Abstract
Objectives
Vascular networks respond to chronic alterations in blood supply by structural remodeling. Previously, we showed that blood flow changes in the mouse gracilis artery lead to transient diameter increases, which can generate large increases in circumferential wall stress. Here, we examine the associated changes in the medial area of the arterial wall and the effects on circumferential wall stress.
Methods
To induce blood flow changes, one of the two feeding vessels to the gracilis artery was surgically removed. At 7 to 56 days after blood flow interruption, the vasculature was perfused with India ink for morphological measurements, and processed for immuno-cytochemistry to mark the medial cross-section area. Theoretical simulations of hemodynamics were used to analyze the data.
Results
During adaptive increases in vessel diameter, increases in medial area were observed, most strongly in the middle region of the artery. Simulations showed that this increase in medial area limits the increase in estimated circumferential stress during vascular adaptation to less than 50%, in contrast to an increase of up to 250% if the medial area had remained unchanged.
Conclusions
During vascular adaptation, increases in circumferential stress are limited by growth of the media coordinated with diameter changes.
Keywords: vascular adaptation, blood flow, ischemia, smooth muscle cells, endothelial cells
INTRODUCTION
In the circulation, vascular elements are capable of structural remodeling in response to local stimuli including shear stress, intravascular pressure and metabolic state of the tissue [1, 2, 7, 8, 11, 15, 18, 19, 26]. When blood flow to a part of a vascular network is interrupted, the remaining vessels may adapt to compensate for the decrease of blood supply [3, 9, 21–23]. This process may lead to arteriogenesis, in which pre-existing arterioles enlarge to form collateral arteries in the presence of arterial occlusions [10, 20, 24].
In a previous study, we showed that the diameter of the mouse gracilis artery increased transiently and in a spatially dependent manner following permanent removal of one of its blood supplies [8]. The aim of the present study was to investigate whether these diameter increases are accompanied by increases in the area of the vessel wall media in the same experimental setting and whether the morphological changes are coordinated with hemodynamic parameters (blood flow and intravascular pressure) and mechanical (shear and circumferential) stresses. Such behavior might be expected because a structural increase in vessel diameter (D) at constant medial mass or area results in a proportional decrease of medial thickness (h) and increase of medial tension (T = PD/2) for a given transmural pressure (P). For fixed wall mass, the circumferential stress in the media, which is given by σ = T/h therefore increases approximately in proportion to D2. It is known that increased circumferential stress stimulates growth of the vessel wall [14, 19], which in turn tends to restore the stress to its initial level. In the present study, we therefore used experimental and theoretical approaches to examine the relationship between the diameter, wall area and circumferential stress along the vessel length following alterations in hemodynamic and metabolic conditions caused by flow interruption.
Vessel remodeling following partial ischemia involves proliferation of endothelial cells (EC) and smooth muscle cells (SMC) [4, 20–22, 24]. We therefore also assessed the contributions of growth of new SMC and EC (proliferation) and hypertrophy of the existing cells to the overall increase in wall area. Changes in tortuosity, reflecting longitudinal vessel growth, have been reported following femoral artery occlusion [20, 21] or removal [22]. A further objective of this work was to evaluate changes in tortuosity between the control and remodeled gracilis artery.
Previous studies, as referenced above, have provided extensive information about the structural responses of vascular elements to various stimuli and about the underlying cellular processes. However, understanding of how these responses are integrated during the formation of functional and efficient vascular structures and during adaptation to injury and other stimuli remains limited. The present study is intended to contribute to such understanding by providing quantitative information about spatially dependent growth and regression of the medial layer occurring in response to diameter changes driven by changes in hemodynamic conditions. The experimental system using the mouse gracilis artery was chosen because the artery is fed from both ends, such that its perfusion can be reduced in a controlled and repeatable way by removing one of the two blood supplies.
MATERIAL AND METHODS
Experimental animals and surgical procedure
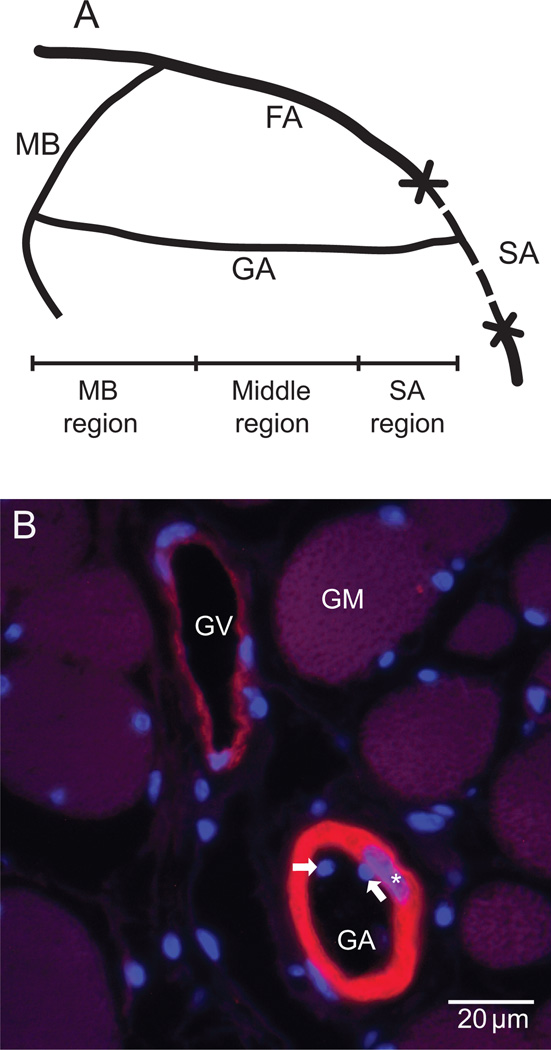
Male FVB/n mice (7–11 wk old with a body mass of 27.4 ±3 g at the time of data collection) were used for all experiments according to procedures approved by the University of Arizona Institutional Animal Care and Use Committee. Surgical procedures were described in detail previously [8]. Briefly, the blood supply from the saphenous artery to the gracilis artery was interrupted by ligating the saphenous artery and vein and then removing a 5 mm portion above and below the point where the gracilis artery branches off the saphenous artery (Figure1A). The gracilis artery and vein were not ligated. Prior to skin closure, bleeding from collateral vessels was stopped and blood was removed from the exposed muscle and skin tissues. The blood supply to the gracilis artery from the muscular branch of the femoral artery remained intact. The mice were euthanized at days 7, 14, 21, 28 or 56 after surgery.
Figure 1.
A. Schematic of the surgical procedure and the three regions of the gracilis artery. FA: femoral artery. MB: muscular branch of FA. SA: saphenous artery. GA: gracilis artery. B. Micrograph of a paraffin section stained for smooth muscle and cell nuclei by immunohistochemistry using fluorescently labeled anti smooth muscle α-actin (red/yellow) antibody and bisbenzimidazole (BBI, blue), respectively. The medial cross-section area was estimated from measurements of the α-actin positive area. Endothelial cell nuclei are represented by the BBI positive structures inside the vessel lumen (arrows), while the smooth muscle cell nuclei are the BBI structures overlapping the α-actin positive areas (stars). GA: gracilis artery, GV: gracilis vein, GM: gracilis muscle fibers.
Morphometric analysis of the gracilis artery inner diameter
The inner diameter of the gracilis artery was measured from ink-filled specimens as described before [8]. The entire mouse vasculature was perfused with India ink (Hunt Manufacturing Co., No. 3232) to the level of capillaries and the gracilis muscle was dehydrated in a series of ethanol solutions and cleared in methyl salicylate. The inner diameter was measured every 300µm from the ink cast in images of cleared tissue using a stereomicroscope and the image analysis software Sigma Scan (SPSS Science, Chicago, IL). Variations in diameter of 1 µm or more are readily resolved by this technique. The artery length was divided into three regions as follows: 1) 'MB’ (3/8 of total length adjacent to the intact muscular branch of the femoral artery), 2) 'middle' (following 3/8 of total length), and 3) 'SA' (2/8 of total length adjacent to the injury end proximal to the saphenous artery) (Figure 1A). Values for morphological variables were obtained as averages for each region for each animal.
Immunohistological analysis of the gracilis artery wall
Vessel media cross-section area and SMC and EC nuclei were evaluated from paraffin sections of control (right side artery) and remodeled (left side artery) samples of the previously analyzed ink filled specimens. Among the original specimens that were previously prepared for microvascular network visualization [8] four samples from each of days 7, 14, 21, 28 and 56 had an intact vascular network and therefore were considered appropriate for further histological preparations. Diameter measurements and the immunohistological analysis reported in this study were performed on this subset of the original samples. The gracilis muscles were rehydrated prior to histological processing to remove the methyl salicylate. Additional fixation was not necessary. The entire gracilis muscle was embedded vertically in paraffin and serial sections were collected at locations 600 µm apart along the length of the muscle. The sections were fluorescently labeled with anti smooth muscle a-actin antibody (Sigma, St. Louis, MO) using the Zenon® Alexa Fluor 555 Mouse IgG2a labeling kit (Molecular Probes, Eugene, OR) to mark the media of the gracilis artery. All nuclei were counterstained with bisbenzimidazole (BBI) (Molecular Probes, Eugene, OR). Any nucleus lying entirely within the stained SMC was assumed to be an SMC nucleus, and any nucleus lying within or overlapping the region enclosed by the inner edge of the stained SMC was assumed to be an EC nucleus.
At each location, the gracilis artery medial cross-section area was measured from three consecutive sections using the image analysis software package MetaMorph® (Molecular Devices, Sunnyvale, CA). Values were averaged for the MB, middle and SA regions as described above. To correct for the biological differences and possible processing variations for data from different animals used at different time points, the ratios between normal and remodeled values from the two legs of each animal were calculated and reported for the morphometric measurements (diameter, medial cross-section area and number of nuclei) and the calculated hemodyamic parameters (shear stress, circumferential stress, blood flow and pressure). The morphometric values are reported as mean ± SE. Differences were considered significant at a p-value < 0.05 for paired t-tests with equal-variance.
Hemodynamic parameter estimation
Hemodynamic parameters (gracilis artery blood flows, transverse arteriole blood flows, wall shear stresses, intravascular pressures) were calculated from measured gracilis artery diameter and length values as described before [8]. Based on the previous morphometric measurements, the gracilis artery was assumed to feed 16 transverse arterioles, which are evenly spaced along the artery’s length (6.6 mm). The end of the artery supplied by the muscular branch of the femoral artery (MB end) and the end supplied by the saphenous artery (SA end) were assumed to be at arterial pressure, PA = 100 mmHg. The flow rate entering the transverse arteriole connected to node i was assumed to depend on the pressure Pi at that node according to QTA,i = (Pi − PV)/RTA, where PV = 2 mmHg is venous pressure, and RTA is the flow resistance of the pathway connecting the transverse arteriole to the venous pressure given by RTA = (PA − PV)/qref where qref is a reference value of the transverse arteriole flow estimated previously as qref = 29 nl/min, based on available published data [8].
The flow rate in segment i of the gracilis artery is given by Qi = (Pi − Pi+1)/Ri, where the flow resistance of the segment is Ri = 8Liη/(πDi4), Li and Di are its length and diameter, and η = 3 cP is the approximate apparent viscosity of blood for vessels in the diameter range of 10–30 µm [17]. The equation for conservation of blood flow at node i is then
The resulting system of linear equations was solved to obtain the pressures Pi at each node. The wall shear stress and the mean pressure in segment i are given by τw,i = Di(Pi − Pi+1)/(4Li) and Pm,i = (Pi + Pi+1)/2.
Circumferential wall stress
The medial thickness for any given time point and region was calculated as w = ½[(D2 + 4A/π)1/2 − D] where A is the medial cross-section area as estimated from anti smooth muscle α-actin antibody staining of sections, and D is the internal diameter estimated from ink-filled specimens prior to sectioning. The circumferential stress in each segment was estimated as σ = ½P(D/w + 1) where P is the mean pressure in each gracilis artery segment [8].
Because the arteries were not straight and precisely aligned with the specimens, it cannot be assumed that the cross-sections examined were perpendicular to the sectioning planes. However, it can be shown [27] that the cross-section area of structures observed in multiple transverse sections provides an unbiased estimate of the volume of the structures, independent of their orientation relative to the sectioning planes.
Tortuosity index
The index of tortuosity for the entire vessel was computed as the ratio between the total vessel length and the length of the straight line between the vessel end points.
RESULTS
The gracilis artery smooth muscle layer was identified from digitized photographs of paraffin sections stained for smooth muscle α-actin (Figure 1, red structures). The gracilis artery was differentiated from the gracilis vein, in which the α-actin stain is much less extensive due to a thinner smooth muscle layer (Figure 1B). The BBI stain allowed the identification of the smooth muscle cell nuclei (overlapped with the α-actin stain, Figure 1B, stars) and the endothelial cell nuclei inside the vessel lumen (Figure 1B, arrows).
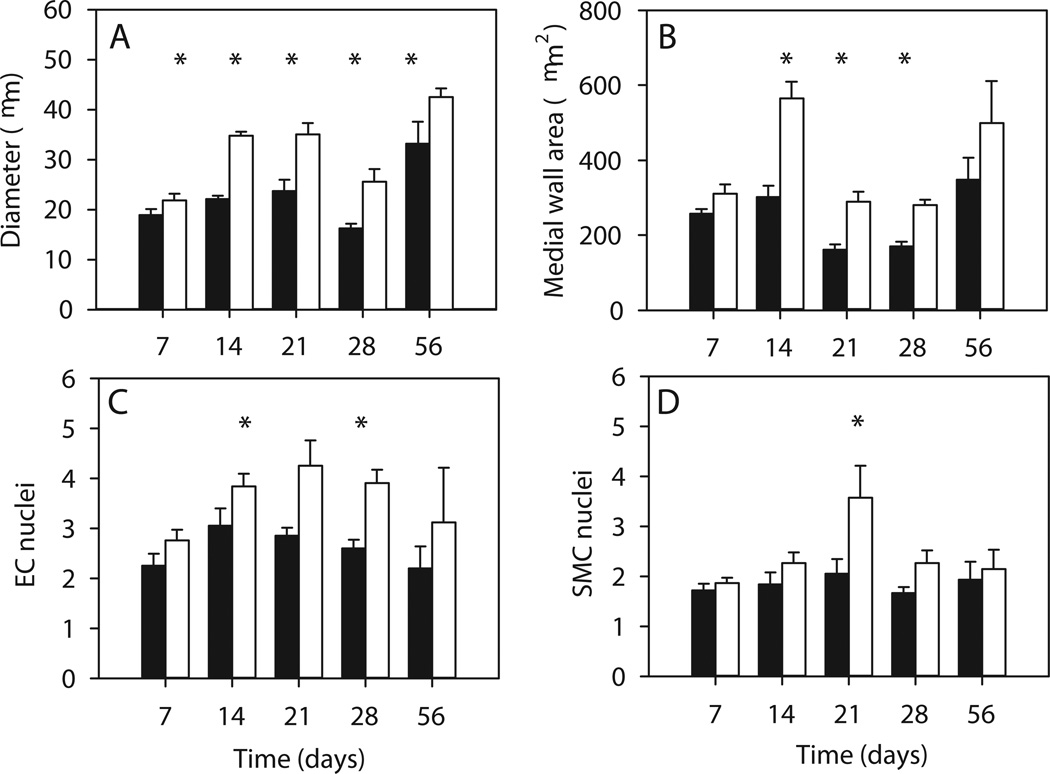
Variations with time after surgery of gracilis artery diameter, medial cross-section area and numbers of EC and SMC nuclei are summarized in Figure 2, averaged over the entire length of the artery for the remodeled and control vessels. The diameter increased overall starting with day 7 (Figure 2A). The medial cross-section area also increased significantly, but with a 7-day delay, and was not significantly different at day 56 when compared to normal values (Figure 2B). The numbers of EC and SMC nuclei generally increased; the change was significant at some time points (Figure 2C and D).
Figure 2.
Measured quantities averaged over the entire length of the artery. Days 7 through 56 denote days after surgery. Solid bars: contralateral normal control. Open bars: remodeled with a portion of the saphenous artery removed. A. Gracilis artery internal diameter. B. Gracilis artery medial cross-section area. C. Number of endothelial cell nuclei (EC). D. Number of smooth muscle cell nuclei (SMC). * indicates significantly different values from the normal contralateral values, p<0.05.
An increase in diameters can be expected following an increase in blood flow in collateral vessel during ischemic revascularization [9, 10, 12, 13, 28]. In the present study, however, removal of one of the two blood supplies of the gracilis artery led to a spatially dependent alteration of blood flow in the artery, with a decrease in flow in the region close to the saphenous artery, a large relative increase of flow in the middle region, and a smaller relative increase in the region close to the muscular branch. Therefore, for the rest of the study we divided the artery into three distinct regions, and examined the relative changes occurring in each region separately. The average baseline values of the morphological and hemodynamic parameters in each region are shown in Table 1 for the control values in the contralateral GAs.
Table 1.
Baseline Parameters*
| Parameter | MB | Middle | SA | Mean |
|---|---|---|---|---|
| Diameter (µm) | 28.5±1.7 | 18.5±1.4 | 21.3±2 | 22.8±1.6 |
| Medial wall area (µm2) | 331.6±26.6 | 179.9±16.1 | 230±24.6 | 247±20.8 |
| EC nuclei | 2.9±0.2 | 2.5±0.2 | 2.4±0.2 | 2.5±0.2 |
| SMC nuclei | 2.1±0.2 | 1.6±0.1 | 1.6±0.1 | 1.7±0.1 |
| GA flow (nl/min) | 202.8±22.6 | 68.4±19.1 | 71.2±11.2 | 119.5±17.6 |
| Wall shear stress (dyn/cm2) | 50.2±4.9 | 46.0±3.0 | 53.6±7.4 | 49.5±4.0 |
| Circumferential stress (105 dyn/cm2) | 6.6±0.8 | 4.6±0.8 | 4.7±0.7 | 5.1±0.7 |
Average of all normal contralateral GA values (n=20) ± SE.
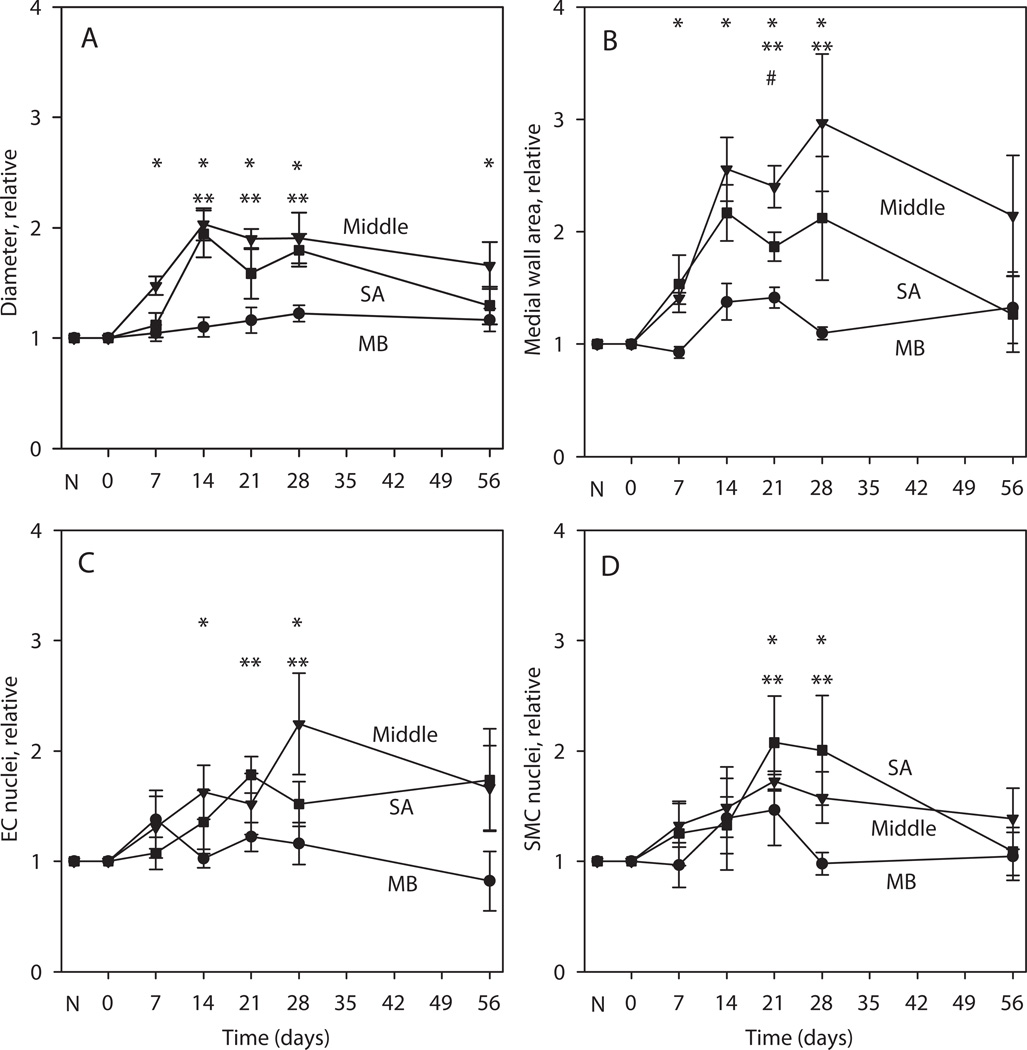
Variations with time after surgery of gracilis artery diameter, medial cross-section area and numbers of EC and SMC nuclei in each of the three regions are summarized in Figure 3. Results are presented as ratios of remodeled/control values to control for processing and biological variation between samples collected at different time points. Vessel diameters were significantly elevated relative to contralateral controls at all time points in the middle region and at days 14, 21 and 28 for the SA region (Figure 3A). Similarly, the results for medial cross-section area (Figure 3B) show a significant increase at days 7 to 28 in the middle and at 14 and 21 days close to the injured end (SA region). A similar increase in the number of EC and SMC nuclei is observed (Figure 3C–D), although at a lower level than diameter and medial area, suggesting that the observed remodeling results from a combination of hyperplasia (increased number of cells) and hypertrophy (increased cell mass). By day 56, all measured parameters return towards control values. In the MB, the changes in these parameters are relatively small and significant only in the case of medial area at day 21.
Figure 3.
Measured quantities as relative values of remodeled/normal values in the MB, middle and SA regions of the gracilis arteries. N denotes condition before surgery. Days 0–56 denote days after surgery. A. Diameter. B. Medial cross-section area. C. EC nuclei. D. SMC nuclei. #, * and ** indicate significantly different values from no change (1.0) in the MB, middle and SA regions respectively.
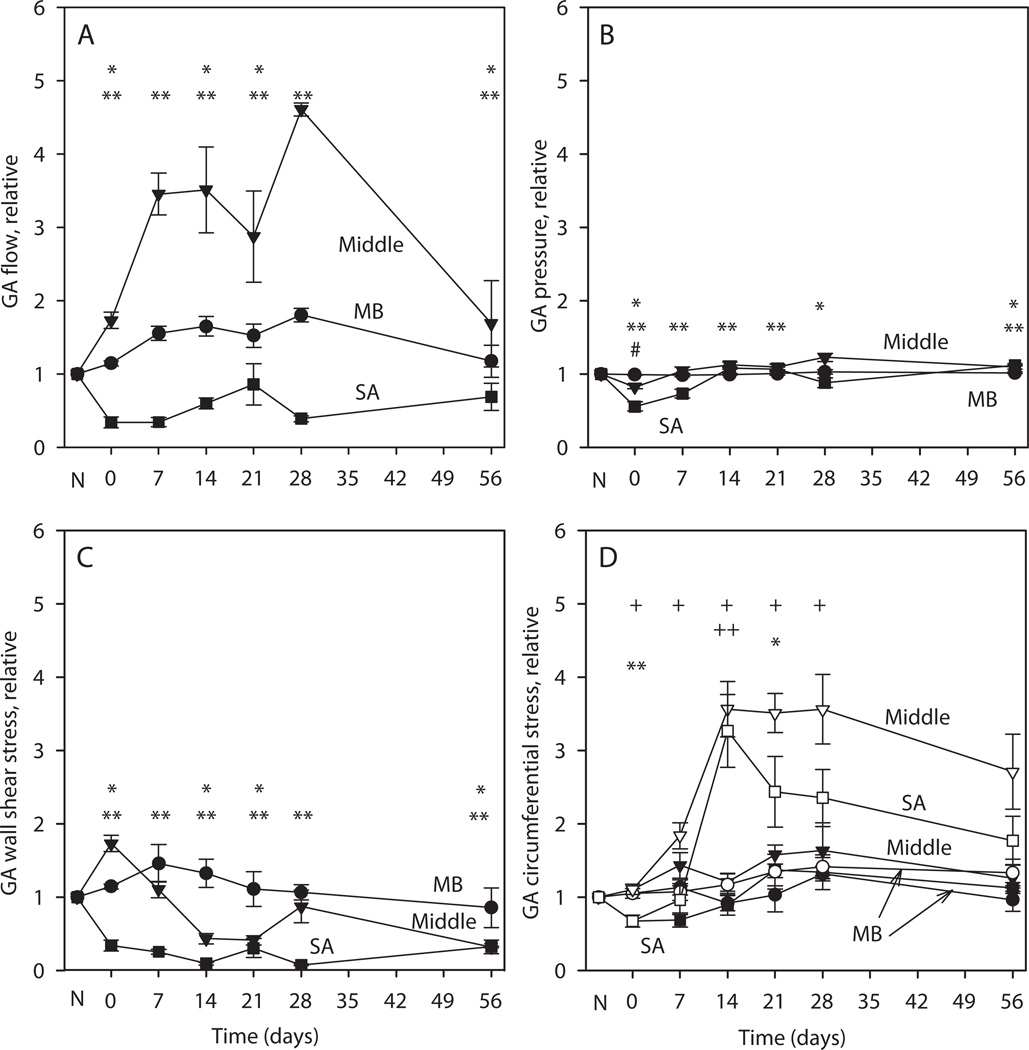
In order to explore the factors contributing to structural adaptation, we examined the spatially dependent changes in blood flow, intravascular pressure, wall shear stress and circumferential stresses occurring during the experimental period. As direct measurement of these values in all segments of the gracilis artery is not experimentally practical, we employed theoretical modeling to estimate these values based on the measured values and published values of gracilis muscle volume blood flow and murine systemic pressure. The results are also expressed as remodeled/control ratios (Figure 4). At day zero, immediately following the removal of the SA blood supply, blood flow almost doubles in the middle region (Figure 4A). At later times, it increases further as a consequence of increasing diameters in the middle region. The flow in the SA region is decreased compared to controls throughout the observation period. After surgery, the pressures in the middle and SA regions decrease (Figure 4B). The subsequent increases in SA and middle region diameters reduce flow resistance and the pressures return close to, although significantly below, control values. Immediately after removal of SA supply, the wall shear stress increases in the middle region due to the increase in flow (Figure 4C). The subsequent increase in diameter causes the wall shear stress to drop below control levels. The wall shear stress in the SA region remains below control.
Figure 4.
Simulated hemodynamic parameters and mechanical stresses on the GA wall. All values are expressed as ratios of remodeled to contralateral control values, averaged over MB, middle and SA regions. N denotes condition before surgery. Days 0–56 denote days after surgery. A. Flow in gracilis artery. B. Pressure in artery, which drives flow in transverse arterioles. Immediately after surgery, the driving pressure in the injury region is reduced. C. Wall shear stress in artery. Immediately after surgery, wall shear stress is elevated in the middle region D. Circumferential wall shear stress. Open symbols: stress that would be generated with no change in medial area. Solid symbols: stress generated including the effect of changes in medial area. In A–D, #, * and ** indicate significantly different values from no change (1.0) in the MB, middle and SA regions respectively, including changes in medial area. In D, + and ++ denote significantly different values from no change (1.0) in the circumferential stress in the middle and SA regions respectively with no change in medial area.
To investigate the role of the wall remodeling in restoring the circumferential stress, we estimated the stress both with and without changes in medial area (Figure 4D). In the latter case (open symbols), the control values for wall area were used in the calculation of circumferential stresses, which are substantially increased throughout the remodeling period in the middle and SA regions. However, when wall area changes are taken into account, the circumferential stresses are maintained close to control levels in all three regions.
Tortuosity did not differ significantly between the control and remodeled vessels. The overall tortuosity coefficient was 1.058±0.001 in the normal and 1.096±0.027 in remodeled arteries. No evidence of reduction in length or compression of the gracilis muscle after removal of the SA was observed.
DISCUSSION
In previous work [8], we examined the spatially and temporally dependent structural changes in the internal diameter of the gracilis artery following the removal of one of its blood supplies. We developed a theoretical model for structural remodeling, in which the diameter of each segment varies in response to stimuli derived from local metabolic and hemodynamic conditions. For this purpose, a previously developed model [17] was extended to include effects of a time-delayed metabolic stimulus in regions of reduced perfusion. This time-delayed response, which may result from a gradual release of cytokines in response to injury, was considered to be responsible for the approximately 14-day delay between surgery and the maximum diameter response, and also for the overshoot and subsequent reduction in vessel diameters. The observed time-dependent diameter changes in all three regions of the artery (MB, middle and SA) following surgery were shown to be consistent with this model. In the MB (intact) region, a slight outward remodeling occurred in response to increased wall shear stress. The middle region experienced increased wall shear stress and increased metabolic signal, both of which stimulate outward remodeling. In the SA region, the wall shear stress decreased, which would tend to cause a decrease in diameter. However, this tendency was overcome, according to the model, by the fact that the transverse arterioles branching from this section of the gracilis artery experienced a severe reduction in flow, leading to hypoxia and inflammation, and generating growth signals that were propagated by upstream conducted responses to the gracilis artery.
Our previous study [8] did not include measurements or analysis of changes in wall thickness. In the present study, we used experimental and theoretical approaches to study the relationship between circumferential stress and changes in the cross-section area of the medial layer during vascular remodeling. To this purpose, the medial cross-section area was measured every 600 µm along the length of the gracilis artery following interruption of one of its two feed arteries. The measurements were made on the same tissue specimens for which changes in gracilis artery diameter were previously measured and reported [8]. Several previous studies have examined the effect of chronic changes in blood flow on the diameter and medial cross-section area of small arteries [5, 16, 25]. In those studies, the vessels examined had baseline diameters of about 400 µm and showed increases of diameter of up to about 35% and increases of medial cross-section area of up to about 45%. In the present study, the baseline diameters of the gracilis artery were much smaller, around 20 µm, and the relative increases in diameter and medial cross-section were much larger, ranging up to 100% in diameter and 200% in medial cross-section. A unique feature of the present study is that temporal variations of diameter and medial area were systematically examined along the entire length of the vessel, allowing observations of different responses in the SA region of the injury caused by the saphenous artery removal, in the middle region which has the smallest initial diameter, and the MB region of the intact blood supply.
Our findings are consistent with the concept that vascular segments are capable of continuous structural remodeling in response to altered conditions [19]. Following removal of a feed to the gracilis artery, the vessel is exposed to an ischemic environment at the injured end and increased blood flow in the middle region, whereas no significant alterations in local environment are expected at the intact end. As a result, the middle and SA regions of the vessel experience outward remodeling and increasing medial area up to day 14, followed by inward remodeling and loss of medial area by day 56.
The observed transient remodeling occurred by a combination of hypertrophy of existing vascular cells and hyperplasia. Proliferation of EC and SMC during remodeling was previously reported [4, 16, 20–22, 24, 25]. The increase in relative numbers of EC nuclei was approximately proportional to vessel circumference, suggesting that the increase in endothelial surface area is achieved mainly by proliferation of EC. We observed generally small increases in the number of SMC nuclei. This may be because the experimental model used here involves removal of a smaller portion of the femoral artery than other reported models, resulting in a less severe perturbation. Similarly, this difference in experimental procedure may be the reason that significant changes in tortuosity, typically seen in other hindlimb ischemia models, were not observed in the present study. Further work, including for instance BrdU staining of proliferating cells, is needed to determine more precisely the contributions of proliferation and hypertrophy to the observed medial remodeling.
To explore the interaction between structural remodeling and hemodynamic stimuli and stresses, we used the experimental morphometric results, published literature values for blood flow, and fluid mechanical equations to estimate levels of gracilis artery flow, pressure, wall shear stress and circumferential stress. The reference value of the blood flow in the transverse arterioles was estimated by dividing a published value for total gracilis muscle volume flow by the total number of transverse arterioles [8]. For the simulation of the remodeled gracilis artery blood flow we used the same level of TA flow. Experimental in vivo measurement of blood flow in individual arteriolar segments, although possible in other vascular beds using for example fluorescent tracers, is not feasible in all segments of the gracilis artery because it dives under the gracilis muscle towards the saphenous artery end [6].
If vessel diameter were to increase without increase in wall area, wall thickness would decrease and wall tension would increase according to the law of Laplace. The average circumferential stress, approximately the quotient of tension and wall thickness, would therefore undergo a relatively large increase, in proportion to D2, of approximately 250% in the middle region (Figure 4D, open symbols). However, due to the observed increase in medial cross-section area (Figure 3D), this increase is strongly attenuated with a maximum value of about 50% increase over control values (Figure 4D, solid symbols). Thus, the observed changes in medial area, occurring in coordination with the changes in vessel diameter, tend to maintain circumferential stress close to its control levels.
Any time lag between the increase in diameter and the increase in medial area would result in a transient increase in circumferential stress at the time of the diameter increase. No such increase was seen (Figure 4D, solid symbols), implying that the increase in medial area is a relatively rapid response to increased circumferential stress, with a time lag of less than 7 days which is the maximum temporal resolution of the experimental data (7 days).
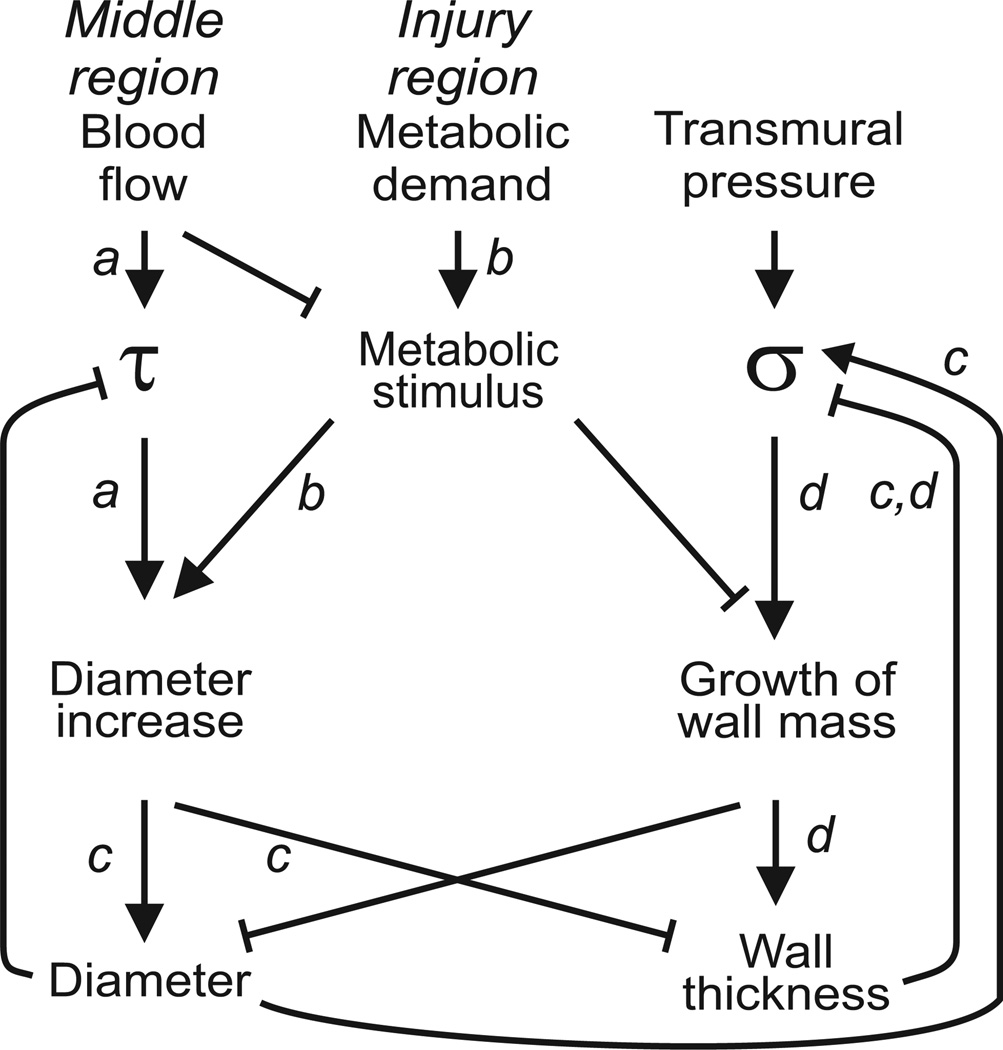
The present results are consistent with the concept that increased circumferential tension, resulting from increased diameter, stimulates wall growth to maintain a constant level of circumferential stress [14, 19, 24]. The observed structural adaptation process can be broken down into several steps, as illustrated schematically in Figure 5: a) the increase in blood flow in the middle region causes an increase in shear stress (τ) in this region, which causes the diameter to increase; b) the increased metabolic demand in the SA region close to the injury causes the release of a metabolic stimulus most likely mediated by release of growth factors and other cytokines which causes an overshoot of diameter increase in the upstream middle region and growth of wall volume as discussed in our earlier study; c) diameter increase causes the wall shear stress to return to normal but also causes an increase in the circumferential stress (σ); d) the increase in circumferential stress causes growth of wall volume which decreases σ.
Figure 5.
Schematic representation of interactions between stimuli driving remodeling and resulting modes of structural response. a: Blood flow increases in the middle region when supply at one end is removed, causing increased wall shear stress (τ) and diameter increase. b: Reduced perfusion pressure in injury region results in decreased TA flow, increased metabolic signal and diameter increase. c: Increased diameter causes reduced wall thickness and increased wall tension. Both factors lead to increased circumferential wall stress (σ) for a given value of transmural pressure. d: Increased circumferential stress drives increase in medial area, which returns circumferential stress toward its original level.
In summary small arteries undergo structural remodeling according to spatially varying stimuli resulting from removal of one blood supply. Changes in diameter and medial area are coordinated so that a relatively constant level of circumferential stress is maintained.
PERSPECTIVES.
This work combines experimental observations and theoretical computations to correlate the changes in vessel diameter and wall thickness with the hemodynamic stresses experienced by remodeling blood vessels. The results corroborate the concept of dynamic adaptation of wall structure of small vessels to hemodynamic and metabolic stimuli and the role of maintenance of circumferential wall stress in these reactions. Such results have implications for vascular remodeling in normal and pathological conditions. The theoretical framework could be used in a translational medicine context for in silico simulation of vascular responses to agents that target vascular remodeling (i.e. anti-angiogenic therapy in cancer).
Acknowledgements
Supported by AHA grant 0010189Z and NIH grants HL034555 and HL063732.
LIST OF ABREVIATIONS
- EC
endothelial cells
- SMC
smooth muscle cells
- BBI
bisbenzimidazole
- GA
gracilis artery
- GV
gracilis vein
- GN
gracilis nerve
- MB
muscular branch of the femoral artery
- SA
saphenous artery
REFERENCES
- 1.Bakker EN, Versluis JP, Sipkema P, VanTeeffelen JW, Rolf TM, Spaan JA, VanBavel E. Differential structural adaptation to haemodynamics along single rat cremaster arterioles. J Physiol. 2003;548(Pt 2):549–555. doi: 10.1113/jphysiol.2002.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bakker EN, Buus CL, VanBavel E, Mulvany MJ. Activation of resistance arteries with endothelin-1: from vasoconstriction to functional adaptation and remodeling. J Vasc Res. 2004;41(2):174–182. doi: 10.1159/000077288. [DOI] [PubMed] [Google Scholar]
- 3.Buschmann I, Schaper W. Arteriogenesis Versus Angiogenesis: Two Mechanisms of Vessel Growth. News Physiol Sci. 1999:14121–14125. doi: 10.1152/physiologyonline.1999.14.3.121. [DOI] [PubMed] [Google Scholar]
- 4.Buschmann I, Heil M, Jost M, Schaper W. Influence of inflammatory cytokines on arteriogenesis. Microcirculation. 2003;10(3–4):371–379. doi: 10.1038/sj.mn.7800199. [DOI] [PubMed] [Google Scholar]
- 5.Buus CL, Pourageaud F, Fazzi GE, Janssen G, Mulvany MJ, De Mey JG. Smooth muscle cell changes during flow-related remodeling of rat mesenteric resistance arteries. Circ Res. 2001;89(2):180–186. doi: 10.1161/hh1401.093575. [DOI] [PubMed] [Google Scholar]
- 6.Cardinal TR, Hoying JB. A modified fluorescent microsphere-based approach for determining resting and hyperemic blood flows in individual murine skeletal muscles. Vascul Pharmacol. 2007;47(1):48–56. doi: 10.1016/j.vph.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gruionu G, Hoying JB, Gruionu LG, Laughlin MH, Secomb TW. Structural adaptation increases predicted perfusion capacity after vessel obstruction in arteriolar arcade network of pig skeletal muscle. Am J Physiol Heart Circ Physiol. 2005;288(6):H2778–H2784. doi: 10.1152/ajpheart.00917.2004. [DOI] [PubMed] [Google Scholar]
- 8.Gruionu G, Hoying JB, Pries AR, Secomb TW. Structural remodeling of mouse gracilis artery after chronic alteration in blood supply. Am J Physiol Heart Circ Physiol. 2005;288(5):H2047–H2054. doi: 10.1152/ajpheart.00496.2004. [DOI] [PubMed] [Google Scholar]
- 9.Heil M, Ziegelhoeffer T, Wagner S, Fernandez B, Helisch A, Martin S, Tribulova S, Kuziel WA, Bachmann G, Schaper W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ Res. 2004;94(5):671–677. doi: 10.1161/01.RES.0000122041.73808.B5. [DOI] [PubMed] [Google Scholar]
- 10.Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10(1):83–97. doi: 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- 11.Langille BL, Bendeck MP. Arterial responses to compromised blood flow. Toxicol Pathol. 1990;18(4 Pt 1):618–622. [PubMed] [Google Scholar]
- 12.Limbourg A, Korff T, Napp LC, Schaper W, Drexler H, Limbourg FP. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat Protoc. 2009;4(12):1737–1746. doi: 10.1038/nprot.2009.185. [DOI] [PubMed] [Google Scholar]
- 13.Luo MY, Yang BL, Ye F, Wu X, Peng S, Yi B, Wang W, Zhu W, Luo H, Wen JG, Cai WJ, Schaper J, Schaper W. Collateral vessel growth induced by femoral artery ligature is impaired by denervation. Mol Cell Biochem. 2011;354(1–2):219–229. doi: 10.1007/s11010-011-0821-6. [DOI] [PubMed] [Google Scholar]
- 14.Mulvany MJ. Small artery remodeling and significance in the development of hypertension. News Physiol Sci. 2002:17105–17109. doi: 10.1152/nips.01366.2001. [DOI] [PubMed] [Google Scholar]
- 15.Pistea A, Bakker EN, Spaan JA, VanBavel E. Flow inhibits inward remodeling in cannulated porcine small coronary arteries. Am J Physiol Heart Circ Physiol. 2005;289(6):H2632–H2640. doi: 10.1152/ajpheart.00205.2005. [DOI] [PubMed] [Google Scholar]
- 16.Pourageaud F, De Mey JG. Structural properties of rat mesenteric small arteries after 4-wk exposure to elevated or reduced blood flow. Am J Physiol. 1997;273(4 Pt 2):H1699–H1706. doi: 10.1152/ajpheart.1997.273.4.H1699. [DOI] [PubMed] [Google Scholar]
- 17.Pries AR, Secomb TW, Gaehtgens P. Structural adaptation and stability of microvascular networks: theory and simulations. Am J Physiol. 1998;275(2 Pt 2):H349–H360. doi: 10.1152/ajpheart.1998.275.2.H349. [DOI] [PubMed] [Google Scholar]
- 18.Pries AR, Reglin B, Secomb TW. Structural adaptation of microvascular networks: functional roles of adaptive responses. Am J Physiol Heart Circ Physiol. 2001;281(3):H1015–H1025. doi: 10.1152/ajpheart.2001.281.3.H1015. [DOI] [PubMed] [Google Scholar]
- 19.Pries AR, Reglin B, Secomb TW. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension. 2005;46(4):725–731. doi: 10.1161/01.HYP.0000184428.16429.be. [DOI] [PubMed] [Google Scholar]
- 20.Schaper W, Scholz D. Factors regulating arteriogenesis. Arterioscler Thromb Vasc Biol. 2003;23(7):1143–1151. doi: 10.1161/01.ATV.0000069625.11230.96. [DOI] [PubMed] [Google Scholar]
- 21.Scholz D, Ziegelhoeffer T, Helisch A, Wagner S, Friedrich C, Podzuweit T, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. J Mol Cell Cardiol. 2002;34(7):775–787. doi: 10.1006/jmcc.2002.2013. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan CJ, Doetschman T, Hoying JB. Targeted disruption of the Fgf2 gene does not affect vascular growth in the mouse ischemic hindlimb. J Appl Physiol. 2002;93(6):2009–2017. doi: 10.1152/japplphysiol.00451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sullivan CJ, Hoying JB. Flow-dependent remodeling in the carotid artery of fibroblast growth factor-2 knockout mice. Arterioscler Thromb Vasc Biol. 2002;22(7):1100–1105. doi: 10.1161/01.atv.0000023230.17493.e3. [DOI] [PubMed] [Google Scholar]
- 24.Tulis DA, Unthank JL, Prewitt RL. Flow-induced arterial remodeling in rat mesenteric vasculature. Am J Physiol. 1998;274(3 Pt 2):H874–H882. doi: 10.1152/ajpheart.1998.274.3.H874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuttle JL, Nachreiner RD, Bhuller AS, Condict KW, Connors BA, Herring BP, Dalsing MC, Unthank JL. Shear level influences resistance artery remodeling: wall dimensions, cell density, and eNOS expression. Am J Physiol Heart Circ Physiol. 2001;281(3):H1380–H1389. doi: 10.1152/ajpheart.2001.281.3.H1380. [DOI] [PubMed] [Google Scholar]
- 26.Unthank JL, Nixon JC, Burkhart HM, Fath SW, Dalsing MC. Early collateral and microvascular adaptations to intestinal artery occlusion in rat. Am J Physiol. 1996;271(3 Pt 2):H914–H923. doi: 10.1152/ajpheart.1996.271.3.H914. [DOI] [PubMed] [Google Scholar]
- 27.Weibel E. Stereological Methods. New York: Academic Press; 1979. [Google Scholar]
- 28.Ziegler MA, Distasi MR, Bills RG, Miller SJ, Alloosh M, Murphy MP, George Akingba A, Sturek M, Dalsing MC, Unthank JL. Marvels, mysteries, and misconceptions of vascular compensation to peripheral artery occlusion. Microcirculation. 2010;17(1):3–20. doi: 10.1111/j.1549-8719.2010.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]