallel experiments suggested that phosphatidylinositol 3,4,5-trisphosphate [PI(3,4,5)P3] might be involved, because stimulation of cells with platelet-derived growth factor (PDGF) led to a small increase in PM localization of the Rit tail and this small effect could be reversed by addition of an inhibitor of phosphoinositide 3-kinase (PI 3-kinase), LY294002 (LY29) (Fig. 2C; control experiments in fig. S3). Strikingly, the combined reduction of both PI(4,5)P2 and PI(3,4,5)P3 concentration triggered a dissociation of most Rit tail protein from the PM (Fig. 2, D and E). The effect of reducing either PI(4,5)P2 or PI(3,4,5)P3 concentration alone on Rit tail localization was relatively small (Fig. 2E).
Fig. 2.
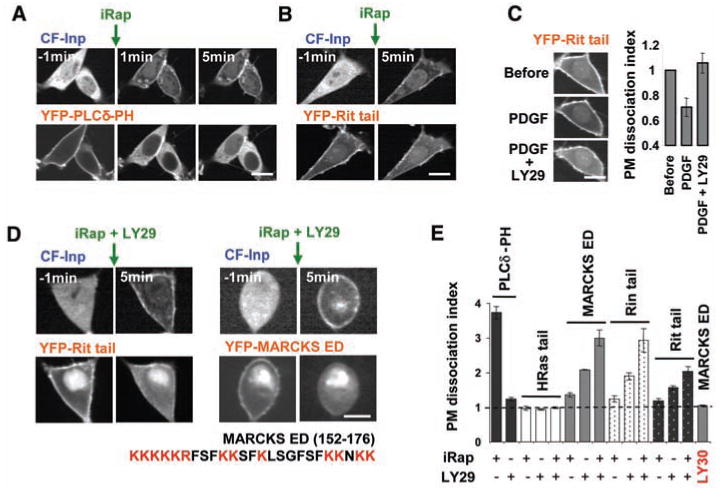
Depletion of PI(4,5)P2 and PI(3,4,5)P3 dissociates Rit, Rin, and MARCKS ED with polybasic-nonlipid targeting motifs from the PM. (A) Development of a chemically inducible translocation method to deplete PI(4,5)P2 from the inner leaflet of the PM (22, 23). The PI(4,5)P2 biosensor YFP-PLCd-PH was cotransfected to monitor depletion of PI(4,5)P2. (B) Depletion of PI(4,5)P2 caused only a small reduction in the PM localization of YFP-conjugated Rit tail. (C) A small, but significant, PDGF receptor–mediated increase in Rit tail PM localization can be reversed by addition of the PI3-kinase inhibitor LY29 (before and 9 min after addition of 5 μM iRap). The bar graph shows a quantification of the same experiment. The PM dissociation index is the relative ratio of internal over PM fluorescence; that is, F1cyt/F1PM * F0PM/F0cyt, with F0 and F1 as the fluorescent intensities before and after PI(4,5)P2 and PI(3,4,5)P3 depletion. (D) Joint reduction in PI(4,5)P2 and PI(3,4,5)P3 triggered a near-complete dissociation of Rit tail and MARCKS ED from the PM. (E) Quantitative analysis of the CF-Inp and/or LY29-triggered PM dissociation of MARCKS ED, and Rit and Rin tails. PLCδ-PH and HRas tails are shown as controls. The inactive LY29 analog LY30 was used as a control (24). Scale bars, 10 μm.
We also found that both PI(4,5)P2 and PI(3,4,5)P3 have to be lowered to significantly dissociate a tail fragment of Rin and a polybasic effector domain peptide from myristoylated alaninerich C kinase substrate (MARCKS) protein (MARCKS ED) from the PM (Fig. 2, D and E). This MARCKS ED peptide was included because it has been extensively used for in vitro biochemical studies of the interaction between polybasic amino acids and phosphatidylserine and PI(4,5)P2 (7, 14, 18, 19). HRas, which has a prenyl-palmitoyl PM-targeting motif without a polybasic cluster, did not dissociate from the PM after depletion of PI(4,5)P2 and PI(3,4,5)P3 (Fig. 2E). Control experiments with the same constructs in HeLa cells showed similar results (fig. S4), and an analysis of the dissociation kinetics showed that PM dissociation occurs within minutes after CF-Inp activation and LY29 addition (fig. S5A). We also verified that PM dissociation of polybasic proteins occurred when we combined PI(4,5)P2 depletion with addition of wortmannin, an alternative PI 3-kinase inhibitor, or with expression of a dominant negative PI 3-kinase inhibitory construct (20) (figs. S6 and S7). Additional control experiments are shown in figs. S8 to S12, including in vitro lipid-blot assays that showed enhanced affinity of proteins with polybasic clusters for PI(3,4,5)P3 over PI(4,5)P2. These control experiments strengthen the argument that PI(4,5)P2 and the less abundant PI(3,4,5)P3 both serve as PM anchors for proteins with polybasic clusters.
Insights into the electrostatic binding mechanism between positively charged polybasic clusters and negatively charged polyphospho-inositides came from a sequence comparison of nonprenylated PM-targeted small GTPases. Most of these proteins contain two or three subclusters of polybasic residues in the C-terminal tail. Each subcluster spans about four or five amino acids, and the mean distance between subclusters is nine amino acids (Fig. 3A). Consistent with a need for multiple subclusters, the removal of a flanking subcluster in the Rit tail was sufficient to abolish PM targeting (Rit tail 199 to 219) (Fig. 3B). This suggests that PM targeting results from additive binding energy of individual subclusters that each electrostatically interact with a PI(4,5)P2 or PI(3,4,5)P3 lipid. Selectivity for polyphos-phoinositides over phosphatidylserine may occur because of opposing high relative-charge densities of polyphosphoinositides and polybasic subclusters (7).
Fig. 3.
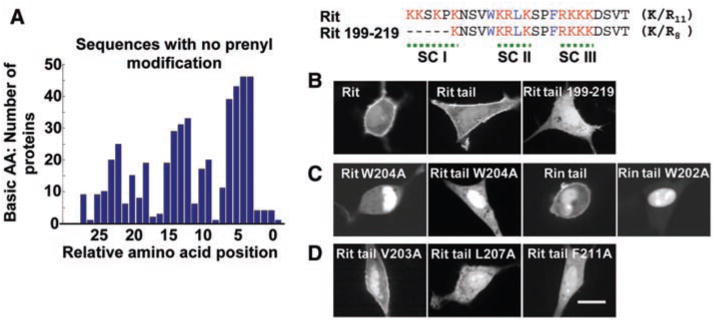
Polybasic subclusters and hydrophobic amino acids are required for PM targeting by polybasic-nonlipid targeting motifs. (A) Statistical analysis of the relative sequence location of positively charged amino acids in nonprenylated small GTPases. (B) Loss of PM targeting of Rit tail fragment after removal of a single subcluster with positively charged amino acids. (C) Identification of a tryptophan residue in the polybasic regions of Rit and Rin that mediates PM over nuclear localization. Full-length small GTPases, as well as tail fragment mutants, are shown. (D) Identification of two additional hydrophobic amino acid residues that contribute to the PM targeting of Rit. Scale bars, 10 μm.
We then investigated differences between the targeting mechanisms of the three polybasic-nonlipid, polybasic-myristoyl, and polybasic-prenyl PM-targeting motifs. A distinct feature of the polybasic-nonlipid targeting motifs is their similarity to nuclear localization sequences. We found hydrophobic amino acids to be important for selective PM localization, because site-directed mutagenesis of a single hydrophobic amino acid (Trp204Ala in Rit or Trp202Ala in Rin) led to a complete loss of PM targeting and a strong nuclear localization (Fig. 3C). A loss of PM targeting but with less nuclear targeting could also be observed for Leu207Ala and Phe211Ala Rit mutants (Fig. 3D) and for hydrophobic amino acid mutants of the small GTPases GEM and RAD (fig. S13). Thus, hydrophobic amino acids strengthen PM binding of polybasic-nonlipid motifs and prevent the polybasic cluster from functioning as a nuclear localization sequence.
The polybasic-myristoyl PM-targeting motifs have the distinct feature of a separated N-terminal myristoylation consensus sequence and a C-terminal polybasic cluster. Mutant constructs showed that effective PM targeting of Arl7 required an N-terminal myristoyl motif (left panel, Fig. 4A), as well as a flexible C-terminal polybasic tail (middle versus right panel, Fig. 4A), which suggests that the two ends of the protein synergistically support PM targeting.
Fig. 4.

Depletion of PI(4,5)P2 and PI(3,4,5)P3 dissociates proteins with polybasic-myristoyl and polybasic-prenyl targeting motifs from the PM. (A) Aligned sequences of the N terminus (6 amino acids) and C terminus (20 amino acids) of six ARF family members. Confocal images of an Arl7 mutant construct lacking the N-terminal myristoylation motif (left), a C-terminal tagged Arl7 control construct (middle), and a mutant construct with an internal CFP tag and a flexible C-terminal polybasic Arl7 tail (right). (B) PM localization motifs in geranylgeranylated Rab35. Confocal images from left to right: localization of wild-type Rab35, wild-type tail fragment, wild-type tail lacking geranylgeranylation motif (ΔCC), Rab35 tail ΔCC mutant with KRas caax (Rab35 tail caax), and Rab35 tail caax mutant lacking polybasic amino acids (Rab35 tail caax ΔpB). (C) Depletion of PI(4,5)P2 by CF-Inp activation without L29 addition causes only a minimal PM dissociation of Arl7, KRas tail fragment, and Rab35. (D) Quantitative analysis of the much larger PM dissociation of polybasic-lipid modified proteins after depletion of PI(4,5)P2 and PI(3,4,5)P3 by CF-Inp activation and addition of LY29. Scale bars, 10 μm.
The polybasic-prenyl PM-targeting motif includes proteins such as KRas for which a 20– amino acid tail sequence is sufficient for farnesylation and PM targeting (Fig. 1D), as well as Rab35 for which an intact GTPase domain is required for geranylgeranylation (21) and for PM targeting (second and third panels, Fig. 4B). We further compared the roles of farnesylation and geranylgeranylation by creating a Rab35 mutant with a consensus CAAX farnesylation sequence in place of the geranylgeranylation sequence. This mutant showed PM localization indistinguishable from that of the geranylgeranylated Rab35 (fourth panel, Fig. 4B). We also confirmed that the PM targeting of RAB35 requires a polybasic cluster (last panel, Fig. 4B). This shows that the polybasic-geranylgeranyl motifs of Rab35 can be equally effective in PM targeting as the polybasic-farnesyl motif of KRas, which supports the notion that both types of prenylation motifs can be grouped into a single polybasicprenyl PM-targeting motif.
We then tested whether PI(4,5)P2 and PI(3,4,5)P3 also regulate polybasic-myristoyl and polybasic-prenyl targeting motifs. As for the polybasic-nonlipid targeting motif, depletion of PI(4,5)P2 alone triggered only a minor reduction in PM localization of the Arl7 polybasic-myristoyl targeting motif and the KRas and Rab35 polybasic-prenyl motifs (Fig. 4C). Depletion of both PI(4,5)P2 and PI(3,4,5)P3 triggered significant PM dissociation of all polybasic-myristoyl and polybasic-prenyl constructs tested (Fig. 4D) with a kinetics similar to that of Rit (fig. S5), which suggests that PI(4,5)P2 and PI(3,4,5)P3 have the same role for PM localization for all three types of polybasic PM-targeting motifs. HRas was again included as a control protein without a polybasic cluster.
Our study shows that polybasic PM-targeting motifs are built from two parts, an unspecific membrane-targeting part that can be hydrophobic amino acids, myristoyl groups, or prenyl groups and a polybasic targeting part that provides PM specificity by binding of positively charged amino acid clusters to negatively charged PI(4,5)P2 and PI(3,4,5)P3 lipids in the PM. This gives PI(4,5)P2 and PI(3,4,5)P3 a ubiquitous role in regulating signaling, cytoskeletal, and transport proteins and argues that these lipid second messengers function as signaling hubs in cellular control systems.
Supplementary Material
Acknowledgments
This work was supported by grants from National Institute of Mental Health and National Institute of General Medical Sciences, NIH, to T.M.
We thank A. Salmeen, M. F. Teruel, M. Fivaz, and other members of the Meyer laboratory for support; A. R. Koh and S. H. Ryu (POSTECH) for critical reading the manuscript; S. McLaughlin (SUNY Stonybrook) and B. Hille (U. Washington) for discussions; and James Whalen for assisting with the in vitro lipid-binding assays. T.I. is a recipient of a fellowship from the Quantitative Chemical Biology Program. B.O.P. was supported by a grant from KOSEF/MOST EB-NCRC (grant no. R15-2003-012-01001-0) and by scholarships from the Brain Korea 21 program.
Footnotes
Supporting Online Material: www.sciencemag.org/cgi/content/full/1134389/DC1, Materials and Methods, Figs. S1 to S13, References
References and Notes
- 1.Fivaz M, Meyer T. Neuron. 2003;40:319. doi: 10.1016/s0896-6273(03)00634-2. [DOI] [PubMed] [Google Scholar]
- 2.Teruel MN, Meyer T. Cell. 2000;103:181. doi: 10.1016/s0092-8674(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 3.Guan JL. Science. 2004;303:773. doi: 10.1126/science.1094376. [DOI] [PubMed] [Google Scholar]
- 4.Michaelson D, et al. J Cell Biol. 2001;152:111. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadwallader KA, Paterson H, Macdonald SG, Hancock JF. Mol Cell Biol. 1994;14:4722. doi: 10.1128/mcb.14.7.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghomashchi F, Zhang X, Liu L, Gelb MH. Biochemistry. 1995;34:11910. doi: 10.1021/bi00037a032. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin S, Murray D. Nature. 2005;438:605. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 8.Heo WD, Meyer T. Cell. 2003;113:315. doi: 10.1016/s0092-8674(03)00315-5. [DOI] [PubMed] [Google Scholar]
- 9.Schurmann A, et al. J Biol Chem. 1994;269:15683. [PubMed] [Google Scholar]
- 10.Choy E, et al. Cell. 1999;98:69. doi: 10.1016/S0092-8674(00)80607-8. [DOI] [PubMed] [Google Scholar]
- 11.Lee CH, Della NG, Chew CE, Zack DJ. J Neurosci. 1996;16:6784. doi: 10.1523/JNEUROSCI.16-21-06784.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rocks O, et al. Science. 2005;307:1746. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 13.Radhakrishna H, Donaldson JG. J Cell Biol. 1997;139:49. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McLaughlin S, Wang J, Gambhir A, Murray D. Annu Rev Biophys Biomol Struct. 2002;31:151. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Heo WD, Grimley JS, Wandless TJ, Meyer T. Nat Methods. 2005;2:415. doi: 10.1038/nmeth763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raucher D, et al. Cell. 2000;100:221. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 17.Stauffer TP, Ahn S, Meyer T. Curr Biol. 1998;8:343. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Arbuzova A, Hangyas-Mihalyne G, McLaughlin S. J Biol Chem. 2001;276:5012. doi: 10.1074/jbc.M008355200. [DOI] [PubMed] [Google Scholar]
- 19.Chapline C, Ramsay K, Klauck T, Jaken S. J Biol Chem. 1993;268:6858. [PubMed] [Google Scholar]
- 20.Poser S, Impey S, Trinh K, Xia Z, Storm DR. EMBO J. 2000;19:4955. doi: 10.1093/emboj/19.18.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seabra MC, Wasmeier C. Curr Opin Cell Biol. 2004;16:451. doi: 10.1016/j.ceb.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 22.Suh BC, Inoue T, Meyer T, Hille B. Science. 2006;314:1454. doi: 10.1126/science.1131163. published online 21 September 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Correspondence about the iRap inducible enzyme systems should be directed to T. Inoue (e-mail: jctinoue@stanford.edu)
- 24.Poh TW, Pervaiz S. Cancer Res. 2005;65:6264. doi: 10.1158/0008-5472.CAN-05-0152. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


