Abstract
We examined the effects of angiotensin II AT1–receptor blockade with olmesartan on high fat (HF) diet–induced vascular oxidative stress and endothelial dysfunction in normal salt (NS) diet–fed Dahl salt-sensitive (DSS) rats. Treatment with NS + HF diet (32% crude fat, 0.3% NaCl) for 20 weeks significantly increased blood pressure in DSS rats. NS + HF diet–fed DSS rats also showed higher plasma levels of thiobarbituric acid–reactive substances, aortic superoxide production, and mRNA levels of p22phox and gp91phox in aortic tissues than NS diet–fed DSS rats. Furthermore, acetylcholine-induced vasorelaxation of aorta from NS + HF diet–fed DSS rats was significantly reduced. In NS + HF diet–fed DSS rats, treatment with olmesartan medoxomil (10 mg/kg per day, p.o.) and hydralazine (25 mg/kg per day, p.o.) similarly decreased blood pressure. However, in these animals, only olmesartan normalized plasma levels of thiobarbituric acid–reactive substances, vascular superoxide in aortic tissues, and acetylcholine-induced vasorelaxation. These data indicate that HF diet–induced hypertension is associated with vascular oxidative stress and endothelial dysfunction in NS diet–treated DSS rats. Inhibition of angiotensin II AT1 receptors may elicit beneficial effects on HF-induced hypertension and vascular injury in subjects that have genetically enhanced sodium-sensitive blood pressure.
Keywords: high fat (HF) diet, Dahl salt-sensitive (DSS) rats, oxidative stress, endothelial dysfunction, angiotensin II (AngII)
Introduction
It has been suggested that a diet rich in fat and salt can have a profound influence on blood pressure (1). Salt restriction decreased blood pressure in obese subjects more significantly than in non-obese subjects, and subjects that undergo weight loss demonstrated a suppression in salt-induced blood pressure increases (2). In rats, long treatment with a high fat (HF) diet caused greater salt-induced increases in blood pressure (3 – 5). It has also been demonstrated that endothelial dysfunction closely contributes to the development of HF diet–induced hypertension (6 – 8). Both human and animal studies have indicated that a HF diet induces endothelial dysfunction through the generation of oxidative stress (9, 10). Nagae et al. (4) have shown that HF loading with a normal salt (NS) diet (0.3% NaCl) increased blood pressure in Dahl salt-sensitive (DSS) rats. However, it remains to be determined why a HF diet increases blood pressure in DSS rats that are not treated with a high-salt diet.
In the present study, we hypothesized that HF diet augments the development of hypertension in subjects that have genetically enhanced sodium-sensitive blood pressure through vascular oxidative stress and endothelial dysfunction. To test this hypothesis, we examined the effects of a HF diet in NS diet–treated DSS rats. Since it has been indicated that angiotensin II (AngII) contributes to HF diet–induced vascular oxidative stress and injury (11, 12), we also wanted to elucidate the effects of olmesartan medoxomil, an AngII AT1–receptor antagonist (12 – 14), in these animals. In particular, we investigated the changes in NADPH oxidase activity and its components, p22phox and gp91phox in aortic tissues, as potential sources of vascular oxidative stress (15).
Materials and Methods
Animals
All experimental procedures were performed according to guidelines for the care and use of animals established by the Kagawa University Medical School. Male 6-week-old DSS rats (Seak-Yoshitomi Co., Ltd., Fukuoka) were treated with a NS diet (4.8% crude fat, 0.3% NaCl; n = 8; Clea Japan, Inc., Tokyo), a NS + HF diet (32% crude fat, 0.3% NaCl; n = 8; Clea Japan, Inc.), a NS + HF diet treated with olmesartan medoxomil (10 mg/kg per day, p.o.; n = 9; Daiichi-Sankyo Co., Ltd., Tokyo) or a NS + HF diet treated with hydralazine (25 mg/kg per day, p.o.; n = 7; Wako Co., Ltd., Osaka) for 20 weeks. Control Dahl salt-resistant (DSR, Seak-Yoshitomi Co., Ltd.) rats were also treated with NS or NS + HF diets (n = 6 in each case). The treatment period for the HF diet was determined on the basis of previous studies (16, 17). Preliminary studies also showed that HF diet treatment for 20 weeks resulted in vascular oxidative stress in NS diet–fed DSS rats (data not shown). Systolic blood pressure (SBP) was measured using tail-cuff plethysmography. After an observation period, rats were killed by decapitation and trunk blood was collected. Thoracic aorta was then carefully removed, and acetylcholine- and sodium nitroprusside-induced vasorelaxation, aortic superoxide production, or gene expression of p22phox and gp91phox were evaluated, as described previously (18 – 20).
Endothelial function
Endothelial function was determined as described previously (19). Briefly, thoracic aortas were removed, freed from fat, and adherent connective tissue and then cut into strips taking special care to preserve the endothelium. Approximately 2-mm aortic segments of the thoracic aorta were suspended in organ chambers containing 10 mL of Krebs-Ringer-bicarbonate solution (118.5 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 25 mmol/L NaHCO3, and 10 mmol/L glucose) under a resting tension of 1.5 g at 37°C and gassed with 95% O2 – 5% CO2. Contractions and relaxations were measured as changes in isometric tension using a force displacement transducer (TB-612T; Nihon Kohden, Osaka) coupled to a polygraph (RM 6000, Nihon Kohden). After a 1.5-h equilibration period, vessels were contracted with 1 μmol/L phenylephrine and subsequently treated with 1 μmol/L acetylcholine to test the tissue viability. The endothelium was considered functional when the relaxation of precontracted vessels to 1 μmol/L acetylcholine was at least 90%.
Aortic superoxide production
Superoxide anion production in aortic segments was determined with the use of lucigenin-enhanced chemiluminescence, as described previously (20, 21). Briefly, perivascular tissue was carefully removed, the vessels were repeatedly washed to remove adherent blood cells, and then cut into 5-mm ring segments. The rings were placed in chilled bicarbonate buffer that was composed of 118.3 mmol/L NaCl, 4.7 mmol/L KCl, 2.5 mmol/L CaCl2, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 25.0 mmol/L NaHCO3, 5.5 mmol/L glucose, and 0.026 mmol/L EDTA and was bubbled continuously with 95% O2 – 5% CO2 to maintain pH 7.4 and were allowed to equilibrate for 30 min at 37°C. After equilibration, the rings were rinsed with prewarmed (37°C) modified Krebs-HEPES buffer composed of 119 mmol/L NaCl, 20 mmol/L HEPES, 4.6 mmol/L KCl, 1.0 mmol/L MgSO4, 0.15 mmol/L Na2HPO4, 0.4 mmol/L KH2PO4, 25 mmol/L NaHCO3, 1.2 mmol/L CaCl2, and 5.5 mmol/L glucose (pH 7.4). Rings were placed in 1 mL of Krebs-HEPES buffer containing lucigenin (250 μmol/L) and equilibrated in the dark for 10 min at 37°C. The chemiluminescence was then recorded every 30 s for 15 min with a luminescence reader (BLR-301, Hitachi-Aloka Medical, Ltd., Tokyo). Lucigen chemiluminescence was expressed as counts per minute per milligram of dry tissue weight.
Quantitative PCR
The mRNA expression of glyceraldehydes-3-phosphate dehydrogenase (GAPDH), p22phox and gp91phox were analyzed by real-time PCR using a LightCycler FastStart DNA Master SYBR Green I kit or TaqMan Gene Expression Assay kits (Applied Biosystems, Foster City, CA, USA). The oligonucleotide primer sequences of GAPDH, p22phox, and gp91phox and PCR conditions were as described previously (13, 20). All data were expressed as the relative difference between NS diet–fed DSS rats and other groups after normalization to GAPDH expression.
Other analytical procedures
Plasma thiobarbituric acid–reactive substances (TBARS) (13, 20) and AngII levels were measured (22), as described previously in detail. Postprandial plasma glucose levels, insulin, total cholesterol, and triglycerides were determined using commercially available assay kits (all kits from Wako Co.).
Statistical analyses
Values are presented as means ± S.E.M. Statistical comparisons of differences were performed using one- or two-way analysis of variance combined with the Newmankeuls post-hoc test. A P-values of less than 0.05 was considered statistically significant.
Results
General characteristics
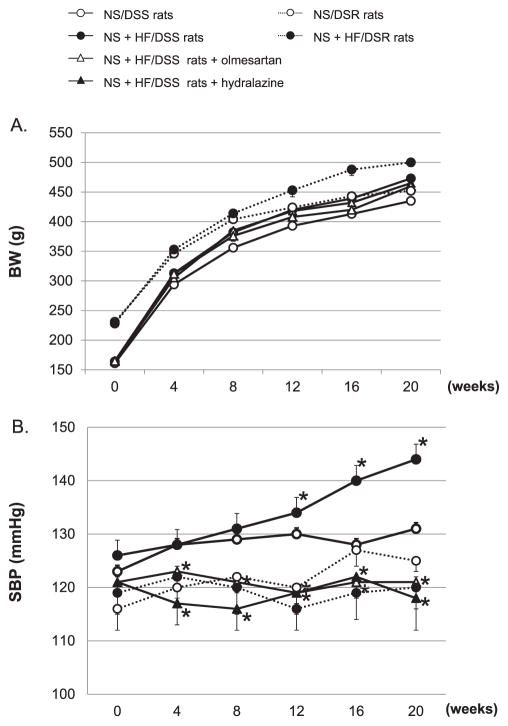
The profiles of body weight (BW) and SBP are shown in Fig. 1: A and B, respectively. At baseline (6 weeks of age), DSR rats showed higher BW than DSS rats. In both DSR and DSS rats, NS + HF diet significantly increased BW. In NS + HF diet–fed DSS rats, neither olmesartan nor hydralazine changed BW (Fig. 1A). At baseline, SBP was not different among the groups, and a NS diet for 12 weeks did not change the SBP in either DSR or DSS rats. However, a NS + HF diet did significantly increase the SBP in DSS. On the other hand, the SBP of NS + HF diet–fed DSR rats tended to be lower as compared with that of NS diet–fed DSR rats at 16 and 20 weeks; however, these changes were not statistically significant. Olmesartan and hydralazine similarly decreased SBP in NS + HF diet–fed DSS rats (Fig. 1B). No significant differences in postprandial plasma glucose levels were observed between the groups. HF-diet tended to increase postprandial plasma insulin levels in NS diet–fed DSS and DSR rats, but these changes were not statistically significant (data not shown). At 20 weeks, NS + HF diet–fed DSS rats showed increased postprandial plasma total cholesterol and triglyceride levels (113 ± 19 and 108 ± 24 mg/dL, respectively) as compared with NS/DSS rats (83 ± 12 and 61 ± 15 mg/dL, respectively). Similarly, NS + HF diet–fed DSR rats showed increased postprandial plasma cholesterol and triglyceride levels (106 ± 22 and 98 ± 19 mg/dL, respectively) as compared with NS + DSR rats (73 ± 13 and 59 ± 11 mg/dL, respectively).
Fig. 1.
The profiles of BW and SBP in DSR and DSS rats. A NS + HF diet significantly increased BW in both DSR and DSS rats (A), but increased the SBP in only DSS rats (B). Olmesartan and hydralazine similarly decreased the SBP in NS + HF diet–fed DSS rats. BW, body weight. SBP, systolic blood pressure. NS, normal salt. HF, high fat. DSS rats, Dahl salt-sensitive rats. DSR rats, Dahl salt-resistant rats. *P < 0.05: NS/DSS rats vs. NS + HF/DSS rats, NS + HF/DSS rats + olmesartan or NS + HF/DSS rats + hydralazine. For clarity, asterisks have been removed from Fig. 1A.
Acetylcholine- and sodium nitroprusside-induced vasorelaxation
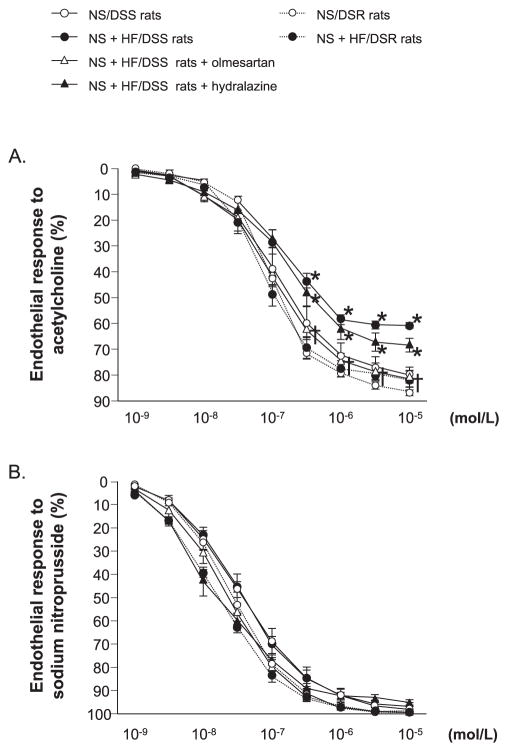
Acetylcholine-induced relaxation was not different among NS or NS + HF diet–fed DSR rats and NS diet–fed DSS rats (Fig. 2A). However, acetylcholine-induced relaxation was significantly reduced in NS + HF diet–fed DSS rats. In these animals, impairment of acetylcholine-induced relaxation was recovered by treatment with olmesartan but not by hydralazine. There was no difference in sodium nitroprusside-induced vasorelaxation among any of the groups (Fig. 2B).
Fig. 2.
Dose–response curve for acetylcholine (A)- and sodium nitroprusside (B)-induced vasorelaxation of the thoracic aorta. Acetylcholine-induced relaxation was significantly reduced in NS + HF diet–fed DSS rats. In these animals, impairment of acetylcholine-induced relaxation was normalized by treatment with olmesartan but not with hydralazine. Sodium nitroprusside–induced vasorelaxation was not different among the groups. *P < 0.05: NS/DSS rats vs. NS + HF/DSS rats, NS + HF/DSS rats + olmesartan or NS + HF/DSS rats + hydralazine; †P < 0.05: NS + HF/DSS rats vs. NS + HF/DSS rats + olmesartan or NS + HF/DSS rats + hydralazine.
Plasma AngII and TBARS levels
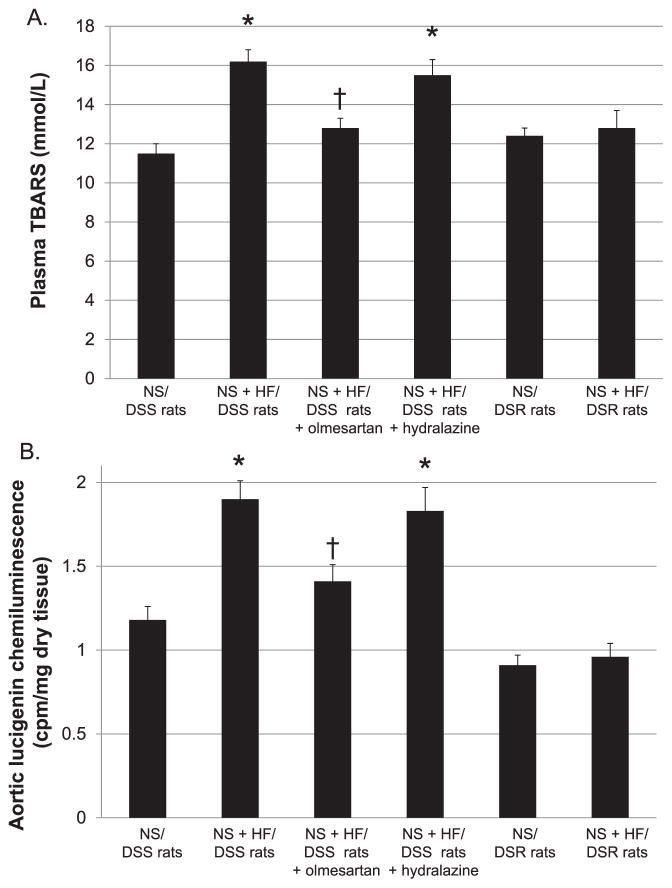
Plasma AngII concentrations were not different between NS and NS + HF diet–fed DSR (37 ± 6 vs. 41 ± 9 pmol/L) or DSS rats (27 ± 4 vs. 30 ± 5 pmol/L). In NS + HF diet–fed DSS rats, both olmesartan and hydralazine significantly increased plasma AngII levels (53 ± 4 and 61 ± 10 pmol/L, respectively). As shown in Fig. 3A, plasma TBARS levels were not different between NS and NS + HF diet–fed DSR rats (12.4 ± 0.4 and 12.8 ± 0.9 mmol/L, respectively). However, NS + HF diet–fed DSS rats showed higher plasma TBARS levels than NS diet–fed DSS rats (16.2 ± 0.6 vs. 11.5 ± 0.5 mmol/L). In NS + HF diet–fed DSS rats, treatment with olmesartan prevented the increases in plasma TBARS levels (12.8 ± 0.5 mmol/L) but hydralazine did not (15.5 ± 0.8 mmol/L).
Fig. 3.
Plasma TBARS levels (A) and aortic superoxide production (B). NS + HF diet–fed DSS rats showed higher plasma TBARS levels and aortic superoxide production (lucigenin chemiluminescence) than NS diet–fed DSS rats. In NS + HF diet–fed DSS rats, treatment with olmesartan, but not hydralazine, prevented the increases in plasma TBARS levels and aortic superoxide production. TBARS, thiobarbituric acid reactive substances. *P < 0.05: NS/DSS rats vs. NS + HF/DSS rats, NS + HF/DSS rats + olmesartan or NS + HF/DSS rats + hydralazine; †P < 0.05: NS + HF/DSS rats vs. NS + HF/DSS rats + olmesartan or NS + HF/DSS rats + hydralazine.
Aortic superoxide production
As shown in Fig. 3B, aortic lucigenin chemiluminescence in NS + HF diet–fed DSR rats tended to be higher than NS diet–fed DSR rats, but these changes were not statistically significant (0.91 ± 0.06 vs. 0.96 ± 0.08 cpm/mg dry tissue). In NS + HF diet–fed DSS rats, we observed an approximately 2-fold increase in aortic lucigenin chemiluminescence compared with NS diet–fed DSS rats (1.90 ± 0.11 vs. 1.18 ± 0.08 cpm/mg dry tissue). Again, olmesartan treatment but not hydralazine, prevented the increases in aortic lucigenin chemiluminescence in NS + HF diet–fed DSS rats (1.41 ± 0.1 and 1.83 ± 0.14 cpm/mg dry tissue, respectively).
Aortic p22phox and gp91phox mRNA levels
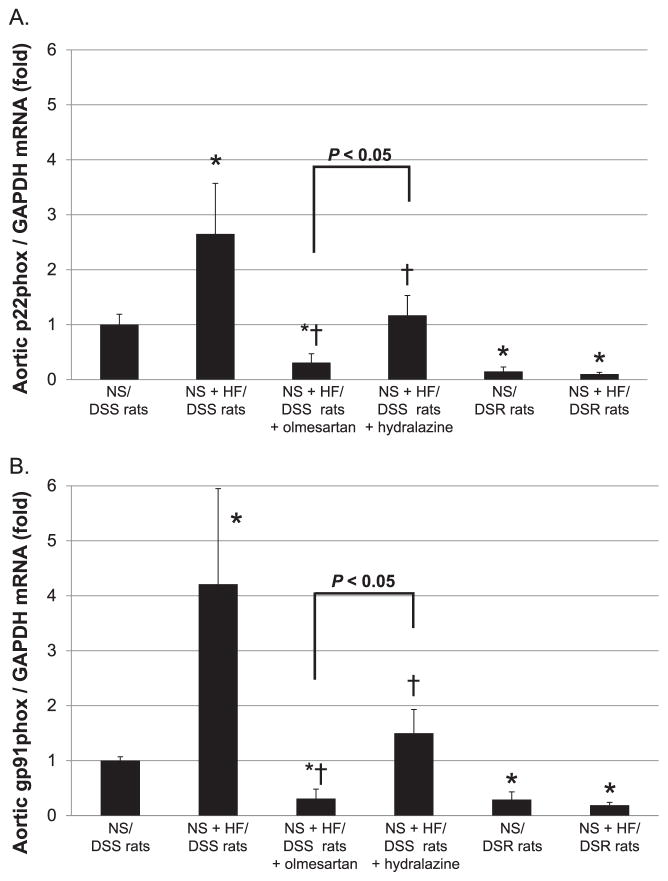
The levels of p22phox and gp91phox mRNA in aortic tissues were not significantly different between NS and NS + HF diet–fed DSR rats (Fig. 4: A and B). However, NS + HF diet–fed DSS rats showed significantly higher gp91phox and p22phox mRNA levels in aortic tissues than NS diet–fed DSS rats (2.65 ± 0.92- and 4.21 ± 1.74-fold, respectively). In NS + HF diet–fed DSS rats, treatment with olmesartan, and to lesser extent hydralazine, significantly decreased p22phox and gp91phox mRNA levels.
Fig. 4.
The levels of p22phox and gp91phox mRNA in aortic tissues. All data were expressed as the relative differences between NS diet–fed DSS rats and other groups after normalization to GAPDH expression. NS + HF diet–fed DSS rats showed significantly higher p22phox (A) and gp91phox (B) mRNA levels in aortic tissues than NS diet–fed DSS rats. In NS + HF diet–fed DSS rats, treatment with olmesartan or hydralazine significantly decreased p22phox and gp91phox mRNA levels. However, the effect of olmesartan was significantly greater than that of hydralazine. *P < 0.05: NS/DSS rats vs. NS + HF/DSS rats, NS + HF/DSS rats + olmesartan or NS + HF/DSS rats + hydralazine: †P < 0.05; NS + HF/DSS rats vs. NS + HF/DSS rats + olmesartan or NS + HF/DSS rats + hydralazine.
Discussion
In agreement with previous studies (4), the present study shows that a HF diet augments the development of hypertension in NS diet–fed DSS rats but not in NS diet–fed control DSR rats. The present study also showed that HF diet–induced hypertension is associated with endothelial dysfunction as well as increases in the values of vascular oxidative stress markers, such as plasma TBARS levels, aortic superoxide production (lucigenin chemiluminescence), and mRNA levels of p22phox and gp91phox in aortic tissues of NS diet–fed DSS rats. While olmesartan (an AngII AT1–receptor antagonist) and hydralazine (a non-specific vasodilator) similarly decreased blood pressure in HF + NS diet–fed DSS rats, only olmesartan rescued plasma levels of TBARS, aortic superoxide, and acetylcholine-induced vasorelaxation. These data suggest that HF diet–induced hypertension is accompanied by AngII-dependent vascular oxidative stress–induced endothelial dysfunction in subjects that have genetically enhanced sodium-sensitive blood pressure.
Endothelial dysfunction significantly contributes to the development of HF-induced hypertension (3, 6). Several studies have indicated that a HF diet induces endothelial dysfunction through the generation of oxidative stress (9, 10). It has also been shown that a HF + high salt diet induces endothelial dysfunction through oxidative stress in mice (23). Galili et al. (24) have shown that long treatment with a HF diet induced coronary oxidative stress and endothelial dysfunction long before the development of insulin resistance in swine. In healthy subjects, endothelial dysfunction and oxidative stress have been observed immediately after consuming a HF meal (10, 25 – 27). In the present study, HF diet–induced hypertension is associated with vascular oxidative stress (as indicated by increases in plasma TBARS levels, superoxide production, and mRNA levels of p22phox and gp91phox in aortic tissues) and endothelial dysfunction in NS diet–fed DSS rats. Collectively, these data support the hypothesis that HF diet–induced vascular oxidative stress contributes to the pathogenesis of endothelial dysfunction leading to the development of hypertension in DSS rats. It has been shown that the HF diet increases oxidative stress through the activation of NAPDH oxidase, and NADPH oxidase activation is associated with increases in mRNA levels of NADPH oxidase components, such as p22phox and gp91phox (15). However, transcriptional regulation of NADPH oxidase components remains unclear. Several studies have shown that treatment with HF diet induces vascular oxidative stress in normal rats (28) and mice (11, 12). However, in the present study, the HF-induced oxidative stress was likely to occur more readily in NS diet–fed DSS rats than NS diet–fed DSR rats. Although its precise mechanism(s) are not clear, it may be due to the differences in the experimental conditions (strain, observation period food composition, or consumption, etc.) and/or low sensitivity for the detection of vascular oxidative stress. Nagae et al. (4) have suggested that HF increases blood pressure in DSS rats through sympathetic excitation. Thus, it is also possible that HF diet–induced activation of the sympathetic nerve system and associated vascular oxidative stress are likely to occur more readily in DSS rats.
The present study also shows that a non-specific vasodilator, hydralazine, decreases blood pressure, but does not improve the oxidative stress and endothelial dysfunction observed in NS + HF diet–fed DSS rats. Therefore, hypertension is unlikely to be the primary cause for HF-induced vascular oxidative stress and endothelial dysfunction in these animals. Currently, we cannot address the issues regarding the role of insulin resistance in the development of hypertension in HS + NS diet–fed DSS rats, although there are several preclinical and clinical studies indicating that olmesartan improves insulin resistance and decreases blood pressure in hypertensive subjects (29 – 32). Hydralazine did not alter oxidative stress markers, although 22phox and gp91phox mRNA levels were partially but significantly decreased by hydralazine. These data suggest that NADPH oxidase–independent mechanism is also involved. In this regard, recent studies reported that AngII stimulates oxidative stress through mitochondrial and xanthine oxidase pathways (33). Another possibility is that oxidative stress is stimulated by sympathetic nerve activation during hydralazine treatment (34).
We have previously shown that AngII-induced hypertension is accompanied by an increase in vascular superoxide in rats (21). In this study, we have shown that inhibition of AngII AT1–receptor with olmesartan improves HF diet–induced hypertension, vascular oxidative stress, and endothelial dysfunction in NS diet–fed DSS rats. Thus, AngII may be involved in HF-induced vascular oxidative stress and associated endothelial dysfunction and hypertension. However, we failed to detect significant differences in plasma AngII levels between NS and NS + HF diet–fed DSS rats. Since the local renin–angiotensin system (RAS) is regulated independently by the circulating RAS (35), further studies will be needed to determine whether the vascular RAS is activated during the development of HF-induced hypertension. It is also important to note that although olmesartan is a highly selective AT1-receptor antagonist (14), it also possesses anti-oxidative properties (36) and inverse agonistic action (37).
There is a growing body of evidence that supports that AngII-induced release of vascular superoxide radicals inactivates nitric oxide (NO) and thereby induces endothelial dysfunction and associated hypertension (11, 12, 38). Studies have shown that increased vascular superoxide production is associated with an impaired response to acetylcholine-induced vasorelaxation in AngII-infused hypertensive rats (6, 9) and 2K1C Goldblatt hypertensive rats (8). It has also been shown that impaired endothelial function is associated with reduced NO production in high salt–fed DSS hypertensive rats (6) and HF diet–fed normotensive rats (7 – 9). To investigate the role of NO, further studies are needed to examine the effects of antioxidants and NO inhibitors on acetylcholine-induced vasodilation. It has also been reported that oxidative stress enhances the contractile response to AngII in the aortic ring of HF diet–fed rats (38).
In conclusion, the present study indicates for the first time that HF diet–dependent hypertension is associated with vascular oxidative stress and endothelial dysfunction in NS diet–fed DSS rats. Since NS + HF diet–fed DSR rats do not show any changes in blood pressure, vascular oxidative stress, and endothelial function, such HF-induced changes are likely to occur more readily in subjects with genetically enhanced sodium-sensitive blood pressure. Finally, inhibition of the AngII AT1–receptor may have beneficial effects on HF-induced hypertension in these subjects.
Acknowledgments
This work was supported in part by a Grant-in-Aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by a supporting International Joint Research Project from the Japan Society of the Promotion Science (to Akira Nishiyama). Ludek Cervenka was also supported by the project of Ministry of Health of the Czech Republic for the development of research organization 00023001 (IKEM), institutional support. We are grateful to Daiichi-Sankyo Co., Ltd. for supplying olmesartan medoxomil.
References
- 1.Dimsdale JE, Ziegler M, Mills P, Berry C. Prediction of salt sensitivity. Am J Hypertens. 1990;3:429–435. doi: 10.1093/ajh/3.6.429. [DOI] [PubMed] [Google Scholar]
- 2.Rocchini AP, Key J, Bondie D, Chico R, Moorehead C, Katch V, et al. The effect of weight loss on the sensitivity of blood pressure to sodium in obese adolescents. N Engl J Med. 1989;321:580–585. doi: 10.1056/NEJM198908313210905. [DOI] [PubMed] [Google Scholar]
- 3.Morrison RG, Mills C, Moran AL, Walton CE, Sadek MH, Mangiarua EI, et al. A moderately high fat diet promotes salt-sensitive hypertension in obese zucker rats by impairing nitric oxide production. Clin Exp Hypertens. 2007;29:369–381. doi: 10.1080/10641960701578360. [DOI] [PubMed] [Google Scholar]
- 4.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Effect of high fat loading in Dahl salt-sensitive rats. Clin Exp Hypertens. 2009;31:451–461. doi: 10.1080/10641960902825487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang HY, Reddy S, Kotchen TA. A high sucrose, high linoleic acid diet potentiates hypertension in the Dahl salt sensitive rat. Am J Hypertens. 1999;12:183–187. doi: 10.1016/s0895-7061(98)00238-6. [DOI] [PubMed] [Google Scholar]
- 6.Reil TD, Barnard RJ, Kashyap VS, Roberts CK, Gelabert HA. Diet-induced changes in endothelial-dependent relaxation of the rat aorta. J Surg Res. 1999;85:96–100. doi: 10.1006/jsre.1999.5666. [DOI] [PubMed] [Google Scholar]
- 7.Lockette W, Otsuka Y, Carretero O. The loss of endothelium-dependent vascular relaxation in hypertension. Hypertension. 1986;8:II61–II66. doi: 10.1161/01.hyp.8.6_pt_2.ii61. [DOI] [PubMed] [Google Scholar]
- 8.Crespo MJ, Escobales N, Rodriguez-Sargent C. Endothelial dysfunction in the San Juan hypertensive rat: possible role of the nitric oxide synthase. J Cardiovasc Pharmacol. 1996;27:802–808. doi: 10.1097/00005344-199606000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Roberts CK, Vaziri ND, Sindhu RK, Barnard RJ. A high-fat, refined-carbohydrate diet affects renal NO synthase protein expression and salt sensitivity. J Appl Physiol. 2003;94:941–946. doi: 10.1152/japplphysiol.00536.2002. [DOI] [PubMed] [Google Scholar]
- 10.Tsai WC, Li YH, Lin CC, Chao TH, Chen JH. Effects of oxidative stress on endothelial function after a high-fat meal. Clin Sci (Lond) 2004;106:315–319. doi: 10.1042/CS20030227. [DOI] [PubMed] [Google Scholar]
- 11.Doran DE, Weiss D, Zhang Y, Griendling KK, Taylor WR. Differential effects of AT1 receptor and Ca2+ channel blockade on atherosclerosis, inflammatory gene expression, and production of reactive oxygen species. Atherosclerosis. 2007;195:39–47. doi: 10.1016/j.atherosclerosis.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimada K, Murayama T, Yokode M, Kita T, Fujita M, Kishimoto C. Olmesartan, a novel angiotensin II type 1 receptor antagonist, reduces severity of atherosclerosis in apolipoprotein E deficient mice associated with reducing superoxide production. Nutr Metab Cardiovasc Dis. 2011;21:672–678. doi: 10.1016/j.numecd.2009.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Nagai Y, Yao L, Kobori H, Miyata K, Ozawa Y, Miyatake A, et al. Temporary angiotensin II blockade at the prediabetic stage attenuates the development of renal injury in type 2 diabetic rats. J Am Soc Nephrol. 2005;16:703–711. doi: 10.1681/ASN.2004080649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koike H, Sada T, Mizuno M. In vitro and in vivo pharmacology of olmesartan medoxomil, an angiotensin II type AT1 receptor antagonist. J Hypertens Suppl. 2001;19:S3–S14. doi: 10.1097/00004872-200106001-00002. [DOI] [PubMed] [Google Scholar]
- 15.Hitomi H, Kiyomoto H, Nishiyama A. Angiotensin II and oxidative stress. Curr Opin Cardiol. 2007;22:311–315. doi: 10.1097/HCO.0b013e3281532b53. [DOI] [PubMed] [Google Scholar]
- 16.Fardin NM, Oyama LM, Campos RR. Changes in baroreflex control of renal sympathetic nerve activity in high-fat-fed rats as a predictor of hypertension. Obesity (Silver Spring) 2012;20:1591–1597. doi: 10.1038/oby.2012.4. [DOI] [PubMed] [Google Scholar]
- 17.Yan MX, Ren HB, Kou Y, Meng M, Li YQ. Involvement of nuclear factor kappa B in high-fat diet-related pancreatic fibrosis in rats. Gut Liver. 2012;6:381–387. doi: 10.5009/gnl.2012.6.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishiyama A, Kobori H, Fukui T, Zhang GX, Yao L, Rahman M, et al. Role of angiotensin II and reactive oxygen species in cyclosporine A-dependent hypertension. Hypertension. 2003;42:754–760. doi: 10.1161/01.HYP.0000085195.38870.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakano D, Kwak CJ, Fujii K, Ikemura K, Satake A, Ohkita M, et al. Sesamin metabolites induce an endothelial nitric oxide-dependent vasorelaxation through their antioxidative property-independent mechanisms: possible involvement of the metabolites in the antihypertensive effect of sesamin. J Pharmacol Exp Ther. 2006;318:328–335. doi: 10.1124/jpet.105.100149. [DOI] [PubMed] [Google Scholar]
- 20.Rahman M, Nishiyama A, Guo P, Nagai Y, Zhang GX, Fujisawa Y, et al. Effects of adrenomedullin on cardiac oxidative stress and collagen accumulation in aldosterone-dependent malignant hypertensive rats. J Pharmacol Exp Ther. 2006;318:1323–1329. doi: 10.1124/jpet.106.105106. [DOI] [PubMed] [Google Scholar]
- 21.Nishiyama A, Fukui T, Fujisawa Y, Rahman M, Tian RX, Kimura S, et al. Systemic and regional hemodynamic responses to tempol in angiotensin II-infused hypertensive rats. Hypertension. 2001;37:77–83. doi: 10.1161/01.hyp.37.1.77. [DOI] [PubMed] [Google Scholar]
- 22.Nishiyama A, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of renal interstitial fluid angiotensin II in angiotensin II-induced hypertension. J Hypertens. 2003;21:1897–1903. doi: 10.1097/00004872-200310000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Dobrian AD, Schriver SD, Lynch T, Prewitt RL. Effect of salt on hypertension and oxidative stress in a rat model of diet-induced obesity. Am J Physiol Renal Physiol. 2003;285:F619–F628. doi: 10.1152/ajprenal.00388.2002. [DOI] [PubMed] [Google Scholar]
- 24.Galili O, Versari D, Sattler KJ, Olson ML, Mannheim D, McConnell JP, et al. Early experimental obesity is associated with coronary endothelial dysfunction and oxidative stress. Am J Physiol Heart Circ Physiol. 2007;292:H904–H911. doi: 10.1152/ajpheart.00628.2006. [DOI] [PubMed] [Google Scholar]
- 25.Gaenzer H, Sturm W, Neumayr G, Kirchmair R, Ebenbichler C, Ritsch A, et al. Pronounced postprandial lipemia impairs endothelium-dependent dilation of the brachial artery in men. Cardiovasc Res. 2001;52:509–516. doi: 10.1016/s0008-6363(01)00427-8. [DOI] [PubMed] [Google Scholar]
- 26.Vogel RA, Corretti MC, Plotnick GD. Effect of a single high-fat meal on endothelial function in healthy subjects. Am J Cardiol. 1997;79:350–354. doi: 10.1016/s0002-9149(96)00760-6. [DOI] [PubMed] [Google Scholar]
- 27.Marchesi S, Lupattelli G, Schillaci G, Pirro M, Siepi D, Roscini AR, et al. Impaired flow-mediated vasoactivity during postprandial phase in young healthy men. Atherosclerosis. 2000;153:397–402. doi: 10.1016/s0021-9150(00)00415-9. [DOI] [PubMed] [Google Scholar]
- 28.Li R, Wang WQ, Zhang H, Yang X, Fan Q, Christopher TA, et al. Adiponectin improves endothelial function in hyperlipidemic rats by reducing oxidative/nitrative stress and differential regulation of eNOS/iNOS activity. Am J Physiol Endocrinol Metab. 2007;293:E1703–E1708. doi: 10.1152/ajpendo.00462.2007. [DOI] [PubMed] [Google Scholar]
- 29.De Luis DA, Conde R, Gonzalez Sagrado M, Aller R, Izaola O, Perez Castrillon JL, et al. Effects of olmesartan vs irbesartan on metabolic parameters and visfatin in hypertensive obese women. Eur Rev Med Pharmacol Sci. 2010;14:759–763. [PubMed] [Google Scholar]
- 30.Dohi T, Narui K, Kasai T, Takaya H, Inoshita A, Maeno K, et al. Effects of olmesartan on blood pressure and insulin resistance in hypertensive patients with sleep-disordered breathing. Heart Vessels. 2011;26:603–608. doi: 10.1007/s00380-010-0104-2. [DOI] [PubMed] [Google Scholar]
- 31.Mizukawa M, Ohmori K, Obayashi A, Ishihara Y, Yoshida J, Noma T, et al. Effects of combined olmesartan and pravastatin on glucose intolerance and cardiovascular remodeling in a metabolic-syndrome model. Hypertens Res. 2009;32:617–624. doi: 10.1038/hr.2009.63. [DOI] [PubMed] [Google Scholar]
- 32.Rizzoni D, Pasini E, Flati V, Rodella LF, Paiardi S, Assanelli D, et al. Angiotensin receptor blockers improve insulin signaling and prevent microvascular rarefaction in the skeletal muscle of spontaneously hypertensive rats. J Hypertens. 2008;26:1595–1601. doi: 10.1097/HJH.0b013e328304b060. [DOI] [PubMed] [Google Scholar]
- 33.Dikalov S. Cross talk between mitochondria and NADPH oxidases. Free Radic Biol Med. 2011;51:1289–1301. doi: 10.1016/j.freeradbiomed.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rafiq K, Noma T, Fujisawa Y, Ishihara Y, Arai Y, Nabi AH, et al. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation. 2012;125:1402–1413. doi: 10.1161/CIRCULATIONAHA.111.064097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 36.Izuhara Y, Nangaku M, Inagi R, Tominaga N, Aizawa T, Kurokawa K, et al. Renoprotective properties of angiotensin receptor blockers beyond blood pressure lowering. J Am Soc Nephrol. 2005;16:3631–3641. doi: 10.1681/ASN.2005050522. [DOI] [PubMed] [Google Scholar]
- 37.Miura S, Fujino M, Hanzawa H, Kiya Y, Imaizumi S, Matsuo Y, et al. Molecular mechanism underlying inverse agonist of angiotensin II type 1 receptor. J Biol Chem. 2006;281:19288–19295. doi: 10.1074/jbc.M602144200. [DOI] [PubMed] [Google Scholar]
- 38.Viswanad B, Srinivasan K, Kaul CL, Ramarao P. Effect of tempol on altered angiotensin II and acetylcholine-mediated vascular responses in thoracic aorta isolated from rats with insulin resistance. Pharmacol Res. 2006;53:209–215. doi: 10.1016/j.phrs.2005.11.002. [DOI] [PubMed] [Google Scholar]