Abstract
There is strong and consistent evidence from in vitro studies that disturbed blood flow produces a pro-atherogenic vascular endothelial phenotype. However, data from human studies are lacking. To address this, a 220 mmHg occlusion cuff was placed on the distal forearm of 10 young, healthy men to induce a localized region of disturbed blood flow in the proximal vasculature for 20 min. We hypothesized that disturbed blood flow would induce endothelial activation and apoptosis as indicated by increases in local concentrations of CD62E+ and CD31+/CD42b− endothelial microparticles, respectively. Distal cuff occlusion induced reductions in mean blood flow, mean shear and antegrade shear, and increases in retrograde flow, retrograde shear and oscillatory shear stress, confirming that our protocol produced a disturbed blood flow stimulus in the experimental arm. Relative to baseline (0 min), CD62E+ endothelial microparticles increased by ~3 fold at 10 min and ~4 fold at 20 min in the experimental arm (P < 0.05). CD31+/CD42b− endothelial microparticles were elevated by ~9 fold at the 20 min time point (P < 0.05). There were no changes in the concentrations of either endothelial microparticle population throughout the experiment in the contralateral arm, exposed to normal resting blood flow (no cuffs). These findings indicate that disturbed blood flow acutely induces endothelial activation and apoptosis in humans, as reflected by release of microparticles from activated (CD62E+) and apoptotic (CD31+/CD42b−) endothelial cells. These data provide the first in vivo experimental evidence of disturbed blood flow-induced endothelial injury in humans.
Keywords: shear stress, atherosclerosis, microparticles
INTRODUCTION
It is well-established that geometrically irregular arterial regions such as curvatures, branches, and bifurcations are characterized by overall low shear stress combined with high retrograde flow and oscillatory shear stress.1 Collectively, such flow patterns represent a state of disturbed blood flow. Importantly, these arterial regions also display marked susceptibility to the development of atherosclerosis compared to segments exposed to laminar flow and moderate to high shear.2, 3 Insights into the mechanisms underlying this association between disturbed blood flow and disease susceptibility have come primarily from in vitro studies. For example, early studies with cultured endothelial cells indicated that disturbed flow induces endothelial activation and cell turnover,4 whereas laminar shear stress mitigates pro-atherogenic processes such as leukocyte adhesion.5 Similarly, recent studies indicated that pro-atherogenic genes are upregulated in cultured endothelial cells exposed to shear patterns of in vivo atheroprone regions relative to cells exposed to patterns observed in protected regions.6–8 These cell culture data are consistent with data indicating that oscillatory shear stress induces pathologic remodeling and impairments in endothelium-dependent vasodilation in isolated arteries.9 Thus, the evidence from in vitro preparations that disturbed flow patterns produce an unfavorable vascular endothelial cell phenotype is strong and consistent.
Although the data are still limited, recently the association between disturbed blood flow and vascular dysfunction has begun to receive support from experimental in vivo studies. For example, in mice, induction of disturbed blood flow via partial carotid ligation produces rapid and profound endothelial dysfunction and atherosclerosis.10 In humans, our group and others have recently shown that there is evidence of increased pro-atherogenic blood flow and shear patterns in peripheral conduit arteries of subjects at increased risk for cardiovascular diseases and atherosclerosis.11–14 Surprisingly, only one study has tested whether disturbed blood flow can acutely influence the human vasculature in vivo. In an elegant experiment, Thijssen et al.15 used 25, 50, and 75 mmHg distal forearm cuff occlusion pressures to elicit progressive increases in brachial artery retrograde flow and shear and concomitant reductions in mean flow and shear. This maneuver induced a dose-dependent reduction in brachial artery flow-mediated dilation (FMD), providing the first indication that disturbances in blood flow result in functional impairment of the human endothelium in vivo. However, while FMD is a well-established index of vascular function, it does not allow for insights into the cellular/molecular underpinnings of the functional impairment.
Microparticles are small (≤ 1 μm diameter) vesicles formed from plasma membranes and released into the circulation upon cellular activation, apoptosis, and/or injury of many cell types16. Elevated circulating levels of endothelial microparticles (EMPs), first detected in the in vivo human circulation in 1999,17 are now well-established as systemic markers of dysfunctional and diseased vascular endothelium.18, 19 For example, EMPs are elevated in patients with atherosclerosis,20 coronary artery disease,21 hypertension,22 cerebrovascular disease23 and the metabolic syndrome,24 among other cardiovascular and metabolic abnormalities. Interestingly, surface protein marker expression analysis of EMPs can reveal insights into the insult that triggered their release from the vascular wall.25 Constitutive markers such as CD31 are expressed on EMPs shed from apoptotic endothelial cells, whereas expression of inducible markers such as CD62E (E-selectin) indicates endothelial activation induced by a pro-inflammatory event.25, 26 Thus, the examination of multiple EMP populations may provide novel information about the in vivo nature of disturbed flow-induced endothelial injury.
Therefore, the purpose of this study was to determine the acute effect of disturbed blood flow on the release of EMPs from the human vascular endothelium. We adapted the distal forearm cuff occlusion model introduced by Thijssen et al.15 to create a localized region of disturbed blood flow, with the contralateral arm serving as a control. We hypothesized that disturbed blood flow would induce endothelial activation and apoptosis as indicated by increases in local concentrations of CD62E+ and CD31+/CD42b− EMPs, respectively.
METHODS
Subjects
Ten healthy men with a mean age of 29 ± 1 yr, height of 176 ± 1, and weight of 83 ± 2 kg (means ± SEM) participated. All subjects were free of known cardiovascular, metabolic and neurological diseases and were nonsmokers, normotensive (resting blood pressure: 115 ± 64) and were non-obese (body mass index: 26.7 ± 0.5 kg/m2). The investigators provided verbal and written explanations of the study procedures and the risks and benefits associated with participation, and subjects provided their written informed consent. All procedures adhered to the principles of the Declaration of Helsinki, and the study was approved by the University of Missouri-Columbia Health Sciences Institutional Review Board.
Experimental Design and Protocols
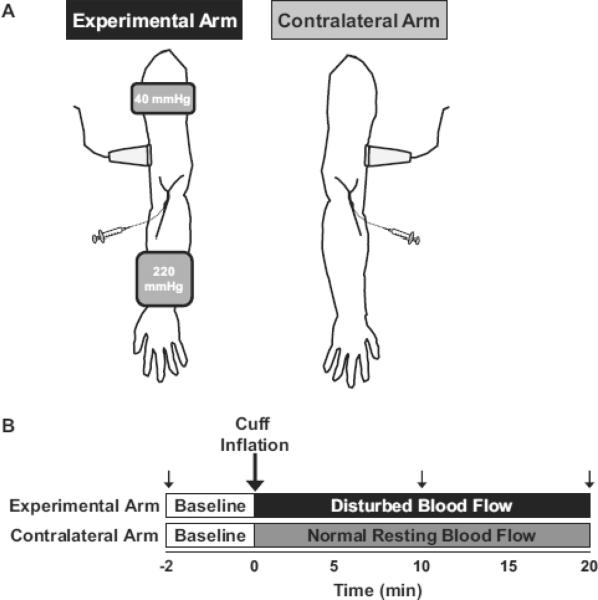
Subjects reported to the laboratory following an overnight fast and abstained from caffeine and strenuous exercise for a minimum of 12 hr. Indwelling venous catheters were placed in a superficial antecubital vein of both arms, followed by a 20 min quiet rest period in the supine position in a dimly lit, temperature-controlled (20–22°C) room. The experimental setup and design are depicted in Fig 1A and Fig 1B, respectively. Two pneumatic cuffs (Hokanson) were placed on the experimental arm for 20 min: (i) a distal cuff, inflated to 220 mmHg to produce a localized environment of disturbed blood flow (low mean flow and shear, low antegrade flow and shear, high retrograde flow and shear, and high oscillatory shear stress) in the proximal vasculature, and (ii) a proximal cuff positioned ~3 cm distal form the axilla, inflated to 40 mmHg to partially occlude venous flow from the arm, thereby facilitating the trapping of EMPs that would be released during the experiment (Fig 1). The distal occlusion pressure of 220 mmHg was selected to maximize a disturbed blood flow state and also to occlude backflow from the ischemic downstream region, which could contribute to increases in EMPs being measured upstream. The absence of contamination of blood from the distal ischemic region was assessed by measuring plasma lactate concentrations (YSI 2700, Yellow Springs, Ohio) in 0 and 20 min samples from the experimental arm (mean ± SEM: 0.63 ± 0.05 vs. 0.61 ± 0.04 mmol/L, P = 0.69). Two separate control experiments were carried out in the contralateral arm. First, simultaneous blood flow (n = 3) and EMP (n = 7) measures were performed in the absence of any cuffs (see Fig 1A; n = 3). Second, blood flow (n = 3) and EMP (n = 2) measures were obtained with the application of a proximal 40 mmHg occlusion cuff.
Figure 1. Experimental set-up (A) and design (B).
Refer to methods for details.
Brachial Artery Blood Flow Measures
Brachial artery blood velocities and diameters were measured using duplex Doppler ultrasound, as described in detail previously.27, 28 Briefly, images of the brachial artery diameter and blood velocity profiles were acquired ~5 cm proximal to the antecubital fossa in the anterior-medial plane at 30 Hz using a custom Labview program interfaced to the video output of the Doppler ultrasound. Offline analyses of brachial artery diameters and Doppler blood velocity profiles were performed using custom-designed edge-detection and wall-tracking software (Labview, National Instruments). Blood flow was calculated as VmπD2/4 × 60, where Vm is time-average mean blood velocity (cm/s), D is arterial diameter (cm), and mean shear rate (s–1) was defined as 4×VmD–1.27, 28 Antegrade and retrograde time-averaged blood velocities were used to calculate their respective shear rates. The oscillatory shear index, an indicator of the magnitude of shear oscillations, was calculated as follows: |retrograde shear|/(antegrade shear + |retrograde shear|).11, 27–31 The oscillatory shear index values range from 0 to 0.5, where a value of 0 corresponds to pure unidirectional shear and 0.5 indicates pure oscillation with a time-average shear of 0.
Plasma Endothelial Microparticle (EMP) Measures
Blood samples were obtained from both the experimental and control arm at 0, 10, and 20 min into 8 mL vials containing acid citrate dextrose. Microparticles were isolated from plasma samples and CD62E+ and CD31+/CD42b− EMP populations were analyzed by flow cytometry as described previously.32 Briefly, plasma samples (500 μl aliquots) were thawed at room temperature and centrifuged at 1500g for 20 min at room temperature to obtain platelet poor plasma. The top two-thirds (i.e., 335 μl) of platelet-poor plasma was further centrifuged at 1500g for 20 min at room temperature to obtain cell free plasma.33 The top 100 μl of cell-free plasma was incubated with fluorochrome-labeled antibodies for 20 min in the dark at room temperature. The following antibody combinations were used to assess concentrations of two EMP subpopulations: (i) CD62E-PE (15 μl/sample) for assessment of EMPs from activated endothelium (i.e., CD62E+); and (ii) phycoerythrin (PE)-CD31 (20 μl/sample) and fluorescein-isothiocyanate (FITC)-CD42b (20 μl/sample) for assessment of EMPs from apoptotic endothelium (i.e., CD31+/CD42b−). Samples were fixed with 93 μl 2% paraformaldehyde and were diluted with 500 μl of sterile PBS before flow cytometric analysis.
Samples were analyzed using a Beckman Coulter CyAn ADP flow cytometer in the University of Missouri Cell and Immunobiology Core Facility. EMPs were defined as CD31+/CD42b− or CD62E+ events smaller than 1.0 μm. Size calibration was performed with 0.9 μm standard precision NIST Traceable polystyrene particle beads (Polysciences, Inc.). Fluorescence minus one controls and unstained samples were used to discriminate true events from noise. The flow rate was set on medium and all samples were run for 3 min. Using calibrator beads (BD Truecount), we calculated that, on medium flow rate, the average rate of microparticle event analysis was 140 μl/min. EMP counts per μl plasma were determined using the formula: [(number of EMP events/volume of sample analyzed) × (final sample volume)/(volume of cell-free plasma)].32, 34
Statistics
Data were analyzed using a 2-factor (arm × time) repeated measures ANOVA with SPSS software (version 18). In the event of significant interaction, Fisher's LSD post hoc analysis was used to examine differences within arms (i.e., baseline vs. 10 min and baseline vs. 20 min) and between arms at 10 and 20 min. Data are presented as mean ± SE. P ≤ 0.05 was considered statistically significant.
RESULTS
CD62E+ EMPs increased substantially at 10 min and 20 min compared to baseline in the experimental arm (P < 0.05, Fig 2A), indicating endothelial activation. Likewise, CD31+/CD42b− EMP concentrations were elevated at the 20 min time point (P < 0.05, Fig 2B), suggestive of endothelial apoptosis. There were no changes in the concentrations of either EMP population over the same time period in the contralateral arm when no cuffs were applied (n = 7; Fig 2A and 2B). Additionally, in three subjects, EMPs were analyzed in samples obtained with a proximal 40 mmHg venous occlusion cuff inflated on the contralateral arm. However, samples from one of these subjects appeared hemolyzed and were therefore not used for EMP analysis.34 The proximal 40 mmHg cuff did not produce major changes in CD62E+ or CD31+/CD42b− EMP concentrations in either subject from which acceptable samples were obtained (means ± SE: 1.7 ± 0.1, 1.7 ± 0.2, and 1.9 ± 0.1 CD62E+ EMPs/μl plasma at 0, 10, and 20 min, respectively; 0.9 ± 0.2, 1.0 ± 0.1, and 1.6 ± 0.2 CD31+/CD42b− EMPs/μl plasma at 0, 10, and 20 min, respectively). These data suggest that the increases in EMPs observed in the experimental arm were driven by the disturbed blood flow patterns induced by the distal 220 mmHg cuff.
Figure 2. Effect of disturbed flow on local concentrations of CD62E+ (A) and CD31+/CD42b− (B) endothelial microparticles (EMPs).
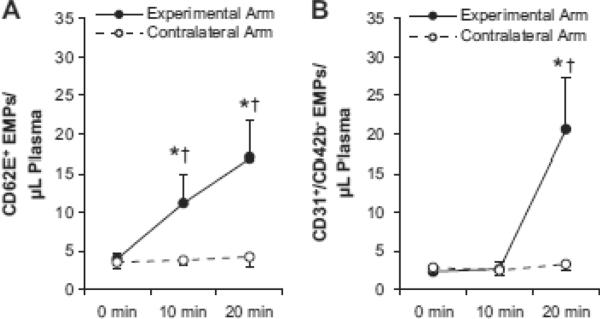
*Significantly different from within-arm baseline (0 min) value (P < 0.05). †Significantly different from contralateral arm (exposed to normal resting blood flow, i.e. no cuffs) at the same time point (P < 0.05).
As expected, the distal forearm cuff reduced brachial artery blood flow, mean shear and antegrade shear, and increased retrograde shear the oscillatory shear index (all P < 0.05, Fig 3). These results confirm that the cuff successfully induced a localized disturbance in blood flow in the experimental arm. Representative Doppler velocity profiles are provided in Fig 3D. Brachial artery diameter did not change throughout the protocol in the experimental arm (0.40 ± 0.01, 0.41 ± 0.01, and 0.41 ± 0.01 cm at 0, 10, and 20 min, respectively; P > 0.05). There were no statistically significant changes in blood flow or shear patterns in the contralateral arm when no cuff was applied (Table 1A). When the proximal 40 mmHg cuff was applied (Table 1B), there were slight but significant increases in retrograde shear and the oscillatory shear index (P < 0.001), although the magnitude of these increases were not as robust as those observed in the experimental arm (Fig 2).
Figure 3. Characterization of the disturbed flow stimulus induced by the experimental protocol.
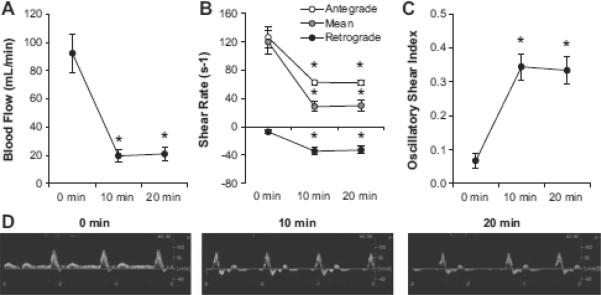
(A) Mean blood flow, (B) Antegrade, mean, and retrograde shear rates, (C) Oscillatory shear index, (D) Representative Doppler brachial artery blood velocity traces from the experimental arm. *Significantly different from baseline (0 min) value (P < 0.05).
Table 1.
Blood flow and shear patterns in the contralateral arm
| Time | ||||
|---|---|---|---|---|
|
|
||||
| Variable | 0 min | 10 min | 20 min | P for trend |
|
|
|
|
|
|
| A. No cuffs, n = 3 | ||||
| Diameter (cm) | 0.40 ± 0.01 | 0.41 ± 0.02 | 0.41 ± 0.02 | NS |
| Mean Flow (mL/min) | 60.4 ± 15.4 | 69.7 ± 20.4 | 73.2 ± 23.1 | NS |
| Mean Shear (s−1) | 82.6 ± 23.6 | 89.2 ± 49.8 | 90.1 ± 29.5 | NS |
| Antegrade Shear (s−1) | 86.5 ± 21.0 | 94.0 ± 25.4 | 91.3 ± 26.7 | NS |
| Retrograde Shear (s−1) | −3.5 ± 2.3 | −4.3 ± 3.6 | −2.3 ± 1.6 | NS |
| Ossilatory Shear Index | 0.05 ± 0.04 | 0.07 ± 0.06 | 0.04 ± 0.03 | NS |
| B. Proximal cuff only (40 mmHg), n = 3 | ||||
| Diameter (cm) | 0.42 ± 0.02 | 0.45 ± 0.02 | 0.45 ± 0.02 | NS |
| Mean Flow (mL/min) | 106.5 ± 20.5 | 57.2 ± 7.5 | 51.2 ± 5.3 | NS |
| Mean Shear (s−1) | 131.9 ± 40.8 | 49.8 ± 8.2 | 42.9 ± 5.6 | NS |
| Antegrade Shear (s−1) | 138.5 ± 38.6 | 70.2 ± 8.0 | 67.5 ± 4.8 | NS |
| Retrograde Shear (s−1) | −8.0 ± 1.1 | −19.9 ± 0.6 | −24.1 ± 1.3 | P < 0.001 |
| Ossilatory Shear Index | 0.07 ± 0.03 | 0.23 ± 0.03 | 0.27 ± 0.02 | P < 0.001 |
NS, not statistically significant (P > 0.05).
DISCUSSION
The major finding of this study is that disturbed blood flow acutely induces endothelial activation and apoptosis in humans, as reflected by release of microparticles from activated (CD62E+) and apoptotic (CD31+/CD42b−) endothelial cells. These data provide the first in vivo experimental evidence of disturbed blood flow-induced endothelial injury in humans.
The two EMP populations we examined are well-established markers of endothelial activation (CD62E+) and apoptosis (CD31+/CD42b−).16, 26 These cellular processes are important components of a damaged endothelium. Our findings are consistent with previous in vitro data indicating that disturbed flow upregulates the expression of E-selectin as well as other adhesion molecules and markers of inflammation on the vascular endothelium.6, 35, 36 Additionally, in vivo experimental conditions that chronically produce disturbed blood flow, such as that seen in atheroprone regions, have been shown to induce substantial vascular dysfunction, injury, remodeling, and atherosclerosis in rodents.10, 37 It has also been reported that low in vivo mean shear rates are associated with elevated circulating EMP levels in humans.38 Our data extend these previous findings by providing experimental evidence that disturbed blood flow confers an injurious stimulus to the endothelium in humans. In addition, from our data, it seems reasonable to expect that circulating EMPs could preferentially originate from classically atheroprone segments of the vasculature (e.g., branch points, curvatures, bifurcations), as these regions are characterized by disturbances in flow. It will be interesting for future studies to examine the extent to which elevations in pro-atherogenic shear patterns are responsible for the consistently-observed phenomenon of elevations in basal circulating EMP levels in patients with cardiovascular diseases.18, 19, 21
Our data complement and extend the important study of Thijssen et al,15 which demonstrated that increased retrograde flow and shear can acutely impair arterial function in humans. One potential caveat is that we used a complete (220 mmHg) distal occlusion rather than partial (e.g. 75 mmHg)15 because we were concerned that with partial occlusion, venous pressure downstream of the occlusion cuff (i.e., in the hand) might eventually exceed that of the cuff, resulting in contamination of sampling region with blood that had been exposed to a partially ischemic and high pressure environment. These stimuli could theoretically promote the release of EMPs independent of any changes in blood flow patterns. Nevertheless, despite our slight deviation from the protocol employed by Thijssen et al.,15 our findings concur with theirs by demonstrating that disturbed blood flow confers a deleterious signal at the cellular level resulting in endothelial activation and apoptosis in humans. Furthermore, we also observed no changes in EMPs in the contralateral arm exposed to normal resting blood flow, further supporting that our observations in the experimental arm were due to local disturbances in blood flow.
Although we have provided evidence that disturbed blood flow can induce the release of distinct EMP populations, the heterogeneity of circulating EMPs should be kept in mind. Peterson et al.39 demonstrated that EMPs induced by in vitro exposure to tumor necrosis factor-α were dramatically different from both control and plasminogen activator inhibitor-1-induced EMPs using a comparative proteomics approach. It seems likely that if EMPs produced by disturbed blood flow were subjected to similar comparative proteomics, the phenotype might be somewhat distinct from EMPs released upon other insults. Additionally, although our experimental protocol was successful in inducing a localized region of disturbed blood flow, we were not able to attribute our observations to specific component(s) of the disturbed flow profile. This will be an important issue for future studies to address, as recent data indicate that low time average-shear stress and fluid flow reversal produce somewhat distinct pro-atherogenic responses at the molecular level.40 Furthermore, our control experiments with a proximal 40 mmHg cuff on the contralateral arm induced a moderately disturbed blood flow stimulus. However, this condition was not sufficient to induce an increase in CD62E+ or CD31+/CD42b− EMPs in the contralateral limb. It was somewhat surprising that (i) the proximal low pressure cuff altered arterial flow and shear patterns, and (ii) we did not detect any changes in EMP concentrations in spite of this moderate disturbance in flow. These data might suggest the existence of a disturbed flow “threshold” for the acute induction of endothelial injury.
A few limitations of our study warrant discussion. First, although the flow and shear data using the proximal cuff in the contralateral arm indicated minimal changes in EMPs, these data should be interpreted with caution given the small sample size. Overall, future studies designed to determine a dose-response relationship between magnitude of flow disturbance and release of EMPs from the vascular wall are needed. In addition, it might have been advantageous to incorporate measurements of brachial artery FMD into our study, as this would have allowed a direct comparison with the study of Thijssen et al.,15 and it would have been interesting to correlate our changes in EMPs with changes in FMD. However, an unavoidable caveat to an assessment of FMD with our protocol was that there would have been substantial reactive hyperemia upon release of the 220 mmHg occlusion cuff prior to a post-intervention FMD assessment. Thus, we would not have been able to link our EMP findings with FMD data because the reactive hyperemia (i.e., increases in flow and shear), and not disturbed blood flow, would have been the final stimulus to which the brachial artery endothelium was exposed. Exposing the limb to a reactive hyperemia would confound our experimental design, making any interpretation difficult.
Perspectives
Our findings provide clear in vivo experimental evidence from human subjects to corroborate ~30 years of in vitro work supporting the close association between disturbed flow patterns and a damaged/dysfunctional vascular endothelium. We also confirm and extend recent work indicating that disturbed blood flow can reduce arterial function in humans as assessed by FMD.15 Our data suggest that the previously-reported functional impairments may be related to pro-inflammatory and pro-apoptotic effects of disturbed blood flow. Additionally, the broader implications of our study relate to the growing understanding of the role of disturbed flow in the pathogenesis of vascular disease. Taken together with the accumulating evidence of pro-atherogenic shear patterns in conduit arteries of subjects with increased risk for cardiovascular diseases,11–14 our data stimulate the hypothesis that disturbed blood flow contributes to increased concentrations of circulating EMPs in these subjects.19 Notably, our study was conducted in the forearm, which is generally resistant to atherosclerosis. Thus, we speculate that disturbed blood flow-induced increases in EMP release might be even more pronounced in atheroprone arteries. Future studies of experimentally-induced disturbed blood flow in atherosclerosis-susceptible vascular beds, e.g. in conduit arteries of the leg, are clearly warranted.
Importantly, EMPs not only serve as biomarkers of damaged endothelium and diagnostic/prognostic biomarkers of cardiovascular diseases,41–44 they also deliver inflammatory cytokines (e.g. C-reactive protein),45 carry regulatory microRNAs,46 reduce endothelial nitric oxide synthesis,47 and promote thrombosis, inflammation and the production of reactive oxygen species.26 Thus, our findings raise the idea that disturbed blood flow induces EMPs which may further promote endothelial dysfunction at distal sites, resulting in a feed-forward “vicious cycle” of vascular injury. Finally, it is important to bear in mind that our study volunteers were young healthy men. It is possible that the acutely altered blood flow patterns in the current study that resulted in endothelial activation and apoptosis could be matched by equal repair processes such that it may not lead to long term endothelial damage in these subjects. However, it seems reasonable to hypothesize that in subjects with diseases or risk factors associated with impaired endogenous endothelial repair mechanisms (e.g. aging, obesity, type 2 diabetes, etc.), disturbances in blood flow could lead to irreparable endothelial damage. This hypothesis warrants future testing.
In summary, our study indicates that experimental induction of disturbed blood flow in the human forearm acutely increases local concentrations of CD62E+ and CD31+/CD42b− EMPs. As these EMP populations are well-validated markers of endothelial cell damage, our findings indicate that disturbed flow acutely induces activation and apoptosis of the human vascular endothelium in vivo.
Novelty and Significance.
-
1)
What is new?
Disturbed blood flow has previously been shown to produce damage to vascular cells in cell culture and animal models.
Here, we extend these findings to humans demonstrating that vascular wall endothelial cells release microparticles (markers of cellular injury) in response to an acute disturbed blood flow stimulus.
-
2)
What is relevant?
Disturbed blood flow is a hallmark characteristic of atherosclerosis-susceptible arterial regions. However, there are few previously published experimental studies providing solid evidence that disturbed flow actually causes endothelial injury at the cellular/molecular level in vivo. Ours is the first such study in humans.
Endothelial microparticles, which we find are released in response to a disturbed flow stimulus, can themselves promote the development of vascular disease.
Recent studies also indicate that increased circulating levels of endothelial microparticles are strongly related to a number of cardiovascular diseases and risk factors, including hypertension.
-
3)
Summary: We demonstrate that disturbed blood flow acutely induces endothelial injury in humans, as reflected by local release of endothelial cell microparticles in response to an experimental disturbed blood flow stimulus.
Acknowledgments
Sources of Funding This work was supported by National Institutes of Health grants T32-AR048523 (N.T.J. and L.J.B.) and R01-HL093167 (P.J.F.). J.P. was supported by a postdoctoral fellowship from the American Heart Association (AHA 11POST5080002).
Footnotes
Disclosures None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: Molecular, cellular, and vascular behavior. J Am Coll Cardiol. 2007;49:2379–2393. doi: 10.1016/j.jacc.2007.02.059. [DOI] [PubMed] [Google Scholar]
- 2.Caro CG, Fitz-Gerald JM, Schroter RC. Arterial wall shear and distribution of early atheroma in man. Nature. 1969;223:1159–1160. doi: 10.1038/2231159a0. [DOI] [PubMed] [Google Scholar]
- 3.Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53:502–514. doi: 10.1161/01.res.53.4.502. [DOI] [PubMed] [Google Scholar]
- 4.Davies PF, Remuzzi A, Gordon EJ, Dewey CF, Jr., Gimbrone MA., Jr. Turbulent fluid shear stress induces vascular endothelial cell turnover in vitro. Proc Natl Acad Sci U S A. 1986;83:2114–2117. doi: 10.1073/pnas.83.7.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lawrence MB, McIntire LV, Eskin SG. Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion. Blood. 1987;70:1284–1290. [PubMed] [Google Scholar]
- 6.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol Genomics. 2002;9:27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 7.O'Keeffe LM, Muir G, Piterina AV, McGloughlin T. Vascular cell adhesion molecule-1 expression in endothelial cells exposed to physiological coronary wall shear stresses. J Biomech Eng. 2009;131:081003. doi: 10.1115/1.3148191. [DOI] [PubMed] [Google Scholar]
- 8.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–1833. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 9.Gambillara V, Chambaz C, Montorzi G, Roy S, Stergiopulos N, Silacci P. Plaque-prone hemodynamics impair endothelial function in pig carotid arteries. Am J Physiol Heart Circ Physiol. 2006;290:H2320–2328. doi: 10.1152/ajpheart.00486.2005. [DOI] [PubMed] [Google Scholar]
- 10.Nam D, Ni CW, Rezvan A, Suo J, Budzyn K, Llanos A, Harrison D, Giddens D, Jo H. Partial carotid ligation is a model of acutely induced disturbed flow, leading to rapid endothelial dysfunction and atherosclerosis. Am J Physiol Heart Circ Physiol. 2009;297:H1535–1543. doi: 10.1152/ajpheart.00510.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla J, Simmons GH, Fadel PJ, Laughlin MH, Joyner MJ, Casey DP. Impact of aging on conduit artery retrograde and oscillatory shear at rest and during exercise: Role of nitric oxide. Hypertension. 2011;57:484–489. doi: 10.1161/HYPERTENSIONAHA.110.165365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casey DP, Padilla J, Joyner MJ. Alpha-adrenergic vasoconstriction contributes to the age-related increase in conduit artery retrograde and oscillatory shear. Hypertension. 2012;60:1016–1022. doi: 10.1161/HYPERTENSIONAHA.112.200618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Credeur DP, Dobrosielski DA, Arce-Esquivel AA, Welsch MA. Brachial artery retrograde flow increases with age: Relationship to physical function. Eur J Appl Physiol. 2009;107:219–225. doi: 10.1007/s00421-009-1117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young CN, Deo SH, Padilla J, Laughlin MH, Fadel PJ. Pro-atherogenic shear rate patterns in the femoral artery of healthy older adults. Atherosclerosis. 2010;211:390–392. doi: 10.1016/j.atherosclerosis.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thijssen DH, Dawson EA, Tinken TM, Cable NT, Green DJ. Retrograde flow and shear rate acutely impair endothelial function in humans. Hypertension. 2009;53:986–992. doi: 10.1161/HYPERTENSIONAHA.109.131508. [DOI] [PubMed] [Google Scholar]
- 16.Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet JM, Tedgui A. Endothelial microparticles in diseases. Cell and tissue research. 2009;335:143–151. doi: 10.1007/s00441-008-0710-9. [DOI] [PubMed] [Google Scholar]
- 17.Combes V, Simon AC, Grau GE, Arnoux D, Camoin L, Sabatier F, Mutin M, Sanmarco M, Sampol J, Dignat-George F. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest. 1999;104:93–102. doi: 10.1172/JCI4985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horstman LL, Jy W, Jimenez JJ, Ahn YS. Endothelial microparticles as markers of endothelial dysfunction. Front Biosci. 2004;9:1118–1135. doi: 10.2741/1270. [DOI] [PubMed] [Google Scholar]
- 19.Jimenez JJ, Jy W, Mauro LM, Horstman LL, Bidot CJ, Ahn YS. Endothelial microparticles (emp) as vascular disease markers. Adv Clin Chem. 2005;39:131–157. doi: 10.1016/s0065-2423(04)39005-0. [DOI] [PubMed] [Google Scholar]
- 20.Pirro M, Schillaci G, Paltriccia R, Bagaglia F, Menecali C, Mannarino MR, Capanni M, Velardi A, Mannarino E. Increased ratio of cd31+/cd42− microparticles to endothelial progenitors as a novel marker of atherosclerosis in hypercholesterolemia. Arterioscler Thromb Vasc Biol. 2006;26:2530–2535. doi: 10.1161/01.ATV.0000243941.72375.15. [DOI] [PubMed] [Google Scholar]
- 21.Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating cd31+/annexin v+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:112–116. doi: 10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- 22.Preston RA, Jy W, Jimenez JJ, Mauro LM, Horstman LL, Valle M, Aime G, Ahn YS. Effects of severe hypertension on endothelial and platelet microparticles. Hypertension. 2003;41:211–217. doi: 10.1161/01.hyp.0000049760.15764.2d. [DOI] [PubMed] [Google Scholar]
- 23.Jung KH, Chu K, Lee ST, Park HK, Bahn JJ, Kim DH, Kim JH, Kim M, Kun Lee S, Roh JK. Circulating endothelial microparticles as a marker of cerebrovascular disease. Ann Neurol. 2009;66:191–199. doi: 10.1002/ana.21681. [DOI] [PubMed] [Google Scholar]
- 24.Arteaga RB, Chirinos JA, Soriano AO, Jy W, Horstman L, Jimenez JJ, Mendez A, Ferreira A, de Marchena E, Ahn YS. Endothelial microparticles and platelet and leukocyte activation in patients with the metabolic syndrome. Am J Cardiol. 2006;98:70–74. doi: 10.1016/j.amjcard.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 25.Jimenez JJ, Jy W, Mauro LM, Soderland C, Horstman LL, Ahn YS. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb Res. 2003;109:175–180. doi: 10.1016/s0049-3848(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 26.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 27.Simmons GH, Padilla J, Young CN, Wong BJ, Lang JA, Davis MJ, Laughlin MH, Fadel PJ. Increased brachial artery retrograde shear rate at exercise onset is abolished during prolonged cycling: Role of thermoregulatory vasodilation. J Appl Physiol. 2011;110:389–397. doi: 10.1152/japplphysiol.00936.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padilla J, Simmons GH, Vianna LC, Davis MJ, Laughlin MH, Fadel PJ. Brachial artery vasodilatation during prolonged lower limb exercise: Role of shear rate. Exp Physiol. 2011;96:1019–1027. doi: 10.1113/expphysiol.2011.059584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu SP, Ringgaard S, Oyre S, Hansen MS, Rasmus S, Pedersen EM. Wall shear rates differ between the normal carotid, femoral, and brachial arteries: An in vivo mri study. J Magn Reson Imaging. 2004;19:188–193. doi: 10.1002/jmri.10441. [DOI] [PubMed] [Google Scholar]
- 30.Newcomer SC, Sauder CL, Kuipers NT, Laughlin MH, Ray CA. Effects of posture on shear rates in human brachial and superficial femoral arteries. Am J Physiol Heart Circ Physiol. 2008;294:H1833–1839. doi: 10.1152/ajpheart.01108.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padilla J, Young CN, Simmons GH, Deo SH, Newcomer SC, Sullivan JP, Laughlin MH, Fadel PJ. Increased muscle sympathetic nerve activity acutely alters conduit artery shear rate patterns. Am J Physiol Heart Circ Physiol. 2010;298:H1128–1135. doi: 10.1152/ajpheart.01133.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins NT, Landers RQ, Thakkar SR, Fan X, Brown MD, Prior SJ, Spangenburg EE, Hagberg JM. Prior endurance exercise prevents postprandial lipaemia-induced increases in reactive oxygen species in circulating cd31+ cells. J Physiol. 2011;589:5539–5553. doi: 10.1113/jphysiol.2011.215277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah MD, Bergeron AL, Dong JF, Lopez JA. Flow cytometric measurement of microparticles: Pitfalls and protocol modifications. Platelets. 2008;19:365–372. doi: 10.1080/09537100802054107. [DOI] [PubMed] [Google Scholar]
- 34.van Ierssel SH, Van Craenenbroeck EM, Conraads VM, Van Tendeloo VF, Vrints CJ, Jorens PG, Hoymans VY. Flow cytometric detection of endothelial microparticles (emp): Effects of centrifugation and storage alter with the phenotype studied. Thromb Res. 2010;125:332–339. doi: 10.1016/j.thromres.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 35.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–14876. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins NT, Martin JS, Laughlin MH, Padilla J. Exercise-induced signals for vascular endothelial adaptations: Implications for cardiovascular disease. Curr Cardiovasc Risk Rep. 2012;6:331–346. doi: 10.1007/s12170-012-0241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 38.Boulanger CM, Amabile N, Guerin AP, Pannier B, Leroyer AS, Mallat CN, Tedgui A, London GM. In vivo shear stress determines circulating levels of endothelial microparticles in end-stage renal disease. Hypertension. 2007;49:902–908. doi: 10.1161/01.HYP.0000259667.22309.df. [DOI] [PubMed] [Google Scholar]
- 39.Peterson DB, Sander T, Kaul S, Wakim BT, Halligan B, Twigger S, Pritchard KA, Jr., Oldham KT, Ou JS. Comparative proteomic analysis of pai-1 and tnf-alpha-derived endothelial microparticles. Proteomics. 2008;8:2430–2446. doi: 10.1002/pmic.200701029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conway DE, Williams MR, Eskin SG, McIntire LV. Endothelial cell responses to atheroprone flow are driven by two separate flow components: Low time-average shear stress and fluid flow reversal. Am J Physiol Heart Circ Physiol. 2010;298:H367–374. doi: 10.1152/ajpheart.00565.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nozaki T, Sugiyama S, Koga H, Sugamura K, Ohba K, Matsuzawa Y, Sumida H, Matsui K, Jinnouchi H, Ogawa H. Significance of a multiple biomarkers strategy including endothelial dysfunction to improve risk stratification for cardiovascular events in patients at high risk for coronary heart disease. J Am Coll Cardiol. 2009;54:601–608. doi: 10.1016/j.jacc.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 42.Amabile N, Guerin AP, Leroyer A, Mallat Z, Nguyen C, Boddaert J, London GM, Tedgui A, Boulanger CM. Circulating endothelial microparticles are associated with vascular dysfunction in patients with end-stage renal failure. J Am Soc Nephrol. 2005;16:3381–3388. doi: 10.1681/ASN.2005050535. [DOI] [PubMed] [Google Scholar]
- 43.Amabile N, Guerin AP, Tedgui A, Boulanger CM, London GM. Predictive value of circulating endothelial microparticles for cardiovascular mortality in end-stage renal failure: A pilot study. Nephrol Dial Transplant. 2012;27:1873–1880. doi: 10.1093/ndt/gfr573. [DOI] [PubMed] [Google Scholar]
- 44.Koga H, Sugiyama S, Kugiyama K, Watanabe K, Fukushima H, Tanaka T, Sakamoto T, Yoshimura M, Jinnouchi H, Ogawa H. Elevated levels of ve-cadherin-positive endothelial microparticles in patients with type 2 diabetes mellitus and coronary artery disease. J Am Coll Cardiol. 2005;45:1622–1630. doi: 10.1016/j.jacc.2005.02.047. [DOI] [PubMed] [Google Scholar]
- 45.Habersberger J, Strang F, Scheichl A, Htun N, Bassler N, Merivirta RM, Diehl P, Krippner G, Meikle P, Eisenhardt SU, Meredith I, Peter K. Circulating microparticles generate and transport monomeric c-reactive protein in patients with myocardial infarction. Cardiovasc Res. 2012;96:64–72. doi: 10.1093/cvr/cvs237. [DOI] [PubMed] [Google Scholar]
- 46.Diehl P, Fricke A, Sander L, Stamm J, Bassler N, Htun N, Ziemann M, Helbing T, El-Osta A, Jowett JB, Peter K. Microparticles: Major transport vehicles for distinct micrornas in circulation. Cardiovasc Res. 2012;93:633–644. doi: 10.1093/cvr/cvs007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol. 2008;173:1210–1219. doi: 10.2353/ajpath.2008.080228. [DOI] [PMC free article] [PubMed] [Google Scholar]