Abstract
Objective
No studies have compared the response to selective serotonin reuptake inhibitors and atypical antipsychotics in anorexia nervosa. This case study examines such a comparison.
Method
This report describes a case of 12-year-old identical twins with anorexia nervosa, one of whom was treated with olanzapine and the other with fluoxetine, while undergoing family therapy.
Results
Twin A treated with fluoxetine went from 75% to 84.4% ideal body weight, while Twin B treated with olanzapine went from 72% to 99.9% ideal body weight over the course of 9 months.
Discussion
This case supports the need for adequately powered, controlled clinical trials to test the efficacy of olanzapine in adolescents presenting with anorexia nervosa.
Introduction
Anorexia nervosa (AN) is associated with substantial morbidity and mortality, yet, little in the way of proven treatments exists.1 The role of medications in the treatment of low-weight patients with AN has typically been limited.2–4 In contrast to the effectiveness of antidepressant medications in patients with bulimia nervosa (BN), several studies have failed to demonstrate a beneficial effect for the addition of selective serotonin reuptake inhibitors (SSRIs) in the inpatient treatment of malnourished AN patients.5–7 Recently, there has been interest in the use of atypical antipsychotics for the treatment of AN, prompted in part by observations of weight gain in other patient groups. This has resulted in case reports and open trials that raise the possibility that olanzapine,8–21 quetiapine,22–24 risperidone 25, 26 and most recently aripiprazole 27 may have some efficacy in AN. A randomized, placebo-controlled trial of olanzapine in 34 individuals with AN treated in a day hospital setting showed improved rate of weight gain and reduced obsessional symptoms28.
These “antidepressant” and atypical antipsychotic trials have several limitations. First, they include small numbers of AN patients. Second, they were largely uncontrolled and not conducted in outpatient settings, confounding medication effects with the efficacy of inpatient or partial hospitalization programs. Finally, no study has directly compared the response of SSRIs and atypical antipsychotics in AN. Thus, it is not known whether one type of medication is superior to the other.
This article describes twins similarly ill with AN presenting to an outpatient eating disorders clinic and the differential impact of using fluoxetine versus olanzapine during weight restoration.
Clinical course
The subjects were born to a healthy mother as identical twins without any complications during delivery. The twins attained appropriate developmental milestones until age 10. They were referred by their pediatrician at age 12 for weight loss by food restriction and associated behavioral concerns and when assessed, both twins met criteria for anorexia nervosa. Twins A and B both reported hand washing rituals associated with an eating disorder preoccupation that they would ingest calories and gain weight by touching surfaces that had food in it. Both twins also indicated preoccupations about the fat, calorie, and sugar content of food, eating from a full plate of food, and had excessive regional concerns about the appearance of their stomachs. Twin A reported a fear of being fat and a fear of weighing outside a narrow weight range, while Twin B reported exercise preoccupations and a fear of consuming liquids. Neither twin had binging or purging behaviors.
The twins were referred by their pediatrician to an outpatient eating disorder clinic at age 12. The flattening of the twins’ growth curves began at age 10 ½. From age 10 ½ to 11 ½, twin A reached a weight of 60 lbs and twin B’s weight plateaued at 61.5 lbs. By age 12, twin A’s weight dropped to 51 lbs and twin B’s weight dropped to 52 lbs, both registering a 9 lb weight loss during the 3 months prior to reaching age 12. At the time of initial evaluation, twin A was at 69% ideal body weight (IBW) and twin B was at 67% IBW. They presented with sinus bradycardia below 45 bpm and low blood pressure from severe caloric restriction that required hospitalization for 17 days. At hospital discharge (and prior to entering outpatient treatment), twin A was at 75% IBW and twin B was at 72% IBW.
Results
Outpatient treatment was begun after acute medical stabilization in a pediatric inpatient unit, where liaison was maintained through consultation with a child psychiatrist. After medical stabilization, the twins and their family signed informed consent to receive outpatient family therapy within a research study and were randomized to family-based therapy (described below). In addition, the twins were offered medical management services through the clinic. Medical necessity combined with consultation with the parents, determined the relative timing and choice of medications. At the outset, parents were open to medications but wanted to wait and see if behavioral interventions were effective. Although further observation in a baseline pre-medication state may have been scientifically desirable, Twin B suffered weight loss to 70% IBW within a week of post-hospital discharge with worsening caloric restriction but maintained medical stability. This prompted parental request for medication and the start of a trial of olanzapine. Twin A maintained or increased weight for several weeks prior to plateauing, but displayed increased anxiety, emotional dysregulation and OCD-like behaviors, which resulted in parental request to start medication.
Twin A was started on fluoxetine 10 mg taken by mouth every morning (week 8) and titrated up in 10 mg weekly increments to a final dose of 60 mg, which was maintained through week 36. She tolerated this regimen well without side effects. Twin B was started on olanzapine 2.5 mg taken by mouth at bedtime (week 2) and titrated further to 3.75 mg (week 6), which she tolerated well. The olanzapine dose was adjusted to 2.5 mg with the emergence of food cravings reported by twin B in the presence of steady weight gain (week 25). Olanzapine dose adjustment resulted in resolution of food cravings and was maintained through week 36. Monitoring of lipid profile and glucose levels did not reveal any abnormalities.
Both twins received family therapy for weight restoration in the same sessions over 9 months. This approach, identified as family-based treatment (FBT), was originally developed at the Maudsley Hospital in England and has shown significant promise for treating adolescents with AN. In FBT, the family is explicitly trained in regaining parental control over the adolescent patient’s eating behavior to achieve weight gain.29 Once weight and eating behavior are normalized, control is gradually returned to the adolescent while moving the focus to rebuilding relationships within the family and pursuing developmental milestones. Studies have shown short term and long-term efficacy for this therapeutic approach.30–33
Upon completing 9 months of outpatient treatment on separate medications while attending the same family therapy sessions, both twins had reduced eating disorder preoccupations or rituals, obsessions and compulsions. Throughout the course of treatment, Twin A was less communicative and forthcoming about her symptoms than Twin B, which was evident with collateral information from the parents. Interviewing Twin A was also complicated by her responses which mirrored those of Twin B, or at other times, she remained silent if interviewed separately. At the end of treatment assessment, per parents, both twins did not display obsessions, compulsions or rituals but Twin A continued to require prompting to maintain food intake and as opposed to Twin B who expressed hunger at meal times. Additionally, Twin A did not regain her normal eating pattern whereas Twin B did. After 9 months, Twin B was remarkably weight restored at 99.9% IBW, while Twin A had yet to weight restore at 84.4% IBW, meeting weight criteria for Anorexia Nervosa.
Discussion
This report of AN treatment in twins provides the first evidence of improved weight restoration on olanzapine versus fluoxetine. Interestingly, both medications were associated with reported reductions in OCD-like symptoms. Although this is a single subject, the findings are consistent with the notion that SSRIs do not appear to be useful in stimulating renourishment in the ill, underweight state 5–7. In contrast, these data are consistent with the publications cited in the introduction that suggest that some atypicals may be more effective.
There are several limitations that need to be noted. It appeared the twins were identical, however, this was not confirmed by genetic testing. We did not confirm the parent’s report that the patients were taking the medication by measuring blood levels.
Considering these limitations, this is the first case study of which we are aware, where fluoxetine and olanzapine are compared in individuals or twins with AN. The data presented in this report suggests that further trials are needed to examine the efficacy of olanzapine compared to fluoxetine, and in combination with family therapy.
Figure 1.
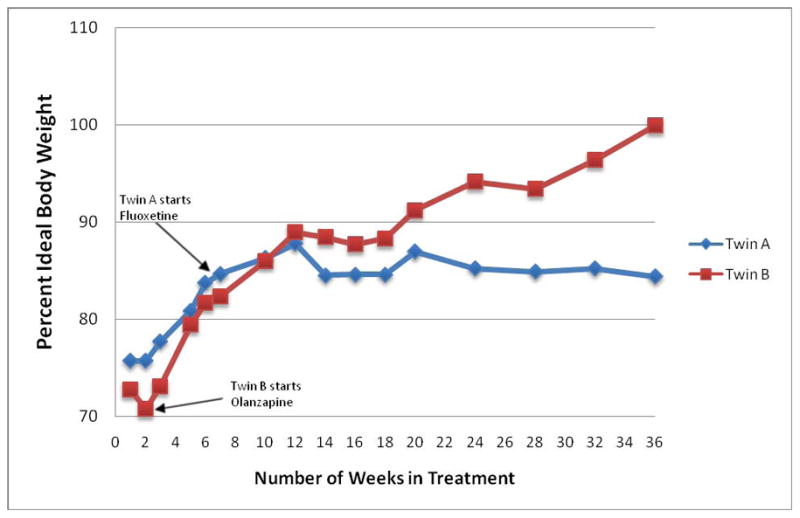
Differential weight restoration on olanzapine versus fluoxetine during treatment
Acknowledgments
Dr. Duvvuri was supported by the Davis Foundation Fellowship in Eating Disorders, MH074508 and 5T32MH018399. Dr Kaye has received salary support from the University of Pittsburgh and the University of California, San Diego; Research funding/support from the NIMH; Research funding for an investigator initiated treatment study from Astra-Zeneca and consulting fees from Lundbeck and Merck. In addition, there are honoraria for presentations from academic institutions and meetings, and compensation for grant review activities from the National Institutes of Health.
Footnotes
The authors have no other conflicts of interests to disclose.
References
- 1.Duvvuri V, Kaye W. Clinical Synthesis. Anorexia Nervosa. Focus. 2009;7:455–62. [Google Scholar]
- 2.Jimerson DC, Wolfe BE, Brotman AW, Metzger ED. Medications in the treatment of eating disorders. Psychiatr Clin North Am. 1996 Dec 1;19(4):739–54. doi: 10.1016/s0193-953x(05)70378-6. [DOI] [PubMed] [Google Scholar]
- 3.Attia E, Wolk S, Cooper T, Glasofer D, Walsh B. Plasma tryptophan during weight restoration in patients with anorexia nervosa. Biol Psychiatry. 2005;57:674–8. doi: 10.1016/j.biopsych.2004.11.045. [DOI] [PubMed] [Google Scholar]
- 4.Bulik C, Berkman N, Brownley K, Sedway J, Lohr K. Anorexia nervosa treatment: a systematic review of randomized controlled trials. Int J Eat Disord. 2007;40:310–20. doi: 10.1002/eat.20367. [DOI] [PubMed] [Google Scholar]
- 5.Attia E, Haiman C, Walsh BT, Flater SR. Does fluoxetine augment the inpatient treatment of anorexia nervosa? Am J Psychiatry. 1998 Apr 1;155(4):548–51. doi: 10.1176/ajp.155.4.548. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson CP, La Via MC, Crossan PJ, Kaye WH. Are serotonin selective reuptake inhibitors effective in underweight anorexia nervosa? Int J Eat Disord. 1999 Jan 1;25(1):11–7. doi: 10.1002/(sici)1098-108x(199901)25:1<11::aid-eat2>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 7.Strober M, Pataki C, Freeman R, DeAntonio M. No effect of adjunctive fluoxetine on eating behavior or weight phobia during the inpatient treatment of anorexia nervosa: an historical case- control study. J Child Adolesc Psychopharmacol. 1999;9(3):195–201. doi: 10.1089/cap.1999.9.195. [DOI] [PubMed] [Google Scholar]
- 8.Barbarich N, McConaha C, Gaskill J, LaVia M, Frank GK, Brooks S, Plotnicov K, Kaye WH. An open trial of olanzapine in anorexia nervosa. J Clin Psychiatry. 2004;65:1480–2. doi: 10.4088/jcp.v65n1106. [DOI] [PubMed] [Google Scholar]
- 9.Bosanac P, Burrows G, Norman T. Olanzapine in anorexia nervosa. Aust N Z J Med. 2003;37(4):494. doi: 10.1046/j.1440-1614.2003.01221.x. [DOI] [PubMed] [Google Scholar]
- 10.Brambilla F, Garcia CFS, Daga GFA, Santonastaso P, Ramaciotti C, Bondi E, Mellado C, Borriello R, Monteleone P. Olanzapine therapy in anorexia nervosa: psychobiological effects. Int Clin Psychopharmacol. 2007;22:197–204. doi: 10.1097/YIC.0b013e328080ca31. [DOI] [PubMed] [Google Scholar]
- 11.Dennis K, Le Grange D, Bremer J. Olanzapine use in adolescent anorexia nervosa. Eat Weight Disord. 2006;11:e53–6. doi: 10.1007/BF03327760. [DOI] [PubMed] [Google Scholar]
- 12.Ercan E, Copkunol H, Cykoethlu S, Varan A. Olanzapine treatment of an adolescent girl with anorexia nervosa. Hum Psychopharmacol. 2003;18(5):401–3. doi: 10.1002/hup.492. [DOI] [PubMed] [Google Scholar]
- 13.Mondraty N, Birmingham C, Touyz SW, Sundakov V, Chapman L, Beaumont P. Randomized controlled trial of olanzapine in the treatment of cognitions in anorexia nervosa. Australas Psychiatry. 2005;13:72–5. doi: 10.1080/j.1440-1665.2004.02154.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang T, Chou Y, Shiah I. Combined treatment of olanzapine and mirtazapine in anorexia nervosa associated with major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:306–9. doi: 10.1016/j.pnpbp.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 15.Boachie A, Goldfield G, Spettigue W. Olanzapine use as an adjunctive treatment for hospitalized children with anorexia nervosa: case reports. Int J Eat Disord. 2003;33:98–103. doi: 10.1002/eat.10115. [DOI] [PubMed] [Google Scholar]
- 16.Jensen VS, Mejlhede A. Anorexia nervosa: treatment with olanzapine. Br J Psychiatry. 2000 Jul 1;177:87. doi: 10.1192/bjp.177.1.87. [DOI] [PubMed] [Google Scholar]
- 17.Malina A, Gaskill J, McConaha C, Frank GK, LaVia M, Scholar L, Kaye WH. Olanzapine treatment of anorexia nervosa: a restrospective study. Int J Eat Disord. 2003;33(2):234–7. doi: 10.1002/eat.10122. [DOI] [PubMed] [Google Scholar]
- 18.Mehler C, Wewetzer C, Schulze U, Warnke A, Theisen F, Dittmann RW. Olanzapine in children and adolescents with chronic anorexia nervosa. A study of five cases. Eur Child Adolesc Psychiatry. 2001 Jun;10(2):151–7. doi: 10.1007/s007870170039. [DOI] [PubMed] [Google Scholar]
- 19.Powers PS, Santana CA, Bannon YS. Olanzapine in the treatment of anorexia nervosa: an open label trial. International Journal of Eating Disorders. 2002;32:146–54. doi: 10.1002/eat.10084. [DOI] [PubMed] [Google Scholar]
- 20.Hansen L. Olanzapine in the treatment of anorexia nervosa. Br J Psychiatry. 1999 Dec 1;175:592. doi: 10.1192/s000712500026354x. [DOI] [PubMed] [Google Scholar]
- 21.La Via MC, Gray N, Kaye WH. Case reports of olanzapine treatment of anorexia nervosa. Int J Eat Disord. 2000 Apr 27;3:363–6. doi: 10.1002/(sici)1098-108x(200004)27:3<363::aid-eat16>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Bosanac P, Kurlender S, Norman T, Hallasm K, Wesnes K, Manktelow T, Burrows G. An open-label study of quetiapine in anorexia nervosa. Hum Psychopharmacol. 2007;22:223–30. doi: 10.1002/hup.845. [DOI] [PubMed] [Google Scholar]
- 23.Mehler-Wex C, romanos M, Kirchheiner J, Schulze U. Atypical antipsychotics in severe anorexia nervosa in children and adolescents-review and case reports. Eur Eat Disord Rev. 2008;16:100–8. doi: 10.1002/erv.843. [DOI] [PubMed] [Google Scholar]
- 24.Powers P, Bannon Y, Eubanks R, McCormick T. Quetiapine in anorexia nervosa patients: an open label outpatient pilot study. Int J Eat Disord. 2007;40:21–6. doi: 10.1002/eat.20325. [DOI] [PubMed] [Google Scholar]
- 25.Fisman S, Steele M, Short J, Byrne T, Short J, Lavalle C. Case study: anorexia nervosa and autistic disorder in an adolescent girl. J Am Acad Child Adolsc Psychiatry. 1996;35:937–40. doi: 10.1097/00004583-199607000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Newman-Toker J. Risperidone in anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 2000;39(8):941–2. doi: 10.1097/00004583-200008000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Trunko M, Schwartz TDV, Kaye W. Aripiprazole in anorexia nervosa and low-weight bulimia nervosa: CASE REPORTS. Int J Eat Disord. 2010 Feb 22; doi: 10.1002/eat.20807. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Bissada H, Tasca G, Barber A, Bradwejn J. Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: A randomized, double-blind, placebo-controlled trial. Am J Psych. 2008;165(10):1281–8. doi: 10.1176/appi.ajp.2008.07121900. [DOI] [PubMed] [Google Scholar]
- 29.Russell G, Szmukler G, Dare C, Eisler I. An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry. 1987;44(12):1047–56. doi: 10.1001/archpsyc.1987.01800240021004. [DOI] [PubMed] [Google Scholar]
- 30.Eisler I, Dare C, Hodes M, Russell G, Dodge E, Le Grange D. Family therapy for adolescent anorexia nervosa: the results of a controlled comparison of two family interventions. J Child Psychol Psychiat. 2000;41(6):727–36. [PubMed] [Google Scholar]
- 31.Eisler I, Simic M, Russell G, Dare C. A randomised controlled treatment trial of two forms of family therapy in adolescent anorexia nervosa: a five year follow-up. J Child Psychol Psychiatry. 2007;48(6):552–60. doi: 10.1111/j.1469-7610.2007.01726.x. [DOI] [PubMed] [Google Scholar]
- 32.Lock J, Agras W, Bryson S, Kraemer H. A comparison of short- and long-term family therapy for adolescent anorexia nervosa. J Am Acad Child Adolsc Psychiatry. 2005;44(7):632–9. doi: 10.1097/01.chi.0000161647.82775.0a. [DOI] [PubMed] [Google Scholar]
- 33.Lock J, Couturier J, Agras W. Comparison of long-term outcomes in adolescents with anorexia nervosa treated with family therapy. J Am Acad Child Adoles Psychiatry. 2006;45(6):666–72. doi: 10.1097/01.chi.0000215152.61400.ca. [DOI] [PubMed] [Google Scholar]


